Abstract
Polycystic ovary syndrome (PCOS) is a common endocrine disorder that is characterized by chronic hyperandrogenic anovulation leading to symptoms of hirsutism, acne, irregular menses, and infertility. Multiple metabolic and cardiovascular risk factors are associated with PCOS, including insulin resistance, obesity, type 2 diabetes, hypertension, inflammation, and subclinical atherosclerosis. However, current treatments for PCOS are only moderately effective at controlling symptoms and preventing complications. This article describes how the physiological effects of major complementary and alternative medicine (CAM) treatments could reduce the severity of PCOS and its complications. Acupuncture reduces hyperandrogenism and improves menstrual frequency in PCOS. Acupuncture's clinical effects are mediated via activation of somatic afferent nerves innervating the skin and muscle, which, via modulation of the activity in the somatic and autonomic nervous system, may modulate endocrine and metabolic functions in PCOS. Chinese herbal medicines and dietary supplements may also exert beneficial physiological effects in PCOS, but there is minimal evidence that these CAM treatments are safe and effective. Mindfulness has not been investigated in PCOS, but it has been shown to reduce psychological distress and exert positive effects on the central and autonomic nervous systems, hypothalamic-pituitary-adrenal axis, and immune system, leading to reductions in blood pressure, glucose, and inflammation. In conclusion, CAM treatments may have beneficial endocrine, cardiometabolic, and reproductive effects in PCOS. However, most studies of CAM treatments for PCOS are small, nonrandomized, or uncontrolled. Future well-designed studies are needed to further evaluate the safety, effectiveness, and mechanisms of CAM treatments for PCOS.
Keywords: acupuncture, mindfulness meditation, herbs, dietary supplements, traditional Chinese medicine
polycystic ovary syndrome (PCOS), a common endocrine disorder that affects 5–10% of reproductive-age women, is characterized by chronic hyperandrogenic anovulation leading to symptoms of hirsutism, acne, irregular menses, and infertility (86). The exact etiology of PCOS remains unclear, but it is believed to result from complex interactions between genetic, behavioral, and environmental factors. Multiple metabolic and cardiovascular risk factors are associated with PCOS, including insulin resistance (IR), obesity, type 2 diabetes (DM-2), hypertension, dyslipidemia, inflammation, and subclinical cardiovascular disease (32, 62, 63, 87, 107). Anxiety, depression, and reduced quality of life are also common in PCOS (5, 19, 45, 107).
Current treatments for PCOS are only moderately effective at controlling symptoms and preventing complications. In fact, when 648 women were asked, “If your PCOS could be safely and effectively helped by something else besides fertility drugs or birth control pills, would that interest you?”, 99% responded yes (95). Although the prevalence of complementary and alternative medicine (CAM) used by women with PCOS is not known, a landmark study showed that one in three Americans use CAM (23). Recent studies suggest that several CAM treatments could be beneficial as an adjunct to conventional medical management of PCOS. This article describes how the physiological effects of CAM treatments could reduce the severity of PCOS and its endocrine, cardiometabolic, and reproductive complications.
Acupuncture
Acupuncture is a form of sensory stimulation in which thin needles are placed in the skin and muscles. It is of great importance to describe the needling “dose”, because the intensity, frequency, and type of stimulation, manual or electrical, with high or low frequency, and the interval between stimulations directly influence the kind of receptors activated and thus the therapeutic effect (114). Patients' expectations may also influence the results, as acupuncture may have strong psychological effects (94).
The primary mechanism for acupuncture's clinical effects is activation of somatic afferent nerves innervating the skin and muscles, which may modulate somatic and autonomic nervous system activity and endocrine and metabolic functions. The efficacy of acupuncture in treating pain and disease has been studied from a Western scientific perspective. Systematic reviews have concluded that there is no evidence for acupuncture point specificity and suggest that needles can be inserted anywhere in appropriate segments (80, 122). Here, we will use a neurophysiological approach to describe how acupuncture, specifically electro-acupuncture (EA), where needles are electrically stimulated, may work in women with PCOS, because it has good support from experimental and clinical studies (36, 79).
Etiology of PCOS.
The etiology of PCOS is poorly understood (83). Ovarian hyperandrogenemia, the most consistent endocrine feature, probably plays a key role (30), but hyperinsulinemia/insulin resistance and abdominal obesity are also thought to be important (4). Whether hyperandrogenism results from the hyperinsulinemia of IR or vice versa is unclear (91). Moreover, neuroendocrine defects can contribute to persistently rapid luteinizing hormone (LH) pulsatility and increased amplitude, which further augment ovarian androgen production (7). In addition, PCOS women have high sympathetic nervous system activity compared with controls, and circulating testosterone is the strongest factor explaining the high activity (105). Furthermore, high activity in the sympathetic neurons innervating the ovaries precedes the development of ovarian cysts in rats (58), and women with PCOS may have increased ovarian nerve fiber density (37).
Clinical effects of acupuncture in PCOS.
The clinical effect of acupuncture on menstrual dysfunction in PCOS has been evaluated in several case control studies (12, 29, 101, 117) and in one randomized controlled trial (RCT) (n = 84) (44). In the RCT, women were randomized in a 2:2:1 ratio to receive 14 treatments with low-frequency EA (n = 33) for 16 wk or physical exercise (n = 34) at least three times per week for 16 wk, or no intervention during the study period, which served as a control group (n = 17). This RCT demonstrated that low-frequency EA was superior to physical exercise and improved hyperandrogenism and menstrual frequency more effectively than no intervention (44). Whether the improved menstrual frequency reflected ovulation induction remains to be elucidated.
The effect of acupuncture (manual or electrical stimulation) on metabolic variables in women with PCOS has never been evaluated in clinical trials. Low-frequency EA, with repetitive muscle contraction, may activate physiological processes similar to those resulting from physical exercise and could influence metabolic variables. Experimental evidence is described below.
The effect of acupuncture on mental health and health-related quality of life in women with PCOS has not been evaluated in clinical trials. Acupuncture has been used to treat depressive disorders, but its effectiveness and safety are not well defined (124). Because women with PCOS are prone to develop symptoms of anxiety and depression and decreased health-related quality of life, this is an important area for exploration.
Placebo and limitations in acupuncture studies.
Acupuncture treatment is associated with particularly potent placebo effects, and there are indications that acupuncture treatment may be associated with larger effects than pharmacological and other physical placebos (52, 53, 67). The characteristics of acupuncture treatment are relevant in the context of placebo effects, including frequent patient-practitioner contacts and the procedure of needling (51). Placebo effects result in true psychobiological events and exist in clinical practice (27).
Acupuncture studies are difficult to design for many reasons, including the variety of sham procedures, the number of acupuncture points used, the number and duration of acupuncture treatments, and differences in stimulation techniques (114). Studies involving acupuncture in which both the patient and the therapist are unaware of the treatment are practically impossible to conduct. Thus, many variables affect the outcome of an acupuncture study. The so called placebo-acupuncture needle (102) has been used in many trials, and most often it has similar effects to true acupuncture but is superior to no treatment if a no-treatment group is included. Thus, the placebo-acupuncture needle is not inert and may not be used as a sham (72). Instead, it is of importance to control for the increased amount of attention, and the control/comparison group should meet with a therapist the same amount of times as in the acupuncture group.
With this in mind, standardized study protocols to increase the validity of acupuncture studies by following the new revised STandards for Reporting Interventions in Clinical Trials of Acupuncture (STRICTA) checklist in conjunction with CONSORT will improve critical appraisal, analysis, and replication of trials (73). Furthermore, given the uncertainties about physiological effects of sham controls and the question of enhanced placebo effects, it is crucial that direct, head-to-head comparisons of acupuncture and gold-standard treatment be conducted.
Physiological basis of acupuncture in PCOS: peripheral mechanisms.
Insertion and manual or electrical stimulation of needles in skin and muscle activates Aα, β, δ, and C fibers (49). In particular, activation of Aδ and C fibers may be essential for modulating autonomic nervous system activity (90). Manual and probably electrical stimulation causes release of neuropeptides from peripheral nerve terminals into the area surrounding the needle, increasing blood flow (42). Low-frequency (2 Hz) EA also increases skeletal muscle glucose uptake (39). In insulin-resistant rats with dihydrotestosterone (DHT)-induced PCOS, peripheral insulin sensitivity is improved by low-frequency EA for 4–5 wk with three treatments per week (75) and normalized by five treatments per week (47). This normalization may reflect increased expression of soleus muscle glucose transporter (GLUT)4 protein, including the plasma membrane of muscle cells (47). Moreover, there is a dose-response relationship between the number of EA treatments and improvement in insulin sensitivity (74, 75). Similarly, in rats with prednisolone-induced IR, low-frequency EA acutely increases protein expression of insulin receptor substrate-1 and GLUT4 in skeletal muscle (65). Low-frequency EA improved insulin sensitivity in DM-2 db/db mice, and the effect was mediated, at least partly, via regulation of skeletal muscle Sirtuin-1 (SIRT1) and peroxisome proliferator-activated receptor-γ coactivator-1α (PGC-1α) (64). The increased peripheral blood flow and glucose uptake in skeletal muscle are most likely mediated by a reflex response from muscle twitches during manual or electrical stimulation, as the response is abolished after transection of somatic afferent nerve fibers (38) (Fig. 1).
Fig. 1.
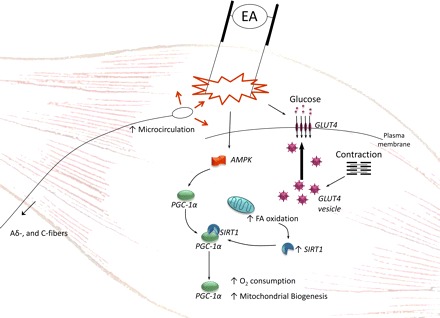
Stimulation of needles activates Aδ and C fibers and causes release of neuropeptides from peripheral nerve terminals, increasing blood flow locally. Low-frequency electro-acupuncture (EA) caused muscle contraction and increased GLUT4 expression and most likely translocation to plasma membrane. Low-frequency EA also increased Sirtuin-1 (SIRT1) and peroxisome proliferator-activated receptor-γ coactivator-1α (PGC-1α).
Segmental (spinal) mechanisms.
Needles in abdominal and leg muscles with somatic innervations corresponding to the sympathetic innervations of the ovaries, so-called segmental acupuncture points, may alter ovarian function by modulating sympathetic efferent activity (96, 98, 100). This is of particular interest since the PCOS ovary has been shown to have denser innervation (37) and high concentrations of nerve growth factor (NGF), a marker of sympathetic activity (20). Needles placed in the abdominal and hindlimb muscles of female rats and stimulated with low-frequency EA modulate the activity of ovarian sympathetic nerves, as reflected by increased ovarian blood flow (96, 98, 99). The response was demonstrated to be mediated by ovarian sympathetic nerves as a reflex response and was controlled by supraspinal pathways [i.e., central nervous system (CNS)] (96, 98). Further evidence that low-frequency EA modulates ovarian sympathetic nerve activity comes from studies in estradiol valerate-induced PCOS. Gene and protein expression of markers of sympathetic activity (α1a-, α1b-, α1d-, and β2-adrenoceptors, NGF, the p75 neurotropin receptor, and tyrosine hydroxylase) were normalized after 4 wk of low-frequency EA (76, 100). In rats with DHT-induced PCOS, ovarian morphology was improved by thrice weekly treatment for 4–5 wk, as reflected by a higher proportion of healthy antral follicles and a thinner theca interna cell layer than in untreated PCOS rats (74, 75). When treatment was increased to five times per week, low-frequency EA normalized estrus cyclicity, indicating a clear dose-response relationship (25). It is not known whether manual stimulation of acupuncture needles induces similar effects.
Central mechanisms of acupuncture.
When needles are placed, the peripheral nervous system transfers signals to the brain, which contributes to the effect of acupuncture. Since the CNS regulates pituitary hormone release, acupuncture may also modulate endocrine and metabolic function.
Many brain areas, especially the hypothalamic nucleus, are involved in the effect of acupuncture. Acupuncture-induced release of CNS neuropeptides seems to be essential for inducing functional changes in organ systems (36). The central hypothalamic β-endorphin system is a key mediator of changes in autonomic functions, such as effects on the vasomotor center, which decreases sympathetic tone and is manifested as improved blood pressure regulation and decreased muscle sympathetic nerve activity (120). Both exercise and low-frequency EA increase hypothalamic β-endorphin secretion and decrease blood pressure and sympathetic nerve activity; these effects are reversed by μ-opiod receptor antagonists (48). Interestingly, repeated low-frequency EA plus physical exercise significantly decrease high sympathetic nerve activity measured by microneurography in women with PCOS (97). Decreased sympathetic nerve activity, possibly mediated by modulation of hypothalamic β-endorphin secretion, may partly explain the decrease in circulating testosterone and improved menstrual frequency after low-frequency EA plus physical exercise in women with PCOS (44).
Hypothalamic β-endorphin interacts with the hypothalamic-pituitary-ovarian axis by exerting a tonic inhibitory effect on the gonadotropin-releasing hormone (GnRH) pulse generator and on pituitary LH release (46). In PCOS, growing evidence suggests that the opioid system is dysregulated both centrally and peripherally, with complex interactions (24). Indeed, opioid receptor antagonists improve menstrual cyclicity, induce ovulation, and decrease testosterone, insulin, and LH levels and the LH/FSH ratio (1, 15, 28). Acupuncture might affect the hypothalamic-pituitary-ovarian axis by modulating central β-endorphin production and secretion, thereby influencing release of hypothalamic GnRH and pituitary secretion of gonadotropins, as shown by the decrease in LH/FSH ratio after low-frequency EA (101). Furthermore, in rats with DHT-induced PCOS, five low-frequency EA treatments per week for 4–5 wk restored high hypothalamic androgen receptor and GnRH protein expression, which may help explain the beneficial neuroendocrine effects of low-frequency EA in women with PCOS (25).
β-Endorphin is also released into peripheral blood from the hypothalamus via the anterior pituitary, a process regulated by CRF. Circulating β-endorphin is thought to be related to the hyperinsulinemic response (70). It may also decrease hyperinsulinemia by lowering high concentrations of circulating β-endorphin (101). Interestingly, low-frequency EA lowers high circulating β-endorphin concentrations in women with PCOS and may decrease hyperinsulinemia and increase insulin clearance or insulin sensitivity (12, 101).
In sum, clinical and experimental evidence indicates that acupuncture with electrical muscle stimulation may be a suitable alternative or complement to improving endocrine and reproductive function in women with PCOS without adverse side effects. More experimental mechanistic studies and RCTs to further explore the use of acupuncture to treat PCOS-related symptoms are warranted.
Chinese Herbal Medicine
Chinese herbal medicine (CHM) is an integral part of traditional Chinese medicine (TCM). In China today, TCM is often administered as a complement to Western medicine. While TCM traces its roots back thousands of years, it rests, from the view of evidence-based medicine, more on a philosophy than a science. Much of the central philosophy involves maintaining the balanced flow of life energy (qi). TCM views organ systems as contributing to mind-body states and tries to address imbalances of these organ systems. TCM views PCOS as linked to disorders of the kidneys, liver, and spleen. Reproductive abnormalities, especially anovulation, are believed to be linked to the kidney, and a deficit in kidney is viewed as the primary problem in PCOS (82, 110).
Traditionally, CHMs are combined in varying preparations. Although some preparations are regulated by the government, there remains concern about quality control of individual formulations, given the variation in plant quality from harvest to harvest, and concerns about harmful supplements or byproducts of preparation such as heavy metals, herbicides, pesticides, microorganisms, mycotoxins, insects, pharmaceuticals, etc. (6, 68). The FDA has published guidelines to ensure better quality control of manufactured products from plants, but many of these products fall outside of regulation by the FDA, as they are not pharmaceuticals. CHMs also include many animal byproducts that we will not discuss in detail in this review. For example, a common preparation used to induce ovulation in women with PCOS is Di Long (Earth Dragon), which is made from abdominal extracts of the red earthworm Lumbricus rubellus.
Safety and efficacy of CHMs.
Unfortunately, there is minimal evidence that CHMs are safe and efficacious. Most of the trials have been small and thus inadequately powered to detect true differences. Most, not surprisingly, have been conducted mainly in Chinese populations and published in Chinese and thus are not easily accessible. The studies have also tested a large number of varying preparations (most containing multiple components), and thus there has been little to no replication for individual preparations. Above all, the studies have been of poor methodological quality without adherence to CONSORT guidelines.
This is well illustrated by systematic reviews of CHM in subfertile women with PCOS (123) and patients with impaired glucose tolerance (IGT) (34) and DM-2 (68), disorders related to PCOS because of the common underlying link of IR (Table 1).
Table 1.
List of systematic reviews of CHM for treatment of PCOS and disorders of glucose metabolism including type 2 diabetes
| Topic | Total No. of Studies Retrieved | Total Studies Included in Cochrane Review | Total No. of Studies in Chinese | Total No. of Subjects in Included Studies | Total No. of Preparations Tested in Trials | Main Conclusions |
|---|---|---|---|---|---|---|
| Subfertile PCOS (123) | 267 | 4 | 4 | 334 | 6 | Limited evidence that addition of CHM to clomiphene is associated with improved clinical pregnancy outcomes and no other evidence of any other effect. Methodology of RCTs was not adequately reported. |
| Impaired fasting glucose (IFG) or impaired glucose tolerance (IGT) (34) | 1,926 | 16 | 15 | 1,391 | 15 | Some positive evidence favors CHM for treatment of IGT or IFG. Limited by the following factors: lack of trials that tested the same herbal medicine, lack of details on cointerventions, unclear methods of randomization, poor reporting, and other risks of bias |
| Type 2 diabetes (68) | 713 | 66 | 61 | 8,302 | 69 | Some herbal medicines show hypoglycemic effect in type 2 diabetes. However, these findings are limited by low methodological quality, small sample size, and limited number of trials. |
PCOS, polycystic ovary syndrome; CHM, Chinese herbal medicine; RCT, randomized controlled trial. Nos. in parentheses are references.
Here, we also review the evidence for CHM in IGT and DM-2 because women with PCOS are at markedly increased risk for both IGT and DM-2 due to IR (63). Furthermore, these disorders of glucose dysregulation may represent later stages in the pathophysiological progression of PCOS. Finally, many drugs used to prevent or treat PCOS have been borrowed from DM-2, most notably metformin, but also the thiazolidinediones, acarbose, but also newer agents such as incretin mimetics such as exenatide. Similarly, many of the CHMs used to treat DM-2 may be useful for PCOS. However, the evidence for the benefit of CHM in all three disorders is weak (Table 1).
There are the fewest articles for PCOS, which is perhaps understandable from a public health perspective, as IGT and DM-2 are more prevalent than PCOS. The large drop-off from published articles to those meeting the minimum quality standard for a systematic review is staggering. None of the studies documented allocation concealment (the method by which the order of treatment assignment was generated and implemented in the study) or blinding, none of the studies used an intention to treat method analysis, a standard method to account for drop-out in a randomized trial (123).
The authors of the review of CHM in PCOS (123) did note that in two studies where multiple formulations of Chinese herbs were given as adjuvant therapy to clomiphene, there was a significantly increased odds of pregnancy with CHM (OR 2.97, 95% CI 1.71 to 5.17) (123). The reviews (34, 68, 123) share a common theme, a hint of efficacy clouded by poor methodology and lack of replication. But all reviews were eager to see larger better designed studies carried out.
There are multiple hurdles to adapting CHM into Western medicine. One is the lack of scientific justification from a hypothesis-driven perspective for the use of these medications. Many herbs contain multiple active substances, and combinations are exponentially more problematic for determining what is doing what to what. Much research currently is focusing on compound analysis through such technologies as high-performance liquid chromatography-mass spectrometry to identify specific bioactive agents.
Physiological mechanisms of CHMs.
Currently, the physiological mechanisms for efficacy of most CHMs are unknown in PCOS. Table 2 lists several CHMs used in PCOS and their proposed mechanism of action. Many may have selective estrogenic effects and function like clomiphene to induce ovulation. Risks, however, must be carefully determined, and these have not been well delineated. Gancao or licorice, given chronically or in excess, can cause an acquired form of apparent mineralocorticoid excess, as it is a potent inhibitor of 11β-hydroxysteroid dehydrogenease. This enzyme inactivates cortisol to cortisone, and decreased inactivation, especially in the kidney, can lead to excess cortisol cross-reacting with the mineralocorticoid receptor, which induces fluid retention, hypokalemia, and hypertension (66). In addition, CHMs may interfere with the metabolism of other drugs used to treat PCOS. For example, plantain has been proposed to interfere with many commonly prescribed medications such as digitoxin and tricyclic antidepressants, although at least one study shows no clinical interactions (17).
Table 2.
Partial list of CHMs used to treat PCOS, their proposed mechanisms of action, and their reported side effects
| Mechanism | Chinese Name | Latin Name | English Name | Adverse Effects |
|---|---|---|---|---|
| Improve insulin sensitivity | Baishao | Radix paeoniae Alba | White peony root | Uterine contractions, interfere with blood clotting |
| Danggui | Radix angelicae Sinensis | Angelica | Uterine contractions | |
| Danshen | Salvia miltiorrhiza Bunge | Red sage | May interact and potentiate effects of warfarin | |
| Huang Lian | Rhizoma coptidis | Goldenthread | Hypertension, respiratory failure, paresthesias | |
| Induce ovulation (through estrogenic effects) | Luole | Ocimum basilicum | Basil | Contains a chemical, estragole, which has caused liver cancer in mice |
| Sanqi | Radix no-toginseng | Panax pseudoginseng | Dry mouth, flushed skin, nervousness, sleep problems, nausea, and vomiting. | |
| Zelan | Herba lycopi | Bugleweed | Enlarged thyroid gland, hypoglycemia | |
| Zexie | Rhizoma alismatis | Water plantain | Fresh rootstock may be poisonous | |
| Inhibit androgen synthesis | Gancao | Radix glycytthizae | Licorice | Hypertension, fluid retention, hypokalemia, exacerbate kidney disease |
Mechanisms of action should be explored in cell culture and animal models by basic scientists. For example, Rhizoma alismatis has been found in an in vitro tissue model to inhibit intestinal glucose absorption and stimulate glucose uptake in fibroblasts and adipocytes (59). Furthermore, in a streptozotocin-induced diabetes mouse model, it lowers plasma glucose and triglycerides and improves insulin levels (119). Other CHMs, such as Radix notoginseng, have been found to have similar antidiabetic effects in mouse models, improving not only glucose tolerance and insulin action in a dose-response fashion but also ameliorating obesity (13). Similarly, Salvia miltiorrhiza Bunge has been shown to significantly improve glucose tolerance in a prenatally androgenized rat model of PCOS and to favorably impact insulin signaling in treated animals (118). Berberine, a component of Rhizoma coptidis, has been shown to improve glucose uptake and insuln action in human thecal cells with dexamethasone-induced insulin resistance (125). These favorable changes in glucose metabolism have also been shown to favorably alter sex steroid feedback or production, ameliorating hyperandrogenism in these models (22, 125). Additionally, antioxidant activity has been noted in vitro for a number of these substances (61, 116).
Furthermore, extensive investigation of promising drugs should be established in preliminary studies, first exploring safety and secondarily exploring proof of concept through larger dose ranging studies. This is certainly the FDA model for new drugs; i.e., begin with Phase I studies, progress to dose ranging and further safety Phase II studies, and finally choose a dose and perform a large-scale, adequately powered and designed Phase III efficacy study. Second, there remains, at least in the US, a great gulf between practitioners of TCM and allopathic physicians and a general skepticism of the Weltanschauung of the other. Along these lines, there is a lack of adequately trained investigators who are familiar both with CHM and with the design and implementation of RCTs. This latter element is especially lacking in China, where most of these trials are conducted, as the systematic reviews document.
A group of international authors has recognized the unique challenges of RCTs of CHMs and made the following summary recommendations to improve the quality of these trials by ensuring the stability, consistency, and purity of CHMs. These suggested guidelines, replicated here, are a positive step toward the exploration of CHMs for the treatment of PCOS and related disorders. At present, their potential is untapped, although this represents a tremendous opportunity for researchers on both sides of the ocean.
Dietary Supplements
Several dietary supplements may have benefical effects on PCOS. However, most studies are small or uncontrolled. Therefore, larger, better-designed studies are needed to further evaluate the risks and benefits of these supplements in PCOS. In addition, it is important to note that the supplements discussed here are not FDA approved for the treatment of PCOS.
Vitamin D.
Accumulating evidence suggests that vitamin D deficiency may be a causal factor in the pathogenesis of IR and the metabolic syndrome in PCOS (35). Furthermore, 25-hydroxyvitamin D levels are closely associated with impaired β-cell function, IGT, and the metabolic syndrome in PCOS women (113). Two small, uncontrolled studies demonstrate that vitamin D may improve IR and lipid profiles in PCOS patients (56, 92). One of these studies demonstrated a significant reduction in homeostatic model assessment of insulin resistance (HOMA-IR) 3 wk after a single oral vitamin D3 dose of 300,000 IU in 11 obese, insulin-resistant women with PCOS (56, 92). Moreover, vitamin D supplementation may also improve anovulation in PCOS. A pilot RCT of 60 infertile PCOS patients showed that the number of dominant follicles (≥14 mm) during 2–3 mo of follow-up was higher in the calcium (1,000 mg/day) plus vitamin D (400/day) plus metformin (1,500 mg/day) group than in the calcium + vitamin D-only group or the metformin-only group (88).
Vitamin B12 and folate.
Two recent studies suggest that B vitamins may be important in PCOS. In the first study, IR, obesity, and elevated homocysteine were associated with lower serum vitamin B12 concentrations in PCOS patients (55). The second study was a nonrandomized, placebo-controlled, double-blind trial that demonstrated that supplementation with folate (400 μg daily) for 6 mo increases the beneficial effect of metformin on the vascular endothelium in women with PCOS (84). However, the mechanisms are still unclear.
Green tea and spearmint tea.
Tea, next only to water, is the most popularly consumed beverage in the world, with a per capita consumption of 120 ml/day (78). Green tea has been shown to exert beneficial effects on glucose and lipid metabolism (11, 21) and the hormonal system (26, 50) in rats and humans, which are all very relevant in the management of PCOS patients. In addition, herbal tea reduces body weight and induced ovulation in androgen-sterilized rats (103). However, there are only two RCTs of herbal tea in PCOS, one using green tea (10) and the other spearmint tea (33).
The principal component of green tea, (−)-epigallocatechin-3-gallate (EGCG), significantly reduced body weight and circulating testosterone, estradiol, leptin, insulin, IGF-I, LH, glucose, cholesterol, and triglyceride in Sprague-Dawley rats and lean and obese Zucker rats (50). In vitro studies demonstrate that green tea extract and EGCG inhibit basal and stimulated testosterone production in rat Leydig cells. The mechanisms underlying the effects of EGCG involve the in vitro inhibition of the PKA/PKC signaling pathways as well as the inhibition of P-450 side-chain cleavage enzyme and 17β-hydroxysteroid dehydrogenase function during testicular steroidogenesis (26).
In an RCT of 34 obese Chinese women with PCOS, the body weight of the green tea capsule group (540 mg EGCG/day) decreased by a nonsignificant 2.4% after treatment, whereas the body weight, body mass index (BMI), and body fat content of the control group were significantly higher after 3 mo (10). However, there were no significant differences in glucose, lipid metabolism, or any of the hormone levels between the two groups. The lack of a positive finding in this study may be due to an inadequate dose of green tea and the small sample size of the study. Furthermore, the response to EGCG may be greater in other ethnic groups, especially those groups who do not already have a strong habit of taking tea in their daily lives (121).
In regard to spearmint tea, an RCT of 41 PCOS women showed that spearmint tea twice a day for 1 mo significantly decreased free and total testosterone levels, improved patients' subjective assessments of their hirsutism, and increased LH and FSH compared with a placebo herbal tea (33). Further studies are needed to confirm these findings and further elucidate the mechanisms underlying the antiandrogenic effects of spearmint tea.
Cinnamon extract.
Cinnamon extract (a traditional herb) has been shown to potentiate the insulin effect through upregulation of glucose uptake in cultured adipocytes (3, 8, 43). Cinnamon extract also improves insulin action via increasing glucose uptake in vivo, as it has been shown to enhance the insulin-signaling pathway in skeletal muscle in rats (85). An RCT of 15 women with PCOS showed significant reductions in IR in the cinnamon group (333 mg of cinnamon extract, 3 times a day) but not in the placebo group (111).
ω-3 and other polyunsaturated fatty acids.
A small RCT of 25 PCOS women demonstrated that dietary supplementation with ω -3 fatty acid 4 g/day (4 × 1,000-mg capsules of 56% docosahexaenoic acid and 27% eicosapentaenoic acid; Ocean Nutrition, Halifax, NS, Canada) for 8 wk has beneficial effects on liver fat content and other cardiovascular risk factors in women with PCOS (16). Another small study, of 17 women with PCOS, showed that increased dietary polyunsaturated fatty acid (PUFA) intake from walnuts (48 g walnuts per 800 kcal energy intake) for 3 mo increased glucose levels in women with PCOS (54). Forty-eight grams of walnuts contain 311 kcal (70 kcal from 30 g fat, 28 kcal from 7 g protein, and 36 kcal from 9 g carbohydrates) and provide 19 g of linolenic acid and 3.3 g of α-linolenic acid. Further studies are needed to determine the risks and benefits of ω-3 fatty acids and other PUFAs in PCOS.
Qi Gong and Tai Chi
Exercise is an important component of a healthy lifestyle, and it reduces metabolic and reproductive disturbances in PCOS. Qi gong and Tai chi are the two most popular Chinese medical exercises worldwide. Qi gong may be beneficial for individuals with DM-2 or metabolic syndrome, for its favorable effects on hemoglobin (Hb) A1c, glucose levels, and insulin sensitivity, reported by several RCTs (69, 104, 109). Tai chi has been shown to have similar energy expenditure to other moderate-intensity activities, such as walking at a speed of 6 km/h (2). It also has favorable effects on glucose control, lipid profile, and anxiety in patients with DM-2 (41, 108, 112). Therefore, Qi gong and Tai chi may be effective adjunct treatments for PCOS women. However, to date no studies have evaluated the effects of Qi gong or Tai chi in PCOS.
Mindfulness Meditation
Mindfulness, a component of ancient meditative practices such as Vipassana meditation and Zen meditation, is increasingly being applied to Western medicine to enhance psychological health and overall well-being (14, 71). In contemporary Western psychology, mindfulness has been described as the awareness that emerges through intentionally paying attention to one's present thoughts, emotions, and bodily sensations moment to moment in a nonjudgmental manner (93). Mindfulness has not been investigated in women with PCOS; however, studies in non-PCOS populations, including patients with DM-2 (89), suggest that mindfulness has psychological and physiological effects that could be beneficial in PCOS. Mindfulness-based stress reduction (MBSR), the most researched mindfulness-based program, reduces psychological distress (71) and may also reduce blood pressure, glucose, and inflammation (9, 77, 89, 115). These physiological effects appear to be mediated by changes in brain activity (18, 31) and structure (40, 60) leading to improvements in the autonomic nervous system (57, 81, 106) and hypothalamic-pituitary-adrenal (HPA) axis (9, 77, 115). It is hypothesized that these beneficial effects of mindfulness might ultimately lower the risk for diabetes and cardiovascular disease in PCOS. However, most of the evidence comes from small, uncontrolled, nonrandomized studies in non-PCOS populations. Therefore, well-designed RCTs of mindfulness in women with PCOS are needed before definitive conclusions can be drawn regarding the effects and mechanisms of mindfulness in PCOS.
Conclusions
In conclusion, several CAM treatments may have beneficial endocrine, cardiometabolic, and reproductive effects in women with PCOS. However, most studies are small, nonrandomized, or uncontrolled. Therefore, larger, well-designed RCTs are needed to further evaluate the safety, effectiveness, and mechanisms of CAM treatments for PCOS.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1. Ahmed MI, Duleba AJ, El Shahat O, Ibrahim ME, Salem A. Naltrexone treatment in clomiphene resistant women with polycystic ovary syndrome. Hum Reprod 23: 2564–2569, 2008. [DOI] [PubMed] [Google Scholar]
- 2. Ainsworth BE, Haskell WL, Whitt MC, Irwin ML, Swartz AM, Strath SJ, O'Brien WL, Bassett DR, Jr, Schmitz KH, Emplaincourt PO, Jacobs DR, Jr, Leon AS. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc 32: S498–S504, 2000. [DOI] [PubMed] [Google Scholar]
- 3. Anderson RA, Broadhurst CL, Polansky MM, Schmidt WF, Khan A, Flanagan VP, Schoene NW, Graves DJ. Isolation and characterization of polyphenol type-A polymers from cinnamon with insulin-like biological activity. J Agric Food Chem 52: 65–70, 2004. [DOI] [PubMed] [Google Scholar]
- 4. Barber TM, McCarthy MI, Wass JA, Franks S. Obesity and polycystic ovary syndrome. Clin Endocrinol (Oxf) 65: 137–145, 2006. [DOI] [PubMed] [Google Scholar]
- 5. Barnard L, Ferriday D, Guenther N, Strauss B, Balen AH, Dye L. Quality of life and psychological well being in polycystic ovary syndrome. Hum Reprod 22: 2279–2286, 2007. [DOI] [PubMed] [Google Scholar]
- 6. Bian ZX, Moher D, Dagenais S, Li YP, Wu TX, Liu L, Miao JX, Song L, Zhang HM. Improving the quality of randomized controlled trials in Chinese herbal medicine, part IV: applying a revised CONSORT checklist to measure reporting quality. Zhong Xi Yi Jie He Xue Bao 4: 233–242, 2006. [DOI] [PubMed] [Google Scholar]
- 7. Blank SK, McCartney CR, Helm KD, Marshall JC. Neuroendocrine effects of androgens in adult polycystic ovary syndrome and female puberty. Semin Reprod Med 25: 352–359, 2007. [DOI] [PubMed] [Google Scholar]
- 8. Broadhurst CL, Polansky MM, Anderson RA. Insulin-like biological activity of culinary and medicinal plant aqueous extracts in vitro. J Agric Food Chem 48: 849–852, 2000. [DOI] [PubMed] [Google Scholar]
- 9. Carlson LE, Speca M, Faris P, Patel KD. One year pre-post intervention follow-up of psychological, immune, endocrine and blood pressure outcomes of mindfulness-based stress reduction (MBSR) in breast and prostate cancer outpatients. Brain Behav Immun 21: 1038–1049, 2007. [DOI] [PubMed] [Google Scholar]
- 10. Chan CC, Koo MW, Ng EH, Tang OS, Yeung WS, Ho PC. Effects of Chinese green tea on weight, and hormonal and biochemical profiles in obese patients with polycystic ovary syndrome—a randomized placebo-controlled trial. J Soc Gynecol Investig 13: 63–68, 2006. [DOI] [PubMed] [Google Scholar]
- 11. Chantre P, Lairon D. Recent findings of green tea extract AR25 (Exolise) and its activity for the treatment of obesity. Phytomedicine 9: 3–8, 2002. [DOI] [PubMed] [Google Scholar]
- 12. Chen BY, Yu J. Relationship between blood radioimmunoreactive beta-endorphin and hand skin temperature during the electro-acupuncture induction of ovulation. Acupunct Electrother Res 16: 1–5, 1991. [DOI] [PubMed] [Google Scholar]
- 13. Chen ZH, Li J, Liu J, Zhao Y, Zhang P, Zhang MX, Zhang L. Saponins isolated from the root of Panax notoginseng showed significant anti-diabetic effects in KK-Ay mice. Am J Chin Med 36: 939–951, 2008. [DOI] [PubMed] [Google Scholar]
- 14. Chiesa A, Serretti A. A systematic review of neurobiological and clinical features of mindfulness meditations. Psychol Med 40: 1239–1252, 2010. [DOI] [PubMed] [Google Scholar]
- 15. Ciampelli M, Fulghesu AM, Guido M, Murgia F, Muzj G, Belosi C, Fortini A, Cento R, Lanzone A. Opioid blockade effect on insulin beta-cells secretory patterns in polycystic ovary syndrome. Oral glucose load versus intravenous glucagon bolus. Horm Res 49: 263–268, 1998. [DOI] [PubMed] [Google Scholar]
- 16. Cussons AJ, Watts GF, Mori TA, Stuckey BG. Omega-3 fatty acid supplementation decreases liver fat content in polycystic ovary syndrome: a randomized controlled trial employing proton magnetic resonance spectroscopy. J Clin Endocrinol Metab 94: 3842–3848, 2009. [DOI] [PubMed] [Google Scholar]
- 17. Dasgupta A, Davis B, Wells A. Effect of plantain on therapeutic drug monitoring of digoxin and thirteen other common drugs. Ann Clin Biochem 43: 223–225, 2006. [DOI] [PubMed] [Google Scholar]
- 18. Davidson RJ, Kabat-Zinn J, Schumacher J, Rosenkranz M, Muller D, Santorelli SF, Urbanowski F, Harrington A, Bonus K, Sheridan JF. Alterations in brain and immune function produced by mindfulness meditation. Psychosom Med 65: 564–570, 2003. [DOI] [PubMed] [Google Scholar]
- 19. Deeks AA, Gibson-Helm ME, Teede HJ. Anxiety and depression in polycystic ovary syndrome: a comprehensive investigation. Fertil Steril 93: 2421–2423, 2010. [DOI] [PubMed] [Google Scholar]
- 20. Dissen GA, Garcia-Rudaz C, Paredes A, Mayer C, Mayerhofer A, Ojeda SR. Excessive ovarian production of nerve growth factor facilitates development of cystic ovarian morphology in mice and is a feature of polycystic ovarian syndrome in humans. Endocrinology 150: 2906–2914, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dulloo AG, Duret C, Rohrer D, Girardier L, Mensi N, Fathi M, Chantre P, Vandermander J. Efficacy of a green tea extract rich in catechin polyphenols and caffeine in increasing 24-h energy expenditure and fat oxidation in humans. Am J Clin Nutr 70: 1040–1045, 1999. [DOI] [PubMed] [Google Scholar]
- 22. Dvorak Z, Vrzal R. Berberine reduces insulin resistance: the roles for glucocorticoid receptor and aryl hydrocarbon receptor. Fertil Steril 95: e7; author reply e8–e9, 2011. [DOI] [PubMed] [Google Scholar]
- 23. Eisenberg DM, Kessler RC, Foster C, Norlock FE, Calkins DR, Delbanco TL. Unconventional medicine in the United States. Prevalence, costs, and patterns of use. N Engl J Med 328: 246–252, 1993. [DOI] [PubMed] [Google Scholar]
- 24. Eyvazzadeh AD, Pennington KP, Pop-Busui R, Sowers M, Zubieta JK, Smith YR. The role of the endogenous opioid system in polycystic ovary syndrome. Fertil Steril 92: 1–12, 2009. [DOI] [PubMed] [Google Scholar]
- 25. Feng Y, Johansson J, Shao R, Manneras L, Fernandez-Rodriguez J, Billig H, Stener-Victorin E. Hypothalamic neuroendocrine functions in rats with dihydrotestosterone-induced polycystic ovary syndrome: effects of low-frequency electro-acupuncture. PLoS One 4: e6638, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Figueiroa MS, Cesar Vieira JS, Leite DS, Filho RC, Ferreira F, Gouveia PS, Udrisar DP, Wanderley MI. Green tea polyphenols inhibit testosterone production in rat Leydig cells. Asian J Androl 11: 362–370, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Finniss DG, Kaptchuk TJ, Miller F, Benedetti F. Biological, clinical, and ethical advances of placebo effects. Lancet 375: 686–695, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fruzzetti F, Bersi C, Parrini D, Ricci C, Genazzani AR. Effect of long-term naltrexone treatment on endocrine profile, clinical features, and insulin sensitivity in obese women with polycystic ovary syndrome. Fertil Steril 77: 936–944, 2002. [DOI] [PubMed] [Google Scholar]
- 29. Gerhard I, Postneek F. Auricular acupuncture in the treatment of female infertility. Gynecol Endocrinol 6: 171–181, 1992. [DOI] [PubMed] [Google Scholar]
- 30. Gilling-Smith C, Story H, Rogers V, Franks S. Evidence for a primary abnormality of thecal cell steroidogenesis in the polycystic ovary syndrome. Clin Endocrinol (Oxf) 47: 93–99, 1997. [DOI] [PubMed] [Google Scholar]
- 31. Goldin PR, Gross JJ. Effects of mindfulness-based stress reduction (MBSR) on emotion regulation in social anxiety disorder. Emotion 10: 83–91, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gonzalez F, Rote NS, Minium J, Kirwan JP. Evidence of proatherogenic inflammation in polycystic ovary syndrome. Metabolism 58: 954–962, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Grant P. Spearmint herbal tea has significant anti-androgen effects in polycystic ovarian syndrome. A randomized controlled trial. Phytother Res 24: 186–188, 2010. [DOI] [PubMed] [Google Scholar]
- 34. Grant SJ, Bensoussan A, Chang D, Kiat H, Klupp NL, Liu JP, Li X. Chinese herbal medicines for people with impaired glucose tolerance or impaired fasting blood glucose. Cochrane Database Syst Rev CD006690, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hahn S, Haselhorst U, Tan S, Quadbeck B, Schmidt M, Roesler S, Kimmig R, Mann K, Janssen OE. Low serum 25-hydroxyvitamin D concentrations are associated with insulin resistance and obesity in women with polycystic ovary syndrome. Exp Clin Endocrinol Diabetes 114: 577–583, 2006. [DOI] [PubMed] [Google Scholar]
- 36. Han JS. Acupuncture: neuropeptide release produced by electrical stimulation of different frequencies. Trends Neurosci 26: 17–22, 2003. [DOI] [PubMed] [Google Scholar]
- 37. Heider U, Pedal I, Spanel-Borowski K. Increase in nerve fibers and loss of mast cells in polycystic and postmenopausal ovaries. Fertil Steril 75: 1141–1147, 2001. [DOI] [PubMed] [Google Scholar]
- 38. Higashimura Y, Shimoju R, Maruyama H, Kurosawa M. Electro-acupuncture improves responsiveness to insulin via excitation of somatic afferent fibers in diabetic rats. Auton Neurosci 150: 100–103, 2009. [DOI] [PubMed] [Google Scholar]
- 39. Holmang A, Mimura K, Lönnroth P. Involuntary leg movements affect interstitial nutrient gradients and blood flow in rat skeletal muscle. J Appl Physiol 92: 982–988, 2002. [DOI] [PubMed] [Google Scholar]
- 40. Holzel BK, Ott U, Gard T, Hempel H, Weygandt M, Morgen K, Vaitl D. Investigation of mindfulness meditation practitioners with voxel-based morphometry. Soc Cogn Affect Neurosci 3: 55–61, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hung JW, Liou CW, Wang PW, Yeh SH, Lin LW, Lo SK, Tsai FM. Effect of 12-wk Tai Chi Chuan exercise on peripheral nerve modulation in patients with type 2 diabetes mellitus. J Rehabil Med 41: 924–929, 2009. [DOI] [PubMed] [Google Scholar]
- 42. Jansen G, Lundeberg T, Kjartansson J, Samuelson UE. Acupuncture and sensory neuropeptides increase cutaneous blood flow in rats. Neurosci Lett 97: 305–309, 1989. [DOI] [PubMed] [Google Scholar]
- 43. Jarvill-Taylor KJ, Anderson RA, Graves DJ. A hydroxychalcone derived from cinnamon functions as a mimetic for insulin in 3T3-L1 adipocytes. J Am Coll Nutr 20: 327–336, 2001. [DOI] [PubMed] [Google Scholar]
- 44. Jedel E, Labrie F, Odén A, Holm G, Nilsson L, Janson P, Lind AK, Ohlsson C, Stener-Victorin E. Impact of electroacupuncture and physical exercise on hyperandrogenism and oligo/amenorrhoea in women with polycystic ovary syndrome: a randomized controlled trial. Am J Physiol Endocrinol Metab 300: E37–E45, 2011. [DOI] [PubMed] [Google Scholar]
- 45. Jedel E, Waern M, Gustafson D, Landen M, Eriksson E, Holm G, Nilsson L, Lind AK, Janson PO, Stener-Victorin E. Anxiety and depression symptoms in women with polycystic ovary syndrome compared with controls matched for body mass index. Hum Reprod 25: 450–456, 2010. [DOI] [PubMed] [Google Scholar]
- 46. Jenkins PJ, Grossman A. The control of the gonadotrophin releasing hormone pulse generator in relation to opioid and nutritional cues. Hum Reprod 8, Suppl 2: 154–161, 1993. [DOI] [PubMed] [Google Scholar]
- 47. Johansson J, Feng Y, Shao R, Lonn M, Billig H, Stener-Victorin E. Intense electroacupuncture normalizes insulin sensitivity, increases muscle GLUT4 content, and improves lipid profile in a rat model of polycystic ovary syndrome. Am J Physiol Endocrinol Metab 299: E551–E559, 2010. [DOI] [PubMed] [Google Scholar]
- 48. Jonsdottir I. Neuropeptides and their interaction with exercise and immune function. Immunol Cell Biol 78: 562–570, 2000. [DOI] [PubMed] [Google Scholar]
- 49. Kagitani F, Uchida S, Hotta H, Aikawa Y. Manual acupuncture needle stimulation of the rat hindlimb activates groups I, II, III and IV single afferent nerve fibers in the dorsal spinal roots. Jpn J Physiol 55: 149–155, 2005. [DOI] [PubMed] [Google Scholar]
- 50. Kao YH, Hiipakka RA, Liao S. Modulation of endocrine systems and food intake by green tea epigallocatechin gallate. Endocrinology 141: 980–987, 2000. [DOI] [PubMed] [Google Scholar]
- 51. Kaptchuk TJ. The placebo effect in alternative medicine: can the performance of a healing ritual have clinical significance? Ann Intern Med 136: 817–825, 2002. [DOI] [PubMed] [Google Scholar]
- 52. Kaptchuk TJ, Goldman P, Stone DA, Stason WB. Do medical devices have enhanced placebo effects? J Clin Epidemiol 53: 786–792, 2000. [DOI] [PubMed] [Google Scholar]
- 53. Kaptchuk TJ, Kelley JM, Conboy LA, Davis RB, Kerr CE, Jacobson EE, Kirsch I, Schyner RN, Nam BH, Nguyen LT, Park M, Rivers AL, McManus C, Kokkotou E, Drossman DA, Goldman P, Lembo AJ. Components of placebo effect: randomised controlled trial in patients with irritable bowel syndrome. Br Med J 336: 999–1003, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kasim-Karakas SE, Almario RU, Gregory L, Wong R, Todd H, Lasley BL. Metabolic and endocrine effects of a polyunsaturated fatty acid-rich diet in polycystic ovary syndrome. J Clin Endocrinol Metab 89: 615–620, 2004. [DOI] [PubMed] [Google Scholar]
- 55. Kaya C, Cengiz SD, Satiroglu H. Obesity and insulin resistance associated with lower plasma vitamin B12 in PCOS. Reprod Biomed Online 19: 721–726, 2009. [DOI] [PubMed] [Google Scholar]
- 56. Kotsa K, Yavropoulou MP, Anastasiou O, Yovos JG. Role of vitamin D treatment in glucose metabolism in polycystic ovary syndrome. Fertil Steril 92: 1053–1058, 2009. [DOI] [PubMed] [Google Scholar]
- 57. Kubota Y, Sato W, Toichi M, Murai T, Okada T, Hayashi A, Sengoku A. Frontal midline theta rhythm is correlated with cardiac autonomic activities during the performance of an attention demanding meditation procedure. Brain Res Cogn Brain Res 11: 281–287, 2001. [DOI] [PubMed] [Google Scholar]
- 58. Lara HE, Ferruz JL, Luza S, Bustamante DA, Borges Y, Ojeda SR. Activation of ovarian sympathetic nerves in polycystic ovary syndrome. Endocrinology 133: 2690–2695, 1993. [DOI] [PubMed] [Google Scholar]
- 59. Lau CH, Chan CM, Chan YW, Lau KM, Lau TW, Lam FC, Che CT, Leung PC, Fung KP, Ho YY, Lau CB. In vitro antidiabetic activities of five medicinal herbs used in Chinese medicinal formulae. Phytother Res 22: 1384–1388, 2008. [DOI] [PubMed] [Google Scholar]
- 60. Lazar SW, Kerr CE, Wasserman RH, Gray JR, Greve DN, Treadway MT, McGarvey M, Quinn BT, Dusek JA, Benson H, Rauch SL, Moore CI, Fischl B. Meditation experience is associated with increased cortical thickness. Neuroreport 16: 1893–1897, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Lee MJ, Lee HS, Park SD, Moon HI, Park WH. Protective effects of luteolin-7-O-beta-d-glucuronide methyl ester from the ethyl acetate fraction of Lycopi Herba against pro-oxidant reactive species and low-density lipoprotein peroxidation. J Enzyme Inhib Med Chem 25: 702–707, 2010. [DOI] [PubMed] [Google Scholar]
- 62. Legro RS, Kunselman AR, Dodson WC, Dunaif A. Prevalence and predictors of dyslipidemia in women with polycystic ovary syndrome. Am J Med 111: 607–613, 2001. [DOI] [PubMed] [Google Scholar]
- 63. Legro RS, Kunselman AR, Dodson WC, Dunaif A. Prevalence and predictors of risk for type 2 diabetes mellitus and impaired glucose tolerance in polycystic ovary syndrome: a prospective, controlled study in 254 affected women. J Clin Endocrinol Metab 84: 165–169, 1999. [DOI] [PubMed] [Google Scholar]
- 64. Liang F, Chen R, Nakagawa A, Nishizawa M, Tsuda S, Wang H, Koya D. Low-frequency electroacupuncture improves insulin sensitivity in obese diabetic mice through activation of sirt1/pgc-1alpha in skeletal muscle. Evid Based Complement Altern Med 2011: 735297, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Lin RT, Tzeng CY, Lee YC, Ho WJ, Cheng JT, Lin JG, Chang SL. Acute effect of electroacupuncture at the Zusanli acupoints on decreasing insulin resistance as shown by lowering plasma free fatty acid levels in steroid-background male rats. BMC Complement Altern Med 9: 26, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Lin SH, Chau T. A puzzling cause of hypokalaemia. Lancet 360: 224, 2002. [DOI] [PubMed] [Google Scholar]
- 67. Linde K, Niemann K, Meissner K. Are sham acupuncture interventions more effective than (other) placebos? A re-analysis of data from the Cochrane review on placebo effects. Forsch Komplementmed 17: 259–264, 2010. [DOI] [PubMed] [Google Scholar]
- 68. Liu JP, Zhang M, Wang WY, Grimsgaard S. Chinese herbal medicines for type 2 diabetes mellitus. Cochrane Database Syst Rev CD003642, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Liu X, Miller YD, Burton NW, Brown WJ. A preliminary study of the effects of Tai Chi and Qigong medical exercise on indicators of metabolic syndrome, glycaemic control, health-related quality of life, and psychological health in adults with elevated blood glucose. Br J Sports Med 44: 704–709, 2010. [DOI] [PubMed] [Google Scholar]
- 70. Lobo RA, Granger LR, Paul WL, Goebelsmann U, Mishell DR., Jr Psychological stress and increases in urinary norepinephrine metabolites, platelet serotonin, and adrenal androgens in women with polycystic ovary syndrome. Am J Obstet Gynecol 145: 496–503, 1983. [DOI] [PubMed] [Google Scholar]
- 71. Ludwig DS, Kabat-Zinn J. Mindfulness in medicine. JAMA 300: 1350–1352, 2008. [DOI] [PubMed] [Google Scholar]
- 72. Lund I, Lundeberg T. Are minimal, superficial or sham acupuncture procedures acceptable as inert placebo controls? Acupunct Med 24: 13–15, 2006. [DOI] [PubMed] [Google Scholar]
- 73. MacPherson H, Altman DG, Hammerschlag R, Youping L, Taixiang W, White A, Moher D. Revised STandards for Reporting Interventions in Clinical Trials of Acupuncture (STRICTA): extending the CONSORT statement. PLoS Med 7: e1000261, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Manneras L, Cajander S, Lonn M, Stener-Victorin E. Acupuncture and exercise restore adipose tissue expression of sympathetic markers and improve ovarian morphology in rats with dihydrotestosterone-induced PCOS. Am J Physiol Regul Integr Comp Physiol 296: R1124–R1131, 2009. [DOI] [PubMed] [Google Scholar]
- 75. Manneras L, Jonsdottir IH, Holmang A, Lonn M, Stener-Victorin E. Low-frequency electro-acupuncture and physical exercise improve metabolic disturbances and modulate gene expression in adipose tissue in rats with dihydrotestosterone-induced polycystic ovary syndrome. Endocrinology 149: 3559–3568, 2008. [DOI] [PubMed] [Google Scholar]
- 76. Manni L, Lundeberg T, Holmang A, Aloe L, Stener-Victorin E. Effect of electro-acupuncture on ovarian expression of alpha(1)- and beta(2)-adrenoceptors, and p75 neurotrophin receptors in rats with steroid-induced polycystic ovaries. Reprod Biol Endocrinol 3: 21, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Matchim Y, Armer JM, Stewart BR. Effects of mindfulness-based stress reduction (MBSR) on health among breast cancer survivors. West J Nurs Res, 2010 Oct 18 [Epub ahead of print] PMID: 20956583. [DOI] [PubMed] [Google Scholar]
- 78. McKay DL, Blumberg JB. The role of tea in human health: an update. J Am Coll Nutr 21: 1–13, 2002. [DOI] [PubMed] [Google Scholar]
- 79. Moffet HH. How might acupuncture work? A systematic review of physiologic rationales from clinical trials. BMC Complement Altern Med 6: 25, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Moffet HH. Sham acupuncture may be as efficacious as true acupuncture: a systematic review of clinical trials. J Altern Complement Med 15: 213–216, 2009. [DOI] [PubMed] [Google Scholar]
- 81. Nagai M, Hoshide S, Kario K. The insular cortex and cardiovascular system: a new insight into the brain-heart axis. J Am Soc Hypertens 4: 174–182, 2010. [DOI] [PubMed] [Google Scholar]
- 82. Ni HY, Gong J. [ Research progress on Chinese herbal medicine in treating PCOS]. Liaoning J Trad Chinese Med 34: 123–124, 2007. [Google Scholar]
- 83. Norman RJ, Dewailly D, Legro RS, Hickey TE. Polycystic ovary syndrome. Lancet 370: 685–697, 2007. [DOI] [PubMed] [Google Scholar]
- 84. Palomba S, Falbo A, Giallauria F, Russo T, Tolino A, Zullo F, Colao A, Orio F. Effects of metformin with or without supplementation with folate on homocysteine levels and vascular endothelium of women with polycystic ovary syndrome. Diabetes Care 33: 246–251, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Qin B, Nagasaki M, Ren M, Bajotto G, Oshida Y, Sato Y. Cinnamon extract (traditional herb) potentiates in vivo insulin-regulated glucose utilization via enhancing insulin signaling in rats. Diabetes Res Clin Pract 62: 139–148, 2003. [DOI] [PubMed] [Google Scholar]
- 86. Raja-Khan N, Legro RS. Diagnosis and management of polycystic ovary syndrome. J Clin Outcomes Manage 12: 218–227, 2005. [Google Scholar]
- 87. Raja-Khan N, Shuja SA, Kunselman AR, Hogeman CS, Demers LM, Gnatuk CL, Legro RS. Brachial artery conductance during reactive hyperemia is increased in women with polycystic ovary syndrome. Eur J Obstet Gynecol Reprod Biol 155: 49–53, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Rashidi B, Haghollahi F, Shariat M, Zayerii F. The effects of calcium-vitamin D and metformin on polycystic ovary syndrome: a pilot study. Taiwan J Obstet Gynecol 48: 142–147, 2009. [DOI] [PubMed] [Google Scholar]
- 89. Rosenzweig S, Reibel DK, Greeson JM, Edman JS, Jasser SA, McMearty KD, Goldstein BJ. Mindfulness-based stress reduction is associated with improved glycemic control in type 2 diabetes mellitus: a pilot study. Altern Ther Health Med 13: 36–38, 2007. [PubMed] [Google Scholar]
- 90. Sato A, Sato Y. Regulation of regional cerebral blood flow by cholinergic fibers originating in the basal forebrain. Neurosci Res 14: 242–274, 1992. [DOI] [PubMed] [Google Scholar]
- 91. Schuring AN, Schulte N, Sonntag B, Kiesel L. Androgens and insulin—two key players in polycystic ovary syndrome. Recent concepts in the pathophysiology and genetics of polycystic ovary syndrome. Gynakol Geburtshilfliche Rundsch 48: 9–15, 2008. [DOI] [PubMed] [Google Scholar]
- 92. Selimoglu H, Duran C, Kiyici S, Ersoy C, Guclu M, Ozkaya G, Tuncel E, Erturk E, Imamoglu S. The effect of vitamin D replacement therapy on insulin resistance and androgen levels in women with polycystic ovary syndrome. J Endocrinol Invest 33: 234–238, 2010. [DOI] [PubMed] [Google Scholar]
- 93. Shapiro SL. The integration of mindfulness and psychology. J Clin Psychol 65: 555–560, 2009. [DOI] [PubMed] [Google Scholar]
- 94. Sherman KJ, Cherkin DC, Ichikawa L, Avins AL, Delaney K, Barlow WE, Khalsa PS, Deyo RA. Treatment expectations and preferences as predictors of outcome of acupuncture for chronic back pain. Spine (Phila Pa 1976) 35: 1471–1477, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Sills ES, Perloe M, Tucker MJ, Kaplan CR, Genton MG, Schattman GL. Diagnostic and treatment characteristics of polycystic ovary syndrome: descriptive measurements of patient perception and awareness from 657 confidential self-reports. BMC Womens Health 1: 3, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Stener-Victorin E, Fujisawa S, Kurosawa M. Ovarian blood flow responses to electroacupuncture stimulation depend on estrous cycle and on site and frequency of stimulation in anesthetized rats. J Appl Physiol 101: 84–91, 2006. [DOI] [PubMed] [Google Scholar]
- 97. Stener-Victorin E, Jedel E, Janson PO, Sverrisdottir YB. Low-frequency electroacupuncture and physical exercise decrease high muscle sympathetic nerve activity in polycystic ovary syndrome. Am J Physiol Regul Integr Comp Physiol 297: R387–R395, 2009. [DOI] [PubMed] [Google Scholar]
- 98. Stener-Victorin E, Kobayashi R, Kurosawa M. Ovarian blood flow responses to electro-acupuncture stimulation at different frequencies and intensities in anaesthetized rats. Autonom Neurosci Basic Clin 108: 50–56, 2003. [DOI] [PubMed] [Google Scholar]
- 99. Stener-Victorin E, Kobayashi R, Watanabe O, Lundeberg T, Kurosawa M. Effect of electro-acupuncture stimulation of different frequencies and intensities on ovarian blood flow in anaesthetised rats with steroid-induced polycystic ovaries. Reprod Biol Endocrinol 2: 16, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Stener-Victorin E, Lundeberg T, Waldenstrom U, Manni L, Aloe L, Gunnarsson S, Janson PO. Effects of electro-acupuncture on nerve growth factor and ovarian morphology in rats with experimentally induced polycystic ovaries. Biol Reprod 63: 1497–1503, 2000. [DOI] [PubMed] [Google Scholar]
- 101. Stener-Victorin E, Waldenstrom U, Tagnfors U, Lundeberg T, Lindstedt G, Janson PO. Effects of electro-acupuncture on anovulation in women with polycystic ovary syndrome. Acta Obstet Gynecol Scand 79: 180–188, 2000. [PubMed] [Google Scholar]
- 102. Streitberger K, Kleinhenz J. Introducing a placebo needle into acupuncture research. Lancet 352: 364–365, 1998. [DOI] [PubMed] [Google Scholar]
- 103. Sun F, Yu J. The effect of a special herbal tea on obesity and anovulation in androgen-sterilized rats. Proc Soc Exp Biol Med 223: 295–301, 2000. [DOI] [PubMed] [Google Scholar]
- 104. Sun GC, Lovejoy JC, Gillham S, Putiri A, Sasagawa M, Bradley R. Effects of Qigong on glucose control in type 2 diabetes: a randomized controlled pilot study. Diabetes Care 33: e8, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Sverrisdottir YB, Mogren T, Kataoka J, Janson PO, Stener-Victorin E. Is polycystic ovary syndrome associated with high sympathetic nerve activity and size at birth? Am J Physiol Endocrinol Metab 294: E576–E581, 2008. [DOI] [PubMed] [Google Scholar]
- 106. Takahashi T, Murata T, Hamada T, Omori M, Kosaka H, Kikuchi M, Yoshida H, Wada Y. Changes in EEG and autonomic nervous activity during meditation and their association with personality traits. Int J Psychophysiol 55: 199–207, 2005. [DOI] [PubMed] [Google Scholar]
- 107. Teede H, Deeks A, Moran L. Polycystic ovary syndrome: a complex condition with psychological, reproductive and metabolic manifestations that impacts on health across the lifespan. BMC Med 8: 41, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Tsai JC, Wang WH, Chan P, Lin LJ, Wang CH, Tomlinson B, Hsieh MH, Yang HY, Liu JC. The beneficial effects of Tai Chi Chuan on blood pressure and lipid profile and anxiety status in a randomized controlled trial. J Altern Complement Med 9: 747–754, 2003. [DOI] [PubMed] [Google Scholar]
- 109. Tsujiuchi T, Kumano H, Yoshiuchi K, He D, Tsujiuchi Y, Kuboki T, Suematsu H, Hirao K. The effect of Qi-gong relaxation exercise on the control of type 2 diabetes mellitus: a randomized controlled trial. Diabetes Care 25: 241–242, 2002. [DOI] [PubMed] [Google Scholar]
- 110. Wang BQ, Ling M. [ Research development of Chinese herbal medicine for PCOS]. Shandong J Trad Chinese Med 27: 138–140, 2008. [Google Scholar]
- 111. Wang JG, Anderson RA, Graham GM, 3rd, Chu MC, Sauer MV, Guarnaccia MM, Lobo RA. The effect of cinnamon extract on insulin resistance parameters in polycystic ovary syndrome: a pilot study. Fertil Steril 88: 240–243, 2007. [DOI] [PubMed] [Google Scholar]
- 112. Wang JH. Effects of Tai Chi exercise on patients with type 2 diabetes. Med Sport Sci 52: 230–238, 2008. [DOI] [PubMed] [Google Scholar]
- 113. Wehr E, Pilz S, Schweighofer N, Giuliani A, Kopera D, Pieber TR, Obermayer-Pietsch B. Association of hypovitaminosis D with metabolic disturbances in polycystic ovary syndrome. Eur J Endocrinol 161: 575–582, 2009. [DOI] [PubMed] [Google Scholar]
- 114. White A, Cummings M, Barlas P, Cardini F, Filshie J, Foster NE, Lundeberg T, Stener-Victorin E, Witt C. Defining an adequate dose of acupuncture using a neurophysiological approach—a narrative review of the literature. Acupunct Med 26: 111–120, 2008. [DOI] [PubMed] [Google Scholar]
- 115. Witek-Janusek L, Albuquerque K, Chroniak KR, Chroniak C, Durazo-Arvizu R, Mathews HL. Effect of mindfulness based stress reduction on immune function, quality of life and coping in women newly diagnosed with early stage breast cancer. Brain Behav Immun 22: 969–981, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Xia W, Sun C, Zhao Y, Wu L. Hypolipidemic and antioxidant activities of Sanchi (Radix Notoginseng) in rats fed with a high fat diet. Phytomedicine 18: 516–520, 2010. [DOI] [PubMed] [Google Scholar]
- 117. Xiaoming MO, Ding LI, Yunxing PU, Guifang XI, Xiuzhen LE, Zhimin FU. Clinical studies on the mechanism for acupuncture stimulation of ovulation. J Trad Chinese Med 13: 115–119, 1993. [PubMed] [Google Scholar]
- 118. Yang X, Zhang Y, Wu X, Bae CS, Hou L, Kuang H, Wang Y, Stener-Victorin E. Cryptotanshinone reverses reproductive and metabolic disturbances in prenatally androgenized rats via regulation of signaling mechanisms and androgen synthesis. Am J Physiol Regul Integr Comp Physiol 300: R869–R875, 2011. [DOI] [PubMed] [Google Scholar]
- 119. Yang XB, Huang ZM, Cao WB, Chen HY, Wang JH, Xu L. [ Therapeutic and protective effects of water-ethanolic extract from Rhizoma alismatis on streptozotocin-induced diabetic mice]. Xhongguo Shi Yian Fang Ji Xue Za Zhi 18: 336–350, 2002. [Google Scholar]
- 120. Yao T, Andersson S, Thoren P. Long-lasting cardiovascular depression induced by acupuncture-like stimulation of the sciatic nerve in unanaesthetized spontaneously hypertensive rats. Brain Res 240: 77–85, 1982. [DOI] [PubMed] [Google Scholar]
- 121. Yu Ng EH, Ho PC. Polycystic ovary syndrome in asian women. Semin Reprod Med 26: 14–21, 2008. [DOI] [PubMed] [Google Scholar]
- 122. Zhang H, Bian Z, Lin Z. Are acupoints specific for diseases? A systematic review of the randomized controlled trials with sham acupuncture controls. Chin Med 5: 1, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Zhang J, Li T, Zhou L, Tang L, Xu L, Wu T, Lim DC. Chinese herbal medicine for subfertile women with polycystic ovarian syndrome. Cochrane Database Syst Rev 9: CD007535, 2010. [DOI] [PubMed] [Google Scholar]
- 124. Zhang ZJ, Chen HY, Yip KC, Ng R, Wong VT. The effectiveness and safety of acupuncture therapy in depressive disorders: Systematic review and meta-analysis. J Affect Disord 124: 9–21, 2009. [DOI] [PubMed] [Google Scholar]
- 125. Zhao L, Li W, Han F, Hou L, Baillargeon JP, Kuang H, Wang Y, Wu X. Berberine reduces insulin resistance induced by dexamethasone in theca cells in vitro. Fertil Steril 95: 461–463, 2011. [DOI] [PubMed] [Google Scholar]


