Abstract
Compelling evidence supports the role of oxidative stress and renal interstitial inflammation in the pathogenesis of hypertension. Resveratrol is a polyphenolic stilbene, which can lower oxidative stress by activating the transcription factor nuclear factor-E2-related factor-2 (Nrf2), the master regulator of numerous genes encoding antioxidant and phase II-detoxifying enzymes and molecules. Given the role of oxidative stress and inflammation in the pathogenesis of hypertension, we conducted this study to test the hypothesis that long-term administration of resveratrol will attenuate renal inflammation and oxidative stress and, hence, progression of hypertension in the young spontaneously hypertensive rats (SHR). SHR and control [Wistar-Kyoto (WKY)] rats were treated for 9 wk with resveratrol or vehicle in their drinking water. Vehicle-treated SHR exhibited renal inflammatory injury and oxidative stress, as evidenced by glomerulosclerosis, tubulointerstitial injury, infiltration of inflammatory cells, and increased levels of renal 8-isoprostane and protein carbonylation. This was associated with reduced antioxidant capacity and downregulations of Nrf2 and phase II antioxidant enzyme glutathione-S-transferase (GST). Resveratrol treatment mitigated renal inflammation and injury, reduced oxidative stress, normalized antioxidant capacity, restored Nrf2 and GST activity, and attenuated the progression of hypertension in SHR. However, resveratrol had no effect on these parameters in WKY rats. In conclusion, development and progression of hypertension in the SHR are associated with inflammation, oxidative stress, and impaired Nrf2-GST activity in the kidney. Long-term administration of resveratrol restores Nrf2 expression, ameliorates inflammation, and attenuates development of hypertension in SHR. Clinical studies are needed to explore efficacy of resveratrol in human hypertension.
Keywords: oxidative stress, hypertension, proximal tubules
oxidative stress is a condition in which generation of reactive oxygen species (ROS) exceeds the capacity of the antioxidant defense system. ROS triggers inflammation, which confounds the deleterious effects of oxidative stress. In renal and vascular tissues, oxidative stress and its constant companion inflammation play a major role in the pathogenesis of all forms of hypertension by complex mechanisms that involve ROS-mediated inactivation and reduced production of NO, generation of vasoconstrictive prostanoids, augmentation of sympathetic activity, and sodium retention (31). Numerous studies have demonstrated increased production of ROS, upregulation of ROS-generating enzymes, and accumulation of biomarkers of oxidative stress in the kidney and vascular tissues of animals with acquired and genetic hypertension (23). Under normal conditions, nuclear factor-erythroid-2-related factor 2 (Nrf2) plays a central role in basal activity and coordinated induction of more than 250 genes, including those encoding antioxidant and phase II-detoxifying enzymes and substrates (13, 32). Thus, impaired activation of Nrf2 can contribute to the development or amplification of oxidative stress, and inflammation and strategies aimed at restoration of Nrf2 activity should help to contain these pathological conditions and their adverse consequences in hypertensive states.
Resveratrol is a polyphenolic stilbene derivative (3,4′,5-trihydroxy-trans-stilbene) found in several edible plants and in red wine that has received considerable attention because of its accessibility to the general population and a myriad of potential health benefits (18). Resveratrol has been found to counteract proinflammatory cytokines and improve chemically induced inflammation and confer numerous salutary effects (30). Nevertheless, most of these effects have not been confirmed in rigorous studies (5).
With respect to the beneficial cardiovascular effects of resveratrol, animal studies have shown amelioration of endothelial dysfunction and arteriolar remodeling (3, 28). These protective effects are largely mediated by its ability to activate Nrf2 and, hence, upregulate endogenous production of antioxidant and cytoprotective enzymes and substrates that attenuate oxidative stress and inflammation (29). Experimental and human studies have provided strong support for the role of renal inflammation in the pathogenesis of hypertension (11, 20, 21, 25). The complex interaction between immune cell infiltration, inflammatory cytokines, oxidative stress, and intrarenal ANG II raise the blood pressure, in part, by blunting pressure natriuresis (9). Resveratrol has been shown to ameliorate hypertension in double transgenic rats harboring human renin and human angiotensinogen genes (27), in estradiol-induced overproduction of superoxide in the rostral ventrolateral medulla, in animals with DOCA-salt hypertension, in obese Zucker rats, and in fructose-fed rats (4, 6, 16, 19). In addition, resveratrol has been reported to ameliorate hypertension, improve compliance and remodeling of the small arteries, and prevent development of pathological cardiac hypertrophy and contractile dysfunction in the spontaneously hypertensive rats (SHR) (3, 22, 28). To our knowledge, the effect of resveratrol on renal inflammation in SHR has not been previously demonstrated. The present study was undertaken to determine the protective effects of resveratrol against renal inflammation, oxidative stress, and development of hypertension in the SHR.
MATERIALS AND METHODS
Male 3–4-wk-old spontaneously hypertensive rats (SHR) and Wistar Kyoto (WKY) rats were purchased from Charles River Laboratories (Wilmington, MA) and kept in institutional facilities with free access to tap water and standard rat chow (Purina Mills, St. Louis, MO). All experiments were performed according to University of Houston guidelines and protocols and were approved by the Institutional Animal Care and Use Committee.
SHR and WKY rats were randomly divided into groups receiving either resveratrol (R) (50 mg/l) or tap water. Resveratrol was prepared by first dissolving 50 mg resveratrol in 1 ml ethanol. This solution was then diluted with tap water (4). Rats were supplied with either fresh solution of resveratrol (SHR-R and WKY-R; n = 8–12 each) or tap water (SHR and WKY; n = 8–12 each) every day starting at 3–4 wk of age and maintained until 12 wk of age.
Animal surgery.
Blood pressure was determined in unrestrained conscious animals by radiotelemetry. Sensor radio transmitters (TA11PA-C40; Data Sciences International, St. Paul, MN) were implanted in the aorta at 6 wk of age with sterile precautions under general 5% isoflurane anesthesia. To secure the catheter to the aorta, tissue adhesive (Vetbond, 3M Animal Care Products, St. Paul, MN) was used. The body of the telemetric device was placed in the abdominal cavity and sutured to the abdominal musculature. Postsurgical analgesia was achieved by the administration of buprenorphine (0.05 mg/kg sc) every 12 h for 2 days. Animals were allowed 1 wk for recovery before blood pressure measurements were recorded.
Proximal tubule preparation.
Rats were anesthetized with pentobarbital sodium (50 mg/kg ip), and a midline abdominal incision was made to cannulate the aorta below the kidneys and initiate an in situ digestion with iso-osmotic collagenase and hyaluronidase (Sigma, St. Louis, MO). Density gradient centrifugation with 25% Ficoll in Krebs-Henseleit buffer was used to enrich and purify proximal tubules, as detailed previously (2). Proximal tubules were homogenized by routine lab methods, as detailed elsewhere (2), and protein concentration was determined by using a BCA kit with BSA as a standard.
Measurement of oxidative and antioxidative markers.
Oxidative markers like renal proximal tubular 8-isoprostane and protein carbonylation (Millipore, Billerica, MA) were measured by using commercially available assay kits. Renal proximal tubular antioxidant capacity and the activities of antioxidative enzymes glutathione-S-transferase (GST) and superoxide dismutase (SOD) were measured by using commercially available kits from Cayman Chemicals (Ann Arbor, MI).
Immunoblotting.
Renal proximal tubular homogenate and nuclear fractions for GST and Nrf2, respectively, were resolved by SDS-PAGE and transferred to PVDF membrane (Millipore, Bedford, MA) followed by overnight blocking with 5% BSA (Pierce, Rockford, IL). The membranes were incubated with anti-GST rabbit polyclonal antibody (Santa Cruz Biotechnology, Santa Cruz, CA) or anti-Nrf2 antibody (Ab53019; AbCam, Cambridge, MA), and Western blot analysis was performed as detailed previously (1).
Light microscopy and immunohistology.
Rats were killed by exsanguination under general anesthesia, and kidneys were harvested for light microscopy and immunohistology studies. Light microscopy was done in the formalin-fixed sections stained with periodic acid-Schiff and hematoxylin and eosin. Glomerulosclerosis index score and tubulointerstitial damage were evaluated as described in previous studies (17–19). The sclerosis in the glomeruli were graded from 0 to +4: grade 0 = normal, grade +1 = <25% involvement of the glomerular tuft, grade +2 = 25–50% involvement of the glomerular tuft, grade +3 = 50–75% involvement of the glomerular tuft, and grade +4 = sclerosis occupying >75% of the glomerular tuft. The glomerulosclerosis score was obtained as follows: [(1 × n glomeruli with +1) + (2 × n glomeruli with +2) + (3 × n glomeruli with +3) + (4 × n glomeruli with +4)] × 100/total number of glomeruli examined. Tubulointerstitium was evaluated in successive fields examined at ×20 magnification in the entire cortical and juxtamedullary areas of each specimen using computer-assisted image analysis (Olympus BX51 System Microscope and DP70 microscope digital camera with software, Sigma Pro, Leesburg, VA). Tubulointerstitial damage (tubular dilatation, interstitial infiltration, and fibrosis) was graded in a 0–5 scale: 0 = no changes, grade 1 = <10%, grade 2 = 10–25%, grade 3 = 25–50%, grade 4 = 50–75%, grade 5 = 75–100%. Immunoperoxidase methodology was used to identify lymphocytes and macrophages in the glomeruli (positive cells/glomerular cross section) and tubulointerstitial regions (positive cells per mm2). All histological evaluations were done without prior knowledge of the experimental group being studied.
Antisera for immunohistology.
Macrophages were identified with mouse anti-ED1 (Biosource, Camarillo, CA), lymphocytes with mouse anti-rat CD5 (Biosource) and ANG II-positive cells with rabbit anti-ANG II-human IgG (Peninsula Laboratories). Peroxidase-conjugated goat anti-mouse IgG (Stressgen Bioreagents Victoria, BC Canada) was used as a secondary antibody.
Nrf2 assay.
Nuclear Nrf2 translocation was determined by following the protocol described by Wang et al. (33, 34). Briefly, Nrf2 double-stranded DNA probe was commercially synthesized by using the published ARE sequence. Strand one was labeled at 5′ end with 6-FAMTM Fluorescein fluorophore and the complementary strand was labeled at 3′ end with Iowa Black FQ, a quencher. A single-stranded DNA competitor with sequence similar to quencher sequence was synthesized as well. The binding of Nrf2 to double-stranded ARF sequence stabilized the fluorophore-quencher complex and delayed the competitor from separating the quencher from the fluorophore. Therefore, the higher the Nrf2 content, the lower the fluorescence intensity, allowing quantitative measurement of Nrf2 protein concentration based on its inverse relation to the fluorescence intensity. Proximal tubular nuclear fractions, isolated by using commercially NE-PER extraction kit (Thermo Fisher), was incubated with double-stranded ARE sequence in the presence and absence of competitor single-stranded sequence as detailed by Wang et al. (33). Fluorescence was measured by BioTek plate reader (absorption 495/emission 520).
Statistical analysis.
Results are presented as means ± SE. Two-way ANOVA with Newman-Keuls post hoc tests were used for evaluation of statistical significance of difference between means. P < 0.05 was considered significant. Commercially available statistical software (GraphPad Prism, version 5, San Diego, CA) was used for analysis.
RESULTS
Effect of resveratrol on body weight, food and water intake, and blood pressure.
Resveratrol treatment was started at the age of 3 wk and continued until 12 wk of age. Blood pressure measurements were recorded from the age of 7–12 wk. Body weight (g, WKY: 312.3 ± 5.1, WKY-R: 318.8 ± 5.1, SHR: 327.5 ± 8.1, SHR-R: 316.3 ± 9.2), food consumption (g/day, WKY: 19.3 ± 3.7, WKY-R: 17.5 ± 3.4, SHR: 21.1 ± 4.7, SHR-R: 20.5 ± 3.0) and water intake (ml/day, WKY: 46.0 ± 6.3, WKY-R: 47.3 ± 5.1, SHR: 42.8 ± 6.1, SHR-R: 47.3 ± 5.8) were similar in all the experimental groups. As expected, the mean arterial pressure was significantly higher in SHR compared with WKY rats (Fig. 1). Compared with untreated SHR, resveratrol-treated SHR showed a significant reduction in blood pressure from week 9 onward (Fig. 1). Moreover, unlike the untreated SHR, which showed a steady rise in blood pressure, the resveratrol-treated group showed no further rise in blood pressure beyond 7 wk of age. Untreated and resveratrol-treated WKY showed similar MAP (Fig. 1).
Fig. 1.
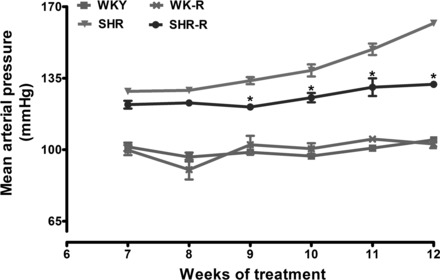
Effect of resveratrol (R) on blood pressure in spontaneously hypertensive rats (SHR) and Wistar-Kyoto (WKY) rats. Blood pressure was measured continuously from 7 to 12 wk of age. Data are expressed as means ± SE from 6–8 rats. *P < 0.05 vs. untreated SHR at respective time point.
Effect of resveratrol on markers of oxidative stress and antioxidant capacity.
The renal proximal tubular 8-isoprostane level was significantly higher in SHR compared with WKY rats (Fig. 2A). Resveratrol treatment significantly decreased proximal tubular 8-isoprostane levels in SHR, while having no effect in WKY rats (Fig. 2A). Also, proximal tubular protein carbonyl levels were significantly higher in SHR compared with WKY rats (Fig. 2B). Resveratrol treatment significantly decreased protein carbonyl levels in SHR but not in WKY rats (Fig. 2B). Proximal tubular total antioxidant activity was significantly lower in SHR than in WKY rats (Fig. 2C). Resveratrol treatment restored total antioxidant activity in SHR, while having no effect in WKY rats (Fig. 2C).
Fig. 2.
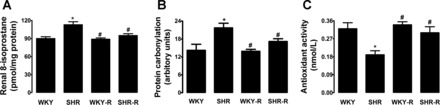
Effect of resveratrol (R) on oxidative stress in renal proximal tubules from SHR and WKY rats. Proximal tubular 8-isoprostane (A), protein carbonylation (B) and total antioxidant activity (C). Data are expressed as means ± SE from 6–8 rats. *P < 0.05 vs. WKY rats and #P < 0.05 vs. SHR.
Effect of resveratrol on renal injury and inflammation.
Both treated and untreated SHR groups exhibited mild, but significant, increments in the glomerulosclerosis index (scores: WKY: 4.0 ± 0.65, SHR: 7.1 ± 1.37**, WKY-R: 4.1 ± 0.78, SHR-R: 6.2 ± 0.77*; **P < 0.01 vs. WKY, *P < 0.05 vs. WKY), and tubulointerstitial injury (scores: WKY: 1.28 ± 0.48, SHR: 2.12 ± 0.33**, WKY-R: 1.11 ± 0.11, SHR-R: 1.89 ± 0.06*; **P < 0.01 vs. WKY, *P < 0.05 vs. WKY) scores compared with the corresponding values in the WKY rats. Resveratrol treatment did not modify renal histology in the WKY rats but led to a minor improvement in renal lesions in SHR. However, the difference between SHR and SHR-R did not reach the statistical significance.
Immune cells were rarely present in the glomeruli in any experimental group (<3 cells/glomerular cross section). In contrast, tubulointerstitial areas showed significant infiltration of inflammatory cells. Resveratrol treatment ameliorated the renal infiltration of lymphocytes, macrophages, and ANG II-positive cells in tubulointerstitial areas of the kidney (Fig. 3, A–C and microphotographs in Fig. 4).
Fig. 3.
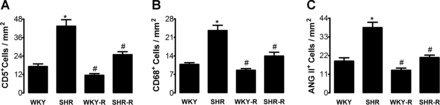
Effect of resveratrol (R) on kidney tubulointerstitial inflammation in SHR and WKY rats. Lymphocytes: CD5+ cells (A), macrophages: CD68+ cells (B), and ANG II-positive cells (C) are all increased in the SHR and reduced by resveratrol treatment. Data are expressed as means ± SE. *P < 0.05 vs. WKY rats and #P < 0.05 vs. SHR.
Fig. 4.
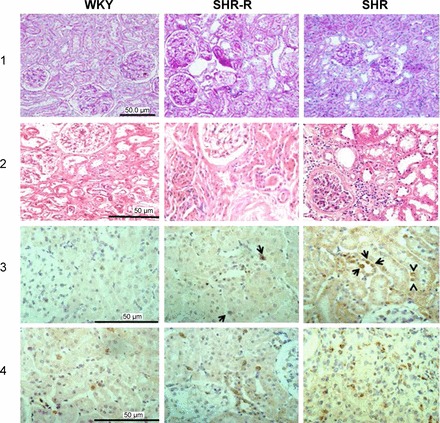
Representative kidney microphotographs of the histologic (periodic acid-Schiff staining, row 1), (hematoxylin-and-eosin staining, row 2) and immunohistologic findings (immunoperoxidase; rows 3 and 4) in WKY, SHR treated with resveratrol (SHR-R) and SHR. Light histology is normal in WKY rats and show focal areas of tubular dilation and atrophy in the SHR-R, while SHR rats (untreated) show more extensive tubulointerstitial damage and one glomerulus with a focal (<10%) are of sclerosis. ANG II-positive cells (row 3) are not found in the WKY rats, a small number of tubular cells stain positive for ANG II (arrows) in the SHR-R group and are increased in number, in association with positive infiltrating cells in the SHR control. Lymphocytes (row 4) are scarce in the WKY group and are increased in number in the SHR-R group, and higher numbers of lymphocytes are present in the SHR group.
Effect of resveratrol on Nrf2 activity and its downstream antioxidant enzymes.
As shown in Fig. 5A, the fluorescence intensity was higher in SHR proximal tubular nuclear extracts, suggesting a decrease in Nrf2 concentration as fluorescence intensity is inversely proportional to Nrf2 concentration. The decrease in nuclear Nrf2 was also observed with Western blot analysis, as Nrf2 immunoreactivity was significantly lower in SHR compared with WKY rats (Fig. 5, B and C). Resveratrol treatment significantly increased nuclear Nrf2 expression in the proximal tubules of SHR but had no effect in WKY rats (Fig. 5, A–C). GST message level, abundance, and activity were significantly lower in the proximal tubules of SHR compared with WKY rats, and resveratrol treatment normalized GST function in SHR (Fig. 6, A–C). Despite the presence of oxidative stress, SOD activity in SHR was similar to those found in the WKY, pointing to a maladaptive response in the SHR kidney (Fig. 6D). Resveratrol administration significantly increased SOD activity in SHR (Fig. 6D). Resveratrol had no effect on GST and SOD activity or expression in WKY rats (Fig. 6, A–D).
Fig. 5.

Effect of resveratrol (R) on nuclear Nrf2 expression in renal proximal tubules form SHR and WKY rats. Nrf2 nuclear fluorescence intensity (A), bars representing nuclear Nrf2 immunoreactivity (B), and three representative nuclear Nrf2 blots and a histone blot. lane 1, WKY; lane 2, SHR; lane 3, WKY-R; lane 4, SHR-R (C). MW, molecular weight; V, vehicle. Data are expressed as means ± SE from 6–8 rats. *P < 0.05 vs. WKY rats, and #P < 0.05 vs. SHR.
Fig. 6.
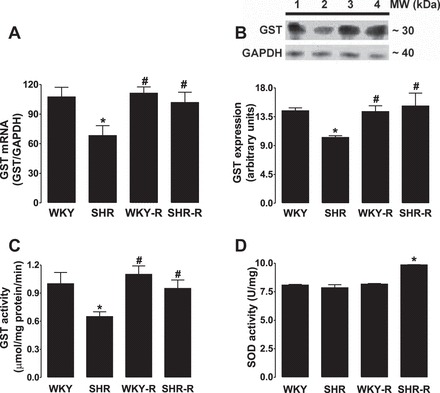
Effect of resveratrol (R) phase II antioxidant enzymes in renal proximal tubules form SHR and WKY rats. A: glutathione-S-transferase (GST) protein mRNA level. B: bars representing protein content; top: a representative immunoblot: lane 1, WKY; lane 2, SHR; lane 3, WKY-R; and lane 4, SHR-R. Enzymatic activity of GST (C) and superoxide dismutase (SOD) in proximal tubules (D). Data are expressed as means ± SE from 6–8 rats. *P < 0.05 vs. WKY rats, and #P < 0.05 vs. SHR.
DISCUSSION
In confirmation of earlier studies, the untreated SHR exhibited interstitial immune cell infiltration in the renal tissue (22, 24). This was associated with marked elevation of by-products of lipid and protein oxidation in the isolated renal proximal tubular epithelial cells, suggesting oxidative stress in these cells. The present study demonstrated, for the first time, involvement of proximal tubular epithelial cells in this pathological process. This is not surprising since proximal tubular epithelial cells are very rich in mitochondria, which generate massive amounts of ATP to accommodate reabsorption of filtered sodium by the Na+/K+-ATPase pump. Since mitochondria are the main source of ROS production, the antioxidant defense system is critical for proper functioning and viability of proximal tubular epithelial cells. Despite the presence of oxidative stress, which should have led to activation (nuclear translocation) of Nrf2, the untreated SHR paradoxically exhibited a significant reduction of nuclear Nrf2 and diminished or unchanged expression of its target gene products in the proximal tubular epithelial cells. These findings point to the role of impaired Nrf2 activity in the pathogenesis of oxidative stress and inflammation in the SHR kidney.
The central findings of this study are the demonstration that early treatment with the natural Nrf2 activator, resveratrol, reduced oxidative stress in proximal tubular epithelial cells, lowered the number of interstitial inflammatory cells and ANG II-positive cells in the kidney, and attenuated progression of hypertension in SHR. Attenuation of oxidative stress in the proximal tubular epithelial cells with resveratrol administration was accompanied by and, in part, due to restoration of the Nrf2 activity, and expression of the antioxidant enzymes in the treated SHR. Previous studies have shown that one of the mechanisms of antioxidant effect of resveratrol is via activation of transcription factor Nrf2. Normally, ROS trigger activation (nuclear translocation) of the redox-sensitive transcription factor Nrf2, which leads to transcriptional upregulation of numerous target genes encoding detoxifying and antioxidant enzymes and related proteins (15). Despite the presence of oxidative stress, Nrf2 activation was significantly lower in the SHR kidney compared with the WKY rats. Resveratrol treatment caused a significant increase in nuclear Nrf2 abundance in the proximal tubular epithelial cells of the SHR group. It is noteworthy that resveratrol treatment did not alter the constitutive activity of Nrf2 in the WKY rats, reflecting their ability to maintain redox homeostasis.
Nrf2-mediated activation of antioxidant enzymes, such as GST and SOD, plays an important role in protecting the cells against oxidative injury and in the aging process (10). We found that GST protein expression and activity was significantly decreased in proximal tubules of SHR, and resveratrol treatment normalized both abundance and activity of GST in these rats. Despite oxidative stress, proximal tubular SOD activity in the SHR was similar to those found in the WKY rats, indicating failure of these rats to counter increased oxidative stress. Long-term resveratrol administration enhanced SOD and GST levels in the SHR but not in the WKY rats. Taken together, these findings illustrate the role of impaired Nrf2 activity in the pathogenesis of oxidative stress in the proximal tubular epithelial cells of SHR and its amelioration with long-term administration of resveratrol.
Oxidative stress, renal interstitial inflammation, and activation of intrarenal ANG II are intimately interrelated and participate in a vicious cycle, which promotes hypertension by augmenting vascular resistance and reducing pressure natriuresis (9, 31). Therefore, it is not surprising that amelioration of oxidative stress with resveratrol administration resulted in the reduction of renal inflammation and intrarenal ANG II expression and lowering of blood pressure in SHR. The suppression of renal inflammation obtained with resveratrol has been previously shown in experimental models of obesity, diabetes, monocrotaline-induced pulmonary hypertension and ANG II-induced hypertension (6, 7, 12, 14, 17, 26, 35). Although we did not study renal ANG II activity, we found that increased ANG II-positive cells in the untreated SHR kidney tissue, which were reduced by resveratrol treatment. Previous studies from our laboratories have shown that in tubulointerstitial inflammation, the fluctuations of ANG II-positive cells are directly correlated with the existing renal ANG II activity (8).
Limitations.
Although resveratrol treatment, in part, via Nrf2 signaling, ameliorated renal oxidative stress and inflammation, the blood pressure in SHR remained significantly higher than WKY rats. This is not surprising given the fact that hypertension is a multifactorial disease. While renal oxidative stress and inflammation are major contributors to etiology of hypertension, many other organs via a plethora of mechanisms also significantly contribute to the development and maintenance of long-term high blood pressure.
Perspectives and Significance
The development and progression of hypertension in the young SHR are associated with impaired Nrf2 signaling, inflammation, and oxidative stress in renal proximal tubular epithelial cells. Long-term administration of resveratrol, a natural Nrf2 activator, restores Nrf2 activity, improves oxidative milieu and inflammation, and attenuates severity and progression of hypertension in SHR. Further studies are needed to explore the effects resveratrol as an adjunct in the treatment of hypertension in humans.
GRANTS
The study was supported by National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-098509 to M. F. Lokhandwala, a Scientist Development Grant (0835428N, National Center) from the American Heart Association to A. A. Banday, and Grant FONACYT- LOCTI 1306 to B. Rodoriguez-Iturbe.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: A.A.J. and Y.Q. performed experiments; A.A.J. and Y.Q. analyzed data; A.A.J. and Y.Q. interpreted results of experiments; A.A.J. and Y.Q. prepared figures; A.A.J. and N.D.V. drafted manuscript; A.A.J., Y.Q., B.R.-I., N.D.V., M.F.L., and A.A.B. approved final version of manuscript; B.R.-I., N.D.V., M.F.L., and A.A.B. edited and revised manuscript; A.A.B. conception and design of research.
REFERENCES
- 1.Banday AA, Fazili FR, Marwaha A, Lokhandwala MF. Mitogen-activated protein kinase upregulation reduces renal D1 receptor affinity and G-protein coupling in obese rats. Kidney Int 71: 397–406, 2007. [DOI] [PubMed] [Google Scholar]
- 2.Banday AA, Marwaha A, Tallam LS, Lokhandwala MF. Tempol reduces oxidative stress, improves insulin sensitivity, decreases renal dopamine D1 receptor hyperphosphorylation, and restores D1 receptor-G-protein coupling and function in obese Zucker rats. Diabetes 54: 2219–2226, 2005. [DOI] [PubMed] [Google Scholar]
- 3.Behbahani J, Thandapilly SJ, Louis XL, Huang Y, Shao Z, Kopilas MA, Wojciechowski P, Netticadan T, Anderson HD. Resveratrol and small artery compliance and remodeling in the spontaneously hypertensive rat. Am J Hypertens 23: 1273–1278, 2010. [DOI] [PubMed] [Google Scholar]
- 4.Bhatt SR, Lokhandwala MF, Banday AA. Resveratrol prevents endothelial nitric oxide synthase uncoupling and attenuates development of hypertension in spontaneously hypertensive rats. Eur J Pharmacol 667: 258–264, 2011. [DOI] [PubMed] [Google Scholar]
- 5.Biala A, Tauriainen E, Siltanen A, Shi J, Merasto S, Louhelainen M, Martonen E, Finckenberg P, Muller DN, Mervaala E. Resveratrol induces mitochondrial biogenesis and ameliorates ANG II-induced cardiac remodeling in transgenic rats harboring human renin and angiotensinogen genes. Blood Press 19: 196–205, 2010. [DOI] [PubMed] [Google Scholar]
- 6.Chan V, Fenning A, Iyer A, Hoey A, Brown L. Resveratrol improves cardiovascular function in DOCA-salt hypertensive rats. Curr Pharm Biotechnol 12: 429–436, 2011. [DOI] [PubMed] [Google Scholar]
- 7.Csiszar A, Labinskyy N, Olson S, Pinto JT, Gupte S, Wu JM, Hu F, Ballabh P, Podlutsky A, Losonczy G, de Cabo R, Mathew R, Wolin MS, Ungvari Z. Resveratrol prevents monocrotaline-induced pulmonary hypertension in rats. Hypertension 54: 668–675, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Franco M, Martinez F, Quiroz Y, Galicia O, Bautista R, Johnson RJ, Rodriguez-Iturbe B. Renal angiotensin II concentration and interstitial infiltration of immune cells are correlated with blood pressure levels in salt-sensitive hypertension. Am J Physiol Regul Integr Comp Physiol 293: R251–R256, 2007. [DOI] [PubMed] [Google Scholar]
- 9.Franco M, Tapia E, Bautista R, Pacheco U, Santamaria J, Quiroz Y, Johnson RJ, Rodriguez-Iturbe B. Impaired pressure natriuresis resulting in salt-sensitive hypertension is caused by tubulointerstitial immune cell infiltration in the kidney. Am J Physiol Renal Physiol 304: F982–F990, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.George L, Lokhandwala MF, Asghar M. Exercise activates redox-sensitive transcription factors and restores renal D1 receptor function in old rats. Am J Physiol Renal Physiol 297: F1174–F1180, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harrison DG, Guzik TJ, Lob HE, Madhur MS, Marvar PJ, Thabet SR, Vinh A, Weyand CM. Inflammation, immunity, and hypertension. Hypertension 57: 132–140, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Inanaga K, Ichiki T, Matsuura H, Miyazaki R, Hashimoto T, Takeda K, Sunagawa K. Resveratrol attenuates angiotensin II-induced interleukin-6 expression and perivascular fibrosis. Hypertens Res 32: 466–471, 2009. [DOI] [PubMed] [Google Scholar]
- 13.Kobayashi A, Kang MI, Watai Y, Tong KI, Shibata T, Uchida K, Yamamoto M. Oxidative and electrophilic stresses activate Nrf2 through inhibition of ubiquitination activity of Keap1. Mol Cell Biol 26: 221–229, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kumar A, Sharma SS. NF-κB inhibitory action of resveratrol: a probable mechanism of neuroprotection in experimental diabetic neuropathy. Biochem Biophys Res Commun 394: 360–365, 2010. [DOI] [PubMed] [Google Scholar]
- 15.Mattson MP, Son TG, Camandola S. Mechanisms of action and therapeutic potential of neurohormetic phytochemicals. Dose Response 5: 174–186, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miatello R, Vazquez M, Renna N, Cruzado M, Zumino AP, Risler N. Chronic administration of resveratrol prevents biochemical cardiovascular changes in fructose-fed rats. Am J Hypertens 18: 864–870, 2005. [DOI] [PubMed] [Google Scholar]
- 17.Palsamy P, Subramanian S. Ameliorative potential of resveratrol on proinflammatory cytokines, hyperglycemia mediated oxidative stress, and pancreatic β-cell dysfunction in streptozotocin-nicotinamide-induced diabetic rats. J Cell Physiol 224: 423–432, 2010. [DOI] [PubMed] [Google Scholar]
- 18.Rahman I, Ungvari Z. Resveratrol and Longevity. In: Resveratrol—2014. Proceedings of the 3rd International Conference on Resveratrol and Health. www.Resveratrol2014.hawaii-conference.com. [Google Scholar]
- 19.Rivera L, Moron R, Zarzuelo A, Galisteo M. Long-term resveratrol administration reduces metabolic disturbances and lowers blood pressure in obese Zucker rats. Biochem Pharmacol 77: 1053–1063, 2009. [DOI] [PubMed] [Google Scholar]
- 20.Rodriguez-Iturbe B, Franco M, Tapia E, Quiroz Y, Johnson RJ. Renal inflammation, autoimmunity and salt-sensitive hypertension. Clin Exp Pharmacol Physiol 39: 96–103, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rodriguez-Iturbe B, Johnson RJ. The role of renal microvascular disease and interstitial inflammation in salt-sensitive hypertension. Hypertens Res 33: 975–980, 2010. [DOI] [PubMed] [Google Scholar]
- 22.Rodriguez-Iturbe B, Quiroz Y, Nava M, Bonet L, Chavez M, Herrera-Acosta J, Johnson RJ, Pons HA. Reduction of renal immune cell infiltration results in blood pressure control in genetically hypertensive rats. Am J Physiol Renal Physiol 282: F191–F201, 2002. [DOI] [PubMed] [Google Scholar]
- 23.Rodriguez-Iturbe B, Vaziri ND, Herrera-Acosta J, Johnson RJ. Oxidative stress, renal infiltration of immune cells, and salt-sensitive hypertension: all for one and one for all. Am J Physiol Renal Physiol 286: F606–F616, 2004. [DOI] [PubMed] [Google Scholar]
- 24.Rodriguez-Iturbe B, Zhan CD, Quiroz Y, Sindhu RK, Vaziri ND. Antioxidant-rich diet relieves hypertension and reduces renal immune infiltration in spontaneously hypertensive rats. Hypertension 41: 341–346, 2003. [DOI] [PubMed] [Google Scholar]
- 25.Schiffrin EL. T lymphocytes: a role in hypertension? Curr Opin Nephrol Hypertens 19: 181–186, 2010. [DOI] [PubMed] [Google Scholar]
- 26.Sharma S, Chopra K, Kulkarni SK. Effect of insulin and its combination with resveratrol or curcumin in attenuation of diabetic neuropathic pain: participation of nitric oxide and TNF-α. Phytother Res 21: 278–283, 2007. [DOI] [PubMed] [Google Scholar]
- 27.Subramanian M, Balasubramanian P, Garver H, Northcott C, Zhao H, Haywood JR, Fink GD, MohanKumar SM, MohanKumar PS. Chronic estradiol-17β exposure increases superoxide production in the rostral ventrolateral medulla and causes hypertension: reversal by resveratrol. Am J Physiol Regul Integr Comp Physiol 300: R1560–R1568, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thandapilly SJ, Wojciechowski P, Behbahani J, Louis XL, Yu L, Juric D, Kopilas MA, Anderson HD, Netticadan T. Resveratrol prevents the development of pathological cardiac hypertrophy and contractile dysfunction in the SHR without lowering blood pressure. Am J Hypertens 23: 192–196, 2010. [DOI] [PubMed] [Google Scholar]
- 29.Ungvari Z, Bagi Z, Feher A, Recchia FA, Sonntag WE, Pearson K, de Cabo R, Csiszar A. Resveratrol confers endothelial protection via activation of the antioxidant transcription factor Nrf2. Am J Physiol Heart Circ Physiol 299: H18–H24, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vang O, Ahmad N, Baile CA, Baur JA, Brown K, Csiszar A, Das DK, Delmas D, Gottfried C, Lin HY, Ma QY, Mukhopadhyay P, Nalini N, Pezzuto JM, Richard T, Shukla Y, Surh YJ, Szekeres T, Szkudelski T, Walle T, Wu JM. What is new for an old molecule? Systematic review and recommendations on the use of resveratrol. PLoS One 6: e19881, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vaziri ND, Rodriguez-Iturbe B. Mechanisms of disease: oxidative stress and inflammation in the pathogenesis of hypertension. Nat Clin Pract Nephrol 2: 582–593, 2006. [DOI] [PubMed] [Google Scholar]
- 32.Wakabayashi N, Slocum SL, Skoko JJ, Shin S, Kensler TW. When NRF2 talks, who's listening? Antioxid Redox Signal 13: 1649–1663, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang Z, Gidwani V, Sun Z, Zhang D, Wong PK. Development of a molecular assay for rapid screening of chemopreventive compounds targeting Nrf2. J Assoc Lab Autom 13: 243–248, 2008. [Google Scholar]
- 34.Wang Z, Gidwani V, Zhang DD, Wong PK. Separation-free detection of nuclear factor-κB with double-stranded molecular probes. The Analyst 133: 998–1000, 2008. [DOI] [PubMed] [Google Scholar]
- 35.Zhang H, Morgan B, Potter BJ, Ma L, Dellsperger KC, Ungvari Z, Zhang C. Resveratrol improves left ventricular diastolic relaxation in type 2 diabetes by inhibiting oxidative/nitrative stress: in vivo demonstration with magnetic resonance imaging. Am J Physiol Heart Circ Physiol 299: H985–H994, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]


