Abstract
Causes of lower induction of Hsp70 in neurons during heat shock are still a matter of debate. To further inquire into the mechanisms regulating Hsp70 expression in neurons, we studied the activity of Heat Shock Factor 1 (HSF1) and histone posttranslational modifications (PTMs) at the hsp70 promoter in rat cortical neurons. Heat shock induced a transient and efficient translocation of HSF1 to neuronal nuclei. However, no binding of HSF1 at the hsp70 promoter was detected while it bound to the hsp25 promoter in cortical neurons during heat shock. Histone PTMs analysis showed that the hsp70 promoter harbors lower levels of histone H3 and H4 acetylation in cortical neurons compared to PC12 cells under basal conditions. Transcriptomic profiling data analysis showed a predominant usage of cryptic transcriptional start sites at hsp70 gene in the rat cerebral cortex, compared with the whole brain. These data support a weaker activation of hsp70 canonical promoter. Heat shock increased H3Ac at the hsp70 promoter in PC12 cells, which correlated with increased Hsp70 expression while no modifications occurred at the hsp70 promoter in cortical neurons. Increased histone H3 acetylation by Trichostatin A led to hsp70 mRNA and protein induction in cortical neurons. In conclusion, we found that two independent mechanisms maintain a lower induction of Hsp70 in cortical neurons. First, HSF1 fails to bind specifically to the hsp70 promoter in cortical neurons during heat shock and, second, the hsp70 promoter is less accessible in neurons compared to non-neuronal cells due to histone deacetylases repression.
Introduction
Heat, free radicals, bacterial infections, heavy metals, among other stresses, turn on the heat shock response in cells. This program consists of a fast and transitory increase of heat shock proteins (hsp) favoring cells survival [1]. The induction of hsp genes is regulated by the transcription factor Heat Shock Factor 1 (HSF1). Under basal conditions, HSF1 rests in the cells as an inactive monomer. Stressful stimuli induce HSF1 trimerization and its nuclear permanency. HSF1 binds the Heat Shock Element (HSE) present in the promoter of hsp genes, where it is finally activated by phosphorylation allowing competence for transcriptional activation [2].
Hsp70 is one of the most conserved proteins in nature [3] characterized by being one of the most highly induced in response to stress [4]. Even though the stress response is a general conserved cellular program, different cell populations present differential capacity to induce Hsp70 expression during stress. Neuronal cells do not induce or induce lower levels of Hsp70 in response to stressful stimuli [5–10]. Moreover, neuronal differentiation programs decrease heat shock response. For instance, PC12 differentiation to pseudo sympathetic neuronal phenotype by neuronal growth factor (NGF) treatment [11] decreases their capacity to induce Hsp70 in response to heat shock and ethanol treatments [12,13]. The lower capacity of neurons to induce Hsp70 during stress may have important implications for the vulnerability to neurodegenerative diseases [14–16]. For, instance, overexpression of Hsp70 reduces neuronal dystrophy in a mouse model of Parkinson’s disease [17] and Hsp70 reduction by miR-61-1 increases α-synuclein aggregation in SH-SY5Y cells [18].
Several mechanisms have been studied to clarify why neurons display lower induction of Hsp70 in stress. Marcuccilli et al. [19] suggested a more important role for HSF2 since HSF1 was barely detected in neurons. It has been shown that even though HSF1 is present in neurons, there is a lack of proper activation during heat shock [20]. Other scientists have suggested a negative role for chromatin on hsp70 gene expression in neuronal cells, thus preventing the access of HSF1 and other transcription factors to the promoter [7,10]. Moreover, it was shown that HSF1 does not bind DNA under stress in cell lines with neuronal phenotype [7,21]. This proposal has been reinforced by data showing that histone deacetylase (HDAC) inhibitors increase hsp70 transcription in neurons [22,23]. Additionally, Guertin and Lis [24] showed in Drosophila by genome-wide analysis that an active chromatin landscape around HSEs is required for HSF1 binding elicited by heat shock. These data indicate that the regulation of Hsp70 expression and neuroprotection mechanisms during stress in neurons are still poorly understood.
Post-translational modifications (PTMs) of the N-terminal tail of histones underlie chromatin status regulating gene expression. Acetylation of histones marks actively transcribed chromatin while closed chromatin is characterized by unacetylated histones. On the other hand, methylation on specific residues distinguishes actively transcribed genes from repressed ones. Di- and tri-methylated lysine 4 on histone H3 (H3K4me2, H3K4me3) and unmethylated K9 on histone 3 (H3K9me0) are features of actively transcribed genes, and the opposite marks are found in repressed genes [25]. The importance of PTMs of histones on hsp70 promoter in response to stress has been shown in yeast and Drosophila and increasing data is available in mammalian genomes [26].
In this work, rat transcriptomic databases and cultured rat cortical neurons were used to study HSF1 expression, nuclear translocation and binding to DNA. In addition, chromatin PTMs at the hsp70 promoter, in basal and stress conditions, were analyzed to inquire further into the mechanisms that decrease stress-dependent induction of Hsp70 in neurons. Altogether, the data show that cortical neurons display lower response to heat shock even though HSF1 is present and activated. Strong HDAC-dependent repression and a specific failure of HSF1 binding to the hsp70 promoter weakens Hsp70 induction in cortical neurons.
Materials and Methods
Primary Culture of Cortical Neurons
Primary cultures of rat cortical neurons were prepared from E18 embryos, obtained from timed pregnant Sprague-Dawley rats (Animal Care Facility of the Faculty of Biological Sciences, Pontificia Universidad Catolica de Chile) decapitated using a guillotine. Every effort was made to reduce the chance of pain or suffering. The procedures were approved by the Bioethical Committee of the Faculty of Biological Sciences of the Pontificia Universidad Católica de Chile and were performed in strict accordance with the guidelines published in ‘‘NIH Guide for the Care and Use of Laboratory Animals” and “Guidelines for the Use of Animals in Neuroscience Research” by the Society for Neuroscience.
Cortical cells prepared as described [27] were plated on poly-L-lysine-coated wells and maintained in Neurobasal medium supplemented with B27, 100 U/ml penicillin and 100 μg/ml streptomycin for 7 days in vitro (DIV), before any experimental manipulation. To inhibit glial proliferation, 2 μM Cytosine-Arabinoside [28] was added on the second day of culture and removed by changing the medium 24h later. Unless otherwise indicated, all cell culture reagents were acquired from Invitrogen Corporation.
PC12 differentiation protocol
PC12 cells (ATCC) were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% horse serum, 5% fetal bovine serum, 1% penicillin/streptomycin and maintained at 37°C, 10% CO2. For neuronal differentiation experiments, PC12 cells were plated at 5x103 cells/cm2. Twenty four hours later, the medium was replaced by DMEM supplemented with 2% horse serum plus 50 ng/ml of NGF (Alomone Labs, Ltd). Cells were maintained for 7 days, changing the media with fresh NGF every two days, prior to heat shock treatment. Undifferentiated PC12 cells were cultured under low serum conditions but without NGF.
Heat Shock and Drug Treatments
Heat shock was induced by incubating PC12 and cortical neuron cultures for 2h in a preheated water bath at 42°C. Any modification to this heat shock protocol is indicated in figure legends. Trichostatin A (TSA; Sigma-Aldrich) was directly added to the culture medium at the desired concentration ranging from 2.5–40 nM. Cells were incubated with the drug for 15h and harvested afterward.
Preparation of Nuclear Fractions and Western Blotting
PC12 cells and cortical neurons were washed three times and scrapped in ice-cold phosphate-buffered saline (PBS). Whole-cell extracts were obtained by resuspending the cells with 1ml syringe in Triton X-100 lysis buffer (50mM Tris-HCl pH 7.5, 1% Triton X-100, 150mM NaCl, 1mM PMSF plus protease inhibitors) and left on ice for 20min. Homogenates were centrifuged at 14000 rpm for 20 min at 4°C and supernatants were saved for further analysis. Samples were resolved on SDS-PAGE and proteins detected by western blot with a mouse monoclonal antibody against Hsp70 (Stressmarq) and a rabbit polyclonal antibody against HSF1 (Cell Signaling Technology, Inc). Quantification of Hsp70 induction was carried out as described before [29] using α-tubulin (mouse monoclonal antibody; Sigma) and GAPDH (mouse monoclonal antibody; Zymed, Millipore) as loading controls. The nuclear fraction from cortical neurons was obtained as described previously [30] with one modification. After the wash of the nuclear fraction, the pellet was resuspended in Triton X-100 lysis buffer as described above, and 25μg of nuclear protein was used for western blotting.
Quantitative Real-Time PCR
Total RNA was extracted from cells using TRIzol reagent (Invitrogen Corporation) following manufacturer’s instructions; 500ng of RNA were subjected to reverse transcription using MMLV-RT (Fermentas International Inc). Quantitative Real-Time PCR analysis was performed using a LightCycler (Roche Applied Science) as described before [31]. Cyclophilin A (CYC) was used as the internal reference gene. The following primers were used to amplify target cDNAs: Hsp70c-F (5’-AACTACAAGGGCGAGAACCGGTC-3’)
Hsp70c-R (5’-GATGATCCGCAGCACGTTCAGA-3’)
Hsp25c-F (5’- ACTCAGCAGCGGTGTCTCAGAGATCC-3’)
Hsp25c-R (5’-GGTGAAGCACCGAGAGATGTAGCCA-3’)
CYC-F: (5’-TGCTCTGAGCACTGGGGAGAAA-3’)
CYC-R: (5’-CATGCCTTCTTTCACCTTCCCAAAGAC-3’).
Plasmids
Myc/His-hHSF1 expression vector encoding full-length human HSF1 was previously described [29,32]. Hsp70B-luc reporter plasmid was generated by subcloning a BglII-HindIII fragment (1.44kb) containing human Hsp70B gene promoter from p2500CAT vector (Stressgen Biotechnologies Corp; [33]) into pGL3-basic vector (Promega).
Reporter Gene Assays
Transient transfection and reporter gene assays were performed as previously described [34]. Briefly, PC12 cells (1.5x105 cells/well; 24 wells plate) were transfected with 515ng of total DNA by using Lipofectamine 2000 reagent (Invitrogen). Hsp70B-luc reporter plasmid (149.3ng) was used in 1:1 molar ratio respect to Myc/His-hHSF1 expression plasmid. DNA was kept constant by adding pBLUescript (Stratagene), and in every experiment 25ng of the pCMX-β-gal reporter vector was cotransfected as control of transfection efficiency.
Chromatin Immunoprecipitation Assay
ChIP assays from PC12 cells (7x106 cells) and cortical neurons (1x107 cells) were performed as previously described [29]. Immunoprecipitations were carried out with the following rabbit polyclonal antibodies: anti-H3Ac, anti-H4Ac, anti-H3K4me2, H3K9me2 (Upstate, Millipore); anti-H3K4me3, anti-H3 (Abcam) and anti-HSF1 (Santa Cruz, Biotech). Rabbit Pre-immune IgG (Santa Cruz, Biotech) and no antibody were used as a control of immunoprecipitation specificity. Quantitative real-time PCR (qPCR) analysis was performed using a LightCycler (Roche Applied Science). One μl of each sample was subjected to qPCR using the following primers: rHsp70pr-F (5’-ACACTTGTCACAACCGGAACAAGC-3’), rHsp70pr-R (5’-TCTCTGCGAGTGGAACCAGAAACT-3’), rHsp25-F1 (5’-GACAGTGGGAACTGCTCCAG-3') and rHsp25-R1: (5'-TGGCAATGACCGTCTAAGGG-3'). Standard curves were generated by using serial dilutions of previously quantified genomic DNA. The amount of immunoprecipitated DNA in each sample was calculated using fit point analysis with no baseline adjustment. Data was normalized to the Percentage of the Input Method for further analysis. When comparing histone PTMs of the hsp70 (hspa1b) or the hsp25 (hspb1) rat gene promoters between cortical neurons and PC12 cells, ChIP signals were normalized relative to nucleosome density. In this case, the ChIP-qPCR signals obtained with every antibody were divided by the signal obtained with anti-H3 [35].
Bioinformatic Analysis
RNA-seq data of rat whole brain (SRR594428, male breeding age) and cerebral cortex (SRR388230, SRR388232 and SRR388234, adult male) were used. Whole brain data were sequenced by Illumina HiSeq 2000 [36], and the reads were processed to remove the adapters and low-quality sequences with FASTX toolkit (http://hannonlab.cshl.edu/fastx_toolkit/index.html). The resultant reads were aligned to the rat reference genome (rn5) using TopHat [37]. Cerebral cortex data were sequenced by AB SOLiD 4 System using a strand-specific library preparation protocol [38]. The 50 nt reads were trimmed to 36 nt. The resultant reads were aligned using Bowtie [39] to the rat reference genome (rn5). We used CAGE data from rat brain (8–12 weeks) and cerebral cortex (DRP000155, 3–28 days) [40] and PolyA-seq data from rat brain (SRX080235) [41]. The reads were processed to remove the adapters and low-quality sequences and were mapped to the rat reference genome (rn5) using Bowtie. All the alignments were visualized as the UCSC Genome Browser tracks [42]. An integration of RNA-seq, CAGE-seq, and PolyA-seq data were used to propose a more accurate annotation of Hsp70.
Statistical analysis
Results are presented as mean ± SEM from at least three independent experiments. The results were analyzed for statistical significance as described in each figure legend, using GraphPad PRISM version 6.0.
Results
Neuronal cells show reduced induction of Hsp70 in response to heat shock
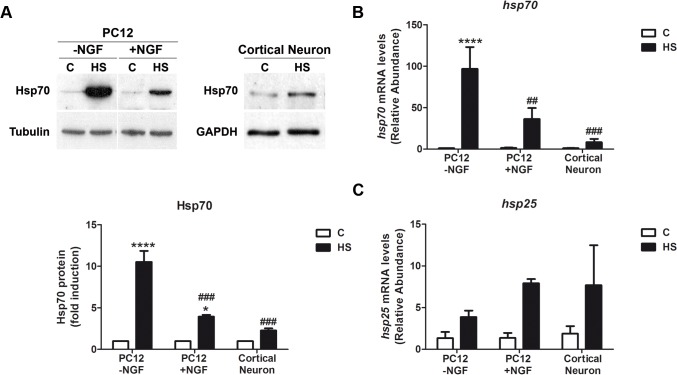
Primary cultures of rat cortical neurons, and undifferentiated and NGF-treated PC12 cells were exposed to 42°C for 2h. Hsp70 protein levels are low under basal conditions in every cell type analyzed. The amount of Hsp70 protein increased in the three cell types in response to heat shock, even though the data confirm that the increment of Hsp70 in cells with neuronal phenotype, such as NGF-treated PC12 cells (PC12 plus NGF, 3.9 ± 0.3 fold of induction) and cortical neurons (2.4 ± 0.6 fold of induction) is significantly lower than in non-neuronal cells (undifferentiated PC12 cells; 8.2 ± 0.2 fold of induction) (Fig 1A). This lower induction of Hsp70 by heat shock in neurons is also observed at transcriptional level. After 2h of heat shock, the mRNA of hsp70 is induced 96.8 times in undifferentiated PC12 cells while in cortical neurons is induced only 8.9 times (Fig 1B). This effect is specific for hsp70 gene since hsp25 mRNA is induced to a similar extent in cortical neurons and, NGF-treated and naïve PC12 cells (Fig 1C). These data suggest that the neuronal differentiation process decreases the capability of inducing hsp70 gene transcription by heat stress.
Fig 1. Neuronal cells show weaker induction of Hsp70 in response to heat shock.
(A) Upper panels: Representative immunoblots of Hsp70 protein in undifferentiated (-NGF) and differentiated (+NGF for 7d) PC12 cells, and cortical neurons (E18.5, 7div) subjected to heat shock (42°C, 2h) or kept under control conditions (C, 37°C). Lower panel: Quantification of the relative Hsp70 protein levels showed in the upper panels. Tubulin and GAPDH were used as loading controls. Statistical analyses were performed by Two-way ANOVA followed by Tukey’s multiple comparisons test. ****p < 0.0001, *p < 0.05 compared with control; ###p <0.001, compared to Hsp70 fold induction in undifferentiated PC12 cells during heat shock. (B, C) Relative abundance of hsp70 and hsp25 transcripts in PC12 cells and cortical neurons. hsp70 and hsp25 mRNA levels were calculated comparing the abundance of each cDNA in cells under control and heat shock conditions. For each sample, cyclophilin mRNA was used as a reference gene. Data are expressed as mean plus SEM of at least three independent experiments. Statistical analyses were performed by Two-way ANOVA followed by Tukey's multiple comparisons test. ****p < 0.0001, *p < 0.05 compared with control; ##p < 0.01, ###p <0.001, compared to Hsp70 mRNA abundance in undifferentiated PC12 cells during heat shock.
Transcriptomic profiling data of whole brain and cerebral cortex show a differential transcriptional start sites usage of hsp70 gene
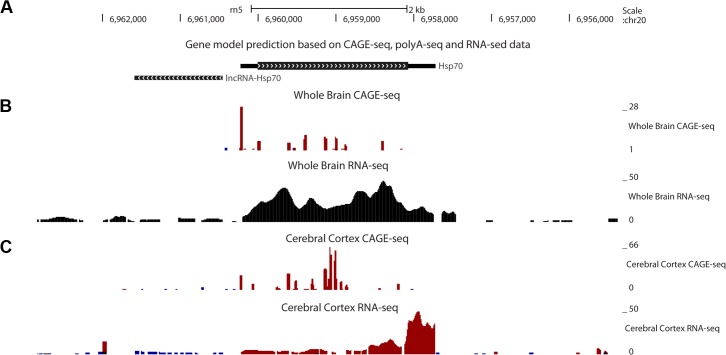
To look for in vivo data of hsp70 transcriptional pattern, we studied the transcriptomic profile of rat hsp70 gene in the cerebral cortex and whole brain. Based on RNA-seq, CAGE-seq and PolyA-seq data, we annotated a rat hsp70 gene model (Fig 2A). Transcriptome profiling data show a predominant usage of the bidirectional promoter of hsp70 in the whole brain but not in the cerebral cortex. In cerebral cortex the highest CAGE-seq peaks are located inside the hsp70 open-reading frame (ORF), which is coincident with a RNA-seq coverage increase at the 3’ end of hsp70 (Fig 2B and 2C), indicating that differential transcriptional start sites usage occurs in cortical cells. This data suggests that cryptic promoters are activated inside hsp70 ORF in cortical cells, and this is due to a weaker activation of the canonical promoter of hsp70.
Fig 2. The hsp70 gene shows predominant cryptic transcription initiation sites usage in rat cerebral cortex.
(A) Model of rat hsp70 gene based on our transcriptome profiling data analysis. (B) Whole brain transcriptome profiling data shows a predominant transcription start site at the beginning of the gene. (C) Cerebral cortex transcriptome profiling data shows predominant transcriptional start sites inside the codifying sequence of hsp70 gene. The coverage for reads aligned in the positive and negative strand are shown in red and blue, respectively.
Heat shock induces HSF1 nuclear translocation; although HSF1 does not bind to hsp70 promoter in cortical neurons
HSF1 is the main transcription factor responsible for inducing Hsp70 expression during the heat shock response. Thus, a lower induction of Hsp70 in neurons could be due to a lower expression of HSF1 or a failure of its activation in response to stress. Cortical neurons and undifferentiated and NGF-treated PC12 cells were exposed to heat shock to evaluate the following aspects of HSF1: protein levels, transcriptional activity, heat shock-induced nuclear localization and DNA binding ability to the endogenous hsp70 gene promoter. Western blot assays, performed with an anti-HSF1 antibody, which recognizes both the hypo- and hyper-phosphorylated forms, showed that cortical neurons, and undifferentiated and NGF-treated PC12 cells displayed equivalent amounts of both faster and heat shock-induced slower HSF1 bands (Fig 3A) indicating that HSF1 level is not the limiting factor inducing Hsp70 during heat shock in neuronal cells. Supporting the activation of HSF1 in cortical neurons, one hour after heat shock a strong signal of HSF1 was observed in the nuclear fraction (Fig 3B), showing that HSF1 is efficiently translocated to cell nuclei in response to stress. These results indicate that the initial steps required for HSF1 activation are operative in neurons. To test the transactivation ability of HSF1 in neuronal cells, gene reporter assays were performed using 1.44 Kb of the hsp70 gene promoter. The graph in Fig 3C shows that following a short stimulation with NGF (24 hours), HSF1 lost 50% of its transactivation ability, confirming that neuronal differentiation process inhibits Hsp70 induction by this transcription factor. The lower reporter activity in NGF-treated PC12 cells could be due to either a failure of the transcriptional activity of HSF1 or a failure of its DNA binding ability. ChIP assays were carried out to compare HSF1 binding to hsp70 and hsp25 promoters in cortical neurons and PC12 cells under basal and heat shock conditions. There was no detectable interaction of HSF1 on the hsp70 gene promoter in cortical neurons under basal or after one hour of heat shock (Fig 3D, upper panel). By contrast, heat shock induced a significant HSF1 binding to the hsp70 promoter in undifferentiated PC12 cells (Fig 3D, upper panel). Remarkably, HSF1 bound efficiently to the hsp25 promoter under heat shock conditions in both PC12 and cortical neurons during heat shock response (Fig 3D lower panel). Altogether, the data indicate that the mechanisms activating HSF1 in neurons work properly during heat shock. Moreover, the data suggest that neuronal differentiation may induce changes on chromatin landscape at the hsp70 gene promoter, preventing HSF1 binding specifically to this promoter in neurons.
Fig 3. HSF1 does not bind to the hsp70 promoter in neurons during heat shock.
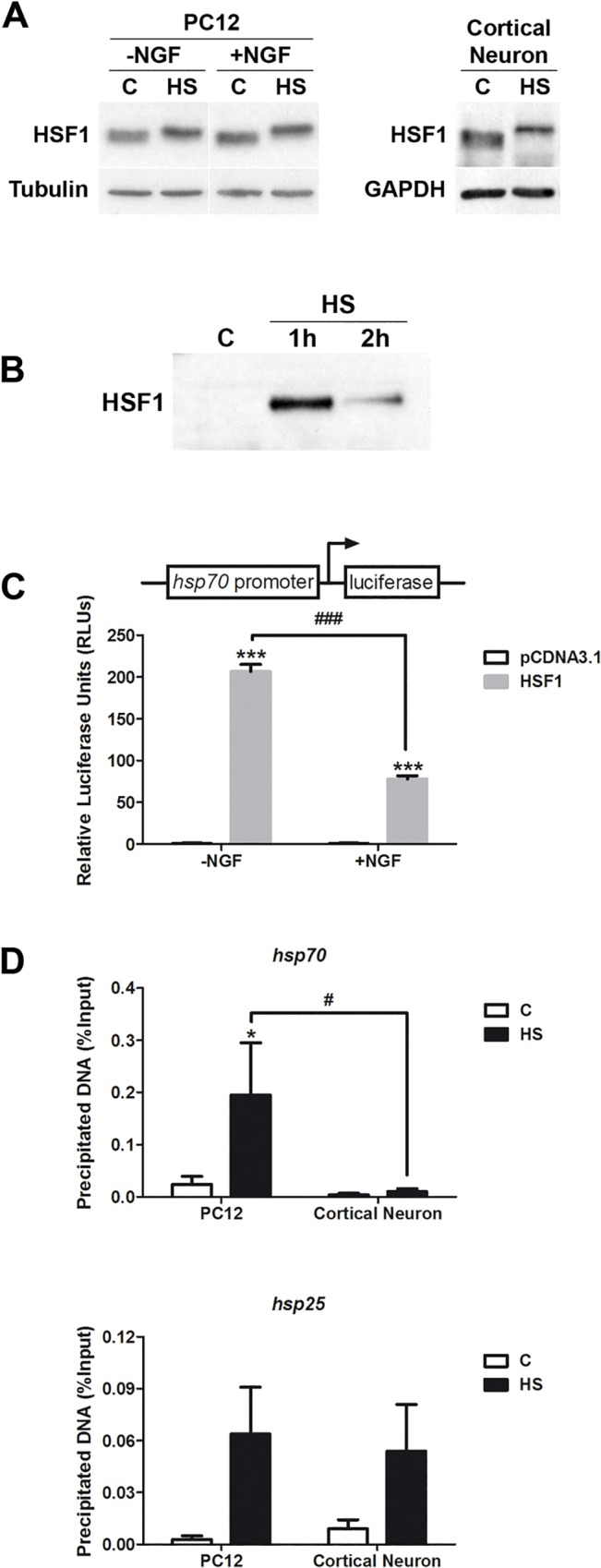
(A) Representative immunoblots of HSF1 protein expression in undifferentiated (-NGF) and differentiated (+NGF for 7d) PC12 cells, and in cortical neurons (E18.5, 7div) subjected to heat shock (42°C, 2h) or kept at control conditions (C, 37°C). (B) Nuclear fractions from cortical neurons at control or, after 1h or 2h of heat shock were analyzed for HSF1 protein detection. (C) HSF1 ability to activate hsp70 promoter in PC12 cells was assayed after 24 h of NGF (50 ng/ml) treatment. Data correspond to the mean plus SEM of three independent experiments. Statistical analyses were performed by Two-way ANOVA followed by Tukey's multiple comparisons test: ***p <0.001, compared to pCDNA3.1; ###p< 0.001 (D) PC12 cells and cortical neurons were heat shocked at 42°C for 1h or kept unstressed. Chromatin was immunoprecipitated with an anti-HSF1 antibody and amplified by quantitative real-time PCR using primers flanking the promoter area of both hsp70 (upper panel) and hsp25 (lower panel) genes. Data are expressed as the percentage of the immunoprecipitated DNA in relation to the Input and correspond to the mean plus SEM of at least three independent experiments. Statistical analyses were performed by Two-way ANOVA followed by Tukey's multiple comparisons test: *p < 0.05 compared with control; #p < 0.05 compared to heat shocked PC12 cells.
Histone PTM features on hsp70 gene promoter in cortical neurons and PC12 cells
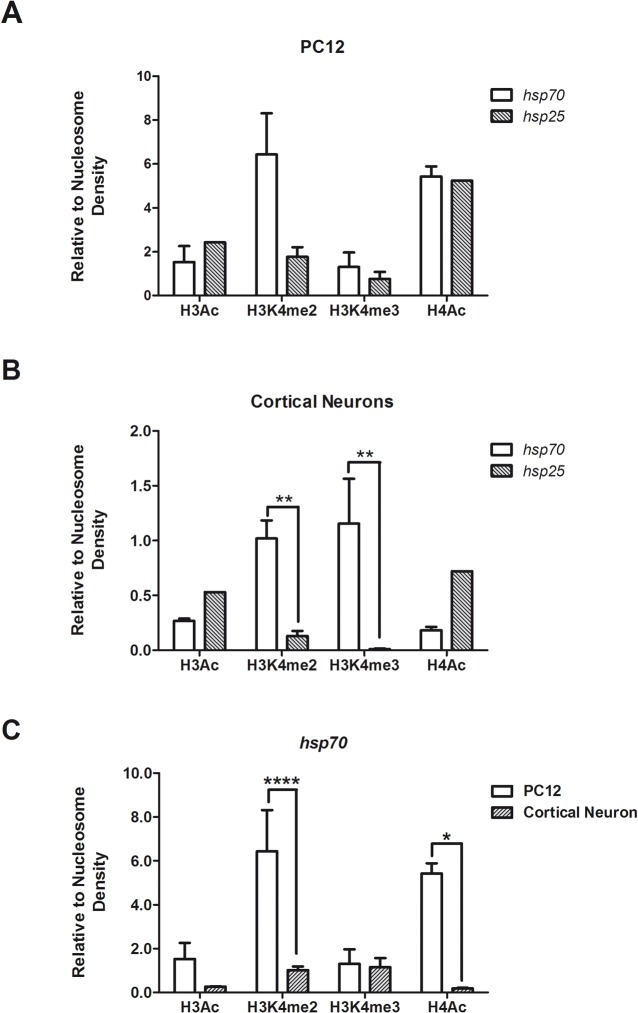
Transcriptional activators such as HSF1 recognize and bind specific DNA sequence elements depending on chromatin context [43]. To assess whether HSEs on the hsp70 gene promoter are in a permissive or repressed chromatin context in neurons, ChIP experiments were performed to evaluate H3Ac (K9, K14), H4Ac (K5, K8, K12, K16), H3K4me2, H3K4me3 and H3K9me2 levels. In addition, histone PTMs of hsp25 promoter in PC12 cells (Fig 4A) and cortical neurons (Fig 4B) were compared. ChIP data show that at basal conditions in PC12 cells, histone PTMs of hsp70 promoter were similar to those of hsp25 promoter, except for H3K4me2 that was 3.6 times higher for hsp70 (Fig 4A). H3K4me2 defines Transcription Factors Binding Regions (TFBRs) [44], associating this feature of hsp70 promoter with higher binding of HSF1. Surprisingly, higher levels of H3K4me2 were also observed in the hsp70 promoter in cortical neurons (Fig 4B), while no binding of HSF1 occurred (Fig 3D). Thus, hsp70 promoter maintains its accesibility to other transcription factors in neurons. Moreover, a higher level of H3K4me3 was also observed in hsp70 gene promoter in cortical neurons compared with hsp25 (Fig 4B). H3K9me2, which is associated with transcriptional repression [43], was not detected in the promoter of hsp70 in any cell type (data not shown). Regarding histone acetylation, hsp70 promoter acetylation was significantly lower in cortical neurons compared to PC12 cells (Fig 4C). Furthermore, levels of H3Ac and H4Ac in the hsp25 promoter were higher than in the hsp70 promoter in cortical neurons (Fig 4B). Altogether, the data suggest that the hsp70 promoter is more closed in cortical neurons than in non-neuronal cells, even though it presents features of an active promoter.
Fig 4. Analysis of the chromatin environment of rat promoter regions of hsp70 and hsp25 genes.
H3Ac, H4Ac, H3K4me2 and H3K4me3 were analyzed in the proximal promoter region of the hsp70 and hsp25 rat genes in undifferentiated PC12 cells (A) and cortical neurons (B), under basal conditions. The graphs show the data from the ChIP assays and correspond to the mean plus SEM of the percentage of DNA immunoprecipitated by each antibody (% of the Input), normalized by the percentage of DNA immunoprecipated by anti-H3 antibody (Relative to Nucleosome Density). Statistical analyses were performed by Two-way ANOVA followed by Bonferroni post hoc multiple comparison test. **p < 0.01. (C) Comparative analysis of the hsp70 gene promoter between PC12 and cortical neurons. The graph shows the data as described above for (A) and (B). Statistical analyses were performed by Two-way ANOVA followed by Sidak's multiple comparison test. ****p < 0.0001; *p < 0.05.
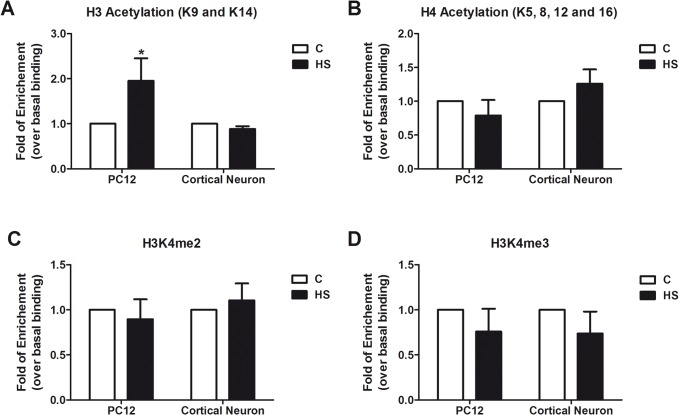
To assess whether heat shock induces changes on histone PTMs that may account for the different degree of Hsp70 induction between neuronal and non-neuronal cells, histone PTMs on hsp70 promoter during heat shock in cortical neurons and PC12 cells were evaluated. ChIP results showed that heat shock significantly increased H3Ac levels on the hsp70 promoter in PC12 cells, but not in neurons (Fig 5A). Levels of H4Ac, H3K4me2 and H3K4me3, remained equal during heat shock in cortical neurons and PC12 cells (Fig 5B–5D).
Fig 5. Effect of heat shock on histone PTMs of the rat hsp70 promoter in PC12 cells and cortical neurons.
Comparison of four histone PTM levels: H3Ac (A) and H4Ac (B), H3K4me2 (C) and H3K4me3 (D), at the hsp70 gene promoter under control (white bars) and heat shock (black bars) conditions, in cortical neurons and PC12 cells. Cells were heat shocked at 42°C for 1h or kept unstressed. Data is expressed as fold of enrichment over basal condition (unstressed) and correspond to the mean plus SEM of three independent experiments. Statistical analyses were performed by Two-way ANOVA followed by Sidak's multiple comparisons test: *p < 0.05 compared to control.
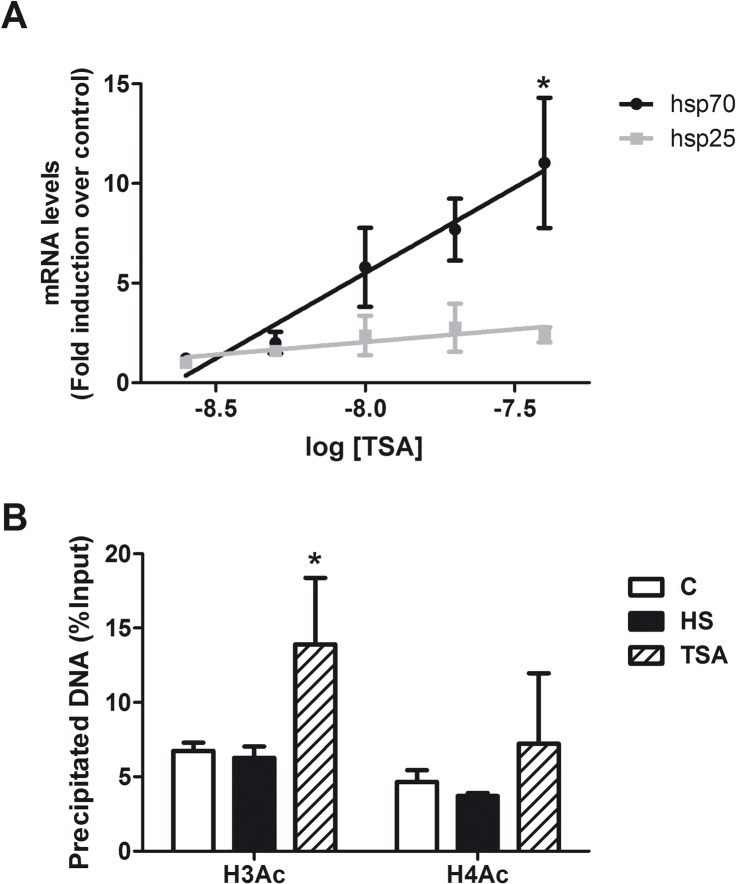
Considering the previous results indicating an association between histone acetylation and Hsp70 induction, we studied the effect of increasing histone acetylation by HDAC inhibition with Trichostatin A (TSA) on hsp70 and hsp25 induction. TSA treatment specifically augmented hsp70 transcript in a dose-dependent manner, while it did not change hsp25 mRNA expression (Fig 6A). Similar results were observed for Hsp70 protein that reached 4.9 times higher levels at 40 nM TSA, compared to the control condition (not shown). The hsp70 expression induced by TSA correlated with a significant increase of H3Ac level at hsp70 promoter (Fig 5B). TSA treatment did not alter the methylation status of H3K4 (data not shown). These results suggest a direct relationship between H3Ac levels and Hsp70 induction. No HSF1 was detected at the hsp70 gene promoter in cortical neurons treated with TSA (data not shown), indicating that TSA-induction of Hsp70 in neurons is independent of HSF1 binding to the hsp70 promoter.
Fig 6. Augmented H3Ac mediated by HDACs inhibition is associated with increased expression of Hsp70 in cortical neurons.
(A) Fold Induction of hsp70 (black circles) and hsp25 (grey squares) transcripts in cortical neurons by different doses of TSA. Data are expressed as mean plus SEM of at least three independent experiments. Statistical analyses were performed by best fit Linear Regression and Spearman’s r correlation analysis where *p < 0.05 for hsp70 mRNA. (B) Comparison of histone acetylation of H3 and H4 at the hsp70 gene promoter in cortical neurons under control, heat shock (42°C, 1h) or TSA (50 nM, 15h) treatment. Data is expressed as the percentage of the immunoprecipitated DNA in relation to the input and correspond to the mean plus SEM of at least three independent experiments. Statistical analyses were performed by Two-way ANOVA followed by Tukey's multiple comparisons test: *p < 0.05 compared to control.
Discussion
For long time, the decreased stress-dependent induction of Hsp70 in neurons has been subject of debate, with most of the hypotheses pointing to a failure on HSF1 expression or activation [19,20,45,46]. In this report, the evidence indicates that the decreased induction of Hsp70 is related to the failure of HSF1 binding specifically to the hsp70 promoter in neurons. This data supports a role for changes in chromatin context during neuronal differentiation that hamper HSF1 binding to this gene. The data also suggest a role for histone PTMs on the hsp70 promoter in this phenomenon. In particular, reduced levels of H4Ac were associated with absence of HSF1 binding to the hsp70 promoter in neurons.
The results allow discarding a lower abundance of HSF1 as the limiting factor explaining the lower induction of Hsp70 in neurons during heat shock stress. As shown by Western blots, HSF1 is detected in equivalent amounts in undifferentiated and NGF-differentiated PC12 cells, as well as in cultured cortical neurons. Furthermore, heat shock induced HSF1 translocation to the nuclei of neurons, suggesting that HSF1 activation is operative in neurons. However, heat shock failed inducing HSF1 binding at the hsp70 promoter in neurons, as was showed by ChIP assays, and as it was previously reported in cell lines with neuronal phenotype [7,21].
The analysis of the chromatin landscape showed that the promoter of hsp70 does not have epigenetic marks for repression in cortical neurons, but present a profile of PTMs indicative of a more closed promoter compared to PC12 cells. Indeed, the results showed that the hsp70 promoter in neurons exhibited higher levels of H3K4m2 and H3K4me3, compared with hsp25, indicative of sites prone for binding of transcription factors and transcriptional activation [25]. However, significant lower histone H4Ac levels were observed in the hsp70 promoter in neurons compared to non-neuronal cells. Moreover, levels of acetylation of H3 and H4 in hsp70 promoter are lower than in hsp25 promoter in cortical neurons. Bioinformatic data also indicated that the hsp70 promoter is weaker in cortex compared to the whole brain. It has been shown that transcription elongation factors repress transcription initiation from cryptic sites [47–49]. Therefore, a decreased hsp70 transcription in rat cortex would generate a permissive environment for transcription initiation from within hsp70 coding region.
The influence of H4Ac in HSF1 DNA-binding affinity has been previously showed. Binding profiles of HSF1 to every HSE in the Drosophila genome revealed that H4Ac is a critical feature modulating HSF1 binding in vivo [24,50]. Moreover, disease progression in a genetic mouse model of Huntington, leads to decreased H4Ac in the brain, which correlates with a reduced ability of HSF1 to bind hsp70 gene promoter despite its activation [51]. Thus, levels of H4Ac correlate with HSF1 binding to target elements.
Lower H3K4me2 in the hsp70 promoter in cortical neurons compared with PC12 cells predicts a less prone binding site for transcription factors. Recent analysis of the ENCODE (Encyclopedia of DNA Elements) Consortium data-base revealed that H3K4me2 consistently defines TFBRs, with an overlapping score of ~90% between H3K4me2 and TFBRs in three different human cell lines. Likewise, regions with higher levels of H3K4me2 exhibit a higher overlapping percentage with TFBRs than regions with lower levels of H3K4me2 [44]. Thus, the data suggest that high H3K4me2 levels in the hsp70 promoter in non-neuronal cells correlate with the strong potency of inducing Hsp70 during stress.
Expression of Hsp70 in the presence of TSA also indicates an association between H3 acetylation and efficient induction of Hsp70 expression. H3 acetylation was the only histone PTM significantly modified by heat shock in non-neuronal cells replicated in cortical neurons by TSA treatment, which associate with induction of Hsp70. The contribution of histone acetylation on Hsp70 expression induced by treatment with HDACs inhibitors in neurons is widely reported [22,23,28,52] and neuroprotective effects mediated by Hsp70 induction has been associated to Sp1 activation [23].
There is no doubt that HSF1 controls Hsp induction. HSF1 knockout mice present no induction of any Hsp in brain tissue [53]. However, the contribution of HSF1 to Hsp70 induced expression in neural tissue remains controversial. The possibility that HSF1 controls the heat shock response and survival in neurons in a non-canonical way is supported by a recent work. In this work by Verma et al, it was demonstrated that HSF1 effectively promotes neuroprotection through an Hsp-independent mechanism that does not require either HSF1 trimerization or the classical transactivation pathway [54].
Another interesting observation is that the induction of other Hsp proteins by heat shock, also dependent on the HSF1 activity, is not affected by neuronal differentiation. As we showed, no significant differences of Hsp25 mRNA induction were observed between differentiated and untreated PC12 cells or cortical neurons. These data strongly indicate that rather than something special preventing HSF1 action in neurons, the hsp70 gene undergoes a modification during neuronal differentiation that prevents the action of HSF1 in this particular gene. Our studies on the transcriptomic profile of rat hsp70 gene in the cerebral cortex, showing differential transcriptional start sites usage, support this idea. This fact added to particular differences on histone PTMs on hsp70 gene promoter may also explain that distinct neuronal cell types induce different extents of Hsp70 [16,20,21,52,55].
In conclusion, we have confirmed that cells with neuronal phenotype exhibit a weaker induction of Hsp70 in response to stress. Our data indicate that this particular feature is owed mainly to the lack of binding of HSF1 to hsp70 gene promoter determined by a less receptive chromatin landscape with a repressive contribution of HDACs.
Data Availability
All relevant data are within the paper.
Funding Statement
This work was supported by the Fondo Nacional de Desarrollo Científico y Tecnológico (http://www.conicyt.cl/fondecyt/sobre-fondecyt/que-es-fondecyt/) Grant # 1110352 to MEA and Millennium Scientific Initiative (http://www.iniciativamilenio.cl) grant N P10/063-F. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Lindquist S (1986) The heat-shock response. Annu Rev Biochem 55: 1151–1191. [DOI] [PubMed] [Google Scholar]
- 2. Anckar J, Sistonen L (2011) Regulation of HSF1 function in the heat stress response: implications in aging and disease. Annu Rev Biochem 80: 1089–1115. 10.1146/annurev-biochem-060809-095203 [DOI] [PubMed] [Google Scholar]
- 3. Gupta RS (1998) Protein phylogenies and signature sequences: A reappraisal of evolutionary relationships among archaebacteria, eubacteria, and eukaryotes. Microbiol Mol Biol Rev 62: 1435–1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Akerfelt M, Morimoto RI, Sistonen L (2010) Heat shock factors: integrators of cell stress, development and lifespan. Nat Rev Mol Cell Biol 11: 545–555. 10.1038/nrm2938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Marini AM, Kozuka M, Lipsky RH, Nowak TS Jr (1990) 70-kilodalton heat shock protein induction in cerebellar astrocytes and cerebellar granule cells in vitro: comparison with immunocytochemical localization after hyperthermia in vivo. J Neurochem 54: 1509–1516. [DOI] [PubMed] [Google Scholar]
- 6. Nishimura RN, Dwyer BE, Clegg K, Cole R, de Vellis J (1991) Comparison of the heat shock response in cultured cortical neurons and astrocytes. Brain Res Mol Brain Res 9: 39–45. [DOI] [PubMed] [Google Scholar]
- 7. Mathur SK, Sistonen L, Brown IR, Murphy SP, Sarge KD, Morimoto RI (1994) Deficient induction of human hsp70 heat shock gene transcription in Y79 retinoblastoma cells despite activation of heat shock factor 1. Proc Natl Acad Sci U S A 91: 8695–8699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nishimura RN, Dwyer BE (1996) Evidence for different mechanisms of induction of HSP70i: a comparison of cultured rat cortical neurons with astrocytes. Brain Res Mol Brain Res 36: 227–239. [DOI] [PubMed] [Google Scholar]
- 9. Walsh D, Li Z, Wu Y, Nagata K (1997) Heat shock and the role of the HSPs during neural plate induction in early mammalian CNS and brain development. Cell Mol Life Sci 53: 198–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Drujan D, De Maio A (1999) Expression of HSP70 is impaired at the transcriptional level in stressed murine neuroblastoma cells. Shock 12: 443–448. [DOI] [PubMed] [Google Scholar]
- 11. Greene LA, Tischler AS (1976) Establishment of a noradrenergic clonal line of rat adrenal pheochromocytoma cells which respond to nerve growth factor. Proc Natl Acad Sci U S A 73: 2424–2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dwyer DS, Liu Y, Miao S, Bradley RJ (1996) Neuronal differentiation in PC12 cells is accompanied by diminished inducibility of Hsp70 and Hsp60 in response to heat and ethanol. Neurochem Res 21: 659–666. [DOI] [PubMed] [Google Scholar]
- 13. Hatayama T, Takahashi H, Yamagishi N (1997) Reduced induction of HSP70 in PC12 cells during neuronal differentiation. J Biochem 122: 904–910. [DOI] [PubMed] [Google Scholar]
- 14. Tonkiss J, Calderwood SK (2005) Regulation of heat shock gene transcription in neuronal cells. Int J Hyperthermia 21: 433–444. [DOI] [PubMed] [Google Scholar]
- 15. Chen S, Brown IR (2007) Neuronal expression of constitutive heat shock proteins: implications for neurodegenerative diseases. Cell Stress Chaperones 12: 51–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tagawa K, Marubuchi S, Qi ML, Enokido Y, Tamura T, Inagaki R, et al. (2007) The induction levels of heat shock protein 70 differentiate the vulnerabilities to mutant huntingtin among neuronal subtypes. J Neurosci 27: 868–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Moloney TC, Hyland R, O'Toole D, Paucard A, Kirik D, O'Doherty A, et al. (2014) Heat shock protein 70 reduces alpha-synuclein-induced predegenerative neuronal dystrophy in the alpha-synuclein viral gene transfer rat model of Parkinson's disease. CNS Neurosci Ther 20: 50–58. 10.1111/cns.12200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhang Z, Cheng Y (2014) miR-16-1 promotes the aberrant alpha-synuclein accumulation in parkinson disease via targeting heat shock protein 70. ScientificWorldJournal 2014: 938348 10.1155/2014/938348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Marcuccilli CJ, Mathur SK, Morimoto RI, Miller RJ (1996) Regulatory differences in the stress response of hippocampal neurons and glial cells after heat shock. J Neurosci 16: 478–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Batulan Z, Shinder GA, Minotti S, He BP, Doroudchi MM, Nalbantoglu J, et al. (2003) High threshold for induction of the stress response in motor neurons is associated with failure to activate HSF1. J Neurosci 23: 5789–5798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yang J, Oza J, Bridges K, Chen KY, Liu AY (2008) Neural differentiation and the attenuated heat shock response. Brain Res 1203: 39–50. 10.1016/j.brainres.2008.01.082 [DOI] [PubMed] [Google Scholar]
- 22. Ren M, Leng Y, Jeong M, Leeds PR, Chuang DM (2004) Valproic acid reduces brain damage induced by transient focal cerebral ischemia in rats: potential roles of histone deacetylase inhibition and heat shock protein induction. J Neurochem 89: 1358–1367. [DOI] [PubMed] [Google Scholar]
- 23. Marinova Z, Ren M, Wendland JR, Leng Y, Liang MH, Yasuda S, et al. (2009) Valproic acid induces functional heat-shock protein 70 via Class I histone deacetylase inhibition in cortical neurons: a potential role of Sp1 acetylation. J Neurochem 111: 976–987. 10.1111/j.1471-4159.2009.06385.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Guertin MJ, Lis JT (2010) Chromatin landscape dictates HSF binding to target DNA elements. PLoS Genet 6: e1001114 10.1371/journal.pgen.1001114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Suganuma T, Workman JL (2011) Signals and combinatorial functions of histone modifications. Annu Rev Biochem 80: 473–499. 10.1146/annurev-biochem-061809-175347 [DOI] [PubMed] [Google Scholar]
- 26. Calderwood SK, Xie Y, Wang X, Khaleque MA, Chou SD, Murshid A, et al. (2010) Signal Transduction Pathways Leading to Heat Shock Transcription. Sign Transduct Insights 2: 13–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Blanco EH, Zuniga JP, Andres ME, Alvarez AR, Gysling K (2011) Corticotropin-releasing factor binding protein enters the regulated secretory pathway in neuroendocrine cells and cortical neurons. Neuropeptides 45: 273–279. 10.1016/j.npep.2011.05.002 [DOI] [PubMed] [Google Scholar]
- 28. Faraco G, Pancani T, Formentini L, Mascagni P, Fossati G, Leoni F, et al. (2006) Pharmacological inhibition of histone deacetylases by suberoylanilide hydroxamic acid specifically alters gene expression and reduces ischemic injury in the mouse brain. Mol Pharmacol 70: 1876–1884. [DOI] [PubMed] [Google Scholar]
- 29. Gomez AV, Galleguillos D, Maass JC, Battaglioli E, Kukuljan M, Andrés ME (2008) CoREST represses the heat shock response mediated by HSF1. Mol Cell 31: 222–231. 10.1016/j.molcel.2008.06.015 [DOI] [PubMed] [Google Scholar]
- 30. Wang H, Yu SW, Koh DW, Lew J, Coombs C, Bowers W, et al. (2004) Apoptosis-inducing factor substitutes for caspase executioners in NMDA-triggered excitotoxic neuronal death. J Neurosci 24: 10963–10973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Galleguillos D, Fuentealba JA, Gomez LM, Saver M, Gomez A, Nash K, et al. (2010) Nurr1 regulates RET expression in dopamine neurons of adult rat midbrain. J Neurochem 114: 1158–1167. 10.1111/j.1471-4159.2010.06841.x [DOI] [PubMed] [Google Scholar]
- 32. Hietakangas V, Ahlskog JK, Jakobsson AM, Hellesuo M, Sahlberg NM, Holmberg CI, et al. (2003) Phosphorylation of serine 303 is a prerequisite for the stress-inducible SUMO modification of heat shock factor 1. Mol Cell Biol 23: 2953–2968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Schiller P, Amin J, Ananthan J, Brown ME, Scott WA, Voellmy R (1988) Cis-acting elements involved in the regulated expression of a human HSP70 gene. J Mol Biol 203: 97–105. [DOI] [PubMed] [Google Scholar]
- 34. Barrios AP, Gomez AV, Saez JE, Ciossani G, Toffolo E, Battaglioli E et al. (2014) Differential properties of transcriptional complexes formed by the CoREST family. Mol Cell Biol 34: 2760–2770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Haring M, Offermann S, Danker T, Horst I, Peterhansel C, Stam M (2007) Chromatin immunoprecipitation: optimization, quantitative analysis and data normalization. Plant Methods 3: 11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Merkin J, Russell C, Chen P, Burge CB (2012) Evolutionary dynamics of gene and isoform regulation in Mammalian tissues. Science 338: 1593–1599. 10.1126/science.1228186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Trapnell C, Pachter L, Salzberg SL (2009) TopHat: discovering splice junctions with RNA-Seq. Bioinformatics 25: 1105–1111. 10.1093/bioinformatics/btp120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wood SH, Craig T, Li Y, Merry B, de Magalhaes JP (2013) Whole transcriptome sequencing of the aging rat brain reveals dynamic RNA changes in the dark matter of the genome. Age (Dordr) 35: 763–776. 10.1007/s11357-012-9410-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Langmead B, Trapnell C, Pop M, Salzberg SL (2009) Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol 10: R25 10.1186/gb-2009-10-3-r25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Atanur SS, Birol I, Guryev V, Hirst M, Hummel O, Morrissey C, et al. (2010) The genome sequence of the spontaneously hypertensive rat: Analysis and functional significance. Genome Res 20: 791–803. 10.1101/gr.103499.109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Derti A, Garrett-Engele P, Macisaac KD, Stevens RC, Sriram S, Chen R, et al. (2012) A quantitative atlas of polyadenylation in five mammals. Genome Res 22: 1173–1183. 10.1101/gr.132563.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Meyer LR, Zweig AS, Hinrichs AS, Karolchik D, Kuhn RM, Wong M. et al. (2013) The UCSC Genome Browser database: extensions and updates 2013. Nucleic Acids Res 41: D64–69. 10.1093/nar/gks1048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. John S, Sabo PJ, Thurman RE, Sung MH, Biddie SC, et al. (2011) Chromatin accessibility pre-determines glucocorticoid receptor binding patterns. Nat Genet 43: 264–268. 10.1038/ng.759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wang Y, Li X, Hu H (2014) H3K4me2 reliably defines transcription factor binding regions in different cells. Genomics 103: 222–228. 10.1016/j.ygeno.2014.02.002 [DOI] [PubMed] [Google Scholar]
- 45. Taylor DM, De Koninck P, Minotti S, Durham HD (2007) Manipulation of protein kinases reveals different mechanisms for upregulation of heat shock proteins in motor neurons and non-neuronal cells. Mol Cell Neurosci 34: 20–33. [DOI] [PubMed] [Google Scholar]
- 46. Oza J, Yang J, Chen KY, Liu AY (2008) Changes in the regulation of heat shock gene expression in neuronal cell differentiation. Cell Stress Chaperones 13: 73–84. 10.1007/s12192-008-0013-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Cheung V, Chua G, Batada NN, Landry CR, Michnick SW, Hughes TR, et al. (2008) Chromatin- and transcription-related factors repress transcription from within coding regions throughout the Saccharomyces cerevisiae genome. PLoS Biol 6: e277 10.1371/journal.pbio.0060277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kaplan CD, Laprade L, Winston F (2003) Transcription elongation factors repress transcription initiation from cryptic sites. Science 301: 1096–1099. [DOI] [PubMed] [Google Scholar]
- 49. Mason PB, Struhl K (2003) The FACT complex travels with elongating RNA polymerase II and is important for the fidelity of transcriptional initiation in vivo. Mol Cell Biol 23: 8323–8333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Guertin MJ, Martins AL, Siepel A, Lis JT (2012) Accurate prediction of inducible transcription factor binding intensities in vivo. PLoS Genet 8: e1002610 10.1371/journal.pgen.1002610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Labbadia J, Cunliffe H, Weiss A, Katsyuba E, Sathasivam K, Seredenina T, et al. (2011) Altered chromatin architecture underlies progressive impairment of the heat shock response in mouse models of Huntington disease. J Clin Invest 121: 3306–3319. 10.1172/JCI57413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Marinova Z, Leng Y, Leeds P, Chuang DM (2011) Histone deacetylase inhibition alters histone methylation associated with heat shock protein 70 promoter modifications in astrocytes and neurons. Neuropharmacology 60: 1109–1115. 10.1016/j.neuropharm.2010.09.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Zhang Y, Huang L, Zhang J, Moskophidis D, Mivechi NF (2002) Targeted disruption of hsf1 leads to lack of thermotolerance and defines tissue-specific regulation for stress-inducible Hsp molecular chaperones. J Cell Biochem 86: 376–393. [DOI] [PubMed] [Google Scholar]
- 54. Verma P, Pfister JA, Mallick S, D'Mello SR (2014) HSF1 protects neurons through a novel trimerization- and HSP-independent mechanism. J Neurosci 34: 1599–1612. 10.1523/JNEUROSCI.3039-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Morrison-Bogorad M, Pardue S, McIntire DD, Miller EK (1994) Cell size and the heat-shock response in rat brain. J Neurochem 63: 857–867. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.