Abstract
Objective
Concerns persist that deep brain stimulation (DBS) for Parkinson’s disease (PD) increases impulsivity and/or induces excessive reward-seeking. We report here the performance of PD patients with implanted subthalamic nucleus electrodes, with stimulation on and off, on three laboratory tasks of risk-taking and decision-making. They are compared to PD patients maintained on medication and normal control subjects.
Methods and Results
In the Game of Dice Task, a test of “risky” decision-making, PD patients with or without DBS made highest-risk bets more often, and ended up with less money, than normal controls. There was a trend for DBS stimulation to ameliorate this effect.
Deal or No-Deal is an “ambiguous” decision-making task that assessed preference for risk (holding on to one’s briefcase) over a “sure thing” (accepting the banker’s offer). Here, DBS patients were more conservative with stimulation on than off. They accepted smaller offers from the banker and won less money in the DBS-on condition. Overall, the two PD groups won less money than healthy participants.
The Framing Paradigm assessed willingness to gamble on a fixed (unambiguous) prize depending on whether the reward was “framed” as a loss or a gain. Nonsurgical PD patients tended to be more risk-averse than normal subjects, whereas DBS patients were more willing to gamble for gains as well as losses both on and off stimulation.
Conclusions
On “risky” decision-making tasks, DBS patients were more risk-taking than normal, but stimulation may temper this tendency. In contrast, in an “ambiguous risk” situation, DBS patients were more risk-averse (conservative) than normal, and this tendency was greatest with stimulation.
Keywords: decision-making, risk-taking, deep brain stimulation, Parkinson’s disease
Introduction
Patients with Parkinson’s disease (PD) sometimes develop abnormalities of primary or secondary (i.e., conditioned) drives and engage compulsively in problematic pleasure-seeking behaviors (Evans, Strafella, Weintraub, & Stacy, 2009; McKeon et al., 2007; Voon, Hassan, Zurowski, de Souza, et al., 2006). For example, maladaptive gambling, excessive shopping/spending, reckless driving and hypersexuality are all more prevalent in PD patients than in the general population (Avanzi et al., 2008; Cooper et al., 2009; O'Sullivan & Lees, 2007; Voon, Hassan, Zurowski, Duff-Canning, et al., 2006).
It has been proposed that these pathological, driven behaviors are largely medication-related (Voon, Gao, et al., 2011). The levodopa used to treat PD lessens cognitive inflexibility by boosting dopamine levels in depleted dorsolateral prefrontal cortex, but may increase impulsivity by “overdosing” the relatively intact ventral frontal areas (Cools, Barker, Sahakian, & Robbins, 2003). PD patients taking dopamine agonist medications appear to be particularly prone to impulsivity and risk-taking (Antonini et al., 2011; Garcia-Ruiz et al., 2014; McKeon et al., 2007; Voon, Gao, et al., 2011). These phenomena are especially vivid in the dopamine dysregulation syndrome, a medication overuse disorder (Avanzi et al., 2008; O'Sullivan, Evans, & Lees, 2009; Solla, Cannas, Corona, Marrosu, & Marrosu, 2013).
Although these pleasure-seeking behaviors often are conceptualized as symptomatic of an impulse control disorder (Callesen, Scheel-Kruger, Kringelbach, & Moller, 2013; Ceravolo, Frosini, Rossi, & Bonuccelli, 2009; Garcia-Ruiz et al., 2014; Pontone, Williams, Bassett, & Marsh, 2006; Voon, Sohr, et al., 2011; Weintraub et al., 2010), they may also be seen as manifestations of altered risk-reward appraisal and decision-making, and hence a fundamentally cognitive disorder. The burgeoning fields of decisional neuroscience and neuroeconomics (Glimcher, 2008; Loewenstein, Rick, & Cohen, 2008; Sanfey, Loewenstein, McClure, & Cohen, 2006) have provided new models and experimental paradigms for studying the neurological basis of decision-making and choice behavior. Procedures such as the Iowa Gambling Test (IGT) (Bechara, Damasio, Tranel, & Damasio, 1997, 2005) and the Game of Dice Task (GDT) (Brand, Labudda, & Markowitsch, 2006) allow the assessment of financial decision-making in the laboratory setting, during functional brain imaging, etc. Several studies show that PD patients perform more poorly than neurologically normal subjects on both the IGT (Gescheidt et al., 2012; Mapelli, Di Rosa, Cavalletti, Schiff, & Tamburin, 2014; Mimura, Oeda, & Kawamura, 2006; Pagonabarraga et al., 2007) and the GDT (Brand et al., 2004; Labudda et al., 2010). However, other research has found dissociations between these tasks. These studies reveal that PD patients make significantly poorer decisions in situations in which probabilities of success are knowable (i.e., “risky” decision-making, such as in the GDT), but are only marginally impaired, or frankly normal, in situations in which the odds of winning and the risk/reward ratio are unknown (i.e., “ambiguous risk” decision-making, such as in the IGT) (Euteneuer et al., 2009; Poletti et al., 2010; Thiel et al., 2003). This may suggest a failure or inability to perform the mental calculus that determines the likelihood of success, or an overriding of the outcome of that calculus, rather than insensitivity to the risk-reward payoff matrix.
High-frequency deep brain stimulation (DBS) of the subthalamic nucleus (STN) is an effective treatment for PD in cases where motor fluctuations or medication-induced dyskinesias are disabling (The Deep-Brain Stimulation for Parkinson's Disease Study Group, 2001). Although DBS often permits the reduction of dopamine-enhancing medications, concern has been raised that it too may contribute to impulsivity and faulty decision-making (Broen, Duits, Visser-Vandewalle, Temel, & Winogrodzka, 2011; Lim et al., 2009; Smeding et al., 2007). In fact, patients with DBS have been shown to produce faster responses with stimulation turned on than when off (Plessow, Fischer, Volkmann, & Schubert, 2014; Wylie, Stout, & Bashore, 2005), particularly when making decisions under high-conflict conditions (Coulthard et al., 2012; Frank, Samanta, Moustafa, & Sherman, 2007a). They also perform better on tests of processing speed, working memory, and conceptualization with their DBS stimulators turned on, but have difficulty inhibiting responses and show poorer conditional associative learning (Jahanshahi et al., 2000; Swann et al., 2011). A meta-analysis of studies describing the cognitive outcome of STN DBS revealed slight impairment in verbal learning and memory and executive function (Parsons, Rogers, Braaten, Woods, & Troster, 2006). Another prospective randomized trial found that DBS produced only a small impairment in verbal list generation (fluency) (Witt et al., 2008). Thus, STN DBS appears to produce improvements in motor symptoms and selected cognitive processes, but may interfere with inhibitory control and effective decision-making.
The primary goal of the present study was to determine whether DBS of the STN is associated with faulty decision-making due to increased risk-taking among patients with PD. To this end, patients receiving DBS for at least six months were compared on several gambling-type laboratory tasks with their stimulators turned on and with them turned off. Of secondary interest was whether patients with chronic DBS stimulation differ from clinically similar PD patients maintained on medication and from neurologically healthy subjects on these same risk-taking tasks. Based on previous studies, the primary hypotheses were that stimulation of the STN would: 1) increase the subjective reward value of incentives and thereby make high-risk bets more attractive in situations with readily-knowable risks, but 2) not lead to increased risk-taking or reward-seeking in more ambiguous conditions.
Methods
Participants
Three groups of participants were studied: 1) 15 PD patients treated with bilateral STN DBS, 2) 15 PD patients treated with medication only (i.e., no surgical intervention), and 3) 15 neurologically healthy persons. The three groups were constructed to be similar in mean age and education, as well as sex distribution. Potential participants with cognitive impairment (see below) or a reported history of pathological gambling were excluded.
Patients with idiopathic PD meeting U.K. Brain Bank criteria (Hughes, Daniel, Kilford, & Lees, 1992) were recruited from the Movement Disorders Clinic at Johns Hopkins Hospital and from the practices of community neurologists specializing in movement disorders. All were in Hoehn & Yahr stage 3 or less in their “on” state (i.e., at levodopa medication peak or with DBS on). All but four patients were treated with dopamine replacement medication (levodopa/carbidopa). Patients were excluded if they had had undergone changes in their DBS configuration or addition/elimination of anti-parkinsonian medications in the previous three months. All PD patients were required to be accompanied by a companion, who provided transportation and completed informant questionnaires.
All PD patients with DBS were studied at least six months after implantation and programming of bilateral DBS electrodes. Surgical targeting was accomplished using both brain anatomy (MR imaging) and electrophysiology (microelectrode recording). Electrode settings and stimulation parameters (see Table 1) were chosen solely by motor response. DBS patients were excluded if, in the clinical judgment of the lead study neurologist (Z.M.), they would not be able to tolerate the “off” stimulation state.
Table 1.
DBS programming configuration for patients receiving deep brain stimulation.
| Subject # | Polarity Left STN |
Polarity Right STN |
Pulse Width (ms) Left STN |
Pulse Width (ms) Right STN |
Frequency (Hz) Left STN |
Frequency (Hz) Right STN |
Voltage Left STN |
Voltage Right STN |
|---|---|---|---|---|---|---|---|---|
| 1 | 3−/2+ | 7−/6+, | 90 | 90 | 185 | 185 | 3.9 | 4 |
| 2 | 2−/3+ | 10−/11+ | 90 | 90 | 180 | 180 | 2.9 | 2.36 |
| 3 | 2−/C+ | 9−/C+ | 90 | 90 | 180 | 180 | 3.33 | 1.17 |
| 4 | 1+/0− | 6+/5− | 90 | 120 | 185 | 185 | 3.1 | 3 |
| 5 | 3+, 2−, 1− | 7+/6− | 120 | 180 | 185 | 185 | 5.3 | 2 |
| 6 | C+/1− | C+/9− | 90 | 90 | 185 | 185 | 2.8 | 3.5 |
| 7 | 6+/7− | 2−/3+ | 90 | 90 | 185 | 185 | 3.2 | 2.5 |
| 8 | 3−/C+ | 7−/C+ | 90 | 90 | 185 | 185 | 4.4 | 4.4 |
| 9 | 2−/C+ | 10−/C+ | 90 | 90 | 180 | 180 | 2.42 | 2.12 |
| 10 | 0−/1+ | 11−/C+ | 90 | 90 | 180 | 180 | 4.17 | 3.34 |
| 11 | 2+/3− | 3−/C+ | 60 | 60 | 145 | 135 | 3.5 | 3 |
| 12 | 1+/2−/3− | 6−/7− | 180 | 90 | 185 | 185 | 4.8 | 3.9 |
| 13 | 3−/C+ | 11−/C+ | 90 | 90 | 170 | 170 | 2 | 2 |
| 14 | 2−1+ | 9−C+ | 90 | 90 | 180 | 180 | 3.5 | 2 |
| 15 | 0−1−2+ | 8−9−10+ | 90 | 90 | 185 | 185 | 2.5 | 3.7 |
PD patients maintained on medication (“PD-med”) were selected based on the presence of motor fluctuations that caused at least some disability. Some were interested in surgical options for their PD, but had not yet exhausted medication options.
Cognitively and neurologically normal persons, matched to the patient groups for age, sex, and education, were recruited from among the relatives of patients and from the general public. A brief screening interview was conducted by telephone at the time of enrollment to exclude those with neurologic disorders, major mental illnesses, cognitive impairment, or problematic gambling.
Procedures
Patients recruited at the Movement Disorders Clinic at the Johns Hopkins Hospital completed the Montreal Cognitive Assessment (MoCA) (Nasreddine et al., 2005) during their screening visit and were excluded from the study if they scored 21 or lower. Patients recruited from other clinics were screened with the Telephone Interview for Cognitive Status (TICS) (Brandt & Folstein, 2003), with delayed recall of the 10-item list (Lee, Kawachi, Berkman, & Grodstein, 2003). Those who scored 31 or lower were excluded.
Normal control subjects and PD-med patients participated in one experimental session lasting 1–2 hours. Patients with DBS took part in two sessions, approximately one month apart. In one session, their DBS stimulators remained active (“DBS-on”). In the other, their DBS stimulators were de-activated by a study neurologist 15 minutes prior to the start of the neurological exam and cognitive testing, and kept off for the duration of the session (“DBS-off”). [Although the motor effects of DBS are virtually immediate (Lopiano et al., 2002), the time course for the cognitive effect is unknown. The “wash out” period we chose – 15 minutes – is generally consistent with other studies in the literature (Castrioto et al., 2014; Frank, Samanta, Moustafa, & Sherman, 2007b; Pillon et al., 2000).] The study neurologist remained in the laboratory or was available within 5 minutes (via pager or cell phone) to re-activate patients’ DBS if they requested. No such requests were made; all patients tolerated the “off” state well. The order of conditions (DBS-on vs. DBS-off) was haphazard.
No medications were altered for this study. All patients remained on their usual antiparkinsonian medications, which were recorded. Persons in the DBS group were taking the same PD medications, at the same dosages, at their two study visits.
Score on Part III (motor scale) of the Unified Parkinson’s Disease Rating Scale (UPDRS) (Goetz et al., 2007; Movement Disorder Society Task Force on Rating Scales for Parkinson's, 2003) during the “on” state from the clinic visit closest in time to the research session was excerpted from each patient’s medical record as a measure of disease severity. In addition, most of the DBS patients had the UPDRS motor scale rated at the outset of each of their research sessions, with their stimulators on and after being off for 15 minutes.
The following cognitive tests and rating scales were then administered to characterize the participants:
Hopkins Adult Reading Test (HART) (Schretlen et al., 2009): This test of single-word reading provides an estimate of premorbid intellect.
Montreal Cognitive Assessment (MoCA) (Nasreddine et al., 2005): This is a brief global cognitive screening test. To be included in the study, each participant had to achieve a score of 22 or higher.
Geriatric Depression Scale, 15-item version (GDS-15) (Sheikh & Yesavage, 1986): This brief self-report questionnaire screened for depressive symptoms.
Questionnaire for Impulsive/Compulsive Disorders in Parkinson’s Disease–short form (QUIP) (Papay et al., 2011): All PD patients and neurologically normal control subjects completed the self-report version of this questionnaire, which assesses the presence of impulsive and compulsive behaviors.
Experimental Tasks
During each testing session, participants completed a number of experimental cognitive tasks, three of which are reported here. All three tasks assess risk-taking, but they vary in the degree to which subjects can compute the likelihood of success and the magnitude of the reward.
Game of Dice Task (GDT) (Brand et al., 2004; Brand et al., 2006)
This task has been used previously to study risky decision-making; i.e., when the odds of success are known, or at least knowable (Brand et al., 2005; Brand & Schiebener, 2013; Delazer, Sinz, Zamarian, & Benke, 2007). Participants were allotted $20 of virtual money and told that they could win or lose money by rolling a single electronic die. On each of 18 trials, they bet on which sides would come up. They could choose to bet on 1, 2, 3, or 4 of the 6 sides. If they bet on a single side and it came up (1/6, or 7% probability), they won $10; if it didn’t come up, they lost $10. Choosing 2 sides (2/6, or 33% probability) was associated with a $5 win or loss; 3 sides (3/6, or 50% probability), a $2 win or loss; and 4 sides (4/6, or 67% probability), a $1 win or loss. Thus, participants could easily discern that choosing 1 side was the most risky and disadvantageous choice in the long run, and choosing 4 sides was the most conservative and advantageous choice. The frequency of each choice and the total amount of money accumulated at the end of 18 trials were the variables of interest.
Deal or No-Deal (DND) (De Roos, 2010; Post, van den Assem, Baltussen, & Thaler, 2008)
This task is a computer version of the popular television game show (Take-Two-Interactive-Software, 2009). Participants attempted to win a monetary prize that ranged from $.01 to $1,000,000 inside a closed briefcase. The amounts were positively skewed; the mean prize was $131,478. Initially, the participant selected one of 26 briefcases, which became his/her case. It was removed from play and remained unopened until the end of the game. Thus, the “baseline value” of the participant’s briefcase was $131,478, although this was never made explicit. In each of nine rounds, the participant opened a specified number of the remaining cases (first 6, then 5, 4, 3, 2, 1, 1, 1, and 1), revealing and eliminating from play the prize inside. Thus, the number of prizes remaining in play decreased in each round (20, 15, 11, 8, 6, 5, 4, 3, and 2). After each round, a “banker” offered to buy the participant’s briefcase. The banker’s offers followed a set of rules that are unknown to the participant. Each successive offer was an increasing percentage of the mean value of the remaining prizes (Wolstenholme, 2006). This rule is never made explicit to the players but is inherent in the offers made. On each round, the participant decided whether to accept the banker’s offer (“Deal”) or to continue to the next round of opening briefcases (“No Deal”). If the participant rejected all nine offers, he/she received the prize in his/her own briefcase. The variables of interest in DND are: 1) the round on which the subject accepts an offer (“deals”), which assesses risk propensity versus risk aversion, 2) the largest monetary offer rejected, and 3) the amount ultimately won [i.e., offer accepted or amount in initial suitcase (if subject never deals)].
The probability of success in DND (i.e., winning a large prize) is largely a matter of luck rather than knowledge, skill or judgment. However, the participant’s choice on each round to accept or reject the banker’s offer is based on his/her subjective probability of success and reward value of the offer. It is unlikely, especially in early rounds, that the participant actually calculates the aggregate expected values for all of the remaining cases. In addition, a risk-averse participant may “take the money and run” on an early round, while a risk-inclined participant may continue to gamble in the hopes of a larger payoff. Finally, participants are not informed of the banker’s decision rules regarding the offers made. Thus, this task assesses decision-making in a relatively ambiguous situation.
Framing Paradigm (Kahneman & Tversky, 1984; Tversky & Kahneman, 1986)
This was the last experimental task in the research session. Performance on it determined the amount of actual money subjects received for their participation in the research session. Subjects were presented with one of two scenarios, randomly selected. (Patients with DBS received alternative scenarios in each of their two experimental sessions). In scenario A, they were paid $50 in cash for participating in the study, and then offered the choice of accepting a $25 bonus or taking a 50% chance, by toss of a coin, for a $50 bonus (versus no bonus). Under these conditions, most people opt for the sure-thing (the $25 bonus). In scenario B, subjects were paid $100 in cash, and then had to choose between returning $25 or taking a 50% chance on having to return $50 (versus returning nothing). Under these conditions, most people take the gamble. Note that the marginal value of all four alternatives is precisely $75, but that alternative “frames” (as gains or losses) lead to predictably different choices. This task is similar to the GDT in that the probability of success and the consequences of choices are clearly articulated by the researcher. Thus, it is close to a pure test of “risky” decision-making.
The study protocol was fully reviewed and approved by the Johns Hopkins Medicine Institutional Review Board. The risks and benefits of participation in the study were fully explained to participants, and written informed consent was obtained.
Data Analysis
Due to the small sample sizes and the non-normal distributions of most of the outcome variables, task data were subjected to nonparametric statistical analyses. The comparisons of primary interest were between the DBS-on and DBS-off conditions. These within-subjects differences were evaluated with the Wilcoxin sign-rank test. Of secondary interest were differences among three groups: PD patients with chronic STN stimulation, PD patients maintained on medication, and neurologically normal subjects. For these, the Kruskal-Wallis one-way analysis of variance was employed.
Results
The demographic and clinical characteristics of the three groups are shown in Table 2. The groups were well matched on age, education, and sex distribution. The DBS patients had slightly lower scores than the other groups on the HART (premorbid I.Q. estimate) and MoCA (general cognitive function) (p<.05). The DBS group and the PD-med group had generally equivalent symptom severity (UPDRS motor score, GDS-15 score, and QUIP score). Although the PD-med patients were more often taking dopamine agonist medications (p=.01), there was no difference between them and the DBS patients in levodopa equivalent daily doses (Tomlinson et al., 2010). Given the nonparametric analyses and the very few significant correlations between scores on these clinical scales and on the decision-making tasks (see below), no effort was made to “covary” or adjust for these minor differences among the groups.
Table 2.
Demographic and clinical characteristics of three groups of participants. Means (±SDs), except as noted. Group differences tested with analysis of variance (for means) and chi-squared tests (for frequencies).
| DBS Patients | PD-Med Patients | Normal Controls | p | ||
|---|---|---|---|---|---|
| # Men: # Women | 7:8 | 8:7 | 9:6 | .765 | |
| Age, years | 67.15 ± 6.28 | 64.78 ± 8.09 | 62.39 ± 10.04 | .299 | |
| Education, highest grade | 16.00 ± 2.73 | 16.27 ± 2.96 | 16.00 ± 2.20 | .951 | |
| MoCA, score | 25.67 ± 1.78 | 26.87 ± 1.69 | 27.60 ± 1.43 | .030 | |
| UPDRS Motor Scale | 18.57 ± 12.55 | 14.43 ± 10.14 | -- | .345 | |
| HART (premorbid IQ est.) | 108.93 ± 12.46 | 117.67 ± 6.04 | 117.14 ± 9.49 | .031 | |
| GDS-15 (depression score) | 1.87 ± 1.25 | 3.33 ± 3.54 | 1.33 ± 1.18 | .054 | |
| QUIP Self-Rating, total | 1.00 ±1.69 | 0.80 ±1.32 | 0.33 ±.488 | .346 | |
| (# endorsing) | Gambling | 2 | 1 | 0 | .343 |
| Sex | 2 | 4 | 2 | .544 | |
| Buying | 3 | 1 | 1 | .407 | |
| Eating | 2 | 2 | 0 | .334 | |
| Hobbying | 2 | 2 | 2 | 1.00 | |
| Punding | 2 | 2 | 0 | .334 | |
| Walking About | 1 | 0 | 0 | .360 | |
| Medication | 1 | 0 | 0 | .360 | |
| Medications, number taking: | PD with and without DBS only: | ||||
| Dopamine replacement (levodopa/carbidopa) | 14 | 12 | 0 | .283 | |
| Dopamine agonists (e,g., pramipexole, bromocriptine, ropinirole) | 5 | 12 | 0 | .010 | |
| COMT inhibitors (e.g., entacapone, tolcapone) | 1 | 1 | 0 | 1.00 | |
| MAOIs (e.g., selegiline, deprenyl, rasagiline) | 7 | 8 | 0 | .715 | |
| Other PD medications (e.g., amantadine) | 7 | 3 | 0 | .121 | |
| Other psychoactive medications | 8 | 6 | 1 | .464 | |
| Medications, levodopa equivalent dose (mg.): | 1,351.02 ± 868.61 | 1,262.98 ± 829.83 | -- | .776 | |
Deactivating patients’ DBS stimulators 15 minutes prior to the test session clearly affected their neurological state. Their mean UPDRS motor scale in the “on” state was 18.57 (SD=12.55), while in the “off” state it was 32.45 (SD=16.79) (Wilcoxin z=2.31, p=.02).
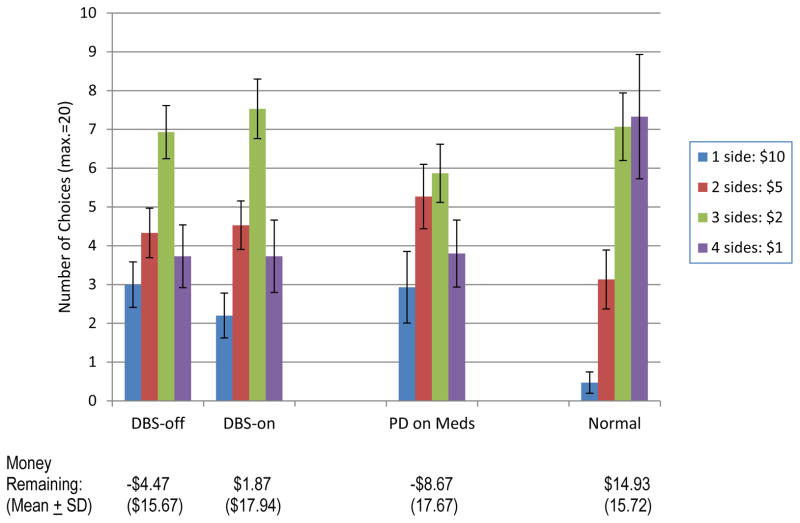
On the GDT, the probability of winning on each trial increases as a function of the number of sides of the die on which a participants bets. Results from the normal control group mirrored this pattern, such that these subjects chose to bet on four sides of the die (4/6 probability of winning) most often; they almost never chose the riskiest option of betting on a single side of the die (1/6 probability of winning) (see Figure 1). This was not the case among PD patients. They chose the riskiest option more often and the safest option less often. After 18 trials, the DBS patients tested with stimulation on retained an average of only $1.87 of their initial $20. When tested with stimulation off, they had lost all of their allocation and went into debt to the tune of −$4.47. The difference between the DBS-on and DBS-off performances did not reach statistical significance (Wilcoxin z=1.11, p=.27).
Figure 1.
Results from the Game of Dice Task. Means (± standard errors). Note that the frequency at which the normal control group chooses each option is proportional to the amount of risk; i.e., the riskiest option is chosen least often, followed by the second riskiest, and so on. This is not the case for patients with PD, with or without DBS. They chose the riskiest option 15–16% of the time, compared to only 2–3% of the time for the normal control subjects.
The three groups (DBS-on, PD-med, and normal) differed markedly in the number of 1-side choices (Kruskal-Wallis H=9.90, df=2, p=.007). After 18 trials, the normal subjects were left with an average of $14.93, while the PD-med patients lost all $20 and went into debt to the amount of −$8.67 and the DBS patients with stimulation on still had an average of +$1.87 (H=13.12, df=2, p=.001).
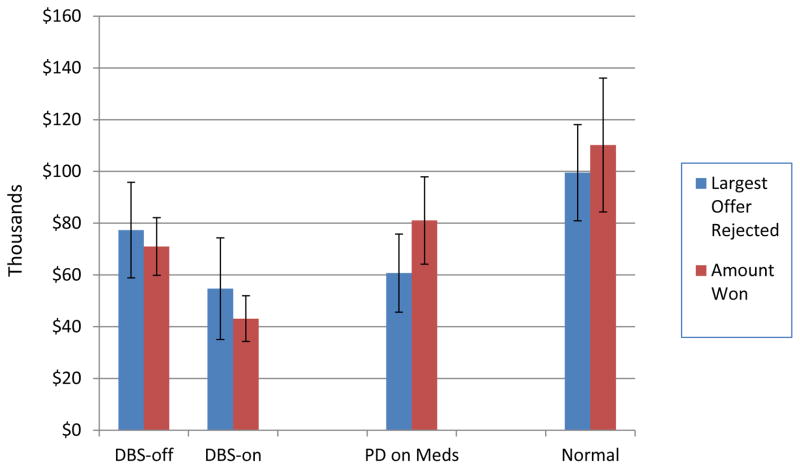
On DND, DBS patients won less money with the stimulation on than off (z=2.10, df=2, p=.04) (see Figure 2). They tended to “deal” on an earlier round (data not shown), and the largest offer they rejected was marginally smaller (z=1.80, p=.07). Thus, stimulation of the STN made patients more risk-averse on this task. However, such a “take the money and run” bias was associated with smaller winnings.
Figure 2.
Results from Deal or No-Deal. The three groups differed significantly in largest banker’s offer rejected (p=.042) and the amount won (p=.046). In addition, DBS patients settled for significantly smaller offers with their stimulators turned on than off (p=.036). Means (± standard errors).
The three groups differed in the maximum banker’s offer rejected (H=6.33, df=2, p=.04) and the final amount won (H=6.14, df=2, p=.05). The PD groups settled for lower offers than did the normal subjects. The two PD groups tended to “deal” on an earlier round than the normal control subjects, but the difference did not reach statistical significance (H=4.05, df=2, p=.13).
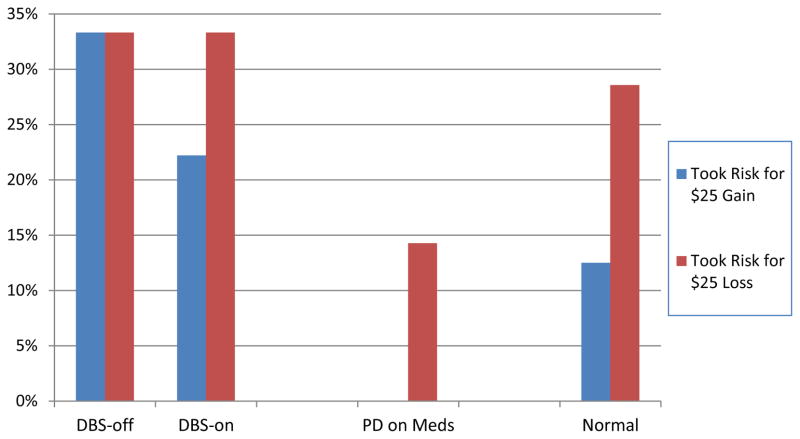
In the Framing Paradigm that determined subjects’ payments for participation in the study, participants were generally risk-averse. They preferred the sure gain or loss over the chance for a larger gain or loss. However, DBS patients appeared to take the most risks (see Figure 3). There was no significant difference between the decisions made by DBS patients depending on whether stimulation was on or off (log-linear G2=1.62, df=4, p=.81).
Figure 3.
Results from the Framing Paradigm. Percentage of participants in each group and condition choosing to gamble on their remuneration for the study session as opposed to accepting a sure payment of $75.
Among the three groups, the medically managed PD patients were the most risk-averse; they rarely opted for the gamble. Due to the very small number of observations in each group-by-scenario condition, frequency of risk-taking did not differ among the four conditions (χ2=3.43, df=3, p=0.33).
The relationship of each of the decision-making outcome variables with scores on the five clinical scales (UPDRS Motor Scale, HART, MoCA, QUIP, and GDS-15) was examined in the pooled sample of 45 participants (30 for UPDRS). There were only two statistically significant correlations: between MoCA score and number of 1-side bets in the GDT (Spearman ρ = −0.45, df=42, p=.003) and between MoCA score and final accumulation in the GDT (Spearman ρ = 0.33, df=42, p=.03). Among these same participants (with stimulation “on” for the DBS group), there were few significant correlations among the decision-making tasks: Opting to take the risk for a gain in the Framing Paradigm was positively correlated with the DND round on which subjects dealt (Spearman ρ = 0.43, df=25, p=.03) and negatively correlated with amount they won on this task (Spearman ρ = −0.40, df=25, p=.05). In addition, the largest banker’s offer rejected in DND was inversely related to the number of riskiest (1-side) bets in the GDT (Spearman ρ = −0.30, df=44, p=.05).
Discussion
Human decision-making engages multiple cognitive processes. “Cold” calculations (e.g., probabilities of success) are supplemented by a large number of “warm” (social/emotional) factors, such as prior history, superstitions, heuristics, and desires (Tversky & Kahneman, 1986). Prior research has revealed dissociations between patients’ performance on tasks with explicitly-defined risks and rewards (i.e., “risky” situations) and ones in which these parameters are not readily known (i.e., “ambiguous” situations) (Euteneuer et al., 2009). The present study extends upon previous research by examining PD patients receiving DBS with decision-making tasks that varied in their ambiguity.
In the GDT, the probabilities and payoffs are readily calculable or estimable, and the consequences of success or failure of each roll of the die are explicit. It has been argued that the dorsal corticostriatothalamic loop that mediates higher-order cognition is principally engaged during this task (Brand et al., 2006; Euteneuer et al., 2009). We found that, on average, patients with PD placed the lowest-probability, highest-reward bet five times more often than neurologically normal participants. PD patients were half as likely to place the only bet that was likely to win (albeit with the smallest payout), and they finished the game with significantly less money. Our finding, in both PD groups, confirms several previous studies using variations of this task (Brand et al., 2004; Euteneuer et al., 2009). PD patients have been shown consistently to make riskier bets and achieve worse outcomes on tasks with explicit parameters, like the GDT (Cools et al., 2003). This could be due to impairment in the ability to perform the reward-size by probability-of-success calculation, or relative disregard of probabilities because of the overriding attraction of the highest reward. The significant negative correlation between score on the MoCA and number of 1-side bets on the GDT suggests that the former of these potential explanations may be more important than the latter. In any event, there is no indication that stimulation of the STN caused patients to make riskier bets. If anything, patients tended to make fewer high-risk bets and lose less money when their DBS was on than when it was off.
Success in DND requires a fair amount of luck (i.e., chance working in your favor) in selecting as one’s own a briefcase containing a large prize and opening (thereby eliminating from play) briefcases containing small prizes on each round. Compared to the GDT, the risks here are more ambiguous. Like the IGT, DND requires decision-making under uncertainty. It is impossible to calculate the likely outcome of each round, or to discern how the banker’s offer will change as the rounds progress. The only decisions to be made are whether to accept the banker’s offers. Presumably, this is done by estimating the value of one’s briefcase based on the magnitude of the prizes remaining on the game board. The participants are never told that the banker’s offer, as a percentage of remaining prizes, increases over time, but is never as high as the expected value. As a result, it is always rational to continue (“no deal”). However, it is also risky, since eliminating a briefcase with a large prize leads to a substantial decline in the next offer. On this task, we observed a tendency for PD patients to “deal” on an earlier round and win smaller prizes, which suggests a form of “myopic risk aversion” (Thaler, Tversky, Kahneman, & Schwartz, 1997). This morbid conservatism was more pronounced when the STN was being stimulated, resulting in DBS patients winning less money with the stimulator on than off. Thus, stimulation of the STN nominally lessened the risk-taking and reward-maximizing behavior of PD patients on both the GDT (which improved their financial outcome) and DND (which worsened their financial outcome).
Results from the Framing Task were less definitive, due in part to the small number of observations, but may be particularly important. On this task, participants are given the opportunity to take a risk with a clear probability of success (50%) for a certain gain ($25 more than the “sure thing” option). Thus, it is a test of risky decision-making, similar to the GDT. Patients with DBS electrodes implanted were somewhat more likely to accept gambles than PD-med controls, whether stimulation was on or off. Although the differences in choices among groups and conditions were not statistically significant, the performance of PD patients on this task is clearly in the direction opposite that on the GDT. On the GDT, PD-med subjects made riskier bets and this tendency was ameliorated somewhat by DBS. On the Framing Paradigm, PD-med patients were least likely to choose to gamble, and more patients with DBS opted to gamble, regardless of whether the rewards were framed as gains or losses.
Virtually all decisions represent some amount of risk, and neurologically normal people often make decisions that are statistically sub-optimal. After all, most people would agree that if one were offered a prize of $499,000, it would be unwise to trade it for a 50% chance at $1,000,000. Our findings indicate that PD patients made decisions that were both different from the norm and led to worse outcomes (smaller prizes). Stimulation of the STN appears to exacerbate the tendency of PD patients to accept a relatively smaller offer on the ambiguous-risk DND and slightly ameliorated their maladaptive risk-taking on the explicit-risk GDT. However, chronic stimulation increased risk-taking proclivity (albeit not significantly) on the Framing Paradigm, another explicit risk task. These findings cause us to rethink the simple dichotomy of decision-making tasks as “risky” or “ambiguous.”
Several limitations of this study should be considered. First, although our sample sizes were comparable to those in many other studies, they were small. Thus, we are underpowered statistically to observe all but the largest differences among groups or between stimulation conditions. Second, one could argue that there is a certain selection bias inherent in group membership. PD patients who are receiving treatment with DBS have opted to undergo brain surgery rather than continued medical therapy. In selecting a newer and more invasive treatment, they have displayed a willingness to engage in risk-taking. This makes the within-group comparisons of the DBS patients (i.e., with their stimulators on and off) particularly informative of the effects of STN stimulation. A related limitation is the absence of a pre-operative assessment of the DBS patients. This was dictated by logistical considerations, and we acknowledge that having a separate non-DBS PD control group is less than ideal. Fourth, almost all of the patients were taking dopamine replacement medications, which was necessary to provide appropriate medical care. These medications are thought to blunt patients’ responses to negative outcomes (Frank et al., 2007a). Thus, differences noted between the PD groups and the neurologically normal group may be due to PD, dopamine replacement, or a combination of both. However, attempting to equate the DBS and PD-med groups for l-dopa dosage or bioavailability would probably result in clinically atypical groups. Fifth, more patients in the PD-med group were taking dopamine agonist medications than those in the DBS group. However, there were no significant differences between the PD-med group and the DBS patients in the off condition, which suggests (but does not prove) that differential use of agonist medications does not account for our findings. Sixth, although the DBS patients were on the same medication regimen at their two study visits, we have no way of knowing whether they were tested at the same point in their dopamine response curves. Therefore, we cannot rule out the possibility that some of the difference between DBS-on and DBS-off conditions was due to medications. Finally, discontinuing STN stimulation for 15 minutes may not be sufficient to eliminate the cognitive effects of chronic stimulation. It is recognized that DBS has both phasic (transient) and tonic (enduring) effects on the motor system, and it is indeed possible that there are tonic effects on cognition that persist long after stimulation is discontinued.
Like our PD patients, animals with lesions of the STN also appear to display selective alterations in behaviors related to impulsivity and decision-making. Rats with STN lesions display decreased “impulsive choice” (in a delayed discounting paradigm), but increased “impulsive action” (behavioral disinhibition) (Baunez et al., 2001; Phillips & Brown, 1999; Uslaner & Robinson, 2006; Winstanley, Baunez, Theobald, & Robbins, 2005). Future studies might borrow or adapt testing paradigms used in these elegant animal studies to the assessment of the effects of STN DBS in patients.
The precise neural networks underpinning various decision tasks remain to be elucidated. Excessive dopamine release in the ventral striatum has been theorized to lead to subjectively greater reward value of stimuli (Housden, O'Sullivan, Joyce, Lees, & Roiser, 2010). Activity in the ventral striatum also has been associated with decreased reaction times (Schroeder et al., 2002). Separate neural pathways have been proffered for decisions that are made in “risky” situations (i.e., dorsolateral prefrontal-dorsal striatal loop) and “ambiguous” ones (i.e., limbic-orbitofrontal-ventral striatal loop) (Euteneuer et al., 2009). On our tasks, patients with PD performed aberrantly on both risky and ambiguous tasks, which may indicate dysfunction in both the dorsal and ventral loops. The STN has been described as delaying responses in order to allow for further signal integration. DBS has been described as “releasing the brake” of the STN, improving some cognitive functions and leading to faster responses, particularly in high-conflict situations (Ballanger et al., 2009; Frank et al., 2007a). Our findings on the DND task may suggest that patients with DBS stimulation made the choice to “deal” in response to smaller offers in order to terminate their decisional conflict. Further research with these and related tasks would be valuable in elucidating the contributions of various frontal cortical and basal ganglia structures in decision-making, and in identifying how patients with disorders and treatments affecting those structures might best be supported.
Acknowledgments
The authors thank Stephen Reich, M.D., Steven Grill, M.D., Joseph Savitt, M.D., Shawn Smyth, M.D., Liana Rosenthal, M.D., Susan R. Dunlop, R.N. for referring patients to this study, and Matthew Cohen, Ph.D. for helpful comments on the manuscript. E. Ray Dorsey, M.D., the Johns Hopkins Institute for Clinical and Translational Research (grant # UL1 RR025005 from the NIH National Center for Research Resources), and the Saudi Arabian Cultural Mission provided financial support of this research.
Literature Cited
- Antonini A, Siri C, Santangelo G, Cilia R, Poletti M, Canesi M, Barone P. Impulsivity and compulsivity in drug-naive patients with Parkinson's disease. Mov Disord. 2011;26(3):464–468. doi: 10.1002/mds.23501. [DOI] [PubMed] [Google Scholar]
- Avanzi M, Baratti M, Cabrini S, Uber E, Brighetti G, Bonfa F. The thrill of reckless driving in patients with Parkinson's disease: an additional behavioural phenomenon in dopamine dysregulation syndrome? Parkinsonism Relat Disord. 2008;14(3):257–258. doi: 10.1016/j.parkreldis.2007.04.006. [DOI] [PubMed] [Google Scholar]
- Ballanger B, van Eimeren T, Moro E, Lozano AM, Hamani C, Boulinguez P, Strafella AP. Stimulation of the subthalamic nucleus and impulsivity: release your horses. Annals of Neurology. 2009;66:817–824. doi: 10.1002/ana.21795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baunez C, Humby T, Eagle DM, Ryan LJ, Dunnett SB, Robbins TW. Effects of STN lesions on simple vs choice reaction time tasks in the rat: preserved motor readiness, but impaired response selection. Eur J Neurosci. 2001;13(8):1609–1616. doi: 10.1046/j.0953-816x.2001.01521.x. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Tranel D, Damasio AR. Deciding advantageously before knowing the advantageous strategy. Science. 1997;275(5304):1293–1295. doi: 10.1126/science.275.5304.1293. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Tranel D, Damasio AR. The Iowa Gambling Task and the somatic marker hypothesis: some questions and answers. Trends Cogn Sci. 2005;9(4):159–162. doi: 10.1016/j.tics.2005.02.002. discussion 162–154. [DOI] [PubMed] [Google Scholar]
- Brand M, Fujiwara E, Borsutzky S, Kalbe E, Kessler J, Markowitsch HJ. Decision-making deficits of korsakoff patients in a new gambling task with explicit rules: associations with executive functions. Neuropsychology. 2005;19(3):267–277. doi: 10.1037/0894-4105.19.3.267. [DOI] [PubMed] [Google Scholar]
- Brand M, Labudda K, Kalbe E, Hilker R, Emmans D, Fuchs G, Markowitsch HJ. Decision-making impairments in patients with Parkinson's disease. Behavioral Neurology. 2004;15(3–4):77–85. doi: 10.1155/2004/578354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand M, Labudda K, Markowitsch HJ. Neuropsychological correlates of decision-making in ambiguous and risky situations. Neural Netw. 2006;19(8):1266–1276. doi: 10.1016/j.neunet.2006.03.001. [DOI] [PubMed] [Google Scholar]
- Brand M, Schiebener J. Interactions of age and cognitive functions in predicting decision making under risky conditions over the life span. J Clin Exp Neuropsychol. 2013;35(1):9–23. doi: 10.1080/13803395.2012.740000. [DOI] [PubMed] [Google Scholar]
- Brandt J, Folstein FM. Telephone Interview for Cognitive Status: Professional Manual. Lutz, FL: Psychological Assessment Resources, Inc; 2003. [Google Scholar]
- Broen M, Duits A, Visser-Vandewalle V, Temel Y, Winogrodzka A. Impulse control and related disorders in Parkinson's disease patients treated with bilateral subthalamic nucleus stimulation: a review. Parkinsonism Relat Disord. 2011;17(6):413–417. doi: 10.1016/j.parkreldis.2011.02.013. [DOI] [PubMed] [Google Scholar]
- Callesen MB, Scheel-Kruger J, Kringelbach ML, Moller A. A systematic review of impulse control disorders in Parkinson's disease. J Parkinsons Dis. 2013;3(2):105–138. doi: 10.3233/JPD-120165. [DOI] [PubMed] [Google Scholar]
- Castrioto A, Funkiewiez A, Debu B, Cools R, Lhommee E, Ardouin C, Krack P. Iowa gambling task impairment in Parkinson's disease can be normalised by reduction of dopaminergic medication after subthalamic stimulation. J Neurol Neurosurg Psychiatry. 2014 doi: 10.1136/jnnp-2013-307146. [DOI] [PubMed] [Google Scholar]
- Ceravolo R, Frosini D, Rossi C, Bonuccelli U. Impulse control disorders in Parkinson's disease: definition, epidemiology, risk factors, neurobiology and management. Parkinsonism Relat Disord. 2009;15(Suppl 4):S111–115. doi: 10.1016/S1353-8020(09)70847-8. [DOI] [PubMed] [Google Scholar]
- Cools R, Barker RA, Sahakian BJ, Robbins TW. L-Dopa medication remediates cognitive inflexibility, but increases impulsivity in patients with Parkinson's disease. Neuropsychologia. 2003;41(11):1431–1441. doi: 10.1016/s0028-3932(03)00117-9. [DOI] [PubMed] [Google Scholar]
- Cooper CA, Jadidian A, Paggi M, Romrell J, Okun MS, Rodriguez RL, Fernandez HH. Prevalence of hypersexual behavior in Parkinson's disease patients: Not restricted to males and dopamine agonist use. Int J Gen Med. 2009;2:57–61. doi: 10.2147/ijgm.s4674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulthard EJ, Bogacz R, Javed S, Mooney LK, Murphy G, Keeley S, Whone AL. Distinct roles of dopamine and subthalamic nucleus in learning and probabilistic decision making. Brain. 2012;135(Pt 12):3721–3734. doi: 10.1093/brain/aws273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Roos N, Sarafidis Y. Decision making under risk in Deal or No Deal. J Applied Econometrics. 2010;25:987–1027. [Google Scholar]
- Delazer M, Sinz H, Zamarian L, Benke T. Decision-making with explicit and stable rules in mild Alzheimer's disease. Neuropsychologia. 2007;45(8):1632–1641. doi: 10.1016/j.neuropsychologia.2007.01.006. [DOI] [PubMed] [Google Scholar]
- Euteneuer F, Schaefer F, Stuermer R, Boucsein W, Timmermann L, Barbe MT, Kalbe E. Dissociation of decision-making under ambiguity and decision-making under risk in patients with Parkinson's disease: a neuropsychological and psychophysiological study. Neuropsychologia. 2009;47(13):2882–2890. doi: 10.1016/j.neuropsychologia.2009.06.014. [DOI] [PubMed] [Google Scholar]
- Evans AH, Strafella AP, Weintraub D, Stacy M. Impulsive and compulsive behaviors in Parkinson's disease. Mov Disord. 2009;24(11):1561–1570. doi: 10.1002/mds.22505. [DOI] [PubMed] [Google Scholar]
- Frank MJ, Samanta J, Moustafa AA, Sherman SJ. Hold your horses: Impulsivity, deep brain stimulation, and medication in parkinsonism. Science. 2007a;318:1309–1312. doi: 10.1126/science.1146157. [DOI] [PubMed] [Google Scholar]
- Frank MJ, Samanta J, Moustafa AA, Sherman SJ. Hold your horses: impulsivity, deep brain stimulation, and medication in parkinsonism. Science. 2007b;318(5854):1309–1312. doi: 10.1126/science.1146157. [DOI] [PubMed] [Google Scholar]
- Garcia-Ruiz PJ, Martinez Castrillo JC, Alonso-Canovas A, Herranz Barcenas A, Vela L, Sanchez Alonso P, Mahillo Fernandez I. Impulse control disorder in patients with Parkinson's disease under dopamine agonist therapy: a multicentre study. J Neurol Neurosurg Psychiatry. 2014 doi: 10.1136/jnnp-2013-306787. [DOI] [PubMed] [Google Scholar]
- Gescheidt T, Czekoova K, Urbanek T, Marecek R, Mikl M, Kubikova R, Bares M. Iowa Gambling Task in patients with early-onset Parkinson's disease: strategy analysis. Neurol Sci. 2012;33(6):1329–1335. doi: 10.1007/s10072-012-1086-x. [DOI] [PubMed] [Google Scholar]
- Glimcher PW, Fehr E, Camerer C, Poldrack RA, editors. Neuroeconomics: Decision making and the brain. Amsterdam: Elsevier; 2008. [Google Scholar]
- Goetz CG, Fahn S, Martinez-Martin P, Poewe W, Sampaio C, Stebbins GT, LaPelle N. Movement Disorder Society-sponsored revision of the Unified Parkinson's Disease Rating Scale (MDS-UPDRS): Process, format, and clinimetric testing plan. Mov Disord. 2007;22(1):41–47. doi: 10.1002/mds.21198. [DOI] [PubMed] [Google Scholar]
- Housden CR, O'Sullivan SS, Joyce EM, Lees AJ, Roiser JP. Neuropsychopharmacology. 2010. Intact reward learning but elevated delay discounting in Parkinson's disease patients with impulsive-compulsive spectrum behaviors. Electronic publication ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson's disease: a clinico-pathological study of 100 cases. Journal of Neurology, Neurosurgery, and Psychiatry. 1992;55:181–184. doi: 10.1136/jnnp.55.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahanshahi M, Ardouin CMA, Brown RG, Rothwell JC, Obeso J, Albanese A, Limousin-Dowsey P. The impact of deep brain stimulation on executive function in Parkinson's disease. Brain. 2000;123:1142–1154. doi: 10.1093/brain/123.6.1142. [DOI] [PubMed] [Google Scholar]
- Kahneman D, Tversky A. Choices, values, and frames. American Psychologist. 1984;39(4):341–350. [Google Scholar]
- Labudda K, Brand M, Mertens M, Ollech I, Markowitsch HJ, Woermann FG. Decision making under risk condition in patients with Parkinson's disease: a behavioural and fMRI study. Behav Neurol. 2010;23(3):131–143. doi: 10.3233/BEN-2010-0277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Kawachi I, Berkman LF, Grodstein F. Education, other socioeconomic indicators, and cognitive function. Am J Epidemiol. 2003;157(8):712–720. doi: 10.1093/aje/kwg042. [DOI] [PubMed] [Google Scholar]
- Lim SY, O'Sullivan SS, Kotschet K, Gallagher DA, Lacey C, Lawrence AD, Evans AH. Dopamine dysregulation syndrome, impulse control disorders and punding after deep brain stimulation surgery for Parkinson's disease. J Clin Neurosci. 2009;16(9):1148–1152. doi: 10.1016/j.jocn.2008.12.010. [DOI] [PubMed] [Google Scholar]
- Loewenstein G, Rick S, Cohen JD. Neuroeconomics. Annual Review of Psychology. 2008;59:647–672. doi: 10.1146/annurev.psych.59.103006.093710. [DOI] [PubMed] [Google Scholar]
- Lopiano L, Rizzone M, Bergamasco B, Tavella A, Torre E, Perozzo P, Lanotte M. Deep brain stimulation of the subthalamic nucleus in PD: an analysis of the exclusion causes. J Neurol Sci. 2002;195(2):167–170. doi: 10.1016/s0022-510x(02)00008-4. [DOI] [PubMed] [Google Scholar]
- Mapelli D, Di Rosa E, Cavalletti M, Schiff S, Tamburin S. Decision and dopaminergic system: an ERPs study of Iowa gambling task in Parkinson's disease. Front Psychol. 2014;5:684. doi: 10.3389/fpsyg.2014.00684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeon A, Josephs KA, Klos KJ, Hecksel K, Bower JH, Michael Bostwick J, Eric Ahlskog J. Unusual compulsive behaviors primarily related to dopamine agonist therapy in Parkinson's disease and multiple system atrophy. Parkinsonism Relat Disord. 2007;13(8):516–519. doi: 10.1016/j.parkreldis.2007.04.004. [DOI] [PubMed] [Google Scholar]
- Mimura M, Oeda R, Kawamura M. Impaired decision-making in Parkinson's disease. Parkinsonism and Related Disorders. 2006;12:169–175. doi: 10.1016/j.parkreldis.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Movement Disorder Society Task Force on Rating Scales for Parkinson's D. The Unified Parkinson's Disease Rating Scale (UPDRS): status and recommendations. Mov Disord. 2003;18(7):738–750. doi: 10.1002/mds.10473. [DOI] [PubMed] [Google Scholar]
- Nasreddine ZS, Phillips NA, Bedirian V, Charbonneau S, Whitehead V, Collin I, Chertkow H. The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. Journal of the American Geriatrics Society. 2005;53:695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- O'Sullivan SS, Evans AH, Lees AJ. Dopamine dysregulation syndrome: an overview of its epidemiology, mechanisms and management. CNS Drugs. 2009;23(2):157–170. doi: 10.2165/00023210-200923020-00005. [DOI] [PubMed] [Google Scholar]
- O'Sullivan SS, Lees AJ. Pathological gambling in Parkinson's disease. Lancet Neurol. 2007;6(5):384–386. doi: 10.1016/S1474-4422(07)70089-3. [DOI] [PubMed] [Google Scholar]
- Pagonabarraga J, Garcia-Sanchez C, Llebaria G, Pascual-Sedano B, Gironell A, Kulisevsky J. Controlled study of decision-making and cognitive impairment in Parkinson's disease. Mov Disord. 2007;22(10):1430–1435. doi: 10.1002/mds.21457. [DOI] [PubMed] [Google Scholar]
- Papay K, Mamikonyan E, Siderowf AD, Duda JE, Lyons KE, Pahwa R, Weintraub D. Patient versus informant reporting of ICD symptoms in Parkinson's disease using the QUIP: validity and variability. Parkinsonism Relat Disord. 2011;17(3):153–155. doi: 10.1016/j.parkreldis.2010.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons TD, Rogers SA, Braaten AJ, Woods SP, Troster AI. Cognitive sequelae of subthalamic nucleus deep brain stimulation in Parkinson's disease: a meta-analysis. Lancet Neurol. 2006;5(7):578–588. doi: 10.1016/S1474-4422(06)70475-6. [DOI] [PubMed] [Google Scholar]
- Phillips JM, Brown VJ. Reaction time performance following unilateral striatal dopamine depletion and lesions of the subthalamic nucleus in the rat. Eur J Neurosci. 1999;11(3):X1003–1010. [PubMed] [Google Scholar]
- Pillon B, Ardouin C, Damier P, Krack P, Houeto JL, Klinger H, Agid Y. Neuropsychological changes between “off” and “on” STN or GPi stimulation in Parkinson's disease. Neurology. 2000;55(3):411–418. doi: 10.1212/wnl.55.3.411. [DOI] [PubMed] [Google Scholar]
- Plessow F, Fischer R, Volkmann J, Schubert T. Subthalamic deep brain stimulation restores automatic response activation and increases susceptibility to impulsive behavior in patients with Parkinson's disease. Brain Cogn. 2014;87:16–21. doi: 10.1016/j.bandc.2014.02.009. [DOI] [PubMed] [Google Scholar]
- Poletti M, Frosini D, Lucetti C, Del Dotto P, Ceravolo R, Bonuccelli U. Decision making in de novo Parkinson's disease. Mov Disord. 2010;25(10):1432–1436. doi: 10.1002/mds.23098. [DOI] [PubMed] [Google Scholar]
- Pontone G, Williams JR, Bassett SS, Marsh L. Clinical features associated with impulse control disorders in Parkinson disease. Neurology. 2006;67(7):1258–1261. doi: 10.1212/01.wnl.0000238401.76928.45. [DOI] [PubMed] [Google Scholar]
- Post T, van den Assem MJ, Baltussen G, Thaler RH. Deal or no deal? Decision making under risk in a large-payoff game show. American Economic Review. 2008;98:38–71. [Google Scholar]
- Sanfey AG, Loewenstein G, McClure SM, Cohen JD. Neuroeconomics: cross-currents in research on decision-making. Trends in Cognitive Sciences. 2006;10(3):108–116. doi: 10.1016/j.tics.2006.01.009. [DOI] [PubMed] [Google Scholar]
- Schretlen DJ, Winicki JM, Meyer SM, Testa SM, Pearlson GD, Gordon B. Development, psychometric properties, and validity of the Hopkins Adult Reading Test (HART) Clinical Neuropsychologist. 2009;23(6):926–943. doi: 10.1080/13854040802603684. 908454850 [pii] [DOI] [PubMed] [Google Scholar]
- Schroeder U, Kuehler A, Haslinger B, Erhard P, Fogel W, Tronnier VM, Ceballos-Baumann AO. Subthalamic nucleus stimulation affects striato-aneterior cingulate cortex circuit in a response conflict task: A PET study. Brain. 2002;125:1995–2004. doi: 10.1093/brain/awf199. [DOI] [PubMed] [Google Scholar]
- Sheikh JI, Yesavage JA. Geriatric Depression Scale (GDS): Recent evidence and development of a shorter version. In: Brink TL, editor. Clinical gerontology: A guide to assessment and intervention. New York: The Haworth Press; 1986. pp. 165–173. [Google Scholar]
- Smeding HM, Goudriaan AE, Foncke EM, Schuurman PR, Speelman JD, Schmand B. Pathological gambling after bilateral subthalamic nucleus stimulation in Parkinson disease. J Neurol Neurosurg Psychiatry. 2007;78(5):517–519. doi: 10.1136/jnnp.2006.102061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solla P, Cannas A, Corona M, Marrosu MG, Marrosu F. Dopamine dysregulation syndrome in Parkinson's disease patients with unsatisfactory switching from immediate to extended release pramipexole: a further clue to incentive sensitization mechanisms? Behav Neurol. 2013;27(4):563–566. doi: 10.3233/BEN-129026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swann N, Poizner H, Houser M, Gould S, Greenhouse I, Cai W, Aron AR. Deep brain stimulation of the subthalamic nucleus alters the cortical profile of response inhibition in the beta frequency band: a scalp EEG study in Parkinson's disease. J Neurosci. 2011;31(15):5721–5729. doi: 10.1523/JNEUROSCI.6135-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Take-Two-Interactive-Software. Deal or No Deal. New York: Endemol International B.V; 2009. [Google Scholar]
- Thaler RH, Tversky A, Kahneman D, Schwartz A. The effect of myopia and loss aversion on risk taking: an experimental test. Quarterly Journal of Economics. 1997;112(2):647–661. [Google Scholar]
- The Deep-Brain Stimulation for Parkinson's Disease Study Group. Deep-brain stimulation of the subthalamic nucleus or the pars interna of the globus pallidus in Parkinson's disease. New England Journal of Medicine. 2001;345(13):956–963. doi: 10.1056/NEJMoa000827. [DOI] [PubMed] [Google Scholar]
- Thiel A, Hilker R, Kessler J, Habedank B, Herholz K, Heiss WD. Activation of basal ganglia loops in idiopathic Parkinson's disease: a PET study. J Neural Transm. 2003;110(11):1289–1301. doi: 10.1007/s00702-003-0041-7. [DOI] [PubMed] [Google Scholar]
- Tomlinson CL, Stowe R, Patel S, Rick C, Gray R, Clarke CE. Systematic review of levodopa dose equivalency reporting in Parkinson's disease. Mov Disord. 2010;25(15):2649–2653. doi: 10.1002/mds.23429. [DOI] [PubMed] [Google Scholar]
- Tversky A, Kahneman D. Rational choice and the framing of decisions. Journal of Business. 1986;59:S251–S278. [Google Scholar]
- Uslaner JM, Robinson TE. Subthalamic nucleus lesions increase impulsive action and decrease impulsive choice - mediation by enhanced incentive motivation? Eur J Neurosci. 2006;24(8):2345–2354. doi: 10.1111/j.1460-9568.2006.05117.x. [DOI] [PubMed] [Google Scholar]
- Voon V, Gao J, Brezing C, Symmonds M, Ekanayake V, Fernandez H, Hallett M. Dopamine agonists and risk: impulse control disorders in Parkinson's disease. Brain. 2011;134(Pt 5):1438–1446. doi: 10.1093/brain/awr080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voon V, Hassan K, Zurowski M, de Souza M, Thomsen T, Fox S, Miyasaki J. Prevalence of repetitive and reward-seeking behaviors in Parkinson disease. Neurology. 2006;67(7):1254–1257. doi: 10.1212/01.wnl.0000238503.20816.13. [DOI] [PubMed] [Google Scholar]
- Voon V, Hassan K, Zurowski M, Duff-Canning S, de Souza M, Fox S, Miyasaki J. Prospective prevalence of pathologic gambling and medication association in Parkinson disease. Neurology. 2006;66(11):1750–1752. doi: 10.1212/01.wnl.0000218206.20920.4d. [DOI] [PubMed] [Google Scholar]
- Voon V, Sohr M, Lang AE, Potenza MN, Siderowf AD, Whetteckey J, Stacy M. Impulse control disorders in Parkinson disease: a multicenter case--control study. Ann Neurol. 2011;69(6):986–996. doi: 10.1002/ana.22356. [DOI] [PubMed] [Google Scholar]
- Weintraub D, Koester J, Potenza MN, Siderowf AD, Stacy M, Voon V, Lang AE. Impulse control disorders in Parkinson disease: a cross-sectional study of 3090 patients. Arch Neurol. 2010;67(5):589–595. doi: 10.1001/archneurol.2010.65. [DOI] [PubMed] [Google Scholar]
- Winstanley CA, Baunez C, Theobald DE, Robbins TW. Lesions to the subthalamic nucleus decrease impulsive choice but impair autoshaping in rats: the importance of the basal ganglia in Pavlovian conditioning and impulse control. Eur J Neurosci. 2005;21(11):3107–3116. doi: 10.1111/j.1460-9568.2005.04143.x. [DOI] [PubMed] [Google Scholar]
- Witt K, Daniels C, Reiff J, Krack P, Volkmann J, Pinsker MO, Deuschl G. Neuropsychological and psychiatric changes after deep brain stimulation for Parkinson's disease: a randomised, multicentre study. Lancet Neurol. 2008;7(7):605–614. doi: 10.1016/S1474-4422(08)70114-5. [DOI] [PubMed] [Google Scholar]
- Wolstenholme J. Deal or no deal? Significance. 2006;3:191–192. [Google Scholar]
- Wylie SA, Stout JC, Bashore TR. Activation of conflicting responses in Parkinson's disease: evidence for degrading and facilitating effects on response time. Neuropsychologia. 2005;43(7):1033–1043. doi: 10.1016/j.neuropsychologia.2004.10.008. [DOI] [PubMed] [Google Scholar]