Abstract
α-Synuclein has been studied in numerous cell types often associated with secretory processes. In pancreatic β-cells, α-synuclein might therefore play a similar role by interacting with organelles involved in insulin secretion. We tested for α-synuclein localizing to insulin-secretory granules and characterized its role in glucose-stimulated insulin secretion. Immunohistochemistry and fluorescent sulfonylureas were used to test for α-synuclein localization to insulin granules in β-cells, immunoprecipitation with Western blot analysis for interaction between α-synuclein and KATP channels, and ELISA assays for the effect of altering α-synuclein expression up or down on insulin secretion in INS1 cells or mouse islets, respectively. Differences in cellular phenotype between α-synuclein knockout and wild-type β-cells were found by using confocal microscopy to image the fluorescent insulin biosensor Ins-C-emGFP and by using transmission electron microscopy. The results show that anti-α-synuclein antibodies labeled secretory organelles within β-cells. Anti-α-synuclein antibodies colocalized with KATP channel, anti-insulin, and anti-C-peptide antibodies. α-Synuclein coimmunoprecipitated in complexes with KATP channels. Expression of α-synuclein downregulated insulin secretion at 2.8 mM glucose with little effect following 16.7 mM glucose stimulation. α-Synuclein knockout islets upregulated insulin secretion at 2.8 and 8.4 mM but not 16.7 mM glucose, consistent with the depleted insulin granule density at the β-cell surface membranes observed in these islets. These findings demonstrate that α-synuclein interacts with KATP channels and insulin-secretory granules and functionally acts as a brake on secretion that glucose stimulation can override. α-Synuclein might play similar roles in diabetes as it does in other degenerative diseases, including Alzheimer's and Parkinson's diseases.
Keywords: ATP-sensitive K+ channels, glucose-sensitive insulin secretion, diabetes, Parkinson's disease, Alzheimer's disease, Kir6.2, SUR1, vesicle
glucose-stimulated insulin secretion (GSIS) is the chief function of β-cells of the endocrine pancreas, which is compromised in all cases of diabetes (16). Insulin secretion involves a series of complex processes whereby changing blood glucose levels regulate insulin synthesis, trafficking, and exocytic release into the blood to maintain low blood glucose levels. After meals, the increased glucose load stimulates insulin secretion, rapidly restoring the normal low levels of blood glucose. One hallmark of the diabetic state is failure of the β-cell to properly secrete sufficient insulin to restore and maintain the low blood glucose levels. We do not know the molecular and cellular mechanisms underlying the dose dependence of the sustained phase of GSIS (24, 41, 48), and until we do we are limited in how to more effectively treat and cure diabetes (3, 10, 17, 33, 40, 50).
The insulin-secretory granule is the primary organelle underlying GSIS of the pancreatic β-cell. The insulin granule has hundreds of different resident proteins (6, 54); however, a far smaller number are likely to be specialized for proper coupling of glucose metabolism to granule functions supporting insulin release. To fundamentally understand insulin secretion and diabetes, it will be necessary to identify not only the protein receptors of the granule specialized for coupling to glucose metabolism but also their interacting cytoplasmic partners in the β-cell.
Synucleins are a family of small cytoplasmic proteins that share an NH2-terminal α-helix that binds phospholipid vesicles and are found only in vertebrates, predominantly in presynaptic nerve terminals (11). The first synuclein gene sequence was identified by using antibodies raised against presynaptic cholinergic vesicles to screen an electric ray expression library (34). The synucleins are now known to be encoded from three genes, α-, β-, and γ-synuclein (21). Most remarkably, α-synuclein plays a central role in the pathogenesis of neurodegenerative diseases. For example, α-synuclein misfolding in Lewy bodies is a hallmark of all Parkinson's disease subjects, and α-synuclein mutations cause rare, inherited forms of Parkinson's disease (49). Interestingly, α-synuclein is expressed in the pancreas (55) and in neurons. In neurons, α-synuclein has been associated with neurotransmitter synthesis and exocytic release (18, 30, 32, 46, 47). These observations raise the possibility that α-synuclein expression in the pancreas originates from insulin-secreting β-cells, where α-synuclein might be associated with the cellular machinery underlying insulin biogenesis and its exocytic release.
From its biophysical and biochemical properties alone, α-synuclein does not appear to be specialized to confer glucose dependency on insulin secretion but might interact with a protein having such functions. Designed as an exquisite sensor of nucleotide levels that signal changes in glucose metabolism, one candidate-binding partner is the ATP-sensitive potassium (KATP) channel, a key component of the insulin granule membrane (8, 19, 20, 23, 43, 56). The interactions of the cytoplasmic ligands ATP, MgADP, and anionic phospholipids on the KATP channel account for its regulation by glucose and are well studied (39, 51). However, little is known about protein ligands that interact with the secretory granule KATP channel in β-cells; yet this knowledge could be critical to the organelle basis of GSIS.
In this study, we combined immunohistochemistry and coimmunoprecipitation approaches to identify proteins of the β-cell cytoplasm that associate with insulin-secretory granules. Furthermore, we sought to characterize the molecular basis of such interactions and their functional consequences. We show that α-synuclein is expressed as a cytoplasmic protein of the pancreatic β-cell, localizes to insulin-secretory granules through its interactions with the KATP channel, and functions to downregulate insulin secretion.
MATERIALS AND METHODS
Pancreatic islets and β-cells.
For histochemistry and coimmunoprecipitation experiments, murine islets were isolated from C57Bl/6 wild-type (WT) mouse pancreata by collagenase digestion and purified by hand-picking islets using a stereo microscope (20). For the secretory assays, INS1-832/13 cells (27), C57Bl/129 α-synuclein knockout (ASKO) mice (1), C57Bl/129 (WT littermates), and C57Bl/6 WT mice were used, with each control showing similar differences compared with the knockouts. Briefly, islets were washed using serum-free culture medium. Medium was nearly completely removed by pipetting, leaving 50 μl. Recombinant virus was added at an MOI of 200 and applied by gently pipetting to lift islets off the bottom of the tube and by incubating and gently flicking (every 15 min for 1 h). The islets were then washed twice in complete medium (containing 10% FBS). Native and infected cells were cultured in RPMI 1640 medium containing 7.5 mM glucose, FBS 10% and PS (100 U/ml) and incubated at 37°C, 5% CO2. INS1-832/13 cells were cultured in d-glucose-free RPMI 1640 medium (GIBCO-BRL) supplemented with 10% heat-inactivated fetal calf serum (Bio-Whittaker), 7.5 mM glucose, 10 mM HEPES, 2 mM l-glutamine, 1 mM sodium pyruvate, 0.05 mM β-mercaptoethanol, 100 μg/ml streptomycin, and 100 U/ml penicillin in a humidified 5% CO2 incubator at 37°C. Lentivirus described in Alerte et al. (2) was used according to Environmental Health and Safety guidelines and mice according to Division of Laboratory Animal Resources guidelines on protocols approved at the University of Pittsburgh.
Immunohistochemistry.
The following antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz, CA) and used according to provided instructions. Catalog numbers and previous publications using antibodies are indicated in parentheses. Rabbit polyclonal anti-α-synuclein antibody [sc-7011 (C20); 1, 13, 57], goat polyclonal anti-α/β-synuclein [sc-7012 (N19)], mouse monoclonal anti-C-peptide [sc-57046 (5D3); 26], goat polyclonal anti-SUR-1 [sc-5789 (C16); 36], goat polyclonal anti-Kir6.2 [sc-11226 (N18); 14], and goat polyclonal anti-Kir6.2 [sc-20809 (H55); 61]. For the two different anti-synuclein antibodies, similar results were obtained and those obtained with sc-7011 (C20) are shown. Similar results were also obtained with the two anti-Kir6.2 antibodies and shown as indicated. Guinea pig anti-insulin was obtained from Dako (A0564; Carpentaria, CA). Alexa fluor 594 donkey anti-rabbit IgG (H+L), Alexa fluor 488 goat anti-rabbit IgG (H+L), Alexa fluor 488 goat anti-guinea pig IgG (H+L), Alexa fluor 594 goat anti-guinea pig IgG (heavy + light chain, H+L), Alexa fluor 488 donkey anti-goat IgG (H+L), Alexa fluor 594 donkey anti-goat IgG (H+L), and Alexa fluor 488 donkey anti-mouse IgG (H+L), and red fluorescent Bodipy®TR-glibenclamide were from Molecular Probes (Invitrogen, Carlsbad, CA). When indicated, dissociated islet cells were obtained by brief (<5 min) incubation in Ca2+-free buffer and plated; otherwise, intact islets were used. Mouse islet cells or INS1-832/13 cells were fixed with 2% paraformaldehyde in PBS for 20 min, washed in PBS three times, and blocked by incubation in 2% BSA in PBS (pH 7.5) overnight at 4°C. The cells were then incubated with primary antibodies (as indicated, each at 5 μg/ml) in blocking buffer overnight at 4°C. The islets were washed three times with PBS, incubated with labeled secondary antibodies in blocking buffer for 2 h at room temperature, and then washed three times with PBS. Confocal microscopy was used to detect the immunofluorescence as described below.
Confocal fluorescence microscopy.
Cells were placed into an optical recording chamber (Harvard Apparatus, Holliston, MA) at 37°C. Single-photon confocal microscopy was performed using an Olympus Fluoview 300 Confocal Laser Scanning head with an Olympus IX70 inverted microscope (Olympus, Melville, NY). Excitation of GFP or green Alexa fluor 488 conjugated to secondary antibody was by the 488-nm argon laser line and emission detected using sharp cutoff 510IF long-pass and BA530RIF short-pass filters for PMT 1. Excitation of red Alexa fluor 594 conjugated to secondary antibody was by the 543-nm green HeNe laser line and emission detected using a sharp cutoff BA610IF long-pass filter for PMT 2. colocalization experiments using two different fluorophores were done by sequential excitation and detection of the two channels as above. Control experiments showed no cross-talk detectable under the conditions of the dual-imaging experiments by comparing images from sequential acquisition of controls using single fluorophore labeling. For immunohistochemistry experiments, blocking peptide used to preadsorb the antibody, or omission of primary antibody, resulted in little if any fluorescence and no punctate fluorescence (data not shown). For islets, β-cells were identified by expression of insulin detected by anti-insulin antibodies. For all the fluorescently labeled secondary antibodies used, in the absence of primary antibody application, the fluorescently labeled secondary antibody fluorescent signals were indistinguishable from background. All images were obtained using an Olympus PlanApo ×60 oil, NA 1.4 objective lens. Images were recorded beginning from the bottom plasma membrane of a β-cell. The plane was identified as the first plane above the fluorescence reflection coverslip artifact. Images were acquired from this bottom z section and from sequential z sections above (typically 0.5 μm apart). For measuring the diameters of fluorescent puncta, maximum diameters were acquired from 3-D projections of the cells. Image analysis used MetaMorph v. 6.1 analysis software from Molecular Devices (Downingtown, PA) and IgorPro v. 5.5A from Wavemetrics (Lake Oswego, OR). For the determination of insulin granule density along the perimeter of ASKO and WT β-cells, the live-cell fluorescent insulin reporter Ad.Ins-C-emGFP (20) was expressed in the islets cultured in 5.5 mM glucose medium. Morphometric analysis with Metamorph v. 6.1 was used to count fluorescent granules within 1.5 μm of the surface membrane in merged fluorescent/DIC images per micrometer of perimeter membrane.
Coimmunoprecipitation.
All steps were carried out at 4°C as previously described (46, 47), with minor modifications. Briefly, mouse pancreas or mouse islet cells were prepared by homogenization in ice-cold coimmunoprecipitation buffer containing 0.03% Triton X-100, 50 mM Tris, pH 7.4, 100 mM NaCl, 40 mM β-glycerolphosphate, 20 mM sodium fluoride, 5 mM EDTA, 1 mM benzamidine, and 10% glycerol. Particulates were cleared by centrifugation (15 min, 10,000 g), and supernatant fractions were precleared overnight with 50 μl of protein G beads (Zymed) plus 10 μl of 5% BSA. Coimmunoprecipitation was performed by incubating equal total protein amounts of the precleared supernatants in primary antibodies [1:30, anti-α-synuclein antibody, 610787; BD Transduction Labs 31; 1:75 anti-Kir6.2 antibody, sc-20809 (H55), Santa Cruz; 61], plus 25 μl of protein G beads or with beads plus preimmune serum as a control. Supernatants were removed and beads were washed 3× in coimmunoprecipitation buffer, resuspended in 2× Laemmli buffer, and boiled, and immune complexes were separated on 12% Tris-glycine SDS-PAGE gels and transferred to nitrocellulose. Immunoblots were blocked in fish serum (LI-COR Biotechnologies, Lincoln, NE) diluted in an equal volume of 1× PBS at 4°C overnight. Blots were reacted in anti-Kir6.2 anti-body [1:1,000; sc-11228 (G16), Santa Cruz; 36] or anti-α-synuclein antibody [1:1,000, sc-7011-R (C20), Santa Cruz; 1, 13, 57] in fish serum-PBS for 1 h at room temperature. Blots were washed 4× in PBS containing 0.1% Tween (PBS-T) followed by 1 h of incubation, protected from light exposure, in infrared secondary antibodies for the appropriate species. Blots were washed 4× in PBS-T and then visualized at 700 and 800 nm using the Odyssey Infrared Imager (LI-COR). Negative control coimmunoprecipitations used α-synuclein antibody preadsorbed with blocking peptide, or preimmune serum in place of Kir6.2 antibody.
Immunoprecipitation tests for the presence or absence of α-synuclein in ASKO and WT pancreata were performed similarly using rabbit α-synuclein antibody (sc7011-R, 1:500) for immunoblots.
PCR analysis of genomic DNA from ASKO and WT pancreata.
Mouse pancreata were digested at 55°C for 48 h in 500 μl of buffer containing 5 mM Tris·HCl pH 8.0, 20 mM EDTA pH 8.0, 10 mM NaCl, 0.1% SDS, and 10 mg/ml proteinase K. Samples were then mixed with 700 μl of neutralized phenol-chloroform-isoamyl alcohol and spun. DNA was collected and precipitated in 50 μl of 3 M sodium acetate and 1 ml of 100% ethanol. DNA pellets were washed with 70% ethanol and air dried 30 min prior to resuspension in 100 μl of Milli-Q water overnight at 4°C. DNA concentrations were determined using 260/280-nm absorbance ratios, and samples were diluted to 5 μg/μl for PCR, which was performed using a Taq PCR kit (New England Biolabs). Genomic α-synuclein was amplified using the forward primer 5′-GGCGACGTGAAGGAGCCAGG-3′ and the reverse primer 5′-CAGCGAAAGGAAAGCCGAGTGATGTACT-3′. As an internal control, genomic actin was amplified using 5′-ACTGTGTTGGCATAGAGGTC-3′ forward primer and 5′-TTCTACAATGAGCTGCGTGTG-3′ reverse primer. PCR products were separated on 1% agarose-TAE gels.
Secretion assays.
For heterologous expression of α-synuclein, three populations of INS1-832/13 cells (27) were assayed in parallel in six experiments: cells transduced with mouse α-synuclein lentivirus (2), cells transfected with GFP lentivirus, and nontransduced cells. Cells (0.5 × 106) were aliquoted and plated per well in six-well plates and allowed to grow to ∼70% confluence. Two hours before experiments, medium in each well was switched from RPMI to prewarmed (37°C) Krebs secretion buffer with 2.8 mM glucose. The basal secretion assay was begun by washing the cells with fresh Krebs buffer with 2.8 mM glucose (basal condition). After a 1-h incubation, the medium was collected, and the cells were washed and then incubated for a second hour in prewarmed Krebs buffer with 16.7 mM glucose (stimulated condition). The medium was collected and protein extracts were prepared. Insulin remaining in the cells and in the secretory fractions was assayed using an insulin ELISA kit (Mercodia). Cell insulin content and the average stimulated glucose rate of 10.3 ng insulin·min−1·mg−1 total protein were indistinguishable across cells under these experimental conditions. For the ASKO islet secretory assays, the same procedure was used except that each assay used 20 size-matched islets isolated from female C57Bl/129 ASKO mice (1), female C57Bl/129 WT littermates, or female C57Bl/6 WT mice. WT average islet secretory rates in 16.7 mM glucose were 43 pg·min−1·islet−1 and represented 0.21% min−1 of total insulin content.
Transmission electron microscopy.
Pancreata were immersion fixed in 2.5% glutaraldehyde plus 2% paraformaldehyde in PBS overnight at 4°C. Fixed samples were washed 3× in PBS and then postfixed in aqueous 1% OsO4, 1% K3Fe(CN)6 for 1 h. Following the three PBS washes, the pellet was dehydrated through a graded series of 30–100% ethanol, 100% propylene oxide and then infiltrated in a 1:1 mixture of propylene oxide-Polybed 812 epoxy resin (Polysciences, Warrington, PA) for 1 h. After several changes of 100% resin over 24 h, pellet was embedded in molds and cured at 37°C overnight, followed by additional hardening at 65°C for two more days. Ultrathin (70 nm) sections were collected on 200 mesh copper grids and stained with 2% uranyl acetate in 50% methanol for 10 min, followed by 1% lead citrate for 7 min. Sections were imaged using a JEOL JEM 1011 transmission electron microscope (Peabody, MA) at 80 kV fitted with a side mount AMT 2k digital camera (Advanced Microscopy Techniques, Danvers, MA). Insulin granules near the β-cell surface facing a capillary vessel were identified within islets by their characteristic electron-lucent halo surrounding an electron-opaque dense core.
Statistical analysis.
Student's t-test and ANOVA with Bonferroni pairwise posttests were used accordingly to evaluate differences (Prism v. 5.0; GraphPad Software, La Jolla, CA). P < 0.05 was considered significant.
RESULTS
Anti-α-synuclein antibodies label secretory granules in β-cells of the islets.
Because α-synuclein has been implicated in secretion of chromaffin granules (30), we tested, using an antibody to α-synuclein, whether α-synuclein is also expressed in isolated islet cells. Figure 1 shows that anti-α-synuclein staining results in a punctate pattern of fluorescence in isolated mouse islet cells (n = 11 animals). The fluorescent puncta in both fixed and live cells [330 ± 70 nm and ranging up to 1 μm in diameter (1,850 puncta from 10 cells)] were indistinguishable in size and shape from those of insulin-secretory granules. We first tested for the identity of the punctate organelles by colocalization with antibodies against KATP channel subunits, which are known to localize to insulin-secretory granules (20, 23, 56). Anti-α-synuclein colocalized with anti-Kir6.2 at puncta similar in size and shape to insulin-secretory granules.
Fig. 1.
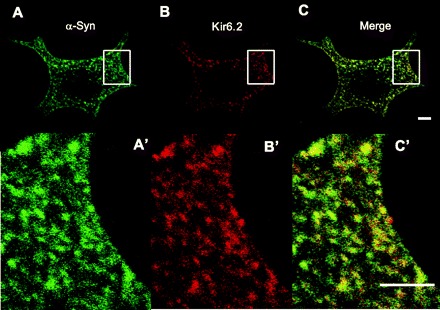
Colocalization of α-synuclein and Kir6.2 at punctate structures. Antibodies for α-synuclein and Kir6.2 were used to stain cells isolated from mouse islets as detected using secondary antibodies conjugated with Alexa fluor 488 and Alexa fluor 594 dyes, respectively and confocal microscopy. A: anti-α-synuclein (sc-7011, C20) was used to stain cells isolated from mouse islets and detected by Alexa 488-labeled secondary antibody. A′: magnified image of the region indicated by the rectangle in A. B: anti-Kir6.2 (sc-11226, N18) was used to stain the same cells but detected using secondary antibody conjugated to Alexa fluor 594. B′: magnification of the region indicated by the rectangle in B. C: merge of images in A and B. C′: magnification of the region indicated by the rectangle in C. Images are single optical section ∼0.5 μm thick. Shape and pattern of fluorescent puncta in the images were typical of all sections through the cell. Scale bar, 2 μm.
Given that KATP channels comprise both Kir6.2 and SUR1 subunits, we further tested for colocalization with antibodies against SUR1. Figure 2 shows that anti-α-synuclein colocalized with anti-SUR1 antibodies within similar punctate structures. To further test that α-synuclein and KATP channels colocalized in the same puncta, a fluorescent probe independent of antibodies, but nevertheless specific for the KATP channel was found to colocalize with α-synuclein antibody staining. Glibenclamides target insulin-secretory granules by binding SUR1 (23). Figure 2, D–F, also shows that 40 nM red fluorescent Bodipy®TR-glibenclamide colocalized with α-synuclein immunostaining. Thus, independent and distinctly different histochemical approaches indicate that KATP channels and α-synuclein colocalize to the same punctain islet cells.
Fig. 2.
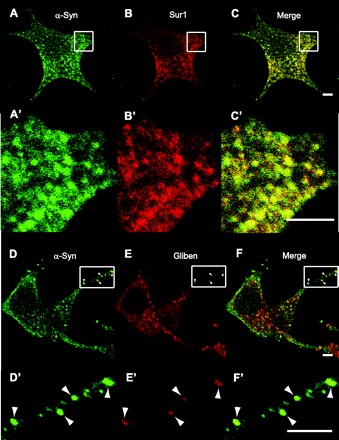
Colocalization of α-synuclein with SUR1 or fluorescent glibenclamide at punctate structures. A: anti-α-synuclein (sc-7011, C20) was used to stain cells isolated from mouse islets and detected by Alexa 488-labeled secondary antibody, as before. A′: magnification of the region indicated by the rectangle in A. B: anti-SUR1 (sc-5789, C16) was used to stain the same cells but detected using secondary antibody conjugated to Alexa fluor 594. B′: magnification of the region indicated by the rectangle in B. C: merge of images in A and B. C′: magnification of the region indicated by the rectangle in C. D–F and D′–F′: same as for A and B and A′ and B′, except that red fluorescence is due to red Bodipy®TR-glibenclamide, rather than SUR1 antibody staining. Shape and pattern of green and red fluorescent puncta show incomplete but clear coincidence patterns, which were typical of all sections through the cell. Scale bars, 2 μm.
We confirmed that α-synuclein and the KATP channel subunits colocalized at insulin granules by costaining with antibodies either to insulin or to the C-peptide segment of proinsulin. Figure 3 shows striking patterns of colocalization of anti-insulin with anti-α-synuclein, as well as with anti-Kir6.2 and anti-SUR1. Figure 4 further indicates that α-synuclein is associated with insulin granules by showing colocalization of anti-C-peptide with anti-α-synuclein immunoreactivity. The observations using several independent approaches demonstrate that α-synuclein and KATP channels colocalize to insulin-secretory granules in the endocrine pancreas. While the KATP channels traffic through the secretory pathway to insulin granules, α-synuclein is known to be cytoplasmically expressed (8). One possibility is that α-synuclein might localize to secretory granules by binding to the cytoplasmic face of their KATP channels. This hypothesis predicts that the proteins may physically associate with one another in β-cells.
Fig. 3.
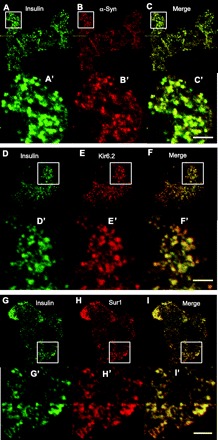
α-Synuclein, Kir6.2, and SUR1 localized to insulin-secretory granules. Anti-insulin (A0564) immunohistochemistry was detected using secondary antibody conjugated to Alexa fluor 488. Anti-α-synuclein (sc-7011, C20), anti-Kir6.2 (sc-11226, N18), and anti-SUR1 (sc-5789, C16) were detected by secondary antibody conjugated to Alexa fluor 594. A–C: anti-insulin (green), anti-α-synuclein (red), and merged staining of isolated islet cells. D–F: anti-insulin (green), anti-Kir6.2 (red), and merged staining of isolated islet cells. G–I: anti-insulin (green), anti-SUR1 (red), and merged staining of isolated islet cells. A′–I′: magnifications of regions indicated by the rectangle in the panel of the corresponding letter. Scale bars, 2 μm.
Fig. 4.
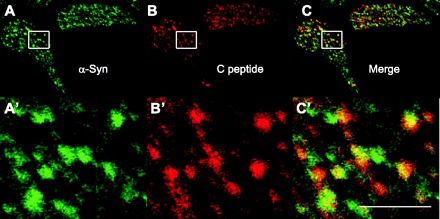
Colocalization of α-synuclein and C-peptide at punctate structures. A: anti-α-synuclein (sc-7011, C20) was used to stain cells isolated from mouse islets and detected by Alexa 488-labeled secondary antibody, as before. B: anti-C-peptide (sc-57046, 5D3) was used to stain the same cells but detected using secondary antibody conjugated to Alexa fluor 594. C: merge of images in A and B. A′–C′: magnifications of regions indicated by the rectangle in the panel of the corresponding letter. In panels labeled by prime letters, scale bar is 2 μm.
α-Synuclein interacts with the insulin granule KATP channel.
Coimmunoprecipitation experiments were used to test for α-synuclein interacting with the KATP channel in a complex. Figure 5 shows that, in mouse pancreatic tissue, anti-α-synuclein antibody precipitated α-synuclein, as expected, and also Kir6.2, as predicted, in results analyzed by Western blotting. Control experiments showed that the anti-α-synuclein antibody not only depleted α-synuclein but also Kir6.2 from the lysate. In subsequent coimmunoprecipitation experiments shown in Fig. 6, these observations were further supported by results with mouse islets, whether immunoprecipitating with anti-α-synuclein or with anti-Kir6.2 directed against a cytoplasmic peptide segment of the channel protein. Control experiments demonstrated that preadsorbing with serum or peptide used to make the antibody resulted in little immunoprecipitation. Additional controls using beads alone without any primary antibody failed to pull down α-synuclein or Kir6.2, demonstrating specificity of the assay (not shown). Our results taken together indicate that α-synuclein associates with insulin-secretory granules by physically interacting in a complex with the KATP channel.
Fig. 5.
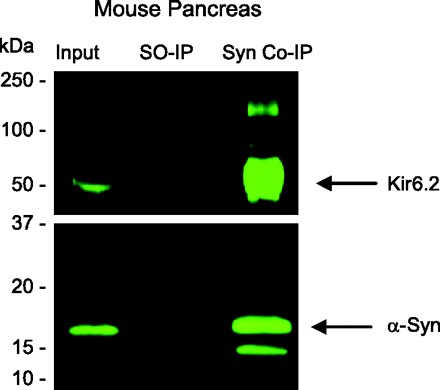
Coimmunoprecipitation of Kir6.2 with α-synuclein from mouse pancreas. Anti-α-synuclein antibody (BD610787) was used to immunoprecipitate α-synuclein from mouse pancreatic tissue extracts in a coimmunoprecipitation experiment. Immunocomplexes were characterized on Western blots using anti-Kir6.2 (sc-11228) and anti-α-synuclein (sc-7011-R). Equivalent aliquots of the initial input of each homogenate (Input) were analyzed. Homogenates in which secondary antibody only was used (SO-IP) served as a negative control. Both α-synuclein and Kir6.2 were coimmunoprecipitated using the anti-α-synuclein antibody (Syn IP).
Fig. 6.
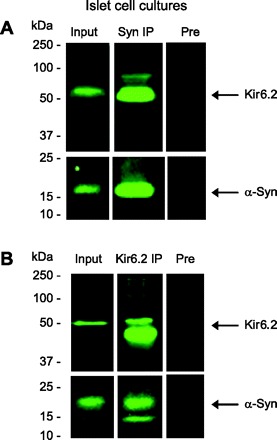
Binding interactions between Kir6.2 and α-synuclein demonstrated in mouse islets. Data from a representative coimmunoprecipitation experiment from mouse islet cells showing immunoblots reacted with an anti-α-synuclein antibody (610787; A and B, bottom) or anti-Kir6.2 antibody (sc-20809, H55; A and B, top). A: immunoblots showing Kir6.2 and α-synuclein in initial homogenate (Input), which was enriched in the coimmunoprecipitation using an anti-α-synuclein antibody (Syn-1, BD 610786; Syn IP). Specificity was confirmed using preadsorbed Syn-1 antibody for coimmunoprecipitation (Pre), which efficiently reduced the level of the proteins coimmunoprecipitated. B: immunoblots reveal the presence of Kir6.2 and α-synuclein in the initial homogenate (Input) as well as in the coimmunoprecipitation performed with anti-Kir6.2 antibody (sc-20809, H55; Kir6.2 IP), and specificity was demonstrated by coimmunoprecipitation using preimmune serum and beads (Pre). Molecular weights, determined from prestained standards, are shown on the left.
Exogenous overexpression of α-synuclein inhibits insulin secretion.
α-Synuclein might play a role in insulin secretion through its association with the secretory granules in pancreatic β-cells. Lentivirus was used to express either α-synuclein or GFP in insulin-secreting INS1-832/13 cells (27). α-Synuclein, GFP, or mock-transduced INS1-832/13 cells were incubated for 1 h in basal 2.8 mM glucose followed by stimulatory 16.7 mM glucose for an additional 1 h, and secretion was assayed by insulin ELISA. The expression of α-synuclein significantly decreased the rate of insulin secretion in 2.8 mM glucose compared with the GFP or mock-transduced controls in each of the six independent experiments performed. The basal rates for α-synuclein, GFP, and nontransduced cells were 1.7 ± 0.12, 2.9 ± 0.16, and 2.8 ± 0.06 ng insulin·min−1·mg−1 total protein, respectively. Thus, excess α-synuclein significantly decreased basal insulin secretion by 41% compared with the controls (P < 0.05), which were indistinguishable from each other, indicating that the transduction procedure itself had no effect. In each experiment, the assay of basal insulin secretion was followed by the assay of stimulated insulin secretion. Surprisingly, the expression of α-synuclein did not significantly inhibit the rate of insulin secretion when stimulated by 16.7 mM glucose compared with the GFP and mock-transduced controls. The stimulatory rates were 9.0 ± 0.10, 10.4 ± 0.12, and 10.2 ± 0.08 pg insulin·min−1·mg−1 total protein, respectively, a decrease in stimulated insulin secretion of 13% compared with controls, but was not significant (P > 0.05).
Loss of α-synuclein expression potentiaties insulin secretion.
The inhibitory effect observed by exogenous expression of α-synuclein predicts that loss of α-synuclein should upregulate GSIS. We tested for this by using ASKO islets (1). ASKO pancreata have the α-synuclein gene deletion and, as expected, fail to express α-synuclein protein (Fig. 7, A and B). The absence of α-synuclein in the ASKO islets significantly increased the rate of insulin secretion in 2.8 and 8.4 but not 16.7 mM glucose compared with WT islets. The insulin release rates for the ASKO and WT islets were 3.8 ± 0.86 and 13.4 ± 1.7 pg−1·min−1·islet−1 at 2.8 mM glucose, 16.2 ± 1.9 and 25.8 ± 3.1 pg−1·min−1·islet−1 at 8.4 mM glucose, and 43.1 ± 4.5 and 44.8 ± 5.1 pg−1·min−1·islet−1 at 16.7 mM glucose (n = 15), respectively. The secretory rates were significantly increased in ASKO compared with WT islets at basal 2.8 mM glucose (P < 0.01) and at 8.4 mM glucose (P < 0.05) but not at 16.7 mM glucose (P > 0.05). The potentiation of insulin secretion in islets with α-synuclein knocked out is entirely consistent with the inhibition of insulin secretion observed in the INS1-832/13 cells overexpressing α-synuclein and provide independent experimental confirmation indicating that α-synuclein acts as negative regulator of insulin secretion.
Fig. 7.
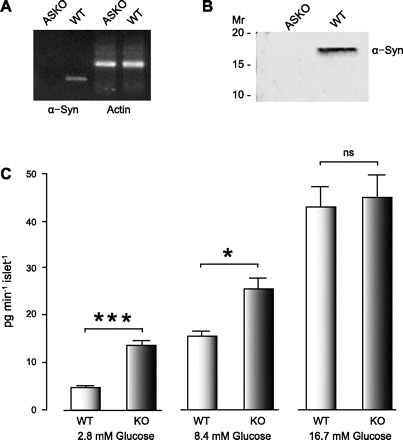
Absence of α-synuclein in α-synuclein knockout (ASKO) islets potentiates insulin secretion. α-Synuclein is not expressed in ASKO mouse pancreata. A: with PCR, the absence of α-synuclein genomic DNA was demonstrated in pancreata of ASKO mice, and its presence was confirmed in wild-type (WT) pancreata. The actin gene was present in pancreata of both ASKO and WT mice. B: immunoprecipitation confirmed a lack of α-synuclein protein in pancreata of ASKO mice and its presence in WT mouse pancreata. Insulin secretory rate was determined by incubating WT islets vs. islets from ASKO (KO) mice at indicated glucose conditions for 1 h. C: loss of α-synuclein significantly potentiated the insulin secretory rate at basal and intermediate but not at high stimulatory glucose. For each condition, 15 independent experiments were performed. *P < 0.05. ***P < 0.001.
ASKO islets show decreased density of insulin granules along the β-cell surface membrane.
By fluorescently labeling insulin-secretory granules with Ins-C-emGFP (59), we noticed a greater frequency of clusterings of fluorescent insulin granules along the β-cell surface membranes in WT (Fig. 8, A and B) compared with the ASKO islets (Fig. 8, D and E). Ultrastructural analysis by transmission electron microscopy showed similar results, with the large dense core insulin granules more organized into clusters near the plasmalemma in WT islets compared with ASKO islets (Fig. 8, C and F). When we quantified the differences in fluorescence density of the granules labeled by Ins-C-emGFP in five islets cultured in 5.6 mM glucose from each of five WT and five ASKO mice studied in parallel, the mean number of green fluorescent granules per micrometer of surface membrane was 0.93 ± 0.04 granules in WT islets and 0.54 ± 0.02 granules in ASKO islets (Fig. 8G). The 42% insulin granule density at the β-cell surface membrane is consistent with the more rapid throughput to exocytic sites and the augmented insulin secretion occurring in ASKO islets, as reported above.
Fig. 8.
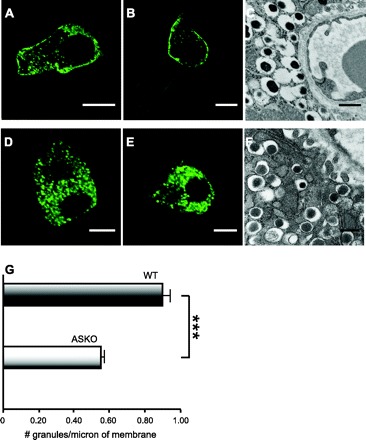
Differences in density of surface insulin granules between ASKO and WT islets. Islets from ASKO and WT mice were isolated and cultured in 5.5 mM glucose for 24 h in parallel. A: Ins-C-emGFP expressed in WT islet β-cell 1. B: Ins-C-emGFP expressed in WT islet β-cell 2. C: ultrastructure of WT pancreatic β-cell showing organized clustering of insulin-secretory granules along surface membrane. D: Ins-C-emGFP expressed in ASKO islet β-cell 1. E: Ins-C-emGFP expressed in ASKO islet β-cell 2. F: ultrastructure of ASKO pancreatic β-cell showing disorganized insulin-secretory granules away from surface membrane. G: In electron micrographs, perimeter membrane regions in β-cells were identified facing an islet capillary with red blood cells and compared between ASKO and controls (n = 4 islets each). Density of insulin granules along the surface membrane of β-cells from Ins-C-emGFP-expressing WT (A and B) and ASKO (D and E) islets. Densities were measured by counting the number of fluorescent insulin granules within 1.5 μm of the perimeter membrane of a central optical section, identified by the large oval nuclear region devoid of fluorescence. ×60, oil. *P < 0.05, ***P < 0.001. Scale bars in confocal fluorescent images are 5 μm and in EM images 500 nm.
DISCUSSION
The main findings of this study are that α-synuclein is a cytoplasmic ligand of the insulin-secretory granule, interacts with the KATP channel, and has an inhibitory action on insulin secretion. By immunostaining, α-synuclein was localized with the insulin granule luminal cargo markers C-peptide and insulin and with insulin-secretory granule membrane markers Kir6.2 and SUR1. These observations provide further positive evidence for the insulin-secretory identity of the organelle to which α-synuclein (as shown here), glibenclamide, Kir6.2, and SUR1 (8, 20, 23, 43, 56) colocalize and make untenable proposed models in which insulin and KATP channel granules are mutually exclusive populations of comparable abundance (62). Coimmunoprecipitation assays demonstrated that α-synuclein physically interacts, either directly or indirectly, in a complex with the insulin granule KATP channel. In the following, we discuss the implications of these findings for understanding the interactions of α-synuclein and the KATP channel in governing insulin secretion and how that might be altered in chronic diseases like diabetes.
Previous studies of α-synuclein in dopaminergic (1) and hippocampal neurons (18, 37) as well as in chromaffin cells (30) are consistent with models in which α-synuclein plays a role as a brake, maintaining a low basal secretory rate, or as an inhibitory ligand that can downregulate secretion (22). These results, together with the findings reported here for pancreatic β-cells, support models in which α-synuclein functions as a negative regulator of secretion in a variety of secretory cell types.
The cytoplasmic termini of Kir6.2 form the inhibitory ATP site (39, 51) of the KATP channel. Its interaction with α-synuclein then raises the question whether α-synuclein could function in an ATP-dependent manner with the KATP channel and whether other cytosolic proteins could be involved. Osterova et al. (42) showed that a small region of α-synuclein shares over 40% homology with 14-3-3 proteins (35). Moreover, they demonstrated that α-synuclein binds 14-3-3 and can thereby associate with 14-3-3 interacting proteins (60). These observations are important because 14-3-3 proteins, too, have recently been shown to interact with the cytoplasmic COOH termini of Kir6.2 in the forward transport of KATP channels to the cell membrane in HEK 293 cells (5, 12, 25). We therefore searched Kir6.2 by using the web-based Minimotif Miner (4) and found candidate sites for interaction of 14-3-3 at positions 177 and 222. These observations suggest that α-synuclein, 14-3-3, and other cytoplasmic proteins might interact with KATP channels to regulate the traffic or priming of insulin-secretory granules being resupplied to release sites at the β-cell surface membrane.
The model is supported by our finding that exogenous expression of α-synuclein significantly decreased basal insulin secretion. Moreover, the ability of 16.7 mM glucose stimulation to overcome the inhibition by α-synuclein in basal conditions suggests that ATP binding to Kir6.2 can antagonize α-synuclein binding to the KATP channel and thereby remove the brake on insulin secretion. The negative regulatory role of α-synuclein was further tested by determining the effect of its removal in ASKO islets. At 2.8 and 8.4 but not at 16.7 mM glucose, α-synuclein islets exhibited a significantly upregulated rate of insulin secretion compared with WT islet controls. The inability of changes in α-synuclein levels to alter insulin secretion in response to maximal glucose stimulation suggests that α-synuclein becomes functionally uncoupled from KATP channels strongly inhibited by glucose metabolism.
To investigate the cellular phenotype associated with altered expression of α-synuclein in the endocrine pancreas, we studied the subcellular distribution of fluorescently labeled insulin granules in ASKO compared with WT islets in 5.6 mM glucose conditions. We found that the surface density of the secretory granules decreased by over 40% in the ASKO compared with the WT islets, consistent with the augmented exocytic rates found by ELISA. This was also observed at the ultrastructural level, where large dense-core insulin granules in ASKO islets were not as abundant or tightly organized proximal to the surface membrane as they were in WT islets. Previous results in neurons suggested that α-synuclein interacts with vesicles that are proximal to the plasma membrane but not those directly docked at release sites (7, 11, 37). Functionally, the absence of α-synuclein in ASKO mice resulted in a decreased density of insulin granules proximal to the plasmalemma, suggesting that α-synuclein normally functions to limit insulin secretion at later steps occurring near or at the β-cell surface membrane. However, we found that α-synuclein interacts with interior as well as surface insulin granules. Therefore, α-synuclein inhibitory actions might also be initiated by its action on interior granules, which might have delayed effects later in the secretory pathway. Further experiments will be required to specify the steps of the insulin-secretory pathway, trafficking to release sites, priming, docking, and exocytic fusion of the insulin granules to determine where α-synuclein exerts its inhibitory effects. The results support models in which α-synuclein interacts with secretory vesicles to retard their progression or priming through the secretory pathway to exocytic sites for release, including interactions with the actin cytoskeleton and SNARE proteins (9, 15, 29, 38, 44, 58, 63).
The findings reported here for pancreatic β-cells extend previous results in which α-synuclein had a negative effect on secretion in dopaminergic neurons (1), hippocampal neurons (7, 37), and chromaffin cells (30). The effects of increasing and decreasing α-synuclein levels on the glucose dose response of insulin secretion demonstrates that α-synuclein plays an inhibitory role in insulin secretion that can be overridden by strong glucose stimulation. The findings are consistent with models where glucose metabolism drives changes in ATP/ADP binding to the KATP channel to functionally uncouple it from α-synuclein and its inhibitory action on insulin secretion. The findings further suggest models for degenerative diseases of secretory cell types in which overexpression of WT α-synuclein or expression of mutant α-synuclein may result in excessive blockade of secretion with consequent cellular stress as an initiating event (22).
Such secretory blockade, together with other precipitating events, leading to cell stress and apoptosis, might provide a common mechanism underlying diseases such as Parkinson's disease, Alzheimer's disease, and diabetes (28, 53). In Parkinson's disease, dominantly inherited mutant forms of α-synuclein or abnormal conformers are involved (49). Importantly, Chandra et al. (9) provide evidence for a physiological neuroprotective role for α-synuclein interactions with exocytic SNARE proteins in neurons. These observations suggest a role for α-synuclein in β-cells normally playing an endoprotective role against cellular degeneration. In diabetes, however, α-synuclein interactions with insulin-secretory granule components, including the KATP channel, could become excessive, as β-cells inappropriately adapt to chronically unmet insulin-secretory demand or other insults (45),further compromising insulin secretion. Additional problems might involve the abnormal formation of Lewy body-like structures or abnormal secretion of α-synuclein and IAPP plaques, leading to apoptosis of β-cells (33). These problems might be common in secretory cell types and account, at least in part, for the significant association of abnormal glucose tolerance in Parkinson's disease patients (52).
GRANTS
This study was funded in part by the American Diabetes Association RA-1-06-39 (to P. Drain) and the Pittsburgh Foundation Medical Research Fund M2009-0041 (to P. Drain and R. G. Perez), and start-up funding from the Department of Neurology (to R. G. Perez).
DISCLOSURES
No conflicts of interest are reported by the authors.
ACKNOWLEDGMENTS
We thank Dr. Chang-Sook Hong for making the lentivirus used in this study, Emily E. Friedrich for excellent technical support, Dr. Chris Newgard for the kind gift of INS1-832/13 cells, William Gosset for statistics, and Dr. Linton Traub for critical discussions.
REFERENCES
- 1. Abeliovich A, Schmitz Y, Farinas I, Choi-Lundberg D, Ho WH, Castillo PE, Shinsky N, Verdugo JM, Armanini M, Ryan A, Hynes M, Phillips H, Sulzer D, Rosenthal A. Mice lacking α-synuclein display functional deficits in the nigrostriatal dopamine system. Neuron 25: 239–252, 2000. [DOI] [PubMed] [Google Scholar]
- 2. Alerte TN, Akinfolarin AA, Friedrich EE, Mader SA, Hong CS, Perez RG. α-Synuclein aggregation alters tyrosine hydroxylase phosphorylation and immunoreactivity: lessons from viral transduction of knockout mice. Neurosci Lett 435: 24–29, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Alquier T, Peyot ML, Latour MG, Kebede M, Sorensen CM, Gesta S, Ronald Kahn C, Smith RD, Jetton TL, Metz TO, Prentki M, Poitout V. Deletion of GPR40 impairs glucose-induced insulin secretion in vivo in mice without affecting intracellular fuel metabolism in islets. Diabetes 58: 2607–2615, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Balla S, Thapar V, Verma S, Luong T, Faghri T, Huang CH, Rajasekaran S, del Campo JJ, Shinn JH, Mohler WA, Maciejewski MW, Gryk MR, Piccirillo B, Schiller SR, Schiller MR. Minimotif Miner: a tool for investigating protein function. Nat Methods 3: 175–177, 2006. [DOI] [PubMed] [Google Scholar]
- 5. Booij PP, Roberts MR, Vogelzang SA, Kraayenhof R, De Boer AH. 14-3-3 Proteins double the number of outward-rectifying K+ channels available for activation in tomato cells. Plant J 20: 673–683, 1999. [DOI] [PubMed] [Google Scholar]
- 6. Brunner Y, Coute Y, Iezzi M, Foti M, Fukuda M, Hochstrasser DF, Wollheim CB, Sanchez JC. Proteomics analysis of insulin-secretory granules. Mol Cell Proteomics 6: 1007–1017, 2007. [DOI] [PubMed] [Google Scholar]
- 7. Cabin DE, Shimazu K, Murphy D, Cole NB, Gottschalk W, McIlwain KL, Orrison B, Chen A, Ellis CE, Paylor R, Lu Bai Nussbaum RL. Synaptic vesicle depletion correlates with attenuated synaptic responses to prolonged repetitive stimulation in mice lacking α-synuclein. J Neurosci 22: 8797–8807, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Carpentier JL, Sawano F, Ravazzola M, Malaisse WJ. Internalization of 3H-glibenclamide in pancreatic islet cells. Diabetologia 29: 259–261, 1986. [DOI] [PubMed] [Google Scholar]
- 9. Chandra S, Gallardo G, Fernández-Chacón R, Schlüter OM, Südhof TC. α-Synuclein cooperates with CSPα in preventing neurodegeneration. Cell 123: 383–396, 2005. [DOI] [PubMed] [Google Scholar]
- 10. Choi D, Radziszewska A, Schroer SA, Liadis N, Liu Y, Zhang Y, Lam PP, Sheu L, Hao Z, Gaisano HY, Woo M. Deletion of Fas in the pancreatic β-cells leads to enhanced insulin secretion. Am J Physiol Endocrinol Metab 297: E1304–E1312, 2009. [DOI] [PubMed] [Google Scholar]
- 11. Clayton DF, George JM. Synucleins in synaptic plasticity and neurodegenerative disorders. J Neurosci Res 58: 120–129, 1999. [PubMed] [Google Scholar]
- 12. Coblitz B, Shikano S, Wu M, Gabelli SB, Cockrell LM, Spieker M, Hanyu Y, Fu H, Amzel LM, Li M. C-terminal recognition by 14-3-3 proteins for surface expression of membrane receptors. J Biol Chem 280: 36263–36272, 2005. [DOI] [PubMed] [Google Scholar]
- 13. Conway KA, Rochet JC, Bieganski RM, Lansbury PT., Jr Kinetic stabilization of the α-synuclein protofibril by a dopamine-α-synuclein adduct. Science 294: 1346–1349, 2001. [DOI] [PubMed] [Google Scholar]
- 14. Crane A, Aguilar-Bryan L. Assembly, maturation, and turnover of KATP channel subunits. J Biol Chem 279: 9080–9090, 2004. [DOI] [PubMed] [Google Scholar]
- 15. Cui N, Kang Y, He Y, Leung YM, Xie H, Pasyk EA, Gao X, Sheu L, Hansen JB, Wahl P, Tsushima RG, Gaisano HY. H3 domain of syntaxin 1A inhibits KATP channels by its actions on the sulfonylurea receptor 1 nucleotide-binding folds-1 and -2. J Biol Chem 279: 53259–53265, 2004. [DOI] [PubMed] [Google Scholar]
- 16. Del Prato S, Marchetti P. β- and α-cell dysfunction in type 2 diabetes. Horm Metab Res 36: 775–781, 2004. [DOI] [PubMed] [Google Scholar]
- 17. Del Prato S, Bianchi C, Marchetti P. β-Cell function and anti-diabetic pharmacotherapy. Diabetes Metab Res Rev 23: 518–527, 2007. [DOI] [PubMed] [Google Scholar]
- 18. Fortin DL, Nemani VM, Voglmaier SM, Anthony MD, Ryan TA, Edwards RH. Neural activity controls the synaptic accumulation of α-synuclein. J Neurosci 25: 10913–10921, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Geng X, Li L, Bottino R, Balamurugan AN, Bertera S, Densmore E, Su A, Chang Y, Trucco M, Drain P. Antidiabetic sulfonylurea stimulates insulin secretion independently of plasma membrane KATP channels. Am J Physiol Endocrinol Metab 293: E293–E301, 2007. [DOI] [PubMed] [Google Scholar]
- 20. Geng X, Li L, Watkins S, Robbins PD, Drain P. The insulin-secretory granule is the major site of K(ATP) channels of the endocrine pancreas. Diabetes 52: 767–776, 2003. [DOI] [PubMed] [Google Scholar]
- 21. George JM. The synucleins. Genome Biol 3: 1–6, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gitler AD, Shorter J. Prime time for α-synuclein. J Neurosci 27: 2433–2434, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Guiot Y, Stevens M, Marhfour I, Stiernet P, Mikhailov M, Ashcroft SJ, Rahier J, Henquin JC, Sempoux C. Morphological localisation of sulfonylurea receptor 1 in endocrine cells of human, mouse and rat pancreas. Diabetologia 50: 1889–1899, 2007. [DOI] [PubMed] [Google Scholar]
- 24. Henquin JC, Ishiyama N, Nenquin M, Ravier MA, Jonas JC. Signals and pools underlying biphasic insulin secretion. Diabetes 51, Suppl 1: S60–S67, 2002. [DOI] [PubMed] [Google Scholar]
- 25. Heusser K, Yuan H, Neagoe I, Tarasov AI, Ashcroft FM, Schwappach B. Scavenging of 14-3-3 proteins reveals their involvement in the cell-surface transport of ATP-sensitive K+ channels. J Cell Sci 119: 4353–4363, 2006. [DOI] [PubMed] [Google Scholar]
- 26. Hilgert I, Stolba P, Kristofova H, Stefanova I, Bendlova B, Lebl M, Horejsi V. A monoclonal antibody applicable for determination of C-peptide of human proinsulin by RIA. Hybridoma 10: 379–386, 1991. [DOI] [PubMed] [Google Scholar]
- 27. Hohmeier HE, Mulder H, Chen G, Henkel-Rieger R, Prentki M, Newgard CB. Isolation of INS-1-derived cell lines with robust ATP-sensitive K+ channel-dependent and -independent glucose-stimulated insulin secretion. Diabetes 49: 424–430, 2000. [DOI] [PubMed] [Google Scholar]
- 28. Klegeris A, McGeer PL. Complement activation by islet amyloid polypeptide (IAPP) and α-synuclein 112. Biochem Biophys Res Commun 357: 1096–1099, 2007. [DOI] [PubMed] [Google Scholar]
- 29. Kwan EP, Gaisano HY. Rescuing the subprime meltdown in insulin exocytosis in diabetes. Ann NY Acad Sci 1152: 154–164, 2009. [DOI] [PubMed] [Google Scholar]
- 30. Larsen KE, Schmitz Y, Troyer MD, Mosharov E, Dietrich P, Quazi AZ, Savalle M, Nemani V, Chaudhry FA, Edwards RH, Stefanis L, Sulzer D. α-Synuclein overexpression in PC12 and chromaffin cells impairs catecholamine release by interfering with a late step in exocytosis. J Neurosci 26: 11915–11922, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Liu Y, Fallon L, Lashuel HA, Liu Z, Lansbury PT., Jr The UCH-L1 gene encodes two opposing enzymatic activities that affect alpha-synuclein degradation and Parkinson's disease susceptibility. Cell 111: 209–218, 2002. [DOI] [PubMed] [Google Scholar]
- 32. Lotharius J, Brundin P. Pathogenesis of Parkinson's disease: dopamine, vesicles and α-synuclein. Nat Rev Neurosci 3: 932–942, 2002. [DOI] [PubMed] [Google Scholar]
- 33. Marchetti P, Dotta F, Lauro D, Purrello F. An overview of pancreatic β-cell defects in human type 2 diabetes: implications for treatment. Regul Pept 146: 4–11, 2008. [DOI] [PubMed] [Google Scholar]
- 34. Maroteaux L, Campanelli JT, Scheller RH. Synuclein: a neuron-specific protein localized to the nucleus and presynaptic nerve terminal. J Neurosci 8: 2804–2815, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mhawech P. 14-3-3 Proteins—an update. Cell Res 15: 228–236, 2005. [DOI] [PubMed] [Google Scholar]
- 36. Morrissey A, Rosner E, Lanning J, Parachuru L, Dhar Chowdhury P, Han S, Lopez G, Tong X, Yoshida H, Nakamura TY, Artman M, Giblin JP, Tinker A, Coetzee WA. Immunolocalization of KATP channel subunits in mouse and rat cardiac myocytes and the coronary vasculature. BMC Physiol 2005. January 12;5(1): 1.PMID: 15647111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Murphy DD, Rueter SM, Trojanowski JQ, Lee VM. Synucleins are developmentally expressed, and α-synuclein regulates the size of the presynaptic vesicular pool in primary hippocampal neurons. J Neurosci 20: 3214–3220, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ng B, Kang Y, Elias CL, He Y, Xie H, Hansen JB, Wahl P, Gaisano HY. The actions of a novel potent islet beta-cell specific ATP-sensitive K+ channel opener can be modulated by syntaxin-1A acting on sulfonylurea receptor 1. Diabetes 56: 2124–2134, 2007. [DOI] [PubMed] [Google Scholar]
- 39. Nichols CG. KATP channels as molecular sensors of cellular metabolism. Nature 440: 470–476, 2006. [DOI] [PubMed] [Google Scholar]
- 40. Nolan CJ, Prentki M. The islet beta-cell: fuel responsive and vulnerable. Trends Endocrinol Metab 19: 285–291, 2008. [DOI] [PubMed] [Google Scholar]
- 41. Nunemaker CS, Wasserman DH, McGuinness OP, Sweet IR, Teague JC, Satin LS. Insulin secretion in the conscious mouse is biphasic and pulsatile. Am J Physiol Endocrinol Metab 290: E523–E529, 2006. [DOI] [PubMed] [Google Scholar]
- 42. Ostrerova N, Petrucelli L, Farrer M, Mehta N, Choi P, Hardy J, Wolozin B. α-Synuclein shares physical and functional homology with 14-3-3 proteins. J Neurosci 19: 5782–5791, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ozanne SE, Guest PC, Hutton JC, Hales CN. Intracellular localization and molecular heterogeneity of the sulphonylurea receptor in insulin-secreting cells. Diabetologia 38: 277–282, 1995. [DOI] [PubMed] [Google Scholar]
- 44. Pasyk EA, Kang Y, Huang X, Cui N, Sheu L, Gaisano HY. Syntaxin-1A binds the nucleotide-binding folds of sulphonylurea receptor 1 to regulate the KATP channel. J Biol Chem 279: 4234–4240, 2004. [DOI] [PubMed] [Google Scholar]
- 45. Paxinou E, Chen Q, Weisse M, Giasson BI, Norris EH, Rueter SM, Trojanowski JQ, Lee VM, Ischiropoulos H. Induction of α-synuclein aggregation by intracellular nitrative insult. J Neurosci 21: 8053–8061, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Peng X, Tehranian R, Dietrich P, Stefanis L, Perez RG. α-Synuclein activation of protein phosphatase 2A reduces tyrosine hydroxylase phosphorylation in dopaminergic cells. J Cell Sci 118: 3523–3530, 2005. [DOI] [PubMed] [Google Scholar]
- 47. Perez RG, Waymire JC, Lin E, Liu JJ, Guo F, Zigmond MJ. A role for α-synuclein in the regulation of dopamine biosynthesis. J Neurosci 22: 3090–3099, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Pigeau GM, Kolic J, Ball BJ, Hoppa MB, Wang YW, Ruckle T, Woo M, Manning-Fox JE, MacDonald PE. Insulin granule recruitment and exocytosis is dependent on p110-gamma in insulinoma and human beta-cells. Diabetes 58: 2084–2092, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, Dutra A, Pike B, Root H, Rubenstein J, Boyer R, Stenroos ES, Chandrasekharappa S, Athanassiadou A, Papapetropoulos T, Johnson WG, Lazzarini AM, Duvoisin RC, Di Iorio G, Golbe LI, Nussbaum RL. Mutation in the α-synuclein gene identified in families with Parkinson's disease. Science 276: 2045–2047, 1997. [DOI] [PubMed] [Google Scholar]
- 50. Prentki M, Nolan CJ. Islet beta cell failure in type 2 diabetes. J Clin Invest 116: 1802–1812, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Proks P, Ashcroft FM. Modeling KATP channel gating and its regulation. Prog Biophys Mol Biol 99: 7–19, 2009. [DOI] [PubMed] [Google Scholar]
- 52. Sandyk R. The relationship between diabetes mellitus and Parkinson's disease. Int J Neurosci 69: 125–130, 1993. [DOI] [PubMed] [Google Scholar]
- 53. Su LJ, Auluck PK, Outeiro TF, Yeger-Lotem E, Kritzer JA, Tardiff DF, Strathearn KE, Liu F, Cao S, Hamamichi S, Hill KJ, Caldwell KA, Bell GW, Fraenkel E, Cooper AA, Caldwell GA, McCaffery JM, Rochet JC, Lindquist S. Compounds from an unbiased chemical screen reverse both ER-to-Golgi trafficking defects and mitochondrial dysfunction in Parkinson's disease models. Dis Model Mech 3: 194–208, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Takamori S, Holt M, Stenius K, Lemke EA, Gronborg M, Riedel D, Urlaub H, Schenck S, Brugger B, Ringler P, Muller SA, Rammner B, Grater F, Hub JS, De Groot BL, Mieskes G, Moriyama Y, Klingauf J, Grubmuller H, Heuser J, Wieland F, Jahn R. Molecular anatomy of a trafficking organelle. Cell 127: 831–846, 2006. [DOI] [PubMed] [Google Scholar]
- 55. Ueda K, Saitoh T, Mori H. Tissue-dependent alternative splicing of mRNA for NACP, the precursor of non-A β component of Alzheimer's disease amyloid. Biochem Biophys Res Commun 205: 1366–1372, 1994. [DOI] [PubMed] [Google Scholar]
- 56. Varadi A, Grant A, McCormack M, Nicolson T, Magistri M, Mitchell KJ, Halestrap AP, Yuan H, Schwappach B, Rutter GA. Intracellular ATP-sensitive K+ channels in mouse pancreatic β-cells: against a role in organelle cation homeostasis. Diabetologia 49: 1567–1577, 2006. [DOI] [PubMed] [Google Scholar]
- 57. Vogiatzi T, Xilouri M, Vekrellis K, Leonidas Stefaniset L. Wildtype α-synuclein is degraded by chaperone-mediated autophagy and macroautophagy in neuronal cells. J Biol Chem 283: 23542–23556, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Wang Z, Thurmond DC. Mechanisms of biphasic insulin-granule exocytosis—roles of the cytoskeleton, small GTPases and SNARE proteins. J Cell Sci 122: 893–903, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Watkins S, Geng X, Li L, Papworth G, Robbins PD, Drain P. Imaging secretory vesicles by fluorescent protein insertion in propeptide rather than mature secreted peptide. Traffic 3: 461–471, 2002. [DOI] [PubMed] [Google Scholar]
- 60. Woods WS, Boettcher JM, Zhou DH, Kloepper KD, Hartman KL, Ladror DT, Qi Z, Rienstra CM, George JM. Conformation-specific binding of α-synuclein to novel protein partners detected by phage display and NMR spectroscopy. J Biol Chem 282: 34555–34567, 2007. [DOI] [PubMed] [Google Scholar]
- 61. Yan FF, Lin CW, Cartier EA, Shyng SL. Role of ubiquitin-proteasome degradation pathway in biogenesis efficiency of β-cell ATP-sensitive potassium channels. Am J Physiol Cell Physiol 289: C1351–C1359, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Yang SN, Wenna ND, Yu J, Yang G, Qiu H, Yu L, Juntti-Berggren L, Kohler M, Berggren PO. Glucose recruits KATP channels via non-insulin-containing dense-core granules. Cell Metab 6: 217–228, 2007. [DOI] [PubMed] [Google Scholar]
- 63. Zhang Y, Kang YH, Chang N, Lam PP, Liu Y, Olkkonen VM, Gaisano HY. Cab45b, a Munc18b-interacting partner, regulates exocytosis in pancreatic beta-cells. J Biol Chem 284: 20840–20847, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]


