Abstract
Inflammation is a recognized antecedent and coincident factor when examining the biology of anxiety. Little is known, however, about how reductions in endogenous anti-inflammatory mediators impact anxiety. Therefore, mood- cognition- and anxiety-associated/like behaviors were examined in IL-4 knock out (KO) mice and wild-type (WT) mice. In comparison to WT mice, IL-4 KO mice demonstrated decreased burrowing and increased social exploration. No differences were seen in forced swim or saccharine preference testing. IL-4 KO mice had similar performance to WT mice in the Morris water maze and during object location and novel object recognition. In the elevated zero-maze, IL-4 KO mice, in comparison to WT mice, demonstrated anxiety-like behavior. Anxiety-like behavior in IL-4 KO mice was not observed, however, during open-field testing. Taken together, these data indicate that IL-4 KO mice display state, but not trait, anxiety suggesting that reductions in endogenous anti-inflammatory bioactives can engender subtypes of anxiety.
Keywords: IL-4, anxiety, immune skew, mice, state anxiety, trait anxiety
Introduction
With a lifetime prevalence of nearly 20% in Americans (Kessler and Wang 2008), anxiety disorders including: panic disorder, obsessive-compulsive disorder, post-traumatic stress disorder, social anxiety disorder and generalized anxiety disorder are among the most common psychiatric conditions suffered and the sixth leading cause of disability (Baxter et al. 2014). In humans, the psychophysiology of anxiety is understood to be a combination of genetic and environmental factors; however, biological triggers are recently described including: overnutrition (Lykouras and Michopoulos 2011), oxidative stress (Bouayed et al. 2009) and inflammation (Pitsavos et al. 2006). Especially with stress-induced anxiety, inflammation is a recognized coincident factor (Maes et al. 1998) and a potential cause of anxiety (Vogelzangs et al. 2013). In general, the mechanisms involved in the development of anxiety remain elusive.
As new pathways linking inflammatory and psychiatric diseases are discovered (Debnath and Venkatasubramanian 2013, Haroon et al. 2012, Hou and Baldwin 2012, Irwin and Rothermundt, Slavich and Irwin 2014), the importance of interactions between the peripheral immune system and brain are continually reassessed and reimagined (Dantzer and Kelley 2007, Kelley and McCusker 2014). Pro-inflammatory cytokines, however, remain key bioactives that regulate psychoneuroimmunology (Dantzer et al. 2008) because they can directly impact neuronal function (Felger and Lotrich 2013, Yirmiya and Goshen 2011). Two of the most recognized peripherally-generated inflammatory cytokines that influence behavior are IL-1β and TNF-α (Gosselin and Rivest 2007, McAfoose and Baune 2009, Wang and Shuaib 2002) with IL-1β specifically possessing anxiogenic properties (Rossi et al. 2012). In contrast, the impacts of anti-inflammatory cytokines on anxiety are not well delineated.
Since naturally occurring states of pure anti- or pro-inflammation are unlikely, examination of mouse models of immune skew are helpful in unraveling how inflammatory status impacts behavior. Within the innate immune system, anti-inflammatory skew is critical to subduing and/or controlling inflammation as happens subsequent to macrophage priming (Opal and DePalo 2000) or with alternative macrophage activation (Dinarello 2000). In rodents, genotype-associated immune skew can blunt lipopolysaccharide (LPS)-dependent sickness symptoms as illustrated when comparing C57BL/6 and BALB/c mice (Bluthé et al. 1999, Bluthé et al. 2002). Since the peripheral immune system and brain communicate in both health and disease, a skew toward pro-inflammation vis-a-vis a reduction in endogenous anti-inflammatory mediators would be expected to result in outcomes similar to those observed due to over-expression or induction of pro-inflammatory bioactives. Since IL-4 is crucial to the development of alternatively activated macrophages and an anti-inflammatory immune skew (Moon et al. 2011), we examined depressive-, cognitive- and anxiety-associated behaviors in IL-4 KO mice to determine if IL-4 deficiency fostered inflammation-associated psychiatric-like maladies in mice.
Methods
Materials
All reagents were purchased from Sigma-Aldrich (St Louis, MO).
Animals
Animal use was performed according to protocols approved by the Institutional Animal Care and Use Committee at the University of Illinois. Wild-type C57BL/6J (WT) and interleukin (IL)-4 knock out (KO) mice (on a C57BL background) were originally purchased from The Jackson Laboratory (Bar Harbor, ME) and bred in-house. Mice were provided food and water ad libitum in standard shoebox cages. Animals were group housed (up to 8 per cage), then moved to individual housing the day prior to testing unless noted otherwise. Housing temperature (72 °F) and humidity (45–55%) were controlled, and a 12/12h reversed dark-light cycle (2100–0900 h) was maintained. Animal behavior was video recorded using a Sony HDR-XR500V Night Shot capable video camera (Tokyo, Japan). Mice used were between 8 and 20 wks of age. All behavior testing was completed under red lights in the dark cycle unless otherwise indicated. A total of 158 mice were used.
qPCR for IL-4
RNA was extracted, cDNA generated, and gene expression was determined as previously described (Moon et al. 2014). In brief, mRNA was reverse transcribed using the High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems). The TaqMan Gene Expression primer for IL-4 (Mm00445259_m1) was used in real-time PCR performed on a 7900 HT Fast Real-Time PCR System (Applied Biosystems) using TaqMan Universal PCR Master Mix. Parallel amplification of β-actin (Mm00607939_s1) was performed to normalize gene expression. Relative fold change of target genes was performed by comparing ΔCts.
Burrowing
As previously described (Kaczmarczyk et al. 2013), mice were individually housed overnight in the presence of a burrow (20 cm long polyvinyl chloride (PVC) piping capped at one end and raised 1.3 cm at the open end). Testing was initiated by filling the burrow with approximately 200 g of food pellets. Food was only available from the burrow, but water was provided ad libitum. Mice were allowed to interact with the filled burrow for 30 min, at which point the amount burrowed was determined from the combined weight of the burrow and food before and after testing.
Social Exploration
Social exploratory behavior was performed as we have described (Sherry et al. 2010). Briefly, a novel male 3- to 4-wk-old conspecific mouse (challenge mouse) was confined to a 7.62 × 7.62 cm wire mesh enclosure placed in the home cage of the test mouse. The test mouse was exposed to the challenge mouse and video recorded for 5 min. Investigation time (nose contact) of the challenge mouse by the test mouse was quantified by a blinded observer.
Forced swim test
A clean white cylindrical PVC container (diameter 16 cm; height 31 cm) was filled with 20 cm of water maintained at 25 ± 1 °C for each mouse. Mice were moved from their home cage to the test container and video recorded for 5 min. Immobility was evaluated from the video as we have described (Park et al. 2011).
Saccharin Preference
Saccharin preference testing was performed as previously described (Lavin et al. 2011). Briefly, mice were singly housed in standard cages with two bottles of water three days prior to testing for acclimation. Testing was initiated at the beginning of the dark cycle by replacing one of the waters (randomized to right verses left) with a 0.4% sodium saccharin solution (Sigma-Aldrich). Bottles were weighed before and 24 hrs after saccharin access and total consumption calculated. Results are represented as % saccharin preference (saccharin volume consumed/total volume consumed).
Morris Water Maze
Mice were tested as previously described (Sparkman et al. 2006). Briefly, in a 100 cm diameter by 30 cm deep circular tank filled with water (21–23°C), a 10 cm diameter transparent round platform was placed 0.5–1cm below the water surface. Animals were trained for 4 days (acquisition phase) at three trials per day. Mice were placed in one of three quadrants other than the one containing the hidden platform and allowed to swim for 60 s or until reaching the platform. If mice failed to locate the platform, they were placed on the platform for 30 s. After three consecutive trials, mice were returned to their home cage and placed under a heat lamp for 10 min. On day 5, the platform was removed and each mouse was tested (probe trial) for 60 s. All phases of training and testing were video recorded and movement determined from the video record using Noldus Information Technology EthoVision XT 7. All training and testing was performed during the dark cycle, with spatial clues illuminated by dim white light.
Novel Object Recognition
Novel object recognition was examined as previously described (Chiu et al. 2012, York et al. 2012). Mice were individually housed overnight in a rat cage-sized arena (26 cm × 48 cm × 21 cm) containing two identical objects (training). After overnight training, objects were removed and sanitized with ethanol. Testing was initiated 1 h after object cleaning. During testing, two objects were returned to the arena, one seen during training (familiar object) and one not seen during training (novel object). Object investigation was video recorded for 5 min and evaluated by a blinded observer. Percent investigation of objects was calculated by dividing the time spent examining each object individually by the total time spent investigating both objects.
Object Location Recognition
Object location recognition testing was performed by methods similar to those used in novel object recognition testing and as previously described (York et al. 2012). In brief, mice were individually housed overnight in a rat cage-sized arena (26 cm × 48 cm × 21 cm) containing two identical objects (training). Each arena had a spatial cue marked on one arena side. During training, the two identical objects were presented to the animal at one cage end (long axis). After overnight training, objects were removed and sanitized with ethanol. Testing was initiated 1 hr after object cleaning. During testing, the two objects used during training were returned to the arena. One object was placed in the same location as during training (familiar location). The second object was placed at the opposite arena end (novel location). Object investigation was video recorded for 5 min and evaluated by a blinded observer. Percent investigation of objects was calculated by dividing the time spent examining each object individually by the total time spent investigating both objects.
Elevated Zero-Maze
As previously described (Kaczmarczyk et al. 2013), an elevated zero-maze was used to examine anxiety-like behaviors. The maze was divided into 4 alternating quadrants, two of which had 14 cm high walls (closed arms) and two of which had no walls (open arms). Testing was performed under white light illumination. To begin testing, mice were placed in a closed arm and movement around the maze video recorded for 5 min. Percent time spent in the closed and open arms was evaluated by a blinded observer and calculated by dividing the time spent in each of these arm types by the total time spent in the maze.
Open Field Test
As we have described (York et al. 2012), mice were placed in a novel open field arena (66 cm length × 45.7 cm width × 22.9 cm height) with lighting positioned to generate a 9 cm shadow from each side wall. Mice were allowed to explore the novel arena and video recorded for 5 min. Noldus Information Technology EthoVision XT 7 was used to track the mouse, determining mouse center point movement and time spent in the shadowed and non-shadowed areas of the arena. Results are presented as raw time (s) and distance moved (cm).
HPLC
As previously described (Moon et al. 2014) mouse brain regions were solubilized using a Sonics & Materials, Inc ultrasonic tissue disruptor (Newtown, CT) in 0.1 N HClO4 and 25 μM ascorbate. Solubilized brains were centrifuged at 12,000 × g for 10 min at 4 °C. Supernatants were collected and diluted in 0.02 N HClO4 for analysis using an ESA Coulochem III detector with a 5041 Enhanced Analytical cell containing a +320 mV Thermo Scientific glassy carbon electrode (Sunnyvale, CA). Norepinephrine (NE), normetanephrine (NME), dopamine (DA), 3,4-dihydroxyphenylacetic acid (DOPAC), homovanillic acid (HVA), 5-hydroxytrypatime (5-HT) and 5-hydroxyindoleacetic acid (5-HIAA) were measured using a pH 3.0 mobile phase (75 mM NaH2PO4, 25 μM EDTA, 0.45 mM octanesulfonic acid and triethylamine in acetonitrile:water (5:95, v:v)) pumped at 0.2 μL/min through a 2 mm × 150 mm Thermo Scientific ODS Hypersil column. Tissue pellets were homogenized in RIPA buffer (150 mM NaCl, 5 mM EDTA, 0.1% SDS, 1% Triton and 10 mM Tris, pH 7.4) and protein concentrations determined using the BioRad DC Protein Assay (Hercules, CA). Results were quantified as ng analyte/mg brain region protein. Values were expressed as raw values and as ratios.
Statistics
Data are expressed as mean ± SEM. Analysis was conducted using Sigma Plot 11.2 (Systat Software, Chicago, IL). One-way ANOVA was run for all experiments except Morris Water Maze, which required two-way repeated measures ANOVA for Task Acquisition. Post-hoc comparison used Tukey’s test. Statistical significance was determined as p<0.05.
Results
IL-4 KO mice display decreased burrowing and increased social exploration
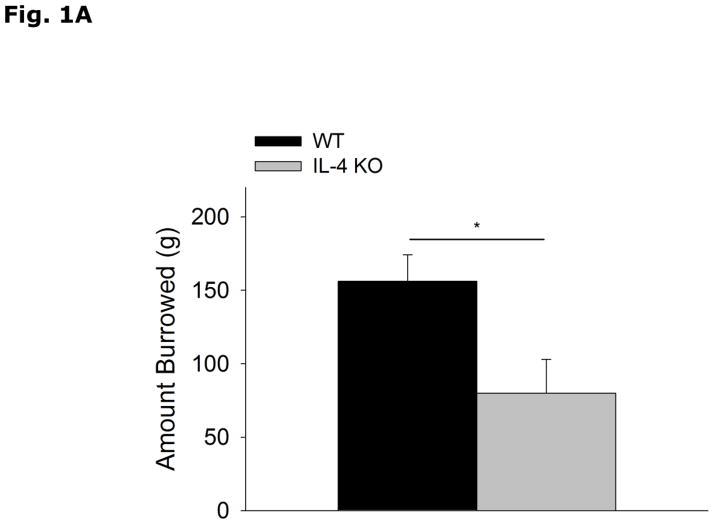
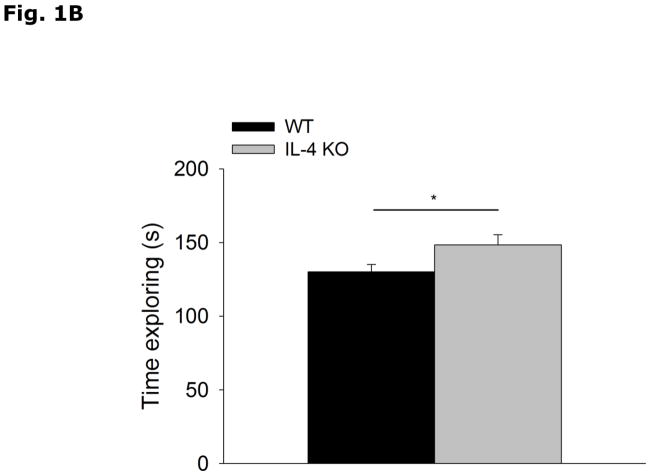
Figs. 1A and 1B show that IL-4 KO mice display decreased food-pellet burrowing (WT vs IL-4 KO, 156.0 ± 18.0 vs 79.8 ± 23.2, p=0.017) and increased social exploration of a novel juvenile (WT vs IL-4 KO, 130.0 ± 5.0 vs 148.4 ± 6.9, p=0.039) when compared to WT mice. Table 1 demonstrates that IL-4 KO mice when compared to WT mice lack IL-4 mRNA.
Figure 1. IL-4 KO mice display decreased burrowing and increased social exploration.
(A) WT and IL-4 KO mice were allowed to burrow for 30 mins. Amount burrowed in grams (g) is expressed as means ± s.e.m.; n=14–16, *p<0.05. (B) WT and IL-4 KO mice were allowed to explore an enclosed conspecific juvenile mouse for 5 mins. Time exploring the juvenile mouse in seconds (s) is expressed as means ± s.e.m.; n=18, *p<0.05.
Table 1.
Gene expression of IL-4 in whole brains of wild type (WT) and IL-4 knock out (KO) mice
| WT | IL-4 KO | |
|---|---|---|
| IL-4 mRNA | 1.00 ± 0.26 | not detected |
Results are expressed as relative fold change in mRNA (ΔmRNA), means ± SEM; statistics not performed due to lack of values in IL-4 KO group; n=8
IL-4 KO mice do not exhibit depressive-like behavior
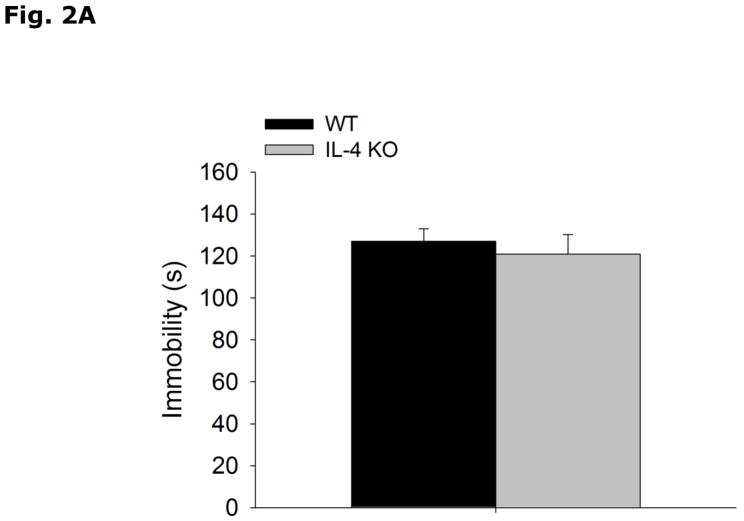
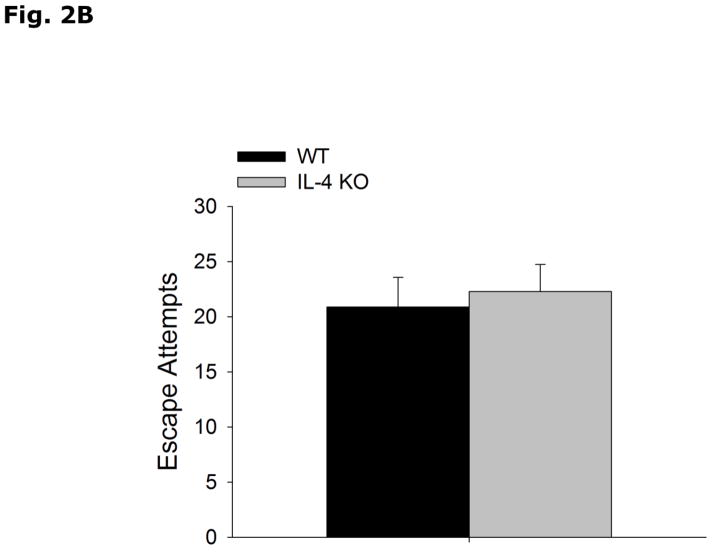
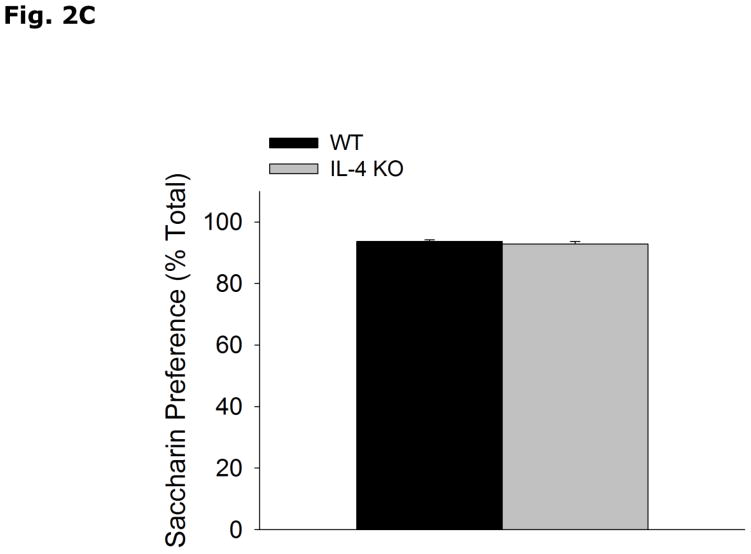
Fig. 2A-C show that WT and IL-4 KO mice have similar immobility (WT vs IL-4 KO, 127.0 ± 6.0 vs 121.0 ± 9.3, p=0.580), and escape attempts (WT vs IL-4 KO, 20.89 ± 2.67 vs 22.29 ± 2.45, p=0.337) in the forced swim test, and preference for saccharin (WT vs IL-4 KO, 93.7 ± 0.5 vs 92.9 ± 0.8, p=0.398).
Figure 2. IL-4 KO mice do not exhibit depressive-like behavior.
(A and B) WT and IL-4 KO mice were subjected to the forced swim test for 5 mins. Time immobile in seconds (s) and escape attempts are expressed as means ± s.e.m.; n=7–9, *p<0.05. (C) WT and IL-4 KO mice were allowed free access to water and a 0.4% saccharin solution for 24 h. Percent saccharine consumption is expressed as means ± s.e.m.; n=17–18, *p<0.05.
Memory is not impaired in IL-4 KO mice
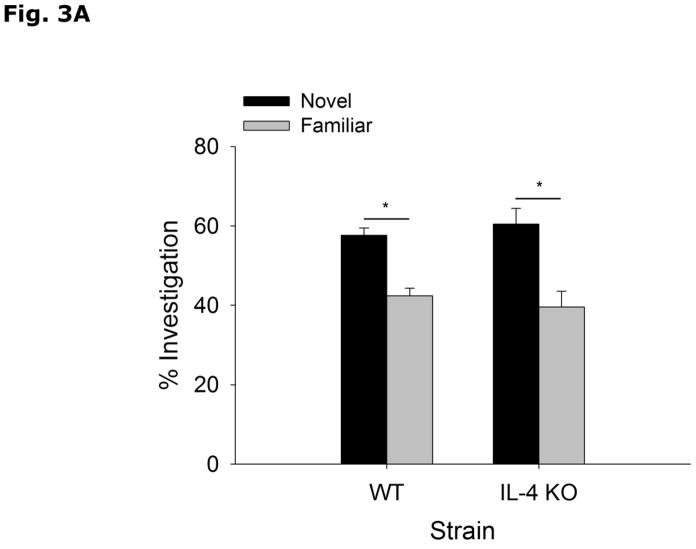
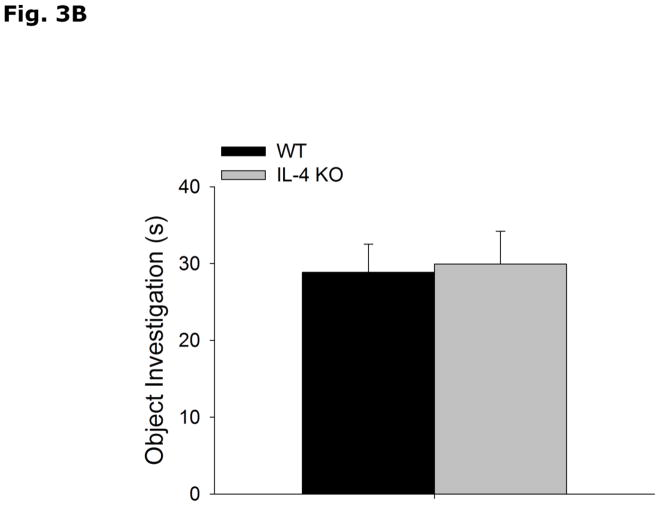
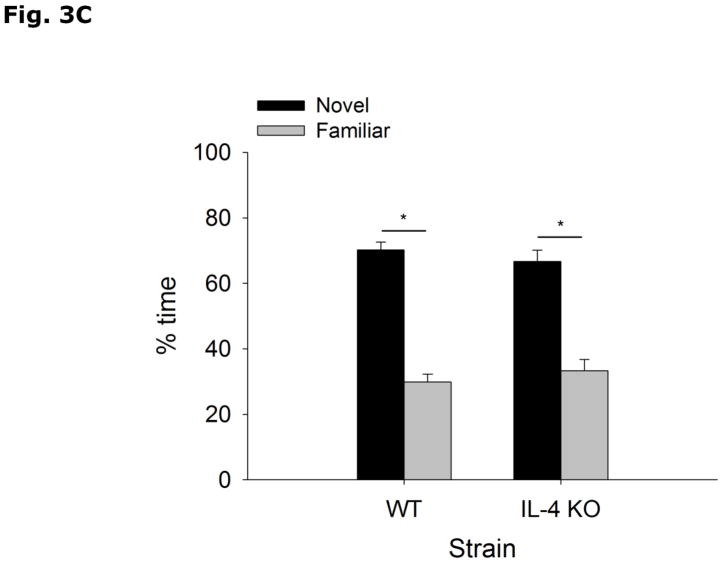
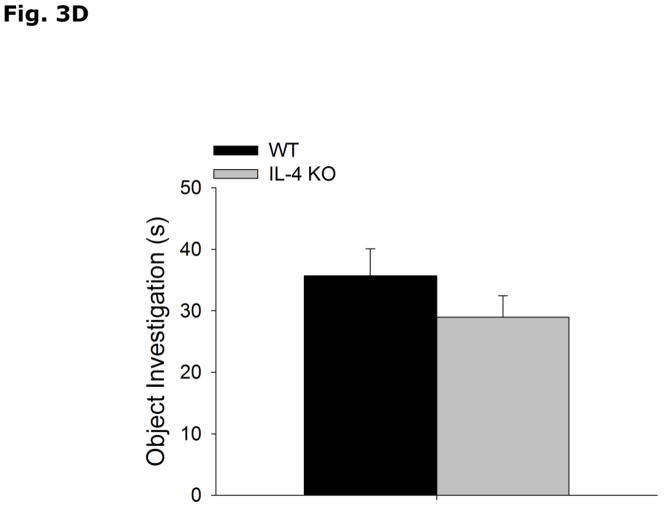
During the acquisition phase of Morris water maze testing (Table 2), WT and IL-4 KO mice both decrease latency to find the hidden platform without a strain interaction (Strain effect p=0.897, Acquisition Day effect p<0.001, Strain x Acquisition Day effect p=0.878; Day 1 vs Day 2, 49.5 ± 2.5 vs 40.1 ± 3.7, p=0.336; Day 1 vs Day 3, 49.5 ± 2.5 vs 29.3 ± 5.3, p=0.004; Day 1 vs Day 4, 49.5 ± 2.5 vs 25.1 ± 4.1, p<0.001; Day 2 vs Day 3, 40.1 ± 3.7 vs 29.3 ± 5.3, p=0.213; Day 2 vs Day 4, 40.1 ± 3.7 vs 25.1 ± 4.1, p=0.043; Day 3 vs Day 4, 29.3 ± 5.3 vs 25.1 ± 4.1, p=0.870). In the probe trial of Morris water maze testing (Table 2), WT and IL-4 KO mice do not differ in % time spent in the platform quadrant (WT vs IL-4 KO, 46.8 ± 6.4 vs 47.1 ± 6.1, p=0.972), latency to platform (WT vs IL-4 KO, 34.9 ± 6.9 vs 29.6 ± 7.2, p=0.603), number of platform crossings (WT vs IL-4 KO, 3.1 ± 0.9 vs 2.3 ± 0.7, p=0.454) and total distance swam during either acquisition (WT vs IL-4 KO, 737.8 ± 51.3 vs 792.5 ± 62.2, p=0.509) or probe (WT vs IL-4 KO, 1054.1 ± 51.1 vs 1010.2 ± 57.1, p=0.573) trials. Figs. 3A and B demonstrate that WT and IL-4 KO mice spend more time investigating an object in a novel location (WT Novel vs Familiar Location, 57.6 ± 1.9 vs 42.4 ± 1.9, p<0.001; IL-4 KO Novel vs Familiar Location, 60.4 ± 4.0 vs 39.6 ± 4.0, p=0.003), with no difference in total time spent investigating the objects (WT vs IL-4 KO, 28.8 ± 3.6 vs 29.9 ± 4.25, p=0.782). Figs. 3C and 3D show that WT and IL-4 KO mice investigate a novel object preferentially over a familiar object (WT Novel vs Familiar Object, 70.2 ± 2.5 vs 29.8 ± 2.5, p<0.001; IL-4 KO Novel vs Familiar Object, 66.7 ± 3.4 vs 33.3 ± 3.4, p<0.001), with no difference in total time spent investigating the objects (WT vs IL-KO, 35.6 ± 4.38 vs 28.9 ± 3.51, p=0.255).
Table 2.
Morris Water Maze performance of WT and IL-4 KO mice
| WT | IL-4 KO | |
|---|---|---|
| Acquisition Phase | ||
| Latency to Platform | ||
| Day 1 | 50.7 ± 2.9 | 48.1 ± 4.15 |
| Day 2 | 38.0 ± 5.8 | 42.2 ± 4.8 |
| Day 3 | 30.6 ± 7.1 | 28.0 ± 8.2 |
| Day 4 | 23.4 ± 6.1 | 26.8 ± 5.7 |
| Distance Swam (cm) | 737.8 ± 51.3 | 792.5 ± 62.2 |
| Probe Phase | ||
| Latency to Platform | 34.9 ± 6.9 | 29.6 ± 7.2 |
| % Time in Target Quadrant | 46.8 ± 6.4 | 47.1 ± 6.1 |
| Number of Platform Crossings | 3.1 ± 0.9 | 2.3 ± 0.7 |
| Distance Swam (cm) | 1054.1 ± 51.1 | 1010.2 ± 57.1 |
Results are expressed as mean ± SEM; no significant results seen with respect to WT vs. IL-4 KO at p<0.05; n=8
Figure 3. Object memory is not impaired in IL-4 KO mice.
(A and B) WT and IL-4 KO mice were examined using object location recognition testing. Exploration of objects was quantified for 5 mins and investigation of each object location as a percent of total investigation time and time spent investigating both objects in seconds (s) are expressed as means ± s.e.m; n=7–9, *p<0.05. (C and D) WT and IL-4 KO mice were examined using novel object recognition testing. Exploration of objects was quantified for 5 mins and investigation of each object as a percent of total investigation time and time spent investigating both objects in seconds (s) are expressed as means ± s.e.m.; n=16, *p<0.05.
IL-4 KO mice demonstrate increased anxiety-like behavior
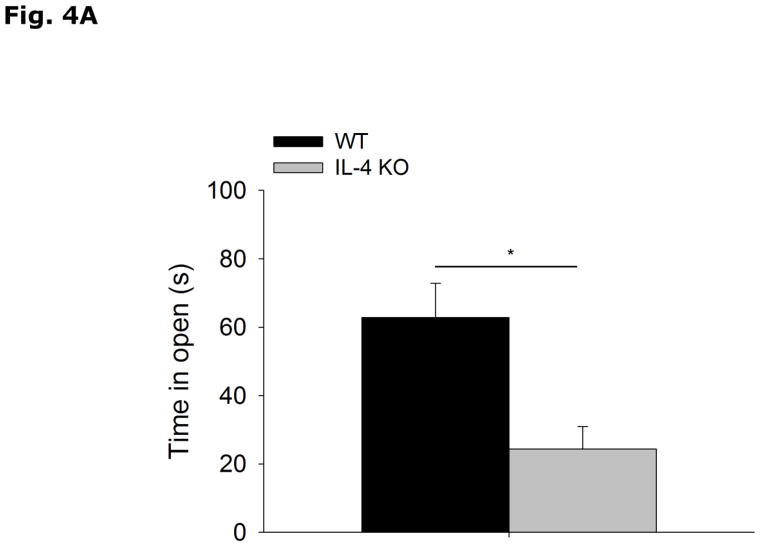
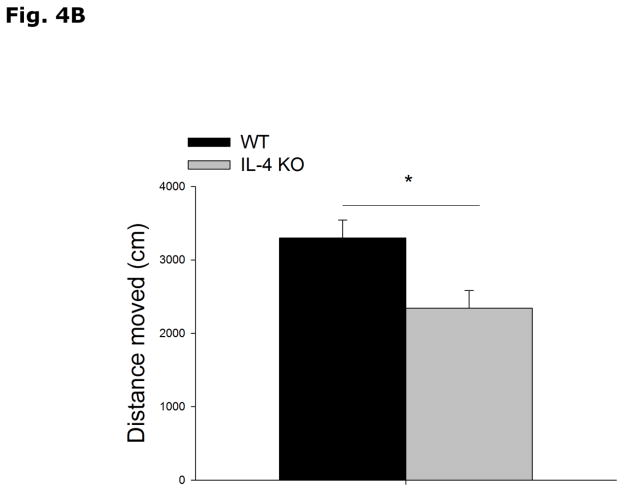
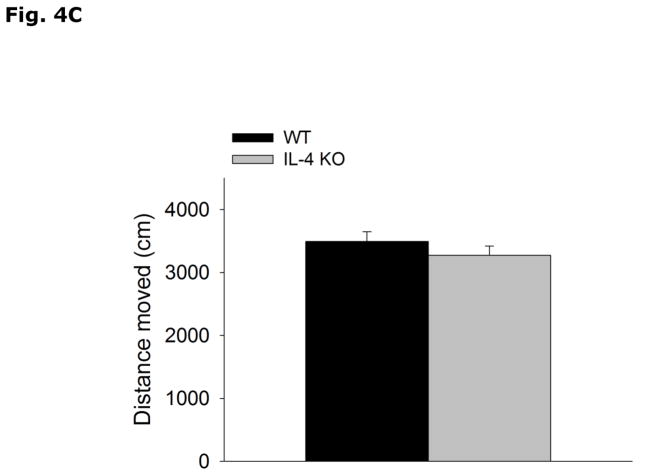
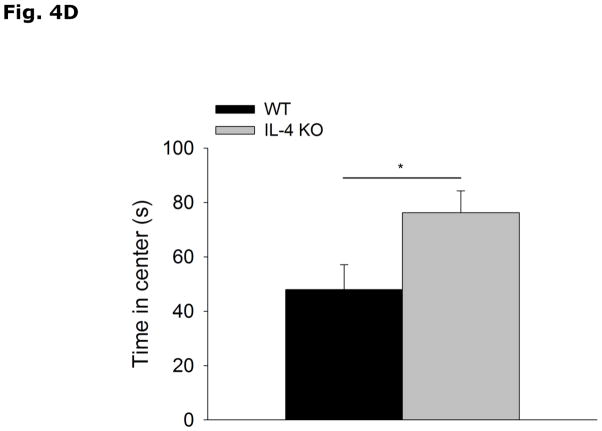
Figs. 4A and 4B show that IL-4 KO mice spend less time in the open arms of the elevated zero-maze than WT controls (WT vs IL-4 KO, 62.8 ± 10.0 vs 24.4 ± 6.5, p=0.003). In addition, IL-4 KO mice move less through the maze than WT mice (WT vs IL-4 KO, 3299.8 ± 245.1 vs 2340.4 ± 241.4, p=0.009). Figs. 4C and 4D demonstrate that IL-4 KOs move the same distance as WT mice in the open field arena (WT vs IL-4 KO, 3491.1 ± 156.0 vs 3275.1 ± 143.9, p=0.338) and spend more time in the area center (non-shadowed) than WT mice (WT vs IL-4 KO, 47.9 ± 9.2 vs 76.2 ± 8.0, p=0.048). Table 3 shows that there is no difference in the levels of NE, NME, DA, DOPAC, HVA, 5-HT or 5-HIAA in the cortex, hippocampus, or amygdala of IL-4 KO mice compared to WT mice.
Fig. 4. IL-4 KO mice demonstrate increased anxiety-like behavior.
(A and B) WT and IL-4 KO mice were tested in the elevated zero-maze for 5 mins. Time spent in the open arms in seconds (s) and distance moved in the maze in centimeters (cm) are expressed as means ± s.e.m; n=15, *p<0.05. (B and C) WT and IL-4 KO mice were allowed to explore an open field arena for 5 mins. Total locomotor activity in centimeters (cm) and time spent in the non-shadowed area in seconds (s) are expressed as means ± s.e.m; n=5, *p<0.05.
Table 3.
NE, NME, DA, DOPAC, HVA, 5-HT and 5-HIAA concentrations in the brain of WT and IL-4 KO mice
| Cortex | Hippocampus | Amygdala | ||||
|---|---|---|---|---|---|---|
| WT | IL-4 KO | WT | IL-4 KO | WT | IL-4 KO | |
| NE | 1.58 ± 0.14 | 1.59 ± 0.11 | 2.36 ± 0.08 | 2.45 ± 0.14 | 2.15 ± 0.06 | 2.09 ± 0.08 |
| NME | 0.17 ± 0.01 | 0.16 ± 0.03 | 0.25 ± 0.03 | 0.26 ± 0.05 | 0.17 ± 0.02 | 0.19 ± 0.04 |
| DA | 6.17 ± 0.71 | 6.75 ± 0.58 | 0.06 ± 0.01 | 0.07 ± 0.01 | 0.87 ± 0.06 | 0.77 ± 0.08 |
| DOPAC | 0.46 ± 0.04 | 0.47 ± 0.03 | 0.09 ± 0.01 | 0.13 ± 0.01 | 0.22 ± 0.02 | 0.21 ± 0.02 |
| HVA | 0.80 ± 0.08 | 0.84 ± 0.07 | 0.18 ± 0.02 | 0.20 ± 0.02 | 0.30 ± 0.01 | 0.27 ± 0.03 |
| 5-HT | 1.34 ± 0.05 | 1.39 ± 0.07 | 2.74 ± 0.12 | 2.96 ± 0.19 | 4.25 ± 0.13 | 4.25 ± 0.15 |
| 5-HIAA | 0.51 ± 0.03 | 0.49 ± 0.03 | 1.89 ± 0.07 | 2.03 ± 0.11 | 1.37 ± 0.03 | 1.39 ± 0.08 |
Results are expressed as means ± SEM; no significant results seen with respect to WT vs. IL-4 KO at p<0.05; n=6
Discussion
Burrowing food or other material is an innate behavior in rodents that is best suited to screen for the presence of behavioral perturbations (Deacon 2006, Deacon 2009, Jirkof et al. 2013). We found that IL-4 KO mice displace less food from a burrow placed in their home cage than WT mice. In general, alterations in burrowing track with a variety of more specific behavioral abnormalities that can include those associated with pain (Jirkof et al. 2010), depression (Lavin et al. 2011) and anxiety (Bailey and Crawley 2009). Our overall results indicate that IL-4 KO mice are behavioral different when compared to WT mice and that this difference appears anxiety-like in nature. Similarly, social exploration is a powerful screening test for behavioral dysfunction (York et al. 2012) but, in contrast to burrowing, IL-4 KO mice are more inclined to socially explore a novel juvenile than WT mice. While best known as a sensitive indication of sickness symptoms (Bluthe et al. 1994), disturbances in social exploration are also seen after generalized hypoxia (Johnson et al. 2008) and starvation (Joesting et al. 2014). Burrowing and social exploration generally parallel each other and are reduced (Luedtke et al. 2014) in conditions tied to reduced motivation like sickness and depression. However, reductions in burrowing and social exploration are also seen in conditions that provoke anxiety (Crawley 1985). While interventions and/or conditions that increase burrowing are well described (including enriched housing (Chamove 1989) and short-term high-fat diet feeding (Kaczmarczyk et al. 2013)), mediations that increase social exploration are unusual. Currently, the best understood cause for increased social exploration appears tied to food limitation/absence paradigms in which the food-deprived animal is thought to increase social interaction so as to be led to food (Tong et al. 2011).
Immunologic skew is implicated in the pathophysiology of depression (Moon et al. 2011) because it may alter the neuronal networks and homeostatic mechanisms responsible for static function (Roman et al. 2013). Hallmarks of a major depressive disorder in humans include despair and anhedonia (loss-of-interest). Therefore, testing for depressive-like behaviors in mice capitalize on species-specific homologues of these aforementioned states (Hales et al. 2014). Assessment of depressive-like behavior in mice is often conducted using the forced swim test (despair) and the sucrose/saccharin preference test (anhedonia) (York et al. 2012). In the forced swim test, mice are placed in a water-filled container without a means of egress. Behavioral despair is defined as reduced drive to escape as quantified by increased immobility and decreased escape attempts (Hales et al. 2014). The sucrose/saccharin preference test evaluates loss-of-interest (anhedonia) by appraising the natural preference of mice for a sweetened solution when compared to water (Hales et al. 2014). Here, we found no impact of IL-4 KO on depressive-like behaviors. This was not surprising since in a meta-analysis of twenty-four studies in which serum concentrations of IL-4 where examined there was no correlation with major depression in humans (Dowlati et al. 2010).
The Morris water maze is used frequently in rodent models to assess spatial learning and memory (York et al. 2012). Animals are trained to use distant visual cues to locate a hidden, submerged escape platform (Terry 2009, Vorhees and Williams 2006). In the acquisition phase, mice are trained in multiple trials per day starting from different positions for each trial. Upon completion of each trial, animals are allowed to remain submerged platform to orient themselves to the spatial cues. Latency to platform is assessed for each trial and impaired spatial memory formation (learning) is defined as a higher time searching for the platform (York et al. 2012). When compared to WT mice, IL-4 KO mice did not differ in latency to platform during the acquisition phase, indicating no deficit in spatial learning. After completion of acquisition, animals were tested for memory recall in a probe trial, where the platform was removed from the maze. Parameters assessed were time spent in the quadrant of the removed platform, latency to the location previously occupied by the platform, and number of platform crossings (York et al. 2012). As above, IL-4 KO mice did not differ from WT mice in these measurements of memory. Interestingly, previous research indicates that IL-4 KO mice display defective spatial memory when assessed using the Morris water maze (Derecki et al. 2010). As demonstrated, we did not observe this phenomenon. However, there appears to be a key difference in how Derecki et al. examined mice when compared to the methods utilized here. Derecki et al. indicated that training and testing of mice was performed during their inactive cycle (sleep period) (Derecki et al. 2010). Recently, Zhu et al found that a single episode of sleep disturbance induces impaired learning and memory in mice (Zhu et al. 2012). Since we trained and tested mice during their active cycle (facilitated by our use of reverse light cycle housing), we sought to avoid sleep disturbance confounds identified by Zhu et al. To support these memory results, we also used object location recognition testing (spatial memory) and novel object recognition testing (non-spatial memory) (York et al. 2012, Weible et al. 2009). Since mice have a preference for novelty, mice spend more time exploring objects whether they are different in position or composition (York et al. 2012). We found that IL-4 KO mice and WT mice have similar performance in these tests supporting our water maze results
Manifesting in rodents as escape and defensive behaviors, anxiety is a highly conserved coping style evolved to respond to real or perceived threats (Steimer 2011). Generally, to test for rodent anxiety, aversive stimuli or approach-avoidance conflict-like situations are used to foster hesitancy to the exploration of things unfamiliar and/or intimidating (Bailey and Crawley 2009). As we show, IL-4 KO mice explore the open arms of an elevated zero-maze less than WT mice and spend more time immobile within the closed arms of the maze. Elevated zero-maze testing is a well-recognized test of mouse anxiety (Singh et al. 2007) because the use of bright lighting, height and open arms creates an unfamiliar/intimidating environment that can induce hesitancy and immobility (York et al. 2012). To further explore this anxious-like phenotype, we then sought to further define the nature of this anxiety-like behavior. Anxiety is considered to be situation-dependent (state anxiety) or situation-independent (trait anxiety). State anxiety is where an unfamiliar or stressful stimulus generates the behavioral response while trait anxiety is an inherent characteristic not defined by a specific precipitating event (Avgustinovich et al. 2000, Bailey and Crawley 2009). The open field test, like the elevated zero-maze, is a common anxiety paradigm (Gould et al. 2009) that uses bright lights, social isolation and a novel environment to evoke fear. Interestingly, IL-4 KO mice appear less anxious than WT during open field testing suggesting that they are prone to state anxiety in regards to the elevated zero-maze. In humans, state anxiety is common and is easily evoked in individuals who are deemed to have low trait anxiety. Importantly, those with greater trait anxiety appear to under-respond neuroendocrinologically to state-anxiety inducing stimuli (Jezova et al. 2004). Therefore, trait and state-anxiety differences in humans may be dependent on endogenous immune skew as our findings in IL-4 KO suggest.
Finally, anxiety is a complex neuropsychiatric disorder, where disturbed amygdala-based serotonin (5-HT) signaling is frequently cited as a mechanism (Asan et al. 2013, Ousdal et al. 2014). We found, however, that WT and IL-4 KO mice have similar basal neurotransmitter levels in the brain. This result was not entirely unexpected given the plethora of endogenous bioactives other than serotonin associated with anxiety including GABAergic pathways and excitatory amino acids. The uncertain neurobiology of anxiety includes mechanisms that impact expression and/or sensitivity of neurotransmitter receptors (Albert et al. 2014, Naumenko et al. 2014) as well as proinflammatory microenvironments within brain regions (Chiu et al. 2012, Fenn et al. 2014). Since significant changes in immune status directly interrupt synaptic transmission (Rostène et al. 2011, Zhang and Oppenheim 2005), immune skew may subtlety disrupt CNS function by selectively impacting brain regions or brain cell types.
In summary, we found that IL-4 KO mice display a state anxiety-like behavior without concomitant depressive-like behavior or cognitive impairment. Our results did not replicate the learning and memory defects demonstrated by Derecki et al. in IL-4 KO animals (Derecki et al. 2010) but as noted above anxiety (Harrison et al. 2009) and sleep disturbance (Zhu et al. 2012) can significantly alter mouse cognitive performance. In addition, different investigative teams can observe phenotype disparities when behavior is examined. As example, IL-1 receptor KO mice were deemed cognitively impaired by Avital et al. (Avital et al. 2003) but anxious without cognitive deficits by Murray et al. (Murray et al. 2013). Future research investigating the neurochemical basis for immune skew related anxiety is warranted so as to better understand the relationship between subtypes of anxiety and the immune-brain axis.
Acknowledgments
Support: This research was supported by the National Institutes of Health: DK064862, NS058525, AA019357 to GGF and DK59802 to the Division of Nutritional Sciences as a Ruth L. Kirchstein National Research Service Award Predoctoral Fellowship to MLM
Abbreviations
- CNS
Central nervous system
- IL
Interleukin
- KO
Knockout
- TNF
tumor necrosis factor
- WT
Wild-type
- LPS
Lipopolysaccharide
References
- Albert PR, Vahid-Ansari F, Luckhart C. Serotonin-prefrontal cortical circuitry in anxiety and depression phenotypes: pivotal role of pre- and post-synaptic 5-HT1A receptor expression. Front Behav Neurosci. 2014;8:199. doi: 10.3389/fnbeh.2014.00199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asan E, Steinke M, Lesch KP. Serontonergic innervation of the amygdala: targets, receptors, and implications for stress and anxiety. Histochem Cell Biol. 2013;139:785–813. doi: 10.1007/s00418-013-1081-1. [DOI] [PubMed] [Google Scholar]
- Avgustinovich DF, Lipina TV, Bondar NP, Aleksevenko OV, Kudryavtseva NN. Features of the genetically defined anxiety in mice. Behav Genet. 2000;30:101–109. doi: 10.1023/a:1001999020138. [DOI] [PubMed] [Google Scholar]
- Avital A, Goshen I, Kamsler A, Segal M, Iverfeldt K, Richter-Levin G, Yirmiya R. Impaired interleukin-1 signaling is associated with deficits in hippocampal memory processes and neural plasticity. Hippocampus. 2003;13:826–834. doi: 10.1002/hipo.10135. [DOI] [PubMed] [Google Scholar]
- Bailey KR, Crawley JN. Chapter 5: Anxiety-Related Behaviors in Mice. In: Buccafusco JJ, editor. Methods of Behavior Analysis in Neuroscience. 2. CRC Press; Boca Raton: 2009. [Google Scholar]
- Baxter AJ, Vos T, Scott KM, Ferrari AJ, Whiteford HA. The global burden of anxiety disorders in 2010. Psychol Med. 2014:1–12. doi: 10.1017/S0033291713003243. [DOI] [PubMed] [Google Scholar]
- Bluthe RM, Pawlowski M, Suarez S, Parnet P, Pittman Q, Kelley KW, Dantzer R. Synergy between tumor necrosis factor alpha and interleukin-1 in the induction of sickness behavior in mice. Psychoneuroendocrinology. 1994;19:197–207. doi: 10.1016/0306-4530(94)90009-4. [DOI] [PubMed] [Google Scholar]
- Bluthé R, Lestage J, Rees G, Bristow A, Dantzer R. Dual Effect of Central Injection of Recombinant Rat Interleukin-4 on Lipopolysaccharide-Induced Sickness Behavior in Rats. Neuropsychopharmacology. 2002;26:86–93. doi: 10.1016/S0893-133X(01)00305-0. [DOI] [PubMed] [Google Scholar]
- Bluthé R, Castanon N, Pousset F, Bristow A, Ball C, Lestage J, Michaud B, Kelley KW, Dantzer R. Central injection of IL-10 antagonizes the behavioural effects of lipopolysaccharide in rats. Psychoneuroendocrinology. 1999;24:301–311. doi: 10.1016/s0306-4530(98)00077-8. [DOI] [PubMed] [Google Scholar]
- Bouayed J, Rammal H, Soulimani R. Oxidative stress and anxiety: relationship and cellular pathways. Oxid Med Cell Longev. 2009;2:63–67. doi: 10.4161/oxim.2.2.7944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamove AS. Cage design reduces emotionality in mice. Laboratory Animals. 1989;23:215–219. doi: 10.1258/002367789780810608. [DOI] [PubMed] [Google Scholar]
- Chiu GS, Chatterjee D, Darmody PT, Walsh JP, Meling DD, Johnson RW, Freund GG. Hypoxia/Reoxygenation Impairs Memory Formation via Adenosine-Dependent Activation of Caspase 1. The Journal of Neuroscience. 2012;32:13945–13955. doi: 10.1523/JNEUROSCI.0704-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawley JN. Exploratory behavior models of anxiety in mice. Neurosci Biobehav Rev. 1985;9:37–44. doi: 10.1016/0149-7634(85)90030-2. [DOI] [PubMed] [Google Scholar]
- Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R, Kelley KW. Twenty years of research on cytokine-induced sickness behavior. Brain Behav Immun. 2007;21:153–160. doi: 10.1016/j.bbi.2006.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deacon RM. Burrowing: a sensitive behavioural assay, tested in five species of laboratory rodents. Behav Brain Res. 2009;200:128–133. doi: 10.1016/j.bbr.2009.01.007. [DOI] [PubMed] [Google Scholar]
- Deacon RM. Burrowing in rodents: a sensitive method for detecting behavioral dysfunction. Nat Protoc. 2006;1:118–121. doi: 10.1038/nprot.2006.19. [DOI] [PubMed] [Google Scholar]
- Debnath M, Venkatasubramanian G. Recent advances in psychoneuroimmunology relevant to schizophrenia therapeutics. Curr Opin Psychiatry. 2013;26:433–439. doi: 10.1097/YCO.0b013e328363b4da. [DOI] [PubMed] [Google Scholar]
- Derecki NC, Cardani AN, Yang CH, Quinnies KM, Crihfield A, Lynch KR, Kipnis J. Regulation of learning and memory by meningeal immunity: A key role for IL-4. J Exp Med. 2010;207:1067–1080. doi: 10.1084/jem.20091419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello CA. Proinflammatory cytokines. Chest. 2000;118:503–508. doi: 10.1378/chest.118.2.503. [DOI] [PubMed] [Google Scholar]
- Dowlati Y, Herrmann N, Swardfager W, Liu H, Sham L, Reim EK, Lanctôt KL. A meta-analysis of cytokines in major depression. Biol Psychiatry. 2010;67:446–457. doi: 10.1016/j.biopsych.2009.09.033. [DOI] [PubMed] [Google Scholar]
- Felger JC, Lotrich FE. Inflammatory cytokines in depression: Neurobiological mechanisms and therapeutic implications. Neuroscience. 2013;246:199–229. doi: 10.1016/j.neuroscience.2013.04.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenn AM, Hall JCE, Gensel JC, Popovich PG, Godbout JP. IL-4 Signaling Drives a Unique Arginase+/IL-1β+ Microglia Phenotype and Recruits Macrophages to the Inflammatory CNS: Consequences of Age-Related Deficits in IL-4Rα after Traumatic Spinal Cord Injury. J Neurosci. 2014;34:8904–8917. doi: 10.1523/JNEUROSCI.1146-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosselin D, Rivest S. Role of IL-1 and TNF in the brain: Twenty years of progress on a Dr. Jekyll/Mr. Hyde duality of the innate immune system. Brain Behav Immun. 2007;21:281–289. doi: 10.1016/j.bbi.2006.12.004. [DOI] [PubMed] [Google Scholar]
- Gould TD, Dao DT, Kovacsics CE. The Open Field Test. In: Gould TD, editor. Mood and Anxiety Related Phenotypes in Mice. Humana Press; 2009. pp. 1–20. [Google Scholar]
- Hales CA, Stuart SA, Anderson MH, Robinson ESJ. Modelling Cognitive Affective Biases in Major Depressive Disorder using Rodents. Br J Pharmacol. 2014;171:4524–38. doi: 10.1111/bph.12603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haroon E, Raison C, Miller A. Psychoneuroimmunology Meets Neuropsychopharmacology: Translational Implications of the Impact of Inflammation on Behavior. Neuropsychopharmacology Reviews. 2012;37:137–162. doi: 10.1038/npp.2011.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison FE, Hosseini AH, McDonald MP. Endogenous anxiety and stress responses in water maze and Barnes maze spatial memory tasks. Behav Brain Res. 2009;198:247–251. doi: 10.1016/j.bbr.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou R, Baldwin DS. A neuroimmunological perspective on anxiety disorders. Hum Psychopharmacol Clin Exp. 2012;27:6–14. doi: 10.1002/hup.1259. [DOI] [PubMed] [Google Scholar]
- Irwin MR, Rothermundt M. Handbook of Clinical Neurology. Elsevier; Chapter 12 - Clinical psychoneuroimmunology; pp. 211–225. [DOI] [PubMed] [Google Scholar]
- Jezova D, Makatsori A, Duncko R, Moncek F, Jakubek M. High trait anxiety in healthy subjects is associated with low neuroendocrine activity during psychosocial stress. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28:1331–1336. doi: 10.1016/j.pnpbp.2004.08.005. [DOI] [PubMed] [Google Scholar]
- Jirkof P, Cesarovic N, Rettich A, Nicholls F, Seifert B, Arras M. Burrowing behavior as an indicator of post-laparotomy pain in mice. Front Behav Neurosci. 2010;4:165. doi: 10.3389/fnbeh.2010.00165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jirkof P, Leucht K, Cesarovic N, Caj M, Nicholls F, Rogler G, Arras M, Hausmann M. Burrowing is a sensitive behavioural assay for monitoring general wellbeing during dextran sulfate sodium colitis in laboratory mice. Laboratory Animals. 2013;47:274–283. doi: 10.1177/0023677213493409. [DOI] [PubMed] [Google Scholar]
- Joesting JJ, Moon ML, Gainey SJ, Tisza BL, Blevins NA, Freund GG. Fasting induces IL-1 resistance and free-fatty acid-mediated up-regulation of IL-1R2 and IL-1RA. Front Immunol. 2014;5:315. doi: 10.3389/fimmu.2014.00315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson DR, Sherry CL, York JM, Freund GG. Acute Hypoxia, Diabetes, and Neuroimmune Dysregulation: Converging Mechanisms in the Brain. The Neuroscientist. 2008;14:235–239. doi: 10.1177/1073858407309544. [DOI] [PubMed] [Google Scholar]
- Kaczmarczyk MM, Machaj AS, Chiu GS, Lawson MA, Gainey SJ, York JM, Meling DD, Martin SA, Kwakwa KA, Newman AF, Woods JA, Kelley KW, Wang Y, Miller MJ, Freund GG. Methylphenidate prevents high-fat diet (HFD)-induced learning/memory impairment in juvenile mice. Psychoneuroendocrinology. 2013;38:1553–1564. doi: 10.1016/j.psyneuen.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley KW, McCusker RH. Getting nervous about immunity. Semin Immunol. 2014;26:389–393. doi: 10.1016/j.smim.2014.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Wang PS. The Descriptive Epidemiology of Commonly Occurring Mental Disorders in the United States*. Annu Rev Public Health. 2008;29:115–129. doi: 10.1146/annurev.publhealth.29.020907.090847. [DOI] [PubMed] [Google Scholar]
- Lavin DN, Joesting JJ, Chiu GS, Moon ML, Meng J, Dilger RN, Freund GG. Fasting induces an anti-inflammatory effect on the neuroimmune system which a high-fat diet prevents. Obesity (Silver Spring) 2011;19:1586–1594. doi: 10.1038/oby.2011.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luedtke K, Bouchard SM, Woller SA, Funk MK, Aceves M, Hook MA. Assessment of depression in a rodent model of spinal cord injury. J Neurotrauma. 2014;31:1107–1121. doi: 10.1089/neu.2013.3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lykouras L, Michopoulos J. Anxiety disorders and obesity. Psychiatriki. 2011;22:307–313. [PubMed] [Google Scholar]
- Maes M, Song C, Lin A, De Jongh R, Van Gastel A, Kenis G, Bosmans E, De Meester I, Benoy I, Neels H, Demedts P, Janca A, Scharpé S, Smith RS. The effects of psychological stress on humans: increased production of pro-inflammatory cytokines and a Th1-like response in stress-induced anxiety. Cytokine. 1998;10:313–318. doi: 10.1006/cyto.1997.0290. [DOI] [PubMed] [Google Scholar]
- McAfoose J, Baune BT. Evidence for a cytokine model of cognitive function. Neuroscience & Biobehavioral Reviews. 2009;33:355–366. doi: 10.1016/j.neubiorev.2008.10.005. [DOI] [PubMed] [Google Scholar]
- Moon ML, McNeil LK, Freund GG. Macrophages make me sick: How macrophage activation states influence sickness behavior. Psychoneuroendocrinology. 2011;36:1431–1440. doi: 10.1016/j.psyneuen.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon ML, Joesting JJ, Lawson MA, Chiu GS, Blevins NA, Kwakwa KA, Freund GG. The saturated fatty acid, palmitic acid, induces anxiety-like behavior in mice. Metabolism. 2014;63:1131–40. doi: 10.1016/j.metabol.2014.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray CL, Obiang P, Bannerman D, Cunningham C. Endogenous IL-1 in cognitive function and anxiety: A study in IL-1R1−/− mice. PloS one. 2013;8:e78385. doi: 10.1371/journal.pone.0078385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naumenko VS, Popova NK, Lacivita E, Leopoldo M, Ponimaskin EG. Interplay between serotonin 5-HT1A and 5-HT7 receptors in depressive disorders. CNS Neurosci Ther. 2014;20:582–590. doi: 10.1111/cns.12247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opal S, DePalo V. Anti-inflammatory cytokines. Chest. 2000;117:1162–1172. doi: 10.1378/chest.117.4.1162. [DOI] [PubMed] [Google Scholar]
- Ousdal OT, Andreassen OA, Server A, Jensen J. Increased amygdala and visual cortex activity and functional connectivity towards stimulus novelty is associated with state anxiety. PloS one. 2014;9:e96146. doi: 10.1371/journal.pone.0096146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park MJ, Yoo SW, Choe BS, Dantzer R, Freund GG. Acute hypoglycemia causes depressive-like behaviors in mice. Metabolism. 2012;61:229–36. doi: 10.1016/j.metabol.2011.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitsavos C, Panagiotakos DB, Papageorgiou C, Tsetsekou E, Soldatos C, Stefanadis C. Anxiety in relation to inflammation and coagulation markers, among healthy adults: the ATTICA study. Atherosclerosis. 2006;185:320–326. doi: 10.1016/j.atherosclerosis.2005.06.001. [DOI] [PubMed] [Google Scholar]
- Roman A, Kreiner G, Nalepa I. Macrophages and depression-a misalliance or well-arranged marriage? Pharmacol Rep. 2013;65:1663–1672. doi: 10.1016/s1734-1140(13)71528-7. [DOI] [PubMed] [Google Scholar]
- Rossi S, Sacchetti L, Napolitano F, De Chiara V, Motta C, Studer V, Musella A, Barbieri F, Bari M, Bernardi G, Maccarrone M, Usiello A, Centonze D. Interleukin-1beta causes anxiety by interacting with the endocannabinoid system. J Neurosci. 2012;32:13896–13905. doi: 10.1523/JNEUROSCI.1515-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rostène W, Dansereau M, Godefroy D, Van Steenwinckel J, Goazigo AR, Mélik-Parsadaniantz S, Apartis E, Hunot S, Beaudet N, Sarret P. Neurochemokines: a menage a trois providing new insights on the functions of chemokines in the central nervous system. J Neurochem. 2011;118:680–694. doi: 10.1111/j.1471-4159.2011.07371.x. [DOI] [PubMed] [Google Scholar]
- Sherry CL, Kim SS, Dilger RN, Bauer LL, Moon ML, Tapping RI, Fahey GC, Jr, Tappenden KA, Freund GG. Sickness behavior induced by endotoxin can be mitigated by the dietary soluble fiber, pectin, through up-regulation of IL-4 and Th2 polarization. Brain Behav Immun. 2010;24:631–640. doi: 10.1016/j.bbi.2010.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh K, Bishnoi M, Kulkarni SK. Elevated Zero-maze: A paradigm to evaluate anti-anxiety effects of drugs. Methods Find Exp Clin Pharmacol. 2007;29:343–348. doi: 10.1358/mf.2007.29.5.1117557. [DOI] [PubMed] [Google Scholar]
- Slavich GM, Irwin MR. From stress to inflammation and major depressive disorder: A social signal transduction theory of depression. Psychol Bull. 2014;140:774–815. doi: 10.1037/a0035302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparkman NL, Buchanan JB, Heyen JRR, Chen J, Beverly JL, Johnson RW. Interleukin-6 Facilitates Lipopolysaccharide-Induced Disruption in Working Memory and Expression of Other Proinflammatory Cytokines in Hippocampal Neuronal Cell Layers. The Journal of Neuroscience. 2006;26:10709–10716. doi: 10.1523/JNEUROSCI.3376-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steimer T. Animal models of anxiety disorders in rats and mice: some conceptual issues. Dialogues Clin Neurosci. 2011;13:495–506. doi: 10.31887/DCNS.2011.13.4/tsteimer. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry AV. Spatial navigation (water maze) tasks. In: Buccafusco JJ, editor. Methods of Behavior Analysis in Neuroscience. 2. CRC Press; Boca Raton (FL): 2009. [PubMed] [Google Scholar]
- Tong J, Mannea E, Aime P, Pfluger PT, Yi CX, Castaneda TR, Davis HW, Ren X, Pixley S, Benoit S, Julliard K, Woods SC, Horvath TL, Sleeman MM, D’Alessio D, Obici S, Frank R, Tschop MH. Ghrelin enhances olfactory sensitivity and exploratory sniffing in rodents and humans. J Neurosci. 2011;31:5841–5846. doi: 10.1523/JNEUROSCI.5680-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogelzangs N, Beekman AT, de Jonge P, Penninx BW. Anxiety disorders and inflamation in a large adult cohort. Transl Psychiatry. 2013;3:e249. doi: 10.1038/tp.2013.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorhees CV, Williams MT. Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat Protoc. 2006;1:848–858. doi: 10.1038/nprot.2006.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang CX, Shuaib A. Involvement of inflammatory cytokines in central nervous system injury. Prog Neurobiol. 2002;67:161–172. doi: 10.1016/s0301-0082(02)00010-2. [DOI] [PubMed] [Google Scholar]
- Weible AP, Rowland DC, Pang R, Kentros C. Neural Corelates of Novel Object and Novel Location Recognition Behavior in the Mouse Anterior Cingulate Cortex. J Neurophys. 2009;102:2055–2068. doi: 10.1152/jn.00214.2009. [DOI] [PubMed] [Google Scholar]
- Yirmiya R, Goshen I. Immune modulation of learning, memory, neural plasticity and neurogenesis. Brain Behav Immun. 2011;25:181–213. doi: 10.1016/j.bbi.2010.10.015. [DOI] [PubMed] [Google Scholar]
- York JM, Blevins NA, Baynard T, Freund GG. Mouse testing methods in psychoneuroimmunology: an overview of how to measure sickness, depressive/anxietal, cognitive, and physical activity behaviors. Methods Mol Biol. 2012;934:243–76. doi: 10.1007/978-1-62703-071-7_13. [DOI] [PubMed] [Google Scholar]
- Zhang N, Oppenheim JJ. Crosstalk between chemokines and neuronal receptors bridges immune and nervous systems. Journal of Leukocyte Biology. 2005;78:1210–1214. doi: 10.1189/jlb.0405224. [DOI] [PubMed] [Google Scholar]
- Zhu B, Dong Y, Xu Z, Gompf HS, Ward SA, Xue Z, Miao C, Zhang Y, Chamberlin NL, Xie Z. Sleep disturbance induces neuroinflammation and impairment of learning and memory. Neurobiol Dis. 2012;48:348–355. doi: 10.1016/j.nbd.2012.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]