Abstract
A role for the dopamine D1-D2 receptor heteromer in the regulation of reward and addiction-related processes has been previously implicated. In the present study, we examined the effects of D1-D2 heteromer stimulation by the agonist SKF 83959 and its disruption by a selective TAT-D1 peptide on amphetamine-induced locomotor sensitization, a behavioural model widely used to study the neuroadaptations associated with psychostimulant addiction. D1-D2 heteromer activation by SKF 83959 did not alter the acute locomotor effects of amphetamine but significantly inhibited amphetamine-induced locomotor responding across the 5 day treatment regimen. In addition, a single injection of SKF 83959 was sufficient to abolish the expression of locomotor sensitization induced by a priming injection of amphetamine after a 72-hour withdrawal. Conversely, inhibition of D1-D2 heteromer activity by the TAT-D1 peptide enhanced subchronic amphetamine-induced locomotion and the expression of amphetamine locomotor sensitization. Treatment solely with the TAT-D1 disrupting peptide during the initial 5 day treatment phase was sufficient to induce a sensitized locomotor phenotype in response to the priming injection of amphetamine. Together these findings demonstrate that the dopamine D1-D2 receptor heteromer exerts tonic inhibitory control on neurobiological processes involved in sensitization to amphetamine, indicating that the dopamine D1-D2 receptor heteromer may be a novel molecular substrate in addiction processes involving psychostimulants.
Keywords: Dopamine D1-D2 Receptor Heteromer, Amphetamine, Locomotor Sensitization
1. Introduction
The dopamine D1-D2 receptor heteromer is a G protein-coupled receptor (GPCR) complex that couples to the Gαq protein to elicit a phospholipase C (PLC)-dependent calcium signal upon its activation (Rashid et al. 2007). It has been reported that a significant proportion of D1 receptor (D1R)-expressing medium spiny neurons (MSNs) in the nucleus accumbens (NAc) co-express the dopamine D1 and D2 receptors (17–25%) (Bertran-Gonzalez et al. 2008; Matamales et al. 2009; Perreault et al. 2010; Gangarossa et al. 2013) and approximately 90% of these MSNs express the D1-D2 heteromer (Hasbi et al. 2009; Perreault et al. 2010). In contrast, only 2–6% of D1R-expressing MSNs in the caudate putamen co-express the D1R and D2R, of which only 25% of the neurons exhibit D1-D2 heteromer formation (Perreault et al. 2010). The D1-D2 co-expressing neurons in the NAc extend efferent projections which directly or indirectly influence the ventral tegmental area (VTA) (Perreault et al. 2012a), a region widely known for its role in mediating addiction-like behaviours and reward through the regulation of mesolimbic dopamine activity (Reviewed: Chen et al., 2010; Koob & Volkow, 2010).
We have previously shown that activation of the D1-D2 receptor heteromer modulated the expression of proteins involved in drug addiction (Hasbi et al. 2009; Ng et al. 2010; Perreault et al. 2010, 2012a), such as brain-derived neurotrophic factor (BDNF) and calcium-calmodulin kinase II (CaMKII) in the NAc and VTA, and D1-D2 heteromer activation in NAc shell enhanced production of the inhibitory neurotransmitter GABA in VTA (Perreault et al. 2012a). These findings thus suggest a potential role for the D1-D2 heteromer in the regulation of neuronal activity in the VTA and possibly as a regulator of brain reward processes.
Since repeated amphetamine treatment was previously shown to enhance the functional activity of the D1-D2 heteromer in rat striatum (Perreault et al. 2010), in this study we aimed to further examine the potential involvement of the D1-D2 heteromer in processes linked with addiction using the amphetamine-induced locomotor sensitization model in rats. Psychostimulant-induced locomotor sensitization was proposed to be an animal model for drug craving, and is characterized by a context-dependent, progressive augmentation of locomotor responsiveness following repeated non-contingent administration of psychostimulants such as cocaine and amphetamine (Robinson and Becker 1986; Kalivas and Stewart 1991; Robinson and Berridge 1993; Anagnostaras and Robinson 1996). amphetamine-induced locomotor sensitization is associated with neuroadaptations of the mesolimbic dopamine system that may enhance the reinforcing properties of cocaine and amphetamine, as animals that were previously sensitized with repeated amphetamine treatment showed increased acquisition of drug self-administration (Mendrek et al. 1998; Suto et al. 2002; Vezina et al. 2002).
Once established, amphetamine locomotor sensitization has been reported to persist for over a year (Paulson and Robinson 1991), which may be a reflection of some of the long-term neurobiological adaptations that accompany the persistent drug-seeking behaviours typically seen in addicted patients (Robinson and Berridge 2000). Similarly, animals that exhibited the reinstatement of cocaine-seeking behaviour induced by a single exposure to amphetamine also expressed locomotor sensitization (De Vries 1998), suggesting that changes in the mesolimbic dopamine system that accompany the expression of amphetamine locomotor sensitization may also contribute to relapse of drug seeking.
The dopamine agonist SKF 83959 is a partial agonist for the D1-D2 heteromer with a number of in vitro and in vivo studies demonstrating its ability to induce D1-D2 heteromer-mediated calcium signaling (Rashid et al. 2007; Verma et al. 2010), Gq and PLC activation (Rashid et al. 2007), CaMKII phosphorylation (Ng et al. 2010) and BDNF production (Hasbi et al. 2009). Validation of selectivity to the D1-D2 heteromer in these studies employed D1 and D2 antagonists and dopamine receptor knockout mice. Recent studies, however, have indicated that SKF 83959 has affinity for, or activates, a number of other receptors, such as the dopamine D5 receptor, the α-adrenergic receptor 2C, and the serotonin 5HT-2C receptor (Sahu et al. 2009; Perreault et al. 2012b; Chun et al. 2013). In addition, there are conflicting reports as to whether SKF 83959 functions as an antagonist (Downes and Waddington 1993; Cools et al. 2002; Jin et al. 2003), a partial agonist (Lee et al. 2014), or has no effect (Lee et al. 2004; Rashid et al. 2007) at the D1R. To assist in elucidating the physiological role of the D1-D2 heteromer, we developed a selective D1-D2 heteromer antagonist, the TAT-D1 peptide, which occludes the interaction site between the two receptors (O'Dowd et al., 2012), thus inhibiting D1-D2 heteromer expression and function and abolishing the physiological effects of D1-D2 heteromer activation by SKF 83959 without affecting other receptor oligomers such as D1-D1 homomers or D2-D5 heteromers (Hasbi et al., 2014). In the present study, we assessed the effects of SKF 83959 on the expression of amphetamine locomotor responses and sensitization. We only attributed an effect to be D1-D2 heteromer-specific when the TAT-D1 peptide produced opposite behavioural output compared to SKF 83959. Our findings showed a novel role for the D1-D2 heteromer in the suppression of amphetamine-induced locomotion and locomotor sensitization.
2. Material and Methods
2.1 Animals
Ninety-six adult male Sprague-Dawley rats (Charles River, Canada), weighing 300–350 g at the start of the experiment, were used. Rats were housed in polyethylene cages in a temperature-controlled colony room, maintained on a 12-h light-dark cycle (lights on at 0700h), with ad libitum access to food and water. Rats were handled daily for 5 days before the start of the experiment. All treatments were performed during the light phase of the day-night cycle. Animals were housed and tested in compliance with the guidelines described in the Guide to the Care and the Use of Experimental Animals (Canadian Council on Animal Care, 1993), and were approved by the Animal Care Ethics Committee of the University of Toronto.
2.2 Drugs
SKF 83959 hydrobromide (Tocris Bioscience) was dissolved in physiological saline containing 5% DMSO, and was administered subcutaneously (s.c.). Amphetamine hydrochloride (Sigma-Aldrich) was dissolved in physiological saline (0.9% NaCl), and was administered intraperitoneally (i.p.). The TAT-D1 disrupting peptide was dissolved in saline and administered into the intracerebroventricular (i.c.v.) space 15 minutes prior to vehicle, SKF 83959, or amphetamine injection. For non-drug injections, an equivalent volume of saline or vehicle was administered. All systemic injections were given at a volume of 1.0 ml/kg just prior to behavioural testing.
2.3 Surgery
Rats were anesthetized with isoflurane (5%), administered analgesic ketoprofen (5 mg/kg, s.c.) and secured in a stereotaxic frame. A cannula (22-gauge, Plastics One) was placed unilaterally into the intracerebroventricular space close to the midline according to the following stereotaxic coordinates: AP −0.8mm, ML + 1.3mm, DV – 3.7mm, and was secured by dental cement anchored with four stainless steel screws (Plastics One) fixed on the dorsal surface of the skull. AP and ML coordinates were taken from bregma, DV coordinate from the dura (Paxinos and Watson 1998). The animals were allowed to recover in their home cage for a minimum of five days before the experiments were performed. Cannulae placement was visually validated postmortem in brain slices.
2.4 Locomotor Activity Apparatus
The testing environment was a non-colony room containing 8 empty activity chambers that are 20cm in height, 25cm in width, and 40cm in length. Two arrays of 16 infrared photocells were attached along the longer sides of the polyethylene cages. The activity chambers were interfaced to a computer that provided automated recording of horizontal locomotor activity when both top and bottom infrared photocells were triggered. Ventilated polyethylene lids were used to cover the activity chambers to prevent animals from escaping.
2.5 Locomotor Sensitization Protocol
The behavioural testing for locomotor sensitization to amphetamine was conducted using a previously described protocol (Hall et al. 2008), which consisted of 3 phases:
Phase I: Habituation
Rats were first habituated to the activity chamber for 2 days for 30 minutes per day.
Phase II: The Sensitizing Regimen
In this phase, we examined the effects of D1-D2 heteromer stimulation and inactivation on locomotion induced by acute and subchronic amphetamine treatment (1.5mg/kg, i.p.). Animals were administered their designated drug treatment, VEH+SAL, SKF 83959+SAL, TAT-D1+SAL, VEH+amphetamine, SKF 83959+amphetamine, TAT-D1+amphetamine, once daily for 5 consecutive days. Immediately following each injection, horizontal locomotor activity was monitored for 60 minutes. The dose of SKF 83959 (0.4 mg/kg, s.c.) given in this phase was chosen based on our previous study showing repeated SKF 83959 treatment significantly enhanced locomotor activity and grooming responses without desensitizing the D1-D2 heteromer (Perreault et al. 2010). The dose of the TAT-D1 peptide (300pmol, i.c.v.) was previously shown to disrupt D1-D2 heteromer formation in vivo as indicated by the loss of co-immunoprecipitation of D1R with D2R in rat NAc tissue (Hasbi et al. 2014).
Phase III: The Expression of Locomotor Sensitization
Here we examined the effect of D1-D2 heteromer stimulation/inactivation alone or with amphetamine during the sensitizing regimen on the expression of locomotor sensitization to amphetamine. All animals from Phase II underwent a 72 hour withdrawal following the 5th injection, which was a time point previously shown to be sufficient to induce the expression of locomotor sensitization in response to amphetamine challenge (Hall et al. 2008). Following withdrawal, all animals were challenged with a priming injection of amphetamine (1.0 mg/kg, i.p.), and their horizontal locomotor activity were measured for 60 minutes. In addition, a subset of animals that were treated solely with amphetamine during Phase II received the co-administration of SKF 83959 (2.5 mg/kg, s.c.) or TAT-D1 peptide (300 pmol, i.c.v.) with the priming amphetamine to examine the effect of acute D1-D2 heteromer stimulation or inactivation on established locomotor sensitization to amphetamine. We increased the dose of SKF 83959 for acute injection in the third phase, which was a dose that we have previously shown to induce changes in CaMKII expression in rat striatum (Ng et al., 2010).
In addition, in order to elucidate the involvement of the D1-D2 heteromer on basal locomotion, we performed a separate experiment in which animals were treated acutely with VEH+SAL, SKF 83959 (0.4mg/kg, s.c.)+SAL, VEH+TAT-D1 (300pmol, i.c.v.), SKF 83959+TAT-D1 without habituation to the test chamber. The horizontal locomotor activity of these animals was measured for 60 minutes immediately following SKF 83959 or VEH treatment.
2.6. Data Analysis
All horizontal locomotor activity values are reported as mean ± s.e.m. All data was analyzed for normality prior to ANOVAs using the Shapiro-Wilk test. The results shown in Figure 1 were analyzed by Two-way ANOVA with D1-D2 heteromer Treatment (VEH, SKF 83959, TAT-D1 peptide) and amphetamine Treatment (SAL, amphetamine) as the between subjects factors. The results shown in Figure 2 were analyzed by Repeated measures of ANOVA with Injection as the within subject factor and Drug Treatments as the between subject factor, followed by post-hoc test (Duncan multiple range test). The results shown in Figure 4 were analyzed by One-way ANOVA with Drug Treatments as the between subjects factor. Planned comparisons between selected groups were performed using Student’s t-test. Computations were performed using the SPSS statistical program. Statistical criteria for significant differences were set at p<0.05.
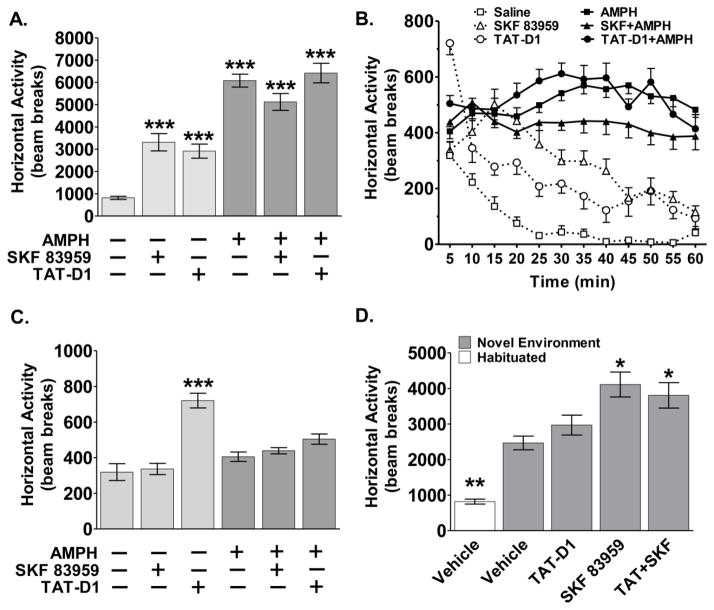
Figure 1. The effects acute D1-D2 heteromer stimulation and inactivation on basal and amphetamine-induced locomotor activity.
(A) The total horizontal activity over 60 minutes in units of beam breaks in response to acute administration of amphetamine (1.5 mg/kg, i.p.), alone or in combination with SKF 83959 (0.4 mg/kg, s.c.) or TAT-D1 peptide (300 pmol, i.c.v.). (B) Time course of the acute locomotor response measured in 5-min intervals over 60 minutes. (C) The total horizontal activity for the first 5 minutes. (D) Total horizontal activity in the absence of habituation in response to SKF 83959, TAT-D1 peptide, or SKF 83959 + TAT-D1. Data represent means ± s.e.m. of n=7–11 rats/group with the exception of amphetamine, n=40 rats/group, and controls n=16 rats/group. AMPH=amphetamine. (*p<0.05, ** p<0.01, *** p<0.001 compared to Saline Control.)
Figure 2. The effects of subchronic D1-D2 heteromer stimulation and inactivation on basal and amphetamine-induced locomotor activity.
Repeated amphetamine treatment (1.5 mg/kg, i.p.) produced a slight increase in total locomotion over the 5 injections. Repeated SKF 83959 co-treatment (0.4 mg/kg, s.c.) attenuated, while repeated TAT-D1 peptide pre-treatment (300pmol, i.c.v.) enhanced, amphetamine-induced locomotion over the 5 injections. Across injections animals treated solely with SKF 83959 or TAT-D1 peptide did not significantly differ from Saline controls. Data represent means ± s.e.m. of n=7–11 rats/group with the exception of amphetamine, n=40 rats/group, and controls n=16 rats/group. AMPH=amphetamine. (*p<0.05, compared to amphetamine group)
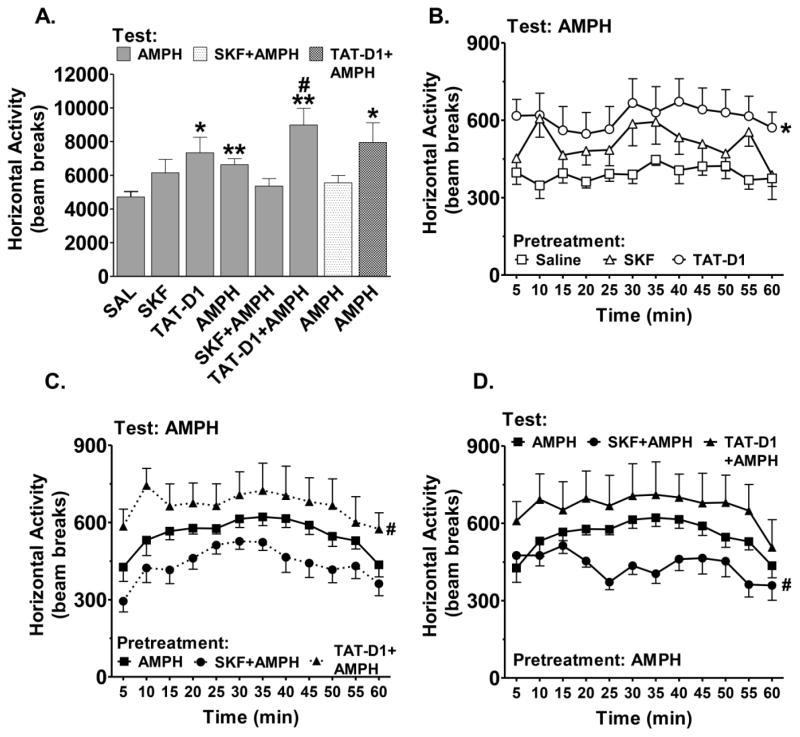
Figure 4. The effects of D1-D2 receptor heteromer stimulation and inactivation on the expression of amphetamine-induced locomotor sensitization.
(A) Total horizontal activity in response to a priming injection of amphetamine (1.0 mg/kg, i.p.) for animals treated daily for 5 days with vehicle, SKF 83959 (0.4 mg/kg, s.c.), TAT-D1 peptide (300 pmol, i.c.v.), amphetamine (1.5 mg/kg, s.c.), AMPH+SKF 83959 or AMPH+TAT-D1 peptide. Total horizontal activity in response to priming amphetamine plus SKF 83959 (2.5mg/kg, s.c.) or TAT-D1 (300pmol, i.c.v.) for amphetamine-sensitized animals are also shown. (B) Time course of the acute locomotor response to priming amphetamine in rats that received subchronic pretreatment with Saline, SKF 83959 or TAT-D1 peptide. (C) The time course showing the effect of subchronic pretreatment with amphetamine, SKF 83959+amphetamine or TAT-D1+amphetamine on priming amphetamine-induced locomotor sensitization (D) Time course of the sensitized locomotor response to priming amphetamine alone, amphetamine+SKF 83959 or amphetamine+TAT-D1 for amphetamine pre-treated rats. Horizontal activity in 5-min intervals is shown and is plotted in units of beam breaks. Data represent means ± s.e.m. of n=8–16 rats/group. AMPH=amphetamine. (*p<0.05, ** p<0.01 compared to SAL group, #p<0.05 compared to amphetamine group.)
3. Results
3.1 The effects of acute D1-D2 heteromer activation and disruption on basal and amphetamine-induced locomotor activity
Using the horizontal locomotor activity data obtained from the 1st injection, we first examined the effect of acute SKF 83959 administration or TAT-D1-induced D1-D2 heteromer disruption on basal and amphetamine-induced locomotor activity (Figure 1A-C). Two-way ANOVA with D1-D2 heteromer Treatment and amphetamine Treatment as between subject factors revealed a significant main effect of D1-D2 Treatment {F(2:82)=3.489, p<0.05} and a significant main effect of amphetamine Treatment {F(1:82)=78.690, p<0.0001}, as well as a significant D1-D2 Treatment X amphetamine Treatment interaction {F(2:82)=7.335, p<0.001}. Planned comparisons between treatment groups were then made using Student’s t-test. As shown in Figure 1A, over the 60 minute period, administration of the agonist SKF 83959 (0.4 mg/kg, s.c.) alone induced a significant increase in locomotion compared to saline-treated controls (bar 1: 818.6 ± 70.8 vs. bar 2: 3314.4 ± 389.6, t(14)=6.30, p<0.001). Amphetamine treatment alone induced a significant increase in locomotion (bar 1: 818.6 ± 70.8 vs. bar 4: 6081.2 ± 291.1, t(46)=8.00, p<0.001), which was not influenced by SKF 83959 co-treatment (bar 5: 5124.9 ± 377.2). Similar to SKF 83959, acute pre-administration (15 min) of the TAT-D1 peptide (300 pmol, i.c.v.) to rats, previously shown to disrupt the D1-D2 heteromer in rat NAc (Hasbi et al. 2014), also elevated total locomotor activity compared to controls (bar 1: 818.6 ± 70.8 vs. bar 3: 2918.1 ± 317.6, t(14)=6.45, p<0.001) with no effect on acute amphetamine-induced locomotion (bar 6: 6420.7 ± 440.2).
Examination of the time course of locomotion revealed that the acute locomotor activating effects of SKF 83959 and TAT-D1 peptide steadily declined across the 60 minutes (Figure 1B, dotted lines). Conversely, the locomotor activity of animals treated with amphetamine alone or in combination with SKF 83959 or TAT-D1 peptide remained elevated throughout the testing period (Figure 1B, solid lines). It is noteworthy that in the first 5 minutes of the testing period (Figure 1C), the animals treated with TAT-D1 peptide alone exhibited a very robust increase in locomotor activity (t(22)=6.15, p<0.001) that was not apparent in the other treatment groups. {D1-D2 Treatment: F(2,82)=17.6, p<0.0001; amphetamine: F(1,82)=0.2, p<0.68; D1-D2 Treatment X amphetamine: F(2,82)=7.7, p<0.001}.
Since D1-D2 heteromer stimulation by SKF 83959 and its inactivation by the TAT-D1 peptide both stimulated basal locomotion in habituated animals, we wanted to further resolve the role of D1-D2 heteromer in modulating basal locomotion. Therefore, we next tested animals in the absence of habituation, a process which may affect total horizontal locomotor activity due to the animal’s natural tendency to explore a novel environment (Eilam and Golani 1989). One-way ANOVA revealed a significant main effect of Drug Treatments {F(4,56)=16.749, p<0.0001}. As shown in Figure 1D, Duncan’s post hoc analysis showed that vehicle-treated rats indeed exhibited significantly higher locomotion in the absence of habituation (bar 1: 818.6 ± 70.8 vs. bar 2: 2466.4 ± 193.4, p<0.01). The exposure to a novel environment also abolished the locomotor-activating effect of acute TAT-D1 peptide pre-treatment (bar 2: 2466.4 ± 193.4 vs. bar 3: 2970.8 ± 280.1), while the locomotion induced by SKF 83959 was maintained (bar 2: 2466.4 ± 193.4 vs. bar 4: 4112.3 ± 350.5, p<0.05). We further showed that pre-treatment with TAT-D1 peptide did not attenuate the locomotor-activating effect of SKF 83959 in the absence of habituation (bar 4: 4112.3 ± 350.5 vs. bar 5: 3806.8 ± 359.0). This demonstrates that locomotor activation induced by SKF 83959 occurs via a mechanism not involving the D1-D2 heteromer.
3.2 The effects of D1-D2 heteromer activation and disruption on locomotor activity induced by repeated amphetamine
To determine whether the D1-D2 heteromer could influence locomotor responding induced by repeated amphetamine treatment, rats were administered 5 daily injections of amphetamine alone or in combination with SKF 83959 or the TAT-D1 peptide, and locomotion was monitored for 60 minutes following each drug injection (Figure 2). Repeated measures of ANOVA showed a significant within subject effect of Injection for the groups that received amphetamine alone {F(4,40)=13.092, p<0.001} and amphetamine plus TAT-D1 {F(4,8)=5.106, p<0.01}. Duncan’s post hoc analysis using Drug Treatment as the between subject factor following repeated measures of ANOVA {F(5,83)=35.410, p<0.0001} further revealed that, compared to the amphetamine alone group, co-administration of SKF 83959 significantly diminished locomotor responsiveness to repeated amphetamine treatment (p<0.05) whereas TAT-D1 co-treated rats exhibited significantly enhanced repeated amphetamine-induced locomotion (p<0.05). Across injections, animals treated solely with SKF 83959 or TAT-D1 peptide did not significantly differ from saline controls.
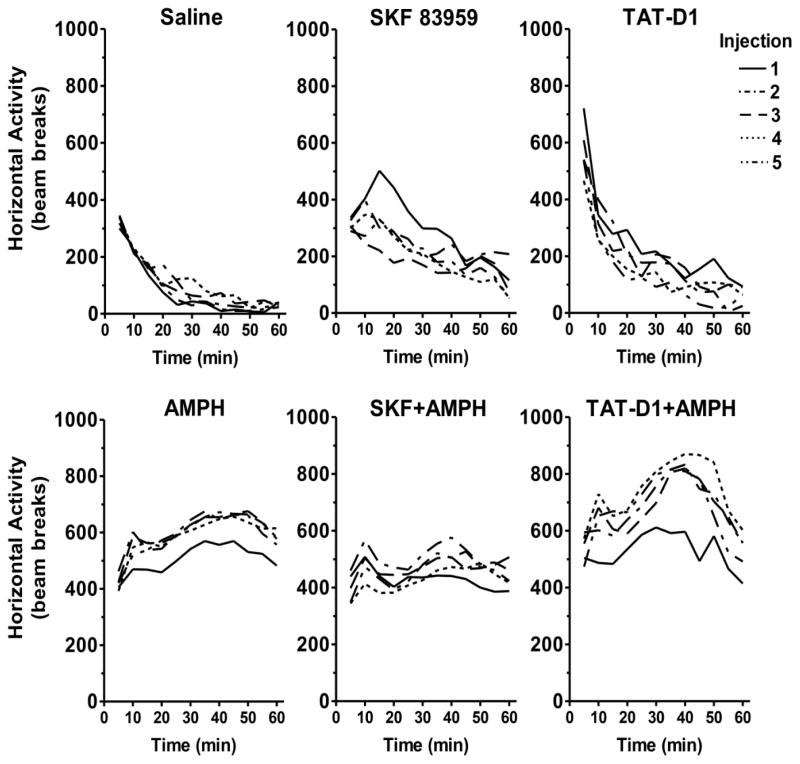
We also examined the change in the temporal pattern of locomotor activity across the 5 injections for each of the treatments described in Figure 2. Repeated saline treatment had no effect on the temporal pattern of locomotor activity, which showed a steady decline over the 60 minutes (Figure 3, upper left panel). SKF 83959 treatment at the first injection produced an initial excitation in locomotor activity that declined after the 15 minute mark (Figure 3, upper middle panel). With subsequent injections, this period of initial excitation gradually diminished and ultimately disappeared. On the other hand, TAT-D1 peptide treatment consistently produced a robust initial increase in locomotor activity within the first 5 minutes across the 5 injections (Figure 3, upper right panel). In contrast to SKF 838959 treatment, repeated injections did not alter the temporal pattern of locomotor activity produced by the TAT-D1 peptide.
Figure 3. The time course of basal or amphetamine-induced locomotor responses following D1-D2 heteromer stimulation or inactivation during the course of repeated treatment.
Horizontal activity is plotted at 5-min intervals. Lines depict overall group means. AMPH=amphetamine. (n=8–11 rats/group with the exception of amphetamine, n=40 rats/group, and controls n=16 rats/group).
The temporal pattern of locomotor activity for the first amphetamine injection steadily increased and peaked at approximately 40 minutes (Figure 3, lower left panel). With successive amphetamine injections, a period of initial excitation emerged at the 10 minute mark, followed by a period of secondary excitation that peaked at 40 minutes, indicating that repeated injections altered the temporal pattern of locomotor activity associated with amphetamine treatment. SKF 83959 co-treatment with amphetamine produced a similar temporal pattern of locomotor activity over the 5 injections as the amphetamine alone group (Figure 3, lower middle panel), although the overall locomotor activity was consistently lower in the SKF 83959 co-treated group. In contrast, TAT-D1 peptide pre-treatment with amphetamine progressively increased the maximum locomotor activity during the secondary excitation over the 5 injections compared to the amphetamine alone group.
3.3 The effects of D1-D2 receptor heteromer activation and disruption on the expression of amphetamine-induced locomotor sensitization
We next wanted to determine whether the D1-D2 heteromer could influence amphetamine-induced expression of locomotor sensitization in response to a priming amphetamine injection following a 72 hour withdrawal from the 5th injection. One-way ANOVA revealed a significant main effect of Drug Treatment {F(7,84)=3.950, p<0.01}. As shown in Figure 4A, Planned comparisons further showed that the group treated with repeated prior amphetamine injections (1.5 mg/kg, i.p.) exhibited significantly higher locomotor activity compared to saline pretreated controls in response to the priming injection of amphetamine (1.0 mg/kg, i.p.) administered on the test day, indicative of locomotor sensitization to amphetamine (bar 4: 6633.3 ± 354.3 vs. bar 1: 4723.0 ± 317.7, t(22)=3.46, p<0.01). Animals previously administered SKF 83959 alone did not differ from saline pretreated controls in locomotor activity following the priming amphetamine injection (bar 2 vs. bar 1). Interestingly, repeated administration of TAT-D1 peptide alone elicited a sensitized locomotor response to the priming amphetamine that was significantly higher than saline pretreated controls (bar 3: 7337.0 ± 920.4 vs. bar 1: 4723.0 ± 317.7, t(14)=2.69, p<0.05), and similar in magnitude to that observed in the amphetamine-sensitized group.
Animals that received SKF 83959 plus amphetamine during the 5 day injection period did not exhibit sensitized responding to the priming amphetamine on the test day (bar 5 vs. bar 1). In contrast, animals that received prior exposure of amphetamine plus TAT-D1 for 5 days not only produced a sensitized locomotor phenotype in response to the priming amphetamine compared to the saline pretreated controls (bar 6: 8989.3 ± 994.0 vs. bar 1: 4723.0 ± 317.7, t(14)=4.09, p<0.01), but the sensitized responding was also further significantly enhanced compared to amphetamine-sensitized animals (bar 6: 8989.3 ± 994.0 vs. bar 4: 6633.3 ± 354.3. t(22)=2.76, p<0.05). Acute co-administration of SKF 83959 with the priming amphetamine on the test day (bar 7) abolished the expression of locomotor sensitization in animals pretreated with amphetamine whereas acute TAT-D1 peptide pre-treatment with the priming amphetamine did not affect the expression of locomotor sensitization to amphetamine (bar 8: 7948.5 ± 1179.5 vs. bar 1: 4723.0 ± 317.7, t(14)=2.64, p<0.05).
Lastly, we also examined the time course of the locomotion induced by the priming amphetamine on the test day for each of the drug treatments described previously in Figure 4A. Prior repeated treatment solely with SKF 83959 or the TAT-D1 peptide did not alter the dynamics of horizontal locomotor activity over the 60 minutes in response to the priming amphetamine on the test day compared to the saline pretreated group (Figure 4B). However, post hoc analysis following repeated measures of ANOVA using Treatment as the between subject factor {F(2,24)=3.31, p<0.056} showed a significant increase in locomotion over the 60 minutes following amphetamine priming for animals treated solely with repeated TAT-D1 peptide during the sensitizing regimen compared to those treated with repeated saline (p<0.05).
Prior SKF 83959 co-treatment or TAT-D1 pre-treatment with amphetamine during the sensitizing regimen also did not affect the dynamics of horizontal locomotor activity over the 60 minutes in response to the priming amphetamine on the test day compared to the amphetamine alone group (Figure 4C). Nevertheless, SKF 83959 co-treatment and TAT-D1 pre-treatment with amphetamine consistently showed an opposing effect on priming amphetamine-induced locomotion at each 5 minute time point throughout the 60 minute period {F(2,34)=5.28, p<0.05}, with the TAT-D1 pre-treated group being significantly higher than the amphetamine alone group (p<0.05) and SKF 83959 co-treatment showing a trend towards a significant decrease (p<0.07). Similarly, acute co-treatment of SKF 83959 and pre-treatment of TAT-D1 with the priming amphetamine also showed an opposing effect on priming amphetamine-induced locomotion at each 5 minute time point throughout the 60 minute period in animals previously sensitized with repeated amphetamine {F(2,36)=6.14, p<0.01} (Figure 4D), with SKF 83959 co-treatment being significantly lower than the amphetamine alone group (p<0.05). Moreover, SKF 83959 co-treatment with the priming amphetamine also altered the dynamics of horizontal locomotor activity starting from the 15 minute mark compared to the amphetamine alone group, while TAT-D1 pre-treatment had no effect.
4. Discussion
In the present study we demonstrated that the dopamine D1-D2 receptor heteromer plays a significant regulatory role in the locomotor activating effects of amphetamine and in locomotor sensitization to amphetamine. Specifically, we showed that SKF 83959 co-treatment reduced the magnitude of the locomotor response induced by repeated amphetamine administration and abolished the expression of locomotor sensitization, with even a single treatment of the agonist SKF 83959 being sufficient to abolish the sensitized phenotype of amphetamine-treated rats. Conversely, inactivation of the D1-D2 receptor heteromer by a selective disrupting TAT-D1 peptide enhanced locomotion induced by repeated amphetamine treatment and augmented the expression of sensitized locomotor responding to amphetamine priming, findings which strongly suggest that the D1-D2 heteromer functions to tonically suppress the neurobiological processes involved in these responses.
Amphetamine-induced locomotor sensitization has been associated with neuroadaptations in the mesolimbic system that are critically involved in the regulation of reward and motivation (Robinson and Becker 1986; Henry and White 1991; Kantor et al. 1999), and which have been proposed to enhance the incentive salience, or “wanting”, of the drug itself, as well as drug-related stimuli such as the environmental context associated with drug use, leading to compulsive drug seeking behaviors that are typically seen in addicted individuals (Robinson and Berridge 2000; Schmidt and Beninger 2006). Thus, these findings provide the first evidence of a possible role for the receptor complex as a negative regulator of the physiological processes associated with amphetamine addiction.
Numerous studies have demonstrated that amphetamine-induced locomotor sensitization is a complex behaviour involving the close interplay between various neurotransmitter systems in regions of the mesocorticolimbic circuitry. The initiation of amphetamine-induced locomotor sensitization is thought to depend on enhanced VTA dopaminergic neuron excitability in the form of long-term potentiation (LTP), which occurs as a consequence of reduced D2 auto-receptor inhibition and reduced inhibitory GABAergic control in the VTA, as well as increased glutamate release from the medial prefrontal cortex, following repeated non-contingent amphetamine administration (White and Wang 1984; Wolf and Xue 1998; Saal et al. 2003). This subsequently results in increased dopamine release in the NAc in response to amphetamine challenge that act on supersensitized D1 receptors (Robinson et al. 1988; Wolf et al. 1994), leading to the expression of amphetamine-induced locomotor sensitization. It should be noted that the increased dopamine release into the NAc was also shown to be dependent on the enhanced activity of CaMKII in the NAc following amphetamine challenge in cocaine-sensitized rats (Pierce and Kalivas 1997). In addition to increased dopamine release, amphetamine challenge also results in increased glutamate release into the NAc from the medial PFC in amphetamine-sensitized animals, the attenuation of which has been shown to block the expression of amphetamine-induced locomotor sensitization (Kim et al. 2005).
There are several potential mechanisms by which the D1-D2 heteromer stimulation may counteract the neuroadaptations associated with amphetamine-induced locomotor sensitization. We have previously demonstrated that D1-D2 heteromer stimulation by a single systemic SKF 83959 injection significantly increased the expression of a major GABA producing enzyme, glutamic acid decarboxylase (GAD67), in the VTA (Perreault et al. 2012a), potentially enhancing GABAergic control in the VTA and preventing LTP of VTA dopaminergic neurons known to be associated with the initiation of amphetamine-induced locomotor sensitization. In addition, we have also shown that repeated stimulation of the D1-D2 heteromer significantly reduced the expression of CaMKII in the NAc (Perreault et al. 2010), which may prevent the enhanced dopamine release in the NAc upon amphetamine challenge and thereby abolish the expression of amphetamine-induced sensitization. Another possibility is that the D1-D2 heteromer may reduce AMPA receptor activity in the NAc, as enhanced glutamatergic activity was shown to be required for the expression of this behaviour (Kim et al. 2005). This idea is supported by our previous work which showed repeated administration of SKF 83959 significantly reduced phosphorylation of AMPA receptor subunit GluA1 at the Ser831 site in the NAc (Perreault et al. 2010), a site shown to be critical for inducing a long-lasting potentiation of acute amphetamine-induced locomotion (Loweth et al. 2010).
Perhaps the most intriguing finding in the present study is the fact that subchronic D1-D2 heteromer inactivation by the TAT-D1 peptide alone was able to produce a sensitized locomotor phenotype following amphetamine priming to a magnitude comparable to that which was observed in amphetamine-sensitized animals, suggesting that the D1-D2 heteromer exerts tonic suppression on the neurological processes associated with the expression of amphetamine-induced locomotor sensitization. Although the mechanism through which the D1-D2 heteromer exerts such effect remains unclear, a study suggests the potential involvement of cyclin-dependent kinase 5 (cdk5). In the study by Taylor et al., animals that received subchronic intra-NAc administration of cdk5 inhibitor roscovitine also exhibited a sensitized locomotor phenotype upon cocaine priming to a degree similar to that produced by cocaine-sensitized animals (Taylor et al. 2007). Furthermore, co-administration of roscovitine with cocaine during the induction phase significantly enhanced the expression of cocaine locomotor sensitization induced by cocaine priming (Taylor et al. 2007), which remarkably mirrors the effect of TAT-D1 peptide co-administration on the expression of amphetamine locomotor sensitization in the current study. Taken together, it is possible that cdk5 may be a downstream effector of the D1-D2 heteromer signaling, and thus D1-D2 heteromer inactivation by the TAT-D1 peptide had similar effect on the expression of psychostimulant-induced locomotor sensitization as the cdk5 inhibitor roscovitine. This is an interesting hypothesis that will be addressed in future studies.
We have previously demonstrated that the D1-D2 heteromer has representations in the mesolimbic, striatonigral and striatopallidal pathways (Perreault et al. 2010, 2011) and that medium spiny neurons that express the D1-D2 heteromer also express both GABA and glutamate (Perreault et al. 2012a). In addition, the acute effects of D1-D2 heteromer activation in the NAc on the expression of proteins involved in GABA and glutamate transmission were shown to be pathway specific, with enhanced GABA-related protein expression in NAc/VTA and increased glutamate-related protein expression in the dorsal striatum (Perreault et al. 2012a). It is therefore possible that the D1-D2 heteromer may exert dual modulation on amphetamine-induced locomotion by differentially regulating glutamate and GABA transmission in several nuclei within the mesocorticolimbic and the basal ganglia circuitry (Perreault et al. 2011, 2012a). This ability of the D1-D2 heteromer to potentially regulate both GABA and glutamate neurotransmission would allow for the “fine tuning” of neuronal activity within the mesolimbic direct and indirect dopamine pathways (Lobb et al. 2010). This line of reasoning is supported by our previous study which showed increased functional activity of the D1-D2 heteromer in the striatum of amphetamine-sensitized rats (Perreault et al. 2010), which may be a potential negative-feedback compensatory process in an attempt to normalize disturbances in dopaminergic transmission induced by subchronic amphetamine treatment.
A potential limitation of the present study is the use of SKF 83959 to examine the behavioural effects of D1-D2 heteromer activation. No selective agonists for the D1-D2 heteromer are available, and while SKF 83959 has been shown to definitively induce the Gq-PLC-linked calcium signaling mediated by the D1-D2 heteromer, its pharmacology has been shown to extend to include a few other receptors including the dopamine D5 receptor (Sahu et al. 2009; Perreault et al. 2012b; Chun et al. 2013; Guo et al. 2013). While the use of the highly selective disrupting TAT-D1 peptide that exclusively targets the specific interaction sites between the D1R and the D2R allows us to conclusively isolate D1-D2 heteromer-specific effects on specific amphetamine-induced locomotor responses, off-target effects of SKF 83959 are also clearly evident. Indeed, we showed herein that acute locomotor activation induced by SKF 83959 was not regulated by the D1-D2 heteromer as the TAT-D1 peptide did not abolish these locomotor effects in the absence of habituation to the testing chamber.
It is also important to note that SKF 83959 attenuated, but did not abolish, amphetamine-induced locomotion during the five day sensitizing phase, indicating that other receptors also likely contribute to locomotor activation by this psychostimulant. In addition, we did not directly demonstrate that TAT-D1 could abolish the effects of SKF 83959 on subchronic amphetamine-induced locomotion as we were concerned that administering all three agents simultaneously may overwhelm the in vivo system, as both SKF 83959 and amphetamine affect various neurotransmitter systems via multiple mechanisms of action (Fleckenstein et al. 2007; Perreault et al. 2012b; Chun et al. 2013; Guo et al. 2013). Nonetheless, the ability of SKF 83959 and the highly specific TAT-D1 peptide to opposingly regulate amphetamine-induced locomotor activation and sensitization argues for a pivotal role for the D1-D2 heteromer in mediating these behaviours.
In the present study, we demonstrated that the dopamine D1-D2 receptor heteromer exerts tonic suppression on the locomotor sensitization behaviour. Despite its lack of clear face validity, psychostimulant-induced locomotor sensitization results in neurobiological alterations in the mesolimbic dopamine system that have been suggested to be the underlying molecular basis for the subsequent manifestation of drug-seeking behaviours, which makes psychostimulant-induced locomotor sensitization an important model for the study of drug addiction (Robinson and Berridge 2000; Steketee and Kalivas 2011). Indeed, not only have behaviorally sensitized animals been shown to concomitantly exhibit conditioned place preference and drug self-administration (Lett 1989; Pierre and Vezina 1997, 1998; Vezina 2004), but the neurocircuitry of psychostimulant-induced locomotor sensitization was also shown to largely overlap with that of the reinstatement model of drug self-administration (Steketee and Kalivas 2011), suggesting that the persistent neuroadaptations associated with psychostimulant sensitization may also contribute to eventual drug relapse. Therefore, the identification of the dopamine D1-D2 receptor heteromer as a negative modulator of locomotor sensitization to amphetamine may have implications in the understanding and treatment of drug addiction.
Highlights.
Activation of the dopamine D1-D2 receptor heteromer inhibits the expression of amphetamine-induced locomotor sensitization.
Disruption of the dopamine D1-D2 receptor heteromer enhances the expression of amphetamine-induced locomotor sensitization
The D1-D2 receptor heteromer may be a novel molecular substrate involved in addiction processes.
Acknowledgments
This work was supported by a grant from the National Institute on Drug Abuse (DA-007223 to S.R.G.). S.R.G. holds a Canada Research Chair in Molecular Neuroscience.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anagnostaras SG, Robinson TE. Sensitization to the psychomotor stimulant effects of amphetamine: modulation by associative learning. Behav Neurosci. 1996;110:1397–414. doi: 10.1037//0735-7044.110.6.1397. [DOI] [PubMed] [Google Scholar]
- Bertran-Gonzalez J, Bosch C, Maroteaux M, Matamales M, Hervé D, Valjent E, et al. Opposing patterns of signaling activation in dopamine D1 and D2 receptor-expressing striatal neurons in response to cocaine and haloperidol. J Neurosci. 2008;28:5671–85. doi: 10.1523/JNEUROSCI.1039-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen BT, Hopf FW, Bonci A. Synaptic plasticity in the mesolimbic system: Therapeutic implications for substance abuse. Ann N Y Acad Sci. 2010:129–39. doi: 10.1111/j.1749-6632.2009.05154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun LS, Free RB, Doyle TB, Huang X-P, Rankin ML, Sibley DR. D1-D2 dopamine receptor synergy promotes calcium signaling via multiple mechanisms. Mol Pharmacol. 2013;84:190–200. doi: 10.1124/mol.113.085175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cools AR, Lubbers L, Van Oosten RV, Andringa G. SKF 83959 is an antagonist of dopamine D1-like receptors in the prefrontal cortex and nucleus accumbens: A key to its antiparkinsonian effect in animals? Neuropharmacology. 2002;42:237–45. doi: 10.1016/s0028-3908(01)00169-1. [DOI] [PubMed] [Google Scholar]
- Downes RP, Waddington JL. Grooming and vacuous chewing induced by SK&F 83959, an agonist of dopamine “D1-like” receptors that inhibits dopamine-sensitive adenylyl cyclase. Eur J Pharmacol. 1993;234:135–6. doi: 10.1016/0014-2999(93)90718-w. [DOI] [PubMed] [Google Scholar]
- Eilam D, Golani I. Home base behavior of rats (Rattus norvegicus) exploring a novel environment. Behav Brain Res. 1989;34:199–211. doi: 10.1016/s0166-4328(89)80102-0. [DOI] [PubMed] [Google Scholar]
- Fleckenstein AE, Volz TJ, Riddle EL, Gibb JW, Hanson GR. New insights into the mechanism of action of amphetamines. Annu Rev Pharmacol Toxicol. 2007;47:681–98. doi: 10.1146/annurev.pharmtox.47.120505.105140. [DOI] [PubMed] [Google Scholar]
- Gangarossa G, Espallergues J, de Kerchove d’Exaerde A, El Mestikawy S, Gerfen CR, Hervé D, et al. Distribution and compartmental organization of GABAergic medium-sized spiny neurons in the mouse nucleus accumbens. Front Neural Circuits. 2013;7:22. doi: 10.3389/fncir.2013.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo L, Zhao J, Jin G, Zhao B, Wang G, Zhang A, et al. SKF83959 Is a Potent Allosteric Modulator of Sigma-1 Receptor. Mol Pharmacol. 2013;83:577–86. doi: 10.1124/mol.112.083840. [DOI] [PubMed] [Google Scholar]
- Hall DA, Stanis JJ, Marquez Avila H, Gulley JM. A comparison of amphetamine- and methamphetamine-induced locomotor activity in rats: Evidence for qualitative differences in behavior. Psychopharmacology (Berl) 2008;195:469–78. doi: 10.1007/s00213-007-0923-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasbi A, Fan T, Alijaniaram M, Nguyen T, Perreault ML, O’Dowd BF, et al. Calcium signaling cascade links dopamine D1-D2 receptor heteromer to striatal BDNF production and neuronal growth. Proc Natl Acad Sci U S A. 2009;106:21377–82. doi: 10.1073/pnas.0903676106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasbi A, Perreault ML, Shen MYF, Zhang L, To R, Fan T, et al. A peptide targeting an interaction interface disrupts the dopamine D1-D2 receptor heteromer to block signaling and function in vitro and in vivo: Effective selective antagonism. FASEB. 2014;28(11):4806–20. doi: 10.1096/fj.14-254037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry DJ, White FJ. Repeated cocaine administration causes persistent enhancement of D1 dopamine receptor sensitivity within the rat nucleus accumbens. J Pharmacol Exp Ther. 1991;258:882–90. [PubMed] [Google Scholar]
- Jin L-Q, Goswami S, Cai G, Zhen X, Friedman E. SKF83959 selectively regulates phosphatidylinositol-linked D1 dopamine receptors in rat brain. J Neurochem. 2003;85:378–86. doi: 10.1046/j.1471-4159.2003.01698.x. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Stewart J. Dopamine transmission in the initiation and expression of drug- and stress-induced sensitization of motor activity. Brain Res Brain Res Rev. 1991;16(3):223–44. doi: 10.1016/0165-0173(91)90007-u. [DOI] [PubMed] [Google Scholar]
- Kantor L, Hewlett GH, Gnegy ME. Enhanced amphetamine- and K+-mediated dopamine release in rat striatum after repeated amphetamine: differential requirements for Ca2+- and calmodulin-dependent phosphorylation and synaptic vesicles. J Neurosci. 1999;19:3801–8. doi: 10.1523/JNEUROSCI.19-10-03801.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Austin JD, Tanabe L, Creekmore E, Vezina P. Activation of group II mGlu receptors blocks the enhanced drug taking induced by previous exposure to amphetamine. Eur J Neurosci. 2005;21:295–300. doi: 10.1111/j.1460-9568.2004.03822.x. [DOI] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–38. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SM, Kant A, Blake D, Murthy V, Boyd K, Wyrick SJ, et al. SKF-83959 is not a highly-biased functionally selective D1 dopamine receptor ligand with activity at phospholipase C. Neuropharmacology. 2014;86:145–54. doi: 10.1016/j.neuropharm.2014.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SP, So CH, Rashid AJ, Varghese G, Cheng R, Lança AJ, et al. Dopamine D1 and D2 receptor Co-activation generates a novel phospholipase C-mediated calcium signal. J Biol Chem. 2004;279:35671–8. doi: 10.1074/jbc.M401923200. [DOI] [PubMed] [Google Scholar]
- Lett BT. Repeated exposures intensify rather than diminish the rewarding effects of amphetamine, morphine, and cocaine. Psychopharmacology (Berl) 1989;98:357–62. doi: 10.1007/BF00451687. [DOI] [PubMed] [Google Scholar]
- Lobb CJ, Wilson CJ, Paladini CA. A dynamic role for GABA receptors on the firing pattern of midbrain dopaminergic neurons. J Neurophysiol. 2010;104:403–13. doi: 10.1152/jn.00204.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loweth JA, Singer BF, Baker LK, Wilke G, Inamine H, Bubula N, et al. Transient overexpression of alpha-Ca2+/calmodulin-dependent protein kinase II in the nucleus accumbens shell enhances behavioral responding to amphetamine. J Neurosci. 2010;30:939–49. doi: 10.1523/JNEUROSCI.4383-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matamales M, Bertran-Gonzalez J, Salomon L, Degos B, Deniau JM, Valjent E, et al. Striatal medium-sized spiny neurons: Identification by nuclear staining and study of neuronal subpopulations in BAC transgenic mice. PLoS One. 2009:4. doi: 10.1371/journal.pone.0004770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendrek A, Blaha CD, Phillips AG. Pre-exposure of rats to amphetamine sensitizes self-administration of this drug under a progressive ratio schedule. Psychopharmacology (Berl) 1998;135:416–22. doi: 10.1007/s002130050530. [DOI] [PubMed] [Google Scholar]
- Ng J, Rashid AJ, So CH, O’Dowd BF, George SR. Activation of calcium/calmodulin-dependent protein kinase IIalpha in the striatum by the heteromeric D1-D2 dopamine receptor complex. Neuroscience. 2010;165:535–41. doi: 10.1016/j.neuroscience.2009.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulson PE, Robinson TE. Sensitization to systemic amphetamine produces an enhanced locomotor response to a subsequent intra-accumbens amphetamine challenge in rats. Psychopharmacology (Berl) 1991;104:140–1. doi: 10.1007/BF02244569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 4. Acad. Press; 1998. [DOI] [PubMed] [Google Scholar]
- Perreault ML, Fan T, Alijaniaram M, O’Dowd BF, George SR. Dopamine D1-D2 receptor heteromer in dual phenotype GABA/glutamate-coexpressing striatal medium spiny neurons: Regulation of BDNF, GAD67 and VGLUT1/2. PLoS One. 2012a:7. doi: 10.1371/journal.pone.0033348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perreault ML, Hasbi A, Alijaniaram M, Fan T, Varghese G, Fletcher PJ, et al. The dopamine D1-D2 receptor heteromer localizes in dynorphin/enkephalin neurons: increased high affinity state following amphetamine and in schizophrenia. J Biol Chem. 2010;285:36625–34. doi: 10.1074/jbc.M110.159954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perreault ML, Hasbi A, O’Dowd BF, George SR. The dopamine d1-d2 receptor heteromer in striatal medium spiny neurons: evidence for a third distinct neuronal pathway in Basal Ganglia. Front Neuroanat. 2011;5:31. doi: 10.3389/fnana.2011.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perreault ML, Jones-Tabah J, O’Dowd BF, George SR. A physiological role for the dopamine D5 receptor as a regulator of BDNF and Akt signalling in rodent prefrontal cortex. Int J Neuropsychopharmacol. 2012b:1–7. doi: 10.1017/S1461145712000685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce RC, Kalivas PW. Repeated cocaine modifies the mechanism by which amphetamine releases dopamine. J Neurosci. 1997;17:3254–61. doi: 10.1523/JNEUROSCI.17-09-03254.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierre PJ, Vezina P. Predisposition to self-administer amphetamine: The contribution of response to novelty and prior exposure to the drug. Psychopharmacology (Berl) 1997;129:277–84. doi: 10.1007/s002130050191. [DOI] [PubMed] [Google Scholar]
- Pierre PJ, Vezina P. D1 dopamine receptor blockade prevents the facilitation of amphetamine self-administration induced by prior exposure to the drug. Psychopharmacology (Berl) 1998;138:159–66. doi: 10.1007/s002130050658. [DOI] [PubMed] [Google Scholar]
- Rashid AJ, So CH, Kong MMC, Furtak T, El-Ghundi M, Cheng R, et al. D1-D2 dopamine receptor heterooligomers with unique pharmacology are coupled to rapid activation of Gq/11 in the striatum. Proc Natl Acad Sci U S A. 2007;104:654–9. doi: 10.1073/pnas.0604049104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Becker JB. Enduring changes in brain and behavior produced by chronic amphetamine administration: a review and evaluation of animal models of amphetamine psychosis. Brain Res. 1986;396:157–98. doi: 10.1016/s0006-8993(86)80193-7. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: An incentive-sensitization theory of addiction. Brain Res Rev. 1993:247–91. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The psychology and neurobiology of addiction: an incentive-sensitization view. Addiction. 2000;95(Suppl 2):S91–S117. doi: 10.1080/09652140050111681. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Jurson PA, Bennett JA, Bentgen KM. Persistent sensitization of dopamine neurotransmission in ventral striatum (nucleus accumbens) produced by prior experience with (+)-amphetamine: a microdialysis study in freely moving rats. Brain Res. 1988;462(2):211–22. doi: 10.1016/0006-8993(88)90549-5. [DOI] [PubMed] [Google Scholar]
- Saal D, Dong Y, Bonci A, Malenka RC. Drugs of abuse and stress trigger a common synaptic adaptation in dopamine neurons. Neuron. 2003;37:577–82. doi: 10.1016/s0896-6273(03)00021-7. [DOI] [PubMed] [Google Scholar]
- Sahu A, Tyeryar KR, Vongtau HO, Sibley DR, Undieh AS. D5 dopamine receptors are required for dopaminergic activation of phospholipase C. Mol Pharmacol. 2009;75:447–53. doi: 10.1124/mol.108.053017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt WJ, Beninger RJ. Behavioural sensitization in addiction, schizophrenia, Parkinson’s disease and dyskinesia. Neurotox Res. 2006;10:161–6. doi: 10.1007/BF03033244. [DOI] [PubMed] [Google Scholar]
- Steketee JD, Kalivas PW. Drug wanting: behavioral sensitization and relapse to drug-seeking behavior. Pharmacol Rev. 2011;63:348–65. doi: 10.1124/pr.109.001933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suto N, Austin JD, Tanabe LM, Kramer MK, Wright DA, Vezina P. Previous exposure to VTA amphetamine enhances cocaine self-administration under a progressive ratio schedule in a D1 dopamine receptor dependent manner. Neuropsychopharmacology. 2002;27:970–9. doi: 10.1016/S0893-133X(02)00379-2. [DOI] [PubMed] [Google Scholar]
- Taylor JR, Lynch WJ, Sanchez H, Olausson P, Nestler EJ, Bibb JA. Inhibition of Cdk5 in the nucleus accumbens enhances the locomotor-activating and incentive-motivational effects of cocaine. Proc Natl Acad Sci U S A. 2007;104:4147–52. doi: 10.1073/pnas.0610288104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma V, Hasbi A, O'Dowd BF, George SR. Dopamine D1-D2 receptor heteromer-mediated calcium release is desensitized by D1 receptor occupancy with or without signal activation: Dual functional regulation by G protein-coupled receptor kinase 2. J Biol Chem. 2010;285:35092–103. doi: 10.1074/jbc.M109.088625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vezina P. Sensitization of midbrain dopamine neuron reactivity and the self-administration of psychomotor stimulant drugs. Neurosci Biobehav Rev. 2004:827–39. doi: 10.1016/j.neubiorev.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Vezina P, Lorrain DS, Arnold GM, Austin JD, Suto N. Sensitization of midbrain dopamine neuron reactivity promotes the pursuit of amphetamine. J Neurosci. 2002;22:4654–62. doi: 10.1523/JNEUROSCI.22-11-04654.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vries TJ. Drug-induced reinstatement of heroin- and cocaine-seeking behaviour following long-term extinction is associated with expression of behavioural sensitization. Eur J Neurosci. 1998;10:3565–71. doi: 10.1046/j.1460-9568.1998.00368.x. [DOI] [PubMed] [Google Scholar]
- White FJ, Wang RY. Electrophysiological evidence for A10 dopamine autoreceptor subsensitivity following chronic D-amphetamine treatment. Brain Res. 1984;309:283–92. doi: 10.1016/0006-8993(84)90594-8. [DOI] [PubMed] [Google Scholar]
- Wolf ME, White FJ, Hu XT. MK-801 prevents alterations in the mesoaccumbens dopamine system associated with behavioral sensitization to amphetamine. J Neurosci. 1994;14(3):1735–45. doi: 10.1523/JNEUROSCI.14-03-01735.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf ME, Xue CJ. Amphetamine and D1 dopamine receptor agonists produce biphasic effects on glutamate efflux in rat ventral tegmental area: modification by repeated amphetamine administration. J Neurochem. 1998;70:198–209. doi: 10.1046/j.1471-4159.1998.70010198.x. [DOI] [PubMed] [Google Scholar]