Summary
Objective
Although analgesic approaches targeting nerve growth factor (NGF) for the treatment of osteoarthritis (OA) pain remain of clinical interest, neurophysiological mechanisms by which NGF contribute to OA pain remain unclear. We investigated the impact of local elevation of knee joint NGF on knee joint, vs remote (hindpaw), evoked responses of spinal neurones in a rodent model of OA pain.
Design
In vivo spinal electrophysiology was carried out in anaesthetised rats with established pain behaviour and joint pathology following intra-articular injection of monosodium iodoacetate (MIA), vs injection of saline. Neuronal responses to knee joint extension and flexion, mechanical punctate stimulation of the peripheral receptive fields over the knee and at a remote site (ipsilateral hind paw) were studied before, and following, intra-articular injection of NGF (10 μg/50 μl) or saline.
Results
MIA-injected rats exhibited significant local (knee joint) and remote (lowered hindpaw withdrawal thresholds) changes in pain behaviour, and joint pathology. Intra-articular injection of NGF significantly (P < 0.05) increased knee extension-evoked firing of spinal neurones and the size of the peripheral receptive fields of spinal neurones (100% increase) over the knee joint in MIA rats, compared to controls. Intra-articular NGF injection did not significantly alter responses of spinal neurones following noxious stimulation of the ipsilateral hind paw in MIA-injected rats.
Conclusion
The facilitatory effects of intra-articular injection of NGF on spinal neurones receiving input from the knee joint provide a mechanistic basis for NGF mediated augmentation of OA knee pain, however additional mechanisms may contribute to the spread of pain to remote sites.
Keywords: Pain, Osteoarthritis, Spinal, Monosodium iodoacetate
Introduction
Osteoarthritis (OA) is a leading cause of chronic pain and disability in the developed world which is often refractory to analgesia. The challenge of finding analgesic strategies which more effectively target the associated pain requires a better understanding of the underlying pain mechanisms. Nerve growth factor (NGF) is widely recognised as a mediator of chronic pain1,2. Evidence for a contribution of NGF to OA pathophysiology includes increased NGF in synovial fluid3, at the osteochondral junction4, synovium5 and cartilage6 of people with OA.
Blockade of NGF with tanzeumab, a humanised IgG2 monoclonal antibody against NGF, had robust analgesic effects in OA sufferers7 and more recently lower back pain8. However, safety issues with this treatment strategy have impacted upon the progress of clinical trials9, and importantly highlight the need for greater mechanistic understanding of the contribution of NGF to OA pain. Models of OA pain mimic both aspects of the clinical pathology and exhibit pain on loading (weight bearing) and lowering of paw withdrawal thresholds (PWT) at remote sites10. Preclinical studies reported a significant correlation between NGFβ levels and pain behaviour in the murine destabilisation of the medial meniscus model of OA pain, in which NGF blockade inhibited OA pain responses11.
OA is not only associated with pain from the site of injury, but also lowered pain pressure thresholds from remote sites12,13 and the presence of central sensitization14. We have shown that one of the functional consequences of a local elevation in levels of NGF within the knee joint is a worsening of the ipsilateral weight bearing distribution (asymmetry), without any further change in pain behaviour from remote sites (ipsilateral hindpaw) in the monosodium iodoacetate (MIA) model of OA pain15, implying pain phenotype specific roles of NGF. This pre-clinical observation is consistent with the demonstration that the anti-NGF treatment fasinumab significantly reduces knee pain on walking in OA patients16. In the context of ongoing clinical evaluation of the analgesic potential of anti-NGF treatment strategies, it is of clinical interest to determine the mechanisms that underlie the impact of elevated knee joint levels of NGF on pain arising from both the knee joint, and potentially remote sites. To address this important question we have used established neurophysiological approaches to determine the influence of local elevation of knee joint NGF on the responses of spinal neurones to noxious stimulation of the knee-joint, vs the hindpaw, in a rodent model of OA pain. The findings of these preclinical studies provide new mechanistic evidence for restricted contributions of knee joint NGF to specific OA pain mechanisms.
Materials and methods
Rats (n = 73) were purchased from Charles River U.K. All studies were carried out in accordance with UK Home Office Animals (Scientific Procedures) Act (1986) and follow the guidelines of the International Association for the Study of Pain.
OA induction
Male Sprague Dawley rats (160–190 g) were briefly anesthetised with isoflurane (1.5–2% in 50% N2O−50% O2). Joint damage was induced by a single intra-articular injection of (MIA; 1 mg/50 μl; Sigma U.K.; n = 24 rats) in saline through the infra-patellar ligament of the left knee10. Control rats received a single injection of saline (50 μl) into the left knee in an identical manner (n = 24 rats). All surgical procedures where performed under sterile conditions in an animal procedure room away from animal holding areas. The experimental unit was a single animal.
Behavioural testing
After intra-articular injection, rats were maintained under the same conditions as during the preoperative period. The posture and behaviour of the rats were carefully monitored following recovery from the anaesthesia and throughout the study. Weight gain, changes in hindpaw weight-distribution and mechanical withdrawal thresholds were recorded immediately prior to intra-articular injection (day 0, baseline) and at regular intervals until day 21 post injection. The tester was blinded to the injury at the time of testing. Behavioural tests were always performed between 8am and noon.
Naïve or saline-injected rats distribute their weight evenly between both paws. Following joint injury, changes in weight distribution are used an indicator of joint discomfort and associated pain in the injured knee17. Weight-distribution through the left (ipsilateral) and right (contralateral) knee were assessed using an incapacitance tester (Linton Instrumentation, U.K.) as previously described18. Mechanical hindpaw withdrawal thresholds were assessed using calibrated von Frey monofilaments (Semmes-Weinstein monofilaments of bending forces 1, 1.4, 2, 4, 6, 8, 10 and 15 g) applied to the plantar surface of the hind paw in ascending order of bending force. Each hair was applied three times for approximately 2–3 s periods or until a withdrawal response was evoked. After a response, the paw was retested with the monofilaments in descending order until no response occurred at which point the monofilaments were again applied in ascending order until the response could once again be evoked. The monofilament that evoked this final reflex was noted as the PWT.
In vivo electrophysiology
Electrophysiological studies of MIA or saline-injected rats (weight at time of study 310–425 g) and naïve control rats (n = 25, weight at time of study 300–405 g) were performed (between 21 and 24 days post-injection for MIA and saline-treated rats, at which time behaviour was stable). Based on published methods10, rats were anaesthetised with inhalation anaesthetic (3% induction, 2% surgery, 1–1.5% maintenance in 60% N2O, 40%O2 (flow rates: 300 cm3 min−1, 200 cm3 min−1, respectively)) and a tracheal cannula was inserted. A laminectomy was performed and segments L1-L4 (knee recordings19,20; or L4-L5 (hind paw recordings10;)) of the spinal cord were exposed depending on the stimulation protocol (see below). The spinal cord was held rigid by clamps rostral and caudal to the exposed section. Extracellular single-unit recordings of deep (500–1000 μm, laminae V–VI) wide dynamic range (WDR) dorsal horn neurones were made with glass-coated tungsten microelectrodes. Laminar V–VI WDR neurones have well characterised responses to noxious stimuli and exhibit graded responses to noxious stimuli21. Electrodes were lowered through the spinal cord in 10 μm steps with a SCAT-01 microdrive (Digitimer, UK) and the depth of recorded neurones from the spinal cord surface were recorded and verified at the end of the experiment. Action potentials were digitised and analysed using a CED micro1401 interface and Spike 2 software (Cambridge Electronic Design, UK). Core body temperature was maintained at 36.5–37.5°C by means of a heating blanket connected to a rectal temperature probe. For knee joint stimulation protocols, the input from cutaneous afferent neurones was minimised by the application of local anaesthetic cream (EMLA: 2.5% lidocaine and 2.5% prilocaine) to the skin over the knee joint for 20–30 min. Following removal of the cream, the skin over the knee joint was excised. Note, it was not possible to remove the skin from the hind paw (as was the case for the knee joint) and therefore evoked responses may reflect a greater proportion of cutaneous input from the hind paw.
Recording of responses to stimulation of the knee joint (knee evoked responses)
Spinal neurones (lumbar segments L1–L4) which had receptive fields predominantly over the knee joint and were responsive to mechanical stimulation, flexion (bend) or extension of the knee joint within a physiological range and did not respond to stimulation of the ankle or hind paw were recorded. A von Frey monofilament (of bending force 26 g (noxious)) was applied to the receptive field over the knee joint for a period of 10 s and the evoked firing of the spinal neurone was recorded (quantified as mean frequency of firing). To mimic the normal movement of the knee joint, responses of spinal neurones to extension of the knee joint and then flexion of the knee joint, within physiological limits, were recorded. To avoid movement or electrical interference confounding the extension or flexion-evoked firing, the knee joint was moved to the new position and then the firing evoked over a period of 5 s was recorded. Once baseline responses had been recorded, the effects of intra-articular NGF vs saline on extension and flexion-evoked firing were determined. Receptive field size of spinal neurones innervating the knee joint mapped with a noxious 26 g von Frey monofilament using established methods22. In order to prevent stimulus driven sensitization of the peripheral tissues, we did not deliver trains of peripheral stimuli to the area over which the receptive field was expanded for quantification purposes, but merely noted threshold responses (present or absent) to stimuli applied to different parts of the receptive field. Trials of evoked responses and receptive field size were recorded at 20 min intervals. Only one neurone was recorded per rat.
Recording of responses of spinal neurons to stimulation of the hind paw (hind paw evoked responses)
In order to investigate the contribution of spinal sensitisation in the models of OA pain, spinal neuronal responses to stimulation of an area remote to the knee joint (the ipsilateral hind paw) were measured in a separate cohort of rats. Spinal neurones (lumbar segments L4–L5) which had receptive fields over the toes and did not respond to movement/stimulation of the ankle or knee were recorded. Responses of spinal neurones to a mechanical punctate stimulus (von Frey monofilament 26 g) applied to the peripheral receptive field over the hind paw were characterised and quantified in a similar fashion as described above. Receptive field size of the neurones over the hindpaw was mapped with a 26 g von Frey monofilament. Trials of evoked responses and receptive field size were recorded at 20 min intervals.
Intra-articular injection of NGF
Following stable control responses of spinal neurones to stimulation of the knee or the hind paw, rats received a single intra-articular injection through the infrapatellar ligament of either NGF (10 μg/50 μl; Sigma UK), dose based on15 or saline (50 μl). Responses of spinal neurones to the stimulation protocols described above were quantified at 20 min intervals for 2 h post-injection. Intra-articular injection of substances required the movement of the joint, thus it was not possible to measure the immediate effects of the injection on neuronal firing. At the end of the experiment rats were killed by anaesthetic overdose and knee joints were collected and a subgroup were processed.
Macroscopic grading of knee joint pathology
Macroscopic scoring of knee joints was based on23. Macroscopic lesions were graded as follows: 0 = normal appearance; 1 = slight yellowish discolouration of the chondral surface; 2 = little cartilage erosions in load-bearing areas; 3 = large erosions extending down to the subchondral bone; and 4 = large erosions with large areas of subchondral bone exposure. Each of the chondral compartments of the knee (the medial and lateral femoral condyles, the medial and lateral tibia plateaux, the patella, and the femoral groove) were graded by an observer blinded to intervention and in vivo data. The six compartment scores were combined (for a maximum possible score of 24) and the mean value calculated for each group.
Data analysis and statistics
Statistical analysis of changes in weight bearing between the hindlimbs and mechanical withdrawal thresholds was performed using a 2-way Analysis of variance (ANOVA) with a Bonferroni post-hoc test. Neuronal responses to stimulation of the knee or hindpaw were normalised as a % of the pre-drug baseline values for individual cells, statistical analyses of neuronal responses to knee or hind paw stimulation (area under curve) were performed using a Mann–Whitney test or a Kruskal Wallis test with a Dunn's post hoc test, according to the number of unmatched groups. In analyses that used the Kruskal–Wallis and Mann–Whitney statistical tests, all observations were independent (data were unpaired) and non-Gaussian; in data analysed using repeated measures two-way ANOVA, data were matched within a group and sphericity was assumed. Peripheral receptive fields to mechanical stimulation of the knee joint or hindpaw were mapped onto a template of the hind limb or hind paw, respectively. Receptive field maps were scanned and converted to 300 dpi JPEG files and the area of the receptive field was quantified using MacBiophotonics Image J software. Baseline receptive field size was calculated as a % of the mean of three receptive field sizes prior to injection of NGF or saline. Statistical analyses of receptive field expansion (area under curve) was performed a Mann Whitney test or a Kruskal Wallis test with a Dunn's post hoc test, where appropriate. Statistical analysis of knee joint pathology was performed using a Mann Whitney test.
Results
Pain behaviour and joint pathology
Consistent with previous studies, intra-articular injection of MIA (1 μg/50 μl) produced a decrease in weight bearing on the ipsilateral hind limb (weight bearing asymmetry), which was significantly different to saline-injected rats up to day 21 post-injection [Fig. 1(A)]. In addition, MIA-injected rats exhibited a significant decrease in mechanical hindpaw withdrawal thresholds, compared to saline-injected rats [Fig. 1(B)]. At 21 days following intra-articular injection of MIA there was an increase in the extent of macroscopic lesions at the level of the articular surface of the ipsilateral knee joint, compared to saline-injected rats [Fig. 1(C)].
Fig. 1.
Intra-articular injection of MIA produces (A) weight bearing asymmetry and (B) decreased hindpaw withdrawal thresholds on the ipsilateral hind paw of MIA rats (n = 24 rats), compared to saline-injected rats (n = 24 rats). The effects of intra-articular MIA on the knee joint pathology were quantified in a sub-set of rats used in this study. There was a significant increase in the appearance of macroscopic lesions (C) on the articular joint surface of MIA-injected rats, compared to saline-injected rats (n = 12 MIA rats; n = 11 saline rats). Statistical analysis of weight bearing asymmetry and PWTs was performed using a 2-way ANOVA with a Bonferroni post-hoc test. Statistical test of knee joint pathology was performed using a Mann Whitney test. *P < 0.05, **P < 0.01, ***P < 0.001 MIA injection vs saline injection.
In vivo spinal electrophysiology
Following characterisation of the pain behaviour associated with intra-articular injection of MIA, electrophysiological recordings of spinal neurones were performed. The mean depths of WDR spinal neurones recorded were similar between the experimental groups (Table I) and corresponded to laminae V and VI of the dorsal horn of the spinal cord. All spinal neurones recorded were responsive to noxious (26 g) mechanical stimulation of the peripheral receptive field, which was either over the ipsilateral knee joint or hindpaw, and had a basal spontaneous firing rate of less than 5 Hz.
Table I.
Summary of the treatment groups and the properties of the dorsal horn spinal neurones in the different groups of rats at baseline before injection of NGF (10 μg/50 μl) or saline into the knee. Neurones had a basal spontaneous activity of less than 5 Hz. Neuronal responses between groups did not significantly differ from each other at baseline. Data are mean ± sem
| Treatment group | Mean depth of neurone (μm) | Baseline 26 g-evoked stimulation (Hz) | Baseline knee flexion evoked firing (Hz) | Baseline knee extension evoked firing (Hz) | |
|---|---|---|---|---|---|
| Baseline neuronal responses: knee joint stimulation | |||||
| Naïve rats | |||||
| n = 7 neurones in seven rats | NGF | 962 ± 74 | 9 ± 4 | 7 ± 2 | 17 ± 6 |
| n = 6 neurones in six rats | Saline | 887 ± 51 | 8 ± 4 | 4 ± 1 | 9 ± 2 |
| MIA rats | |||||
| n = 6 neurones in six rats | NGF | 734 ± 69 | 8 ± 3 | 5 ± 1 | 9 ± 4 |
| n = 6 neurones in six rats | Saline | 776 ± 74 | 12 ± 4 | 6 ± 3 | 7 ± 3 |
| Saline rats | |||||
| n = 6 neurones in six rats | NGF | 722 ± 87 | 9 ± 2 | 6 ± 2 | 9 ± 3 |
| n = 7 neurones in seven rats | Saline | 742 ± 83 | 7 ± 1 | 8 ± 2 | 17 ± 6 |
| Treatment group | Mean depth of neurone (μm) | Baseline 26 g-evoked stimulation (Hz) | |
|---|---|---|---|
| Baseline neuronal responses: hind paw stimulation | |||
| Naïve rats | |||
| n = 6 neurones in six rats | NGF | 842 ± 121 | 37 ± 12 |
| n = 6 neurones in six rats | Saline | 796 ± 34 | 19 ± 4 |
| MIA rats | |||
| n = 6 neurones in six rats | NGF | 780 ± 63 | 22 ± 4 |
| n = 6 neurones in six rats | Saline | 778 ± 51 | 47 ± 7 |
| Saline rats | |||
| n = 6 neurones in six rats | NGF | 735 ± 47 | 32 ± 6 |
| n = 5 neurones in five rats | Saline | 680 ± 32 | 28 ± 7 |
Does intra-articular injection of NGF alter knee joint evoked responses of spinal neurones?
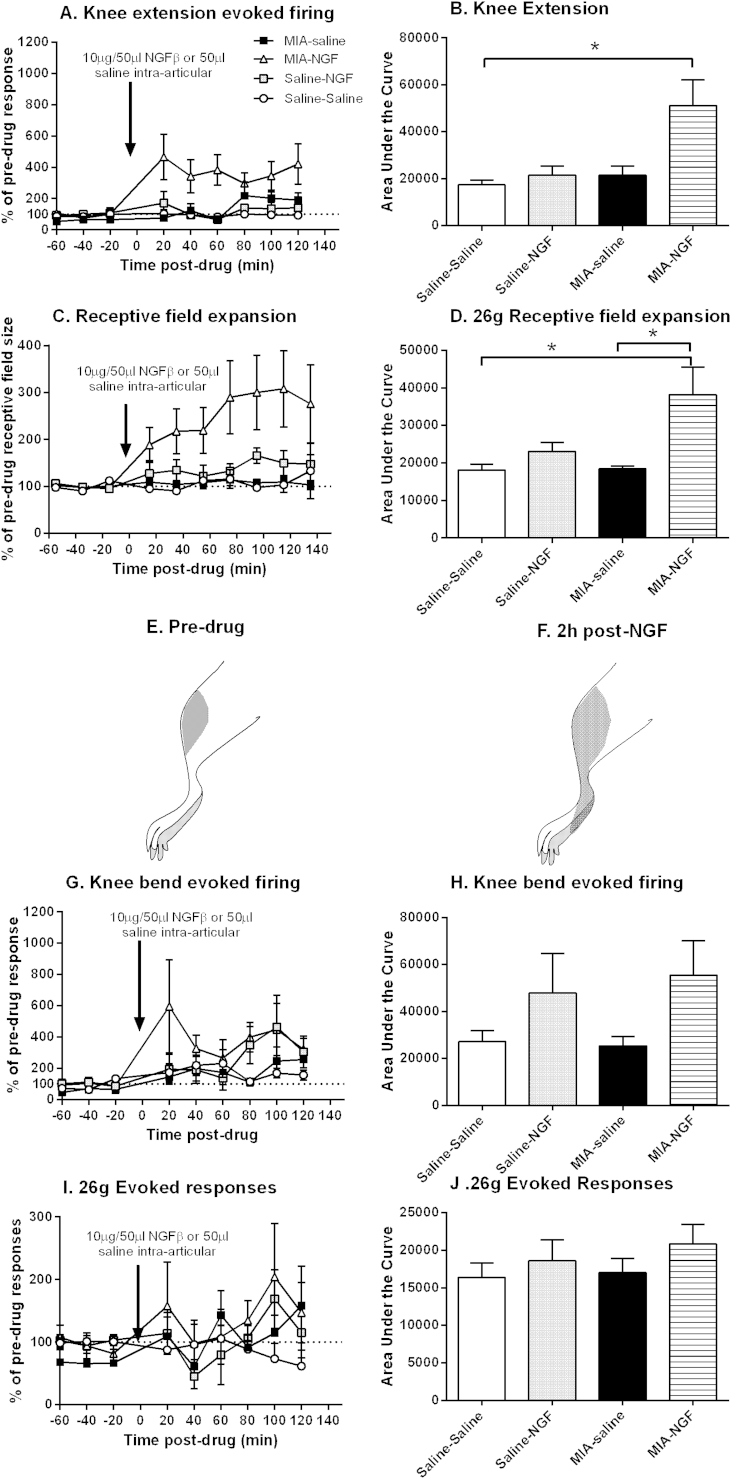
Flexion (bend) and extension of the knee joint within physiological ranges increased the frequency of firing of spinal neurones to a similar extent in MIA-injected and saline-injected rats (Table I). Intra-articular injection of NGF in MIA-injected rats resulted in a significant increase in the firing of spinal neurones following extension of the knee, compared to saline-treated rats [Fig. 2(A), (B), Supplementary Fig. 1]. By contrast, intra-articular injection of NGF did not alter the firing of spinal neurones following extension of the knee in saline-injected rats [Fig. 2(A), (B)]. Intra-articular injection of NGF also resulted in a significant (100% increase) expansion in the size of the receptive fields of the spinal neurones innervating the knee joint in MIA-injected rats, compared to the injection of saline in MIA-injected rats [Fig. 2(C)–(F)]. Intra-articular injection of NGF did not alter the size of the receptive fields of the spinal neurones innervating the knee joint in saline-injected rats [Fig. 2(C)–(F)]. Intra-articular injection of NGF did not alter knee flexion (bend) evoked firing [Fig. 2(G), (H)], nor mechanical punctate evoked firing [Fig. 2(I), (J)], of spinal neurones in MIA-injected, or saline-injected rats. Note the baseline responses of spinal neurones in saline-injected rats were largely comparable to those recorded in naïve rats; similarly the effects of intra-articular injection of NGF in saline-treated rats were largely comparable to effects of intra-articular injection of NGF in naïve rats (Supplementary Fig. 2(A)–(H)).
Fig. 2.
Effects of intra-articular injection of NGF or saline in MIA and saline-injected rats on spinal neuronal responses to (A,B) knee extension, (C–D)26 g receptive field size of spinal neurones innervating the knee joint, in MIA-injected and saline-injected rats. (E–F) A representative image of mapping the peripheral receptive field over the knee joint following stimulation with a 26 g von Frey hair (E) pre- and (F) 2 h post-NGF injection; the shaded area indicates areas over which stimulation was able to evoke single unit activity of a dorsal horn neurone in the spinal cord. Panels show effects of intra-articular injection of NGF or saline in MIA and saline-injected rats on spinal neuronal responses to (G–H) knee flexion (bend) and (I–J) 26 g von Frey punctate stimulation over the knee joint in MIA-injected and saline-injected rats. Data are normalised to baseline values and expressed as mean ± SEM. Statistical analyses comparing the area under curve were performed using a Kruskal Wallis test with a Dunn's post hoc test. *P < 0.05. MIA-NGF: n = 6 neurones in six rats; MIA-saline: n = 6 neurones in six rats, Saline-NGF: n = 6 neurones in six rats, Saline–saline: n = 7 neurones in seven rats.
Does intra-articular NGF alter hind paw evoked responses of spinal neurones?
To investigate whether local knee joint changes in NGF can alter the processing of sensory inputs from remote sites, the effects of an intra-articular injection of NGF or saline on the responses of spinal neurones with receptive fields over the ipsilateral hindpaw, but not the ipsilateral knee joint, were recorded in MIA-treated rats. Neither intra-articular injection of saline, or NGF altered noxious (26 g) hindpaw evoked responses of spinal neurones [Fig. 3(A)–(B)] or the size of the receptive fields of spinal neurones innervating the hindpaw in MIA-injected or saline-injected rats [Fig. 3(C)–(D)], or naïve rats (data not shown).
Fig. 3.
Effects of intra-articular injection of NGF or saline in MIA or saline-injected rats on spinal neuronal responses to (A–B) 26 g von Frey stimulation and (C–D) 26 g receptive field size of neurones innervating the hind paw. Data are normalised to baseline values and expressed as mean ± SEM. Statistical analyses comparing the area under curve were performed using a Kruskal Wallis test. MIA-NGF: n = 6 neurones in six rats; MIA-saline: n = 6 neurones in six rats; saline-NGF: n = 6 neurones in six rats; saline–saline: n = 5 neurones in five rats.
Discussion
Recent work has reported an association between NGF-like immunoreactivity in human synovium and the presence of symptomatic OA5. Herein we demonstrate that elevation of local levels of NGF within the knee joint significantly facilitates knee joint extension-evoked responses of spinal neurones and increases the receptive field size of spinal neurones innervating the knee joint in the MIA model of OA pain, to a greater extent than in naïve or control rats. The effects of NGF on spinal neuronal responses were restricted to those receiving input from the knee joint since ipsilateral hindpaw-evoked responses were not significantly influenced by intra-articular injection of NGF in the model of OA pain. These neurophysiological studies provide new insight into the mechanisms which underpin the contribution of NGF to OA pain, which are relevant the analgesic effects of anti-NGF treatment strategies for OA pain.
Although the MIA model does not mimic the aetiology of clinical OA, the histopathological features of the joint damage and the phenotypes of pain exhibited (pain on loading and spread of pain to sites distant from the joint) provide a clinically relevant model for the investigation of mechanisms driving established OA pain24. Following intra-articular injection of MIA, rats displayed a characteristic decrease in weight bearing and ipsilateral mechanical hind PWTs compared to saline-injected rats, which mimics aspects of the spatial summation and spread of pain evident in OA patients25. Previously we have demonstrated significant increases in numbers of activated microglia in the spinal cord at day 21 in the MIA model26, indicating the presence of features of central sensitization at the time-point used for this neurophysiological investigation. It was noteworthy that the magnitude of mechanically-evoked punctate responses was consistently higher following stimulation of the hindpaw, compared to stimulation of the knee joint, which may reflect the relatively low innervation of the knee joint by primary afferent fibres27 and an additional contribution of cutaneous input from the hind paw to the spinal cord. Macroscopic evaluation of knee joints in a subset of the MIA-treated rats confirmed the presence of histopathological features consistent with clinical OA, compared to saline-injected rats.
Exogenous elevation of NGF within the knee joint, at a dose previously shown to produce pain behaviour15, produced a significant increase in knee extension-evoked firing of spinal neurones and a robust significant increase in the size of the peripheral receptive fields of the spinal neurones innervating the knee joint. These effects, which were specific to the MIA model and significant compared to naïve and saline-injected controls, provide a neurophysiological basis for the robust effects of knee joint NGF on weight bearing measures of pain behaviour in this model, which may be underpinned by the increased mRNA expression of the high affinity receptor for NGF TrkA at the level of the DRG in this model15. Since the present study was unable to distinguish between spinal neurones receiving inputs from intracapsule vs extracapsule afferent fibres, differential effects of NGF on these types of sensory inputs could not be identified. The ability of local elevation of knee joint NGF to facilitate knee extension-evoked firing of spinal neurones may represent changes in sensory inputs from multiple structures within the knee joint, including muscle. Enhanced spinal withdrawal reflexes at the level of the biceps femoris muscle (involved in flexion of the knee) have been demonstrated in the MIA model28 and significant enhancement of pain responses following intramuscular hypertonic saline in OA patients29 is indicative of an enhanced sensitivity of the central processing of muscle inputs following joint damage.
Importantly, intra-articular injection of NGF did not alter the responses of spinal neurones to hind paw stimulation in MIA-treated rats, either in terms of magnitude of evoked firing or the size of the receptive field of neurones innervating the hind paw. Our data suggest that despite MIA-induced knee joint damage driving changes in the spinal processing of sensory inputs from the hindpaw as evidenced by the lowering of hindpaw withdrawal thresholds, elevation of local knee joint levels of NGF is unlikely to contribute to this effect. Indeed, the contribution of knee joint NGF to OA pain may be restricted to peripherally driven events, at least at the time-points investigated in the present study, and any spinally-mediated effects may be restricted to the lumbar segments receiving input predominantly from the joint and not lower segments receiving hind paw input.
Multiple lines of evidence support an association between elevated levels of NGF and OA (see Introduction) and preclinical studies have demonstrated that repeated exposure to NGF drives a long lasting hypersensitivity, including an expansion of receptive fields of spinal neurones in response to stimulation of deep tissues30. Although NGF increased spinal neuronal responses in both arthritic and non-arthritic rats, the magnitude of the effects was two fold greater in the MIA-injected rats. Our neurophysiological data provide evidence that the up-regulation of the NGF/TrkA system in a model of OA15 is associated with a spinal sensitization which facilitates extension-evoked firing of spinal neurones, as well as expanding the area over which sensory inputs from the knee joint can activate individual spinal neurones. Given the complexity of the structures contributing to OA pathology and pain, several mechanisms are likely to contribute to NGF-mediated changes in spinal neuronal responses. These may include peripheral sensitisation, as well as changes in spinal excitability (central sensitisation), as evidenced by the presence of receptive field expansion. The ability of NGF to prime the sensory nervous system is unlikely to occur in isolation as NGF can induce peripheral sensitisation through the release of neuropeptides such as CGRP31 and facilitation of TRPV1 responses32, both of which have established associations with OA pain27,33–35. Indeed, in addition to the effects on thermal hypersensitivity, NGF driven mechanical hyperalgesia is TRPV1 dependant36. Interactions between NGF and other pro-inflammatory substances (e.g., cytokines37), endovanilloids35, infiltrating macrophages and altered channel and receptor expression on sensory nerves may all contribute to these sensitization mechanisms. The observation that the effects of knee joint NGF were limited to spinal neurones receiving input from the knee joint, and did not extend to neurones innervating the hindpaw, suggests that peripheral NGF mechanisms may augment OA knee pain, while additional mechanisms may contribute to the spread of pain to remote sites. This novel insight into the neurophysiological contribution of NGF-to OA pain mechanisms may have important implications for treatment strategies which target NGF signalling.
Author contributions
DRS: Conception and design, collection and analysis of data, drafting of article, critical revision of article and final approval.
LN: Collection and analysis of data, drafting of article and final approval.
DAW: Conception and design, drafting of article, critical revision of article and final approval of manuscript, obtaining funding.
VC: Conception and design, drafting of article, critical revision of article and final approval of manuscript, obtaining funding.
Financial support
DRS was supported by grant number 18769 from Arthritis Research UK. Lillian Nwosu was supported by ARUK and University of Nottingham.
Competing interests
The authors have no competing interests.
Acknowledgements
The authors would like to thank Jenna Turner, Luting Xu, Dr Ian Devonshire, Dr James Burston and Paul Millns for assistance with these studies.
Appendix A. Supplementary data
The following are the supplementary data related to this article:
Supplementary Fig. 1.
Representative traces from MIA-treated rats demonstrating spinal neuronal responses to extension of the knee joint within a physiological range before and 20 min post intra-articular injection with (A–B) saline or (C–D) NGF (10 μg/50 μl). Sorted trace = action potentials in raw trace sorted by Spike 2 Software.
Supplementary Fig. 2.
Effects of an intra-articular injection of NGF or saline on spinal neuronal responses to (A,B) knee bend (C–D) knee extension, (E–F) 26 g von Frey stimulation and (G–H) 26 g receptive field size over the knee joint in naive rats. Data are normalised to pre-intervention baseline values and expressed as mean ± SEM. Statistical analyses comparing the area under curve were performed using a Mann Whitney test. *P < 0.05, **P < 0.01: NGF versus saline. NGF: n = 7 neurones in seven rats; saline: n = 6 neurones in six rats.
References
- 1.Kumar V., Mahal B.A. NGF – the TrkA to successful pain treatment. J Pain Res. 2012;5:279–287. doi: 10.2147/JPR.S33408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pezet S., McMahon S.B. Neurotrophins: mediators and modulators of pain. Annu Rev Neurosci. 2006;29:507–538. doi: 10.1146/annurev.neuro.29.051605.112929. [DOI] [PubMed] [Google Scholar]
- 3.Seidel M.F., Herguijuela M., Forkert R., Otten U. Nerve growth factor in rheumatic diseases. Semin Arthritis Rheum. 2010;40:109–126. doi: 10.1016/j.semarthrit.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 4.Walsh D.A., McWilliams D.F., Turley M.J., Dixon M.R., Franses R.E., Mapp P.I. Angiogenesis and nerve growth factor at the osteochondral junction in rheumatoid arthritis and osteoarthritis. Rheumatology (Oxford) 2010;49:1852–1861. doi: 10.1093/rheumatology/keq188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stoppiello L.A., Mapp P.I., Wilson D., Hill R., Scammell B.E., Walsh D.A. Structural associations of symptomatic knee osteoarthritis. Arthritis Rheum. 2014 Nov;66(11):3018–3027. doi: 10.1002/art.38778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Iannone F., De Bari C., Dell'Accio F., Covelli M., Patella V., Lo Bianco G. Increased expression of nerve growth factor (NGF) and high affinity NGF receptor (p140 TrkA) in human osteoarthritic chondrocytes. Rheumatology (Oxford) 2002;41:1413–1418. doi: 10.1093/rheumatology/41.12.1413. [DOI] [PubMed] [Google Scholar]
- 7.Brown M.T., Murphy F.T., Radin D.M., Davignon I., Smith M.D., West C.R. Tanezumab reduces osteoarthritic knee pain: results of a randomized, double-blind, placebo-controlled phase III trial. J Pain. 2012;13:790–798. doi: 10.1016/j.jpain.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 8.Gimbel J.S., Kivitz A.J., Bramson C., Nemeth M.A., Keller D.S., Brown M.T. Long-term safety and effectiveness of tanezumab as treatment for chronic low back pain. Pain. 2014 Sep;155(9):1793–1801. doi: 10.1016/j.pain.2014.06.004. [DOI] [PubMed] [Google Scholar]
- 9.Bannwarth B., Kostine M. Targeting nerve growth factor (NGF) for pain management: what does the future hold for NGF antagonists? Drugs. 2014;74:619–626. doi: 10.1007/s40265-014-0208-6. [DOI] [PubMed] [Google Scholar]
- 10.Sagar D.R., Staniaszek L.E., Okine B.N., Woodhams S., Norris L.M., Pearson R.G. Tonic modulation of spinal hyperexcitability by the endocannabinoid receptor system in a rat model of osteoarthritis pain. Arthritis Rheum. 2010;62:3666–3676. doi: 10.1002/art.27698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McNamee K.E., Burleigh A., Gompels L.L., Feldmann M., Allen S.J., Williams R.O. Treatment of murine osteoarthritis with TrkAd5 reveals a pivotal role for nerve growth factor in non-inflammatory joint pain. Pain. 2010;149:386–392. doi: 10.1016/j.pain.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 12.Hendiani J.A., Westlund K.N., Lawand N., Goel N., Lisse J., McNearney T. Mechanical sensation and pain thresholds in patients with chronic arthropathies. J Pain. 2003;4:203–211. doi: 10.1016/s1526-5900(03)00557-1. [DOI] [PubMed] [Google Scholar]
- 13.Lluch E., Torres R., Nijs J., Van Oosterwijck J. Evidence for central sensitization in patients with osteoarthritis pain: a systematic literature review. Eur J Pain. 2014 Nov;18(10):1367–1375. doi: 10.1002/j.1532-2149.2014.499.x. [DOI] [PubMed] [Google Scholar]
- 14.Gwilym S.E., Keltner J.R., Warnaby C.E., Carr A.J., Chizh B., Chessell I. Psychophysical and functional imaging evidence supporting the presence of central sensitization in a cohort of osteoarthritis patients. Arthritis Rheum. 2009;61:1226–1234. doi: 10.1002/art.24837. [DOI] [PubMed] [Google Scholar]
- 15.Ashraf S., Mapp P.I., Burston J., Bennett A.J., Chapman V., Walsh D.A. Augmented pain behavioural responses to intra-articular injection of nerve growth factor in two animal models of osteoarthritis. Ann Rheum Dis. 2014;73:1710–1718. doi: 10.1136/annrheumdis-2013-203416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tiseo P.J., Kivitz A.J., Ervin J.E., Ren H., Mellis S.J. Fasinumab (REGN475), an antibody against nerve growth factor for the treatment of pain: results from a double-blind, placebo-controlled exploratory study in osteoarthritis of the knee. Pain. 2014;155:1245–1252. doi: 10.1016/j.pain.2014.03.018. [DOI] [PubMed] [Google Scholar]
- 17.Bove S.E., Calcaterra S.L., Brooker R.M., Huber C.M., Guzman R.E., Juneau P.L. Weight bearing as a measure of disease progression and efficacy of anti-inflammatory compounds in a model of monosodium iodoacetate-induced osteoarthritis. Osteoarthritis Cartilage. 2003;11:821–830. doi: 10.1016/s1063-4584(03)00163-8. [DOI] [PubMed] [Google Scholar]
- 18.Sagar D.R., Ashraf S., Xu L., Burston J.J., Menhinick M.R., Poulter C.L. Osteoprotegerin reduces the development of pain behaviour and joint pathology in a model of osteoarthritis. Ann Rheum Dis. 2013 doi: 10.1136/annrheumdis-2013-203260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Neugebauer V., Vanegas H., Nebe J., Rumenapp P., Schaible H.G. Effects of N- and L-type calcium channel antagonists on the responses of nociceptive spinal cord neurons to mechanical stimulation of the normal and the inflamed knee joint. J Neurophysiol. 1996;76:3740–3749. doi: 10.1152/jn.1996.76.6.3740. [DOI] [PubMed] [Google Scholar]
- 20.Salo P.T., Theriault E. Number, distribution and neuropeptide content of rat knee joint afferents. J Anat. 1997;190:515–522. doi: 10.1046/j.1469-7580.1997.19040515.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dostrovsky J.O., Craig A.D. Wall and Melzack's Textbook of Pain. Elsevier; 2013. Ascending projection systems. [Google Scholar]
- 22.Elmes S.J., Jhaveri M.D., Smart D., Kendall D.A., Chapman V. Cannabinoid CB2 receptor activation inhibits mechanically evoked responses of wide dynamic range dorsal horn neurons in naive rats and in rat models of inflammatory and neuropathic pain. Eur J Neurosci. 2004;20:2311–2320. doi: 10.1111/j.1460-9568.2004.03690.x. [DOI] [PubMed] [Google Scholar]
- 23.Guingamp C., Gegout-Pottie P., Philippe L., Terlain B., Netter P., Gillet P. Mono-iodoacetate-induced experimental osteoarthritis: a dose-response study of loss of mobility, morphology, and biochemistry. Arthritis Rheum. 1997;40:1670–1679. doi: 10.1002/art.1780400917. [DOI] [PubMed] [Google Scholar]
- 24.Vincent T.L., Williams R.O., Maciewicz R., Silman A., Garside P., Arthritis Research UKamwg Mapping pathogenesis of arthritis through small animal models. Rheumatology (Oxford) 2012;51:1931–1941. doi: 10.1093/rheumatology/kes035. [DOI] [PubMed] [Google Scholar]
- 25.Graven-Nielsen T., Wodehouse T., Langford R.M., Arendt-Nielsen L., Kidd B.L. Normalization of widespread hyperesthesia and facilitated spatial summation of deep-tissue pain in knee osteoarthritis patients after knee replacement. Arthritis Rheum. 2012;64:2907–2916. doi: 10.1002/art.34466. [DOI] [PubMed] [Google Scholar]
- 26.Sagar D.R., Burston J.J., Hathway G.J., Woodhams S.G., Pearson R.G., Bennett A.J. The contribution of spinal glial cells to chronic pain behaviour in the monosodium iodoacetate model of osteoarthritic pain. Mol Pain. 2011;7:88. doi: 10.1186/1744-8069-7-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ferreira-Gomes J., Adaes S., Sarkander J., Castro-Lopes J.M. Phenotypic alterations of neurons that innervate osteoarthritic joints in rats. Arthritis Rheum. 2010;62:3677–3685. doi: 10.1002/art.27713. [DOI] [PubMed] [Google Scholar]
- 28.Kelly S., Dobson K.L., Harris J. Spinal nociceptive reflexes are sensitized in the monosodium iodoacetate model of osteoarthritis pain in the rat. Osteoarthritis Cartilage. 2013;21:1327–1335. doi: 10.1016/j.joca.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 29.Bajaj P., Graven-Nielsen T., Arendt-Nielsen L. Osteoarthritis and its association with muscle hyperalgesia: an experimental controlled study. Pain. 2001;93:107–114. doi: 10.1016/S0304-3959(01)00300-1. [DOI] [PubMed] [Google Scholar]
- 30.Hoheisel U., Reuter R., de Freitas M.F., Treede R.D., Mense S. Injection of nerve growth factor into a low back muscle induces long-lasting latent hypersensitivity in rat dorsal horn neurons. Pain. 2013;154:1953–1960. doi: 10.1016/j.pain.2013.05.006. [DOI] [PubMed] [Google Scholar]
- 31.Park K.A., Fehrenbacher J.C., Thompson E.L., Duarte D.B., Hingtgen C.M., Vasko M.R. Signaling pathways that mediate nerve growth factor-induced increase in expression and release of calcitonin gene-related peptide from sensory neurons. Neuroscience. 2010;171:910–923. doi: 10.1016/j.neuroscience.2010.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mills C.D., Nguyen T., Tanga F.Y., Zhong C., Gauvin D.M., Mikusa J. Characterization of nerve growth factor-induced mechanical and thermal hypersensitivity in rats. Eur J Pain. 2013;17:469–479. doi: 10.1002/j.1532-2149.2012.00202.x. [DOI] [PubMed] [Google Scholar]
- 33.Saxler G., Loer F., Skumavc M., Pfortner J., Hanesch U. Localization of SP- and CGRP-immunopositive nerve fibers in the hip joint of patients with painful osteoarthritis and of patients with painless failed total hip arthroplasties. Eur J Pain. 2007;11:67–74. doi: 10.1016/j.ejpain.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 34.Bullock C.M., Wookey P., Bennett A., Mobasheri A., Dickerson I., Kelly S. Increased peripheral calcitonin gene-related peptide receptor signalling drives mechanical sensitization of the joint in rat models of osteoarthritis pain. Arthritis Rheumatol. 2014 doi: 10.1002/art.38656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kelly S., Chapman R.J., Woodhams S., Sagar D.R., Turner J., Burston J.J. Increased function of pronociceptive TRPV1 at the level of the joint in a rat model of osteoarthritis pain. Ann Rheum Dis. 2013 doi: 10.1136/annrheumdis-2013-203413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ota H., Katanosaka K., Murase S., Kashio M., Tominaga M., Mizumura K. TRPV1 and TRPV4 play pivotal roles in delayed onset muscle soreness. PLoS One. 2013;8:e65751. doi: 10.1371/journal.pone.0065751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Allen K.D., Mata B.A., Gabr M.A., Huebner J.L., Adams S.B., Jr., Kraus V.B. Kinematic and dynamic gait compensations resulting from knee instability in a rat model of osteoarthritis. Arthritis Res Ther. 2012;14:R78. doi: 10.1186/ar3801. [DOI] [PMC free article] [PubMed] [Google Scholar]