Abstract
The aging population is increasing dramatically. Aging–associated stress simultaneously drives proinflammatory remodeling, involving angiotensin II and other factors, in both the heart and large arteries. The structural remodeling and functional changes that occur with aging include cardiac and vascular wall stiffening, systolic hypertension and suboptimal ventricular-arterial coupling, features that are often clinically silent and thus termed a silent syndrome. These age-related effects are the result of responses initiated by cardiovascular proinflammatory cells. Local proinflammatory signals are coupled between the heart and arteries due to common mechanical and humoral messengers within a closed circulating system. Thus, targeting proinflammatory signaling molecules would be a promising approach to improve age-associated suboptimal ventricular-arterial coupling, a major predisposing factor for the pathogenesis of clinical cardiovascular events such as heart failure.
Keywords: Aging, Proinflammation, Cardiovascular Remodeling, Ventricular-Arterial Coupling
Introduction
The world population is aging. Aging is a major risk factor for cardiovascular diseases such as hypertension, atherosclerosis and heart failure, due mainly to the increase in allostatic load and subsequent pro-inflammatory processes within the cardiovascular system [1, 2]. Allostatic load represents the consequence of cumulative exposure to physiologic, socioeconomic and psychological stressors across the life span, resulting in heightened neuroendocrine and neural responses, including increase in sympathetic nerve activity, which ultimately have detrimental effects [1, 2]. Augmented angiotensin II (Ang II) signaling appears to be especially important and interestingly, blockade of Ang II signaling alleviates allostatic load and ameliorates inflammatory stress and the incidence of cardiovascular disease in animal models [3, 4].
The steep rise in the incidence of hypertension, atherosclerosis, and chronic heart failure with advancing age is related at least in part to a continuum of adverse structural remodeling and functional changes in the cardiovascular system [4]. These include an increase in stiffening of the heart and vessels, reduced coronary and peripheral blood flow reserve, blood pressure lability, endothelial dysfunction, cardiac hypertrophy, and suboptimal mechanical coupling between the left ventricle and the arterial system (ventricular-arterial coupling) [5–8]. These functional and structural changes are the result of a cellular and extracellular pro-inflammatory phenotypic shift in both the heart and large arteries [4, 9], which lowers the threshold for pathologic stimuli and increases the propensity for cardiovascular disease (Figure 1) [4, 7, 8]. Thus, targeting age-associated pro-inflammatory signaling may be an evidence-based approach to tackle the epidemic of cardiovascular disease in the elderly.
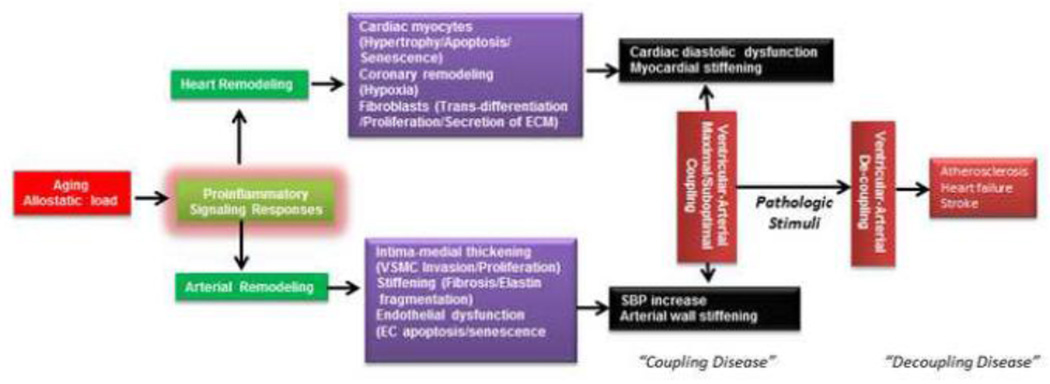
Figure 1. Impact of proinflammatory signaling on heart and arterial phenotype and ventricular-arterial coupling with advancing age.
Aging increases allostatic load, including Ang II signaling, leading to proinflammation. With aging, adverse remodeling develops in both the heart and large arteries, which is the underlying mechanism of abnormal arterial-ventricular coupling. A mismatched or suboptimal arterial-ventricular mechanical coupling, sometimes known as “coupling disease”, is manifest by increases in SBP and left ventricular diastolic dysfunction. Suboptimal arterial-ventricular coupling increases the susceptibility to complications such as heart failure, atherosclerosis and stroke (sometimes termed “de-coupling diseases”), in response to pathologic stimuli such as ischemia.
The cardiovascular functional phenotype with advancing age
Adverse remodeling with aging contributes to a constellation of the related signs of a cardiovascular silent syndrome which occurs in the aging population, including increased systolic blood pressure (SBP), increased pulse wave velocity (PWV), endothelial dysfunction, and cardiac diastolic dysfunction [4]. Cross-sectional measures of SBP show a continuous rise until the eighth or ninth decade, whereas diastolic blood pressure (DBP) increases until the fifth decade, after which it plateaus or decreases [10]. Consequently, pulse pressure continually increases while mean arterial pressure initially increases and ultimately levels off with advancing age [10]. The increase in SBP with increasing age is accompanied by an increase in longitudinal PWV [10, 11]. Carotid-femoral PWV has emerged as a “gold-standard” for the non-invasive measurement of large arterial stiffness. With aging, the left ventricular (LV) wall becomes stiffened with a decrease in compliance; these changes result in significant diastolic LV dysfunction comprising a prolongation of isovolumic relaxation time, and increase in LV end-diastolic pressure and an enlargement of the left atrium but generally no decline in systolic function as assessed by ejection fraction (EF)[5–9, 12, 13].
As well as the stiffening of the heart and the arterial system, the mechanical coupling between the left ventricle and the systemic circulation typically becomes impaired [5–8, 13]. Ventricular-arterial coupling is quantified by the ratio of E(a)/E(lv), an index of the ratio of effective arterial elastance (the net arterial load exerted on the left ventricle) to left ventricle end-systolic elastance (a load–independent measure of left ventricle chamber performance) [5–8, 13]. The ventricular-arterial coupling is a central determinant of cardiovascular mechanical performance and cardiac energetics, and suboptimal ventricular-arterial coupling is a major contributor to cardiovascular dysfunction with aging (systolic hypertension and ventricular diastolic dysfunction) [5–8, 13]. Abnormal ventricular-arterial coupling also greatly increase the propensity to develop cardiovascular morbidities such as heart failure and stroke under pathological stimuli such as ischemia, known as de-coupling diseases (Figure 1) [7, 8].
Proinflammatory signaling in the cardiovascular system with aging
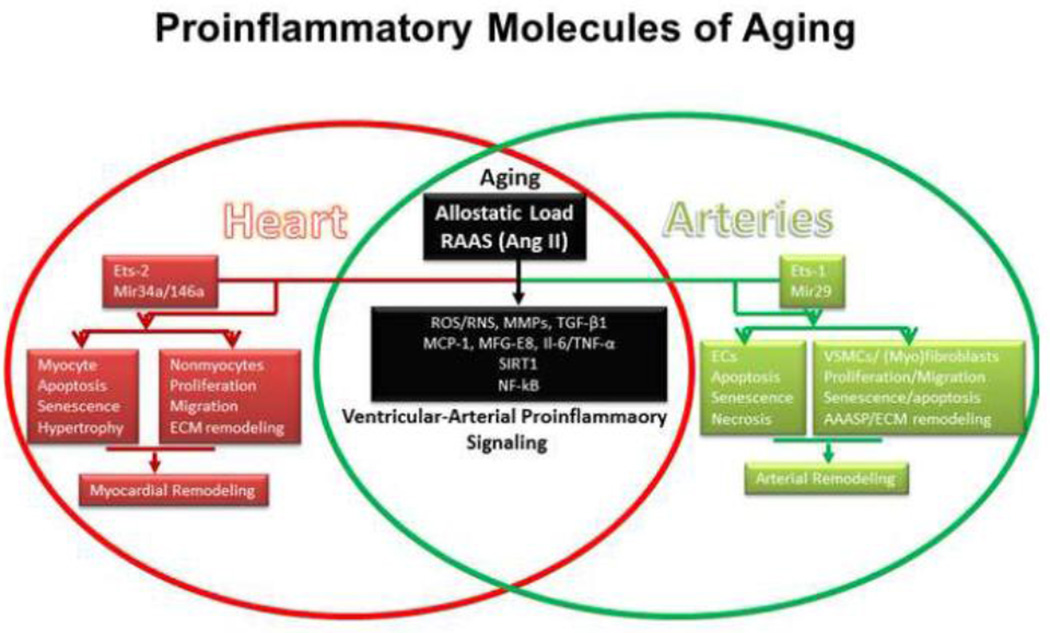
A growing body of evidence indicates that proinflammatory signaling is a key factor driving remodeling in both the heart and large arteries with advancing age. Allostatic–associated proinflammatory signaling molecules, in particular Ang II, are upregulated whereas anti-inflammatory molecules are down-regulated in both the heart and large arteries (Figure 2). In aging-associated proinflammation, aged cardiovascular cells (including endothelial cells, smooth muscle cells and fibroblasts) under stimulation by factors such as Ang II play a key role as opposed to conventional immunological cells such as leukocytes per se [4, 10]. Broadly similar inflammatory and cellular phenotypic changes occur in the heart and the arterial system and from a histologic and conceptual point of view, the heart could be regarded as “a specialized artery” within a closed system. Ang II-associated proinflammatory responses in the myocardium and arterial wall with advancing age appear to be a key molecular mechanism underlying the age-associated cardiovascular cellular phenotypic shift and consequent abnormal ventricular-arterial coupling [4, 9, 10, 13]. Indeed, preliminary studies indicate that blockade of Ang II signaling with angiotensin converting enzyme inhibitors or angiotensin II receptor antagonists improves ventricular-arterial coupling and clinical signs of heart failure[9]. These findings from intervention studies support the concept that aging-associated proinflammation is a fundamental molecular mechanism underlying suboptimal ventricular-arterial coupling and cardiovascular dysfunction.
Figure 2. Proinflammatory signaling mechanisms and effect of cellular phenotype during aging.
Abbreviations and acronyms: Ang II: angiotensin II; Ets-1/2: the v-ets erythroblastosis virus E26 oncogene homolog 1; MMPs: matrix metalloproteases; MFG-E8: milk fat globule epidermal growth factor-8; MCP-1: monocyte chemo-attractant protein-1; NO: nitric oxide; RAAS: renin angiotensin aldosterone system; SIRT1: silent information regulation 2 homolog 1; TGF-β1: transforming growth factor β1; TNF-α: tumor necrosis factor alpha; IL; interleukin; NF-κB: nuclear factor kappa-light-chain-enhancer of activated B cells. ECs: endothelial cells; VSMCs: vascular smooth muscle cells; ECM: extracellular matrix
In the next sections, we review some of the key signaling pathways that are involved in proinflammatory signaling with cardiovascular aging, with a particular focus on Ang II, and also consider the impact on cellular phenotype.
Renin angiotensin system
Components of the renin angiotensin aldosterone system (RAAS) are key elements contributing to aging-associated proinflammation.
In large arteries
The transcription, translation and activity of angiotensin converting enzyme-1 (ACE-1) markedly increase within the aged arterial wall in rodents, nonhuman primates and humans [14–16]. An alternative angiotensin convertase, chymase, also increases within the arterial wall with aging [16]. As expected, levels of the cleavage product, Ang II protein, become markedly increased in rats, nonhuman primates and humans [15–19]. The Ang II AT1 receptor is up-regulated within the old arterial wall [15]. Expression of the mineralocorticoid receptor (MR), a putative receptor of the Ang II downstream target aldosterone (Aldo), is also increased in the aged arterial wall [20, 21]. Aging increases the sensitivity of MR to Aldo, promoting a proinflammatory phenotype [20]. Thus, the activation of the RAAS contributes to proinflammatory remodeling within the arterial wall with aging. Indeed, a chronic administration of Ang II to young rats via minipump produces age-associated arterial remodeling such as wall thickening, fibrosis, and inflammation [17]. Conversely, chronic ACE-1 inhibition or AT1 receptor blockade markedly inhibits the expression of proinflammatory molecules and delays the progression of age-associated aortic remodeling [14, 22]. Furthermore, long-term AT1 blockade improves endothelial function and doubles the lifespan of hypertensive rats, rendering them similar to normotensives [23]. Disruption of the AT1 receptor also retards arterial inflammation and promotes longevity [24].
In the heart
Increased activation of the RAAS cascade is also a prominent feature of age-related cardiac remodeling [25, 26]. Levels of angiotensinogen, ACE-1, AT1, and Aldo are markedly increased in the myocardium in rats with aging [21]. The expression of AT2 receptors is also up-regulated in aging hearts. Notably, AT2 signaling inhibits the signal transduction of AT1 in rat hearts with ageing [27]. Importantly, an infusion of Ang II to young rats generates age-associated cardiac remodeling such as myocyte hypertrophy, fibrosis, and up-regulation of AT1, Aldo, and transforming growth factor beta 1 (TGF-β1)[21]. Further study shows that the down-regulation of the anti-senescence marker protein 30 (SMP30) markedly increases Ang II production from cardiac myocytes [26]; and exacerbates Ang II-induced cardiac hypertrophy, dysfunction and remodeling, and deregulates coronary vascular tone via AT1 signaling with aging [25]. In addition, angiotensin converting enzyme 2 (ACE2) and its product Ang 1–7 function as negative regulators of RAAS signaling with aging. Indeed, ACE2 (−/y) mutant mice develop a progressive age-dependent inflammatory cardiac remodeling via Ang II signalling [28]. The AT1 blocker irbesartan completely prevents adverse remodeling, further confirming a critical role of Ang II-mediated stimulation of AT1 receptors with aging [28]. Thus, a shift in the balance of RAAS towards the ACE2/Ang-(1–7)/Mas receptor axis protects heart function, retards aging-associated proinflammatory remodeling, and prevents the incidence of cardiovascular diseases displayed by this animal model of longevity [29]. In addition, mice received the ACE inhibitor enalapril in their drinking water after weaning significantly resist the age-associated increases in cardiomyocyte apoptosis, mitochondrial reactive oxygen species (ROS), and collagen deposition [30, 31].
These findings suggest that Ang II signaling increases in both the heart and the arteries drives proinflammation and ventricular-arterial remodeling with aging. Thus, control of Ang II signaling may be an important strategy to maintain cardiovascular health with aging.
Reactive oxygen/nitrogen species and nitric oxide signaling
The nicotinamide adenine dinucleotide phosphate (NADPH) oxidase family of proteins (or Nox proteins) are activated and/or upregulated by Ang II signaling in the cardiovascular system with aging [32]. Of the 4 Nox family isoforms that are expressed in the cardiovascular system, Nox1, Nox2 and Nox5 are activated by Ang II and generate the reactive oxygen species (ROS), superoxide and hydrogen peroxide while Nox4 abundance is increased by Ang II. Nox-derived ROS can induce further ROS production by other cellular sources such as mitochondria, as well as have direct effects on intracellular signaling pathways. Superoxide generated by Nox proteins may react in a diffusion-limited manner with the intracellular and intercellular messenger nitric oxide (NO), resulting in loss of physiological NO activities (such as vasodilatation) as well as the formation of peroxynitrite (ONOO-), a powerful reactive nitrogen species (RNS). The latter species may have powerful oxidant activity and alter signaling pathways that play an important role in age-associated cardiovascular remodeling (Figure 2). Furthermore, Nox-derived ROS may also contribute to the uncoupling of NO synthase enzymes so that they generate superoxide instead of NO.
In the large arteries
Aging is associated with a significant imbalance between ROS production on the one hand and antioxidant activities and NO bioactivity on the other. The Nox2 membrane-bound components p22 phox and gp91 phox are increased within the aortae of old vs young rats [33]. Furthermore, arterial wall VSMC cytosolic p47phox (a regulatory subunit for Nox1/2 activity) also increases with aging [34, 35]. In contrast, anti-oxidant Cu/Zn SOD (SOD1), MnSOD (SOD2) and extracellular matrix superoxide dismutase (ECM-SOD/SOD3) levels are reduced in arterial walls of aged vs young rats [36–39]. Indeed, an imbalance of oxidases and dismutases with aging has been observed in the arterial wall of rats, resulting in increased in situ ROS levels [40–43]. For example, MnSOD deficiency produces an exaggerated remodeled arterial wall with aging due to increased ROS production [38]. Habitual exercise and antioxidant agents effectively retard arterial aging via attenuation of ROS production [36, 39, 44, 45].
Vascular endothelial cell NO synthase (eNOS) is the main source of NO in the arterial wall and endothelial production of NO is reduced with increasing age [37, 46–48]. Inflammed vessels express a different NOS isoform, inducible NOS (iNOS), which is prone to uncoupling and generation of peroxynitrite [49, 50]. Age-associated endothelial dysfunction of the aorta has been observed in senescence-accelerated mice, which is causally linked to downregulation of eNOS [46]. Augmented release of ROS and subsequent inactivation of NO is an important mechanism leading to the age-associated decline of endothelium-dependent vasorelaxation, and to vessel stiffening and inflammation [40, 51, 52] .
In the heart
Both ROS and RNS are known to play vital roles in aging-related myocardial dysfunction [53]. The mitochondrial electron transport chain is a major source of ROS during aging. In addition, increased expression of Nox2 NADPH oxidase contributes to ROS formation [54]. Indeed, myocardial Nox2 mRNA and protein expression are markedly increased in rats with aging [21]. Increased levels of Nox2 protein, predominantly located in the cardiomyocytes , are significantly associated with heart dysfunction [54]. Conversely, loss of Nox2 reduces age-associated oxidative stress in the myocardium and protects against the progression to advanced heart dysfunction with aging [55]. Furthermore, levels of myocardial Rac1, an important activator of Nox2 oxidase are substantially increased within hypertrophied cardiomyocytes in aged rats [54]. As expected, in a mouse model, overexpression of Rac1 proteins increasingly produces cardiomyocyte hypertrophy with aging [56]. In addition, MnSOD overexpression reduces fibrosis and pro-apoptotic signaling in the aging mouse heart [57]. Conversely, eNOS knockout mice have a markedly shortened lifespan, heart hypertrophy and cardiomyocyte apoptosis [58].
The RNS marker nitrotyrosine increases in myocardial tissue from young to middle-aged. Notably, increased thioredoxin reductase nitration and post-translational ONOO(-) nitration enhance aging-related myocardial ischemia/reperfusion injury in rats [59].
Taken together, the above findings suggest that ROS and RNS levels increase in both the heart and arteries, simultaneously driving proinflammation and ventricular-arterial remodeling with aging. Thus, attenuation of these radical species may be beneficial in cardiovascular aging.
Matrix metalloproteinases
An important component of age-associated cardiovascular remodeling is the degradation and resynthesis of the ECM, which is mediated by matrix metalloproteinases (MMPs) (Figure 2). Ang II signaling potently activates MMPs [17, 60].
In large arteries
MMP-2 mRNA and protein increase in the aortic walls of aged rodents, non-human primates and humans [15, 17, 61–64]. The increased MMP-2 activity in rodents and monkeys in situ is mainly localized within the thickened intima and the inner media [16, 65]. Enhanced MMP-2/9 activity is also observed in older aortae at human autopsy [15]. An increase of MMP-2/9 activity is attributable not only to an enhanced transcription and translation but also an imbalance of its activators, membrane-type1 matrix metalloproteinase (MT1-MMP), urokinase-like plasminogen activator (uPA), tissue plasminogen activator (tPA) and inhibitors, tissue inhibitor of MMP-2 (TIMP-2) and plasminogen activation inhibitor (PAI-1) [16, 65]. Chronic administration of a broad spectrum MMP inhibitor, PD166739, markedly blunts the age-associated increases in aortic gelatinase and interstitial collagenase activity, and reduces the elastin fiber degeneration, collagen deposition, monocyte chemo-attractant protein-1 (MCP-1) expression, TGF-β1 activation, and SMAD-2/3 phosphorylation in FBN rats [60]. These findings suggest that MMPs play an etiologic role in the process of arterial aging.
In the heart
The expression of both MMP-2 and MT1-MMP mRNA and protein are increased in the intima and media of epicardial coronary arteries with aging [21]. These changes in MMP/TIMP expression are accompanied by an increase in MMP gelatinolytic activity in the intima and media of epicardial coronary arteries with aging [21]. In addition, MMP-3, MMP-8, MMP-9, MMP-12, and MMP-14 are increased in the myocardium of old mice [66]. Cardiac-restricted MT1-MMP expression in mice using the full-length human MT1-MMP gene ligated to the myosin heavy chain promoter yields approximately a 200% increase in myocardial MT1-MMP, and increases myocardial MMP-2/-9 expression, and causes left ventricular remodeling, myocardial fibrosis, dysfunction, and reduces survival after myocardial injury with aging [67]. Expression of cardiac-restricted EMMPRIN, an MT1-MMP inducer, in mice using the full-length human EMMPRIN gene ligated to the myosin heavy chain promoter yields approximately a twofold increase in EMMPRIN and causes increased levels of both MT-MMPs and MMP-9, and myocardial fibrosis with aging [68]. Importantly, MMP-9 deletion attenuates the age-related decline in diastolic function, in part by reducing TGF-β1 signaling-induced periostin (OSF2) and connective tissue growth factor (CTGF) expression to regulate myocardial collagen turnover and deposition [12]. Unexpectedly, MMP-28 deletion further elevates inflammation and extracellular matrix responses, without altering macrophage numbers or collagen content with aging [69].
These experimental studies reveal that the ECM and its turnover are important determinants of cardiovascular aging. Therefore, regulation of MMP activity may be important in maintaining cardiovascular integrity and health with aging.
Transforming growth factor-β1
The activation of TGF-β1, a powerful pro-fibrogenic cytokine and downstream mediator of Ang II signaling, plays a central role in ECM remodeling with aging (Figure 2) [17].
In large arteries
TGF-β1 mRNA and protein are abundantly present in the aged arterial wall [17, 64]. Co-expression of both TGF-β1 and TGF beta type II receptor (TβIIR) protein increases in rat aortae at 30 versus 8 months of age [64]. TGF-β1 plays a dominant role in arterial fibrosis [17, 21, 63, 64]. TGF-β1 expression is temporally and spatially associated with collagen expression and local fibrosis in the aging arterial wall [17, 65]. In vitro studies show that ECs and VSMCs treated with TGF-β1 increase collagen type I and III mRNA, and this is attenuated by a TβIIR blocker [70, 71]. Furthermore, the increased MMP-2 co-localizes with TGF-β1, suggesting that an interaction may exist between MMP-2 activation and TGF-β1 activity [63]. Indeed, active TGF-β1, its receptor, and receptor-mediated signaling increase within the aortic wall with aging, which is dependent, at least in part, on a concomitant age-associated increase in MMP-2 activity [64]. Impressively, blockade of both TGF-β1 and MMP retard and prevent age-associated collagen deposition and arterial stiffening [60, 72].
In the heart
Aging increases TGF-β1 expression, predominantly in the interstitial space and perivascular region, which plays a key role in the synthesis and secretion of collagen from cardiac fibroblasts [21, 73]. Blockade of TGF-β1 signaling markedly decreases the proliferation of cardiac myofibroblats, collagen deposition and myocardial wall stiffening [74]. Interestingly, a member of the TGF-β superfamily of ligands, growth differentiation factor 11 (GDF-11), is able to bind activin receptor type I and II and cause nuclear gene expression reprogramming of cardiomyocyte aging [75]. Recombinant GDF-11 via intravenous administration into old mice reduces heart weight and cardiomyocyte size [75].
These findings suggest that the TGF superfamily plays multiple roles in cardiovascular aging, in particular, ECM deposition and stiffening. Thus, modulation of TGF signaling is a prime approach to de-stiffen the aging cardiovascular system.
Monocyte chemoattractant protein-1
MCP-1 is a potent inflammatory cytokine downstream of Ang II signaling in the cardiovascular system (Figure 2).
In large arteries
MCP-1 mRNA increases within the aortic wall in FXBN rats with aging [76]. The increased MCP-1 protein is predominantly localized in the thickened intima [76]. MCP-1 dimerization is necessary for its activity [77], and in the aged arterial wall MCP-1 forms dimers with high local concentration [18]. MCP-1 increases the activation of TGF-β1 and MMP-2, and is a potent chemoattractant for VSMC invasion/migration with aging [63]. Knockdown of MCP-1 significantly retards activation of MMP-2, and plaque size while increasing the stability of plaques in the ApoE deficiency mice with advanced age [78, 79].
In the heart
Myocardial MCP-1 is markedly increased with advancing age [74]. Interestingly, increased MCP-1 expression in cardiomyocytes promotes hypertrophy and senescence/apoptosis. Increased MCP-1 expression in fibroblasts promotes the migration of monocytes and T lymphocytes away from the endothelial barrier, facilitating the monocyte transition into macrophages and finally into myeloid fibroblasts [80]. Both myeloid and mesenchymal fibroblasts contribute to fibrosis in the aging heart via collagen synthesis [80]. MCP-1 knockout decreases the apoptosis of cardiomyocytes, inhibits the inflammatory response, and shortens the healing process of myocardial infarction in mice [81].
These findings show that MCP-1 is increased in both heart and large arteries in the absence of infection, further confirming that a low grade sterile inflammation occurs with aging.
Milk fat globule EGF-8 and integrins
Milk fat globule EGF-8 (MFG-E8) is another downstream molecule of Ang II signaling in the cardiovascular system, which interacts with integrins (Figure 2) [18].
In large arteries
High-throughput proteomic screening has identified MFG-E8, a cell adhesion protein, as a novel signature protein of the aging arterial wall [18]. With aging, levels of arterial MFG-E8 and its degradation fragment, medin, both increase and accumulate within the aorta of rodents, nonhuman primates and humans [18, 82, 83]. MFG-E8 is induced by Ang II and itself induces the expression of MCP-1 in VSMCs within the old rat aortic wall [18]. Integrins are the primary adhesion receptors and are responsible for conveying information from the ECM to the intracellular pathways in the arterial wall. Increased co-expression and physical interaction of MFG-E8 and integrin αvβ5 occur with aging in both the rat aortic wall in vivo and in VSMC in vitro, facilitating VSMC invasion and proliferation with aging [18, 84]. In addition, aging also alters the expression of other integrin αvβ3 and β1, which directly affect the stiffness of VSMCs [51, 85]. Thus, increased vascular stiffness is attributable to ECM, VSMCs, and their interaction via integrin signaling.
In the heart
MFG-E8 is also a proteomic signaling signature of the aged ventricle, in which electrophysiological and biochemical changes can occur, with the ventricle losing distensibility and impairing diastolic function [86] . These functional and structural changes are associated with activation of the MFG-E8 driven proinflammatory signaling network [86]. In addition, increased MFG-E8 expression in monocytes is closely associated with the development of heart failure [87].
Intergrin protein expression is also altered in the myocardium with aging. Aging increases integrins α1 and α5 as well as integrin-linked kinase [88, 89]. This integrin-related signaling is involved in age-associated ECM assembly and cardiac myocyte senescence [88, 89]. These findings suggest that the matrix-cell interaction via integrin signaling is altered by aging and facilitates proinflanmmatory remodeling in cardiovascular system.
Interleukin-6 and tissue necrosis factor-α
Both interleukin-6 (IL-6) and tissue necrosis factor-alpha (TNF-α) cytokines can be downstream of Ang II signaling within the cardiovascular system (Figure 2) [90–93] and be involved in proinflammatory signaling in the aging cardiovascular system [61, 94–98].
In large arteries
IL-6 is increased in aged aortae, and secretion of IL-6 from old is greater than from young aortic VSMCs [94]. An age-associated up-regulation of innate immune toll-like receptor [TLR] 4 and its signal adaptor, MyD88, are in part, responsible for the age-elevated basal IL-6 response in VSMCs [94]. Both IL-6 and TLR4 are likely involved in proinflammation of arterial aging.
TNF-α is higher in arterial tissue of the old vs young rats and is associated with MMP-2 activation [61]. The age-associated increase in this proinflammatory cytokine is associated with activation of NF-κB in both VSMCs and ECs [99, 100] and MMP-2 in VSMCs [61].
In the heart
Both IL-6 and TNF-α cytokines are increased in aged myocardium, which is exaggerated in response to inflammatory stress in the absence of IL-10 [95, 96]. The age-associated increase in expression of this cytokine promotes myocardial damage and matrix remodeling, including collagen deposition [95]. Interestingly, transgenic mice with cardiac restricted overexpression of TNF develop progressive left ventricle dilation/remodeling from 4 to 12 weeks of age [96]. This remodeling includes the dynamic change of the myofibrillar collagen [96]. Notably, both IL-6 and TNF-α signaling are required for development of the myocardium or healing process [97, 98]. IL-6 knockout mice develop ventricular dysfunction with collagen deposition, reduced capillary density and decreased myocyte population [98]. Aging decreases the expression of TNF-α receptor and subsequently impairs TNF-α receptor mediated expression of PDGF-B in microvascular endothelial cells, contributing to a role of cardio-protection [97]. Thus, restoring TNF-α receptor mediated expression of the PDGF-B pathway is a novel strategy for the prevention and treatment of heart disease in the elderly.
SIRT1
The activity of the evolutionary conserved NAD(+)-dependent deacetylase silent mating type information regulation 2 homolog (SIRT1), a longevity gene, declines with age [35, 38, 40, 101]. Importantly, SIRT1 regulates AT1 signaling in the cardiovascular system (Figure 2) [102–104]
In large arteries
The expression of SIRT1 decreases with aging within the arterial wall, contributing to arterial dysfunction [38]. In aortic segments isolated from young rats, dysregulation of SIRT1 promotes Nox-dependent production of ROS leading to impaired endothelial function [40]. Thus, impaired activity of SIRT1 in young arteries mimics the vascular aging phenotype [40]. Interestingly, SIRT3, a homolog of SIRT1, deacetylates transcription factor fork-head box O3(FOXO3) and acts to protect mitochondria against age-associated oxidative damage [105]. The caloric restriction (CR) effect to retard aging and increase lifespan in rodents is linked to an elevation of SIRT1 activity [106, 107]. Resveratrol, an activator of SIRT1, mimics CR, and improves arterial health in rodents fed a high fat diet [108, 109]. A recent study shows that resveratrol prevents high fat/sucrose diet-induced central arterial wall inflammation and stiffening in nonhuman primates [110].
In the heart
The activation of SIRT1 attenuates age-dependent induction of left ventricular dysfunction. SIRT1 is expressed predominantly as a sumoylated form in cardiomyocyte nuclei. Cardiac overexpression of desumoylase, sentrin-specific protease 2 (SENP2), significantly reduces nuclear sumoylated SIRT1 levels [111]. Myocardial ischemia/reperfusion stress leads to desumoylation and translocation of nuclear SIRT1 into the cytoplasm in aged but not in young hearts [111]. SIRT1 activity in ischemic young hearts is 3.2-fold higher than that detected in ischemic aged hearts [111]. These findings suggest that aging causes impaired nucleocytoplasmic shuttling and activation of SIRT1 during ischemic stress, impairs cardiac SIRT1 activity, and increases susceptibility of the aged heart to ischemia/reperfusion injury [111]. Furthermore, low (2.5-fold) to moderate (7.5-fold) overexpression of SIRT1 in transgenic mouse hearts attenuated age-dependent increases in cardiac hypertrophy, apoptosis/fibrosis, cardiac dysfunction, and expression of senescence markers [112]. In advanced heart failure, low SIRT1 expression, like aging, is a significant contributing factor to the down-regulation of antioxidants and upregulation of pro-apoptotic molecules through the p53, FOXO1, and oxidative stress pathways[102].
These finding show that SIRT1 plays an important role in cardiovascular aging, which is driven by an augmentation of Ang II signaling.
Micro-RNAs
MicroRNAs (miRNAs) have emerged as crucial regulators of Ang II signaling within the cardiovascular system (Figure 2) [91, 113–115].
In large arteries
miR-146a is highly up-regulated in senescent ECs. Interestingly, a 1,000-fold increased expression of miR-146a in the arterial wall of chronic heart failure patients is significantly correlated with telomere length and telomerase activity [116]. miR-146a is a marker of senescence-associated pro-inflammatory status in vascular cells [116]. In addition the increased expression of miR-29 family members is associated with a profound down-regulation of numerous ECM components, facilitating the formation of aortic aneurysms in aged mice [113]. As expected, ECM remodeling is significantly induced in Ang II-treated aged mice, which is substantially reduced by the blockade of miR-29b signaling [113]. More importantly, miR-29b levels are profoundly increased in biopsies of human thoracic aneurysms [113], which is suppressed by TGF-β in a SMAD3-dependent manner in adventitia fibroblasts, thus triggering a profibrotic effect [117].
In the heart
Altered expression of miRNAs in the heart during ageing contributes to the age-dependent decline in cardiac function. miR-34a is increased in the ageing heart and in vivo silencing or genetic deletion of miR-34a reduces age-associated cardiomyocyte cell death [118]. Moreover, miR-34a inhibition reduces cell death and fibrosis following acute myocardial infarction, reduces telomere shortening, DNA damage responses and cardiomyocyte apoptosis, and improves functional recovery after acute myocardial infarction [118]. Further study shows that miR-34a promotes cardiac fibrosis after myocardial infarction dependent SMAD4 signaling [114]. In addition, miR-22 is most prominently up-regulated during cardiac aging. Cardiac expression of its bioinformatically predicted target mimecan (osteoglycin) is gradually decreased with advanced age [119]. Both miR-22 and its target mimecan are co-expressed in cardiac fibroblasts and smooth muscle cells [119]. miR-22 overexpression induces cellular senescence and promotes migratory activity of cardiac fibroblasts[119]. miR-22 upregulation in the aging heart contributed at least partly to accelerated cardiac fibroblast senescence and increased migratory activity and collagen deposition [119]. In addition, changes in miR-21/-29 are involved in age-associated atrial fibrosis and fibrillation in dogs [120]. miR-29 is also involved in ECM secretion by cardiac fibroblasts via TGF-β/SMAD3 signaling [121].
These findings indicate that micro RNAs complexly modulate cardiovascular cell and matrix remodeling at multiple levels during aging.
ETS and NF-kB factors
Both ETSs and NF-kB are inflammatory transcription factors, which are master regulators of important signaling molecules in cardiovascular aging (Figure 2) [4, 90].
In large arteries
Expression of Ets-1 is increased within arterial walls with aging, predominantly located in VSMCs [60]. Ets-1 is a critical factor for Ang II mediated p47phox-associated ROS production and MCP-1 associated arterial inflammation [90]. Infection of VSMCs with an adenovirus harbouring a full-length Ets-1 cDNA increased activities of both TGF-β1 and MCP-1 [60].
Activated NF-κB regulates the activity of MMP-2/-9, calpain-1, MCP-1, TGF-β1, and ROS, which deliver multiple signals to drive proinflammation within the older arterial wall [4, 122]. Interestingly, endothelium-restricted inhibition of NF-kB markedly reduced atherosclerotic plaque formation in ApoE−/− mice fed with a cholesterol-rich diet via an abrogation of adhesive molecule induction and blockade of macrophage recruitment as well as reduced expression of cytokines and chemokines [123].
In the heart
Ets-2 expression is decreased in the myocardium in long-lived rats [124]. Lower Ets-2 prevents the apoptosis of cardiomyocytes and higher Ets-2 levels are associated with promotion of myocyte death [124]. Thus, Ets-2 abundance leads to loss of cardiomycytes, contributing to longevity variability in rats.
Activity of myocardial NF-kB is increased in old vs. young rats, which is associated with impairment of cardiomyocyte relaxation [125]. Importantly, a direct inhibition of NF-kB effectively prevents cardiac hypertrophy and heart failure with aging [126].
Proinflammatory cellular phenotypic changes during cardiovascular aging
Ang II-associated proinflammatory molecular and cellular mechanisms of cardiovascular aging are depicted in Figure 2 [4]. These signaling cascades impact on the phenotype of all cardiovascular cells in an autocrine, paracrine, or juxtacrine manner. ECs, VSMCs, and fibroblasts are dominant cells in the normal arterial wall. With aging, these cells are phenotypically shifted, facilitating endothelial disruption, intimal-medial thickening, elastin fracture, and fibrosis.
Aging increases the vulnerability of ECs. The typical arterial ECs in young animals are generally small and round and have a single small nucleus. Multinucleated ECs with a large cytoplasm, known as multinucleated variant ECs, are increased with age in humans [127] . Additionally, the EC gap junction protein also changes with aging [128]. Plasma membrane fluidity of aged rat ECs is significantly lower than that of young rat ECs [129] . Importantly, long-term stiffening of the cells leads to their decreased survival as observed in disturbed Aldo/sodium homeostasis [130]. The telomere length of ECs obtained from human donors decreases with advancing age [131]. The loss of telomeric DNA in human EC cultures is a function of population doubling, suggesting that as cell turnover increases replicative capability declines [132]. Both miRNA 21 and 34a regulate the expression of p53, p21 and p16, revealing a significant role in EC senescence with aging [133–137].
With advancing age, VSMCs appear as multiple phenotypes. Many medial VSMCs develop a “synthetic phenotype”, characterized by the decreased contractile proteins, α-SMA, SM1 and SM2, and enriched with intermediate filament, desmin and fetal protein SMemb [15, 19, 138]. Subsets of VSMC within the aged aortic wall have an enhanced proliferation capacity and are indicated by an increased replication rate in old cultured VSMCs compared to young cells [138, 139] . In young cultured VSMCs, MFG-E8 triggers phosphorylation of ERK1/2, augments levels of PCNA, CDK4 and PDGF signaling, increases 5-bromo-2'-deoxyuridine (BrdU) incorporation, and promotes proliferation via αvβ5 integrin signaling [84]. MFG-E8 silencing, integrin inhibition, or the blockade of ERK-1/2 phosphorylation in old cells reduces PCNA and CDK4 levels and decelerates the S phase of the cell cycle, conferring a reduction in proliferative capacity [84]. In vitro studies demonstrate substantial heterogeneity among VSMC phenotypes within the old arterial wall. Indeed, some old VSMCs are likely to enter an irreversible growth arrest called cellular senescence. Senescence-associated β-gal activity in the old rat arterial wall is detected in VSMCs enriched in the cyclin-dependent kinase inhibitor p16 and NOX4, which is likely linked to increase Ang II signaling [140, 141]
In addition, the migration/invasion of VSMCs, which is a key cellular event in age-associated diffuse intimal thickening, is induced by proinflammatory molecules [4, 10]. The capacity of young VSMC invasion modulated by increased activation of MMP-2, increases up to that of old cells with advancing passage in culture [15, 18, 19, 63]. The number of VSMCs isolated from old animals that invade a basement membrane is significantly greater than that of young VSMCs, and this age difference is abolished by an MMP-2 inhibitor, GM6001 [63, 76].
VSMC senescence is also driven by abnormal accumulation of the intracellular protein, prelamin A. With aging, prelamin A accumulates in VSMCs and induces DNA damage, leading to mitotic failure, genomic disruption, mitosis instability, and premature senescence [142] . In addition, a mutation in the prelamin A-processing enzyme, FACE1, leads to the accumulation of permanently farnesylated prelamin A in VSMCs, also causing premature senescence [143]. An age-associated arterial secretory phenotype (AAASP) is characterized in the cytokine secretion profile of primary VSMCs derived from old nonhuman primates [99, 144]. Old VSMCs secrete more IL-1β, IL-6, MCP-1, and TNF-α [99]. Interestingly, senescent cells also appear to have a cell-type or cell-strain exclusive senescent phenotype (CESP). One such CESP is the procalcitory phenotype, recently reported in senescent VSMCs [145].
Interestingly, aging increases MMP-2-cleaved elastin and activated TGF-β1/TGF-β receptor type II signaling, contributes to increased production of collagen I, II, III, and the biologic glue, fibronectin, and leads to elastin fragmentation and fibrosis [146, 147]. In addition to increases in MMP-2 activity, activation of the intracellular calcium-dependent proteinase, calpain-1, is also involved in TGF-β1 activation and increased collagen production in old VSMCs [146] .
With advancing age or under inflammatory conditions, fibroblasts become activated, synthesize smooth muscle actin and become myofibroblasts (Figure 2). In response to either Ang II or Aldo, fibroblasts trans-differentiate into myofibroblasts with enhanced activation, migration, proliferation, and secretion of osteopontin (OPN) and osetocalcin, contributing to both intimal and adventitial thickening [148–151].
The cardiomyocytes and nonmyocte fibroblasts are major cell types of the myocardium. The total number of cardiomyocytes is estimated to be one third lower in the old heart than the number of cardiomyocytes at birth [152]. Age increases the signaling of Ang II, ROS, MMPs, TGF-β1, MFG-E8, and miR 34a, and decreases the signaling of SIRT2 and ETS-2 in cardiomyocytes, facilitating the development of hypertrophy, senescence, and apoptosis (Figure 2)[75, 152–156]. Furthermore, the number of myocardial fibroblast cells under these proinflammatory stimuli is increased and also activated as myofibroblasts or as inflammatory senescent cells, promoting ECM remodeling and modifications [73, 80, 119, 121, 156]. These cellular and extracellular changes are underlying mechanisms of myocardial aging.
Taken together, these cellular phenotypic changes in the heart and arterial vasculature are responsible for the functional phenotype of the aged cardiovascular system.
Concluding Remarks and Future Perspectives
Aging increases allostatic load, contributing to proinflammatory responses within the cardiovascular system (Figure 1&2). A chronic increase in the production of Ang II-associated inflammatory signals appears to be especially important for age-associated adverse cardiovascular structural and functional remodelling (Figure 2). Proinflammatory signaling in the aged heart and arteries leads to the functional phenotype of a stiffened cardiovascular system and suboptimal ventricular-arterial mechanical coupling (Figure 1). Adverse cardiovascular, cellular and molecular events that mimic the aged phenotype can be produced in experimental young animals under inflammatory stress conditions (e.g. increased Ang II signaling) and can be attenuated in old animals via interference of proinflammatory signals (Figure 2). Thus, early and effective strategies to control age-associated cardiovascular proinflammatory signaling may be potential novel approaches to tackle cardiovascular aging or age-related disease in the future.
Highlights.
Cardiovascular functional phenotype with aging
Cardiovascular proinflammation with aging
Cardiovascular cellular phenotype with aging
Acknowledgements
The authors would like to thank Robert E. Monticone for his editorial assistance in preparing this document.
Funding sources
AMS is supported by the British Heart Foundation; a Foundation Leducq Transatlantic Network of Excellence Award; and the Department of Health via a National Institute for Health Research (NIHR) Biomedical Research Centre award to Guy’s & St Thomas’ NHS Foundation Trust in partnership with King’s College London and King’s College Hospital NHS Foundation Trust. MW is supported by the Intramural Research Program of the National Institute on Aging, National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest statement
None declared.
References
- 1.Merkin SS, Karlamangla A, Roux AV, Shrager S, Seeman TE. Life course socioeconomic status and longitudinal accumulation of allostatic load in adulthood: multi-ethnic study of atherosclerosis. American journal of public health. 2014;104:e48–e55. doi: 10.2105/AJPH.2013.301841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Seeman TE, Singer BH, Rowe JW, Horwitz RI, McEwen BS. Price of adaptation--allostatic load and its health consequences. MacArthur studies of successful aging. Archives of internal medicine. 1997;157:2259–2268. [PubMed] [Google Scholar]
- 3.Saavedra JM. Angiotensin II AT(1) receptor blockers ameliorate inflammatory stress: a beneficial effect for the treatment of brain disorders. Cellular and molecular neurobiology. 2012;32:667–681. doi: 10.1007/s10571-011-9754-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang M, Jiang L, Monticone RE, Lakatta EG. Proinflammation: the key to arterial aging. Trends in endocrinology and metabolism: TEM. 2014;25:72–79. doi: 10.1016/j.tem.2013.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chantler PD, Lakatta EG. Arterial-ventricular coupling with aging and disease. Frontiers in physiology. 2012;3:90. doi: 10.3389/fphys.2012.00090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chantler PD, Lakatta EG, Najjar SS. Arterial-ventricular coupling: mechanistic insights into cardiovascular performance at rest and during exercise. Journal of applied physiology. 2008;105:1342–1351. doi: 10.1152/japplphysiol.90600.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kass DA. Age-related changes in venticular-arterial coupling: pathophysiologic implications. Heart failure reviews. 2002;7:51–62. doi: 10.1023/a:1013749806227. [DOI] [PubMed] [Google Scholar]
- 8.Najjar SS, Schulman SP, Gerstenblith G, Fleg JL, Kass DA, O'Connor F, et al. Age and gender affect ventricular-vascular coupling during aerobic exercise. Journal of the American College of Cardiology. 2004;44:611–617. doi: 10.1016/j.jacc.2004.04.041. [DOI] [PubMed] [Google Scholar]
- 9.Zouein FA, de Castro Bras LE, da Costa DV, Lindsey ML, Kurdi M, Booz GW. Heart failure with preserved ejection fraction: emerging drug strategies. Journal of cardiovascular pharmacology. 2013;62:13–21. doi: 10.1097/FJC.0b013e31829a4e61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lakatta EG, Wang M, Najjar SS. Arterial aging and subclinical arterial disease are fundamentally intertwined at macroscopic and molecular levels. The Medical clinics of North America. 2009;93:583–604. doi: 10.1016/j.mcna.2009.02.008. Table of Contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.AlGhatrif M, Strait JB, Morrell CH, Canepa M, Wright J, Elango P, et al. Longitudinal trajectories of arterial stiffness and the role of blood pressure: the Baltimore Longitudinal Study of Aging. Hypertension. 2013;62:934–941. doi: 10.1161/HYPERTENSIONAHA.113.01445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chiao YA, Ramirez TA, Zamilpa R, Okoronkwo SM, Dai Q, Zhang J, et al. Matrix metalloproteinase-9 deletion attenuates myocardial fibrosis and diastolic dysfunction in ageing mice. Cardiovascular research. 2012;96:444–455. doi: 10.1093/cvr/cvs275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chantler PD, Nussbacher A, Gerstenblith G, Schulman SP, Becker LC, Ferrucci L, et al. Abnormalities in arterial-ventricular coupling in older healthy persons are attenuated by sodium nitroprusside. American journal of physiology Heart and circulatory physiology. 2011;300:H1914–H1922. doi: 10.1152/ajpheart.01048.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Michel JB, Heudes D, Michel O, Poitevin P, Philippe M, Scalbert E, et al. Effect of chronic ANG I-converting enzyme inhibition on aging processes. II. Large arteries. The American journal of physiology. 1994;267:R124–R135. doi: 10.1152/ajpregu.1994.267.1.R124. [DOI] [PubMed] [Google Scholar]
- 15.Wang M, Zhang J, Jiang LQ, Spinetti G, Pintus G, Monticone R, et al. Proinflammatory profile within the grossly normal aged human aortic wall. Hypertension. 2007;50:219–227. doi: 10.1161/HYPERTENSIONAHA.107.089409. [DOI] [PubMed] [Google Scholar]
- 16.Wang M, Takagi G, Asai K, Resuello RG, Natividad FF, Vatner DE, et al. Aging increases aortic MMP-2 activity and angiotensin II in nonhuman primates. Hypertension. 2003;41:1308–1316. doi: 10.1161/01.HYP.0000073843.56046.45. [DOI] [PubMed] [Google Scholar]
- 17.Wang M, Zhang J, Spinetti G, Jiang LQ, Monticone R, Zhao D, et al. Angiotensin II activates matrix metalloproteinase type II and mimics age-associated carotid arterial remodeling in young rats. The American journal of pathology. 2005;167:1429–1442. doi: 10.1016/S0002-9440(10)61229-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fu Z, Wang M, Gucek M, Zhang J, Wu J, Jiang L, et al. Milk fat globule protein epidermal growth factor-8: a pivotal relay element within the angiotensin II and monocyte chemoattractant protein-1 signaling cascade mediating vascular smooth muscle cells invasion. Circulation research. 2009;104:1337–1346. doi: 10.1161/CIRCRESAHA.108.187088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jiang L, Wang M, Zhang J, Monticone RE, Telljohann R, Spinetti G, et al. Increased aortic calpain-1 activity mediates age-associated angiotensin II signaling of vascular smooth muscle cells. PloS one. 2008;3:e2231. doi: 10.1371/journal.pone.0002231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krug AW, Allenhofer L, Monticone R, Spinetti G, Gekle M, Wang M, et al. Elevated mineralocorticoid receptor activity in aged rat vascular smooth muscle cells promotes a proinflammatory phenotype via extracellular signal-regulated kinase 1/2 mitogen-activated protein kinase and epidermal growth factor receptor-dependent pathways. Hypertension. 2010;55:1476–1483. doi: 10.1161/HYPERTENSIONAHA.109.148783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang M, Zhang J, Walker SJ, Dworakowski R, Lakatta EG, Shah AM. Involvement of NADPH oxidase in age-associated cardiac remodeling. Journal of molecular and cellular cardiology. 2010;48:765–772. doi: 10.1016/j.yjmcc.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Basso N, Cini R, Pietrelli A, Ferder L, Terragno NA, Inserra F. Protective effect of long-term angiotensin II inhibition. American journal of physiology Heart and circulatory physiology. 2007;293:H1351–H1358. doi: 10.1152/ajpheart.00393.2007. [DOI] [PubMed] [Google Scholar]
- 23.Linz W, Heitsch H, Scholkens BA, Wiemer G. Long-term angiotensin II type 1 receptor blockade with fonsartan doubles lifespan of hypertensive rats. Hypertension. 2000;35:908–913. doi: 10.1161/01.hyp.35.4.908. [DOI] [PubMed] [Google Scholar]
- 24.Benigni A, Corna D, Zoja C, Sonzogni A, Latini R, Salio M, et al. Disruption of the Ang II type 1 receptor promotes longevity in mice. The Journal of clinical investigation. 2009;119:524–530. doi: 10.1172/JCI36703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Misaka T, Suzuki S, Miyata M, Kobayashi A, Shishido T, Ishigami A, et al. Deficiency of senescence marker protein 30 exacerbates angiotensin II-induced cardiac remodelling. Cardiovascular research. 2013;99:461–470. doi: 10.1093/cvr/cvt122. [DOI] [PubMed] [Google Scholar]
- 26.Mizukami H, Saitoh S, Machii H, Yamada S, Hoshino Y, Misaka T, et al. Senescence Marker Protein-30 (SMP30) Deficiency Impairs Myocardium-Induced Dilation of Coronary Arterioles Associated with Reactive Oxygen Species. International journal of molecular sciences. 2013;14:9408–9423. doi: 10.3390/ijms14059408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shi ST, Li YF. Interaction of signal transduction between angiotensin AT1 and AT2 receptor subtypes in rat senescent heart. Chinese medical journal. 2007;120:1820–1824. [PubMed] [Google Scholar]
- 28.Oudit GY, Kassiri Z, Patel MP, Chappell M, Butany J, Backx PH, et al. Angiotensin II-mediated oxidative stress and inflammation mediate the age-dependent cardiomyopathy in ACE2 null mice. Cardiovascular research. 2007;75:29–39. doi: 10.1016/j.cardiores.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 29.Giani JF, Miquet JG, Munoz MC, Burghi V, Toblli JE, Masternak MM, et al. Upregulation of the angiotensin-converting enzyme 2/angiotensin-(1–7)/Mas receptor axis in the heart and the kidney of growth hormone receptor knock-out mice. Growth hormone & IGF research : official journal of the Growth Hormone Research Society and the International IGF Research Society. 2012;22:224–233. doi: 10.1016/j.ghir.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ferder L, Romano LA, Ercole LB, Stella I, Inserra F. Biomolecular changes in the aging myocardium: the effect of enalapril. American journal of hypertension. 1998;11:1297–1304. doi: 10.1016/s0895-7061(98)00152-6. [DOI] [PubMed] [Google Scholar]
- 31.Inserra F, Romano L, Ercole L, de Cavanagh EM, Ferder L. Cardiovascular changes by long-term inhibition of the renin-angiotensin system in aging. Hypertension. 1995;25:437–442. doi: 10.1161/01.hyp.25.3.437. [DOI] [PubMed] [Google Scholar]
- 32.Burgoyne JR, Mongue-Din H, Eaton P, Shah AM. Redox signaling in cardiac physiology and pathology. Circulation research. 2012;111:1091–1106. doi: 10.1161/CIRCRESAHA.111.255216. [DOI] [PubMed] [Google Scholar]
- 33.Hamilton CA, Brosnan MJ, McIntyre M, Graham D, Dominiczak AF. Superoxide excess in hypertension and aging: a common cause of endothelial dysfunction. Hypertension. 2001;37:529–534. doi: 10.1161/01.hyp.37.2.529. [DOI] [PubMed] [Google Scholar]
- 34.Kim IJ, Kim YK, Son SM, Hong KW, Kim CD. Enhanced vascular production of superoxide in OLETF rat after the onset of hyperglycemia. Diabetes research and clinical practice. 2003;60:11–18. doi: 10.1016/s0168-8227(02)00278-4. [DOI] [PubMed] [Google Scholar]
- 35.Tang Y, Xu J, Qu W, Peng X, Xin P, Yang X, et al. Resveratrol reduces vascular cell senescence through attenuation of oxidative stress by SIRT1/NADPH oxidase-dependent mechanisms. The Journal of nutritional biochemistry. 2012;23:1410–1416. doi: 10.1016/j.jnutbio.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 36.Fleenor BS, Sindler AL, Marvi NK, Howell KL, Zigler ML, Yoshizawa M, et al. Curcumin ameliorates arterial dysfunction and oxidative stress with aging. Experimental gerontology. 2013;48:269–276. doi: 10.1016/j.exger.2012.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gong X, Ma Y, Ruan Y, Fu G, Wu S. Long-term atorvastatin improves age-related endothelial dysfunction by ameliorating oxidative stress and normalizing eNOS/iNOS imbalance in rat aorta. Experimental gerontology. 2014;52C:9–17. doi: 10.1016/j.exger.2014.01.015. [DOI] [PubMed] [Google Scholar]
- 38.Roos CM, Hagler M, Zhang B, Oehler EA, Arghami A, Miller JD. Transcriptional and phenotypic changes in aorta and aortic valve with aging and MnSOD deficiency in mice. American journal of physiology Heart and circulatory physiology. 2013;305:H1428–H1439. doi: 10.1152/ajpheart.00735.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lund DD, Chu Y, Miller JD, Heistad DD. Protective effect of extracellular superoxide dismutase on endothelial function during aging. American journal of physiology Heart and circulatory physiology. 2009;296:H1920–H1925. doi: 10.1152/ajpheart.01342.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zarzuelo MJ, Lopez-Sepulveda R, Sanchez M, Romero M, Gomez-Guzman M, Ungvary Z, et al. SIRT1 inhibits NADPH oxidase activation and protects endothelial function in the rat aorta: implications for vascular aging. Biochemical pharmacology. 2013;85:1288–1296. doi: 10.1016/j.bcp.2013.02.015. [DOI] [PubMed] [Google Scholar]
- 41.Rice KM, Meduru S, Kakarla SK, Katta A, Mupparaju SP, Kidd B, et al. Chronic paracetamol treatment influences indices of reactive oxygen species accumulation in the aging Fischer 344 X Brown Norway rat aorta. Annals of clinical and laboratory science. 2012;42:152–161. [PubMed] [Google Scholar]
- 42.Ferrini MG, Davila HH, Valente EG, Gonzalez-Cadavid NF, Rajfer J. Aging-related induction of inducible nitric oxide synthase is vasculo-protective to the arterial media. Cardiovascular research. 2004;61:796–805. doi: 10.1016/j.cardiores.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 43.Tanguy S, Boucher F, Toufektsian MC, Besse S, de Leiris J. Aging exacerbates hydrogen peroxide-induced alteration of vascular reactivity in rats. Antioxidants & redox signaling. 2000;2:363–368. doi: 10.1089/ars.2000.2.2-363. [DOI] [PubMed] [Google Scholar]
- 44.Kao CL, Chen LK, Chang YL, Yung MC, Hsu CC, Chen YC, et al. Resveratrol protects human endothelium from H(2)O(2)-induced oxidative stress and senescence via SirT1 activation. Journal of atherosclerosis and thrombosis. 2010;17:970–979. doi: 10.5551/jat.4333. [DOI] [PubMed] [Google Scholar]
- 45.Gu Q, Wang B, Zhang XF, Ma YP, Liu JD, Wang XZ. Chronic aerobic exercise training attenuates aortic stiffening and endothelial dysfunction through preserving aortic mitochondrial function in aged rats. Experimental gerontology. 2014;56:37–44. doi: 10.1016/j.exger.2014.02.014. [DOI] [PubMed] [Google Scholar]
- 46.Novella S, Dantas AP, Segarra G, Vidal-Gomez X, Mompeon A, Garabito M, et al. Aging-related endothelial dysfunction in the aorta from female senescence-accelerated mice is associated with decreased nitric oxide synthase expression. Experimental gerontology. 2013;48:1329–1337. doi: 10.1016/j.exger.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 47.Santhanam L, Tuday EC, Webb AK, Dowzicky P, Kim JH, Oh YJ, et al. Decreased S-nitrosylation of tissue transglutaminase contributes to age-related increases in vascular stiffness. Circulation research. 2010;107:117–125. doi: 10.1161/CIRCRESAHA.109.215228. [DOI] [PubMed] [Google Scholar]
- 48.Steppan J, Sikka G, Jandu S, Barodka V, Halushka MK, Flavahan NA, et al. Exercise, vascular stiffness, and tissue transglutaminase. Journal of the American Heart Association. 2014;3:e000599. doi: 10.1161/JAHA.113.000599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tian J, Yan Z, Wu Y, Zhang SL, Wang K, Ma XR, et al. Inhibition of iNOS protects endothelial-dependent vasodilation in aged rats. Acta pharmacologica Sinica. 2010;31:1324–1328. doi: 10.1038/aps.2010.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.van Oostrom O, Velema E, Schoneveld AH, de Vries JP, de Bruin P, Seldenrijk CA, et al. Age-related changes in plaque composition: a study in patients suffering from carotid artery stenosis. Cardiovascular pathology : the official journal of the Society for Cardiovascular Pathology. 2005;14:126–134. doi: 10.1016/j.carpath.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 51.Galmiche G, Labat C, Mericskay M, Aissa KA, Blanc J, Retailleau K, et al. Inactivation of serum response factor contributes to decrease vascular muscular tone and arterial stiffness in mice. Circulation research. 2013;112:1035–1045. doi: 10.1161/CIRCRESAHA.113.301076. [DOI] [PubMed] [Google Scholar]
- 52.van der Loo B, Labugger R, Skepper JN, Bachschmid M, Kilo J, Powell JM, et al. Enhanced peroxynitrite formation is associated with vascular aging. The Journal of experimental medicine. 2000;192:1731–1744. doi: 10.1084/jem.192.12.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fan Q, Chen M, Fang X, Lau WB, Xue L, Zhao L, et al. Aging might augment reactive oxygen species (ROS) formation and affect reactive nitrogen species (RNS) level after myocardial ischemia/reperfusion in both humans and rats. Age. 2013;35:1017–1026. doi: 10.1007/s11357-012-9421-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kuka S, Tatarkova Z, Racay P, Lehotsky J, Dobrota D, Kaplan P. Effect of aging on formation of reactive oxygen species by mitochondria of rat heart. General physiology and biophysics. 2013;32:415–420. doi: 10.4149/gpb_2013049. [DOI] [PubMed] [Google Scholar]
- 55.Parajuli N, Patel VB, Wang W, Basu R, Oudit GY. Loss of NOX2 (gp91phox) prevents oxidative stress and progression to advanced heart failure. Clinical science. 2014;127:331–340. doi: 10.1042/CS20130787. [DOI] [PubMed] [Google Scholar]
- 56.Elnakish MT, Awad MM, Hassona MD, Alhaj MA, Kulkarni A, Citro LA, et al. Cardiac remodeling caused by transgenic overexpression of a corn Rac gene. American journal of physiology Heart and circulatory physiology. 2011;301:H868–H880. doi: 10.1152/ajpheart.00807.2010. [DOI] [PubMed] [Google Scholar]
- 57.Kwak HB, Lee Y, Kim JH, Van Remmen H, Richardson AG, Lawler JM. MnSOD Overexpression Reduces Fibrosis and Pro-Apoptotic Signaling in the Aging Mouse Heart. The journals of gerontology Series A, Biological sciences and medical sciences. 2014 doi: 10.1093/gerona/glu090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li W, Mital S, Ojaimi C, Csiszar A, Kaley G, Hintze TH. Premature death and age-related cardiac dysfunction in male eNOS-knockout mice. Journal of molecular and cellular cardiology. 2004;37:671–680. doi: 10.1016/j.yjmcc.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 59.Wang K, Zhang J, Wang X, Liu X, Zuo L, Bai K, et al. Thioredoxin reductase was nitrated in the aging heart after myocardial ischemia/reperfusion. Rejuvenation research. 2013;16:377–385. doi: 10.1089/rej.2013.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang M, Zhang J, Telljohann R, Jiang L, Wu J, Monticone RE, et al. Chronic matrix metalloproteinase inhibition retards age-associated arterial proinflammation and increase in blood pressure. Hypertension. 2012;60:459–466. doi: 10.1161/HYPERTENSIONAHA.112.191270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li Z, Froehlich J, Galis ZS, Lakatta EG. Increased expression of matrix metalloproteinase-2 in the thickened intima of aged rats. Hypertension. 1999;33:116–123. doi: 10.1161/01.hyp.33.1.116. [DOI] [PubMed] [Google Scholar]
- 62.Spiers JP, Kelso EJ, Siah WF, Edge G, Song G, McDermott BJ, et al. Alterations in vascular matrix metalloproteinase due to ageing and chronic hypertension: effects of endothelin receptor blockade. Journal of hypertension. 2005;23:1717–1724. doi: 10.1097/01.hjh.0000176787.04753.ee. [DOI] [PubMed] [Google Scholar]
- 63.Wang M, Spinetti G, Monticone RE, Zhang J, Wu J, Jiang L, et al. A local proinflammatory signalling loop facilitates adverse age-associated arterial remodeling. PloS one. 2011;6:e16653. doi: 10.1371/journal.pone.0016653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang M, Zhao D, Spinetti G, Zhang J, Jiang LQ, Pintus G, et al. Matrix metalloproteinase 2 activation of transforming growth factor-beta1 (TGF-beta1) and TGF-beta1-type II receptor signaling within the aged arterial wall. Arteriosclerosis, thrombosis, and vascular biology. 2006;26:1503–1509. doi: 10.1161/01.ATV.0000225777.58488.f2. [DOI] [PubMed] [Google Scholar]
- 65.Wang M, Lakatta EG. Altered regulation of matrix metalloproteinase-2 in aortic remodeling during aging. Hypertension. 2002;39:865–873. doi: 10.1161/01.hyp.0000014506.13322.66. [DOI] [PubMed] [Google Scholar]
- 66.Lindsey ML, Goshorn DK, Squires CE, Escobar GP, Hendrick JW, Mingoia JT, et al. Age-dependent changes in myocardial matrix metalloproteinase/tissue inhibitor of metalloproteinase profiles and fibroblast function. Cardiovascular research. 2005;66:410–419. doi: 10.1016/j.cardiores.2004.11.029. [DOI] [PubMed] [Google Scholar]
- 67.Spinale FG, Escobar GP, Mukherjee R, Zavadzkas JA, Saunders SM, Jeffords LB, et al. Cardiac-restricted overexpression of membrane type-1 matrix metalloproteinase in mice: effects on myocardial remodeling with aging. Circulation Heart failure. 2009;2:351–360. doi: 10.1161/CIRCHEARTFAILURE.108.844845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zavadzkas JA, Plyler RA, Bouges S, Koval CN, Rivers WT, Beck CU, et al. Cardiac-restricted overexpression of extracellular matrix metalloproteinase inducer causes myocardial remodeling and dysfunction in aging mice. American journal of physiology Heart and circulatory physiology. 2008;295:H1394–H1402. doi: 10.1152/ajpheart.00346.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ma Y, Chiao YA, Zhang J, Manicone AM, Jin YF, Lindsey ML. Matrix metalloproteinase-28 deletion amplifies inflammatory and extracellular matrix responses to cardiac aging. Microscopy and microanalysis : the official journal of Microscopy Society of America, Microbeam Analysis Society, Microscopical Society of Canada. 2012;18:81–90. doi: 10.1017/S1431927611012220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chiasson VL, Jones KA, Kopriva SE, Mahajan A, Young KJ, Mitchell BM. Endothelial cell transforming growth factor-beta receptor activation causes tacrolimus-induced renal arteriolar hyalinosis. Kidney international. 2012;82:857–866. doi: 10.1038/ki.2012.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lu P, Wang S, Cai W, Sheng J. Role of TGF-beta1/Smad3 signaling pathway in secretion of type I and III collagen by vascular smooth muscle cells of rats undergoing balloon injury. Journal of biomedicine & biotechnology. 2012;2012:965953. doi: 10.1155/2012/965953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ishida W, Mori Y, Lakos G, Sun L, Shan F, Bowes S, et al. Intracellular TGF-beta receptor blockade abrogates Smad-dependent fibroblast activation in vitro and in vivo. The Journal of investigative dermatology. 2006;126:1733–1744. doi: 10.1038/sj.jid.5700303. [DOI] [PubMed] [Google Scholar]
- 73.Shivakumar K, Dostal DE, Boheler K, Baker KM, Lakatta EG. Differential response of cardiac fibroblasts from young adult and senescent rats to ANG II. American journal of physiology Heart and circulatory physiology. 2003;284:H1454–H1459. doi: 10.1152/ajpheart.00766.2002. [DOI] [PubMed] [Google Scholar]
- 74.Chiao YA, Dai Q, Zhang J, Lin J, Lopez EF, Ahuja SS, et al. Multi-analyte profiling reveals matrix metalloproteinase-9 and monocyte chemotactic protein-1 as plasma biomarkers of cardiac aging. Circulation Cardiovascular genetics. 2011;4:455–462. doi: 10.1161/CIRCGENETICS.111.959981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Loffredo FS, Steinhauser ML, Jay SM, Gannon J, Pancoast JR, Yalamanchi P, et al. Growth differentiation factor 11 is a circulating factor that reverses age-related cardiac hypertrophy. Cell. 2013;153:828–839. doi: 10.1016/j.cell.2013.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Spinetti G, Wang M, Monticone R, Zhang J, Zhao D, Lakatta EG. Rat aortic MCP-1 and its receptor CCR2 increase with age and alter vascular smooth muscle cell function. Arteriosclerosis, thrombosis, and vascular biology. 2004;24:1397–1402. doi: 10.1161/01.ATV.0000134529.65173.08. [DOI] [PubMed] [Google Scholar]
- 77.Zhang Y, Rollins BJ. A dominant negative inhibitor indicates that monocyte chemoattractant protein 1 functions as a dimer. Molecular and cellular biology. 1995;15:4851–4855. doi: 10.1128/mcb.15.9.4851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Liu XL, Zhang PF, Ding SF, Wang Y, Zhang M, Zhao YX, et al. Local gene silencing of monocyte chemoattractant protein-1 prevents vulnerable plaque disruption in apolipoprotein E-knockout mice. PloS one. 2012;7:e33497. doi: 10.1371/journal.pone.0033497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ni W, Kitamoto S, Ishibashi M, Usui M, Inoue S, Hiasa K, et al. Monocyte chemoattractant protein-1 is an essential inflammatory mediator in angiotensin II-induced progression of established atherosclerosis in hypercholesterolemic mice. Arteriosclerosis, thrombosis, and vascular biology. 2004;24:534–539. doi: 10.1161/01.ATV.0000118275.60121.2b. [DOI] [PubMed] [Google Scholar]
- 80.Cieslik KA, Trial J, Crawford JR, Taffet GE, Entman ML. Adverse fibrosis in the aging heart depends on signaling between myeloid and mesenchymal cells; role of inflammatory fibroblasts. Journal of molecular and cellular cardiology. 2014;70:56–63. doi: 10.1016/j.yjmcc.2013.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dewald O, Zymek P, Winkelmann K, Koerting A, Ren G, Abou-Khamis T, et al. CCL2/Monocyte Chemoattractant Protein-1 regulates inflammatory responses critical to healing myocardial infarcts. Circulation research. 2005;96:881–889. doi: 10.1161/01.RES.0000163017.13772.3a. [DOI] [PubMed] [Google Scholar]
- 82.Peng S, Glennert J, Westermark P. Medin-amyloid: a recently characterized age-associated arterial amyloid form affects mainly arteries in the upper part of the body. Amyloid : the international journal of experimental and clinical investigation : the official journal of the International Society of Amyloidosis. 2005;12:96–102. doi: 10.1080/13506120500107006. [DOI] [PubMed] [Google Scholar]
- 83.Peng S, Westermark GT, Naslund J, Haggqvist B, Glennert J, Westermark P. Medin and medin-amyloid in ageing inflamed and non-inflamed temporal arteries. The Journal of pathology. 2002;196:91–96. doi: 10.1002/path.1014. [DOI] [PubMed] [Google Scholar]
- 84.Wang M, Fu Z, Wu J, Zhang J, Jiang L, Khazan B, et al. MFG-E8 activates proliferation of vascular smooth muscle cells via integrin signaling. Aging cell. 2012;11:500–508. doi: 10.1111/j.1474-9726.2012.00813.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Qiu H, Zhu Y, Sun Z, Trzeciakowski JP, Gansner M, Depre C, et al. Short communication: vascular smooth muscle cell stiffness as a mechanism for increased aortic stiffness with aging. Circulation research. 2010;107:615–619. doi: 10.1161/CIRCRESAHA.110.221846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Grant JE, Bradshaw AD, Schwacke JH, Baicu CF, Zile MR, Schey KL. Quantification of protein expression changes in the aging left ventricle of Rattus norvegicus. Journal of proteome research. 2009;8:4252–4263. doi: 10.1021/pr900297f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wang M, Wang HH, Lakatta EG. Milk fat globule epidermal growth factor VIII signaling in arterial wall remodeling. Current vascular pharmacology. 2013;11:768–776. doi: 10.2174/1570161111311050014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Burgess ML, McCrea JC, Hedrick HL. Age-associated changes in cardiac matrix and integrins. Mechanisms of ageing and development. 2001;122:1739–1756. doi: 10.1016/s0047-6374(01)00296-2. [DOI] [PubMed] [Google Scholar]
- 89.Chen X, Li Z, Feng Z, Wang J, Ouyang C, Liu W, et al. Integrin-linked kinase induces both senescence-associated alterations and extracellular fibronectin assembly in aging cardiac fibroblasts. The journals of gerontology Series A, Biological sciences and medical sciences. 2006;61:1232–1245. doi: 10.1093/gerona/61.12.1232. [DOI] [PubMed] [Google Scholar]
- 90.Zhan Y, Brown C, Maynard E, Anshelevich A, Ni W, Ho IC, et al. Ets-1 is a critical regulator of Ang II-mediated vascular inflammation and remodeling. The Journal of clinical investigation. 2005;115:2508–2516. doi: 10.1172/JCI24403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Maegdefessel L, Azuma J, Toh R, Deng A, Merk DR, Raiesdana A, et al. MicroRNA-21 blocks abdominal aortic aneurysm development and nicotine-augmented expansion. Science translational medicine. 2012;4 doi: 10.1126/scitranslmed.3003441. 122ra22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Qian L, Li X, Fang R, Wang Z, Xu Y, Zhang H, et al. Class A scavenger receptor deficiency augments angiotensin II-induced vascular remodeling. Biochemical pharmacology. 2014;90:254–264. doi: 10.1016/j.bcp.2014.05.015. [DOI] [PubMed] [Google Scholar]
- 93.Duerrschmid C, Crawford JR, Reineke E, Taffet GE, Trial J, Entman ML, et al. TNF receptor 1 signaling is critically involved in mediating angiotensin-II-induced cardiac fibrosis. Journal of molecular and cellular cardiology. 2013;57:59–67. doi: 10.1016/j.yjmcc.2013.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Song Y, Shen H, Schenten D, Shan P, Lee PJ, Goldstein DR. Aging enhances the basal production of IL-6 and CCL2 in vascular smooth muscle cells. Arteriosclerosis, thrombosis, and vascular biology. 2012;32:103–109. doi: 10.1161/ATVBAHA.111.236349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Meador BM, Krzyszton CP, Johnson RW, Huey KA. Effects of IL-10 and age on IL-6, IL-1beta, and TNF-alpha responses in mouse skeletal and cardiac muscle to an acute inflammatory insult. Journal of applied physiology. 2008;104:991–997. doi: 10.1152/japplphysiol.01079.2007. [DOI] [PubMed] [Google Scholar]
- 96.Sivasubramanian N, Coker ML, Kurrelmeyer KM, MacLellan WR, DeMayo FJ, Spinale FG, et al. Left ventricular remodeling in transgenic mice with cardiac restricted overexpression of tumor necrosis factor. Circulation. 2001;104:826–831. doi: 10.1161/hc3401.093154. [DOI] [PubMed] [Google Scholar]
- 97.Cai D, Xaymardan M, Holm JM, Zheng J, Kizer JR, Edelberg JM. Age-associated impairment in TNF-alpha cardioprotection from myocardial infarction. American journal of physiology Heart and circulatory physiology. 2003;285:H463–H469. doi: 10.1152/ajpheart.00144.2003. [DOI] [PubMed] [Google Scholar]
- 98.Banerjee I, Fuseler JW, Intwala AR, Baudino TA. IL-6 loss causes ventricular dysfunction, fibrosis, reduced capillary density, and dramatically alters the cell populations of the developing and adult heart. American journal of physiology Heart and circulatory physiology. 2009;296:H1694–H1704. doi: 10.1152/ajpheart.00908.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Csiszar A, Sosnowska D, Wang M, Lakatta EG, Sonntag WE, Ungvari Z. Age-associated proinflammatory secretory phenotype in vascular smooth muscle cells from the non-human primate Macaca mulatta: reversal by resveratrol treatment. The journals of gerontology Series A, Biological sciences and medical sciences. 2012;67:811–820. doi: 10.1093/gerona/glr228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Csiszar A, Wang M, Lakatta EG, Ungvari Z. Inflammation and endothelial dysfunction during aging: role of NF-kappaB. Journal of applied physiology. 2008;105:1333–1341. doi: 10.1152/japplphysiol.90470.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Donato AJ, Magerko KA, Lawson BR, Durrant JR, Lesniewski LA, Seals DR. SIRT-1 and vascular endothelial dysfunction with ageing in mice and humans. The Journal of physiology. 2011;589:4545–4554. doi: 10.1113/jphysiol.2011.211219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gao P, Xu TT, Lu J, Li L, Xu J, Hao DL, et al. Overexpression of SIRT1 in vascular smooth muscle cells attenuates angiotensin II-induced vascular remodeling and hypertension in mice. Journal of molecular medicine. 2014;92:347–357. doi: 10.1007/s00109-013-1111-4. [DOI] [PubMed] [Google Scholar]
- 103.Li L, Gao P, Zhang H, Chen H, Zheng W, Lv X, et al. SIRT1 inhibits angiotensin II-induced vascular smooth muscle cell hypertrophy. Acta biochimica et biophysica Sinica. 2011;43:103–109. doi: 10.1093/abbs/gmq104. [DOI] [PubMed] [Google Scholar]
- 104.Miyazaki R, Ichiki T, Hashimoto T, Inanaga K, Imayama I, Sadoshima J, et al. SIRT1, a longevity gene, downregulates angiotensin II type 1 receptor expression in vascular smooth muscle cells. Arteriosclerosis, thrombosis, and vascular biology. 2008;28:1263–1269. doi: 10.1161/ATVBAHA.108.166991. [DOI] [PubMed] [Google Scholar]
- 105.Tseng AH, Shieh SS, Wang DL. SIRT3 deacetylates FOXO3 to protect mitochondria against oxidative damage. Free radical biology & medicine. 2013;63:222–234. doi: 10.1016/j.freeradbiomed.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 106.Graff J, Kahn M, Samiei A, Gao J, Ota KT, Rei D, et al. A dietary regimen of caloric restriction or pharmacological activation of SIRT1 to delay the onset of neurodegeneration. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013;33:8951–8960. doi: 10.1523/JNEUROSCI.5657-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Corbi G, Conti V, Scapagnini G, Filippelli A, Ferrara N. Role of sirtuins, calorie restriction and physical activity in aging. Frontiers in bioscience. 2012;4:768–778. doi: 10.2741/417. [DOI] [PubMed] [Google Scholar]
- 108.Hubbard BP, Sinclair DA. Small molecule SIRT1 activators for the treatment of aging and age-related diseases. Trends in pharmacological sciences. 2014;35:146–154. doi: 10.1016/j.tips.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Baur JA. Resveratrol, sirtuins, and the promise of a DR mimetic. Mechanisms of ageing and development. 2010;131:261–269. doi: 10.1016/j.mad.2010.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Mattison JA, Wang M, Bernier M, Zhang J, Park SS, Maudsley S, et al. Resveratrol prevents high fat/sucrose diet-induced central arterial wall inflammation and stiffening in nonhuman primates. Cell metabolism. 2014;20:183–190. doi: 10.1016/j.cmet.2014.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Tong C, Morrison A, Mattison S, Qian S, Bryniarski M, Rankin B, et al. Impaired SIRT1 nucleocytoplasmic shuttling in the senescent heart during ischemic stress. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2013;27:4332–4342. doi: 10.1096/fj.12-216473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Alcendor RR, Gao S, Zhai P, Zablocki D, Holle E, Yu X, et al. Sirt1 regulates aging and resistance to oxidative stress in the heart. Circulation research. 2007;100:1512–1521. doi: 10.1161/01.RES.0000267723.65696.4a. [DOI] [PubMed] [Google Scholar]
- 113.Boon RA, Seeger T, Heydt S, Fischer A, Hergenreider E, Horrevoets AJ, et al. MicroRNA-29 in aortic dilation: implications for aneurysm formation. Circulation research. 2011;109:1115–1119. doi: 10.1161/CIRCRESAHA.111.255737. [DOI] [PubMed] [Google Scholar]
- 114.Huang J, Sun W, Huang H, Ye J, Pan W, Zhong Y, et al. miR-34a modulates angiotensin II-induced myocardial hypertrophy by direct inhibition of ATG9A expression and autophagic activity. PloS one. 2014;9:e94382. doi: 10.1371/journal.pone.0094382. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 115.Tu Y, Wan L, Bu L, Zhao D, Dong D, Huang T, et al. MicroRNA-22 downregulation by atorvastatin in a mouse model of cardiac hypertrophy: a new mechanism for antihypertrophic intervention. Cellular physiology and biochemistry : international journal of experimental cellular physiology, biochemistry, and pharmacology. 2013;31:997–1008. doi: 10.1159/000350117. [DOI] [PubMed] [Google Scholar]
- 116.Olivieri F, Lazzarini R, Recchioni R, Marcheselli F, Rippo MR, Di Nuzzo S, et al. MiR-146a as marker of senescence-associated pro-inflammatory status in cells involved in vascular remodelling. Age. 2013;35:1157–1172. doi: 10.1007/s11357-012-9440-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Maegdefessel L, Azuma J, Tsao PS. MicroRNA-29b regulation of abdominal aortic aneurysm development. Trends in cardiovascular medicine. 2014;24:1–6. doi: 10.1016/j.tcm.2013.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Boon RA, Iekushi K, Lechner S, Seeger T, Fischer A, Heydt S, et al. MicroRNA-34a regulates cardiac ageing and function. Nature. 2013;495:107–110. doi: 10.1038/nature11919. [DOI] [PubMed] [Google Scholar]
- 119.Jazbutyte V, Fiedler J, Kneitz S, Galuppo P, Just A, Holzmann A, et al. MicroRNA-22 increases senescence and activates cardiac fibroblasts in the aging heart. Age. 2013;35:747–762. doi: 10.1007/s11357-012-9407-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Xu GJ, Gan TY, Tang BP, Chen ZH, Ailiman M, Zhou XH, et al. Changes in microRNAs expression are involved in age-related atrial structural remodeling and atrial fibrillation. Chinese medical journal. 2013;126:1458–1463. [PubMed] [Google Scholar]
- 121.Abonnenc M, Nabeebaccus AA, Mayr U, Barallobre-Barreiro J, Dong X, Cuello F, et al. Extracellular matrix secretion by cardiac fibroblasts: role of microRNA-29b and microRNA-30c. Circulation research. 2013;113:1138–1147. doi: 10.1161/CIRCRESAHA.113.302400. [DOI] [PubMed] [Google Scholar]
- 122.Ungvari Z, Bailey-Downs L, Gautam T, Sosnowska D, Wang M, Monticone RE, et al. Age-associated vascular oxidative stress, Nrf2 dysfunction, and NF-{kappa}B activation in the nonhuman primate Macaca mulatta. The journals of gerontology Series A, Biological sciences and medical sciences. 2011;66:866–875. doi: 10.1093/gerona/glr092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Gareus R, Kotsaki E, Xanthoulea S, van der Made I, Gijbels MJ, Kardakaris R, et al. Endothelial cell-specific NF-kappaB inhibition protects mice from atherosclerosis. Cell metabolism. 2008;8:372–383. doi: 10.1016/j.cmet.2008.08.016. [DOI] [PubMed] [Google Scholar]
- 124.Sheydina A, Volkova M, Jiang L, Juhasz O, Zhang J, Tae HJ, et al. Linkage of cardiac gene expression profiles and ETS2 with lifespan variability in rats. Aging cell. 2012;11:350–359. doi: 10.1111/j.1474-9726.2012.00794.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Gao ZQ, Yang C, Wang YY, Wang P, Chen HL, Zhang XD, et al. RAGE upregulation and nuclear factor-kappaB activation associated with ageing rat cardiomyocyte dysfunction. General physiology and biophysics. 2008;27:152–158. [PubMed] [Google Scholar]
- 126.Gupta S, Young D, Maitra RK, Gupta A, Popovic ZB, Yong SL, et al. Prevention of cardiac hypertrophy and heart failure by silencing of NF-kappaB. Journal of molecular biology. 2008;375:637–649. doi: 10.1016/j.jmb.2007.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Satoh T, Sasatomi E, Yamasaki F, Ishida H, Wu L, Tokunaga O. Multinucleated variant endothelial cells (MVECs) of human aorta: expression of tumor suppressor gene p53 and relationship to atherosclerosis and aging. Endothelium : journal of endothelial cell research. 1998;6:123–132. doi: 10.3109/10623329809072199. [DOI] [PubMed] [Google Scholar]
- 128.Cheung TM, Ganatra MP, Peters EB, Truskey GA. Effect of cellular senescence on the albumin permeability of blood-derived endothelial cells. American journal of physiology Heart and circulatory physiology. 2012;303:H1374–H1383. doi: 10.1152/ajpheart.00182.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hashimoto M, Hossain S, Masumura S. Effect of aging on plasma membrane fluidity of rat aortic endothelial cells. Experimental gerontology. 1999;34:687–698. doi: 10.1016/s0531-5565(99)00025-x. [DOI] [PubMed] [Google Scholar]
- 130.Kliche K, Jeggle P, Pavenstadt H, Oberleithner H. Role of cellular mechanics in the function and life span of vascular endothelium. Pflugers Archiv : European journal of physiology. 2011;462:209–217. doi: 10.1007/s00424-011-0929-2. [DOI] [PubMed] [Google Scholar]
- 131.Tanaka Y, Moritoh Y, Miwa N. Age-dependent telomere-shortening is repressed by phosphorylated alpha-tocopherol together with cellular longevity and intracellular oxidative-stress reduction in human brain microvascular endotheliocytes. Journal of cellular biochemistry. 2007;102:689–703. doi: 10.1002/jcb.21322. [DOI] [PubMed] [Google Scholar]
- 132.Kurz DJ, Decary S, Hong Y, Trivier E, Akhmedov A, Erusalimsky JD. Chronic oxidative stress compromises telomere integrity and accelerates the onset of senescence in human endothelial cells. Journal of cell science. 2004;117:2417–2426. doi: 10.1242/jcs.01097. [DOI] [PubMed] [Google Scholar]
- 133.Alivernini S, Laura Bosello S, De Luca G, Canestri S, Di Mario C, Rita Gigante M, et al. A3.21 MicroRNA-34a and microRNA-155 in Systemic Sclerosis: possible epigenetic biomarkers of endothelial dysfunction in VEDOSS and long-standing disease. Annals of the rheumatic diseases. 2014;73 Suppl 1:A50. [Google Scholar]
- 134.Dellago H, Preschitz-Kammerhofer B, Terlecki-Zaniewicz L, Schreiner C, Fortschegger K, Chang MW, et al. High levels of oncomiR-21 contribute to the senescence-induced growth arrest in normal human cells and its knock-down increases the replicative lifespan. Aging cell. 2013;12:446–458. doi: 10.1111/acel.12069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Rippe C, Blimline M, Magerko KA, Lawson BR, LaRocca TJ, Donato AJ, et al. MicroRNA changes in human arterial endothelial cells with senescence: relation to apoptosis, eNOS and inflammation. Experimental gerontology. 2012;47:45–51. doi: 10.1016/j.exger.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ito T, Yagi S, Yamakuchi M. MicroRNA-34a regulation of endothelial senescence. Biochemical and biophysical research communications. 2010;398:735–740. doi: 10.1016/j.bbrc.2010.07.012. [DOI] [PubMed] [Google Scholar]
- 137.Qin B, Yang H, Xiao B. Role of microRNAs in endothelial inflammation and senescence. Molecular biology reports. 2012;39:4509–4518. doi: 10.1007/s11033-011-1241-0. [DOI] [PubMed] [Google Scholar]
- 138.Li Z, Cheng H, Lederer WJ, Froehlich J, Lakatta EG. Enhanced proliferation and migration and altered cytoskeletal proteins in early passage smooth muscle cells from young and old rat aortic explants. Experimental and molecular pathology. 1997;64:1–11. doi: 10.1006/exmp.1997.2204. [DOI] [PubMed] [Google Scholar]
- 139.Wang Y, Kuro-o M, Sun Z. Klotho gene delivery suppresses Nox2 expression and attenuates oxidative stress in rat aortic smooth muscle cells via the cAMP-PKA pathway. Aging cell. 2012;11:410–417. doi: 10.1111/j.1474-9726.2012.00796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Craige SE, Keaney JF., Jr Polyploidy and dysregulated ROS signaling: the school of hard Nox? Cell cycle. 2009;8:797. doi: 10.4161/cc.8.6.8336. [DOI] [PubMed] [Google Scholar]
- 141.McCrann DJ, Yang D, Chen H, Carroll S, Ravid K. Upregulation of Nox4 in the aging vasculature and its association with smooth muscle cell polyploidy. Cell cycle. 2009;8:902–908. doi: 10.4161/cc.8.6.7900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Ragnauth CD, Warren DT, Liu Y, McNair R, Tajsic T, Figg N, et al. Prelamin A acts to accelerate smooth muscle cell senescence and is a novel biomarker of human vascular aging. Circulation. 2010;121:2200–2210. doi: 10.1161/CIRCULATIONAHA.109.902056. [DOI] [PubMed] [Google Scholar]
- 143.Reddy S, Comai L. Lamin A, farnesylation and aging. Experimental cell research. 2012;318:1–7. doi: 10.1016/j.yexcr.2011.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Wang M, Monticone RE, Lakatta EG. Arterial aging: a journey into subclinical arterial disease. Current opinion in nephrology and hypertension. 2010;19:201–207. doi: 10.1097/MNH.0b013e3283361c0b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Burton DG, Matsubara H, Ikeda K. Pathophysiology of vascular calcification: Pivotal role of cellular senescence in vascular smooth muscle cells. Experimental gerontology. 2010;45:819–824. doi: 10.1016/j.exger.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 146.Jiang L, Zhang J, Monticone RE, Telljohann R, Wu J, Wang M, et al. Calpain-1 regulation of matrix metalloproteinase 2 activity in vascular smooth muscle cells facilitates age-associated aortic wall calcification and fibrosis. Hypertension. 2012;60:1192–1199. doi: 10.1161/HYPERTENSIONAHA.112.196840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Tae HJ, Marshall S, Zhang J, Wang M, Briest W, Talan MI. Chronic treatment with a broad-spectrum metalloproteinase inhibitor, doxycycline, prevents the development of spontaneous aortic lesions in a mouse model of vascular Ehlers-Danlos syndrome. The Journal of pharmacology and experimental therapeutics. 2012;343:246–251. doi: 10.1124/jpet.112.197020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Fleenor BS, Marshall KD, Durrant JR, Lesniewski LA, Seals DR. Arterial stiffening with ageing is associated with transforming growth factor-beta1-related changes in adventitial collagen: reversal by aerobic exercise. The Journal of physiology. 2010;588:3971–3982. doi: 10.1113/jphysiol.2010.194753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Jin X, Fu GX, Li XD, Zhu DL, Gao PJ. Expression and function of osteopontin in vascular adventitial fibroblasts and pathological vascular remodeling. PloS one. 2011;6:e23558. doi: 10.1371/journal.pone.0023558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Li XD, Chen J, Ruan CC, Zhu DL, Gao PJ. Vascular endothelial growth factor-induced osteopontin expression mediates vascular inflammation and neointima formation via Flt-1 in adventitial fibroblasts. Arteriosclerosis, thrombosis, and vascular biology. 2012;32:2250–2258. doi: 10.1161/ATVBAHA.112.255216. [DOI] [PubMed] [Google Scholar]
- 151.Wang YL, Liu LZ, He ZH, Ding KH, Xue F. Phenotypic transformation and migration of adventitial cells following angioplasty. Experimental and therapeutic medicine. 2012;4:26–32. doi: 10.3892/etm.2012.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Kajstura J, Gurusamy N, Ogorek B, Goichberg P, Clavo-Rondon C, Hosoda T, et al. Myocyte turnover in the aging human heart. Circulation research. 2010;107:1374–1386. doi: 10.1161/CIRCRESAHA.110.231498. [DOI] [PubMed] [Google Scholar]
- 153.Ball AJ, Levine F. Telomere-independent cellular senescence in human fetal cardiomyocytes. Aging cell. 2005;4:21–30. doi: 10.1111/j.1474-9728.2004.00137.x. [DOI] [PubMed] [Google Scholar]
- 154.Papp Z, Czuriga D, Balogh L, Balogh A, Borbely A. How cardiomyocytes make the heart old. Current pharmaceutical biotechnology. 2012;13:2515–2521. [PubMed] [Google Scholar]
- 155.Domenighetti AA, Wang Q, Egger M, Richards SM, Pedrazzini T, Delbridge LM. Angiotensin II-mediated phenotypic cardiomyocyte remodeling leads to age-dependent cardiac dysfunction and failure. Hypertension. 2005;46:426–432. doi: 10.1161/01.HYP.0000173069.53699.d9. [DOI] [PubMed] [Google Scholar]
- 156.Shivakumar K, Sollott SJ, Sangeetha M, Sapna S, Ziman B, Wang S, et al. Paracrine effects of hypoxic fibroblast-derived factors on the MPT-ROS threshold and viability of adult rat cardiac myocytes. American journal of physiology Heart and circulatory physiology. 2008;294:H2653–H2658. doi: 10.1152/ajpheart.91443.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]