Abstract
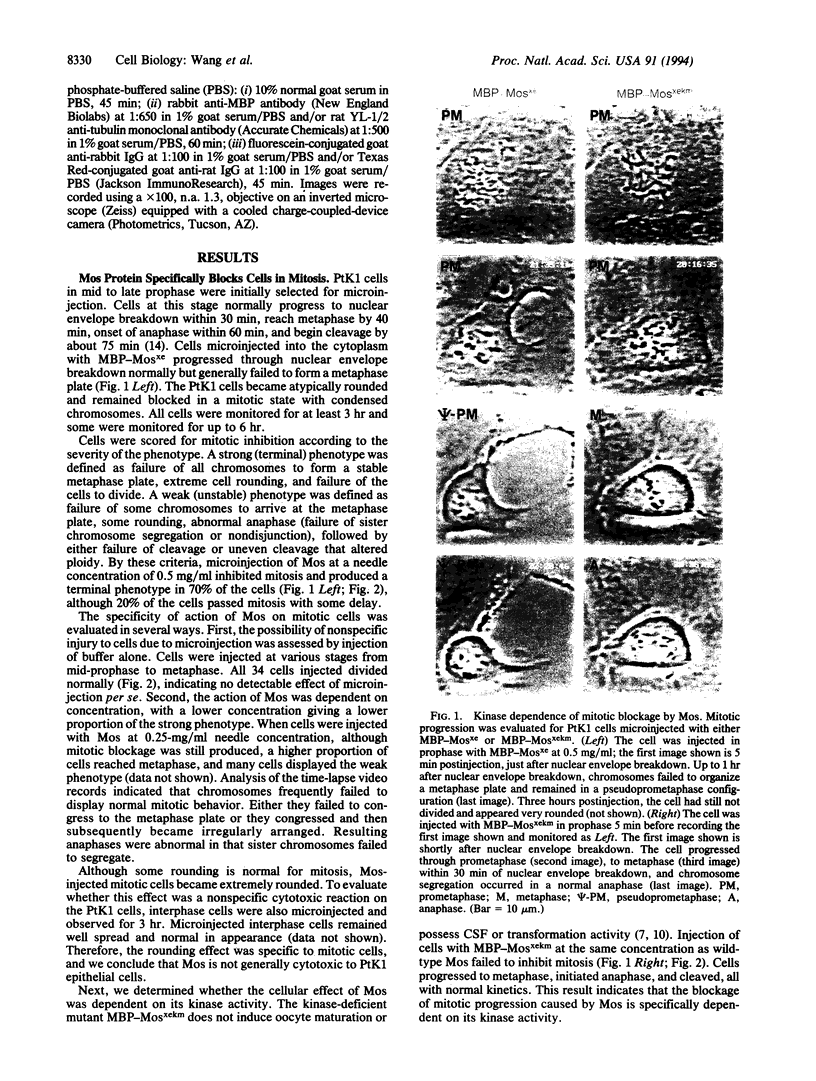
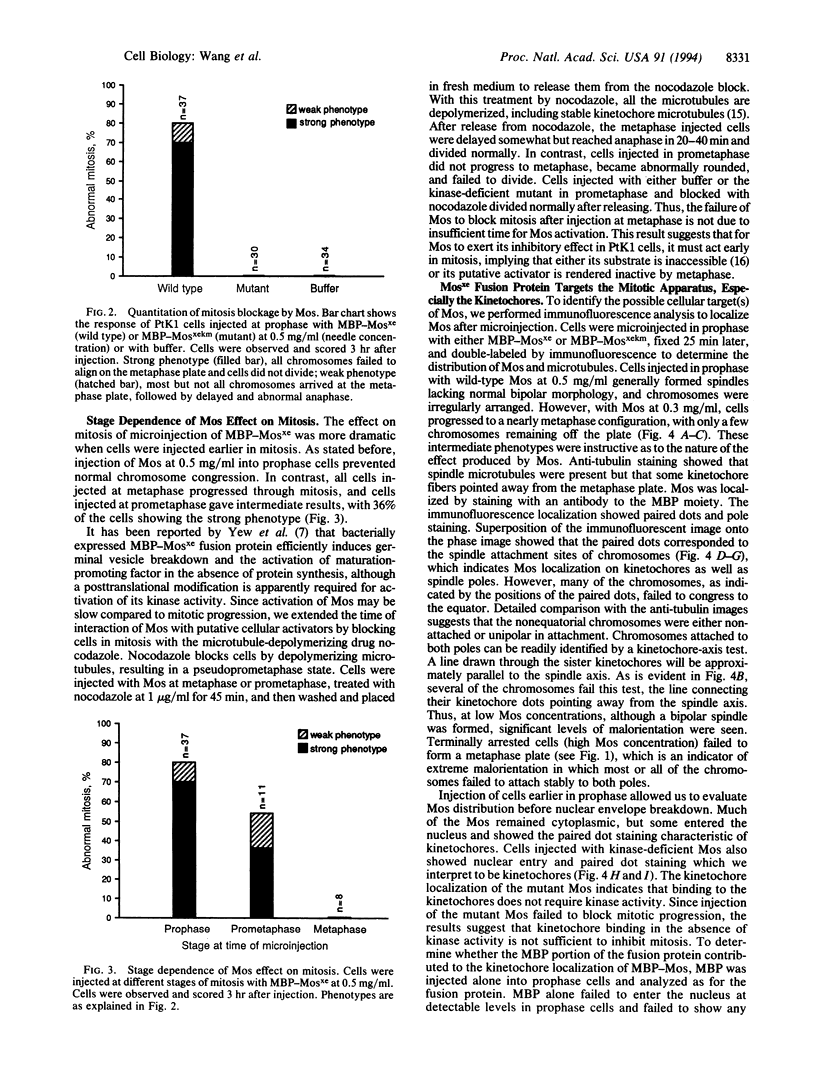
The mos protooncogene has opposing effects on cell cycle progression. It is required for reinitiation of meiotic maturation and for meiotic progression through metaphase II, yet it is an active component of cytostatic factor. mos is a potent oncogene in fibroblasts, but high levels of expression are lethal. The lethality of mos gene expression in mammalian cells could be a consequence of a blockage induced by its cytostatic factor-related activity, which may appear at high dosage in mitotic cells. We have directly tested whether expression of the Mos protein can block mitosis in mammalian cells by microinjecting a fusion protein between Escherichia coli maltose-binding protein and Xenopus c-Mos into PtK1 epithelial cells and analyzing the cells by video time-lapse and immunofluorescence microscopy. Time-course analyses showed that Mos blocked mitosis by preventing progression to a normal metaphase. Chromosomes frequently failed to attain a bipolar orientation and were found near one pole. Injection of a kinase-deficient mutant Mos had no effect on mitosis, indicating that the blockage of mitotic progression required Mos kinase activity. Antitubulin immunostaining of cells blocked by Mos showed that microtubules were present but that spindle morphology was abnormal. Immunostaining for the Mos fusion protein showed that both wild-type and kinase mutant proteins localized at the kinetochores. Our results suggest that mitotic blockage by Mos may result from an action of the Mos kinase on the kinetochores, thus increasing chromosome instability and preventing normal congression.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Fischinger P. J., Haapala D. K. Quantitative interactions of feline leukaemia virus and its pseudotype of murine sarcoma virus in cat cells: requirement for DNA synthesis. J Gen Virol. 1971 Nov;13(2):203–214. doi: 10.1099/0022-1317-13-2-203. [DOI] [PubMed] [Google Scholar]
- Freeman R. S., Pickham K. M., Kanki J. P., Lee B. A., Pena S. V., Donoghue D. J. Xenopus homolog of the mos protooncogene transforms mammalian fibroblasts and induces maturation of Xenopus oocytes. Proc Natl Acad Sci U S A. 1989 Aug;86(15):5805–5809. doi: 10.1073/pnas.86.15.5805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorbsky G. J., Borisy G. G. Microtubules of the kinetochore fiber turn over in metaphase but not in anaphase. J Cell Biol. 1989 Aug;109(2):653–662. doi: 10.1083/jcb.109.2.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorbsky G. J., Ricketts W. A. Differential expression of a phosphoepitope at the kinetochores of moving chromosomes. J Cell Biol. 1993 Sep;122(6):1311–1321. doi: 10.1083/jcb.122.6.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haccard O., Sarcevic B., Lewellyn A., Hartley R., Roy L., Izumi T., Erikson E., Maller J. L. Induction of metaphase arrest in cleaving Xenopus embryos by MAP kinase. Science. 1993 Nov 19;262(5137):1262–1265. doi: 10.1126/science.8235656. [DOI] [PubMed] [Google Scholar]
- Hyman A. A., Mitchison T. J. Two different microtubule-based motor activities with opposite polarities in kinetochores. Nature. 1991 May 16;351(6323):206–211. doi: 10.1038/351206a0. [DOI] [PubMed] [Google Scholar]
- Masui Y., Markert C. L. Cytoplasmic control of nuclear behavior during meiotic maturation of frog oocytes. J Exp Zool. 1971 Jun;177(2):129–145. doi: 10.1002/jez.1401770202. [DOI] [PubMed] [Google Scholar]
- Mitchison T. J. Polewards microtubule flux in the mitotic spindle: evidence from photoactivation of fluorescence. J Cell Biol. 1989 Aug;109(2):637–652. doi: 10.1083/jcb.109.2.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchison T., Evans L., Schulze E., Kirschner M. Sites of microtubule assembly and disassembly in the mitotic spindle. Cell. 1986 May 23;45(4):515–527. doi: 10.1016/0092-8674(86)90283-7. [DOI] [PubMed] [Google Scholar]
- Nicklas R. B. Mitosis. Adv Cell Biol. 1971;2:225–297. doi: 10.1007/978-1-4615-9588-5_5. [DOI] [PubMed] [Google Scholar]
- Papkoff J., Verma I. M., Hunter T. Detection of a transforming gene product in cells transformed by Moloney murine sarcoma virus. Cell. 1982 Jun;29(2):417–426. doi: 10.1016/0092-8674(82)90158-1. [DOI] [PubMed] [Google Scholar]
- Pfarr C. M., Coue M., Grissom P. M., Hays T. S., Porter M. E., McIntosh J. R. Cytoplasmic dynein is localized to kinetochores during mitosis. Nature. 1990 May 17;345(6272):263–265. doi: 10.1038/345263a0. [DOI] [PubMed] [Google Scholar]
- Posada J., Yew N., Ahn N. G., Vande Woude G. F., Cooper J. A. Mos stimulates MAP kinase in Xenopus oocytes and activates a MAP kinase kinase in vitro. Mol Cell Biol. 1993 Apr;13(4):2546–2553. doi: 10.1128/mcb.13.4.2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rattner J. B., Lew J., Wang J. H. p34cdc2 kinase is localized to distinct domains within the mitotic apparatus. Cell Motil Cytoskeleton. 1990;17(3):227–235. doi: 10.1002/cm.970170309. [DOI] [PubMed] [Google Scholar]
- Roy L. M., Singh B., Gautier J., Arlinghaus R. B., Nordeen S. K., Maller J. L. The cyclin B2 component of MPF is a substrate for the c-mos(xe) proto-oncogene product. Cell. 1990 Jun 1;61(5):825–831. doi: 10.1016/0092-8674(90)90192-h. [DOI] [PubMed] [Google Scholar]
- Sagata N., Daar I., Oskarsson M., Showalter S. D., Vande Woude G. F. The product of the mos proto-oncogene as a candidate "initiator" for oocyte maturation. Science. 1989 Aug 11;245(4918):643–646. doi: 10.1126/science.2474853. [DOI] [PubMed] [Google Scholar]
- Sagata N., Oskarsson M., Copeland T., Brumbaugh J., Vande Woude G. F. Function of c-mos proto-oncogene product in meiotic maturation in Xenopus oocytes. Nature. 1988 Oct 6;335(6190):519–525. doi: 10.1038/335519a0. [DOI] [PubMed] [Google Scholar]
- Salmon E. D., Leslie R. J., Saxton W. M., Karow M. L., McIntosh J. R. Spindle microtubule dynamics in sea urchin embryos: analysis using a fluorescein-labeled tubulin and measurements of fluorescence redistribution after laser photobleaching. J Cell Biol. 1984 Dec;99(6):2165–2174. doi: 10.1083/jcb.99.6.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawin K. E., Mitchison T. J., Wordeman L. G. Evidence for kinesin-related proteins in the mitotic apparatus using peptide antibodies. J Cell Sci. 1992 Feb;101(Pt 2):303–313. doi: 10.1242/jcs.101.2.303. [DOI] [PubMed] [Google Scholar]
- Simerly C., Balczon R., Brinkley B. R., Schatten G. Microinjected centromere [corrected] kinetochore antibodies interfere with chromosome movement in meiotic and mitotic mouse oocytes. J Cell Biol. 1990 Oct;111(4):1491–1504. doi: 10.1083/jcb.111.4.1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh B., Hannink M., Donoghue D. J., Arlinghaus R. B. p37mos-associated serine/threonine protein kinase activity correlates with the cellular transformation function of v-mos. J Virol. 1986 Dec;60(3):1148–1152. doi: 10.1128/jvi.60.3.1148-1152.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steuer E. R., Wordeman L., Schroer T. A., Sheetz M. P. Localization of cytoplasmic dynein to mitotic spindles and kinetochores. Nature. 1990 May 17;345(6272):266–268. doi: 10.1038/345266a0. [DOI] [PubMed] [Google Scholar]
- Vandenbunder B., Borisy G. G. Decoration of microtubules by fluorescently labeled microtubule-associated protein 2 (MAP2) does not interfere with their spatial organization and progress through mitosis in living fibroblasts. Cell Motil Cytoskeleton. 1986;6(6):570–579. doi: 10.1002/cm.970060605. [DOI] [PubMed] [Google Scholar]
- Vandré D. D., Borisy G. G. Anaphase onset and dephosphorylation of mitotic phosphoproteins occur concomitantly. J Cell Sci. 1989 Oct;94(Pt 2):245–258. doi: 10.1242/jcs.94.2.245. [DOI] [PubMed] [Google Scholar]
- Verlhac M. H., de Pennart H., Maro B., Cobb M. H., Clarke H. J. MAP kinase becomes stably activated at metaphase and is associated with microtubule-organizing centers during meiotic maturation of mouse oocytes. Dev Biol. 1993 Aug;158(2):330–340. doi: 10.1006/dbio.1993.1192. [DOI] [PubMed] [Google Scholar]
- Watanabe N., Vande Woude G. F., Ikawa Y., Sagata N. Specific proteolysis of the c-mos proto-oncogene product by calpain on fertilization of Xenopus eggs. Nature. 1989 Nov 30;342(6249):505–511. doi: 10.1038/342505a0. [DOI] [PubMed] [Google Scholar]
- Yen T. J., Li G., Schaar B. T., Szilak I., Cleveland D. W. CENP-E is a putative kinetochore motor that accumulates just before mitosis. Nature. 1992 Oct 8;359(6395):536–539. doi: 10.1038/359536a0. [DOI] [PubMed] [Google Scholar]
- Yew N., Mellini M. L., Vande Woude G. F. Meiotic initiation by the mos protein in Xenopus. Nature. 1992 Feb 13;355(6361):649–652. doi: 10.1038/355649a0. [DOI] [PubMed] [Google Scholar]
- Yew N., Oskarsson M., Daar I., Blair D. G., Vande Woude G. F. mos gene transforming efficiencies correlate with oocyte maturation and cytostatic factor activities. Mol Cell Biol. 1991 Feb;11(2):604–610. doi: 10.1128/mcb.11.2.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yew N., Strobel M., Vande Woude G. F. Mos and the cell cycle: the molecular basis of the transformed phenotype. Curr Opin Genet Dev. 1993 Feb;3(1):19–25. doi: 10.1016/s0959-437x(05)80336-3. [DOI] [PubMed] [Google Scholar]
- Zhou R. P., Oskarsson M., Paules R. S., Schulz N., Cleveland D., Vande Woude G. F. Ability of the c-mos product to associate with and phosphorylate tubulin. Science. 1991 Feb 8;251(4994):671–675. doi: 10.1126/science.1825142. [DOI] [PubMed] [Google Scholar]
- Zhou R., Daar I., Ferris D. K., White G., Paules R. S., Vande Woude G. pp39mos is associated with p34cdc2 kinase in c-mosxe-transformed NIH 3T3 cells. Mol Cell Biol. 1992 Aug;12(8):3583–3589. doi: 10.1128/mcb.12.8.3583. [DOI] [PMC free article] [PubMed] [Google Scholar]