Abstract
Using a radioactive glutamate uptake assay and immunolabeling, we report that single-walled carbon nanotubes, chemically-functionalized with polyethylene glycol (SWCNT-PEG), delivered as a colloidal solute, cause an increase in the uptake of extracellular glutamate by astrocytes and an increase in the immunoreactivity of the glutamate transporter GLAST on their cell surface, which is likely a consequence of an increase in the immunoreactivity of glial fibrillary acidic protein. Additional corollary is that astrocytes exposed to SWCNT-PEG became larger and stellate, morphological characteristics of maturation and heightened activity of these glial cells. These results imply that SWCNT-PEG could potentially be used as a viable candidate for neural prosthesis applications, perhaps to alleviate the death toll of neurons due to glutamate excitotoxicity, a pathological process observed in brain and spinal cord injuries.
Keywords: Carbon nanotubes, Astrocytes, Glial fibrillary acidic protein, Glutamate excitotoxicity
Introduction
Carbon nanotubes (CNTs) with their unique physical and chemical properties hold much promise in biomedical applications, especially in the field of neuroprosthetics (Bekyarova et al. 2005; Malarkey and Parpura 2007). Single-walled carbon nanotubes, chemically-functionalized with polyethylene glycol (SWCNT-PEG) to render their water solubility, have been used in cell culture to modulate the morpho-functional properties of neurons and astrocytes. This nanomaterial promoted the outgrowth of selected neurites (Ni et al. 2005), due to the exocytotic incorporation of vesicles into the plasma membrane which was unbalanced by their endocytotic retrieval (Malarkey et al. 2008). It also made astrocytes larger and stellate along with an increase in the immunoreactivity of glial fibrillary acidic protein (GFAP) (Gottipati et al. 2012), an astrocyte-specific intermediate filament; the resulting cellular characteristics render a mature astrocyte phenotype. An application of SWCNT-PEG in vivo at the site of an acute spinal cord injury also showed an alteration in neuronal morphology and an improvement in the locomotor recovery in a rat model (Roman et al. 2011). Whilst these findings implicate the potential beneficial effects of CNTs at the injury site, the possible effects on astrocytes and the contribution of these glial cells is this process was not detailed. Namely, an additional concern with an injury to the spinal cord is the ‘secondary injury’ which leads to progressive degenerative events at the site of the injury (Park et al. 2004). One of the prominent mechanisms that lead to secondary injury is excitotoxicity, a pathological process that results in the death of neurons due to excessive stimulation by glutamate. Astrocytes are the main brain element responsible for the uptake of excess glutamate from the extracellular space through their Na+-dependent excitatory amino acid transporters (EAATs), assuring the fidelity of synaptic transmission, and protecting the neighboring neurons. Since the loss of GFAP has shown to be involved in the trafficking of glutamate transporters to the plasma membrane (Hughes et al. 2004), the question arose whether an increase in GFAP caused by the CNTs could be associated with an increase in the trafficking of the glutamate transporters to the cell surface and result in an increased glutamate uptake; this is the very subject of the present study.
CNTs found use in brain machine interface applications as a coating material for implantable electrodes. CNT surface coating has shown to outperform the traditional tungsten and stainless steel wire electrodes by improving the electrical stimulation of neurons and recordings from these cells (Keefer et al. 2008). However, whether CNTs, by acting on astrocytic uptake mechanism, could help in reducing the transient increase in glutamate levels that otherwise occurs following a microelectrode implantation (Chang et al. 2009) is untested.
We performed a radioactive glutamate uptake study and immunolabeling to show that the CNT-treated astrocytes have an enhanced capacity to uptake glutamate from the extracellular space, a functional output of an increased presence of the glutamate transporters on their surface; this CNT-mediated modulation of glutamate uptake was associated with astrocytes assuming a more mature morphological (larger and stellate shape) and functional (increased expression of GFAP) phenotype. Taken together, these findings further emphasize the potential of CNTs as a viable candidate for neuroprosthesis applications.
Materials and methods
Ethical approval
All procedures were in strict accordance with the National Institutes of Health Guide for Care and Use of Laboratory Animals and were approved by the University of Alabama at Birmingham, Birmingham Institutional Animal Care and Use Committee. The procedures also conform to the principles of UK regulations (Drummond 2009)
Purified astrocytic cell culture
Purified astrocytic cultures were made using a modification (Gottipati et al. 2012; Reyes et al. 2011) of the originally described shaking procedure (McCarthy and de Vellis 1980). Briefly, visual cortices were dissected from 0- to 2-day-old C57BL/6 mice pups and treated with papain in the presence of L-cysteine, neutralized with trypsin inhibitor and triturated in cell culture medium containing α-minimum essential medium (without phenol red; Invitrogen) supplemented with fetal bovine serum (10%, Hyclone), sodium bicarbonate (14 mM), sodium pyruvate (1 mM), D-glucose (20 mM), L-glutamine (2 mM), penicillin (100 I.U./ml) and streptomycin (100 μg/ml) (pH 7.35). The resulting cell suspension was applied into 25 cm2 tissue culture flasks and maintained at 37°C in a 5% CO2 /95% air incubator. The cells were allowed to grow and proliferate for 7-14 days, until they reached ~60% confluency, after which the cell cultures were purified for astrocytes using a shaking procedure described elsewhere (Gottipati et al. 2012). The cells attached to the bottom of the flask were detached by adding trypsin and pelleted by centrifugation at 100 g for 10 minutes. The cells were resuspended in cell culture medium and plated onto polyethyleneimine (PEI; 1 mg/ml)-coated glass coverslips (12 mm in diameter) inlayed in tissue culture dishes, except for the glutamate uptake assay where the cells were plated into 24-well tissue culture plates. Cells were returned to the incubator for 1 h to allow for the attachment of astrocytes to the strata. At this juncture, dishes/plates were rinsed, and PEG (1 μg/ml), SWCNT-PEG (5 μg/ml) or Y-27632 (30 μM), diluted in the cell culture medium, was applied to the astrocytes, which were then returned to the incubator until used in the experiments.
Modification of carbon nanotubes
SWCNT-PEG solute was synthesized and characterized as we previously reported in detail elsewhere (Gottipati et al. 2012). The batch of SWCNT-PEG solute used in this study contained 72.3 weight percent (wt%) of the SWCNT backbone, 22.6 wt% of the functional group PEG and 5.1 wt% of metal impurities (nickel and yttrium in ~4:1 weight ratio; Online Resource 1, Fig S1).
In the experiments using the functional group PEG alone, as a control for 5 μg/ml of SWCNT-PEG solute treated group, PEG was added to the cells at 1 μg/ml, i.e., at the concentration corresponding to 20 wt % of the SWCNT-PEG solute.
[3H]-L-Glutamate uptake assay
Glutamate uptake assay was done using a procedure described elsewhere (Aprico et al. 2004). Astrocytes plated in the 24-well plates were washed twice with Na+-dependent uptake buffer composed of (in mM): NaCl (135), KCl (5), CaCl2 (1), MgSO4 (0.6), glucose (6) and Hepes (10) (pH 7.4). After 2 washes, the uptake buffer was supplemented with 100 μM of L-glutamate, a mixture of [3H]-L-glutamate (American Radiolabeled Chemicals, Saint Louis, MO) and nascent L-glutamate in a ratio of 1:1500. After 5 minutes, the solution (containing glutamate) in the wells was replaced with ice-cold phosphate buffered saline (PBS) to stop the uptake of glutamate; PBS solution was composed of (in mM): NaCl (137), KCl (2.7), Na2HPO4 (10) and KH2PO4 (1.8) (pH 7.2). Cells were then lysed with NaOH (0.3 M) for 30 minutes at 37°C, which was neutralized with HCl (0.3 M). The lysate was analyzed for both the [3H]-L-glutamate and protein contents. The radioactivity of [3H]-L-glutamate in an aliquot of each of the samples was measured using a Beckman LS 6500 Scintillation Counter; these measurement were done upon dilution of the sample in the ScintiVerse BD Cocktail (Fisher Scientific, Pittsburgh, PA). The remaining lysate was used to measure the total protein concentration of cells in each of the wells using a spectrophotometer. Proteins were quantified using the Bradford reagent (Pierce Biotechnology, Rockford, IL, USA) and bovine serum albumin as a standard. The radioactivity and the total protein concentration were used to calculate the amount of glutamate (in nmol) taken up by the astrocytes per mg of protein per minute. All the measurements are normalized to the average glutamate uptake obtained from the control/untreated astrocytes and reported.
Live cell imaging
The morphology of live astrocytes was examined as previously described (Gottipati et al. 2012; Hua et al. 2004). Coverslips with astrocytes were incubated with non-fluorescent Calcein acetoxymethyl ester (AM) (1 μg/ml; Invitrogen) at room temperature (RT; 22-25°C) for 15 minutes; 0.025% (w/v) pluronic acid (Invitrogen) was added to aid in the solubilization of Calcein AM in external solution containing (in mM) NaCl (140), KCl (5), CaCl2 (2), MgCl2 (2), glucose (5) and Hepes (10) (pH 7.4). After rinsing, cells were kept for another 15 minutes in external solution to allow for the de-esterification of Calcein AM, resulting in the intracellular accumulation of calcein, a vital fluorescent dye. These coverslips were rinsed in external solution and mounted onto an imaging chamber filled with external solution. We examined cells using an inverted microscope (Nikon TE300) equipped with differential interference contrast (DIC; 100-W halogen lamp) and wide-field epifluorescence illumination (100-W xenon arc lamp). Visualization was accomplished using a standard fluorescein isothiocyanate (FITC; Chroma Technology Corp.) filter set and a 60X Plan Apo objective and imaged using a CoolSNAP®-HQ2 cooled, charge coupled device (CCD) camera (Roper Scientific Inc.) driven by V++ imaging software (Digital Optics, Auckland, New Zealand). Neutral density filters and an electronic shutter (Vincent Associates, Rochester, NY), controlled by the software, were inserted in the excitation pathway to reduce photo-bleaching.
Morphometric analysis
Acquired calcein images were analyzed to get the morphometric parameters of the cells as described in detail elsewhere (Gottipati et al. 2012). Briefly, two or more images were processed by image stitching and auto-leveling, when the cell size exceeded an individual image frame. Images were processed using a 3x3 kernel, which is a low-pass filter, to reduce the effect of noise along the perimeter. A Laplacian of Gaussian (LoG) 3D filter (Sage et al. 2005) was then applied to images to facilitate edge detection. A circle containing octants was centered over the astrocyte in the LoG3D image and regions of interest (10 × 10 pixels) were created at the intersections of the octant radii and the cell edge/perimeter. These regions were transposed onto the kernel-filtered image and the average intensities of the regions were calculated, which were further averaged and the resulting overall mean intensity was used as the intensity threshold value applied to the very image. Based on the intensity threshold, the outline of the cell was obtained and used to calculate the area and perimeter values, which were further used to calculate the form factor (FF) for each of the cells using the equation (Wilms et al. 1997):
| (Equation 1) |
Indirect immunocytochemistry (ICC) and analysis
Immunolabeling of astrocytes was done using previously described procedures (Gottipati et al. 2012; Hua et al. 2004; Montana et al. 2004). Coverslips with astrocytes were fixed at RT for 30 minutes with freshly prepared Dent’s fixative (80% methanol, 20% dimethyl sulfoxide) for GFAP or with 4% paraformaldehyde for L-glutamate/ L-aspartate transporter (GLAST/EAAT1; SLC1A3) and glial-glutamate transporter (GLT-1/EAAT2; SLC1A2); this was followed by permeabilization of the cells using 0.25% Triton X-100 for 10 minutes. To prevent non-specific binding of antibodies, the cells were then incubated with 10% (v/v) goat serum in PBS for 30 minutes followed by an overnight (> 12 h) incubation of the cells at 4°C with primary antibodies against GFAP (1:500; MP Biomedicals), GLAST (1:250; Alomone Labs) or GLT-1 (1:250; Millipore). Cells were then washed thrice with PBS and incubated for 1 h with tetramethylrhodamine isothiocyanate (TRITC) conjugated secondary (goat-anti-mouse for GFAP and GLT-1, or goat-anti-rabbit for GLAST) antibody (1:200; Millipore) at RT. To test for the non-specific binding of the secondary antibody, parallel controls were run in which the primary antibodies were omitted. After multiple washings with PBS and Milli-Q® water, the coverslips were mounted onto glass microscopic slides in ProLong® Gold antifade reagent (Invitrogen) to prevent photo bleaching. For experiments assessing the surface expression of GLAST and a lack of thereof for GFAP, a non-permeabilization procedure was used where the above described ICC procedure was performed, but with the omission of the detergent, Triton X-100 (Malarkey et al. 2008). Of note, anti-GLAST antibody recognizes the extracellular epitope of this transporter when incorporated into the plasma membrane. Correspondingly, this epitope is located in the lumen of GLAST-laden trafficking vesicles; this location can be labeled upon the permeabilization of the cell.
Immunoreactivity (ir) was visualized and imaged using a standard TRITC (Chroma Technology Corp.) filter set and the above mentioned microscope and associated equipment; matching DIC images were also obtained. Analysis was done using Metamorph 7.8 (Molecular Devices, Chicago, IL) to get the total area of the cell, area of the cell occupied by protein-ir and the average intensity or density of protein-ir, which is the intensity of protein-ir per pixel. The DIC images were manually traced along their edge/perimeter to get the total area of the cells. No primary antibody controls were used to calculate the threshold value for the immunolabeled astrocytes, where the background subtracted mean intensity of the control cells (autofluorescence) + 3 standard deviations (for GLAST and GLT-1) or 6 standard deviations (for GFAP) was used as the threshold value; the background fluorescence was obtained from regions of coverslips containing no cells. The pixels exceeding these threshold values were used to obtain the positive signal and calculate the protein-ir occupancy, which is the ratio of the area of the cell occupied by protein-ir (fluorescence) to the total area (DIC) of the cell, as well as the protein-ir content, which is the product of the average intensity of protein-ir and the total area of the cell. Furthermore, based on the number of the pixels above these threshold values, we classified cells as positive for the specific antigen, i.e. the cells were considered to express a given protein if the number of positive pixels in a cell was at least 1 standard deviation greater than the average number of noise/false positive pixels observed in the cells that were processed for ICC without the primary antibody. All raw fluorescence images had pixel intensities within the camera’s dynamic range (0-16383).
Statistical Analysis
All the statistical analysis was done using the GB-Stat v6.5 software (Dynamic Microsystems Inc., Silver Spring, MD) and SAS Software, version 9.4 of the SAS software for Windows (SAS Institute Inc., Cary, NC). All the data are reported as means ± standard errors of means (Figure 1) or medians with their interquartile range (Figures 2-4, and S2). The number of subjects required for individual set of experiments was estimated using power analysis, set at 80% and an α = 0.05. Nonparametric statistics were used for the subgroups that deviated from normality based on the Shapiro-Wilk test for normality. For the glutamate uptake assay (Figure 1), the independent groups were first analyzed using one-way ANOVA followed by the Fisher’s least significant difference (LSD) test for multiple comparisons. For the assay estimating the surface GLAST-ir (Figure 2), as well as for the validation of the (non)permeabilization procedure using GFAP-ir (Figure S2), the two independent groups were compared using the Mann-Whitney U-test. For the other experiments assessing the effects of the various treatments on the morphology and GFAP-ir (Figures 3-4), the independent groups were first analyzed using the Kruskal–Wallis one-way ANOVA (KWA) followed by the Dunn’s test for multiple comparisons.
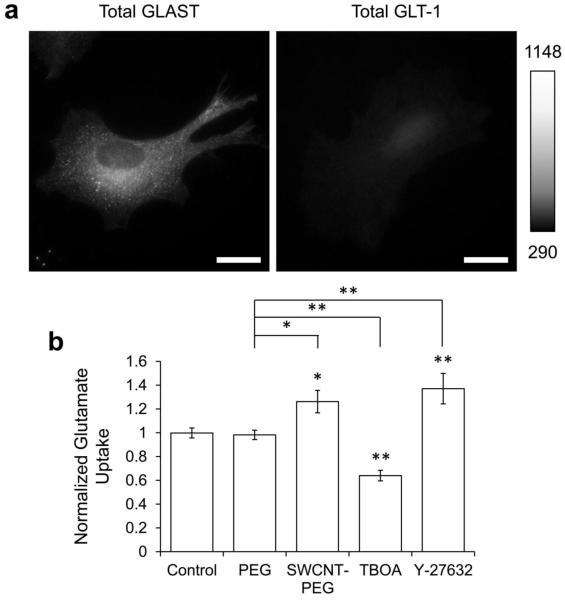
Fig. 1.
SWNCT-PEG solute enhances the uptake of [3H]-L-glutamate by mouse cortical astrocytes in culture. a Images of astrocytes in culture, labeled for the glutamate transporters GLAST (left) and GLT-1 (right), using indirect immunocytochemistry. Scale bar, 20 μm. Gray scale is a linear representation of the fluorescence intensities, expressed in fluorescence intensity units (iu), of the pixels in the images. b Summary graph showing the normalized average effect of 1 μg/ml PEG, 5 μg/ml SWCNT-PEG, 100 μM TBOA and 30 μM Y-27632 on the uptake of [3H]-L-glutamate by cultured astrocytes. Bars represent means with standard error of means. 18 wells containing astrocytes were analyzed per condition. Asterisks indicate a statistical difference compared to the control. Other differences are marked by the brackets. One-way ANOVA followed by Fisher’s LSD test; *p < 0.05, **p < 0.01.
Fig. 2.
SWCNT-PEG solute increases the surface appearance of GLAST on mouse cortical astrocytes. a Images of astrocytes in culture, a subset of which were treated with 5 μg/ml SWCNT-PEG, labeled for GLAST using indirect immunocytochemistry and the non-permeabilization procedure. Scale bar, 20 μm. Gray scale is a linear representation of the fluorescence intensities, expressed in fluorescence intensity units (iu), of the pixels in the images. b Summary graphs showing the median effect of SWCNT-PEG on the quantitative GLAST immunoreactivity (ir) parameters i.e. density, content and occupancy. Density is shown in fluorescence intensity units (iu) per area (pixel). Boxes represent medians with interquartile range. Number of astrocytes studied in each condition is given in parentheses in the density graph. Asterisks indicate a statistical difference when compared to the untreated/control astrocytes. Mann-Whitney U-test. **p < 0.01
Fig. 4.
SWCNT-PEG solute induces morphological changes in mouse cortical astrocytes. a Images of astrocytes in culture, treated with 1 μg/ml PEG, 5 μg/ml SWCNT-PEG or 30 μM Y-27632, and loaded with calcein, a vital fluorescent dye. Scale bar, 20 μm. b Summary graphs showing the median effect of the various treatments on astrocytic morphology, i.e. area, perimeter and form factor. Number of astrocytes studied in each condition is given in parentheses in the area graph. Asterisks indicate a statistical difference when compared to the untreated/control astrocytes. The other differences are marked by the brackets. Kruskal–Wallis one-way ANOVA (KWA) followed by Dunn’s test. *p < 0.05, **p < 0.01.
Fig. 3.
SWCNT-PEG increases GFAP-ir parameters in mouse cortical astrocytes. a Images of astrocytes in culture, treated with 1 μg/ml PEG, 5 μg/ml SWCNT-PEG or 30 μM Y-27632 and labeled for GFAP using indirect immunocytochemistry. Scale bar, 20 μm. b Summary graphs showing the median effect of the various treatments on the quantitative GFAP-ir parameters. Number of astrocytes studied in each condition is given in parentheses in the occupancy graph. Asterisks indicate a statistical difference when compared to the untreated/control astrocytes. Other differences are marked by the brackets. Kruskal–Wallis one-way ANOVA (KWA) followed by Dunn’s test. *p < 0.05, **p < 0.01.
Results
Mouse neonatal astrocytes express GLAST but not GLT-1: confirmation in the culture system
Astrocytes uptake extracellular glutamate primarily through the excitatory amino acid transporters, GLAST/EAAT1 and GLT-1/EAAT2, present on their surface. The transporter type is developmentally regulated, so that early neonatal rat astrocytes (1-day-old) show very low levels of expression of GLT-1 compared to GLAST (Furuta et al. 1997). We confirmed this expression pattern/level in our culture system by labeling mouse astrocytes, plated onto PEI coated coverslips, for GLAST and GLT-1 using ICC and the permeabilization procedure to allow for the detection of total protein levels. We visualized the transporters-ir and found a significant expression of GLAST (n = 20; Fig 1A, left), while only a faint stain of GLT-1 (n = 20; Fig 1A, right). While all astrocytes were positive for GLAST, only 10% of the cells analyzed were positive for GLT-1.
SWCNT-PEG up-regulates the uptake of [3H]-L-glutamate by astrocytes
To assess if the treatment of astrocytes with SWCNT-PEG induces a change in the uptake of glutamate from the extracellular space, we treated astrocytes plated into 24-well tissue culture plates with 5 μg/ml of SWCNT-PEG for 4 days; a part of the wells were treated with 1 μg/ml of PEG alone to determine if the functional group by itself has any effect on the uptake of glutamate. To show that the uptake of glutamate can indeed be upregulated in culture, a part of the wells were treated for 2 days with 30 μM of Y-27632, a Rho-associated protein kinase (ROCK) blocker known to cause rapid and reversible stellation of astrocytes and an increase in the uptake of glutamate (Abe and Misawa 2003; Lau et al. 2011). On the day of the experiment, all the wells were incubated in a Na+-dependent uptake buffer containing a 100 μM of glutamate mixture ([3H]-L-glutamate and nascent glutamate in a ratio of 1:1500) for 5 minutes. A part of the untreated wells incubated with the glutamate mixture were additionally exposed to 100 μM of DL-threo-β-benzyloxyaspartic acid (TBOA), which is a competitive, non-transportable EAAT blocker (Shimamoto et al. 1998). The amount of radioactive glutamate taken up by the astrocytes was estimated using a scintillation counter. Expectedly, we found that TBOA significantly blocked the uptake of glutamate (~40% reduction in the uptake), while the positive control, Y-27632, caused a significant increase compared to the controls (Figure 1B; One-way ANOVA followed by Fisher’s LSD test). The cells treated with SWNCT-PEG, but not PEG alone, showed a significant increase in the uptake of radioactive glutamate (Figure 1B).
SWCNT-PEG causes an increase in the immunoreactivity of GLAST present on the plasma membrane of astrocytes
Since SWCNT-PEG caused an increase in the uptake of glutamate from the extracellular space, and since GLAST is the predominantly expressed glutamate transporter in neonatal astrocytes, we studied if the CNT effect on glutamate uptake could be due to the altered amount of GLAST protein present on the plasma membrane of the astrocytes using ICC and the non-permeabilization procedure. We first validated our non-permeabilization procedure by using an antibody against GFAP, an intracellular antigen, and comparing the GFAP-ir between the permeabilized and non-permeabilized cells (Online Resource 1, Figure S2A). We calculated the density of GFAP-ir and found that the cells permeabilized with Triton X-100 showed a strong staining, while the GFAP stain was essentially absent in the non-permeabilized cells (Online Resource 1, Figure S2B; Mann-Whitney U-test), indicating that the omission of Triton X-100 from the ICC procedure leaves the plasma membrane intact, i.e., impermeable for antibodies.
Next, astrocytes plated onto PEI coated coverslips were treated with 5 μg/ml of SWCNT-PEG for 4 days and labeled for GLAST using ICC and the non-permeabilization procedure (Figure 2A). We quantitatively assessed the surface GLAST-ir parameters, that is, density, content and occupancy and found a significant increase in all the parameters in the presence of SWCNT-PEG (Figure 2B; Mann-Whitney U-test) implying that the CNTs cause an increase in the amount of GLAST protein present on the surface/plasma membrane of the astrocytes, which likely explains the increase in glutamate uptake caused by SWCNT-PEG.
SWCNT-PEG and Y-27632 increase the immunoreactivity of GFAP in astrocytes
The loss of GFAP has been shown to hamper trafficking of EAAT2 to the cell surface (Hughes et al. 2004). Consequently, we investigated if this increase in the surface GLAST presentation by SWCNT-PEG is correlated to an increase in GFAP levels. To assess the effects of the various treatments on GFAP levels, we plated astrocytes onto PEI coated coverslips in all the conditions used above for the glutamate uptake study and labeled them for GFAP using ICC (Figure 3A). We quantitatively assessed the GFAP-ir parameters and found that SWCNT-PEG causes a significant increase in all the ir parameters measured, while PEG alone did not cause any significant changes compared to the untreated astrocytes (Figure 3B; KWA followed by Dunn’s test); these findings are consistent with our previous work (Gottipati et al. 2014). Y-27632 also caused a significant increase in all the GFAP-ir parameters assessed compared to the untreated astrocytes. Taken together, these results indicate that the increase in glutamate uptake and surface GLAST induced by SWCNT-PEG is accompanied by an increase in GFAP-ir; the latter can be emulated by a ROCK inhibitor.
SWCNT-PEG and Y-27632 cause morphological alterations in astrocytes
Changes in GFAP levels have been associated with the morphological plasticity of astrocytes (Brenner 2014). So, we assessed the morphology of astrocytes in all the conditions used above for the glutamate uptake study. The astrocytes were loaded with calcein and imaged live. We found that all the cells imaged, untreated (n = 44) and treated (n = 114), accumulated calcein (Figure 4A), indicating their viability in culture. To assess the morphological parameters, the calcein images were analyzed to obtain the area and perimeter values of the cells and in turn the form factor (FF), a measure of the circularity or roundness of a cell/object; FF = 1 describes a perfectly round/circular object while a FF ≈ 0 describes a line. We found that astrocytes treated with SWCNT-PEG assumed a mature morphological phenotype. i.e., SWCNT-PEG caused a significant increase in the area and perimeter of astrocytes and a significant decrease in the FF (reminiscent of the cell stellation), while PEG alone did not cause any significant changes in the morphological parameters compared to the untreated astrocytes (Figure 4B; KWA followed by Dunn’s test); these findings are in good agreement with our previous work (Gottipati et al. 2014). Y-27632 echoed SWCNT-PEG effects (Figure 4B). Taken together, these results show that SWCNT-PEG solute induces morphological changes in astrocytes, similar to those caused by a ROCK inhibitor.
Discussion
In this study, we show that the graft copolymer SWCNT-PEG, when applied to the astrocytes in culture, can increase astrocytic ability to uptake glutamate from the extracellular space. We also showed that SWCNT-PEG causes an increase in the surface GLAST-ir, which was accompanied by an increase in the GFAP-ir and a change in astrocytic morphology.
The Na+-dependent uptake of glutamate by astrocytes in our culture system predominantly occurs through the glutamate transporter GLAST as per ICC (Figure 1A), a finding consistent with the previous work using rats at comparable development stage (postnatal day 1) (Furuta et al. 1997). It has been shown previously that the ROCK blockers, such as Y-27632 or fasudil, can upregulate astrocytic glutamate transport by remodeling the actin cytoskeleton in cultured astrocytes (Lau et al. 2011). Indeed, we used Y-27632 as a positive control and showed that the glutamate uptake can be enhanced in our culture system (Figure 1B). As cystine was not added to the uptake buffer, the recorded Na+-dependent uptake was not tainted by in parallel operation of the cystine/glutamate antiporter (system xc ), a Cl-dependent system, which would otherwise affect our measurements. Yet, in our experimental conditions we observed ~40% block of glutamate uptake by TBOA, which is substantially less than the expected percentage blockage of ~56%, that we calculated based on a Ki of 42 μM for TBOA and a Km of 57 μM for L-glutamate for EAAT1/GLAST (Shimamoto et al. 1998). It is possible that this discrepancy is a result of an in parallel Ca2+-dependent glutamate release from astrocytes. Namely, as the added tritiated L-glutamate behaves exactly like native L-glutamate in all signaling and metabolic cellular processes, it binds to plasmalemmal glutamate receptors, leading to an increase in intracellular Ca2+ levels and consequential glutamate release (Bezzi et al. 1998). Albeit out of the scope of the present work, such possibility could be discerned by using tritiated D-aspartate which behaves like L-glutamate on plasmalemmal transporters as well as in release from the vesicular pool, but it has a negligible effect on the glutamate receptors compared to that of L-glutamate (Drejer et al. 1986). Nonetheless, SWCNT-PEG enhanced the ability of astrocytes to remove extracellular glutamate (Figure 1B), which could explain some of the previously observed beneficial effects of this material when applied at the site of spinal cord injury (Roman et al. 2011). Perhaps, SWCNTs reduced the accumulation of extracellular glutamate otherwise leading to excitotoxicity and neuronal death that can occur at the site of neural tissue injury (Choi 1994; Nilsson et al. 1990).
SWCNT-PEG also caused an increase in the surface GLAST-ir (Figure 2B) as well as the total GFAP-ir (Figure 3B). Since, the loss of astrocyte-specific intermediate filaments, GFAP and vimentin, have hampered vesicular trafficking (Potokar et al. 2007) and the loss of GFAP in particular led to a reduced trafficking of glutamate transporters to the surface of astrocytes (Hughes et al. 2004), it is plausible that an increase in GFAP-ir due to SWCNT-PEG leads to an increase in the trafficking of glutamate transporters to the plasma membrane, which can in turn increase the uptake of glutamate. In addition to this, an increase in GFAP levels is correlated with an increase in the levels of glutamine synthetase (Wang and Hatton 2009; Wang et al. 2013), an enzyme that converts glutamate to glutamine in astrocytes, and an increased glutamine synthetase expression leads to an increase in glutamate uptake and glutamine release (Zou et al. 2010). Unlike glutamate, glutamine released into the extracellular space does not stimulate the receptors present on the neurons, pointing to an additional possible mechanism by which CNTs may mediate beneficial effects in neuroprosthesis applications, e.g., injury, with glutamate-mediated excitotoxicity settings in place.
Expectedly, along with an increase in GFAP-ir, SWCNT-PEG also caused a change in the morphology of astrocytes, making astrocytes larger and stellate (Figure 4B), findings consistent with our previous work (Gottipati et al. 2014; Gottipati et al. 2012). It should be noted, that, albeit confirmatory, these experiments were a necessary control to accommodate for batch-to-batch variability in SWCNT-PEG synthesis and to make a comparison with the effects caused by Y-27632, which were reminiscent of the effects induced by SWCNT-PEG (Figures 3-4). These similarities might suggest that the mechanism of action of SWCNT-PEG to induce morphological changes in astrocytes could involve the Rho/ROCK pathway.
In the present study, we used SWCNT-PEG at a concentration of 5 μg/ml for 4 days in culture and found no adverse effects on astrocytes. However, the effects of these carbon materials at varying concentrations and exposure times are currently unknown. As some concerns on their possible toxicity and biological incompatibility have been raised (Belyanskaya et al. 2009; Wick et al. 2007), much needed exposure limits need to be set in place in order to bring the use of CNTs into the realm of neural prosthesis.
Supplementary Material
Acknowledgements
We thank Stephanie M. Robert and Dr. Harald Sontheimer, University of Alabama at Birmingham, Birmingham, for their help with the glutamate uptake study and Dr. Vladimir Grubiŝić for his constructive comments on a previous version of this manuscript.
Funding source
Vladimir Parpura acknowledges the support of this work by National Institutes of Health (The Eunice Kennedy Shriver National Institute of Child Health and Human Development award HD078678)
Abbreviations
- CNT
Carbon nanotube
- EAAT
Excitatory amino acid transporter
- GFAP
Glial fibrillary acidic protein
- GLAST
L-glutamate/L-aspartate transporter
- GLT-1
Glial-glutamate transporter
- ICC
Indirect immunocytochemistry
- ir
Immunoreactivity
- PEG
Polyethylene glycol
- ROCK
Rho-associated protein kinase
- SWCNT
Single-walled carbon nanotube
- TBOA
DL-threo-β-benzyloxyaspartic acid
Footnotes
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical standard
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted.
References
- Abe K, Misawa M. Astrocyte stellation induced by Rho kinase inhibitors in culture. Brain Res Dev Brain Res. 2003;143:99–104. doi: 10.1016/s0165-3806(03)00096-8. [DOI] [PubMed] [Google Scholar]
- Aprico K, Beart PM, Crawford D, O'Shea RD. Binding and transport of [3H](2S,4R)-4-methylglutamate, a new ligand for glutamate transporters, demonstrate labeling of EAAT1 in cultured murine astrocytes. J Neurosci Res. 2004;75:751–759. doi: 10.1002/jnr.20013. doi:10.1002/jnr.20013. [DOI] [PubMed] [Google Scholar]
- Bekyarova E, Ni Y, Malarkey EB, Montana V, McWilliams JL, Haddon RC, Parpura V. Applications of Carbon Nanotubes in Biotechnology and Biomedicine. J Biomed Nanotechnol. 2005;1:3–17. doi: 10.1166/jbn.2005.004. doi:10.1166/jbn.2005.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belyanskaya L, Weigel S, Hirsch C, Tobler U, Krug HF, Wick P. Effects of carbon nanotubes on primary neurons and glial cells. Neurotoxicology. 2009;30:702–711. doi: 10.1016/j.neuro.2009.05.005. doi:10.1016/j.neuro.2009.05.005. [DOI] [PubMed] [Google Scholar]
- Bezzi P, et al. Prostaglandins stimulate calcium-dependent glutamate release in astrocytes. Nature. 1998;391:281–285. doi: 10.1038/34651. doi:10.1038/34651. [DOI] [PubMed] [Google Scholar]
- Brenner M. Role of GFAP in CNS injuries. Neurosci Lett. 2014;565:7–13. doi: 10.1016/j.neulet.2014.01.055. doi:10.1016/j.neulet.2014.01.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang SY, Shon YM, Agnesi F, Lee KH. Microthalamotomy effect during deep brain stimulation: potential involvement of adenosine and glutamate efflux. Conf Proc IEEE Eng Med Biol Soc. 2009;2009:3294–3297. doi: 10.1109/IEMBS.2009.5333735. doi:10.1109/IEMBS.2009.5333735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi DW. Glutamate receptors and the induction of excitotoxic neuronal death. Prog Brain Res. 1994;100:47–51. doi: 10.1016/s0079-6123(08)60767-0. [DOI] [PubMed] [Google Scholar]
- Drejer J, Honore T, Meier E, Schousboe A. Pharmacologically distinct glutamate receptors on cerebellar granule cells. Life Sci. 1986;38:2077–2085. doi: 10.1016/0024-3205(86)90206-7. [DOI] [PubMed] [Google Scholar]
- Drummond GB. Reporting ethical matters in the Journal of Physiology: standards and advice. J Physiol. 2009;587:713–719. doi: 10.1113/jphysiol.2008.167387. doi:10.1113/jphysiol.2008.167387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuta A, Rothstein JD, Martin LJ. Glutamate transporter protein subtypes are expressed differentially during rat CNS development. J Neurosci. 1997;17:8363–8375. doi: 10.1523/JNEUROSCI.17-21-08363.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottipati MK, Bekyarova E, Brenner M, Haddon RC, Parpura V. Changes in the morphology and proliferation of astrocytes induced by two modalities of chemically functionalized single-walled carbon nanotubes are differentially mediated by glial fibrillary acidic protein. Nano Lett. 2014;14:3720–3727. doi: 10.1021/nl4048114. doi:10.1021/nl4048114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottipati MK, Kalinina I, Bekyarova E, Haddon RC, Parpura V. Chemically functionalized water-soluble single-walled carbon nanotubes modulate morpho-functional characteristics of astrocytes. Nano Lett. 2012;12:4742–4747. doi: 10.1021/nl302178s. doi:10.1021/nl302178s. [DOI] [PubMed] [Google Scholar]
- Hua X, Malarkey EB, Sunjara V, Rosenwald SE, Li WH, Parpura V. Ca2+-dependent glutamate release involves two classes of endoplasmic reticulum Ca2+ stores in astrocytes. J Neurosci Res. 2004;76:86–97. doi: 10.1002/jnr.20061. [DOI] [PubMed] [Google Scholar]
- Hughes EG, Maguire JL, McMinn MT, Scholz RE, Sutherland ML. Loss of glial fibrillary acidic protein results in decreased glutamate transport and inhibition of PKA-induced EAAT2 cell surface trafficking. Brain Res Mol Brain Res. 2004;124:114–123. doi: 10.1016/j.molbrainres.2004.02.021. doi:10.1016/j.molbrainres.2004.02.021. [DOI] [PubMed] [Google Scholar]
- Keefer EW, Botterman BR, Romero MI, Rossi AF, Gross GW. Carbon nanotube coating improves neuronal recordings. Nat Nanotechnol. 2008;3:434–439. doi: 10.1038/nnano.2008.174. doi:10.1038/nnano.2008.174. [DOI] [PubMed] [Google Scholar]
- Lau CL, O'Shea RD, Broberg BV, Bischof L, Beart PM. The Rho kinase inhibitor Fasudil up-regulates astrocytic glutamate transport subsequent to actin remodelling in murine cultured astrocytes. Br J Pharmacol. 2011;163:533–545. doi: 10.1111/j.1476-5381.2011.01259.x. doi:10.1111/j.1476-5381.2011.01259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malarkey EB, Ni Y, Parpura V. Ca2+ entry through TRPC1 channels contributes to intracellular Ca2+ dynamics and consequent glutamate release from rat astrocytes. Glia. 2008;56:821–835. doi: 10.1002/glia.20656. doi:10.1002/glia.20656. [DOI] [PubMed] [Google Scholar]
- Malarkey EB, Parpura V. Applications of carbon nanotubes in neurobiology. Neurodegener Dis. 2007;4:292–299. doi: 10.1159/000101885. doi:10.1159/000101885. [DOI] [PubMed] [Google Scholar]
- McCarthy KD, de Vellis J. Preparation of separate astroglial and oligodendroglial cell cultures from rat cerebral tissue. J Cell Biol. 1980;85:890–902. doi: 10.1083/jcb.85.3.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montana V, Ni Y, Sunjara V, Hua X, Parpura V. Vesicular glutamate transporter-dependent glutamate release from astrocytes. J Neurosci. 2004;24:2633–2642. doi: 10.1523/JNEUROSCI.3770-03.2004. doi:10.1523/JNEUROSCI.3770-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni Y, Hu H, Malarkey EB, Zhao B, Montana V, Haddon RC, Parpura V. Chemically functionalized water soluble single-walled carbon nanotubes modulate neurite outgrowth. J Nanosci Nanotechnol. 2005;5:1707–1712. doi: 10.1166/jnn.2005.189. [DOI] [PubMed] [Google Scholar]
- Nilsson P, Hillered L, Ponten U, Ungerstedt U. Changes in cortical extracellular levels of energy-related metabolites and amino acids following concussive brain injury in rats. J Cereb Blood Flow Metab. 1990;10:631–637. doi: 10.1038/jcbfm.1990.115. [DOI] [PubMed] [Google Scholar]
- Park E, Velumian AA, Fehlings MG. The role of excitotoxicity in secondary mechanisms of spinal cord injury: a review with an emphasis on the implications for white matter degeneration. J Neurotrauma. 2004;21:754–774. doi: 10.1089/0897715041269641. doi:10.1089/0897715041269641. [DOI] [PubMed] [Google Scholar]
- Potokar M, et al. Cytoskeleton and vesicle mobility in astrocytes. Traffic. 2007;8:12–20. doi: 10.1111/j.1600-0854.2006.00509.x. [DOI] [PubMed] [Google Scholar]
- Reyes RC, Perry G, Lesort M, Parpura V. Immunophilin deficiency augments Ca2+-dependent glutamate release from mouse cortical astrocytes. Cell Calcium. 2011;49:23–34. doi: 10.1016/j.ceca.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roman JA, Niedzielko TL, Haddon RC, Parpura V, Floyd CL. Single-walled carbon nanotubes chemically functionalized with polyethylene glycol promote tissue repair in a rat model of spinal cord injury. J Neurotrauma. 2011;28:2349–2362. doi: 10.1089/neu.2010.1409. doi:10.1089/neu.2010.1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sage D, Neumann FR, Hediger F, Gasser SM, Unser M. Automatic tracking of individual fluorescence particles: application to the study of chromosome dynamics. IEEE Trans Image Process. 2005;14:1372–1383. doi: 10.1109/tip.2005.852787. [DOI] [PubMed] [Google Scholar]
- Shimamoto K, Lebrun B, Yasuda-Kamatani Y, Sakaitani M, Shigeri Y, Yumoto N, Nakajima T. DL-threo-beta-benzyloxyaspartate, a potent blocker of excitatory amino acid transporters. Mol Pharmacol. 1998;53:195–201. doi: 10.1124/mol.53.2.195. [DOI] [PubMed] [Google Scholar]
- Wang YF, Hatton GI. Astrocytic plasticity and patterned oxytocin neuronal activity: dynamic interactions. J Neurosci. 2009;29:1743–1754. doi: 10.1523/JNEUROSCI.4669-08.2009. doi:10.1523/JNEUROSCI.4669-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YF, Sun MY, Hou Q, Parpura V. Hyposmolality differentially and spatiotemporally modulates levels of glutamine synthetase and serine racemase in rat supraoptic nucleus. Glia. 2013;61:529–538. doi: 10.1002/glia.22453. doi:10.1002/glia.22453. [DOI] [PubMed] [Google Scholar]
- Wick P, et al. The degree and kind of agglomeration affect carbon nanotube cytotoxicity. Toxicol Lett. 2007;168:121–131. doi: 10.1016/j.toxlet.2006.08.019. doi:10.1016/j.toxlet.2006.08.019. [DOI] [PubMed] [Google Scholar]
- Wilms H, Hartmann D, Sievers J. Ramification of microglia, monocytes and macrophages in vitro: influences of various epithelial and mesenchymal cells and their conditioned media. Cell Tissue Res. 1997;287:447–458. doi: 10.1007/s004410050769. [DOI] [PubMed] [Google Scholar]
- Zou J, Wang YX, Dou FF, Lu HZ, Ma ZW, Lu PH, Xu XM. Glutamine synthetase down-regulation reduces astrocyte protection against glutamate excitotoxicity to neurons. Neurochem Int. 2010;56:577–584. doi: 10.1016/j.neuint.2009.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.