Abstract
The purpose of this study was to compare hemodynamic and blood analyte responses to reduced central venous pressure (CVP) and pulse pressure (PP) elicited during graded lower body negative pressure (LBNP) to those observed during graded blood loss (BL) in conscious humans. We hypothesized that the stimulus-response relationships of CVP and PP to hemodynamic responses during LBNP would mimic those observed during BL. We assessed CVP, PP, heart rate, mean arterial pressure (MAP), and other hemodynamic markers in 12 men during LBNP and BL. Blood samples were obtained for analysis of catecholamines, hematocrit, hemoglobin, arginine vasopressin, and blood gases. LBNP consisted of 5-min stages at 0, 15, 30, and 45 mmHg of suction. BL consisted of 5 min at baseline and following three stages of 333 ml of hemorrhage (1,000 ml total). Individual r2 values and linear regression slopes were calculated to determine whether the stimulus (CVP and PP)-hemodynamic response trajectories were similar between protocols. The CVP-MAP trajectory was the only CVP-response slope that was statistically different during LBNP compared with BL (0.93 ± 0.27 vs. 0.13 ± 0.26; P = 0.037). The PP-heart rate trajectory was the only PP-response slope that was statistically different during LBNP compared with BL (−1.85 ± 0.45 vs. −0.46 ± 0.27; P = 0.024). Norepinephrine, hematocrit, and hemoglobin were all lower at termination in the BL protocol compared with LBNP (P < 0.05). Consistent with our hypothesis, LBNP mimics the hemodynamic stimulus-response trajectories observed during BL across a significant range of CVP in humans.
Keywords: hemorrhage, central hypovolemia, heart rate, blood pressure, stroke volume
hemorrhage is one of the main causes of death associated with civilian trauma (16, 33, 35) and is the leading cause of potentially survivable death on the battlefield (3, 14). Therefore, identifying physiological changes in response to blood loss (BL) is important because it can promote timely assessment of patient status and appropriate triage. Clinical studies of BL are difficult to perform due to the heterogeneity of patients, injuries, volume of BL, and resuscitation efforts. Standardized laboratory studies in which graded hypovolemia is induced via BL or dehydration provide a standardized way of measuring the effects of hypovolemia; however, the removal of an adequate volume of blood to mimic clinically relevant hemorrhage in conscious humans in a laboratory is invasive and may not be practical. Therefore, lower body negative pressure (LBNP) is frequently used to simulate BL in conscious humans. The application of LBNP results in a central volume shift to the lower body, which creates central hemodynamic conditions that are believed to mimic those obtained during actual BL (12). Recent evidence indicates that LBNP is a valid surrogate to simulate hemodynamic responses to BL in anesthetized baboons (23). Data obtained from human experiments also suggest that LBNP creates a hemodynamic environment that is similar to BL (20, 30, 36). In this context, it has been proposed on the basis of a review of LBNP and BL studies that LBNP creates similar compensatory and hemodynamic responses as BL (12). However, a direct comparison of physiological responses during LBNP and BL have been conducted in only two previous studies, both of which involved only a mildly reduced blood volume of 450 ml (20, 30). Notably, these studies did not compare hemodynamic responses throughout progressive reductions in circulating blood volume to responses obtained during graded LBNP.
Reductions in central blood volume cause not only changes in hemodynamics, but also blood analyte responses. Central hypovolemia generated by LBNP or BL is a strong activator of the sympathetic nervous system and increases circulating catecholamines (11, 13, 15, 24, 28). Additionally, arterial and atrial mechanical stretch receptors sense the decrease in blood pressure during acute reductions in central blood volume and initiate the release of volume-regulating hormones such as arginine vasopressin (1, 2, 11, 18, 24, 37). Therefore, it is plausible that blood analyte responses to LBNP are comparable to the blood analyte responses observed during BL. However, similar to the lack of a comparison of hemodynamic adjustments between LBNP and BL, a direct comparison of blood analyte responses to LBNP and BL has not been fully elucidated.
Despite the idea that LBNP mimics BL, a direct comparison of multiple hemodynamic and blood analyte responses to reductions in central venous pressure (CVP) and pulse pressure (PP) obtained by graded LBNP and graded BL has not been performed in conscious humans. The purpose of this study was to compare hemodynamic responses elicited during a bout of graded LBNP to those observed during graded BL in conscious humans. We hypothesized that hemodynamic responses to graded LBNP (0, 15, 30, and 45 mmHg of LBNP) would mimic hemodynamic responses observed during graded BL (0, 333, 667, and 1,000 ml of BL) across a wide range of CVP and PP, and that these responses would be strongly correlated between the two protocols. Additionally, we hypothesized that the blood analyte responses to LBNP and BL would be similar.
METHODS
Subjects
Twelve healthy men [age 32 ± 2 yr; height 181.8 ± 2.0 cm; weight 88.4 ± 2.5 kg; body mass index (BMI) 26.7 ± 0.5 kg/m2] were recruited to participate in this study. All subjects reported being free of any cardiovascular, respiratory, neurologic, or metabolic disease. Subjects were required to be nonobese (BMI <30), nonsmokers, and not taking any medications. Subjects reported to the Clinical Research Unit at Mayo Clinic at 7:00 A.M. following an overnight fast. Subjects then consumed a small breakfast bar (240 kcal, Cliff Bar; Shelton, CT) and drank 250 ml of water. Subjects were studied in the supine position in a temperature-controlled room (20–22°C). Prior to the study day, all subjects provided written informed consent after all procedures and risks of the study were fully explained, and the study was approved by the Institutional Review Board. To ensure subject safety, a board-certified anesthesiologist was present throughout the study day and a member of the Mayo Clinic autologous transfusion team was in attendance during the BL protocol.
Experimental Design
Subjects underwent an LBNP and a BL protocol on the same day in a randomized order. The goal of the experimental design was to create a wide range of CVP in both protocols. On the basis of recommendations for equating LBNP levels to BL (12), we selected the initial stages of the U.S. Army Institute for Surgical Research LBNP protocol and stepwise reductions in blood volume that would closely mirror CVP at each LBNP stage and allow hemodynamic conditions to stabilize. The order of the protocols was randomized; therefore, we were unable to match CVP values between LBNP and BL due to subject safety. Prior to the first perturbation, subjects were supine for 60–90 min (at least 30 min following invasive instrumentation). Subjects rested quietly in the supine position for 45–75 min following the first protocol. A longer duration of rest was required after the BL protocol to allow for the reinfusion of blood prior to the LBNP protocol. Arterial blood samples were obtained at baseline and at the termination of each protocol to measure circulating catecholamines, hematocrit, hemoglobin, blood gases, bicarbonate, and circulating arginine vasopressin. The protocols were terminated early if 1) mean arterial pressure (MAP) fell by 30%, 2) systolic blood pressure dropped below 80 mmHg, or 3) the subject began to experience symptoms of presyncope or syncope. Figure 1 illustrates the study timeline.
Fig. 1.
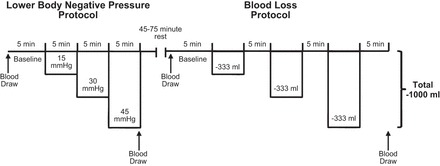
Timeline of the lower body negative pressure (LBNP) and blood loss (BL) protocols. The order of the protocols was randomized. When the LBNP protocol was performed first, 45 min of quiet rest was given between protocols to ensure hemodynamic variables returned to baseline. When BL occurred first, to allow the reinfusion of blood that had been removed, subjects rested quietly for 75 min to allow hemodynamic variables to return to baseline between protocols. Arterial blood was drawn at baseline and during the last stage of each protocol for blood gases, pH, bicarbonate, catecholamines, hematocrit, hemoglobin, and arginine vasopressin.
Measurements and Procedures
Heart rate and arterial oxygen saturation.
A three-lead electrocardiogram (EKG) was used to continuously record heart rate, and arterial oxygen saturation was obtained using a finger pulse oximeter (Cardiocap/5; Datex-Ohmeda, Louisville, CO). The EKG and pulse oximeter tracings were interfaced with a personal computer for continuous measurements.
Central venous pressure.
A 16-gauge peripherally inserted central catheter was introduced into an antecubital vein under local anesthesia (2% lidocaine) using aseptic techniques and advanced until an appropriate CVP waveform was obtained. This catheter was connected to a high-resolution transducer (FloTrac; Edwards Lifesciences, Irvine, CA) positioned at heart level and interfaced with a personal computer for continuous measurement of CVP.
Blood removal.
A second 14-gauge catheter was placed in an antecubital vein to facilitate blood removal for the BL protocol. The catheter was placed under local anesthesia (2% lidocaine) using aseptic techniques. Preservative/anticoagulant bags (63 ml anticoagulant citrate phosphate dextrose solution) were placed below the subject to allow blood to transfer from the subject to the blood collection bags via gravity. In two subjects, a blood pressure cuff was inflated around the upper arm to 40 mmHg to enhance the rate of blood removal and this cuff pressure was released during all measurements. As blood was being collected, it was weighed to determine the volume of blood removed by multiplying the weight of the blood by 1.06 ml/g. The removed blood was kept in the study room (20–22°C) and the temperature of the blood was allowed fluctuate. At the end of the BL protocol, blood was reinfused at a rate of 20 ml/min into the antecubital vein.
Intra-arterial blood pressure, stroke volume, and cardiac output.
A 20-gauge, 5-cm catheter was placed into a brachial artery under local anesthesia (2% lidocaine) using aseptic techniques and ultrasound guidance. The catheter was attached to a high-resolution transducer (FloTrac; Edwards Lifesciences) positioned at heart level and interfaced with a personal computer to obtain continuous beat-by-beat arterial pressure wave forms. PP was calculated as the difference between systolic and diastolic blood pressure. Model flow analysis software (WinCPRS; Absolute Aliens Oy, Turku, Finland) was used to calculate beat-by-beat stroke volume and cardiac output (38). Total peripheral resistance was calculated as MAP divided by cardiac output.
Blood sampling and oxygen delivery.
Arterial blood samples were collected at baseline and at the termination of each protocol for measurement of the partial pressure of oxygen and carbon dioxide, pH, bicarbonate, hematocrit, hemoglobin, catecholamines, and arginine vasopressin. Blood samples were analyzed by the Immunochemistry Core Laboratory of the Clinical Research Unit of the Mayo Clinic Center for Clinical and Translational Science. Oxygen delivery was calculated as follows: [1.39 × hemoglobin concentration × arterial oxygen saturation + (0.003 × partial pressure of oxygen)] × cardiac output.
LBNP protocol.
Subjects laid in an airtight LBNP chamber sealed at the iliac crest. The LBNP protocol was based on the first three stages of the protocol commonly used by the U.S. Army Institute for Surgical Research (5, 7, 9, 22, 31, 32). The protocol consisted of a 5-min baseline period followed by 5 min at 15, 30, and 45 mmHg of suction. Subjects were not allowed to cross their legs and were instructed to refrain from contracting any muscles in the lower body throughout the protocol.
BL protocol.
Following a 5-min baseline period, three aliquots of 333 ml of blood were removed via gravity from an antecubital vein. A 5-min period separated each aliquot to emulate the three stages of LBNP. The removed blood was stored in standard preservative/anticoagulant bags and was periodically agitated to prevent clotting. Subjects were instructed not to cross their legs or contract any muscles in their lower body throughout the protocol.
Data and Statistical Analysis
Data were collected and variables were analyzed offline using signal processing software (WinDaq; DATAZ Instruments, Akron, OH). Data were analyzed and averaged over the last 2 min of each stage for statistical analysis. To explore the relationship between BL and LBNP, individual subject r2 values and linear regression line slopes were calculated for each variable for both protocols. Paired t-tests were used to determine whether the r2 values and slopes of the regression lines of the hemodynamic variables fell on similar trajectories throughout a range of CVP and PP during each protocol. If data were not normally distributed, the Wilcoxon signed rank test was used. The amalgamated r2 value and linear regression line slopes were also calculated using linear regression analysis using group mean values obtained at each stage vs. the group mean CVP and PP obtained during each stage of both protocols. Protocol × stage (2 × 4) repeated measures ANOVA was used to determine whether values obtained during the LBNP protocol were similar to the corresponding stages of the BL protocol. If a significant main or interaction effect was obtained, Tukey's post hoc analysis was performed to determine where differences existed. Group data are presented as mean ± SE. The alpha level was set at 0.05.
RESULTS
Of the 12 subjects who volunteered to participate in this study, 2 did not complete both protocols (both subjects completed 667 ml of BL and 30 mmHg of LBNP); additionally, 1 subject did not complete the LBNP protocol (completed 30 mmHg of LBNP), and 1 subject did not complete the BL protocol (completed 333 ml of BL). These protocols were terminated early due to presyncope symptoms or syncope. Data obtained from subjects who did not complete 667 ml of BL or 30 mmHg of LBNP were excluded from regression analyses. A sample size of 8 subjects (age 32 ± 3 yr; height 185.3 ± 1.8 cm; weight 91.3 ± 3.4 kg; BMI 26.6 ± 0.8 kg/m2) was used for ANOVA analyses due to the missing data points. The mean time for blood removal was 483 ± 163 s (∼41 ml/min) for the three aliquots. The first aliquot took 538 ± 134 s (∼37 ml/min), the second aliquot took 468 ± 160 s (∼43 ml/min), and the final aliquot took 436 ± 194 s (∼46 ml/min). The time to fill each aliquot was not statistically distinguishable (P = 0.068). The correlation of the amalgamated hemodynamic values obtained during BL and LBNP are presented in Figure 2. Tables 1 and 2 display the mean and range of individual r2 values of the hemodynamic variables vs. CVP and PP, respectively. The mean and range of individual regression line slope values of hemodynamic variables vs. CVP and PP are presented in Tables 1 and 2 as well. The mean and individual hemodynamic values generated at each stage across the range of CVP and PP during LBNP and BL are displayed in Figs. 3 and 4, respectively. The mean hemodynamic values obtained at each stage during both protocols are presented in Table 3. The mean and range of individual regression line slope values achieved by plotting blood analyte responses against CVP and PP are displayed in Table 4. The mean blood analyte data obtained at baseline and at protocol termination are presented in Table 5. The mean and individual catecholamine values generated at baseline and at protocol termination plotted against CVP and PP are displayed in Fig. 5.
Fig. 2.
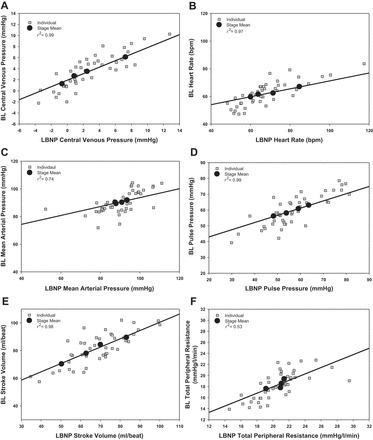
Correlation of amalgamated hemodynamic values obtained during LBNP vs. BL.
Table 1.
Mean and range of individual r2 values and individual regression line slope values of hemodynamic variables vs. central venous pressure
| r2 | r2 Range | Slope | Slope Range | |
|---|---|---|---|---|
| Heart rate | ||||
| LBNP | 0.67 | 0.14–0.95 | −2.42 | −4.58 to −0.46 |
| BL | 0.54 | 0.02–0.98 | −1.53 | −5.29–0.12 |
| MAP | ||||
| LBNP | 0.68 | 0.23–0.99 | 0.93 | 0.08–3.48 |
| BL | 0.35* | <0.01–0.66 | 0.13* | −1.30–2.12 |
| Pulse pressure | ||||
| LBNP | 0.72 | 0.01–0.97 | 1.81 | 0.03–4.37 |
| BL | 0.49 | <0.01–0.92 | 1.23 | 0.09–3.63 |
| SaO2 | ||||
| LBNP | 0.52 | 0.03–0.95 | −0.04 | −0.28–0.23 |
| BL | 0.46 | 0.01–0.98 | −0.04 | −0.68–0.32 |
| Stroke volume | ||||
| LBNP | 0.85 | 0.44–0.99 | 3.69 | 1.86–5.26 |
| BL | 0.73 | <0.01–0.93 | 3.59 | 0.25–5.74 |
| Cardiac output | ||||
| LBNP | 0.68 | 0.18–0.99 | 0.10 | −0.02–0.24 |
| BL | 0.61 | <0.01–0.98 | 0.10 | −0.13–0.31 |
| TPR | ||||
| LBNP | 0.51 | 0.24–0.99 | −0.26 | −0.63–0.17 |
| BL | 0.53 | 0.02–0.99 | 0.07 | −0.75–0.99 |
BL, blood loss; LBNP, lower body negative pressure; MAP, mean arterial pressure; SaO2, arterial oxygen saturation; TPR, total peripheral resistance.
Different from LBNP, P < 0.05.
Table 2.
Mean and range of individual r2 values and individual regression line slope values of hemodynamic variables vs. pulse pressure
| r2 | r2 Range | Slope | Slope Range | |
|---|---|---|---|---|
| Heart rate | ||||
| LBNP | 0.86 | 0.57–0.98 | −1.85 | −6.12 to −0.38 |
| BL | 0.57* | 0.03–0.99 | −0.46* | −2.19–0.76 |
| MAP | ||||
| LBNP | 0.70 | 0.04–1.00 | 0.43 | −0.29–1.95 |
| BL | 0.43 | <0.01–0.66 | 0.67 | −0.20–1.52 |
| SaO2 | ||||
| LBNP | 0.59 | <0.01–0.99 | 0.08 | −0.14–1.05 |
| BL | 0.42 | <0.01–0.99 | −0.01 | −0.40–0.31 |
| Stroke volume | ||||
| LBNP | 0.86 | 0.49–0.99 | 2.76 | 1.16–8.94 |
| BL | 0.86 | 0.29–1.00 | 2.17 | 1.02–4.74 |
| Cardiac output | ||||
| LBNP | 0.69 | <0.01–0.96 | 0.06 | <0.01–0.11 |
| BL | 0.66 | 0.03–0.99 | 0.09 | <−0.01–0.22 |
| TPR | ||||
| LBNP | 0.52 | <0.01–0.96 | −0.19 | −0.68–0.04 |
| BL | 0.64 | <0.01–0.99 | −0.18 | −0.47–0.12 |
Different from LBNP, P < 0.05.
Fig. 3.
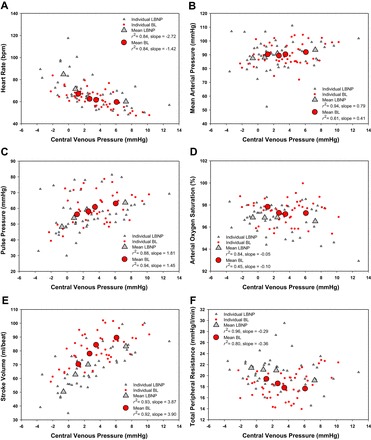
Mean and individual hemodynamic values obtained at each stage across the range of central venous pressures during the LBNP and BL protocols.
Fig. 4.
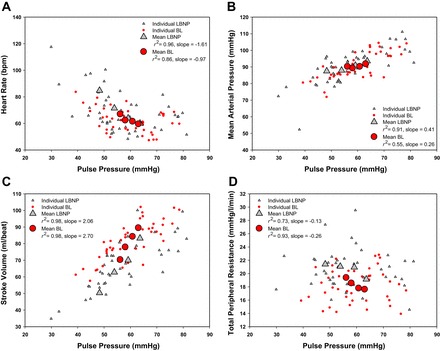
Mean and individual hemodynamic values obtained at each stage across the range of pulse pressures during the LBNP and BL protocols.
Table 3.
Hemodynamic responses during each stage of lower body negative pressure and blood loss
| Baseline | Stage 1 | Stage 2 | Stage 3 | |
|---|---|---|---|---|
| CVP (mmHg) | ||||
| LBNP | 7.1 ± 0.9 | 2.6 ± 0.9a | 0.8 ± 0.8ab | −0.6 ± 0.8abc |
| BL | 5.9 ± 0.8 | 3.6 ± 0.9a | 2.2 ± 1.1abd | 1.2 ± 1.1abd |
| Heart rate (bpm) | ||||
| LBNP | 62 ± 2.8 | 64 ± 3.8 | 71 ± 5.2ab | 83 ± 6.3abc |
| BL | 59 ± 3.4 | 60 ± 3.0 | 63 ± 2.8d | 67 ± 3.5ad |
| MAP (mmHg) | ||||
| LBNP | 95.1 ± 3.2 | 93.0 ± 3.3 | 92.3 ± 3.3 | 87.3 ± 4.0e |
| BL | 91.4 ± 2.7 | 91.0 ± 2.4 | 90.3 ± 3.0 | 89.2 ± 2.7e |
| PP (mmHg) | ||||
| LBNP | 66.2 ± 4.3 | 62.2 ± 4.3 | 57.4 ± 3.8a | 48.7 ± 4.9abc |
| BL | 63.8 ± 3.9 | 61.9 ± 3.4 | 58.6 ± 4.1 | 55.7 ± 4.1ad |
| SaO2 (%) | ||||
| LBNP | 96.5 ± 0.7 | 97.0 ± 0.5 | 96.9 ± 0.4 | 96.9 ± 0.3 |
| BL | 97.5 ± 0.3 | 97.5 ± 0.3 | 97.4 ± 0.3 | 98.1 ± 0.4d |
| SV (ml/beat) | ||||
| LBNP | 82.8 ± 3.1 | 72.8 ± 3.0a | 63.9 ± 3.4ab | 50.8 ± 3.7abc |
| BL | 88.8 ± 3.1d | 83.8 ± 2.6d | 77.6 ± 3.4ad | 70.6 ± 4.0abcd |
| CO (liter/min) | ||||
| LBNP | 5.2 ± 0.4 | 4.6 ± 0.2 | 4.5 ± 0.3e | 4.1 ± 0.2ef |
| BLd | 5.3 ± 0.4 | 5.1 ± 0.3 | 4.8 ± 0.2e | 4.7 ± 0.2ef |
| TPR (mmHg/l/min) | ||||
| LBNP | 18.9 ± 0.9 | 20.4 ± 0.8 | 21.0 ± 1.1e | 21.6 ± 1.0e |
| BLd | 17.9 ± 1.2 | 18.2 ± 1.0 | 18.9 ± 0.8e | 19.3 ± 0.7e |
bpm, beats per minute; CVP, central venous pressure; PP, pulse pressure; SV, stroke volume; CO, cardiac output. Stage 1 of LBNP = 15 mmHg, Stage 2 of LBNP 30 mmHg, Stage 3 = 45 mmHg. Stage 1 of BL = 333 ml, Stage 2 of BL = 667 ml, Stage 3 of BL = 1,000 ml. Values are means ± SE, n = 8.
Different from baseline, P < 0.05;
different from Stage 1, P < 0.05;
different from Stage 2, P < 0.05;
different from LBNP, P < 0.05;
stage main effect, different from baseline, P < 0.05;
stage main effect, different from Stage 1, P = 0.025.
Table 4.
Mean and range of individual regression line slope values of blood analyte and oxygen delivery responses vs. central venous pressure and pulse pressure
| Central Venous Pressure |
Pulse Pressure |
|||
|---|---|---|---|---|
| Slope | Slope Range | Slope | Slope Range | |
| Norepinephrine | ||||
| LBNP | −27.8 | −80.7–0.6 | −30.3 | −178.1–0.2 |
| BL | −8.7* | −32.7–39.5 | −5.1 | −42.7–45.0 |
| Epinephrine | ||||
| LBNP | −13.5 | −48.3–0.0 | −8.9 | −24.9–0.0 |
| BL | −14.5 | −36.3 to −1.2 | −1.6 | −12.1–45.0 |
| Hematocrit | ||||
| LBNP | −0.22 | −0.58–0.07 | −0.50 | −4.98–0.08 |
| BL | 0.24* | −0.33–1.18 | 0.04* | −1.17–0.73 |
| Hemoglobin | ||||
| LBNP | −0.07 | −0.18–0.05 | −0.16 | −1.53–0.02 |
| BL | 0.08* | −0.17–0.30 | −0.02* | −0.45–0.11 |
| Arginine Vasopressin | ||||
| LBNP | −2.1 | −7.8–0.1 | −1.0 | −3.2–0.2 |
| BL | −4.5 | −20.8–0.6 | −0.9 | −3.6–0.9 |
| Oxygen delivery | ||||
| LBNP | 17.3 | −10.6–74.2 | 1.8 | −91.4–27.8 |
| BL | 38.5 | −25.8–137.3 | 10.2 | −142.8–58.2 |
Different from LBNP, P < 0.05.
Table 5.
Blood analyte and oxygen delivery responses at baseline and at termination of LBNP and blood loss protocols
| Baseline | Termination | |
|---|---|---|
| Norepinephrine (pg/ml) | ||
| LBNP | 148 ± 20 | 354 ± 44† |
| BL | 155 ± 22 | 211 ± 29‡ |
| Epinephrine (pg/ml) | ||
| LBNP | 53 ± 7 | 144 ± 30* |
| BL | 49 ± 7 | 103 ± 19* |
| Hematocrit (%) | ||
| LBNP | 40.8 ± 0.8 | 42.4 ± 0.8† |
| BL | 41.1 ± 0.8 | 40.3 ± 0.9†‡ |
| Hemoglobin (g/dl) | ||
| LBNP | 14.2 ± 0.4 | 14.7 ± 0.4† |
| BL | 14.3 ± 0.4 | 14.0 ± 0.4†‡ |
| Oxygen (mmHg) | ||
| LBNP | 99.5 ± 2.9 | 97.8 ± 3.1 |
| BL | 105.1 ± 3.5 | 103.8 ± 2.7 |
| CO2 (mmHg) | ||
| LBNP | 42.1 ± 0.9 | 41.7 ± 1.0 |
| BL | 41.5 ± 0.6 | 41.0 ± 0.7 |
| pH | ||
| LBNP | 7.42 ± 0.01 | 7.41 ± 0.01 |
| BL | 7.42 ± 0.01 | 7.42 ± 0.01 |
| Bicarbonate (mmol/liter) | ||
| LBNP | 26.2 ± 0.2 | 25.8 ± 0.4* |
| BL | 26.2 ± 0.3 | 25.8 ± 0.3* |
| Arginine vasopressin (pg/ml) | ||
| LBNP | 2.8 ± 0.7 | 19.1 ± 6.2* |
| BL | 3.4 ± 0.7 | 13.5 ± 4.0* |
| Oxygen delivery (ml/min) | ||
| LBNP | 948 ± 57 | 809 ± 32* |
| BL‡ | 1036 ± 59 | 926 ± 37* |
Values are means ± SE, n = 12.
Stage main effect, different from baseline, P < 0.05;
different from baseline, P < 0.05;
different from LBNP, P < 0.05.
Fig. 5.
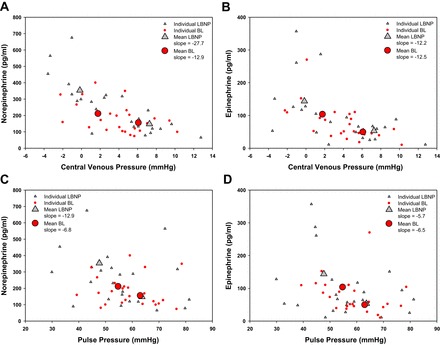
Mean and individual catecholamine values obtained at baseline and protocol termination across the range of central venous pressures and pulse pressures during the LBNP and BL protocols.
Central Venous Pressure
There was a strong correlation of amalgamated CVP values between BL and LBNP (r2 = 0.99) (Fig. 2A).
Heart Rate
There was a strong correlation of amalgamated heart rate values between BL and LBNP (r2 = 0.97) (Fig. 2B). Individual r2 values (P = 0.371) and regression line slopes (P = 0.158) generated from the relationships between heart rate and CVP from each protocol were statistically similar between BL and LBNP (Table 1). Individual r2 values (P = 0.010) and regression line slopes (P = 0.024) produced from the relationships between heart rate and PP from both protocols were statistically greater in LBNP compared with BL (Table 2).
Mean Arterial Pressure
There was a good correlation of amalgamated MAP values between BL and LBNP (r2 = 0.74) (Fig. 2C). Individual r2 values (P = 0.007) and regression line slopes (P = 0.037) produced from the relationships between MAP and CVP from each protocol were statistically greater in LBNP compared with BL (Table 1). Individual r2 values (P = 0.902) and regression line slopes (P = 0.567) produced from the relationships between MAP and PP from each protocol were not statistically similar between BL and LBNP (Table 2).
Pulse Pressure
There was a strong correlation of amalgamated PP values between BL and LBNP (r2 = 0.99) (Fig. 2D). Individual r2 values (P = 0.113) and regression line slopes (P = 0.105) calculated from the relationships between PP and CVP from each protocol were statistically similar between BL and LBNP (Table 1).
Arterial Oxygen Saturation, Blood Gases, Hematocrit, and Hemoglobin
Individual r2 values (P = 0.733) and regression line slopes (P = 0.999) calculated from the relationships between arterial oxygen saturation and CVP from the LBNP and BL protocols were not distinguishable (Table 1). Individual r2 values (P = 0.311) and regression line slopes (P = 0.102) generated from the relationship between arterial oxygen saturation and PP from each protocol were not statistically distinguishable between BL and LBNP (Table 2). The arterial partial pressure of oxygen, partial pressure of carbon dioxide, and pH responses were similar between BL and LBNP at baseline and protocol termination (Table 5). The regression line slopes for the arterial partial pressure of oxygen, partial pressure of carbon dioxide, and pH were not different between BL and LBNP when the responses were plotted against CVP or PP (P > 0.05). The regression line slopes of hematocrit plotted against CVP and PP were different between BL and LBNP (P = 0.002 and P < 0.001, respectively) (Table 4). The regression line slopes of hemoglobin plotted against CVP and PP were also different between protocols (P = 0.001 and P = 0.027, respectively) (Table 4).
Stroke Volume
There was a strong correlation of amalgamated stroke volume values between BL and LBNP (r2 = 0.98) (Fig. 2E). Individual r2 values (P = 0.232) and regression line slopes (P = 0.636) produced from the relationships between stroke volume and CVP from each protocol were statistically similar between BL and LBNP (Table 1). Individual r2 values (P = 0.978) and regression line slopes (P = 0.922) obtained from the relationships between stroke volume and PP from each protocol were statistically similar between BL and LBNP (Table 2).
Cardiac Output
There was a good correlation of amalgamated cardiac output values between BL and LBNP (r2 = 0.80). Individual r2 values (P = 0.433) and regression line slopes (P = 0.642) generated from the relationships between cardiac output and CVP from each protocol were statistically similar between BL and LBNP (Table 1). Individual r2 values (P = 0.945) and regression line slopes (P = 0.121) produced from the relationships between cardiac output and PP from each protocol were statistically similar between BL and LBNP (Table 2).
Oxygen Delivery
The regression line slope generated from the relationship between oxygen delivery and CVP during LBNP was not statistically different from the slope obtained during BL (P = 0.164) (Table 4). The regression line slope produced from the relationship between oxygen delivery and PP was also statistically indistinguishable between LBNP and BL (P = 0.064) (Table 4).
Total Peripheral Resistance
There was a modest correlation of amalgamated total peripheral resistance values between BL and LBNP (r2 = 0.53) (Fig. 2F). Individual r2 values (P = 0.907) and regression line slopes (P = 0.124) produced from the relationships between total peripheral resistance and CVP from each protocol were statistically similar between BL and LBNP (Table 1). Individual r2 values (P = 0.364) and regression line slopes (P = 0.849) generated from the relationships between stroke volume and PP from each protocol were statistically similar between BL and LBNP (Table 2).
Catecholamines
The regression line slope generated from the relationship between norepinephrine and CVP during LBNP was steeper than the slope obtained during BL (P = 0.011) (Table 4). The regression line slopes produced from the relationships between norepinephrine and PP from each of the protocols were not statistically distinguishable (P = 0.129) (Table 4). The regression line slope produced from the relationship between epinephrine and CVP (P = 0.816) and between epinephrine and PP (P = 0.470) were not different between protocols.
Arginine Vasopressin
The regression line slopes obtained from plotting arginine vasopressin against CVP were not statistically distinguishable between BL and LBNP (P = 0.152) (Table 4). Additionally, the regression line slopes generated between arginine vasopressin and PP were not different between protocols (P = 0.936) (Table 4).
DISCUSSION
The overarching results of this investigation indicate that LBNP elicits similar hemodynamic stimulus-response relationships as BL throughout the ranges of CVP and PP that were attained. That is, with the exception of heart rate and MAP, the relationship between indices of central blood volume (i.e., CVP and PP) and hemodynamic responses produced by stepwise decreases in circulating blood volume were mimicked by progressive reductions in LBNP. This is demonstrated by the similar response trajectories across the wide range of CVPs and PPs that were achieved for multiple hemodynamic variables between the two protocols. Therefore, our results provide the first direct comparison of data from human subjects who have undergone more than 450 ml of BL and LBNP. Furthermore, these data support our hypothesis that LBNP models multiple hemodynamic responses induced by hemorrhage.
Heart rate during the LBNP and BL protocols followed similar trajectories throughout the range of CVP. Rea et al. (30) found that heart rate increased 3 beats per minute (bpm) following only 450 ml of BL (reduced CVP by ∼2.4 mmHg), whereas heart rate remained unchanged during 15 mmHg of LBNP (reduced CVP by ∼3.8 mmHg). These small changes in heart rate following a modest volume of BL suggest that heart rate might respond differently to BL compared with LBNP. As a clinical perspective, an elevation in heart rate caused by hemorrhage is a mechanism used to assess patient status in trauma situations. However, the results presented here reinforce the idea that tachycardic heart rates may not necessarily reflect the severity of BL or predict impending hemodynamic collapse (8, 34). Removing up to 17% of total blood volume and reducing CVP to as low as −2 mmHg did not elicit a heart rate greater than 100 bpm in any subject during the BL protocol. Furthermore, only one subject achieved a heart rate greater than 100 bpm during the last two stages of the LBNP protocol. In this context, an increase in total peripheral resistance appears to be a main contributor to the defense of MAP during central hypovolemia. Our data support this idea because we observed an increase in total peripheral resistance while MAP remained unchanged during the first two stages of the LBNP and BL protocols. Additionally, it has been shown that muscle sympathetic nerve activity increases while heart rate and MAP remain stable during low levels of LBNP (30).
Although MAP correlated well between LBNP and BL, the response trajectories across a wide range of CVPs differed statistically between the protocols. MAP was statistically unaltered throughout the early stages of both BL and LBNP and was lower than baseline only during the final stage of both protocols. This observation is consistent with previous reports that indicate that MAP is well defended despite progressive reductions in central blood volume (6, 10). The well-defended MAP during the BL protocol also highlights the concept that using MAP to monitor patients during hemorrhage may not provide accurate information regarding hemodynamic stability (10). In this context, of the three subjects who did not complete the entire BL protocol due to presyncope symptoms or syncope did not exhibit unusually low MAP. The final MAP prior to protocol termination in these subjects was 75 mmHg following 333 ml of BL, and 72 and 85 mmHg following 667 ml of BL. Furthermore, MAP was either unchanged or slightly increased following 1,000 ml of BL in five subjects.
Importantly, stroke volume was well correlated between LBNP and BL, and the stroke volume response trajectories across the range of CVPs were remarkably similar between the LBNP and BL protocol. Reductions in stroke volume are an early indicator that central blood volume has decreased and stroke volume has been shown to fall during progressive reductions in LBNP (4, 5, 7, 20, 22, 29, 31, 32). However, previous studies have not compared stroke volume during graded LBNP to graded BL in humans. In baboons, decreases in stroke volume were nearly identical during LBNP and BL across an extensive range of CVP (23). Our results in humans reinforce the baboon data indicating that stroke volume during graded LBNP accurately models the changes in stroke volume obtained during graded BL. Additionally, aside from CVP, stroke volume was the first hemodynamic variable measured that was statistically different from baseline following 667 ml of BL. In this context, stroke volume also had the greatest decrease from baseline to protocol termination in the subjects who were unable to complete LBNP and BL protocols. In these nonfinishers, stroke volume fell by 10–36% before LBNP protocol termination and stroke volume decreased by 16–25% prior to the cessation of the BL protocol. Therefore, these data support the idea that monitoring stroke volume during BL provides an accurate reflection of decreases in blood volume (26) and tracking stroke volume might provide caregivers vital hemodynamic information that could be used to prevent cardiovascular collapse.
We found statistically different hematocrit and hemoglobin responses to the LBNP and BL protocols. These findings are similar to those observed in anesthetized baboons (23). The reduction in hematocrit and hemoglobin during BL represents a shift of fluid from the extravascular to the intravascular space to counteract the reduction in circulating blood volume (1, 25, 27). Whereas the increase in hematocrit and hemoglobin during LBNP is likely due to a plasma volume shift from the intravascular to extravascular space in the lower body as a result of the large pressure gradient that occurs during LBNP. The differences in hemoglobin, hematocrit, and cardiac output between the protocols generated a lower calculated oxygen delivery during LBNP compared with BL. However, it is doubtful that the disparities in oxygen delivery caused significant physiological changes in tissue oxygenation during LBNP and BL. This is reinforced by our observation that blood pH and the partial pressure of carbon dioxide were unaffected in both protocols.
The circulating catecholamine responses in our LBNP and BL protocols differ from those obtained in baboons (23). We did not observe a statistically significant increase in circulating norepinephrine during the BL protocol but we did observe an increase during LBNP. The mean norepinephrine response in the subjects who completed the BL protocol (65% increase) was nearly 100% lower than the mean values obtained during the LBNP protocol (162% increase). The three subjects who did not complete the BL protocol had abnormally low norepinephrine responses (change from baseline values were −47%, −15%, and 7%). Regardless of the statistically similar epinephrine responses, it is plausible that our BL protocol did not activate the sympathetic nervous system to the same extent as the LBNP protocol. This finding is consistent with our observation that total peripheral resistance was consistently lower during BL compared with the LBNP protocol.
Despite lower CVP and PP during the last LBNP stage compared with the final BL stage, we observed statistically similar arginine vasopressin responses to both protocols. The increase in arginine vasopressin in both protocols is not surprising (1, 2, 11, 18, 24, 37). However, when baboons underwent LBNP and BL protocols, and CVP and PP were matched between protocols, the arginine vasopressin response was lower during LBNP compared with BL (23). It was speculated that arginine vasopressin may be differentially released between the protocols possibly due to a decrease in oxygen delivery during BL as a result of decreases in hematocrit, hemoglobin, and central venous oxygen saturation (23). Our data contrast this idea because the arginine vasopressin response was statistically similar between LBNP and BL even though oxygen delivery was greater in BL compared with LBNP. Previous reports have suggested that arginine vasopressin is associated with presyncope symptoms (2, 11, 18). Therefore, we examined the six individual protocols (three LBNP and three BL) that were not completed and found that five of the six arginine vasopressin responses were considerably large. The mean increase in arginine vasopressin in these individual protocols was nearly five times the mean value obtained from the subjects who completed the protocols. In this context, arginine vasopressin might be an additional marker that can be used in conjunction with the monitoring of other hemodynamic variables such as stroke volume, which would give caregivers insight on patient stability during hemorrhagic trauma.
Limitations
Several limitations pertain to our study. First, we removed blood volume using three equal aliquots that were not based on a percentage of total blood volume. LBNP protocols are also not based on body size, it is difficult to measure the volume of blood that shifts from the thorax to the capacitance vessels in the legs during LBNP, and this volume likely varies from person to person. In this context, LBNP might substantially impede the mobilization of sequestered blood in the leg capacitance vessels to the central circulation via changes in intrathoracic pressure during breathing when compared with BL. That is, the respiratory pump might lose its effectiveness in aiding venous return for a given reduction in central blood volume during LBNP, whereas changes in intrathoracic pressures during BL are not competing against lower body suction for blood volume. This effect might contribute to the divergent stimulus-response trajectories between LBNP and BL in some hemodynamic variables. The physiological consequence of this potential sequestration effect during LBNP needs to be compared with absolute reductions in blood volume during BL to fully elucidate the potential impact it has on the respiratory pump. Second, we did not take all subjects in both protocols to tolerance due to subject safety. Therefore, it remains unclear whether the response trajectories remain similar between LBNP and BL at lower levels of central hypovolemia. Third, we were unable to match the rate of negative pressure change during the LBNP protocol to the rate of blood removal during BL because we randomized the order of the protocols. This temporal difference between protocols may have differentially influenced hemodynamic adjustments to changes in central blood volume during the BL protocol compared with the LBNP protocol. However, we allowed 3 min after progressing to the next LBNP stage and following the removal of each aliquot of blood to reach a stable hemodynamic state prior to data analysis. Fourth, we collected blood only at baseline and at the termination of each protocol. Therefore, we cannot discern whether the blood analyte responses are linear throughout the CVPs and PPs obtained during each protocol. Fifth, we did not test women. Because women have lower total blood volume than men, removing 1,000 ml of blood would likely increase the risk of cardiovascular collapse. We found it interesting that we may have observed differential responses between men and women because women typically have a lower orthostatic stress tolerance than men (17), and hemodynamic responses to orthostatic stress are different between sexes (19). Furthermore, young women appear to regulate blood pressure differently than young men (21), which may influence hemodynamic responses to central hypovolemia.
Conclusions
We observed striking similarities between LBNP and BL in the stimulus-response relationships of CVP and PP to hemodynamic responses. As such, LBNP mimics the trajectories of the hemodynamic responses observed during BL across a significant range of CVP and PP in humans. Therefore, our data support the hypothesis that LBNP adequately reflects the hemodynamic responses observed during BL.
GRANTS
Support for this study was provided by U.S. Army MRMC Combat Casualty Care Research Program Grant W81XWH-11–1-0823, American Heart Association Midwest Affiliate Grant 13POST-14380027 to B.D.J., and by Dutch Heart Foundation E. Dekker Stipend 2012SB013 to N.V.H.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: B.D.J., T.B.C., C.M.v.B., V.A.C., and M.J.J. conception and design of research; B.D.J., N.v.H., T.B.C., C.M.v.B., and M.J.J. performed experiments; B.D.J. and N.v.H. analyzed data; B.D.J., T.B.C., V.A.C., and M.J.J. interpreted results of experiments; B.D.J. prepared figures; B.D.J. drafted manuscript; B.D.J., N.v.H., T.B.C., C.M.v.B., V.A.C., and M.J.J. edited and revised manuscript; B.D.J., N.v.H., T.B.C., C.M.v.B., V.A.C., and M.J.J. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank the subjects for participating in the study. We also thank Margaret McGill-Zimny and David Warren for their assistance with the blood loss protocol.
REFERENCES
- 1.Arnauld E, Czernichow P, Fumoux F, Vincent JD. The effects of hypotension and hypovolaemia on the liberation of vasopressin during haemorrhage in the unanaesthetized monkey (Macaca mulatta). Pflugers Arch 371: 193–200, 1977. [DOI] [PubMed] [Google Scholar]
- 2.Baylis PH, Stockley RA, Heath DA. Influence of lower body negative pressure upon arginine vasopressin release. Clin Endocrinol 9: 89–95, 1978. [DOI] [PubMed] [Google Scholar]
- 3.Champion HR, Bellamy RF, Roberts CP, Leppaniemi A. A profile of combat injury. J Trauma 54, Suppl 5: 13–19, 2003. [DOI] [PubMed] [Google Scholar]
- 4.Convertino VA, Cooke WH, Holcomb JB. Arterial pulse pressure and its association with reduced stroke volume during progressive central hypovolemia. J Trauma 61: 629–634, 2006. [DOI] [PubMed] [Google Scholar]
- 5.Convertino VA, Grudic G, Mulligan J, Moulton S. Estimation of individual-specific progression to impending cardiovascular instability using arterial waveforms. J Appl Physiol 115: 1196–1202, 2013. [DOI] [PubMed] [Google Scholar]
- 6.Convertino VA, Moulton SL, Grudic GZ, Rickards CA, Hinojosa-Laborde C, Gerhardt RT, Blackbourne LH, Ryan KL. Use of advanced machine-learning techniques for noninvasive monitoring of hemorrhage. J Trauma 71, Suppl 1: S25–S32, 2011. [DOI] [PubMed] [Google Scholar]
- 7.Convertino VA, Rickards CA, Lurie KG, Ryan KL. Hyperventilation in response to progressive reduction in central blood volume to near syncope. Aviat Space Environ Med 80: 1012–1017, 2009. [DOI] [PubMed] [Google Scholar]
- 8.Convertino VA, Rickards CA, Ryan KL. Autonomic mechanisms associated with heart rate and vasoconstrictor reserves. Clin Auton Res 22: 123–130, 2012. [DOI] [PubMed] [Google Scholar]
- 9.Convertino VA, Ryan KL, Rickards CA, Cooke WH, Idris AH, Metzger A, Holcomb JB, Adams BD, Lurie KG. Inspiratory resistance maintains arterial pressure during central hypovolemia: implications for treatment of patients with severe hemorrhage. Crit Care Med 35: 1145–1152, 2007. [DOI] [PubMed] [Google Scholar]
- 10.Convertino VA, Ryan KL, Rickards CA, Salinas J, McManus JG, Cooke WH, Holcomb JB. Physiological and medical monitoring for en route care of combat casualties. J Trauma 64, Suppl 4: S342–S353, 2008. [DOI] [PubMed] [Google Scholar]
- 11.Convertino VA, Sather TM. Vasoactive neuroendocrine responses associated with tolerance to lower body negative pressure in humans. Clin Physiol 20: 177–184, 2000. [DOI] [PubMed] [Google Scholar]
- 12.Cooke WH, Ryan KL, Convertino VA. Lower body negative pressure as a model to study progression to acute hemorrhagic shock in humans. J Appl Physiol 96: 1249–1261, 2004. [DOI] [PubMed] [Google Scholar]
- 13.Cvirn G, Schlagenhauf A, Leschnik B, Koestenberger M, Roessler A, Jantscher A, Vrecko K, Juergens G, Hinghofer-Szalkay H, Goswami N. Coagulation changes during presyncope and recovery. PloS One 7: e42221, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eastridge BJ, Mabry RL, Seguin P, Cantrell J, Tops T, Uribe P, Mallett O, Zubko T, Oetjen-Gerdes L, Rasmussen TE, Butler FK, Kotwal RS, Holcomb JB, Wade C, Champion H, Lawnick M, Moores L, Blackbourne LH. Death on the battlefield (2001–2011): implications for the future of combat casualty care. J Trauma Acute Care Surg 73: S431–S437, 2012. [DOI] [PubMed] [Google Scholar]
- 15.Engelke KA, Doerr DF, Crandall CG, Convertino VA. Application of acute maximal exercise to protect orthostatic tolerance after simulated microgravity. Am J Physiol Regul Integr Comp Physiol 271: R837–R847, 1996. [DOI] [PubMed] [Google Scholar]
- 16.Evans J, Wessem KP, McDougall D, Lee K, Lyons T, Balogh Z. Epidemiology of traumatic deaths: comprehensive population-based assessment. World J Surg 34: 158–163, 2010. [DOI] [PubMed] [Google Scholar]
- 17.Franke WD, Johnson CP, Steinkamp JA, Wang R, Halliwill JR. Cardiovascular and autonomic responses to lower body negative pressure. Clin Auton Res 13: 36–44, 2003. [DOI] [PubMed] [Google Scholar]
- 18.Goldsmith SR, Francis GS, Cowley AW, Cohn JN. Response of vasopressin and norepinephrine to lower body negative pressure in humans. Am J Physiol Heart Circ Physiol 243: H970–H973, 1982. [DOI] [PubMed] [Google Scholar]
- 19.Gotshall RW, Tsai PF, Frey MA. Gender-based differences in the cardiovascular response to standing. Aviat Space Environ Med 62: 855, 1991. [PubMed] [Google Scholar]
- 20.Hanson JM, Van Hoeyweghen R, Kirkman E, Thomas A, Horan MA. Use of stroke distance in the early detection of simulated blood loss. J Trauma 44: 128–134, 1998. [DOI] [PubMed] [Google Scholar]
- 21.Hart EC, Charkoudian N, Wallin BG, Curry TB, Eisenach J, Joyner MJ. Sex and ageing differences in resting arterial pressure regulation: the role of the β-adrenergic receptors. J Physiol 589: 5285–5297, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hinojosa-Laborde C, Rickards CA, Ryan KL, Convertino VA. Heart rate variability during simulated hemorrhage with lower body negative pressure in high and low tolerant subjects. Front Physiol 2: 85, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hinojosa-Laborde C, Shade RE, Muniz GW, Bauer C, Goei KA, Pidcoke HF, Chung KK, Cap AP, Convertino VA. Validation of lower body negative pressure as an experimental model of hemorrhage. J Appl Physiol 116: 406–415, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jacobsen J, Søfelt S, Sheikh S, Warberg J, Secher NH. Cardiovascular and endocrine responses to haemorrhage in the pig. Acta Physiol Scand 138: 167–173, 1990. [DOI] [PubMed] [Google Scholar]
- 25.Kashimoto S, Doursout MF, Hartley C, Chelly JE. Effects of thiopental and ketamine on cardiac function during moderate hemorrhage in chronically instrumented rats. J Cardiovasc Pharmacol 21: 829–833, 1993. [DOI] [PubMed] [Google Scholar]
- 26.Leonetti P, Audat F, Girard A, Laude D, Lefrère F, Elghozi JL. Stroke volume monitored by modeling flow from finger arterial pressure waves mirrors blood volume withdrawn by phlebotomy. Clin Auton Res 14: 176–181, 2004. [DOI] [PubMed] [Google Scholar]
- 27.McDonough KH, Giaimo M, Quinn M, Miller H. Intrinsic myocardial function in hemorrhagic shock. Shock 11: 205–210, 1999. [DOI] [PubMed] [Google Scholar]
- 28.Morris MJ, Russell AE, Kapoor V, Cain MD, Elliott JM, West MJ, Wing LM, Chalmers JP. Increases in plasma neuropeptide Y concentrations during sympathetic activation in man. J Auton Nerv Syst 17: 143–149, 1986. [DOI] [PubMed] [Google Scholar]
- 29.Norsk P, Ellegaard P, Videbaek R, Stadeager C, Jessen F, Johansen LB, Kristensen MS, Kamegai M, Warberg J, Christensen NJ. Arterial pulse pressure and vasopressin release in humans during lower body negative pressure. Am J Physiol Regul Integr Comp Physiol 264: R1024–R1030, 1993. [DOI] [PubMed] [Google Scholar]
- 30.Rea RF, Hamdan M, Clary MP, Randels MJ, Dayton PJ, Strauss RG. Comparison of muscle sympathetic responses to hemorrhage and lower body negative pressure in humans. J Appl Physiol 70: 1401–1405, 1991. [DOI] [PubMed] [Google Scholar]
- 31.Rickards CA, Ryan KL, Cooke WH, Convertino VA. Tolerance to central hypovolemia: the influence of oscillations in arterial pressure and cerebral blood velocity. J Appl Physiol 111: 1048–1058, 2011. [DOI] [PubMed] [Google Scholar]
- 32.Ryan KL, Cooke WH, Rickards CA, Lurie KG, Convertino VA. Breathing through an inspiratory threshold device improves stroke volume during central hypovolemia in humans. J Appl Physiol 104: 1402–1409, 2008. [DOI] [PubMed] [Google Scholar]
- 33.Sauaia A, Moore FA, Moore EE, Moser KS, Brennan R, Read RA, Pons PT. Epidemiology of trauma deaths: a reassessment. J Trauma 38: 185–193, 1995. [DOI] [PubMed] [Google Scholar]
- 34.Schafer K, Van Sickle C, Hinojosa-Laborde C, Convertino VA. Physiologic mechanisms underlying the failure of the “shock index” as a tool for accurate assessment of patient status during progressive simulated hemorrhage. J Trauma Acute Care Surg 75, Suppl 2: S197–S202, 2013. [DOI] [PubMed] [Google Scholar]
- 35.Søreide K, Krüger A, Vårdal A, Ellingsen C, Søreide E, Lossius H. Epidemiology and contemporary patterns of trauma deaths: changing place, similar pace, older face. World J Surg 31: 2092–2103, 2007. [DOI] [PubMed] [Google Scholar]
- 36.Thompson CA, Tatro DL, Ludwig DA, Convertino VA. Baroreflex responses to acute changes in blood volume in humans. Am J Physiol Regul Integr Comp Physiol 259: R792–R798, 1990. [DOI] [PubMed] [Google Scholar]
- 37.Wang BC, Sundet WD, Hakumaki MO, Goetz KL. Vasopressin and renin responses to hemorrhage in conscious, cardiac-denervated dogs. Am J Physiol Heart Circ Physiol 245: H399–H405, 1983. [DOI] [PubMed] [Google Scholar]
- 38.Wesseling KH, Jansen JR, Settels JJ, Schreuder JJ. Computation of aortic flow from pressure in humans using a nonlinear, three-element model. J Appl Physiol 74: 2566–2573, 1993. [DOI] [PubMed] [Google Scholar]


