Abstract
Activating mutations of the BRAF gene lead to constitutive activation of the MAPK pathway. Although many human cancers carry the mutated BRAF gene, this mutation has not yet been characterized in canine cancers. As human and canine cancers share molecular abnormalities, we hypothesized that BRAF gene mutations also exist in canine cancers. To test this hypothesis, we sequenced the exon 15 of BRAF, mutation hot spot of the gene, in 667 canine primary tumors and 38 control tissues. Sequencing analysis revealed that a single nucleotide T to A transversion at nucleotide 1349 occurred in 64 primary tumors (9.6%), with particularly high frequency in prostatic carcinoma (20/25, 80%) and urothelial carcinoma (30/45, 67%). This mutation results in the amino acid substitution of glutamic acid for valine at codon 450 (V450E) of canine BRAF, corresponding to the most common BRAF mutation in human cancer, V600E. The evolutional conservation of the BRAF V600E mutation highlights the importance of MAPK pathway activation in neoplasia and may offer opportunity for molecular diagnostics and targeted therapeutics for dogs bearing BRAF-mutated cancers.
Introduction
The RAF proteins are evolutionary conserved serine/threonine kinases that regulate fundamental cellular processes, including growth, differentiation and survival. The RAF family consists of three members: ARAF, BRAF and CRAF. All RAF proteins are activated by RAS and subsequently activate MEK, initiating the signal transduction cascade of the MAPK pathway. Constitutive activation of the MAPK pathway caused by oncogenic mutations of RAF genes results in abnormal proliferation and differentiation. Among the three forms of RAF genes, BRAF gene is most frequently mutated in human cancer [1–3].
The most common (>90%) somatic mutation of the human BRAF gene is a T-to-A transversion in exon 15 at nucleotide 1799 (c.1799T>A), resulting in the amino acid substitution from valine to glutamic acid at codon 600 (V600E) [2]. The V600E mutation occurs within the activation segment of the gene and mimics phosphorylation, drastically elevating kinase activity and activation of the downstream signal [3,4]. This activating mutation has been reported in melanoma (~60%) [4,5], thyroid cancer (20–40%)[6–9], hairy-cell leukemia (~100%)[10] and many other cancers with variable frequency. Coupled with frequent mutations of RAS genes, the presence of BRAF mutations in a wide variety of human cancers underscores the importance of MAPK pathway activation as a common oncogenic molecular pathway.
Dogs develop spontaneous cancers with many similarities to human cancers, including anatomical location, histological appearance and therapeutic response. Cancer in dogs shares not only biological behaviors with humans, but also molecular abnormalities [11,12]. Since activating BRAF mutations are present in a wide variety of human cancer, we hypothesized that BRAF gene mutations are similarly involved in canine cancers, leading to hyperactivation of the MAPK pathway and cell transformation. To test this hypothesis, we screened for the presence of BRAF exon 15 mutations in a cohort of 667 pathologically confirmed canine tumor specimens, comprising a series of hematopoetic tumors (n = 245), sarcomas (n = 160), carcinomas (n = 115), melanocytic tumor (n = 72) as well as other, less common cancer (n = 75).
Materials and Methods
Fresh and formalin fixed tumor specimens of various canine solid tumors, and EDTA blood samples from canine leukemia cases, were submitted from client-owned pet dogs (with informed owner consent) by private veterinary practices across the United States as a part of routine diagnostic procedures (no IACUC required). Additional tumor specimens were recruited via the North Carolina State University (NCSU) Clinical Studies Core, each with informed owner consent and following an NCSU IACUC approved protocol (approval number 13-022-O), which covered the procedure used to obtain the samples and their subsequent use for research application. Hematoxylin and eosin-stained slides of formalin-fixed paraffin-embedded (FFPE) specimens were reviewed by a board-certified veterinary pathologist and confirmed neoplastic in all but leukemia specimens. Leukemia diagnoses were based on the evaluation of cytological and immunophenotypical examination of leukemic cells by a board-certified clinical pathologist.
Genomic DNA was isolated from fresh tissue/blood samples or FFPE tissues. A total of 667 tumor specimens were included in this study. Details of the sample population are shown in Tables 1 and 2. DNA was isolated using a QIAamp FFPE DNA extraction kit (Qiagen, Valencia, CA, USA) or a DNeasy Blood and Tissue Kit (Qiagen). Spectrophotometry (NanoDrop, Thermo Scientific, Wilmington, DE) and agarose gel electrophoresis were used to determine DNA quantity and integrity. For non-neoplastic controls (n = 38), DNA was isolated from canine bladder epithelium of 30 dogs and prostate glands of 8 dogs, obtained by necropsy with no evidence of neoplastic changes upon histopathologic evaluation.
Table 1. Primary cancer samples used in this study.
| Cancer type | N | Pathological classification |
|---|---|---|
| Hematopoietic | 245 | Lymphoma (50), mast cell tumor (50), chronic lymphocytic leukemia (43; 20 B-cell and 23 T-cell origin), histiocytoma (27), plasmacytoma (21), histiocytic sarcoma (20), acute myelgenous leukemia (18), acute lymphoblastic leukemia (16) |
| Sarcoma | 160 | Soft tissue sarcoma (60), hemangiosarcoma (50), osteosarcoma (50) |
| Carcinoma | 115 | Urothelial carcinoma (45), prostatic carcinoma (25), pulmonary carcinoma (18), oral squamous cell carcinoma (18), mammary gland carcinoma (7), anal sac carcinoma (1), renal cell carcinoma (1) |
| Melanocytic | 72 | Melanoma (54; 47 oral, 6 cutaneous and 1 ocular origin), melanocytoma (18) |
| Miscelleneous | 75 | Meningioma (20), ameloblastoma (16), transmissible venereal tumor (14), glioma (13), peripheral nerve sheath tumor (9), nephroblastoma (3) |
Table 2. Signalments of dogs diagnosed with primary cancers.
| Breed | Mixed (83), Labrador Retriever (82), Golden Retriever (72), Boxer (38), German Shepherd Dog (28), Beagle (18), Flat-Coated Retriever (17), Greyhound (17), Australian Shepherd (16), Pug (12), Bernese Mountain Dog (10), Miniature Shnauzer (10), other breeds (< 10 each, 223) |
| Gender | Male (347), female (311), unknown (9) |
| Neutering | Castrated (238), spayed (230), intact (8), unknown (221) |
| Age | < 3 years old (15), 3–7 years old (143), 8–11 years old (248), 11 < years old (124), unknown (137) |
| DNA source | FFPE tissue (458), fresh frozen tissue (132), blood (77) |
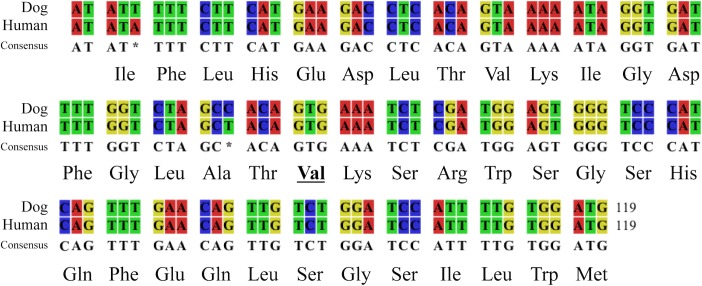
Exon 15 of the human BRAF gene is evolutionally conserved between dogs and humans (Fig 1). Thus, PCR amplification was performed to amplify a 391-bp DNA fragment spanning the genomic canine BRAF sequence corresponding to human BRAF gene exon 15 (CanFam3.1, canine chromosome (CFA) 16: 8,296,227–8,296,345). The following primer pair was designed using Primer-BLAST software (http://www.ncbi.nlm.nih.gov/tools/primer-blast/): forward, AAGCAGGTCACATATGCCAAA (CFA 16: 8,296,007–8,296,027); reverse, ATTTTTGGACCCTGAGGTGC (CFA 16: 8,296,378–8,296,397). Each PCR reaction contained 10–20 ng of genomic DNA, 250 nM of the forward and reverse primer and 1× Taq RED Master Mix Kit (Genesee Scientific, San Diego, CA, USA). PCR cycles consist of initial denaturation of 95°C for 2 min, followed by 40 cycles of 95°C for 30 s, 60°C for 30 s and 72°C for 30 s with a final elongation step at 95°C for 5 min. PCR products were visualized using agarose gel electrophoresis and subjected to targeted Sanger sequencing analysis with the forward and/or reverse primers. Sequence analysis was performed at the North Carolina State University Genome Research Laboratory (http://research.ncsu.edu/gsl/). The sequencing data were analyzed using 4peaks software (http://nucleobytes.com/index.php/4peaks) and compared with the reference sequence (XM_005629550.1) using CLC Sequence Viewer version 7 (CLC bio, Aarthus, Denmark).
Fig 1. DNA and amino acid sequences of human (NM_004333) BRAF exon 15 and dog BRAF gene (XM_005629550.1).
The sequences are highly conserved between human and dog, including valine at codon 600 in human BRAF (underlined) and at codon 450 in canine BRAF.
The Fisher’s exact test was performed to examine difference of BRAF mutation frequencies between groups stratified by gender, neutering status, age or breeds. All statistical analyses were performed with JMP Pro software version 11 (SAS Institute, Cary, NC). Values of P < 0.05 were considered significant.
Results and Discussion
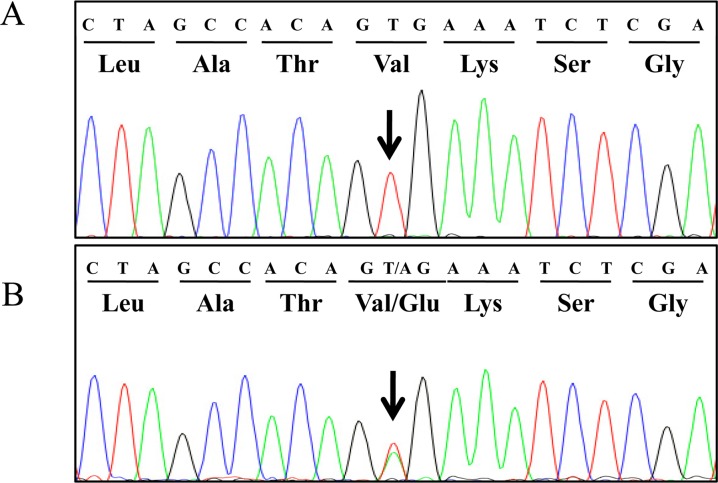
To investigate the presence of BRAF mutations, we sequenced BRAF gene exon 15 in 667 primary tumor samples and 38 control tissue samples. Sequencing analysis revealed a T to A transversion at nucleotide 1349 (c.1349T>A, reference: XM_005629550.1) which occurred in 64 primary tumors, resulting in the amino acid substitution from valine to glutamic acid at codon 450 (V450E) (Fig 2). This amino acid change corresponds to the human V600E mutation (Fig 1). Significant variation exists in the frequency of the V450E mutation across canine cancers: 0% in hematopoietic tumors and sarcomas to 67% and 80% of urothelial carcinoma (UC) and prostatic carcinoma (PC), respectively (Table 3). In all V600E mutants, electropherograms indicated the presence of both mutated and wild-type sequences, suggesting mutation heterozygosity. There was no statistically significant difference in the mutation frequency between different groups of neutering status, age or breeds in UC and PC samples and between the mutational status and gender in UC samples. Details of signalments of dogs with UC and PC are shown in S1 Table.
Fig 2. Sequence analysis of the canine BRAF gene.
(A) Wild-type sequence obtained from a control prostate gland DNA. (B) Mutated sequence mixed with wild-type sequence obtained from a prostatic carcinoma. Arrow indicates the T-to-A nucleotide substitution resulting in the change of valine at codon 450 to glutamic acid.
Table 3. Prevalence of V450E mutation in canine primary cancers.
| Cancer type | N | V450E (frequency) | |
|---|---|---|---|
| Hematopoietic | Lymphoma | 50 | 0 |
| Mast cell tumor | 50 | 0 | |
| Chronic lymphocytic leukemia | 43 | 0 | |
| Histiocytoma | 27 | 0 | |
| Plasmacytoma | 21 | 0 | |
| Histiocytic sarcoma | 20 | 0 | |
| Acute myelogenous leukemia | 18 | 0 | |
| Acute lymphoblastic leukemia | 16 | 0 | |
| Sarcoma | Soft tissue sarcoma | 60 | 0 |
| Hemangiosarcoma | 50 | 0 | |
| Osteosarcoma | 50 | 0 | |
| Carcinoma | Urothelial carcinoma | 45 | 30 (67%) |
| Prostatic carcinoma | 25 | 20 (80%) | |
| Pulmonary carcinoma | 18 | 1 (6%) | |
| Oral squamous cell carcinoma | 18 | 2 (11%) | |
| Other carcinoma | 9 | 0 | |
| Melanocytic | Melanoma | 54 | 3 (6%) |
| Melanocytoma | 18 | 3 (17%) | |
| Miscellaneous | Meningioma | 20 | 0 |
| Ameloblastoma | 16 | 0 | |
| Transmissible venereal tumor | 14 | 0 | |
| Glioma | 13 | 2 (15%) | |
| Peripheral nerve sheath tumor | 9 | 2 (22%) | |
| Nephroblastoma | 3 | 0 | |
In addition to the V450E mutation, a T-to-C transition at nucleotide 1305 (c.1305T>C, silent mutation) was observed in an oral squamous cell carcinoma sample. An intronic deletion of T (c.1292-189delT) was observed in one each of soft tissue sarcoma and melanoma samples. All other tumor and control samples maintained the wild type genomic sequence for BRAF exon 15.
Constitutive activation of MAPK signaling by activating mutations of BRAF (~60%) or NRAS (~15%) genes plays an important role in the pathogenesis of human melanoma [4,5,11,12]. Similarly, constitutive activation of the MAPK pathway is implicated in canine melanoma [13,14], although RAS genes were infrequently mutated [14–16]. In this study, however, only 6% of melanomas (two mucosal and one cutaneous melanoma) and 17% of melanocytomas harbored the BRAF V450E mutation. This mutation was not identified in previous studies of canine melanoma [13,17], likely due the low frequency of the BRAF mutation in canine melanoma. In human melanoma, the presence of BRAF mutation is associated with skin exposure to UV light, and melanomas on mucosal sites or non-UV-exposed skin rarely possess the mutation [18,19]. As canine melanoma occurs mainly on oral mucosa and infrequently on nail beds and non-UV-exposed furred skin, the fact that BRAF is mutated infrequently in canine melanoma is consistent with findings in human counterparts.
Interestingly, canine UC showed much higher frequency of the BRAF mutation than is reported to in human UC tumors [20]. Mutations in genes upstream of the MAPK pathway, including HRAS, KRAS and FGFR3 genes (all of which are upstream molecules of BRAF in MAPK pathway), were found in >82% of human papillary UC, suggesting that activation of the pathway is a main driving factor for the subclass of human UC [21,22]. Although the mutated molecules in the pathway may be different between human and canine UC, the high frequency of BRAF mutation in canine UC suggests that dysregulation of MAPK pathway may play an important role in the pathogenesis of the disease.
Canine PC is characterized by high metastatic potential and local invasiveness, but the factors contributing to aggressive biological behavior are still largely unknown [23]. Although BRAF V600E mutations are infrequent in humans [24–26], accumulating evidence suggests that MAPK pathway plays an important role in the development and progression of human PC, especially in metastatic tumors [27]. Somatic mutations of the RAS genes and copy number gains of BRAF and CRAF genes are observed in human PC at frequencies of ~10, 30% and 15%, respectively [24–27]. These genomic alterations lead to the activation and/or increased expressions of RAF proteins, resulting in the activation of downstream signaling and increasing metastatic properties [26–28]. Additionally, recurrent chromosomal translocations involving RAS and RAF genes, which result in oncogenic fusion genes, were recently discovered in a subset (~5%) of human PC cases [29,30].
A unique feature of canine PC is that the majority of tumors arise in androgen-independent manner, with increased risk in castrated dogs [23]. On the other hand, hormone-deprivation therapy is a mainstay for the treatment of human PC, as androgen plays a critical role in the pathogenesis. Most of human PC, however, progress to a more aggressive, hormone-refractory (castration-resistant) cancer during the clinical course. Activation of BRAF/MAPK signaling makes human PC tumor cells less dependent on androgen for proliferation in vivo and in vitro, contributing to hormone-refractory phenotype [31]. The high incidence of the BRAF mutation and aggressive nature of canine PC may reflect the fact that most canine PC develop independently of androgen stimulation. These clinical and molecular similarities may make canine PC serve as a spontaneously-occurring animal cancer model relevant to hormone-refractory human PC.
Recent advancement in molecular technology enabled us to detect circulating tumor cells in liquid samples such as peripheral blood. Detection of BRAF mutations can be used as a means to diagnose and monitor tumor burden in liquid samples, such as blood or urine, without necessitating biopsy of tumors (called as liquid biopsy, reviewed in [32,33]). Although histopathological examination of a tumor biopsy is the gold standard for the diagnosis of canine UC and PC, the anatomical locations of these tumors often make it difficult to obtain sufficient amount of tissues to diagnose. Additionally, clinicians and owners may be discouraged from choosing this diagnostic workup due to cost and the invasive procedures associated with biopsy. Therefore, access to a non-invasive means of diagnosing these cancers is an unmet need. The high BRAF mutation rate in these tumors makes the BRAF V450E mutation a potential diagnostic marker for affected cancers.
The identification of BRAF mutation in canine cancers raises the possibilities that therapy targeting constitutively-activated MAPK pathway can provide a clinical benefit for those carrying the BRAF V450E mutation, especially UC and PC patients. Recently, vemurafenib and dabrafenib, selective BRAF inhibitors, improved clinical outcomes in patients with melanoma compared to conventional chemotherapy [34,35]. These BRAF inhibitors have also shown therapeutic potentials in other neoplasms harboring BRAF mutations [36–38]. Currently, treatment options for dogs with UC and PC are of limited efficacy. Given the effectiveness of BRAF/MAPK-targeted therapy in human cancers, the BRAF and MAPK pathways may be promising therapeutic targets for these canine cancers. Evaluations of in vitro and in vivo effects of BRAF inhibitors in dogs are warranted for the clinical application of the BRAF inhibitor for dogs bearing cancer with mutated BRAF.
In conclusion, we identified the BRAF V450E mutation in canine cancers with various frequencies. Frequent BRAF mutation in canine UC and PC underscores a potential role of the MAPK signaling pathway in the pathogenesis of these tumors and may offer diagnostic and therapeutic applications for dogs bearing BRAF mutations.
Supporting Information
(XLSX)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This study was funded by the North Carolina State University (NCSU) Cancer Genomics Fund (MB).
References
- 1. Sebolt-Leopold JS, Herrera R. Targeting the mitogen-activated protein kinase cascade to treat cancer. Nature Reviews Cancer. 2004;4(12): 937–947. [DOI] [PubMed] [Google Scholar]
- 2. Dhillon A, Hagan S, Rath O, Kolch W. MAP kinase signalling pathways in cancer. Oncogene. 2007;26(22): 3279–3290. [DOI] [PubMed] [Google Scholar]
- 3. Roskoski R. RAF protein-serine/threonine kinases: structure and regulation. Biochemical and biophysical research communications. 2010;399(3): 313–317. 10.1016/j.bbrc.2010.07.092 [DOI] [PubMed] [Google Scholar]
- 4. Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, et al. Mutations of the BRAF gene in human cancer. Nature. 2002. June 27;417(6892): 949–954. [DOI] [PubMed] [Google Scholar]
- 5. Brose MS, Volpe P, Feldman M, Kumar M, Rishi I, Gerrero R, et al. BRAF and RAS mutations in human lung cancer and melanoma. Cancer research. 2002;62(23): 6997–7000. [PubMed] [Google Scholar]
- 6. Cohen Y, Xing M, Mambo E, Guo Z, Wu G, Trink B, et al. BRAF mutation in papillary thyroid carcinoma. Journal of the National Cancer Institute. 2003;95(8): 625–627. [DOI] [PubMed] [Google Scholar]
- 7. Kimura ET, Nikiforova MN, Zhu Z, Knauf JA, Nikiforov YE, Fagin JA. High Prevalence of BRAF Mutations in Thyroid Cancer Genetic Evidence for Constitutive Activation of the RET/PTC-RAS-BRAF Signaling Pathway in Papillary Thyroid Carcinoma. Cancer research. 2003;63(7): 1454–1457. [PubMed] [Google Scholar]
- 8. Namba H, Nakashima M, Hayashi T, Hayashida N, Maeda S, Rogounovitch TI, et al. Clinical implication of hot spot BRAF mutation, V599E, in papillary thyroid cancers. The Journal of Clinical Endocrinology & Metabolism. 2003;88(9):4393–4397. [DOI] [PubMed] [Google Scholar]
- 9. Xing M. BRAF mutation in thyroid cancer. Endocrine-related cancer. 2005;12(2): 245–262. [DOI] [PubMed] [Google Scholar]
- 10. Tiacci E, Trifonov V, Schiavoni G, Holmes A, Kern W, Martelli MP, et al. BRAF mutations in hairy-cell leukemia. The New England journal of medicine. 2011;364(24): 2305–2315. 10.1056/NEJMoa1014209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tsao H, Goel V, Wu H, Yang G, Haluska FG. Genetic interaction between NRAS and BRAF mutations and PTEN/MMAC1 inactivation in melanoma. Journal of Investigative Dermatology. 2004;122(2): 337–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Goel VK, Lazar AJ, Warneke CL, Redston MS, Haluska FG. Examination of mutations in BRAF, NRAS, and PTEN in primary cutaneous melanoma. Journal of Investigative Dermatology. 2006;126(1): 154–160. [DOI] [PubMed] [Google Scholar]
- 13. Shelly S, Chien MB, Yip B, Kent MS, Theon AP, McCallan JL, et al. Exon 15 BRAF mutations are uncommon in canine oral malignant melanomas. Mammalian genome: official journal of the International Mammalian Genome Society. 2005;16(3): 211–217. [DOI] [PubMed] [Google Scholar]
- 14.Fowles J, Denton C, Gustafson D. Comparative analysis of MAPK and PI3K/AKT pathway activation and inhibition in human and canine melanoma. Veterinary and comparative oncology. In press. [DOI] [PubMed]
- 15. Richter A, Murua Escobar H, Gunther K, Soller JT, Winkler S, Nolte I, et al. RAS gene hot-spot mutations in canine neoplasias. The Journal of heredity. 2005;96(7): 764–765. [DOI] [PubMed] [Google Scholar]
- 16. Campos M, Kool MM, Daminet S, Ducatelle R, Rutteman G, Kooistra HS, et al. Upregulation of the PI3K/Akt pathway in the tumorigenesis of canine thyroid carcinoma. Journal of veterinary internal medicine / American College of Veterinary Internal Medicine. 2014;28(6): 1814–1823. 10.1111/jvim.12435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gillard M, Cadieu E, De Brito C, Abadie J, Vergier B, Devauchelle P, et al. Naturally occurring melanomas in dogs as models for non-UV pathways of human melanomas. Pigment cell & melanoma research. 2014. January;27(1): 90–102. [DOI] [PubMed] [Google Scholar]
- 18. Maldonado JL, Fridlyand J, Patel H, Jain AN, Busam K, Kageshita T, et al. Determinants of BRAF mutations in primary melanomas. Journal of the National Cancer Institute. 2003;95(24): 1878–1890. [DOI] [PubMed] [Google Scholar]
- 19. Edwards R, Ward M, Wu H, Medina C, Brose M, Volpe P, et al. Absence of BRAF mutations in UV-protected mucosal melanomas. Journal of medical genetics. 2004;41(4): 270–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Boulalas L, Zaravinos A, Delakas D, Spandidos DA. Mutational analysis of the BRAF gene in transitional cell carcinoma of the bladder. International Journal of Biological Markers. 2009;24(1): 17–21. [DOI] [PubMed] [Google Scholar]
- 21. Schulz WA. Understanding urothelial carcinoma through cancer pathways. International journal of cancer. 2006;119(7): 1513–1518. [DOI] [PubMed] [Google Scholar]
- 22. Knowles MA, Hurst CD. Molecular biology of bladder cancer: new insights into pathogenesis and clinical diversity. Nat Rev Cancer. 2015;15(1): 25–41. 10.1038/nrc3817 [DOI] [PubMed] [Google Scholar]
- 23. LeRoy BE, Northrup N. Prostate cancer in dogs: comparative and clinical aspects. The Veterinary Journal. 2009;180(2): 149–162. 10.1016/j.tvjl.2008.07.012 [DOI] [PubMed] [Google Scholar]
- 24. Cho NY, Choi M, Kim BH, Cho YM, Moon KC, Kang GH. BRAF and KRAS mutations in prostatic adenocarcinoma. International journal of cancer Journal international du cancer. 2006;119(8): 1858–1862. [DOI] [PubMed] [Google Scholar]
- 25. Kollermann J, Albrecht H, Schlomm T, Huland H, Graefen M, Bokemeyer C, et al. Activating BRAF gene mutations are uncommon in hormone refractory prostate cancer in Caucasian patients. Oncology letters. 2010;1(4): 729–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ren G, Liu X, Mao X, Zhang Y, Stankiewicz E, Hylands L, et al. Identification of frequent BRAF copy number gain and alterations of RAF genes in Chinese prostate cancer. Genes, chromosomes & cancer. 2012;51(11): 1014–1023. [DOI] [PubMed] [Google Scholar]
- 27. Taylor BS, Schultz N, Hieronymus H, Gopalan A, Xiao Y, Carver BS, et al. Integrative genomic profiling of human prostate cancer. Cancer cell. 2010;18(1): 11–22. 10.1016/j.ccr.2010.05.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mulholland DJ, Kobayashi N, Ruscetti M, Zhi A, Tran LM, Huang J, et al. Pten loss and RAS/MAPK activation cooperate to promote EMT and metastasis initiated from prostate cancer stem/progenitor cells. Cancer research. 2012;72(7): 1878–1889. 10.1158/0008-5472.CAN-11-3132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Palanisamy N, Ateeq B, Kalyana-Sundaram S, Pflueger D, Ramnarayanan K, Shankar S, et al. Rearrangements of the RAF kinase pathway in prostate cancer, gastric cancer and melanoma. Nature medicine. 2010;16(7): 793–798. 10.1038/nm.2166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wang X-S, Shankar S, Dhanasekaran SM, Ateeq B, Sasaki AT, Jing X, et al. Characterization of KRAS rearrangements in metastatic prostate cancer. Cancer discovery. 2011;1(1): 35–43. 10.1158/2159-8274.CD-10-0022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gao H, Ouyang X, Banach-Petrosky WA, Gerald WL, Shen MM, Abate-Shen C. Combinatorial activities of Akt and B-Raf/Erk signaling in a mouse model of androgen-independent prostate cancer. Proceedings of the National Academy of Sciences. 2006;103(39): 14477–14482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Crowley E, Di Nicolantonio F, Loupakis F, Bardelli A. Liquid biopsy: monitoring cancer-genetics in the blood. Nature reviews Clinical oncology. 2013;10(8): 472–484. 10.1038/nrclinonc.2013.110 [DOI] [PubMed] [Google Scholar]
- 33. Diaz LA, Bardelli A. Liquid biopsies: genotyping circulating tumor DNA. Journal of Clinical Oncology. 2014;32(6): 579–586. 10.1200/JCO.2012.45.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hauschild A, Grob J-J, Demidov LV, Jouary T, Gutzmer R, Millward M, et al. Dabrafenib in BRAF-mutated metastatic melanoma: a multicentre, open-label, phase 3 randomised controlled trial. The Lancet. 2012;380(9839): 358–365. 10.1016/S0140-6736(12)60868-X [DOI] [PubMed] [Google Scholar]
- 35. Chapman PB, Hauschild A, Robert C, Haanen JB, Ascierto P, Larkin J, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. New England Journal of Medicine. 2011;364(26): 2507–2516. 10.1056/NEJMoa1103782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Follows GA, Sims H, Bloxham DM, Zenz T, Hopper MA, Liu H, et al. Rapid response of biallelic BRAF V600E mutated hairy cell leukaemia to low dose vemurafenib. British journal of haematology. 2013;161(1): 150–153. 10.1111/bjh.12201 [DOI] [PubMed] [Google Scholar]
- 37. Haroche J, Cohen-Aubart F, Emile J-F, Arnaud L, Maksud P, Charlotte F, et al. Dramatic efficacy of vemurafenib in both multisystemic and refractory Erdheim-Chester disease and Langerhans cell histiocytosis harboring the BRAF V600E mutation. Blood. 2013;121(9): 1495–1500. 10.1182/blood-2012-07-446286 [DOI] [PubMed] [Google Scholar]
- 38. Munoz J, Schlette E, Kurzrock R. Rapid response to vemurafenib in a heavily pretreated patient with hairy cell leukemia and a BRAF mutation. Journal of Clinical Oncology. 2013;31(20): e351–e352. 10.1200/JCO.2012.45.7739 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.