Abstract
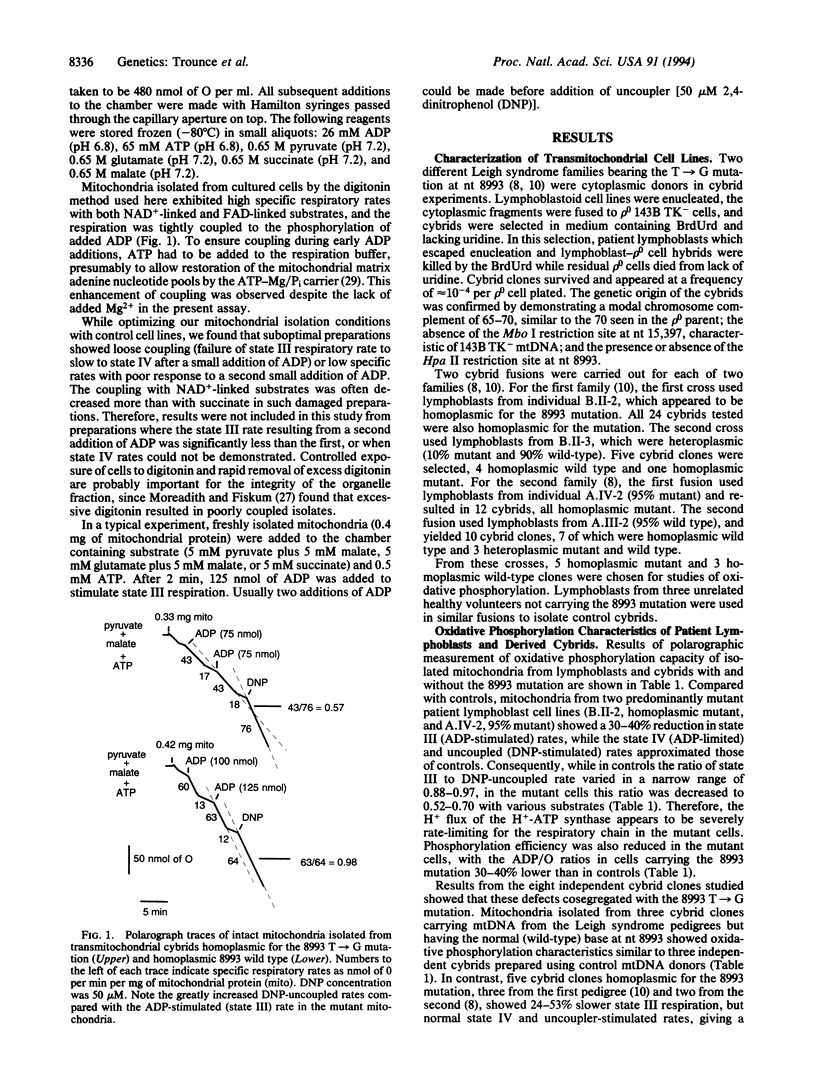
A point mutation in the mtDNA-encoded ATP6 gene (T-->G at nt 8993) associated with Leigh syndrome in two pedigrees was found to decrease ADP-stimulated (state III) respiration and the ratio of ADP molecules phosphorylated to oxygen atoms reduced (ADP/O ratio) but did not affect 2,4-dinitrophenol (DNP)-uncoupled respiration, suggesting a defective mitochondrial H(+)-translocating ATP synthase. Intact mitochondria isolated from patient and control lymphoblastoid cell lines were tested for state III, ADP-limited (state IV), and DNP-uncoupled respiration with various substrates. Mitochondria isolated from patient lymphoblasts harboring 95-100% of mtDNAs carrying the nt 8993 T-->G mutation showed state III respiration rates 26-50% lower than controls while having normal DNP-uncoupled rates. This resulted in state III/DNP ratios of 0.52-0.70 in patient mitochondria versus 0.88-0.97 in controls. The ADP/O ratio was also decreased 30-40% in patient mitochondria. Patient lymphoblasts heteroplasmic for the nt 8993 mutation were enucleated by using Percoll gradients and the cytoplasts were fused to mtDNA-deficient (rho 0) cells by electric shock. Cybrid clones homoplasmic for the wild-type nucleotide (T) at nt 8993 gave state III/DNP and ADP/O ratios similar to those of control cybrids, whereas cybrid clones homoplasmic for the mutant nucleotide (G) showed a 24-53% reduction in state III respiration, a state III/DNP ratio of 0.53-0.64, and a 30% decrease in the ADP/O ratio. Thus, the reduced state III respiration rates and ADP/O ratios are linked to the T-->G mutation at nt 8993.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison W. S., Jault J. M., Zhuo S., Paik S. R. Functional sites in F1-ATPases: location and interactions. J Bioenerg Biomembr. 1992 Oct;24(5):469–477. doi: 10.1007/BF00762364. [DOI] [PubMed] [Google Scholar]
- Anderson S., Bankier A. T., Barrell B. G., de Bruijn M. H., Coulson A. R., Drouin J., Eperon I. C., Nierlich D. P., Roe B. A., Sanger F. Sequence and organization of the human mitochondrial genome. Nature. 1981 Apr 9;290(5806):457–465. doi: 10.1038/290457a0. [DOI] [PubMed] [Google Scholar]
- Aprille J. R. Regulation of the mitochondrial adenine nucleotide pool size in liver: mechanism and metabolic role. FASEB J. 1988 Jul;2(10):2547–2556. doi: 10.1096/fasebj.2.10.3290024. [DOI] [PubMed] [Google Scholar]
- Bunn C. L., Wallace D. C., Eisenstadt J. M. Cytoplasmic inheritance of chloramphenicol resistance in mouse tissue culture cells. Proc Natl Acad Sci U S A. 1974 May;71(5):1681–1685. doi: 10.1073/pnas.71.5.1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomyn A., Martinuzzi A., Yoneda M., Daga A., Hurko O., Johns D., Lai S. T., Nonaka I., Angelini C., Attardi G. MELAS mutation in mtDNA binding site for transcription termination factor causes defects in protein synthesis and in respiration but no change in levels of upstream and downstream mature transcripts. Proc Natl Acad Sci U S A. 1992 May 15;89(10):4221–4225. doi: 10.1073/pnas.89.10.4221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomyn A., Meola G., Bresolin N., Lai S. T., Scarlato G., Attardi G. In vitro genetic transfer of protein synthesis and respiration defects to mitochondrial DNA-less cells with myopathy-patient mitochondria. Mol Cell Biol. 1991 Apr;11(4):2236–2244. doi: 10.1128/mcb.11.4.2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciafaloni E., Santorelli F. M., Shanske S., Deonna T., Roulet E., Janzer C., Pescia G., DiMauro S. Maternally inherited Leigh syndrome. J Pediatr. 1993 Mar;122(3):419–422. doi: 10.1016/s0022-3476(05)83431-6. [DOI] [PubMed] [Google Scholar]
- Clark J. B., Hayes D. J., Byrne E., Morgan-Hughes J. A. Mitochondrial myopathies: defects in mitochondrial metabolism in human skeletal muscle. Biochem Soc Trans. 1983 Dec;11(6):626–627. doi: 10.1042/bst0110626. [DOI] [PubMed] [Google Scholar]
- Cox G. B., Fimmel A. L., Gibson F., Hatch L. The mechanism of ATP synthase: a reassessment of the functions of the b and a subunits. Biochim Biophys Acta. 1986 Apr 2;849(1):62–69. doi: 10.1016/0005-2728(86)90096-4. [DOI] [PubMed] [Google Scholar]
- DiMauro S., Bonilla E., Zeviani M., Nakagawa M., DeVivo D. C. Mitochondrial myopathies. Ann Neurol. 1985 Jun;17(6):521–538. doi: 10.1002/ana.410170602. [DOI] [PubMed] [Google Scholar]
- DiMauro S., Servidei S., Zeviani M., DiRocco M., DeVivo D. C., DiDonato S., Uziel G., Berry K., Hoganson G., Johnsen S. D. Cytochrome c oxidase deficiency in Leigh syndrome. Ann Neurol. 1987 Oct;22(4):498–506. doi: 10.1002/ana.410220409. [DOI] [PubMed] [Google Scholar]
- Fillingame R. H. H+ transport and coupling by the F0 sector of the ATP synthase: insights into the molecular mechanism of function. J Bioenerg Biomembr. 1992 Oct;24(5):485–491. doi: 10.1007/BF00762366. [DOI] [PubMed] [Google Scholar]
- Giles R. E., Blanc H., Cann H. M., Wallace D. C. Maternal inheritance of human mitochondrial DNA. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6715–6719. doi: 10.1073/pnas.77.11.6715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grégoire M., Morais R., Quilliam M. A., Gravel D. On auxotrophy for pyrimidines of respiration-deficient chick embryo cells. Eur J Biochem. 1984 Jul 2;142(1):49–55. doi: 10.1111/j.1432-1033.1984.tb08249.x. [DOI] [PubMed] [Google Scholar]
- Hartzog P. E., Cain B. D. The aleu207-->arg mutation in F1F0-ATP synthase from Escherichia coli. A model for human mitochondrial disease. J Biol Chem. 1993 Jun 15;268(17):12250–12252. [PubMed] [Google Scholar]
- Hayashi J., Ohta S., Kikuchi A., Takemitsu M., Goto Y., Nonaka I. Introduction of disease-related mitochondrial DNA deletions into HeLa cells lacking mitochondrial DNA results in mitochondrial dysfunction. Proc Natl Acad Sci U S A. 1991 Dec 1;88(23):10614–10618. doi: 10.1073/pnas.88.23.10614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holme E., Greter J., Jacobson C. E., Larsson N. G., Lindstedt S., Nilsson K. O., Oldfors A., Tulinius M. Mitochondrial ATP-synthase deficiency in a child with 3-methylglutaconic aciduria. Pediatr Res. 1992 Dec;32(6):731–735. doi: 10.1203/00006450-199212000-00022. [DOI] [PubMed] [Google Scholar]
- Holt I. J., Harding A. E., Petty R. K., Morgan-Hughes J. A. A new mitochondrial disease associated with mitochondrial DNA heteroplasmy. Am J Hum Genet. 1990 Mar;46(3):428–433. [PMC free article] [PubMed] [Google Scholar]
- King M. P., Attardi G. Human cells lacking mtDNA: repopulation with exogenous mitochondria by complementation. Science. 1989 Oct 27;246(4929):500–503. doi: 10.1126/science.2814477. [DOI] [PubMed] [Google Scholar]
- King M. P., Koga Y., Davidson M., Schon E. A. Defects in mitochondrial protein synthesis and respiratory chain activity segregate with the tRNA(Leu(UUR)) mutation associated with mitochondrial myopathy, encephalopathy, lactic acidosis, and strokelike episodes. Mol Cell Biol. 1992 Feb;12(2):480–490. doi: 10.1128/mcb.12.2.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kretzschmar H. A., DeArmond S. J., Koch T. K., Patel M. S., Newth C. J., Schmidt K. A., Packman S. Pyruvate dehydrogenase complex deficiency as a cause of subacute necrotizing encephalopathy (Leigh disease). Pediatrics. 1987 Mar;79(3):370–373. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lott M. T., Voljavec A. S., Wallace D. C. Variable genotype of Leber's hereditary optic neuropathy patients. Am J Ophthalmol. 1990 Jun 15;109(6):625–631. doi: 10.1016/s0002-9394(14)72429-8. [DOI] [PubMed] [Google Scholar]
- Miranda A. F., Ishii S., DiMauro S., Shay J. W. Cytochrome c oxidase deficiency in Leigh's syndrome: genetic evidence for a nuclear DNA-encoded mutation. Neurology. 1989 May;39(5):697–702. doi: 10.1212/wnl.39.5.697. [DOI] [PubMed] [Google Scholar]
- Moreadith R. W., Fiskum G. Isolation of mitochondria from ascites tumor cells permeabilized with digitonin. Anal Biochem. 1984 Mar;137(2):360–367. doi: 10.1016/0003-2697(84)90098-8. [DOI] [PubMed] [Google Scholar]
- Ortiz R. G., Newman N. J., Shoffner J. M., Kaufman A. E., Koontz D. A., Wallace D. C. Variable retinal and neurologic manifestations in patients harboring the mitochondrial DNA 8993 mutation. Arch Ophthalmol. 1993 Nov;111(11):1525–1530. doi: 10.1001/archopht.1993.01090110091031. [DOI] [PubMed] [Google Scholar]
- Puddu P., Barboni P., Mantovani V., Montagna P., Cerullo A., Bragliani M., Molinotti C., Caramazza R. Retinitis pigmentosa, ataxia, and mental retardation associated with mitochondrial DNA mutation in an Italian family. Br J Ophthalmol. 1993 Feb;77(2):84–88. doi: 10.1136/bjo.77.2.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson B. H. Cell culture studies on patients with mitochondrial diseases: molecular defects in pyruvate dehydrogenase. J Bioenerg Biomembr. 1988 Jun;20(3):313–323. doi: 10.1007/BF00769635. [DOI] [PubMed] [Google Scholar]
- Sakuta R., Goto Y., Horai S., Ogino T., Yoshinaga H., Ohtahara S., Nonaka I. Mitochondrial DNA mutation and Leigh's syndrome. Ann Neurol. 1992 Oct;32(4):597–598. doi: 10.1002/ana.410320428. [DOI] [PubMed] [Google Scholar]
- Santorelli F. M., Shanske S., Macaya A., DeVivo D. C., DiMauro S. The mutation at nt 8993 of mitochondrial DNA is a common cause of Leigh's syndrome. Ann Neurol. 1993 Dec;34(6):827–834. doi: 10.1002/ana.410340612. [DOI] [PubMed] [Google Scholar]
- Schotland D. L., DiMauro S., Bonilla E., Scarpa A., Lee C. P. Neuromuscular disorder associated with a defect in mitochondrial energy supply. Arch Neurol. 1976 Jul;33(7):475–479. doi: 10.1001/archneur.1976.00500070017003. [DOI] [PubMed] [Google Scholar]
- Shoffner J. M., Fernhoff P. M., Krawiecki N. S., Caplan D. B., Holt P. J., Koontz D. A., Takei Y., Newman N. J., Ortiz R. G., Polak M. Subacute necrotizing encephalopathy: oxidative phosphorylation defects and the ATPase 6 point mutation. Neurology. 1992 Nov;42(11):2168–2174. doi: 10.1212/wnl.42.11.2168. [DOI] [PubMed] [Google Scholar]
- Tatuch Y., Christodoulou J., Feigenbaum A., Clarke J. T., Wherret J., Smith C., Rudd N., Petrova-Benedict R., Robinson B. H. Heteroplasmic mtDNA mutation (T----G) at 8993 can cause Leigh disease when the percentage of abnormal mtDNA is high. Am J Hum Genet. 1992 Apr;50(4):852–858. [PMC free article] [PubMed] [Google Scholar]
- Tatuch Y., Robinson B. H. The mitochondrial DNA mutation at 8993 associated with NARP slows the rate of ATP synthesis in isolated lymphoblast mitochondria. Biochem Biophys Res Commun. 1993 Apr 15;192(1):124–128. doi: 10.1006/bbrc.1993.1390. [DOI] [PubMed] [Google Scholar]
- Wallace D. C., Bunn C. L., Eisenstadt J. M. Cytoplasmic transfer of chloramphenicol resistance in human tissue culture cells. J Cell Biol. 1975 Oct;67(1):174–188. doi: 10.1083/jcb.67.1.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace D. C. Diseases of the mitochondrial DNA. Annu Rev Biochem. 1992;61:1175–1212. doi: 10.1146/annurev.bi.61.070192.005523. [DOI] [PubMed] [Google Scholar]
- Wallace D. C., Lott M. T., Shoffner J. M., Brown M. D. Diseases resulting from mitochondrial DNA point mutations. J Inherit Metab Dis. 1992;15(4):472–479. doi: 10.1007/BF01799605. [DOI] [PubMed] [Google Scholar]
- Wallace D. C. Mitochondrial diseases: genotype versus phenotype. Trends Genet. 1993 Apr;9(4):128–133. doi: 10.1016/0168-9525(93)90207-x. [DOI] [PubMed] [Google Scholar]
- Wallace D. C., Singh G., Lott M. T., Hodge J. A., Schurr T. G., Lezza A. M., Elsas L. J., 2nd, Nikoskelainen E. K. Mitochondrial DNA mutation associated with Leber's hereditary optic neuropathy. Science. 1988 Dec 9;242(4884):1427–1430. doi: 10.1126/science.3201231. [DOI] [PubMed] [Google Scholar]
- Wallace D. C., Yang J. H., Ye J. H., Lott M. T., Oliver N. A., McCarthy J. Computer prediction of peptide maps: assignment of polypeptides to human and mouse mitochondrial DNA genes by analysis of two-dimensional-proteolytic digest gels. Am J Hum Genet. 1986 Apr;38(4):461–481. [PMC free article] [PubMed] [Google Scholar]
- de Vries D. D., van Engelen B. G., Gabreëls F. J., Ruitenbeek W., van Oost B. A. A second missense mutation in the mitochondrial ATPase 6 gene in Leigh's syndrome. Ann Neurol. 1993 Sep;34(3):410–412. doi: 10.1002/ana.410340319. [DOI] [PubMed] [Google Scholar]



