Abstract
This study used functional MRI (fMRI) to examine a novel aspect of emotion regulation in adolescent development: whether age predicts differences in both the concurrent and lasting effects of emotion regulation on amygdala response. In the first active regulation phase of the testing session, fMRI data was collected while 56 healthy individuals (age range: 10.50–22.92 years) reappraised aversive stimuli so as to diminish negative responses to them. After a short delay, the second re-presentation phase involved passively viewing the aversive images from the reappraisal task. During active regulation, older individuals showed greater drops in negative affect and inverse rostrolateral prefrontal-amygdala connectivity. During re-presentation, older individuals continued to show lasting reductions in the amygdala response to aversive stimuli they had previously reappraised, an effect mediated by rostrolateral PFC. These data suggest that one source of heightened emotionality in adolescence is a diminished ability to cognitively down-regulate aversive reactions.
The average adolescent’s daily emotional experience is filled with higher highs and lower lows than is the average adult’s (R. Larson, Csikszentmihalyi, & Graef, 1980; R. W. Larson, Moneta, Richards, & Wilson, 2002). While this is a normal part of adolescence for most individuals, extreme emotional reactivity and dysregulation during adolescence has been linked to the development of mood and anxiety disorders (Casey et al., 2011). As such, it is critical to understand how emotion regulation develops across adolescence.
Cognitive reappraisal, which involves thinking about a stimulus differently so as to change its emotional impact, provides an ideal test case for examining emotion regulation in adolescents because it is already well-characterized in adults (Buhle et al., 2013; Diekhof, Geier, Falkai, & Gruber, 2011) and is central to treatments like cognitive behavioral therapy (CBT) that are used in adolescent clinical populations (Compton et al., 2004). Prior work suggests that adults are more effective than adolescents at reappraising to reduce negative affect, while data regarding the neural mechanisms underlying this effect remain mixed (McRae, Gross, et al., 2012; Pitskel, Bolling, Kaiser, Crowley, & Pelphrey, 2011; Silvers et al., 2012). One limitation of prior studies examining age-related differences in reappraisal is that they have focused only on the effects of reappraisal in the moment an emotional event unfolds. Characterizing age-related changes in such concurrent effects of emotion regulation is essential, yet understanding whether these effects last over time is important as well. The question of whether age predicts more enduring effects of reappraisal has not been addressed, however.
The present study investigated this question using a novel variant of a reappraisal paradigm that allowed investigation of both concurrent and lasting effects of reappraisal across adolescence. To examine the concurrent effects of reappraisal, participants completed a reappraisal task while undergoing fMRI scanning. Here we examined whether age predicts less amygdala activity or differential recruitment of prefrontal control regions during reappraisal. To examine the lasting effects of reappraisal, after a delay participants passively viewed affective stimuli from the reappraisal task. Here we asked whether older participants would show longer-lasting reappraisal-related drops in the amygdala response given recent work suggesting that reappraising stimuli changes the way healthy adults respond to them in the future (Erk et al., 2010; Macnamara, Ochsner, & Hajcak, 2011), and that adolescents are less capable than adults of extinguishing conditioned fear responses (Pattwell et al., 2012). If age indeed predicts more enduring reappraisal effects, the critical question is how this might occur. One possibility is that for older individuals reappraisal changes the affective meaning of stimuli such that there is a lasting change in bottom-up, affective responses to them, as evidenced by diminished amygdala activity. Alternatively, age-related changes in the durability of amygdala modulation could require continued top-down control, with prefrontal regions (PFC) involved in regulation coming online to support continued amygdala down-regulation for older participants. To test these competing possibilities, two types of analyses were conducted to examine prefrontal-amygdala dynamics. The first used psychophysiological interaction (PPI) analyses to ask whether functional connectivity between PFC and the amygdala was uniquely strengthened during reappraisal, and if so, whether age predicted differences in said connectivity. Given prior work suggesting that PFC-amygdala connectivity underlies successful emotion regulation in adults (Banks, Eddy, Angstadt, Nathan, & Phan, 2007), it was hypothesized that PFC-amygdala connectivity during active regulation would differ as a function of age. The second analysis examined whether between-subject differences in PFC recruitment might mediate age-related differences in the amygdala response during reappraisal. It was hypothesized that during re-presentation of stimuli that had previously been reappraised, older individuals might recruit PFC to a greater extent and that this activation might mediate the relationship between age and sustained reappraisal-related decreases in the amygdala response.
MATERIALS AND METHODS
Participants
Fifty-six healthy individuals (31 female; range = 10.50–22.92 years, mean = 16.45 years, S.D.= 3.84) participated in the present study (see Supplementary Table 1). Informed consent was obtained for all participants 18 and older and informed assent and parental consent were obtained for all participants under 18 in compliance with Institutional Review Board guidelines at Columbia University. All analyses were conducted using a continuous measure of age but age is shown categorically in figures for ease of interpretation.
Participant inclusion and exclusion
The initial sample in this study consisted of 58 healthy volunteers between the ages of 10–22 years (31 female; mean age = 16.27 years, S.D.= 3.9). Two of these participants were excluded from analyses due to excessive head motion (see fMRI data analysis section below for details) resulting in a final sample of 56 individuals. Participants were prescreened prior to participation to ensure that they could read and write in English, had normal or corrected vision, had never been diagnosed with a developmental or psychiatric disorder, had never been prescribed psychotropic medication and did not have any conditions contraindicated for MRI scanning. All participants completed the Wechsler Abbreviated Scale of Intelligence. IQ scores were within the normal range (M=111.58, SD=14.67; range: 83–136) and did not correlate with age (r=.22, p=.11).
Experimental procedure
Phase 1: Active Regulation
Prior to performing the reappraisal task, participants were trained extensively on the immersed (‘close’) and distanced (‘far’) strategies in accordance with procedures previously used in developmental populations (Silvers et al., 2012). On ‘close’ trials, participants were told to imagine standing close to the scene depicted in the photograph and to allow themselves to experience any emotions that the photograph evoked. On ‘far’ trials, participants were told to imagine standing further away from the scene and to focus more on the facts of the photograph than on its emotional details. So as to reduce the likelihood of experimenter demand influencing behavior, participants were not explicitly told to reduce their negative affect on ‘far’ trials. While participants were not told so, ‘close’ trials were used to assess baseline emotional reactivity whereas ‘far’ trials were used to assess regulation. For the remainder of the manuscript, ‘close’ trials will be referred to as ‘reactivity’ trials and ‘far’ trials will be referred to as ‘regulation’ trials for clarity’s sake.
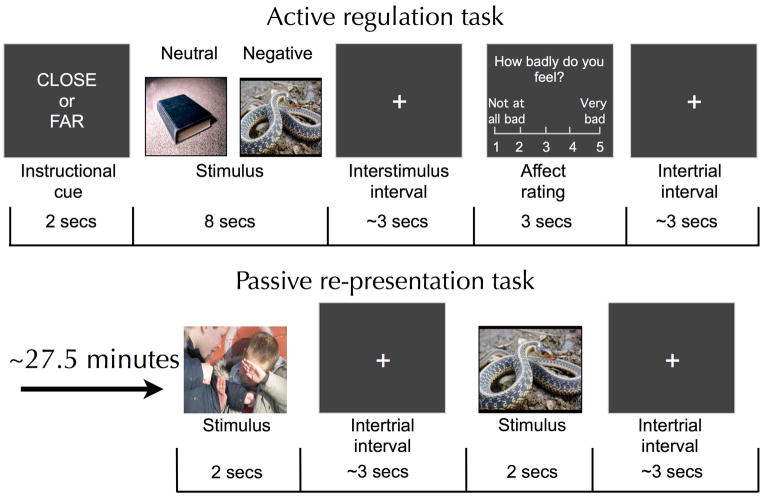
Participants then completed a reappraisal task consisting of 120 experimental trials, 60 of which contained aversive stimuli and 60 of which contained neutral stimuli. Half of all trials were ‘reactivity’ trials and half were ‘regulation’ trials such that in total participants completed 30 reactivity/negative, 30 regulation/negative, 30 reactivity/neutral and 30 regulation/neutral trials. On each trial, participants used the strategy indicated by a cue word (‘reactivity’ or ‘regulation’, shown for 2 seconds) while viewing a photographic stimulus for 8 seconds (Figure 1). After a brief jittered fixation (lasting for an average of 3 seconds), participants rated their negative affect on a five-point scale (1=not feeling badly at all, 5=feeling very badly) via button press. Each trial concluded with another brief jittered fixation (lasting for an average of 3 seconds). Self-report was used as a measure for emotional response for five reasons: (1) self-report provides specific and direct information about the valence and magnitude of an individual’s emotional state that peripheral physiological measures may not be able to provide (e.g., skin conductance reflects gross changes in arousal that could reflect changes in emotion, cognitive effort or both), (2) for peripheral physiological measures that can provide information about the valence of a reaction (e.g. facial EMG, Lang et al, 1993, or potentiated startle, Lang, Bradley, & Cuthbert, 1990), reappraisal has been shown to cause parallel and correlated changes in self-report and physiology (e.g. Ray et al, 2010), (3) self-report is not susceptible to scanner-related electromagnetic artifacts as are peripheral physiological measures, (4) prior work has shown that an individual’s dispositional need to please others (which could be important when being trained by an experimenter) does not predict reappraisal-related changes in self-reported emotion (e.g. Ochsner et al, 2002; Silvers et al., 2012), and (5) in psychiatric contexts self-reports of affective states (of, e.g., depressed mood, anxiety, anhedonia or negative affect more generally) have been validated as a means of assessing symptomology and predicting clinically significant outcomes (Bradley et al., 2011; Edelbrock, Costello, Dulcan, Kalas, & Conover, 1985; Lonigan, Phillips, & Hooe, 2003; Silk, Steinberg, & Morris, 2003).
Figure 1.
Trial structure for the active regulation and passive re-presentation tasks. ‘Close’ instructions were used on reactivity trials while ‘far’ instructions were used on regulation trials.
Stimuli were drawn from the International Affective Picture System (P. J. Lang, Bradley, & Cuthbert, 2001; P. J. Lang, Greenwald, Bradley, & Hamm, 1993) and from a set of similar pictures that had been previously used with participants in this age range (Silvers et al., 2012). Half of the stimuli were social in nature (i.e., they contained images of people) and half were not (e.g. neutral images of objects, like books, or aversive images of insects, attacking dogs, etc.). All participants saw the same neutral stimuli and all participants between the ages of 18–22 saw the same negative stimuli. The assignment of pictures to instruction was counterbalanced between participants.
For ethical reasons concerning the need for a legal guardian to consent to the presentation of aversive stimuli for participants under the age of 18, parents of these participants prescreened one hundred aversive photographic stimuli prior to participation. Parents were permitted to exclude up to 20 negative stimuli so that a pool of 80 negative stimuli remained. Excluded images were substituted with parent-approved stimuli that were closely matched for valence and arousal. Parents typically rejected a small number of pictures (mean=2.41, S.D.=3.68) and 54% of all parents did not reject any pictures at all so that relatively few picture substitutions took place. Neither a child’s age (r=.14, p=.41) nor sex (t(35)=.26, p=.80) predicted how many pictures parents rejected. Rejection rates did not predict differences in baseline emotional reactivity (percent increase in negative affect on reactivity/negative versus reactivity/neutral trials; r=.00, p=.999) or in top-down regulation success (percent decrease in negative affect on regulation/negative versus reactivity/negative trials; r=−.22, p=.19) suggesting that substituting like pictures for excluded ones did not impact task performance in any meaningful way.
Phase 2: Re-presentation
Five minutes after completing the 40-minute reappraisal task, participants were presented with the same aversive images that they previously saw during the reappraisal task so as to assess whether the effects of reappraisal on amygdala activity last. As such, on average stimuli were re-presented 27.5 minutes after being seen in the reappraisal task (20 minutes=mid-point of reappraisal task + 5 minute break + 2.5 minutes=mid-point of re-presentation task). No novel stimuli or neutral stimuli were shown during re-presentation and participants were not told ahead of time that they would be re-presented with stimuli they had previously seen in the reappraisal task. On each of these 60 trials, participants were instructed to passively view stimulus shown for 2 seconds with jittered fixation in between (for an average of 1.5 seconds). This protocol was designed to assess whether prior reappraisals influence relatively rapid and unreflected responses to emotional stimuli. Participants were not asked to rate their emotions while passively viewing stimuli in order to (1) limit the length of scanning and prevent subject fatigue, and 2) to keep methods as similar as possible to a paradigm that was previously validated in adults (Erk et al., 2010). To ensure that participants were attending to the stimuli they were asked to press a button whenever they saw a number (instead of a fixation cross) appear during the intertrial interval period (this occurred on 20 trials).
fMRI acquisition
Whole-brain fMRI data were acquired on a 1.5T GE Signa Twin Speed Excite HD scanner (GE Medical Systems). Functional images were acquired with a T2*-sensitive EPI BOLD sequence. Twenty-eight axial slices were collected with a TR of 2000 ms (TE of 34 ms, flip angle of 84°, field of view of 22.4 cm and 3.5 x 3.5 x 4 mm voxels). High-resolution SPGR structural images were collected with a TR of 19 ms (TE of 5 ms, flip angle of 20°, field of view of 25.6 cm). Stimuli were presented using E-Prime. Stimuli were displayed using an LCD projector and a back-projection screen mounted in the scanner suite. Participants made their responses using a five-finger-button-response unit with a molded hand brace (Avotec Inc. and Resonance Technologies).
Behavioral data analysis
All data were analyzed using SPSS 19.0. A repeated-measures ANOVA was used to assess the effects of valence (within-subjects: negative, neutral), strategy (within-subjects: reactivity, regulation), and age (between-subjects: mean-centered age) on self-reported negative affect. Social content was controlled for in this analysis but given that it did not significantly impact age-related changes in regulation success, it will not be discussed further. Sex was included as a predictor of no interest in this analysis.
fMRI data analysis
Preprocessing and first-level data analysis
The first four volumes of each functional scan were discarded from further analyses to avoid saturation effects. All preprocessing was conducted using the statistical parametric mapping software (SPM8, Wellcome Department of Cognitive Neurology, London, UK). Preprocessing included slice time correction (using the first slice as a reference slice), realignment and coregistration of the functional and structural data. Coregistered anatomical images were then segmented into gray and white matter and normalized (warped) to the standard MNI template brain and warping parameters were applied to all functional images. Normalized functional images were interpolated to 3 x 3 x 3 mm voxels and spatially smoothed with a 6-mm Gaussian filter. A gray-matter mask based on the MNI-standardized Colin-brain was used to constrain the functional data. Given the young age of some of the participants, steps were taken to reduce the impact of head motion. Motion parameters were estimated during preprocessing and any volumes that contained head motion greater than 1.5 mm (translation) or 2 degrees (rotation) relative to the proceeding volume were censored from further analyses. If 10% or more of volumes from within a given run were excluded, the entire run was discarded from further analyses. In order to be included in subsequent group analyses, participants had to have at least three acceptable Reappraisal task runs and an acceptable Re-presentation run. Two participants were excluded due to excessive head motion (Both male; one aged 10.33 years and the other aged 11.17 years).
First-level GLM analyses were implemented in NeuroElf (http://neuroelf.net). Cue, stimulus-viewing and response portions of each trial were modeled as boxcar regressors convolved with a canonical hemodynamic response function. Separate regressors were made for the four different trial types so that neural responses associated with strategy (reactivity vs. regulation) and valence (negative vs. neutral) could be differentiated. For each subject, a robust regression analysis was performed on the conditions of interest. Motion parameters, high-pass temporal filter parameters and an estimate of the subject’s global signal in white matter, gray matter and the ventricles were included as additional regressors of no interest. Following GLM computation for each participant, a second-level random effects analysis was performed on the group data.
Group level analysis plan
Following the computation of subject-level GLMs, three group level analyses were performed:
-
(1a) Hypothesis
Age will predict greater decreases in amygdala responses to negative stimuli on regulation trials in comparison to reactivity trials for both the active regulation and passive re-presentation tasks.
Approach: A targeted region of interest (ROI) analysis was performed to examine amygdala responses during the active regulation and passive re-presentation tasks.
-
(1b) Hypothesis
Hypothesis: Age will predict greater recruitment of prefrontal regions involved in cognitive control and emotion regulation.
Approach: A whole-brain analysis was performed to examine task and age effects on neural responses to negative stimuli during the active regulation and passive representation tasks.
-
(2) Hypothesis
Age will predict greater prefrontal-amygdala coupling for regulation versus reactivity trials during the active regulation task.
Approach: Psychophysiological interaction (PPI) analyses were conducted for both the active regulation and passive re-presentation tasks to examine developmental differences associated with prefrontal-amygdala connectivity.
-
(3) Hypothesis
Lateral PFC activation will mediate the relationship between age and reappraisal-related decreases in the amygdala response during the passive re-presentation task.
Approach: A single-level mediation analysis was performed to identify cortical regions that mediate the effect of age on reappraisal-related decreases in the amygdala response during active regulation and passive re-presentation.
Analysis 1a: Effects of reappraisal and age on amygdala responses
Amygdala ROI definition
In order to independently identify amygdala voxels that were responsive to emotion, a contrast was performed between the reactivity/negative and reactivity/neutral trials from the active regulation task. This contrast was thresholded at a height/extent combination (i.e., a p-value/cluster size combination) that preserved an alpha<.05, as estimated by AlphaSim implemented in NeuroElf (Supplemental Table 2). As expected, negative stimuli elicited a significantly greater bilateral amygdala response than neutral stimuli (Left: −18, −6, −15, 43 voxels; Right: 18, −3, −15, 87 voxels).
Amygdala ROI analysis
To examine how hemisphere, age, time and regulation strategy impacted amygdala responses, NeuroElf was used to extract parameter estimates for each experimental condition in each participant from the two amygdala ROIs. These parameter estimates were subsequently tested offline using a repeated-measures ANOVA in SPSS to examine how hemisphere (left amygdala vs. right amygdala), strategy (reactivity vs. regulation), time (Phase 1: Active regulation vs. Phase 2: Passive re-presentation), and age differentially predicted amygdala responses to negative stimuli. Stimuli social content and gender were controlled for as covariates of no interest.
Analysis 1b: Effects of reappraisal and age on recruitment of control-related regions
In addition to the ROI-based amygdala analysis described above, effects of task and age were also examined using whole-brain mixed-effects models that examined the effects of strategy (reactivity vs. regulation), and mean-centered age on neural responses to negative stimuli. Separate models were constructed for the active regulation and passive re-presentation tasks. Stimuli social content was controlled for in these analyses and those effects are reported in Supplementary Tables 4 and 6. Gender was included as a covariate of no interest. Statistical significance of activation maps was evaluated using the AlphaSim p-value/cluster size combination that preserved an alpha<.05 for multiple comparisons with a voxelwise threshold of p<.005.
Analysis 2: Effects of age on cortical-subcortical functional connectivity underlying reappraisal
To examine developmental differences associated with prefrontal-amygdala connectivity, psychophysiological interaction (PPI) analyses were conducted for both the active regulation and passive re-presentation tasks on the regulation/negative > reactivity/negative contrast. For both tasks, the main effect of task (regulation/negative > reactivity/negative contrast) and the robust correlation between age and task were examined. Given that social content did not interact with age and strategy to predict amygdala activation, social content was not controlled for in this analysis. The left amygdala ROI (−18, −6, −15) that was defined for amygdala ROI analyses was used as a seed region for these PPI analyses. The left amygdala was chosen as a seed region because neural activation to negative stimuli was greater in the left than right amygdala (main effect of hemisphere, F(1,53)=8.05, p<.01) and was thus hypothesized to be a more robust seed for this analysis. Resulting maps were thresholded using p-value/cluster size combinations that preserved an alpha<.05. Given prior work suggesting that prefrontal-amygdala connectivity supports reappraisal in adults (Banks et al., 2007), prefrontal regions identified in PPI analyses are reported for if they exceeded a threshold of p<.005, 20 voxels, for completeness’ sake.
Analysis 3: Neural predictors and mediators of reappraisal-related amygdala modulation
A final analysis was conducted to identify cortical regions that mediate the effect of age on reappraisal-related decreases in the amygdala response. To this end, a reappraisal-related amygdala decrease value was calculated for each participant. To do this, parameter estimates for each experimental condition were extracted from the left amygdala ROI (also used for the PPI analysis) and the estimate from the regulation/negative condition was subtracted from the estimate from the reactivity/negative condition. Participants’ reappraisal-related amygdala decrease values were then robustly correlated (i.e., using robust correlation) with brain activity in the regulation/negative > reactivity/negative contrast. This correlation was performed separately for each task phase (i.e., active regulation, passive re-presentation). Amygdala responses associated with the reactivity/negative condition were used as a covariate of no interest to ensure that large reappraisal-related decreases were not driven by phenomena such as floor effects. Because social content did not interact with age and strategy to predict amygdala activation, social content was not controlled for in this analysis.
In order for a cortical ROI to be tested a potential mediator, it had to (1) predict amygdala modulation while controlling for age, and (2) correlate with age (Baron & Kenny, 1986). ROIs identified in the correlational analysis that met these criteria were subjected to a formal mediation analysis where age was used as a predictor (x), the regulation/negative>reactivity/negative ROI contrast value was used as a mediator (m), reappraisal-related amygdala decrease was used as an outcome variable (y) and statistical significance was assessed with 1000 bootstrapping samples (Hayes, 2013).
RESULTS
Behavioral results
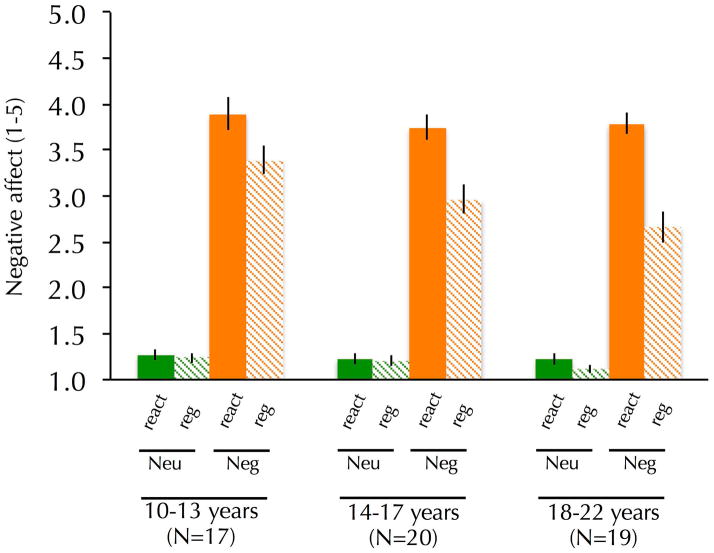
Affect ratings confirmed that the stimuli evoked the intended emotions and the strategy modulated emotion as predicted: during active regulation, participants reported more negative affect on trials with negative as compared to neutral stimuli, F(1, 53)=765.76, p<.001, and less negative affect when reappraising (regulation compared to reactivity trials), F(1, 53)=81.64, p<.001. Additionally, a significant interaction between valence and strategy was observed, F(1, 53)=75.52, p<.001, such that decreases in negative affect were largest when participants reappraised negative stimuli. An interaction between age and strategy, F(1, 53)=4.14, p<.05, was observed such that age was associated with less negative affect on regulation trials but not reactivity trials (Figure 2). A marginally significant interaction between valence, strategy and age was observed, F(1, 53)=3.14, p=.08, such that age predicted greater reappraisal-related decreases in negative affect, even after controlling for affect on reactivity/negative trials (r=.29, p<.05). No other significant age effects were observed. No significant effects of gender were observed. The extra sum-of-squares F-test (Motulsky & Christopoulos, 2004) revealed that a linear model of age was more appropriate for predicting reappraisal-related reductions in negative affect than a quadratic model. These data suggest that participants of all ages used reappraisal to effectively regulate emotional responses, but also that this capacity improved as a function of age.
Figure 2.
Self-reported negative affect as a function of strategy, valence and age. Age was associated with greater reappraisal-related decreases in negative affect. All analyses used a continuous measure of age but age is shown categorically for display purposes.
Imaging results
Result 1a: Effects of reappraisal and age on amygdala responses
Greater amygdala responses were observed on reactivity/negative than reactivity/neutral trials (Supplemental Table 2; Left amygdala coordinates: −18, −6, −15, 43 voxels; Right amygdala coordinates: 18, −3, −15, 87 voxels). A post-hoc examination of the left and right amygdala clusters revealed that reactivity/negative > reactivity/neutral contrast values did not correlate significantly with age (Left amygdala: r=.05, p=.72; Right amygdala: r=−.14, p=.32), suggesting that participants of different ages showed similar baseline amygdala responses to negative stimuli. The left and right amygdala clusters identified in the reactivity/negative > reactivity/neutral contrast were used as ROIs for subsequent analyses.
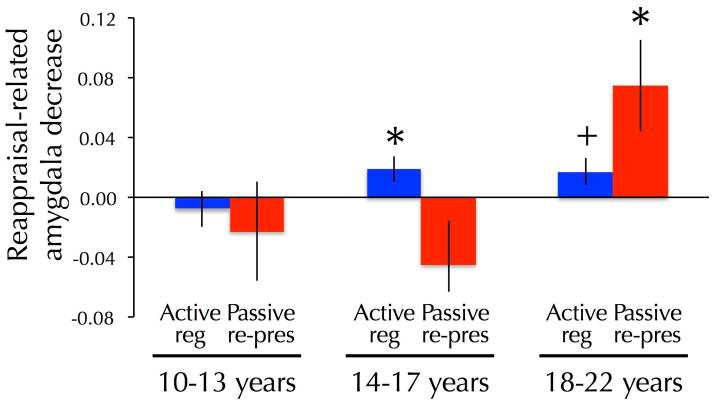
Age predicted larger decreases in the amygdala response on reappraisal versus reactivity trials with negative stimuli (Figure 3), as evidenced by an interaction between age and strategy, F(1, 53)=8.82, p<.005. These effects were marginally stronger in the left than right amygdala, as evidenced by the interaction between amygdala hemisphere, age, and strategy, F(1, 53)=3.56, p=.07. Age continued to predict reappraisal-related reductions in the amygdala response after controlling for the amygdala response on reactivity/negative trials (partial correlation: r=.36, p<.01). An interaction between age, strategy and time was observed such that age predicted larger reappraisal-related decreases in the amygdala response during re-presentation than during active regulation, F(1, 53)=5.62, p<.05. No interaction was observed between amygdala hemisphere (left or right), age, strategy, and time, suggesting that comparable effects were observed in the left and right amygdala, F(1, 53)=.60, p=.44. No other significant age-related effects were observed.
Figure 3.
Bar graphs lines depicting magnitude of left amygdala decrease (Reactivity/negative > Regulation/negative) as a function of age and task phase (Reg=active regulation task, Rep=passive re-presentation task). Analyses were conducted using a continuous measure of age but age is shown categorically to facilitate interpretation. Paired, two-tailed t-tests were performed to compare the reactivity and regulation conditions within each age group to further facilitate interpretation (*p<.05; +p<.08).
Result 1b: Effects of reappraisal and age on recruitment of control-related regions
A whole-brain analysis examining the effects of strategy (reactivity versus regulation trials) and age revealed that during active regulation, reappraisal recruited right dorsolateral prefrontal cortex, bilateral posterior parietal cortex and the precuneus (Supplementary Table 3). No age-related differences in reappraisal-related recruitment were observed during active regulation in the whole-brain analysis (including the amygdala). No cortical brain regions showed differential recruitment as a function of strategy (i.e., stimuli that had previously been reappraised to those that had not) during passive re-presentation (Supplementary Table 5). However, age predicted greater activation of the cuneus in response to previously reappraised stimuli relative to stimuli that had not been reappraised during passive re-presentation.
Result 2: Effects of age on cortical-subcortical functional connectivity underlying reappraisal
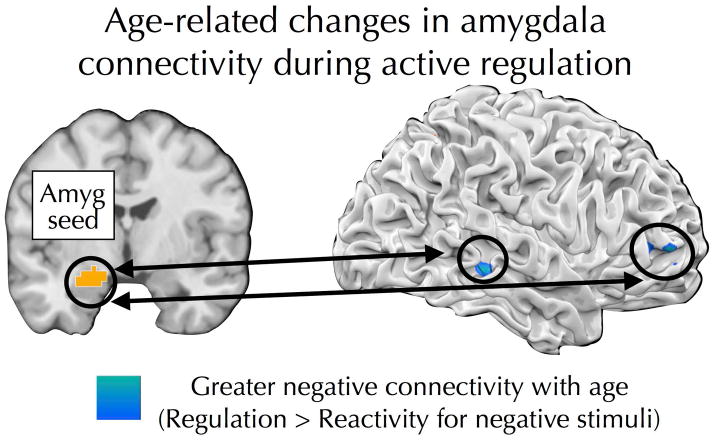
To explore how cortical-subcortical interactions associated with reappraisal may differ as a function of age, PPI analyses were conducted using the left amygdala ROI (defined by the reactivity/negative > reactivity/neutral contrast) as a seed region. The goal of these analyses was to identify brain regions showing greater coupling with the amygdala on regulation/negative trials compared to reactivity/negative trials and to determine whether such connectivity differs as a function of age. Separate PPI analyses were conducted for the active regulation and passive re-presentation tasks. During active regulation, age predicted more positive precuneus-amygdala connectivity and more negative connectivity between the amygdala and right RLPFC (Table 1; Figure 4). No age effects were observed during passive re-presentation (Table 1).
Table 1.
Brain regions identified in PPI analysis
| Region | Hemisphere | # Voxels | t or r | MNI Coordinates | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Active regulation, Regulation/negative > Reactivity/negative t-test | ||||||
| No regions identified | ||||||
| Active regulation, Regulation/negative > Reactivity/negative correlated with age | ||||||
| Middle frontal gyrus* | R | 29 | −.50 | 45 | 51 | 3 |
| Middle temporal gyrus | R | 41 | −.57 | 51 | −33 | −6 |
| Precuneus | R | 46 | .49 | 9 | −51 | 48 |
| Cerebellum | L | 55 | .61 | −15 | −63 | −36 |
| Passive re-presentation, Regulation/negative > Reactivity/negative t-test | ||||||
| Superior frontal gyrus | R | 72 | −3.90 | 18 | 30 | 51 |
| Passive re-presentation, Regulation/negative > Reactivity/negative correlated with age | ||||||
| No regions identified | ||||||
For hemisphere, R=right, L=left, M=medial. t or r=maximum t or r statistic for a given cluster.
p<.005, uncorrected
Figure 4.
Age predicted increasingly negative connectivity between the amygdala and RLPFC and posterior temporal cortex during the active regulation task.
Result 3: Neural predictors and mediators of reappraisal-related amygdala modulation
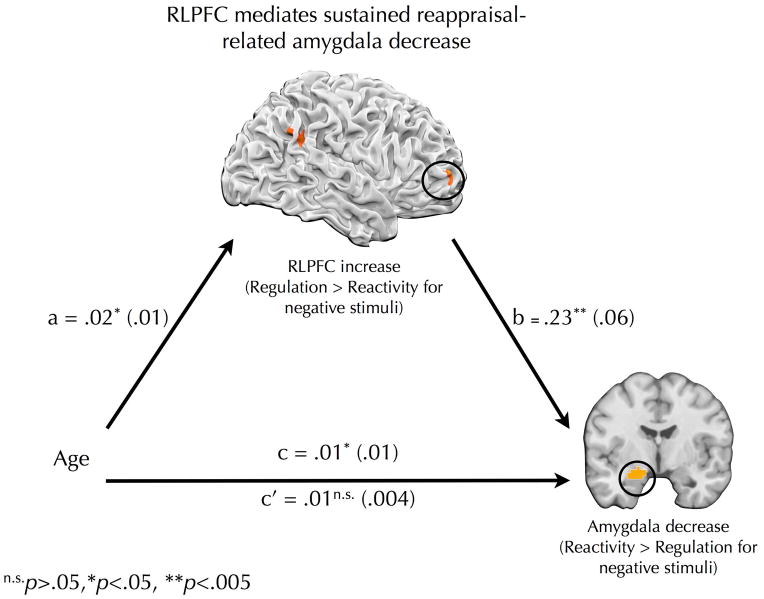
To examine whether different patterns of cortical activation may mediate age-related differences in reappraisal-related amygdala modulation, a three-step approach was adopted. Separate mediation analyses were computed for the active regulation and passive re-interpretation tasks. First, each participant’s mean decrease in activation for regulation/negative trials versus reactivity/negative trials was calculated using the left amygdala ROI. These values were then correlated with the regulation/negative > reactivity/negative contrast to identify brain regions that predicted reappraisal-related decreases in amygdala activity. Second, regulation/negative > reactivity/negative contrast values were extracted from the brain regions identified in Step 1 and correlated with age. Third, if activity in a brain region correlated with reappraisal-related decreases in the amygdala response as well as with age, a formal mediation analysis was conducted to determine whether activity in that brain region mediated the relationship between age and reappraisal-related decreases in the amygdala response. It was revealed (Table 2; Figure 5) that age predicted stronger RLPFC recruitment (a path: β=.02, t(54)=2.46, p<.05) and, before controlling for RLPFC, larger amygdala decreases (c path: β=.01, t(54)=2.61, p<.05). RLPFC predicted the magnitude of reappraisal-related amygdala modulation, even after controlling for age (b path: β=.23, t(53)=4.06, p<.001), while age was unrelated to amygdala decreases after controlling for RLPFC (c’ path: β=.01, t(53)=1.52, p=.13). The indirect effect was significantly greater than zero (β=.006, bias corrected and accelerated 95% confidence intervals=.001, .012), providing further evidence for mediation. Taken together, these data suggest that age predicted stronger inverse connectivity between RLPFC and the amygdala during active regulation and that a neighboring RLPFC region mediated the relationship between age and reappraisal-related amygdala modulation during subsequent passive re-presentation. No brain regions were found to mediate the relationship between age and reappraisal-related decreases in the amygdala response during active regulation.
Table 2.
Neural correlates of reappraisal-related decrease in the amygdala response that were tested as potential mediators of age-amygdala relationship
| Region | Hemisphere | # Voxels | r | MNI Coordinates | Correlate with age? | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Active regulation, Regulation/negative > Reactivity/negative | |||||||
| Amygdala | R | 154 | −.61 | 21 | −3 | −18 | no |
| Passive re-presentation, Regulation/negative > Reactivity/negative | |||||||
| Middle frontal gyrus | R | 55 | .52 | 30 | 66 | 0 | yes |
| Inferior parietal lobule | L | 67 | .47 | 51 | −45 | 30 | no |
For hemisphere, R=right, L=left, M=medial. r=maximum correlation statistic between reappraisal-related amygdala decrease and regulation> reactivity activation for a given cluster. Negative r values indicate that recruitment during reappraisal predicted smaller reappraisal-related amygdala drops while positive r values predict larger reappraisal-related amygdala drops. “Correlate with age?” indicates whether or not region correlated with age.
Figure 5.
Mediation pathway by which age is related to lasting effects of reappraisal on amygdala response. Age predicted recruitment of right rostrolateral prefrontal cortex (RLPFC) for previously reappraised stimuli (Regulation/negative > Reactivity/negative contrast value) during re-presentation. RLPFC recruitment mediated the relationship between age and amygdala decreases (Reactivity/negative > Regulation/negative contrast value) during re-presentation. Beta coefficients are shown for each path with standard error displayed in parentheses. n.s.p>.05, *p<.05, **p<.01, ***p<.001
DISCUSSION
Relative to adults, adolescents experience more extreme and short-lived emotions in their everyday lives and show heightened amygdala reactivity to affective cues (Gee et al., 2013; Hare et al., 2008; R. Larson et al., 1980; Monk et al., 2003). Recent work has suggested that such age-related differences may be partially due to differences in the ability to exert top-down regulation over emotion (McRae, Gross, et al., 2012; Silvers et al., 2012). The present study builds on this prior work by examining whether age predicts differences in the enduring effects of reappraisal across adolescence.
As such, four key results were obtained. First, while actively regulating, adolescents were less effective than young adults at using reappraisal to reduce negative affect and amygdala responses to aversive stimuli. Second, these age-related effects persisted over time such that age predicted enhanced amygdala modulation during the representation of stimuli that had previously been reappraised. Third, during active regulation age predicted greater inverse connectivity between the amygdala and RLPFC regions that are associated with higher order cognitive processing (Christoff & Gabrieli, 2000; Gilbert et al., 2006). Fourth, age predicted greater recruitment of RLPFC during representation of stimuli that had been previously reappraised, and RLPFC recruitment mediated the relationship between age and sustained decreases in amygdala responding. These results suggest that age predicts both immediate and lasting effects of emotion regulation and inform neural models of emotion regulation.
Immediate and lasting effects of emotion regulation across age
The present study builds on prior emotion regulation work and extends it by underscoring the need to examine both concurrent and long-lasting effects of emotion regulation across age. We found that not only are young adults better than adolescents at regulating their emotions in the moment, but also that age-related differences may actually become greater over time. Thus, even if adolescents are somewhat successful at initially reappraising an emotional event, it may be harder for them to “get over” said event because their reappraisals do not endure over time.
In this context, it is important to highlight that age not only predicted greater reappraisal-related reductions in the amygdala response over time but that this effect was mediated by recruitment of RLPFC. RLPFC supports relational integration, prospective memory and autobiographical memory retrieval, and shows differential recruitment across adolescence (Christoff & Gabrieli, 2000; Crone et al., 2009; Dumontheil, Burgess, & Blakemore, 2008; Gilbert et al., 2006; Kim, 2013). As such, it is plausible that RLPFC recruitment during re-presentation supports recall of one’s prior reappraisals, or some other higher-order cognitive process, and that this in turn leads – directly or indirectly – to attenuated amygdala responses. This suggests that – at least over the time interval studied here – age-related differences in sustained amygdala response may be due to differences in the recall of prior reappraisals rather than to changes in the affective meaning of stimuli, per se. Alternatively, given that older participants were more likely to be successful at reappraising in the first place, sustained differences in amygdala and RLPFC recruitment may reflect the fact that younger participants may not have effective reappraisals to recall while older participants do. These possibilities may be further explored in future experiments where participants are queried about whether they actively recalled their prior reappraisals during re-presentation. Additionally, techniques such as transcranial magnetic stimulation could be used in healthy adults to determine whether temporarily interfering with RLPFC activity reduces the sustained effects of reappraisal.
It is intriguing to note that age predicted RLPFC-amygdala functional connectivity during active regulation, while RLPFC activation mediated age effects on reappraisal-related amygdala modulation during re-presentation. Functional connectivity, as assessed by PPI, is thought to index communication or the flow of information between different brain regions (O’Reilly, Woolrich, Behrens, Smith, & Johansen-Berg, 2012). As such, age-related increases in RLPFC-amygdala connectivity during active regulation may reflect increases in prefrontal-subcortical crosstalk that support volitional emotion regulation. By contrast, RLPFC recruitment during re-presentation may attenuate amygdala activity through indirect pathways that are not detectable by PPI. Alternatively, it may be that RLPFC and amygdala recruitment during re-presentation are both neural markers for mature emotion regulation that are highly correlated between-subjects, yet are relatively independent. For example, it may be that those individuals who were older and thus more successful at reappraising in the first place show sustained RLPFC recruitment and diminished amygdala recruitment but this is not because of active communication between these two regions but could instead reflect two parallel and independent streams of neural processing.
From a translational perspective, the fact that age predicted differences in the sustained effects of reappraisal raises important questions with regards to normative and atypical adolescent emotional development. Adolescents (Pattwell et al., 2012), as well as individuals high in trait anxiety (Sehlmeyer et al., 2011), show a diminished capacity to extinguish learned fear responses. Recent work has shown that depressed individuals can successfully reduce amygdala responses during reappraisal but that this reduction does not carry over when they are later exposed to stimuli they previously reappraised (Erk et al., 2010). Anxiety and depression show a marked increase during adolescence (Kessler et al., 2005) and therapies like CBT that rely heavily on reappraisal are commonly used with adolescent patients (Wang et al., 2009). The present findings indicate that the effects of reappraisal last longer in older than younger individuals, which suggests that future work could examine whether CBT may have more long-lasting effects in adults than adolescents.
Neural mechanisms of emotion regulation across age
The present findings build on existing neural models of emotion regulation and provide greater understanding with regards to how brain systems associated with emotion regulation develop during adolescence. Across age, active regulation was associated with recruitment of prefrontal and parietal regions commonly implicated in cognitive and emotional control processes (Buhle et al., 2013; Ochsner, Silvers, & Buhle, 2012). Yet, despite equivalent prefrontal and parietal recruitment, age predicted greater negative prefrontal-amygdala connectivity during reappraisal, greater decreases in negative affect, and more enduring amygdala modulation. This suggests that, relative to adolescents, young adults are more effective at engaging cognitive control to change the affective meaning of a stimulus and to alter amygdala response. While age predicted reduced amygdala activity during regulation, no age related differences were observed when participants immersed themselves in the negative images. Prior work has shown that age predicts reduced amygdala responses during passive viewing of aversive scenes (Vink, Derks, Hoogendam, Hillegers, & Kahn, 2014), yet the present study and one prior study identified age effects exclusively in the context of reappraisal (Pitskel et al., 2011). These results suggest that when left to their own devices, adults spontaneously engage regulatory processes that attenuate amygdala responses to emotional stimuli yet when instructed on when to regulate and when to respond naturally, these age effects emerge only in the context of regulation. While the present study is the first to examine developmental effects associated with prefrontal-amygdala connectivity during reappraisal, these results are consistent with a growing literature suggesting that differences in prefrontal-subcortical connectivity underlie a host of emotional changes that occur during adolescence (Gee et al., 2013; Somerville, Hare, & Casey, 2011; Somerville et al., 2013).
The present results are both consistent and inconsistent with the mixed set of findings that have emerged from prior work examining developmental differences in the behavioral and brain bases of reappraisal. Behaviorally, two studies found evidence for age-related improvements in the ability to use reappraisal to reduce negative affect (McRae, Gross, et al., 2012; Silvers et al., 2012), while one did not (Pitskel et al., 2011). Neurally, one study observed age-related increases in prefrontal recruitment and no age effects on amygdala responding (McRae, Gross, et al., 2012), another decreases in prefrontal recruitment and improved amygdala modulation (Pitskel et al., 2011), and another did not conduct across-age comparisons (Levesque et al., 2004). No prior work has examined reappraisal-related prefrontal-amygdala interactions across age. While it is difficult to isolate the precise reasons for differences between prior results and the present findings, the considerable heterogeneity in methods across studies suggests at least two factors that are important to consider.
The first factor to consider is varying age ranges, with some prior work testing children as young as 7 and others as old as 23, with only some overlap in between. Prior work suggests that the frontal lobes undergo significant structural and functional changes throughout adolescence (Bunge & Wright, 2007; Casey, Tottenham, Liston, & Durston, 2005; Giedd & Rapoport, 2010; Luna, Padmanabhan, & O’Hearn, 2010) and yet only one prior study found age-related differences in reappraisal-related recruitment across multiple prefrontal regions as well as the amygdala (Pitskel et al., 2011). Intriguingly, that study included children as young as 7 (Pitskel et al., 2011), while other studies have not. Together with the present results, this suggests that perhaps young children fail to recruit prefrontal regions and modulate the amygdala altogether during reappraisal, while adolescents show adult-like patterns of prefrontal recruitment but are still maturing with regards to prefrontal-amygdala connectivity.
The second factor is the type of reappraisal tactic used and the way in which participants were instructed to use it (McRae, Ciesielski, & Gross, 2012). In the present study, participants reappraised by distancing – a tactic that is commonly used in both therapeutic and experimental contexts (Beck, 1970; Kross & Ayduk, 2011) – rather than by reinterpreting the meaning of stimuli. In the present study, no age or task effects were observed in left lateral prefrontal cortex, a brain region commonly recruited by reinterpretation but not distancing (Ochsner et al., 2012). In contrast, McRae and colleagues (2012) used a reinterpretation paradigm and found that participants overall recruited left lateral PFC during reappraisal and that this recruitment scaled with age. Additionally, in contrast to most adult neuroimaging studies of reappraisal (e.g., Ochsner et al., 2002), participants were not explicitly told to reduce their negative affect when using the distancing strategy – a decision that was made in light of prior work suggesting that the tendency to give socially desirable responses (i.e., to be susceptible to experimenter demand characteristics) changes as a function of age (Crandall, Crandall, & Katkovsky, 1965). The fact that significant age-related effects were obtained and that participants of all ages reported reduced negative affect speaks to the robustness of the instructed strategy. That said, additional work is needed to directly test precisely how different reappraisal tactics and the explicitness of the reappraisal instruction impact age-related effects.
Supplementary Material
Research highlights.
Young adults are more successful at using cognitive strategies like reappraisal to regulate negative emotion than are adolescents
Age predicts concurrent and sustained reappraisal-related reductions in the amygdala response
Recruitment of prefrontal regions associated with higher-order cognitive processing mediate the relationship between age and sustained decreases in amygdala responding
Acknowledgments
Funding
This work was supported by the National Institutes of Health (R01 NICHD 0691780, F31 NIMH 94056).
Footnotes
Author Contributions
J.A.S. and K.N.O. designed the study. J.A.S., J.S. and A.D.H. collected the data. J.A.S. and J.W. performed data analyses. J.A.S. and K.N.O. wrote the manuscript.
References
- Banks SJ, Eddy KT, Angstadt M, Nathan PJ, Phan KL. Amygdala-frontal connectivity during emotion regulation. Soc Cogn Affect Neurosci. 2007;2:303–312. doi: 10.1093/scan/nsm029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. [Research Support, U.S. Gov’t, Non-P.H.S. Research Support, U.S. Gov’t, P.H.S.] J Pers Soc Psychol. 1986;51(6):1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- Beck AT. Cognitive therapy: Nature and relation to behavior therapy. Behavior Therapy. 1970;1(2):184–200. doi: 10.1016/s0005-7894(70)80030-2. [DOI] [Google Scholar]
- Bradley B, DeFife JA, Guarnaccia C, Phifer J, Fani N, Ressler KJ, Westen D. Emotion dysregulation and negative affect: association with psychiatric symptoms. Journal of Clinical Psychiatry. 2011;72(5):685–691. doi: 10.4088/JCP.10m06409blu. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhle JT, Silvers JA, Wager TD, Lopez R, Onyemekwu C, Kober H, Ochsner KN. Cognitive reappraisal of emotion: A meta-analysis of human neuroimaging studies. Cerebral Cortex. 2013 doi: 10.1093/cercor/bht154. Epub ahead of print June 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunge SA, Wright SB. Neurodevelopmental changes in working memory and cognitive control. [Research Support, U.S. Gov’t, Non-P.H.S. Review] Curr Opin Neurobiol. 2007;17(2):243–250. doi: 10.1016/j.conb.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Ruberry EJ, Libby V, Glatt CE, Hare T, Soliman F, Tottenham N. Transitional and translational studies of risk for anxiety. Depress Anxiety. 2011;28(1):18–28. doi: 10.1002/da.20783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, Tottenham N, Liston C, Durston S. Imaging the developing brain: what have we learned about cognitive development? Trends Cogn Sci. 2005;9(3):104–110. doi: 10.1016/j.tics.2005.01.011. S1364-6613(05)00030-6 [pii] [DOI] [PubMed] [Google Scholar]
- Christoff K, Gabrieli JDE. The frontopolar cortex and human cognition: evidence for a rostrocaudal hierarchical organization in human prefrontal cortex. Psychobiology. 2000;28:168–186. [Google Scholar]
- Compton SN, March JS, Brent D, Albano AMt, Weersing R, Curry J. Cognitive-behavioral psychotherapy for anxiety and depressive disorders in children and adolescents: an evidence-based medicine review. [Review] J Am Acad Child Adolesc Psychiatry. 2004;43(8):930–959. doi: 10.1097/01.chi.0000127589.57468.bf. [DOI] [PubMed] [Google Scholar]
- Crandall VC, Crandall VJ, Katkovsky W. A children’s social desirability questionnaire. Journal of Consulting Psychology. 1965;29(1):27–36. doi: 10.1037/h0020966. [DOI] [PubMed] [Google Scholar]
- Crone EA, Wendelken C, van Leijenhorst L, Honomichl RD, Christoff K, Bunge SA. Neurocognitive development of relational reasoning. Dev Sci. 2009;12(1):55–66. doi: 10.1111/j.1467-7687.2008.00743.x. DESC743 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diekhof EK, Geier K, Falkai P, Gruber O. Fear is only as deep as the mind allows: a coordinate-based meta-analysis of neuroimaging studies on the regulation of negative affect. [Meta-Analysis Review] Neuroimage. 2011;58(1):275–285. doi: 10.1016/j.neuroimage.2011.05.073. [DOI] [PubMed] [Google Scholar]
- Dumontheil I, Burgess PW, Blakemore SJ. Development of rostral prefrontal cortex and cognitive and behavioural disorders. Dev Med Child Neurol. 2008;50(3):168–181. doi: 10.1111/j.1469-8749.2008.02026.x. DMCN02026 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelbrock C, Costello AJ, Dulcan MK, Kalas R, Conover NC. Age differences in the reliability of the psychiatric interview of the child. Child Dev. 1985;56(1):265–275. [PubMed] [Google Scholar]
- Erk S, Mikschl A, Stier S, Ciaramidaro A, Gapp V, Weber B, Walter H. Acute and sustained effects of cognitive emotion regulation in major depression. [Comparative Study Randomized Controlled Trial Research Support, Non-U.S. Gov’t] J Neurosci. 2010;30(47):15726–15734. doi: 10.1523/JNEUROSCI.1856-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee DG, Humphreys KL, Flannery J, Goff B, Telzer EH, Shapiro M, Tottenham N. A developmental shift from positive to negative connectivity in human amygdala-prefrontal circuitry. [Research Support, N.I.H., Extramural Research Support, U.S. Gov’t, Non-P.H.S.] J Neurosci. 2013;33(10):4584–4593. doi: 10.1523/JNEUROSCI.3446-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd JN, Rapoport JL. Structural MRI of pediatric brain development: what have we learned and where are we going? [Review] Neuron. 2010;67(5):728–734. doi: 10.1016/j.neuron.2010.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert SJ, Spengler S, Simons JS, Steele JD, Lawrie SM, Frith CD, Burgess PW. Functional specialization within rostral prefrontal cortex (area 10): a meta-analysis. J Cogn Neurosci. 2006;18(6):932–948. doi: 10.1162/jocn.2006.18.6.932. [DOI] [PubMed] [Google Scholar]
- Hare TA, Tottenham N, Galvan A, Voss HU, Glover GH, Casey BJ. Biological substrates of emotional reactivity and regulation in adolescence during an emotional go-nogo task. [Comparative Study Research Support, N.I.H., Extramural] Biol Psychiatry. 2008;63(10):927–934. doi: 10.1016/j.biopsych.2008.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes AF. Introduction to mediation, moderation and conditional process analysis: A regression-based approach. New York, NY: Guilford Press; 2013. [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- Kim H. Differential neural activity in the recognition of old versus new events: an activation likelihood estimation meta-analysis. [Meta-Analysis Research Support, Non-U.S. Gov’t] Hum Brain Mapp. 2013;34(4):814–836. doi: 10.1002/hbm.21474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kross E, Ayduk O. Making Meaning out of Negative Experiences by Self-Distancing. Current Directions in Psychological Science. 2011;20(3):187–191. doi: 10.1177/0963721411408883. [DOI] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. Emotion, attention, and the startle reflex. Psychological Review. 1990;97(3):377–395. [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. International Affective Picture System (IAPS) Instruction Manual and Affective Ratings, Technical Report. Gainsville, FL: The University of Florida; 2001. [Google Scholar]
- Lang PJ, Greenwald MK, Bradley MM, Hamm AO. Looking at pictures: affective, facial, visceral, and behavioral reactions. Psychophysiology. 1993;30(3):261–273. doi: 10.1111/j.1469-8986.1993.tb03352.x. [DOI] [PubMed] [Google Scholar]
- Larson R, Csikszentmihalyi M, Graef R. Mood variability and the psychosocial adjustment of adolescents. Journal of Youth and Adolescence. 1980;9(6):469–490. doi: 10.1007/BF02089885. [DOI] [PubMed] [Google Scholar]
- Larson RW, Moneta G, Richards MH, Wilson S. Continuity, stability, and change in daily emotional experience across adolescence. [Research Support, U.S. Gov’t, P.H.S.] Child Dev. 2002;73(4):1151–1165. doi: 10.1111/1467-8624.00464. [DOI] [PubMed] [Google Scholar]
- Levesque J, Joanette Y, Mensour B, Beaudoin G, Leroux JM, Bourgouin P, Beauregard M. Neural basis of emotional self-regulation in childhood. [Clinical Trial Research Support, Non-U.S. Gov’t] Neuroscience. 2004;129(2):361–369. doi: 10.1016/j.neuroscience.2004.07.032. [DOI] [PubMed] [Google Scholar]
- Lonigan CJ, Phillips BM, Hooe ES. Relations of positive and negative affectivity to anxiety and depression in children: evidence from a latent variable longitudinal study. J Consult Clin Psychol. 2003;71(3):465–481. doi: 10.1037/0022-006X.71.3.465. [DOI] [PubMed] [Google Scholar]
- Luna B, Padmanabhan A, O’Hearn K. What has fMRI told us about the development of cognitive control through adolescence? Brain Cogn. 2010;72(1):101–113. doi: 10.1016/j.bandc.2009.08.005. S0278-2626(09)00159-6 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macnamara A, Ochsner KN, Hajcak G. Previously reappraised: the lasting effect of description type on picture-elicited electrocortical activity. Soc Cogn Affect Neurosci. 2011;6(3):348–358. doi: 10.1093/scan/nsq053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McRae K, Ciesielski B, Gross JJ. Unpacking cognitive reappraisal: Goals, tactics, and outcomes. Emotion. 2012;12(2):250–255. doi: 10.1037/a0026351. [DOI] [PubMed] [Google Scholar]
- McRae K, Gross JJ, Weber J, Robertson ER, Sokol-Hessner P, Ray RD, Ochsner KN. The development of emotion regulation: an fMRI study of cognitive reappraisal in children, adolescents and young adults. [Research Support, N.I.H., Extramural Research Support, U.S. Gov’t, Non-P.H.S.] Soc Cogn Affect Neurosci. 2012;7(1):11–22. doi: 10.1093/scan/nsr093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monk CS, McClure EB, Nelson EE, Zarahn E, Bilder RM, Leibenluft E, Pine DS. Adolescent immaturity in attention-related brain engagement to emotional facial expressions. [Clinical Trial Comparative Study] Neuroimage. 2003;20(1):420–428. doi: 10.1016/s1053-8119(03)00355-0. [DOI] [PubMed] [Google Scholar]
- Motulsky H, Christopoulos A. Fitting models to biological data using linear and non-linear regression. Oxford: Oxford University Press; 2004. [Google Scholar]
- O’Reilly JX, Woolrich MW, Behrens TE, Smith SM, Johansen-Berg H. Tools of the trade: psychophysiological interactions and functional connectivity. [Research Support, Non-U.S. Gov’t] Soc Cogn Affect Neurosci. 2012;7(5):604–609. doi: 10.1093/scan/nss055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner KN, Bunge SA, Gross JJ, Gabrieli JD. Rethinking feelings: an FMRI study of the cognitive regulation of emotion. [Research Support, Non-U.S. Gov’t Research Support, U.S. Gov’t, Non-P.H.S. Research Support, U.S. Gov’t, P.H.S.] J Cogn Neurosci. 2002;14(8):1215–1229. doi: 10.1162/089892902760807212. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Silvers JA, Buhle JT. Functional imaging studies of emotion regulation: a synthetic review and evolving model of the cognitive control of emotion. Ann N Y Acad Sci. 2012;1251(1):E1–E24. doi: 10.1111/j.1749-6632.2012.06751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattwell SS, Duhoux S, Hartley CA, Johnson DC, Jing D, Elliott MD, Lee FS. Altered fear learning across development in both mouse and human. [Comparative Study Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t] Proc Natl Acad Sci U S A. 2012;109(40):16318–16323. doi: 10.1073/pnas.1206834109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitskel NB, Bolling DZ, Kaiser MD, Crowley MJ, Pelphrey KA. How grossed out are you? The neural bases of emotion regulation from childhood to adolescence. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t] Dev Cogn Neurosci. 2011;1(3):324–337. doi: 10.1016/j.dcn.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehlmeyer C, Dannlowski U, Schoning S, Kugel H, Pyka M, Pfleiderer B, Konrad C. Neural correlates of trait anxiety in fear extinction. [Research Support, Non-U.S. Gov’t] Psychol Med. 2011;41(4):789–798. doi: 10.1017/S0033291710001248. [DOI] [PubMed] [Google Scholar]
- Silk JS, Steinberg L, Morris AS. Adolescents’ emotion regulation in daily fife: Links to depressive symptoms and problem behavior. Child Dev. 2003;74(6):1869–1880. doi: 10.1046/j.1467-8624.2003.00643.x. [DOI] [PubMed] [Google Scholar]
- Silvers JA, McRae K, Gabrieli JD, Gross JJ, Remy KA, Ochsner KN. Age-Related Differences in Emotional Reactivity, Regulation, and Rejection Sensitivity in Adolescence. Emotion. 2012 doi: 10.1037/a0028297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville LH, Hare T, Casey BJ. Frontostriatal maturation predicts cognitive control failure to appetitive cues in adolescents. [Research Support, N.I.H., Extramural] J Cogn Neurosci. 2011;23(9):2123–2134. doi: 10.1162/jocn.2010.21572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville LH, Jones RM, Ruberry EJ, Dyke JP, Glover G, Casey BJ. Medial prefrontal cortex and the emergence of self-conscious emotion in adolesence. Psychological Science. 2013;24(8) doi: 10.1177/0956797613475633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vink M, Derks JM, Hoogendam JM, Hillegers M, Kahn RS. Functional differences in emotion processing during adolescence and early adulthood. Neuroimage. 2014;91:70–76. doi: 10.1016/j.neuroimage.2014.01.035. [DOI] [PubMed] [Google Scholar]
- Wang GJ, Volkow ND, Telang F, Jayne M, Ma Y, Pradhan K, Fowler JS. Evidence of gender differences in the ability to inhibit brain activation elicited by food stimulation. [Clinical Trial Research Support, N.I.H., Extramural Research Support, U.S. Gov’t, Non-P.H.S.] Proc Natl Acad Sci U S A. 2009;106(4):1249–1254. doi: 10.1073/pnas.0807423106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.