Abstract
Most immune responses follow Burnet's rule in that Ag recruits specific lymphocytes from a large repertoire and induces them to proliferate and differentiate into effector cells. However, the phenomenon of “original antigenic sin” stands out as a paradox to Burnet's rule of B cell engagement. Humans, upon infection with a novel influenza strain, produce Abs against older viral strains at the expense of responses to novel, protective antigenic determinants. This exacerbates the severity of the current infection. This blind spot of the immune system and the redirection of responses to the “original Ag” rather than to novel epitopes were described fifty years ago. Recent reports have questioned the existence of this phenomenon. Hence, we revisited this issue to determine the extent to which original antigenic sin is induced by variant influenza viruses. Using two related strains of influenza A virus, we show that original antigenic sin leads to a significant decrease in development of protective immunity and recall responses to the second virus. In addition, we show that sequential infection of mice with two live influenza virus strains leads to almost exclusive Ab responses to the first viral strain, suggesting that original antigenic sin could be a potential strategy by which variant influenza viruses subvert the immune system.
Influenza is the most recurring respiratory disease in humans. During the 20th century, influenza A viruses have afflicted the human race with three pandemics in 1918, 1957, and 1968, and numerous seasonal epidemics (1–3). Every year in the United States, 5–20% of the population gets infected with influenza viruses leading to over 200,000 hospitalizations and 36,000 deaths (4). Although a single influenza infection provides lifelong immunity against the homotypic strain, the public remains susceptible to infection with a novel flu variant (5). This is because the virus constantly undergoes genetic variation to avoid protective immunity of the host. This variation, called antigenic drift, occurs mainly to two surface glycoproteins of the virus, hemagglutinin (HA)3 and neuraminidase, and it leads to seasonal influenza infections (6). Due to continuous antigenic variations and as an effort to minimize the death toll related to influenza virus, annual flu vaccinations are recommended, especially for high-risk groups such as the elderly and immune-compromised patients (7). Significantly more drastic antigenic variation occurs through genetic reassortment of RNA genome segments between two strains of influenza viruses (8). Once this virus acquires transmissibility among the human population, the results can be a devastating pandemic.
Protection against influenza viruses is mediated primarily by neutralizing Abs (9, 10). The host responds to the viral infection by generating lifelong memory cells and neutralizing Abs and the viruses adapt and evolve via antigenic drift. This generates variant viruses that can no longer be neutralized by previous Abs (11). As a result, the variant viruses maintain shared epitopes with the parental strain but also have unique epitopes that allow escape from neutralizing Abs. When an immune host is exposed to this variant influenza virus, two things need to happen to ensure a successful protection: 1) activation of memory B cells that recognize shared epitopes and 2) activation of naive B cells that recognize novel epitopes. In the case of repeated infection with variant influenza viruses, the latter response is not induced and this phenomenon is called original antigenic sin. Original antigenic sin was first discovered ~5 decades ago by Thomas Francis Jr. and several others (12–14). Natural infection in humans with antigenically drifted strains of virus induced Ab production against their childhood strains, but response against the current strain was severely diminished (15). Original antigenic sin is not unique to humans as other studies have reported similar observations in various animal models including mice, ferrets, and rabbits (16–19).
Despite this evidence established in humans as well as lower species, there is still controversy over whether original antigenic sin is a real phenomenon associated with influenza vaccines or infection. Recent studies have raised questions about the existence of original antigenic sin. Gullati et al. (20, 21) showed that immunization of humans with influenza vaccines indicated little evidence of original antigenic sin. In addition, a recent elegant study by Wilson and colleagues (21) showed that the most of the human serum Abs following vaccination bound to the current vaccine strain with greater affinity than to the previous vaccine strain, suggesting insignificant interference of original antigenic sin.
In this report, we revisited the issue of original antigenic sin to determine the extent to which original antigenic sin is induced by variant influenza viruses. We used two H1N1 influenza virus strains A/PR/8/34 (PR8) and A/FM/1/47 (FM1) that appeared in the human population in 1934 and 1947, respectively. In brief, we tested the induction of original antigenic sin in mice using three approaches; sequential immunization with 1) inactivated viruses, 2) HA, and 3) sequential infection with mouse-adapted live viruses. Immunization with inactivated influenza viruses led to minimal original antigenic sin. However, the memory development and recall responses in these animals were compromised, evidenced by a high level of lung viral titers following a lethal challenge with mouse-adapted FM1 virus. Sequential exposure to DNA vaccines encoding HAs led to heightened neutralizing Ab responses against the “original Ag”, PR8, but modest responses to the immunizing strain, FM1. Interestingly, sequential infection with live influenza viruses led to severe original antigenic sin responses. The Ab response was almost exclusively against the original Ag, thereby severely limiting responses against novel epitopes in the drifted strain. These mice developed high viral loads in their lungs upon challenge with FM1 virus. Taken together, our results suggest that the existence of original antigenic sin is reproducibly observed and that induction of original antigenic sin might be a strategy by which drifted strains of influenza evade the host immune system.
Materials and Methods
Mice and immunizations/infections
BALB/c mice were purchased from Charles River Laboratories and housed under specific pathogen-free conditions at the Emory Vaccine Center of the Emory University School of Medicine. We immunized mice i.m. with 1400 hemagglutinin units (HAU) formalin-inactivated virus under anesthesia with isofluorane. For live virus infection, we infected mice intranasally with 25 μl of a 0.1–100 × 50% lethal dose (LD50) dose of mouse-adapted live virus under anesthesia. We collected serum samples at designated time points. To prevent nonspecific virus binding, we treated sera with receptor destroying enzyme II (RDE II; Denka Seiken) overnight, and diluted with PBS for in vitro neutralization and hemagglutination inhibition (HAI) assays. All animal studies had approval of the Emory University's Institutional Animal Care and Use Committee.
Viruses
Madin-Darby canine kidney (MDCK) cells were grown in DMEM containing antibiotics, glutamine, and 10% FBS and serially passed before the cells reaching >90% confluence. Mouse-adapted PR8 and FM1 viruses were provided by Dr. Sang Moo Kang (Emory University, Atlanta, GA) and by Dr. Mark Thompkins (University of Georgia, Athens, GA). For the purpose of immunization with inactivated viruses, we amplified viruses in 11-day-old chicken embryonic eggs for 48 h and harvested the allantoic fluid by centrifugation. We further pelleted the virus from the allantoic fluid supernatants by ultracentrifugation and purified them by sucrose density gradient ultracentrifugation. We then inactivated the virus with 0.1% (v/v) formalin. For in vitro assays, we used viruses freshly grown in MDCK cells. Mouse-adapted viruses were propagated in BALB/c mice by infecting mice intranasally and harvesting their lungs 4 days later. Lung lysates were assessed for viral titers.
HA-encoding DNA vaccines
To generate DNA vaccines encoding HAs from PR8 and FM1, we first infected MDCK cells with these viruses, isolated mRNA, and amplified the two HAs by PCR. We then cloned the two HAs into DNA vaccines. The backbone of all DNA vaccine constructs used was pGA (22). All plasmids were sequenced for cloning accuracy. Sequence analysis of the cloned PR8 and FM1 HAs showed that they are 92% identical at the amino acid level (data not shown). We tested HA expression of different DNA vaccine by in vitro transient transfection into the human embryonic kidney cell line 293T followed by Western blotting (data not shown).
Serum microneutralization assay
RDE II-treated sera were serially diluted in 96-well plates and mixed with viruses freshly grown in MDCK cells at a dose of 2 × 103 TCID50/ml. The plates were incubated at 37°C for 2 h, MDCK cells were added and incubated overnight. Infection of MDCK cells by virus was assessed by the presence of influenza virus nucleoprotein. In brief, cells were fixed with 80% acetone and incubated with biotinylated anti-nucleoprotein Ab (Chemicon International), followed by streptavidin-HRP (Southern Biotechnology Associates). Bound HRP was visualized using 1× TMB substrate solution (eBioscience) and the developed color was assessed using the BioRad microplate reader 550. The highest serum dilution that generated >50% specific signal was considered to be the neutralization titer; 50% specific signal = (OD450 virus control − OD450 cell control)/2 + D450 cell control.
Serum HAI assay
Serial dilutions of RDE II-treated sera were mixed with influenza viruses freshly grown in MDCK cells at a dose of 8 HA/50 μl. Mixtures of virus and serum dilutions were incubated for 15 min, followed by addition of 50 μl 0.5% chicken RBC (Innovative Research). The highest serum dilution inhibiting hemagglutination was taken as the HAI titer.
Plaque assay
Viral titers in mouse lungs were assessed using plaque assays. MDCK cells were grown in six-well plates to >99% confluence. Serial dilutions of lung lysates were added to the cells and allowed to adsorb at 37°C for 1 h for viral infection. The lysates were aspirated and agar containing DMEM, glutamine, antibiotics, DEAE-dextran, nonessential amino acid, TPCK-trypsin, and HEPES buffer was added onto the cells. The plates were incubated for 4–5 days and then the cells were fixed with 0.25% glutaraldehyde. Following fixation, the agar plug was removed, the adherent cell layers were stained with 1% crystal violet, and the plaques were counted.
Statistics
Student's t test was used to generate all statistical values stated. For statistical designations, * denotes p < 0.05; **, denotes p < 0.02; *** denotes p < 0.001.
Results
Minimal original antigenic sin induction yet diminished protective immunity upon sequential immunization with inactivated PR8 and FM1 viruses
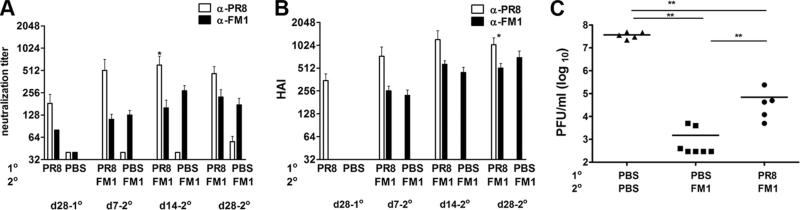
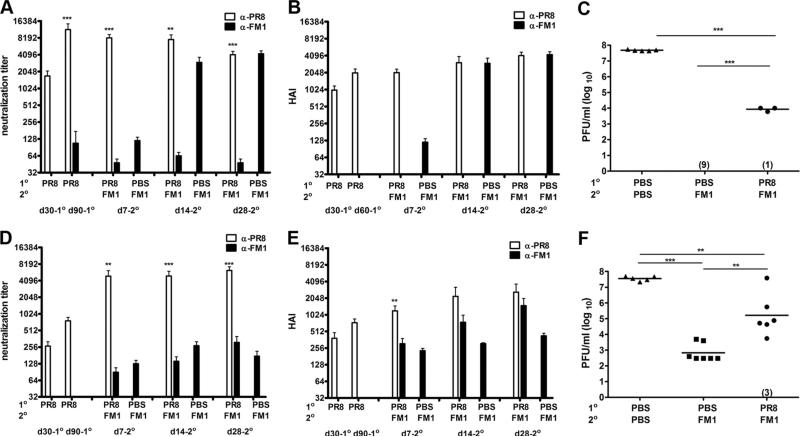
First, we looked at the extent to which sequential immunization with inactivated influenza viruses induce original antigenic sin (Fig. 1A). We immunized cohorts of BALB/c mice with 1400 HA units of formalin-inactivated PR8 and a month later reimmunized them with the same dose of formalin-inactivated FM1. We then collected serum samples at different time points (day 28-post 1°, day 7-post 2°, day 14-post 2°, and day 28-post 2° immunization) to monitor Ab responses against the original vs immunizing virus. We measured the virus neutralization titers against live PR8 and FM1 viruses (Fig. 1A). Upon immunization with FM1, the average neutralization titer against PR8 rose >2-fold at days 7 (p = 0.27) and 14 (p = 0.12) as compared with day 28 post-PR8 immunization. At day 7 following FM1 immunization, the average neutralization titer against PR8 was 3.8-fold higher than against FM1 (p = 0.09), but at day 14, this difference was statistically significant (4.5-fold, p = 0.05). However, at day 28 following FM1 immunization, the difference between neutralization titers against PR8 and FM1 was insignificant (2-fold, p = 0.09).
FIGURE 1.
Sequential immunization with whole inactivated viruses PR8 and FM1 induced minimal original antigenic sin, yet led to diminished protective immunity. BALB/c mice (six mice/group) were i.m. immunized with 1400 HAU of whole formalin-inactivated PR8. Control mice were immunized with PBS. A month later, the PR8-immune and control mice were immunized with 1400 HAU of whole inactivated FM1. Serum samples were collected at memory (day 28) following primary immunization (PR8), and days 7, 14, and 28 following secondary immunization (FM1). Sera were treated with receptor-destroying enzyme II, heat-inactivated, and analyzed for neutralization titers (A) and HAI titers (B) using freshly grown PR8 and FM1 viruses in MDCK cells. A month following FM1 immunization, mice were intranasally challenged with a lethal dose (100 × LD50) of mouse-adapted FM1 virus (C). Mouse lungs were harvested 4 days after challenge and assessed for lung viral titers via plaque assay on MDCK cells; the data are shown as plaque forming units (pfu/ml). Open bars represent serum titers against PR8 and filled bars against FM1. Each data point represents an individual animal. Error bars represent SEM. The data represent three separate experiments. *, p < 0.05; **, p < 0.02.
We also compared the HAI titers against the original vs immunizing Ag (Fig. 1B). One month following immunization with inactivated PR8, the mean HAI titer was 352. However, upon secondary immunization with inactivated FM1, the average HAI titer against PR8 rose more than 2-fold (HI: 736, p = 0.15 at day 7) and continued to increase to 3-fold (1040–1216, p = 0.06 at day 14, p = 0.02 at day 28) as compared with the levels before FM1 immunization. The HAI titers against PR8 were 1.5- to 2-fold higher than FM1 HAI titers at days 7, 14, and 28 following FM1 immunization. Even though the responses to the immunizing FM1 strain was on average 2- to 3-fold lower than the original PR8 strain, the differences were not statistically significant at days 7 (p = 0.07) and 14 (p = 0.14) following FM1 immunization. However, this became significant at day 28 (p = 0.05). Taken together, these observations suggest that in mice sequentially immunized with whole, inactivated viruses, the effects of original antigenic sin, as measured by virus neutralization or HAI, are minimal.
Because the effects of original antigenic sin following immunization with inactivated viruses were minimal, we next determined whether this had any effect on memory/recall responses to a live viral challenge. We hypothesized that there would be no difference in the ability of FM1-immunized vs PR8 and FM1-immunized animals to control the live virus challenge. We first immunized with inactivated PR8, then reimmunized with inactivated FM1 a month later. After another month, the mice were challenged intra nasally with 100 × LD50 live mouse-adapted FM1 virus. Four days later, we harvested the lungs of these mice and assessed virus titers by plaque assay on MDCK cells (Fig. 1C). Lung lysates from naive animals had a high, average viral titer of 4 × 107 pfu/ml while mice immunized a month earlier with FM1 alone had a mean viral titer of 1.5 × 103 pfu/ml. Quite unexpectedly, mice sequentially immunized with PR8 and FM1 had a significantly higher (46-fold) viral titer (7 × 104 pfu/ml) than FM1-immunized mice (1 × 104 pfu/ml) (p = 0.007). These mice had a lower (528-fold) viral titer than naive mice upon challenge; this suggests that cross-reactive Abs raised against PR8 and the reduced Ab levels against FM1 conferred partial protection. Taken together, these data suggest that even though the effects of original antigenic sin are minimal in animals sequentially immunized with whole, inactivated viruses, there is a statistically significant deficit in the ability of these animals to respond to live viral challenge.
Sequential immunization with DNA vaccines encoding HA of PR8 and FM1 induces original antigenic sin
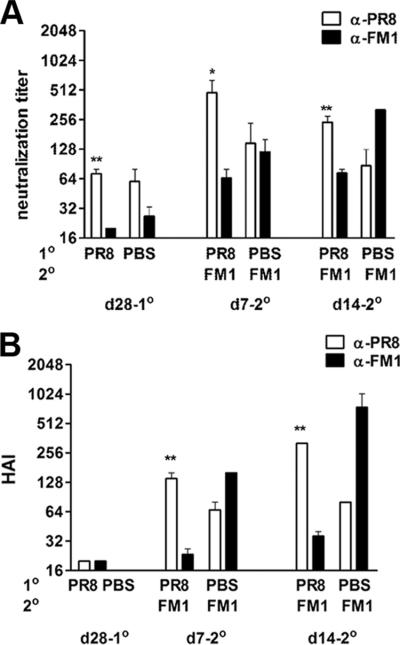
Protection against influenza virus is mediated predominantly by neutralizing Abs against HA. In this line, immunization with purified HA alone induces protective immunity (16). In this study, we tested whether HA protein by itself can induce original antigenic sin. To achieve this, we expressed HA protein in its native membrane-associated form by cloning the PR8- as well as FM1-HA coding sequences into DNA vaccines. We then sequentially immunized, using a gene gun, cohorts of BALB/c mice with 2 μg of DNA vaccine encoding PR8-HA and a month later with 2 μg of DNA encoding FM1-HA. We collected serum samples at the memory time point (day 28-post PR8-HA immunization) and day 7 and 14 post FM1-HA immunization and analyzed the neutralization and HAI titers against the original Ag (PR8) and the immunizing Ag (FM1). As shown in Fig. 2A, 7 days following FM1-HA vaccination in PR8-HA-primed mice, the average neutralization titer against PR8 increased 7-fold higher as compared with the memory time point (day 28, PR8-HA immunized, p = 0.01). The neutralization titers against the immunizing FM1 strain were significantly lower than titers against the original strain PR8 at both day 7 (7-fold; p = 0.03) and day 14 (3.3-fold; p = 0.008). The failure to respond to DNA vaccines encoding FM1-HA was not due to an inherent defect in this vaccine; control mice that received FM1-HA alone had significantly higher neutralization titers against FM1 than mice that received both PR8-HA and FM1-HA vaccines (day 14; 4-fold increase; p = 0.016).
FIGURE 2.
Immunization with DNA vaccines encoding HA of PR8 and FM1 induced original antigenic sin. A cohort of BALB/c (F) mice (5 mice/group) was primed by gene gun with 2 μg of DNA vaccine encoding full length HA from PR8 (PR8-HA). A control group of mice were immunized with PBS. A month later, PR8-HA-immune mice were immunized again with the same dose of DNA vaccine encoding HA of FM1 (FM1-HA). Serum samples were taken at the times described and analyzed for neutralization (A) and HAI titers (B). Open bars represent serum titers against PR8 and filled bars against FM1. Error bars represent SEM. *, p < 0.05; **, p < 0.02. The data represent two separate experiments.
Sequential immunization of PR8-HA and FM1-HA also increased HAI titers significantly against the original Ag (Fig. 2B). PR8-specific HAI titers were 7-fold higher at day 7 and 16-fold higher at day 14 as compared with the memory time point (day 28 following PR8-HA immunization; p < 0.0001). In contrast to immunization with whole, inactivated viruses that led to minimal original antigenic sin, immunization with the FM1-HA-encoding DNA vaccine in PR8-HA-primed mice led to significant original antigenic sin. Following FM1-HA immunization, the HAI titers against PR8 were significantly higher at days 7 (6-fold; p = 0.002) and 14 (9-fold; p = 0.01) than titers against FM1, indicating that the responses were directed predominantly against the original Ag, PR8. Taken together, these observations suggest that sequential immunization with the native form of the HA proteins encoded by DNA vaccines can induce original antigenic sin.
Sequential infection with live, mouse-adapted PR8 and FM1 viruses induces profound original antigenic sin
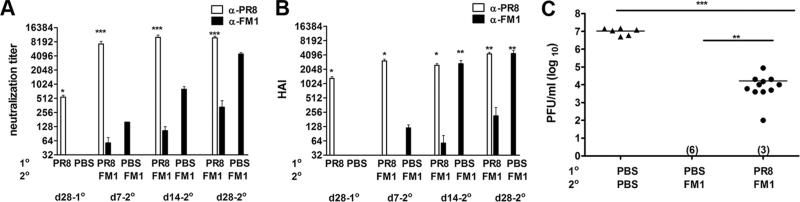
Millions of people in the U.S. get influenza virus infection each year. However, the extent to which sequential live viral infections cause original antigenic sin is unclear. To address this issue, we infected cohorts of BALB/c mice intranasally with 0.1 × LD50 mouse-adapted PR8 virus and a month later infected them again with 0.1 × LD50 mouse-adapted FM1 virus. We collected serum samples periodically and analyzed them for virus neutralization and HAI titers (Fig. 3, A and B). Infection of PR8-immune mice with FM1 virus significantly increased the average PR8-specific neutralization titer (12-fold; p < 0.0001) as compared with the memory time point (day 28-post PR8 infection) (Fig. 3A). Surprisingly, the neutralizing titer against FM1 was considerably lower (>100-fold) than the titers against PR8 (p < 0.0001). Even a month later, these mice had significantly lower neutralization titers against FM1, suggesting that pre-existing immune responses to PR8 led to a diminished response to FM1. In contrast, mice infected with only FM1 virus demonstrated high neutralization titers.
FIGURE 3.
Induction of original antigenic sin was profound upon sequential infection with live mouse-adapted PR8 and FM1 viruses. A cohort of BALB/c (F) mice (14 mice) was intranasally infected with 0.1 × LD50 of mouse-adapted PR8 virus. Control mice (six mice) were injected with PBS. A month later, PR8-infected mice were subsequently infected with 0.1 × LD50 of mouse-adapted FM1 virus. Serum samples were taken at the times described and analyzed for neutralization (A) and HAI titers (B). A month later, these mice were challenged with a lethal dose (100 × LD50) of mouse-adapted FM1 virus (C). Naive mice (six mice) that sequentially received PBS were included as infection control (C). Four days following challenge, lungs of the mice were harvested and assessed for viral titers using plaque assays on MDCK cells, shown as plaque forming units (pfu/ml). Numbers indicate the number of mice with undetectable level of lung viral titers. Open bars represent serum titers against PR8 and filled bars denote titers against FM1. Each data point represents an individual animal. Error bars represent SEM. *, p < 0.05; **, p < 0.02; ***, p < 0.001. The data represent two separate experiments.
We observed a similar trend for the HAI titers in mice sequentially infected with PR8 and FM1. The response against the original Ag, PR8 was heightened while the response to the immunizing strain was severely reduced (Fig. 3B). Infection of PR8-immune mice with FM1 virus significantly increased the average PR8-specific HAI titer (2-fold; p < 0.0001) as compared with the memory time point (day 28 following PR8 infection) (Fig. 3B). In addition, FM1 infection of PR8-immune mice led to significantly higher HAI titers against PR8 at days 7 (608-fold; p = 0.0134), 14 (416-fold; p = 0.003), and 28 (576-fold; p < 0.0001) as compared with the response against FM1.
These data clearly show that sequential infection with live viruses caused much more profound original antigenic sin in mice than immunization with whole inactivated viruses or HA-encoding DNA vaccines. So we tested the impact of reduced FM1 Ab responses on the protective immunity of the host by challenging the mice intranasally with live 100 × LD50 of mouse adapted FM1 virus at day 28 post FM1 infection. Four days later, we harvested their lungs and assessed the viral titers. As controls, we set up cohorts of mice that were infected with FM1 virus alone a month earlier (immune control) and uninfected control mice that received PBS instead of the two viruses (Fig. 3C). The naive, uninfected control mice exhibited lung viral titers of 107 pfu/ml while the immune control mice cleared the virus completely. Interestingly, mice that were sequentially infected with PR8 and FM1 had on average, 4-logs higher lung viral titers (5 × 104 pfu/ml; p < 0.02) than immune control mice. In addition, these sequentially infected mice had on average 2-logs lower viral titers than the uninfected control, indicating cross-reactive anti-PR8 Abs and reduced levels of Abs against FM1 provided protection to some extent. We also conducted identical experiments using a lower dose of live virus (0.01 × LD50) for sequential infections and observed similar results (data not shown). Taken together, our observations demonstrate that sequential infection with antigenically related viruses induce significant original antigenic sin, thereby severely impairing the protective immunity of mice.
Pre-existing immunity against PR8 leads to diminished viral load upon secondary infection with FM1
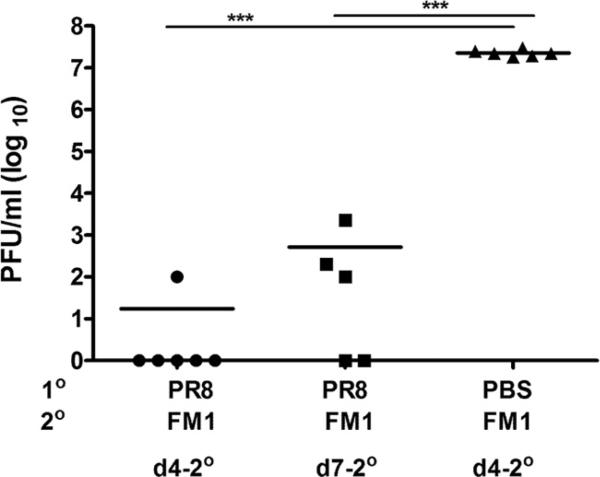
Next, we sought to understand how sequential infection with related viruses induced profound original antigenic sin whereas immunization with inactivated viruses did not. We hypothesized that pre-existing Abs against the original influenza virus bind and lower the antigenic load of the second virus. To directly assess the extent to which anti-PR8 Abs lowered the FM1 viral load in the lungs, we infected mice with 0.1 × LD50 PR8 virus, waited a month and then infected with 0.1 × LD50 FM1 virus. Four days later, we sampled their lungs and assayed them for the presence of live virus (Fig. 4). We detected a low level of virus (102 pfu/ml) in only one of six mice 4 days after FM1 infection. By day 7 following FM1 infection, three of five mice had an average of 8.6 × 102 pfu/ml in their lungs. Control mice that were infected with FM1 alone had an average viral titer of 2.3 × 107 pfu/ml at day 4 following infection. Taken together, these data suggest that pre-existing Abs against PR8 can, to some extent, neutralize the FM1 virus, resulting in a lower viral load in mouse lungs and diminished responses to novel neutralizing epitopes in FM1. This data support the observation in Fig. 3C and suggest that the lowered neutralizing Ab response against FM1 is most likely due to lowered viral load.
FIGURE 4.
Pre-existing immunity to PR8 decreased FM1 viral load. Cohorts of PR8-immune BALB/c mice (five mice/group) were sacrificed at days 4 or 7 following FM1 infection (0.1 × LD50). The viral titers in the lungs were assessed via plaque assay on MDCK cells. Lung viral titers are shown as plaque forming units (pfu/ml). Control mice were infected with FM1 only and assessed for lung viral titer at day 4 following infection. Each data point represents an individual animal. Error bars represent SEM. ***, p < 0.001.
Induction of original antigenic sin is independent of the intervals between exposures to variant influenza viruses
Our data clearly show that induction of original antigenic sin was profound in mice sequentially infected with live viruses (Fig. 3, A and B). It is possible that this could be due to the timing between infections. In the experiments we described, the interval between exposures was 1 mo and perhaps this was not sufficient time for the anti-PR8 response to subside. To test whether the effect of original antigenic sin diminishes if the interval between exposures is longer, we intranasally infected a cohort of BALB/c mice with 0.1 × LD50 mouse-adapted PR8 then waited 3 mo before infecting them with 0.1 × LD50 mouse-adapted FM1. We collected serum samples at the various times and analyzed them for the neutralization and HAI titers (Fig. 5, A and B). As shown in Fig. 5A, 90 days following infection with PR8, the average neutralizing titer against PR8 increased 7-fold as compared with 1 mo (p = 0.01). Upon infection of these 3-mo PR8-immune mice with FM1, the neutralization titer against the original Ag, PR8 increased 5-fold at day 7 (p = 0.0001) as compared with the memory time point, while the neutralization titer against FM1 was significantly reduced (28-fold; p = 0.0002). Even a month later, these mice had significantly lower neutralization titers against FM1, suggesting that pre-existing immune responses to PR8 hinders the development of Ab response to FM1, even when the interval between exposures is long. In contrast, control mice that were infected with FM1 alone maintained high neutralization titers against FM1. Similar to neutralization titers, HAI titers (Fig. 5B) against PR8 increased over 3 mo following infection (2-fold; p = 0.02). Sequential infection of PR8-infected mice with FM1 3 mo later increased HAI titers against PR8 (at day 7, 2-fold: p = 0.01) as compared with the memory time point. Significantly, HAI titers against the second strain, FM1 were under the detection limit throughout the time points.
FIGURE 5.
Induction of original antigenic sin was independent of the interval between exposures to variant viruses. BALB/c mice (four to ten mice/group) were intranasally infected with 0.1 × LD50 of mouse-adapted PR8 (A–C) or i.m. immunized with 1400 HAU of whole inactivated PR8 (D–F). Three months later, PR8-infected or immune mice were infected with 0.1 × LD50 of mouse-adapted FM1 (A–C) or immunized with 1400 HAU of whole inactivated FM1 (D–F). Control mice (nine mice) were injected with PBS. Serum samples were collected at the times described and analyzed for neutralization (A and D) and HAI titers (B and E). A month later, these mice were challenged with a lethal dose (100 × LD50) of mouse-adapted FM1 (C and F). Naive mice (six mice) that sequentially received PBS were included as infection control (C and F). Four days following challenge, lungs of the mice were harvested and assessed for lung viral titers via plaque assay on MDCK cells, shown as plaque forming units (pfu/ml). Numbers indicate the number of mice with undetectable level of lung viral titers. Open bars represent serum titers against PR8 and filled bars against FM1. Each data point represents an individual animal. Error bars represent SEM. *, p < 0.05; **, p < 0.02; ***, p < 0.001. The data represent two separate experiments.
Consistent with reduced neutralization and HAI titers against FM1, these mice had impaired protective immunity following a lethal challenge (Fig. 5C). We challenged these mice with 100 × LD50 mouse-adapted FM1 a month after secondary infection and assessed the lung viral titers via plaque assay 4 days following challenge. As described in Fig. 3C, we also set up cohorts of mice that were infected with FM1 a month earlier (immune control) and uninfected control mice that received PBS instead of the two viruses (Fig. 5C). The immune control group of mice had nondetectable levels of virus in their lungs. In mice sequentially infected with PR8 and FM1 over 3 mo, one of four mice cleared the virus, but the rest of mice had significantly higher lung viral titers (5.2 × 103 pfu/ml) (p < 0.001). As expected, sequentially infected mice with PR8 and FM1 had significantly lower lung viral titers than lethally infected naive control mice (Fig. 5C) suggesting that cross-reactive anti-PR8 Abs and reduced Abs against FM1 confers partial protection. These data indicate that the impact of original antigenic sin is independent of duration between primary and secondary infection, thereby leading to long-lasting impaired immunity against immunizing Ag.
We also determined whether the interval between exposures would alter the original antigenic sin responses in response to inactivated viruses. Our data showed that the effects of original antigenic sin are minimal with sequential immunization with whole, inactivated viruses, yet this caused a significant deficit in protective immunity (Fig. 1). So, we tested whether delayed secondary immunization changes the effects of original antigenic sin (Fig. 5, D–F). In brief, we immunized cohorts of BALB/c mice with 1400 HAU of whole inactivated PR8, then waited 3 mo to immunize them again with 1400 HAU of FM1. We collected serum samples as described above and analyzed them for virus neutralization and HAI titers (Fig. 5, D and E). As shown in Fig. 5D, neutralization titers against PR8 following immunization improved over 3 mo (day 90, 3-fold; p = 0.05) and increased even more following secondary immunization with FM1 (day 7, 19-fold; p = 0.05) as compared with the memory time point (day 30 following PR8). In contrast, the neutralization titers against FM1 were 55-fold lower as compared with the titers against PR8 (p = 0.002). Even a month later, these mice had 20-fold lower neutralization titers against FM1 (p < 0.0001). HAI titers against PR8 also increased 2-fold over 3 mo following immunization (p = 0.05) and 3-fold (p = 0.01) at day 7 following secondary immunization with FM1. In contrast, HAI titers against FM1 were 4-fold lower as compared with titers against PR8 at day 7 (p = 0.01). However, the differences in HAI titers against PR8 and FM1 were insignificant at day 14 and day 28 following FM1 immunization. Nonetheless, lung viral titers following a lethal challenge revealed that these mice had a similar deficit in protective immunity (Fig. 5F). Although mice sequentially immunized with PR8 and FM1 had 67-fold lower viral titers than unimmunized controls (4 × 107 pfu/ml), three of nine mice cleared the virus, yet six of nine had significantly higher viral titers (1.5 × 105 pfu/ml) than the immune control 4 days after challenge (250-fold, p = 0.001, Fig. 5F). Collectively, these data demonstrate that the impaired protective immunity caused by original antigenic sin with sequential immunization is long lasting and independent of duration between primary and secondary exposure.
Induction of original antigenic sin is independent of the order of exposure to variant viruses
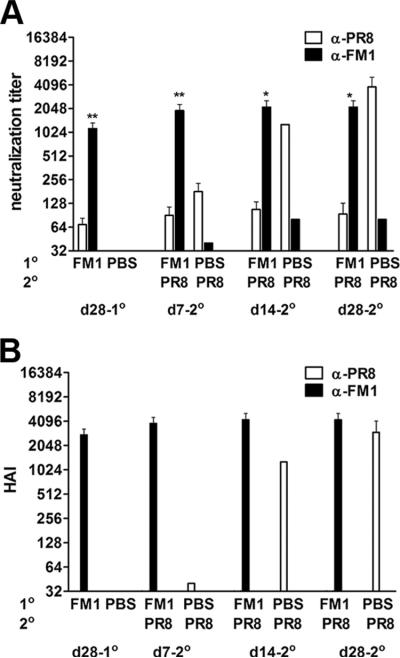
Finally, we determined whether induction of original antigenic sin is dependent upon the order of infection. In brief, we intranasally infected cohorts of BALB/c mice with 0.1 × LD50 mouse-adapted FM1 and 1 mo later, infected them again with 0.1 × LD50 mouse-adapted PR8. We collected serum samples at the various times and analyzed them for virus neutralization and HAI titers (Fig. 6, A and B). As shown in Fig. 6A, infection of FM1-immune mice with PR8 increased the neutralization titer against the original Ag, FM1, 2-fold as compared with the memory time point (day 28 following FM1 infection). As expected, reversing the order also led to the induction of original antigenic sin. The neutralization titer against the immunizing Ag, PR8, was 14-fold lower than the titer against the original Ag, FM1 (p = 0.003) at day 7 following secondary infection. Even a month later, the neutralization titer against FM1 was 27-fold higher than that against PR8 (p = 0.01), indicating that pre-existing immunity against FM1 led to severely reduced Ab response against PR8. Similarly, HAI titers against FM1 increased 1.4 to 2-fold (p = 0.2) upon PR8 infection, whereas HAI titers against PR8 remained under the detection limit throughout the time-points (Fig. 6B). Taken together, these data indicate that regardless of the order of infection, the pre-existing immunity generated by the original influenza virus hinders development of protective immunity against the immunizing variant influenza viruses.
FIGURE 6.
Induction of original antigenic sin was independent of the order of exposure to variant viruses. BALB/c mice (six to ten mice/group) were intranasally infected with 0.1 × LD50 of mouse-adapted FM1. A month later, FM1-infected mice were subsequently infected with 0.1 × LD50 of mouse-adapted PR8 virus. Control mice received PBS and then PR8 virus. Serum samples were taken at the times described and analyzed for neutralization (A) and HAI titers (B). Open bars represent serum titers against PR8 and filled bars against FM1. Error bars represent SEM. *, p < 0.05; **, p < 0.02. The data represent two separate experiments.
Discussion
In 1953, Thomas Francis and colleagues first described the phenomenon of original antigenic sin (12). While analyzing serum samples from field studies of natural influenza infection, they observed that humans produced minimal responses to the current immunizing virus but instead produced higher titer Abs against influenza viruses they encountered as children. This initial observation was expanded upon by a series of elegant studies conducted in the 1960s by Robert Webster and colleagues and others who clearly showed that the phenomenon of original antigenic sin responses to influenza viruses existed not just in humans but in other species as well (18, 19, 23–25). Later in 1979, Cate and colleagues showed that following vaccination with Influenza A/Scotland/74, 82% of the vaccines produced Abs predominantly against the childhood influenza strain, A/HongKong/68 and only 46% produced even low levels of Ab against the vaccine strain, A/Scotland/74 (26). Thus, the phenomenon of original antigenic sin is well established, even though the mechanisms that control it are poorly understood. Despite these results, recent studies have called into question the existence of original antigenic sin. Gillian Air and colleagues analyzed sera from humans immunized with two vaccine strains, A/Philippines/82 and A/Leningrad/86 and showed that 70% of the vaccinees produced Abs against the two vaccine strains with minimal cross reactivity to the epidemic strain A/Victoria/75 (20). They concluded that there was little, if any, evidence for original antigenic sin. In addition, a recent elegant study by Wilson and colleagues concluded that interference of original antigenic sin was insignificant in the human Ab response to various influenza vaccine strains (21). Thus, based upon the observations in these recent studies, we chose to revisit the issue to determine the authenticity of the original antigenic sin phenomenon.
For our study, we chose the two related H1N1 strains, PR8 and FM1, which were the same ones analyzed by Virelizier and colleagues in the 1970s (16, 17). We cloned and sequenced the HAs from the two strains and found that they exhibited 92% identity at the amino acid level (data not shown). Interestingly, most of the changes in HA from FM1 mapped to Ab neutralization sites in HA from PR8, suggesting strong selective pressure on the FM1 virus to render it less susceptible to anti-PR8 Abs (data not shown). We sequentially immunized mice with HA-encoding DNA vaccines or whole formalin-inactivated viruses and observed that the Ab response in PR8-primed and FM1-immunized mice was oriented toward the original Ag (PR8), while the Abs to immunizing Ag (FM1) were reduced (Figs. 1 and 2, A and B). Our data are in agreement with studies done by Schild and colleagues (16) who showed that sequential immunization with purified HAs from two related influenza viruses led to original antigenic sin. These viruses shared cross-reacting antigenic determinants but differed in strain-specific antigenic epitopes. Immunizing mice first with H0 HA (later identified as H1N1) and 2 mo later challenging with homologous (H0), cross-reacting (H1), or unrelated H3 HA proteins led to strong Ab responses to the “original” H0 HA. We also observed that FM1-specific HAI and neutralization titers increased with time and there was a delayed development of FM1 strain-specific responses at later time points (data not shown). These observations conform to the findings by Webster and colleagues (19) who, using Ag adsorption methods, demonstrated the generation of strain-specific responses at later time points.
In contrast to the induction of original antigenic sin by immunization with HA-encoding DNA vaccines, sequential immunization of mice with formalin-inactivated PR8 and FM1 viruses did not show overt evidence for original antigenic sin. The HAI titers and neutralization titers induced against the original Ag, PR8, were only 2–3-fold higher than titers against FM1 at days 7, 14, and 28 following FM1 immunization. These differences, for the most part, were statistically insignificant, indicating that B cell responses to the original Ag as well as the immunizing Ag were generated. This could explain why Wrammert and colleagues (21) found little evidence for original antigenic sin in humans immunized with inactivated influenza virus vaccines. They showed that the interference of original antigenic sin was insignificant in the human Ab response to various vaccine strains. Using human recombinant mAbs generated from sorted single Ab secreting cells, they showed that most recombinant mAbs bound the current vaccine strain with equal or greater affinity than the previous vaccine strain, despite a 10% or less difference of the HA sequence of the current vs previous vaccine strains. Thus, vaccination with inactivated viruses shows minimal evidence of original antigenic sin. Surprisingly, however, despite these minimal differences in serum neutralization as well as HAI titers, these mice were clearly compromised in generating memory responses. When mice sequentially immunized with inactivated PR8 and FM1 were challenged with 100 × LD50 live mouse-adapted FM1 virus, they had 46-fold higher (p = 0.007) viral titer in their lungs than mice immunized with inactivated FM1 virus only (Fig. 1C). Thus, even though the effects of original antigenic sin were minimal in mice sequentially immunized with whole, inactivated viruses, there was a deficit in the establishment of the memory pool and the ability of these animals to respond to subsequent live viral challenge.
Interestingly, in mice sequentially infected with live mouse-adapted influenza viruses, the induction of original antigenic sin was much more profound (Fig. 3, A and B). Sequential infection with live viruses generated severely reduced neutralization Ab responses and compromised memory responses to the second virus. Upon challenge with 100 × LD50 live FM1 virus, these mice exhibited 4 logs higher viral titers in the lungs than mice infected with FM1 virus only (Fig. 3C). The induction of original antigenic sin was not dependent upon the dose of viruses (0.01 or 0.1 LD50) (data not shown) or the order in which the viruses were administered as reversing the order of infection, FM1 followed by PR8, induced preferential Ab production directed toward the original Ag, FM1 (Fig. 6). These data suggest that variant influenza viruses might use original antigenic sin as a potential mode to escape from the host immune system. How live viruses accomplish this while inactivated viruses do not is not entirely clear. It is partially due to neutralization of the second virus, FM1, by preexisting PR8 Abs, resulting in lower antigenic load (Fig. 4). However, it remains unclear whether this cross-neutralization and lowering antigenic load plays a role in mice sequentially immunized with whole, inactivated viruses. The key difference might presumably be the varying degrees of engagement of the innate arm of the immune system by live vs formalin-inactivated viruses (27, 28). Thus, the success of influenza virus’ prevalence stems from the ease of host-to-host transmission, susceptibility to mutational shift/drift and induction of original antigenic sin to escape from the host immune system.
The extent to which antigenic distance is required for induction of original antigenic sin is unknown. However, it is known that the antigenic relationship between the strains must be small. Studies have shown that antigenically distant or dissimilar strains of influenza viruses failed to induce original antigenic sin (16, 18). In line with this, Giliain Air and colleagues (20) have pointed out the significance of antigenic distance between the epidemic strain (A/Victoria/75) and two vaccine strains (A/Philippines/82, A/Leningrad/86) in interpreting the serum responses to influenza vaccines. They analyzed sera from humans immunized with two influenza virus strains A/Philippines/82 and A/Leningrad/86 and showed that 70% of the vaccinees produced Abs against the two vaccine strains with minimal cross reactivity to the epidemic strain A/Victoria/75 (20). The authors concluded that there was no evidence of original antigenic sin in the ten human subjects that they analyzed, but the extent of antigenic distance between the epidemic and vaccine strains was unclear. Furthermore, because the majority of subjects (seven of ten) showed better responses to the vaccine strains, but little cross-reactivity toward the epidemic strains, this might indicate a larger antigenic distance between the epidemic vs vaccine strain.
Original antigenic sin remains a paradox and the mechanisms that invoke it remain elusive. Protection against influenza viruses is predominantly mediated by Ab responses to HA and to a lesser degree, against neuraminidase (9). Because mutations altering these sites occur through antigenic drift, antigenically related viruses have shared common antigenic epitopes as well as unique strain-specific epitopes (17). Thus, we propose a model in which original antigenic sin occurs due to competition between Ag-specific memory and naive B cells for common epitopes. In the context of sequential immunization/infection with PR8 and FM1, the primary exposure induces proliferation of B cells that are 1) specific for PR8 only and 2) cross-reactive with both PR8 and FM1 viruses. Upon exposure to FM1, memory B cells cross-reactive to PR8 and FM1 outcompete naive B cell clones specific for FM1 novel epitopes. This could occur due to the higher frequency of cross-reactive memory B cells and the lower threshold for memory B cell activation as compared with naive B cells. Selective activation of cross-reactive memory B cells leads to heightened differentiation of cross-reactive B cells to plasma cells. In addition, it is possible that another key feature of HA might be at play. Baumgarth and colleagues (29) have demonstrated that influenza viruses can bind to all B cells, irrespective of their BCR specificity, via sialic acid binding. This can lead to Ag uptake and presentation by B cells and the redirection of Ag presentation by B cells instead of dendritic cells may lead to suboptimal activation signals that favor memory over naive B cell activation. Consequently, under conditions where Ag is not limiting, it is conceivable that original antigenic sin could be overcome. This idea is substantiated by studies done by Webster and colleagues (19) showing that in rabbits sequentially immunized with swine influenza virus followed by the antigenically related strain FM1, the suppressed Ab response to FM1 is overcome by administering 30 times more FM1 than swine influenza virus. Although the early response was still dominated by cross-reactive Abs, the later response was a primary response characterized with high avidity and strain-specificity. Therefore, factors including frequency of memory B cells, antigenic load, and quality can shift the competition between cross-reactive memory B cells and strain-specific naive B cells in animals experiencing original antigenic sin.
As we continue to seek protection from seasonal influenza by annual vaccination, the potential for each flu infection or vaccine to induce original antigenic sin remains. Some of the critical questions that remain to be addressed are 1) what is the mechanism of original antigenic sin?, 2) to what extent does the antigenic distance between viruses play a role in induction of original antigenic sin?, and 3) does altered engagement of innate immunity play a role in skewing the Ab responses to the “original” Ag? A better understanding of these issues should enable us to design influenza vaccines that can redeem the host from the lure of original antigenic sin.
Acknowledgments
We acknowledge Dr. David Steinhauer and members of the Jacob Laboratory for helpful discussions. We thank Dr. Mark Tompkins for the gift of mouse-adapted FM1 viruses and Leela Thomas for mouse colony management.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked advertisement in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
This work was supported by contract HHSN266 200700006C from National Institutes of Health/National Institute of Allergy and Infectious Diseases. J.J. is a research scholar of the American Cancer Society.
Abbreviations used in this paper: HA, hemagglutinin; RDE II, receptor destroying enzyme II; HAI, hemagglutination inhibition; LD50, 50% lethal dose; HAU, hemagglutinin unit; MDCK, Madin-Darby canine kidney.
Disclosures
The authors have no financial conflict of interest.
References
- 1.Palese P. Influenza: old and new threats. Nat. Med. 2004;10:S82–S87. doi: 10.1038/nm1141. [DOI] [PubMed] [Google Scholar]
- 2.Doherty PC, Turner SJ, Webby RG, Thomas PG. Influenza and the challenge for immunology. Nat. Immunol. 2006;7:449–455. doi: 10.1038/ni1343. [DOI] [PubMed] [Google Scholar]
- 3.Yewdell J, Garcia-Sastre A. Influenza virus still surprises. Curr. Opin. Microbiol. 2002;5:414–418. doi: 10.1016/s1369-5274(02)00346-6. [DOI] [PubMed] [Google Scholar]
- 4.Smith NM, Bresee JS, Shay DK, Uyeki TM, Cox NJ, Strikas RA. Prevention and control of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP). Morbid. Mortal. Wkly. Rep. Recomm. Rep. 2006;55:1–42. [PubMed] [Google Scholar]
- 5.Webster RG, Bean WJ, Gorman OT, Chambers TM, Kawaoka Y. Evolution and ecology of influenza A viruses. Microbiol. Rev. 1992;56:152–179. doi: 10.1128/mr.56.1.152-179.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carrat F, Flahault A. Influenza vaccine: the challenge of antigenic drift. Vaccine. 2007;25:6852–6862. doi: 10.1016/j.vaccine.2007.07.027. [DOI] [PubMed] [Google Scholar]
- 7.Prevention and control of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP). Morbid. Mortal. Wkly. Rep. Recomm. Rep. 1999;48:1–28. [PubMed] [Google Scholar]
- 8.Cox NJ, Subbarao K. Global epidemiology of influenza: past and present. Annu. Rev. Med. 2000;51:407–421. doi: 10.1146/annurev.med.51.1.407. [DOI] [PubMed] [Google Scholar]
- 9.Murphy BR, Clements ML. The systemic and mucosal immune response of humans to influenza A virus. Curr. Top. Microbiol. Immunol. 1989;146:107–116. doi: 10.1007/978-3-642-74529-4_12. [DOI] [PubMed] [Google Scholar]
- 10.Skehel JJ, Wiley DC. Receptor binding and membrane fusion in virus entry: the influenza hemagglutinin. Annu. Rev. Biochem. 2000;69:531–569. doi: 10.1146/annurev.biochem.69.1.531. [DOI] [PubMed] [Google Scholar]
- 11.Treanor J. Influenza vaccine: outmaneuvering antigenic shift and drift. N. Engl. J. Med. 2004;350:218–220. doi: 10.1056/NEJMp038238. [DOI] [PubMed] [Google Scholar]
- 12.Davenport FM, Hennessy AV, Francis T., Jr. Epidemiologic and immunologic significance of age distribution of antibody to antigenic variants of influenza virus. J. Exp. Med. 1953;98:641–656. doi: 10.1084/jem.98.6.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Francis T., Jr. Influenza: the new acquaintance. Ann. Intern. Med. 1953;39:203–221. doi: 10.7326/0003-4819-39-2-203. [DOI] [PubMed] [Google Scholar]
- 14.Jensen KE, Davenport FM, Hennessy AV, Francis T., Jr. Characterization of influenza antibodies by serum absorption. J. Exp. Med. 1956;104:199–209. doi: 10.1084/jem.104.2.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davenport FM, Hennessy AV. A serologic recapitulation of past experiences with influenza A; antibody response to monovalent vaccine. J. Exp. Med. 1956;104:85–97. doi: 10.1084/jem.104.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Virelizier JL, Allison AC, Schild GC. Antibody responses to antigenic determinants of influenza virus hemagglutinin, II: original antigenic sin: a bone marrow-derived lymphocyte memory phenomenon modulated by thymus-derived lymphocytes. J. Exp. Med. 1974;140:1571–1578. doi: 10.1084/jem.140.6.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Virelizier JL, Postlethwaite R, Schild GC, Allison AC. Antibody responses to antigenic determinants of influenza virus hemagglutinin, I: thymus dependence of antibody formation and thymus independence of immunological memory. J. Exp. Med. 1974;140:1559–1570. doi: 10.1084/jem.140.6.1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Webster RG. Original antigenic sin in ferrets: the response to sequential infections with influenza viruses. J. Immunol. 1966;97:177–183. [PubMed] [Google Scholar]
- 19.de St. Fazekas G, Webster RG. Disquisitions on original antigenic sin, II: proof in lower creatures. J. Exp. Med. 1966;124:347–361. doi: 10.1084/jem.124.3.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gulati U, Kumari K, Wu W, Keitel WA, Air GM. Amount and avidity of serum antibodies against native glycoproteins and denatured virus after repeated influenza whole-virus vaccination. Vaccine. 2005;23:1414–1425. doi: 10.1016/j.vaccine.2004.08.053. [DOI] [PubMed] [Google Scholar]
- 21.Wrammert J, Smith K, Miller J, Langley WA, Kokko K, Larsen C, Zheng NY, Mays I, Garman L, Helms C, et al. Rapid cloning of high-affinity human monoclonal antibodies against influenza virus. Nature. 2008;453:667–671. doi: 10.1038/nature06890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smith JM, Amara RR, McClure HM, Patel M, Sharma S, Yi H, Chennareddi L, Herndon JG, Butera ST, Heneine W, et al. Multiprotein HIV type 1 clade B DNA/MVA vaccine: construction, safety, and immunogenicity in Macaques. AIDS Res. Hum. Retroviruses. 2004;20:654–665. doi: 10.1089/0889222041217419. [DOI] [PubMed] [Google Scholar]
- 23.de St. Fazekas G, Webster RG. Disquisitions of original antigenic sin, I: evidence in man. J. Exp. Med. 1966;124:331–345. doi: 10.1084/jem.124.3.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Deutsch S, Bussard AE. Original antigenic sin at the cellular level, I: antibodies produced by individual cells against cross-reacting haptens. Eur. J. Immunol. 1972;2:374–378. doi: 10.1002/eji.1830020416. [DOI] [PubMed] [Google Scholar]
- 25.Deutsch S, Vinit MA, Bussard AE. Original antigenic sin at the cellular level, II: specificity of the antibodies produced by individual cells. Eur. J. Immunol. 1973;3:235–240. doi: 10.1002/eji.1830030411. [DOI] [PubMed] [Google Scholar]
- 26.Couch RB, Webster RG, Kasel JA, Cate TR. Efficacy of purified influenza subunit vaccines and relation to the major antigenic determinants on the hemagglutinin molecule. J. Infect. Dis. 1979;140:553–559. doi: 10.1093/infdis/140.4.553. [DOI] [PubMed] [Google Scholar]
- 27.Sladkova T, Kostolansky F. The role of cytokines in the immune response to influenza A virus infection. Acta Virol. 2006;50:151–162. [PubMed] [Google Scholar]
- 28.Tovey MG, Lallemand C, Thyphronitis G. Adjuvant activity of type I interferons. Biol. Chem. 2008;389:541–545. doi: 10.1515/bc.2008.051. [DOI] [PubMed] [Google Scholar]
- 29.Doucett VP, Gerhard W, Owler K, Curry D, Brown L, Baumgarth N. Enumeration and characterization of virus-specific B cells by multicolor flow cytometry. J. Immunol. Methods. 2005;303:40–52. doi: 10.1016/j.jim.2005.05.014. [DOI] [PubMed] [Google Scholar]