Abstract
Background
Oral infection of C57BL/6J mice with the protozoan parasite Toxoplasma gondii leads to a lethal inflammatory ileitis.
Principal Findings
Mice lacking the purinergic receptor P2X7R are acutely susceptible to toxoplasmic ileitis, losing significantly more weight than C57BL/6J mice and exhibiting much greater intestinal inflammatory pathology in response to infection with only 10 cysts of T. gondii. This susceptibility is not dependent on the ability of P2X7R-deficient mice to control the parasite, which they accomplish just as efficiently as C57BL/6J mice. Rather, susceptibility is associated with elevated ileal concentrations of pro-inflammatory cytokines, reactive nitrogen intermediates and altered regulation of elements of NFκB activation in P2X7R-deficient mice.
Conclusions
Our data support the thesis that P2X7R, a well-documented activator of pro-inflammatory cytokine production, also plays an important role in the regulation of intestinal inflammation.
Introduction
Toxoplasma gondii is an intracellular protozoan parasite that enters the body through the mouth, invades the intestinal epithelium, crosses into the lamina propria and then disseminates throughout the body. Thus, like many infectious agents, its first point of contact with the immune system is the intestine, where it provokes a potent pro-inflammatory response that is designed to control parasite replication but can result in immunopathology if not properly regulated [1]. Adequate regulation is the result of interactions between T. gondii, immune cells, Th1, Th2 and Th17 cytokines (reviewed in [1, 2]).
In C57BL/6 mice, oral infection with 100 tissue cysts of T. gondii causes a lethal inflammatory ileitis. This is the result of a classic inflammatory cytokine “storm”. Thus, toxoplasmic ileitis, like other inflammatory intestinal diseases, causes necrosis of the villi and mucosa that can be alleviated by depletion of pro-inflammatory cytokines or their receptors; ie, IFN-γ, TNF, IL-12, IL-15, IL-17, IL-18 and IL-23 [3–7]. Deletion of the gene for inducible nitric oxide synthase [8] or depletion of nitric oxide [5] also inhibits inflammatory ileitis. With regard to anti-inflammatory cytokines, toxoplasmic ileitis is associated only circumstantially with defective TGF-β signalling [9, 10] but IL-10 plays a key role in ameliorating ileitis; a normally non-pathogenic dose (20 cysts) of T. gondii can kill mice lacking IL-10 [11].
There are several reasons to believe that the P2X7 receptor (P2X7R) might play an important role in the regulation of intestinal inflammation in response to T. gondii: first, it is an ancient receptor with a well-documented role in innate immunological control of intracellular infections [12] including T. gondii [13]; second, it is expressed on a variety of intestinal cells during inflammation [14–17]; and third, it can affect IL-10 production and activation of inducible nitric oxide synthase [18, 19] including in response to T. gondii [20].
ATP is an important extracellular signal for the immune system, particularly during an inflammatory response. P2X7R, like other purinergic receptors, senses extracellular ATP. However, P2X7R is distinctive from other receptor family members due to its high expression on immune cells and, moreover, its expression is up-regulated by pro-inflammatory cytokines [12]. Not surprisingly, therefore, P2X7R has been implicated in the killing of important intracellular pathogens including Mycobacteria, Chlamydia and Leishmania (reviewed in [12]) and it can also mediate killing of T. gondii, at least in vitro [13]. Furthermore, genetic association studies in diverse human populations show a significant association between resistance to toxoplasmosis and inheritance of a polymorphism in p2rx7 that enhances function [21].
P2X7R is active in several different cells within the intestine [14–17, 22] and there is evidence that it plays both pro-inflammatory as well as regulatory roles during intestinal inflammation. For example, ATP from intestinal bacteria is known to up-regulate the expression of Th17 cytokines and exacerbate colitis [23]. It has also been shown that P2X7R mediates mast cell-dependent intestinal inflammation [17, 24] and inflammation-induced death of enteric neurons via an inflammasome-dependent pathway [16]. P2X7R is also well known as an activator of the inflammasome, a complex of cytosolic proteins that regulates caspase-1 activation and the processing of IL-1β and IL-18 from inactive to active forms. Intestinal expression of IL-1β and IL-18 is enhanced in inflammatory bowel disease (IBD) patients and blocking or deleting IL-18 can reduce intestinal damage in mice [6]. However, the loss-of-function Arg-307-Glu polymorphism of P2X7R is associated with IBD, albeit the association is borderline significant (p = 0.06) from a relatively small number of cases and unaccompanied by any association between IBD and other loss- or gain-of-function polymorphisms [25]. This may indicate that the P2X7R has some role in the dampening of inflammatory responses in the gut as well as in the promotion of pro-inflammatory responses that help manage infections. This idea is supported by observations that intestinal epithelial P2X7R levels are low during active IBD but high in control people and IBD patients in a quiescent phase [14].
In this study, we investigated the role of P2X7R in the regulation of toxoplasmic ileitis. Mice lacking P2X7R had similar parasite loads to, but lost weight faster than, C57BL/6J mice and exhibited higher degrees of intestinal pathology. P2X7R-deficient mice also displayed an inability to regulate their intestinal inflammatory cytokine response.
Results
Mice lacking functional P2X7 receptors are highly susceptible to toxoplasmic ileitis
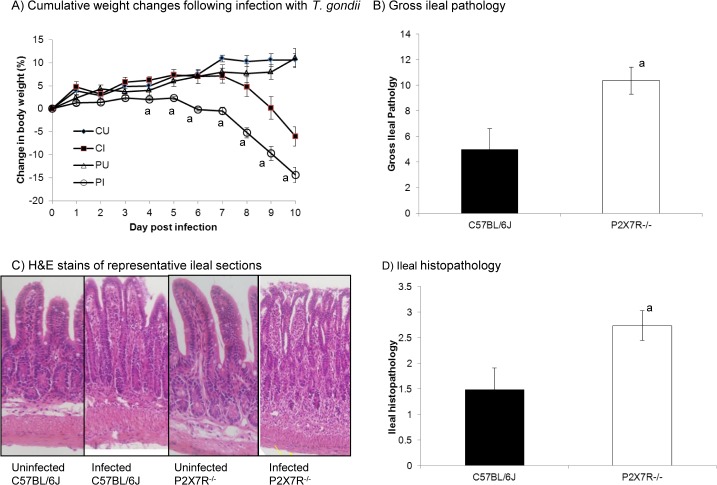
Susceptibility of p2rx7 gene-deleted mice (P2X7R-/-) on a C57BL/6J background to toxoplasmic ileitis was assessed relative to C57BL/6J mice by infecting mice with 10 T. gondii ME49 cysts and monitoring the course of infection. All infected mice exhibited clinical signs of infection and lost weight relative to uninfected mice of the same strain (Fig 1A). P2X7R-/-mice lost significantly more weight than C57BL/6J mice beginning on day 4 p.i. and, by day 10 p.i., P2X7R-/- mice had lost 14 ± 1.66% of their body weight versus 6 ± 2.1% for C57BL/6J mice (P<0.005, assessed by multivariate analysis of variance–MANOVA–with days assigned as the within-subjects variable and mouse strain/infection status assigned to the between subjects variable, followed by the assessment of significant interactions within each time point using planned comparisons, i.e., two-way ANOVA coupled to Tukey’s post-hoc test at each day post-infection)].
Fig 1. Toxoplasmic ileitis is exacerbated in in P2X7R-/- mice compared with wild type.
Male mice (6–8 weeks old) were infected orally with 10 T. gondii ME49 cysts. (A) Mice were weighed daily for 10 days and cumulative weight changes were calculated and the results presented represent the mean ± SEM of the percentage of weight change relative to starting weight per strain per day from one of six experiments that generated similar data. Infected n = 21/strain; uninfected n = 9/strain. P2X7R-/- mice lost significantly more weight than C57BL/6J mice from day 4 post-infection onward (P<0.005, assessed by multivariate analysis of variance (MANOVA) with days assigned as the within-subjects variable and mouse strain/infection status assigned to the between subjects variable, followed by the assessment of significant interactions within each time point using planned comparisons, i.e., two-way ANOVA coupled to Tukey’s post-hoc test at each day post infection). CU, C57BL/6J uninfected; CI, C57BL/6J infected; PU, P2X7R-/- uninfected; PI, P2X7R-/- infected. (B) Gross ileal pathology was scored on day 8 post-infection based on five observations: consistency of the intestinal contents; absence/presence of blood; absence/presence of pus; degree of swelling; and amount of angiogenesis. This system was adapted from Melgar et al. [60] and was based on an ascending scale of severity, for each parameter, as follows: 0 (no abnormality); 1 (minimal); 2 (moderate); or 3 (severe). The score for each parameter was added to give a total out of a maximum possible score of 15. Gross ileal pathology was also assessed in uninfected mice but no pathology was observed in either strain. Results presented are from one of four experiments that generated similar data. (C, D) Ileal histopathology was evaluated based on five parameters: epithelial cell damage; goblet cell loss; crypt dropout; neutrophil and mononuclear cell infiltration in the submucosa and neutrophil and mononuclear cell infiltration in the muscular layers. Three random fields of view at a 40x magnification were graded on an ascending scale of severity: 0 (no abnormality); 0.25 (minimal); 0.5 (mild); 0.75 (moderate); or 1 (severe) giving a total score out of 5 per mouse. Histopathology was also examined in uninfected mice, however, no pathology was observed in any strain. Photomicrographs of histopathology shown (C) are from single mice and are representative of all mice examined in the group. Results are presented as the mean ± SEM (n = 8) for both strains of mice. Results presented are from one of three experiments that generated similar data. a Indicates where the score is significantly different from the score for C57BL/6J mice (P<0.05, one-way ANOVA coupled to Tukey’s post-hoc test).
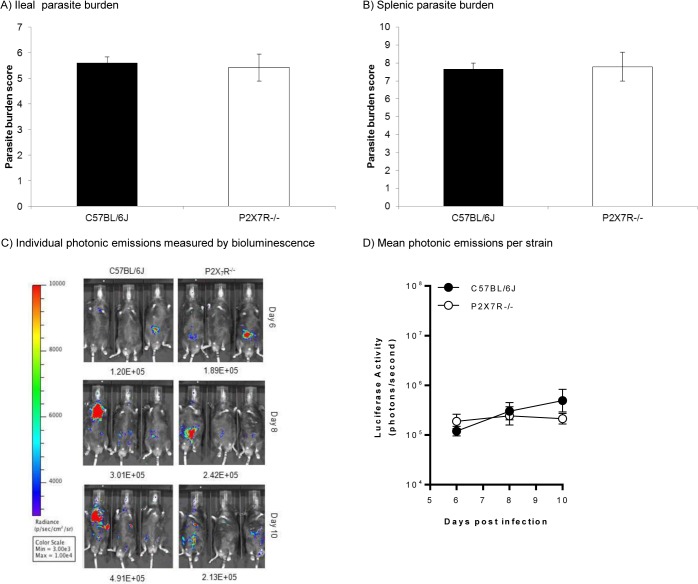
Infected P2X7R-/- mice exhibited significantly higher levels of gross ileal pathology with an average score of 10 compared with average scores of 5 for infected C57BL/6J mice (P<0.05, one-way ANOVA coupled to Tukey’s post-hoc test) (Fig 1B). P2X7R-/- mice had consistently higher scores in the amounts of swelling, angiogenesis and pus present, as well as consistency of the intestinal contents, which were much more liquefied than the intestinal contents of the C57BL/6J mice. Blood was only rarely observed in severe infections in the P2X7R-/- mice and never in the ileum of infected C57BL/6J mice. No pathology was observed in uninfected mice of any strain. Similarly, a significant increase (P<0.05, one-way ANOVA coupled to Tukey’s post-hoc test) in histopathology was observed in infected P2X7R-/- mice compared with infected C57BL/6J mice (Fig 1C and 1D) or uninfected mice of both strains (data not shown). Fig 1C shows representative photomicrograph images of C57BL/6J and P2X7R-/- H&E stained ileal sections from mice that had been infected orally with T. gondii ME49 for 8 days. A representative photomicrograph portraying a healthy uninfected ileal section is included for reference. By 8 days post infection, both strains of mice showed increased cellular infiltration in the sub-mucosa and the muscle layers compared with uninfected ileal sections of each strain. Furthermore, P2X7R-/- mice lost all discernable villus structure due to severe epithelial cell damage and suffered a complete loss of goblet cells as well as severe crypt drop-out in the lamina propria. This all translated into a much higher histopathology score in infected P2X7R-/- mice (Fig 1D). However, at day 8 p.i., despite the significant increase in intestinal pathology in infected P2X7R-/- mice (P<0.05, one-way ANOVA coupled to Tukey’s post-hoc test), there was no significant change in parasite burden in either the intestine or spleen of knockout mice compared with burdens measured in infected C57BL/6J mice (Fig 2A and 2B). Moreover, the dissemination of T. gondii followed similar kinetics in P2X7R-/- and C57BL/6J mice (Fig 2C and 2D).
Fig 2. Control of Toxoplasma gondii is not altered in P2X7R-/- mice compared with wild type.
Male mice (6–8 weeks old) were infected orally with 10 T. gondii ME49 cysts and euthanased 8 days post-infection. Parasite burden in (A) intestines and (B) spleens of C57BL/6J and P2X7R-/- mice was determined by a microtitre limiting dilution assay as detailed in the Materials and Methods. Results are presented as the mean ± SEM (n = 8) for both strains of mice from one of three experiments that generated similar data. Parasite burden measurements from infected P2X7R-/- mice were not significantly different from infected C57BL/6J mice (one-way ANOVA). Identical results were obtained using the classical plaque-forming assay [61] so these are not shown here. (C, D) In a separate series of experiments, three male and three female mice were infected orally with 5 cysts of luciferase-expressing 76KGFP-Luc T. gondii and parasite burden was quantified on day 6, 8 and 10 p.i. by firefly luciferase activity using an IVIS BLI system from Caliper Life Sciences as described previously [32]; images of three infected male C57BL/6J and three infected male P2X7R-/- mice are shown (C). Parasite burden measurements (D) from infected P2X7R-/- mice (n = 6) were not significantly different from infected C57BL/6J mice (n = 6) on any of day 6, 8 or 10 p.i. (two-way ANOVA).
Production of inflammatory cytokines and reactive nitrogen intermediates is elevated in mice lacking functional P2X7 receptors
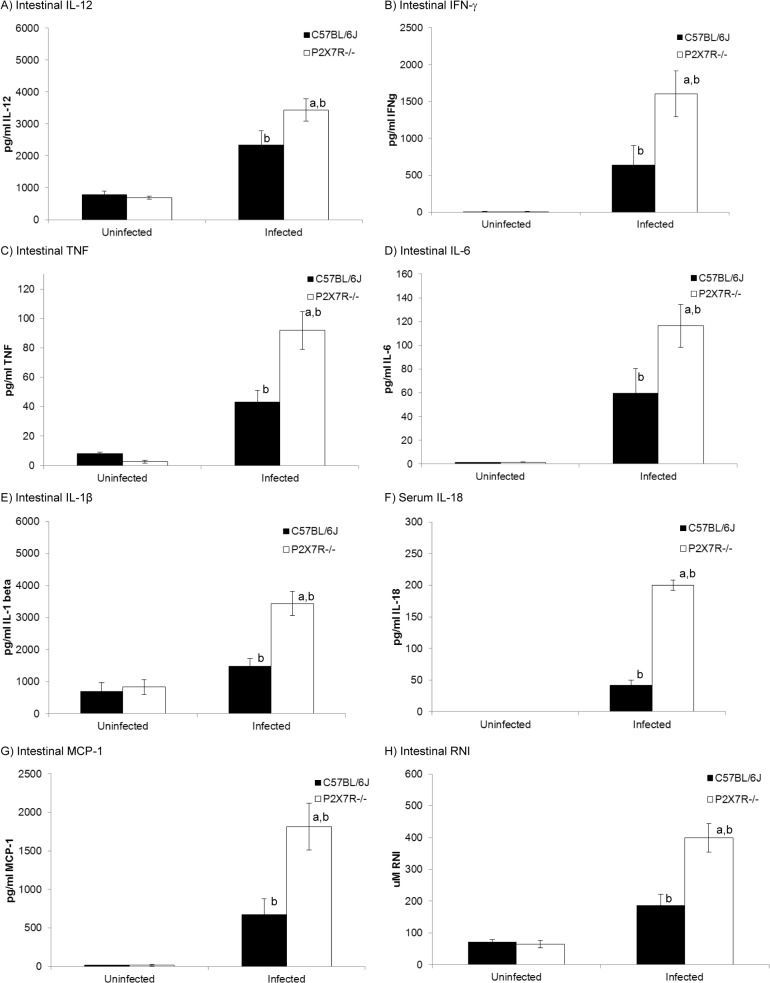
Results in Fig 2 indicated that P2X7R-/- mice were able to control parasite numbers equally as well as C57BL/6J mice, so the more severe pathology observed was not due to lack of control of T. gondii replication. Production of pro-inflammatory cytokines is essential for control of T. gondii but, when not regulated, can lead to immunopathology. Therefore, levels of IL-12, IFN-γ, IL-1β, IL-6, TNF and MCP-1 (CCL2), were measured in ileal homogenates from infected P2X7R-/- mice and compared with levels detected in uninfected P2X7R-/- mice, and infected and uninfected C57BL/6J mice. IL-12, IFN-γ and TNF are all crucial in the development of resistance to T. gondii, however, excessive production of these cytokines has been associated with immunopathology [26–29]. IL-18, IL-1β and IL-6 are also produced in response to T. gondii as part of an inflammatory response [28, 30, 31] and MCP-1 (CCL2) assists the inflammatory response by recruiting macrophages to sites of inflammation and tissue damage [32]. Ileal homogenates from infected P2X7R-/- mice had significantly elevated levels of IL-12, IFN-γ, TNF, IL-1β, IL-6 and MCP-1 (CCL2) (Fig 3) compared with infected C57BL/6J mice or uninfected mice of either strain (P<0.05, two-way ANOVA coupled to Tukey’s post-hoc test). Levels of all these cytokines were similarly elevated in sera of infected P2X7R-/- mice, with the exception of IL-1β, which was not detectable in the sera of any mice studied (data not shown), which is consistent with the findings of others [33]. This may indicate a specific, discrete cellular source for IL-1β, a question that remains unresolved [33]. IL-18 was not detected in the intestinal samples, due to (unresolved) interference of the intestinal samples with the assay. However, IL-18 was detected in sera (Fig 3F) from infected mice and, again, levels of this cytokine were significantly elevated in infected P2X7R-/- mice compared with infected C57BL/6J mice or uninfected mice of either strain (P<0.05, two-way ANOVA coupled to Tukey’s post-hoc test). Levels of reactive nitrogen intermediates in ileal homogenates (Fig 3H) and sera (data not shown) were also significantly elevated in infected P2X7R-/- mice compared with infected C57BL/6J mice or uninfected mice of either strain (P<0.05, two-way ANOVA coupled to Tukey’s post-hoc test).
Fig 3. Inflammatory cytokine production is dysregulated in P2X7R-/- mice compared with wild type in association with toxoplasmic ileitis.
Male mice (6–8 weeks old) were infected orally with 10 T. gondii Me49 cysts and euthanased 8 days post-infection. Ileal homogenates were collected for quantification of cytokine levels by ELISA (IL-1β, IL-6, IL-12, TNF, MCP-1, IFN-γ) or Griess assay (reactive nitrogen intermediates) as described in the Materials and Methods. Sera was analyzed for IL-18. Following infection, P2X7R-/- mice (n = 10) exhibited significantly higher levels of (A) IL-12, (B) IFN-γ, (C) TNF, (D) IL-6 (E) IL-1β, (F) IL-18, (G) MCP-1 and (H) reactive nitrogen intermediates compared with infected C57BL/6J (n = 10) or uninfected mice (n = 5) of either strain (P<0.05). Results are presented as the mean ± SEM for one of three experiments that generated similar data. aindicates a significant difference (P<0.05, two-way ANOVA coupled to Tukey’s post-hoc test at each day post infection) compared with infected C57BL/6J mice, bindicates a significant difference (P<0.05, two-way ANOVA coupled to Tukey’s post-hoc test at each day post infection) between infected and uninfected mice of the same strain.
Elevated levels of reactive nitrogen intermediates are well known to cause pathology, so we attempted to “rescue” mice from toxoplasmic ileitis by inhibiting and/or quenching reactive nitrogen and oxygen species using methods described previously [5, 8, 34, 35]; however, none of these approaches affected weight loss or intestinal pathology in infected P2X7R-/- mice. All mice started losing weight by day 4 p.i. and lost between 13 and 21% of their bodyweight by day 10 p.i., whether untreated or treated with aminoguanidine, N-monomethyl-L-arginine, or N-acetylcysteine. Production of the anti-inflammatory cytokines, TGF-β and IL-10, was also assessed in ileal homogenates of infected P2X7R-/- mice with levels of these two cytokines varying quite widely from mouse to mouse without being significantly different from background levels seen in uninfected mice of either P2X7R-/- or C57BL/6J mice. Thus, mean and standard error levels (pg/ml) of TGF-β were 200 ± 72 (n = 8) and 212 ± 66 (n = 5) for uninfected C57BL/6J and P2X7R-/- mice, respectively, rising (albeit not statistically significantly) to 400 ± 128 (n = 6) and 436 ± 135 (n = 6) in infected mice of the two respective strains. Mean and standard error levels (pg/ml) of IL-10 were 257 ± 86 (n = 6) and 193 ± 57 (n = 6) for uninfected C57BL/6J and P2X7R-/- mice, respectively, versus 205 ± 90 (n = 6) and 275 ± 115 for infected mice (n = 6).
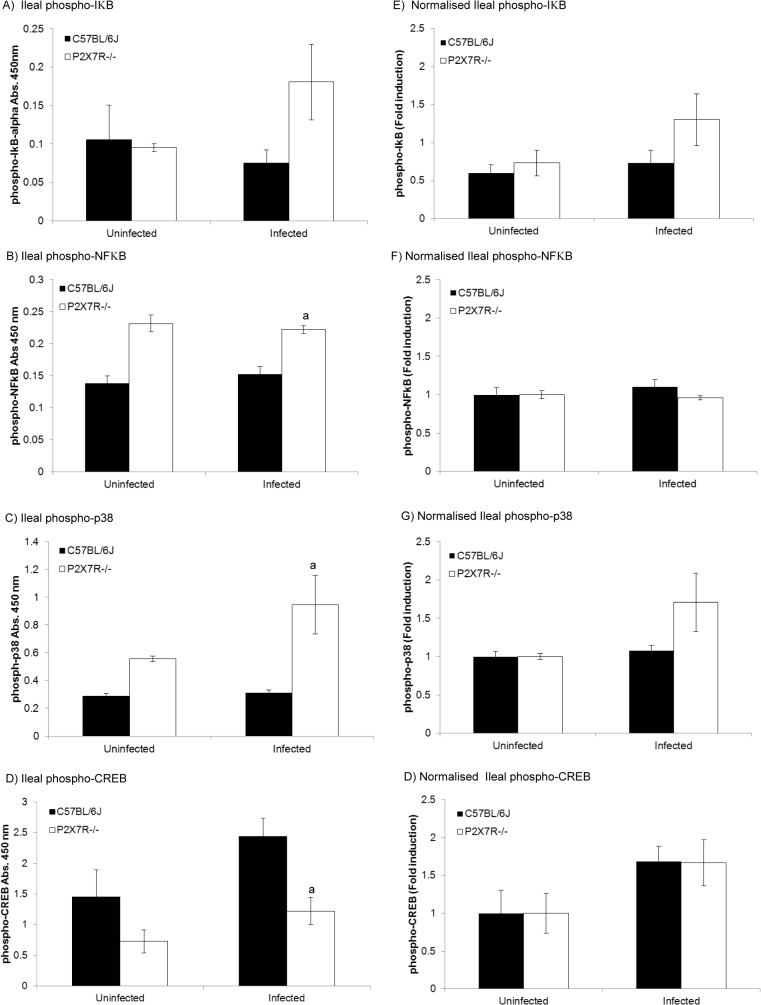
Regulation of the NFκB and MAPK pathways is impaired in mice lacking functional P2X7 receptors
Binding of microbial-derived molecules to cell surface receptors on immune cells triggers activation of both NFκB and the mitogen-activated protein kinase (MAPK) signalling pathways. Triggering of the NFκB pathway results in the translocation of NFκB from the cytoplasm to the nucleus where it induces the expression of its target genes [36, 37]. Full transcriptional activity is achieved when phosphorylated NFκB binds CREB binding protein (CBP). The MAPK pathway results in the phosphorylation of p38 MAPK which, in turn, leads to the phosphorylation of CREB via MSK1 [38]. Phosphorylated CREB sequesters CBP and inhibits transcription of NFκB-controlled genes thus down-regulating pro-inflammatory cytokine production. To investigate whether the elevated levels of pro-inflammatory cytokines observed in infected P2X7R-/- mice (Fig 3) were the result of differential regulation of these pathways between infected C57BL/6J mice and P2X7R-/- mice, activation of key proteins in the NFκB signalling pathway were tested. Although total baseline levels of CREB were not significantly different between C57BL/6J and P2X7R-/- mice, the baseline levels of NFκB were significantly higher in uninfected P2X7R-/- mice compared with C57BL/6J mice (data not shown). Because phosphorylation at specific residues, rather than overall protein levels, is indicative of activation, phosphorylation of proteins that regulate the inflammatory response, specifically IκB, NFκB, p38 MAPK, and CREB, were measured. No baseline difference was detected between uninfected P2X7R-/- versus C57BL/6J mice for phosphorylated IκB (Fig 4A). However, uninfected P2X7R-/- mice possessed higher baseline levels of phosphorylated NFκB (Fig 4B), phosphorylated p38 MAPK (Fig 4C), and lower levels of the negative regulator, phosphorylated CREB (Fig 4D) than uninfected C57BL/6J, although these results did not reach statistical significance. Following infection, P2X7R-/- mice had statistically increased levels of phosphorylated NFκB (Fig 4B) and p38 MAPK (Fig 4C) as well as reduced levels of phosphorylated CREB when compared against infected C57BL/6J mice (P<0.05, two-way ANOVA coupled to Tukey’s post-hoc test). However, when normalized to account for the intrinsic differences observed at baseline between uninfected P2X7R-/- and wild-type C57BL/6J mice, infection did not differentially induce the phosphorylation of NFκB (Fig 4F) or CREB (Fig 4H), but elevated levels of phosphorylated IκB (Fig 4E) and p38 MAPK (Fig 4G) were identified in P2X7R-/- mice, although this did not reach statistical significance using a two-way ANOVA test.
Fig 4. Regulation of transcriptional regulators is impaired in P2X7R-/- mice compared with wild type in association with toxoplasmic ileitis.
Male mice (6–8 weeks old) were infected orally with 10 T. gondii Me49 cysts and euthanased 8 days post-infection. Ileal homogenates were collected for quantification of phospho-IκB, phospho-NFκB, phospho-p38, and phospho-CREB by sandwich ELISA as described in the materials and methods. Following infection, P2X7R-/- mice exhibited significantly higher levels of (A) ileal phospho-IκB, (B) phospho-NFκB, (C) phospho-p38, and significantly lower levels of (D) phospho-CREB compared with infected C57BL/6J or uninfected mice of either strain (a indicates a significant difference, at P<0.05, using a two-way ANOVA coupled to Tukey’s post-hoc test, compared with infected C57BL/6J mice). When the data were normalised against uninfected baseline phospho-protein levels for each mouse strain, no statistically significant differences in phospho-IkB (E) phospho-NFkB (F), phospho-p38 (G) or phospho-CREB (H) were apparent, as measured by fold induction. Results are presented as the mean ± SEM (n = 10 for infected or n = 5 for uninfected mice) for one of three experiments that generated similar data.
Discussion
P2X7R-/- mice are acutely susceptible to toxoplasmic ileitis, losing significantly more weight than C57BL/6J (Fig 1) and exhibiting much greater ileal gross pathology and histopathology in response to infection with only 10 cysts of T. gondii (Fig 1). This susceptibility is not dependent on the ability of P2X7R-/- mice to control the parasite, which they accomplish just as efficiently as C57BL/6J mice. Acute infection with T. gondii is characterised by the proliferation of rapidly dividing tachyzoites that proliferate inside the cell until the cell membrane ruptures. As each tachyzoite can then go on to invade any nucleated cell, the parasite can disseminate through the body quite quickly and, if not controlled, severe pathology can result. Following oral infection, T. gondii forms replicative foci within the epithelia and lamina propria of the intestine before disseminating throughout the host’s body [39] albeit sometimes inconsistently [40]. However, we found no significant difference in parasite burden in either the intestine or spleen in infected P2X7R-/- mice compared with infected C57BL/6J mice and the kinetics of dissemination are similar in the two strains (Fig 2). This indicates, first, that P2X7R is not crucial for limiting parasite replication in vivo and, second, that the burden of infection with T. gondii per se is not responsible for the ensuing ileitis.
The unimportance of P2X7R in the control of T. gondii in vivo is seemingly at odds with demonstrations of P2X7R-dependent killing of T. gondii tachyzoites in vitro [13, 41]. However, it is in keeping with the oft documented observation that parasite burden is rarely associated with toxoplasmic ileitis in a variety of gene knockout mice or mice treated with inhibitors of innate immune effectors [3–11]. Tachyzoites of T. gondii activate a variety of innate immune effector pathways that affect the viability of the parasite to varying degrees (reviewed in [1]). Two recent studies, for example, documented different emphases on the NLRP1 and NLRP3 inflammasome sensors as important factors in detecting and controlling T. gondii [30, 33], a point which is particularly pertinent for the overproduction of IL-1β and IL-18, processing of which can be initiated by different inflammasome pathways in response to T. gondii [30, 33]. Thus, with so many avenues to control T. gondii, it is not totally surprising that elimination of just one line of attack (in this case P2X7R-dependent killing) may have little effect in vivo. Moreover, it is important to remember that a significant feature of toxoplasmic ileitis is the role played by normally commensal bacteria. Oral infection with T. gondii acts as a trigger for changes in the intestinal bacterial population from >95% Gram-positive to >95% Gram-negative [42]. Bacteria accumulate at sites of mucosal damage and translocate into subepithelial tissues [43] and beyond [44] prolonging the pro-inflammatory response provoked by T. gondii and leading to immunopathology [45]. It will be intriguing to see if the intestinal flora in P2X7R-/- mice differs from that in C57BL/6J mice in response to the trigger from infection with T. gondii.
Like T. gondii, P2X7R has been long recognised as an activator of pro-inflammatory responses [46–49], see [50] for a review). Thus, in the absence of P2X7R, we expected to see reduced production of pro-inflammatory cytokines and reactive nitrogen intermediates, accompanied by diminished intestinal inflammation. However, this was not what we observed. Rather, our results fit with other observations in which reduced P2X7R activity is associated with increased intestinal inflammation and IBD [14]. Moreover, recent work [51] provides a possible explanation for this; whilst normal physiological concentrations of ATP (ie, 1-50nm) have little effect on CD4+ T cells or Tregs, and somewhat elevated levels of extracellular ATP (eg, 250nm) stimulate the release of pro-inflammatory cytokines, high levels of extracellular ATP (eg, 1mM) simultaneously inhibit activated CD4+ T cells and enhance the immunosuppressive activity of Tregs. This phenomenon may be particularly pertinent in toxoplasmic ileitis, which is characterised by a collapse in Treg cell numbers and function [52]. Our data support a role for P2X7R in the regulation of intestinal inflammation since we saw a generalised overproduction of pro-inflammatory cytokines and mediators in P2X7R-/- mice (Fig 3).
Our observations on the intestinal levels of regulators of transcription (Fig 4) provide some clues about how the modulatory effects of P2X7R may be mediated, but do not provide a complete explanation. Extracellular ATP is known to induce activation of CREB via the phosphorylation of p38α and MSK-1 [53, 54]. Activated CREB, in turn, binds the transcriptional co-activator, CBP, preventing it from binding with the NFκB subunit, p65, thereby inhibiting expression of pro-inflammatory cytokines [55]. Hence, cells lacking P2X7R may prove less responsive to the sustained build-up of extracellular ATP that could accompany infection with pathogens like T. gondii. The downstream effect of this would be exactly what we see, that is, decreased levels of phospho-CREB and increased levels of phospho-NFκB following infection; however, NF-κB appears to be constitutively over-produced in P2X7R-/- mice, regardless of infection status. Normalization of the phosphorylation of IκB, NFκB, p38 MAPK, and CREB (Fig 4E–4H) to account for observed baseline differences between P2X7R-/- and C57BL/6J mice revealed that intrinsic differences between the strains correlate with differential phosphorylation of inflammatory regulators, independent of infection. The observed baseline differences in the phosphorylation of NFκB, and CREB (Fig 4B and 4D) between P2X7R-/- and C57BL/6J mice without differential baseline levels of inflammatory mediators (Fig 3; uninfected mice) suggests that these intrinsic differences in the phosphorylation state of NFκB and CREB play a key role following perturbation. Thus, the observed over-production of pro-inflammatory cytokines during toxoplasmic ileitis in P2X7R-/- mice may be partially due to continued activation of the NFκB pathway because not enough CBP is being sequestered by CREB to achieve inhibition of NFκB transcription. Further studies are required to support this hypothesis.
Levels of phosphorylated p38α are also elevated in the intestines of P2X7R-/- mice infected with T. gondii (Fig 4C). Phosphorylated p38α can stimulate inflammatory and anti-inflammatory pathways through activation of two different downstream kinases [56]. Thus, in an amplification loop, activated p38α activates mitogen-activated protein kinase-activated protein kinase 2 (MK2) by phosphorylation. Activated MK2 then promotes an increase in TNF, IL-1β and IL-6 production through interactions with NFκB [57]. Increased levels of these cytokines then feedback positively on cells and activate the same pathway, amplifying inflammation [56]. In an anti-inflammatory loop, activated p38α phosphorylates mitogen-and stress-activated kinases 1 and 2 (MSK-1/2). MSK-1 and MSK-2 then phosphorylate CREB allowing it to bind CBP and also stimulate the production of dual-specificity protein phosphatase 1 (DUSP 1), which dephosphorylates and inactivates p38 [58]. The elevation in phospho-p38 seen in P2X7R-/- mice suggests impaired regulation of p38 activation in mice without a functional P2X7R, although further work is needed to elucidate the mechanism involved.
In summary, our data support a thesis in which P2X7R, a well-documented activator of pro-inflammatory cytokine production, also plays an important role in the regulation of inflammation in the intestine. Thus, the lack of a functioning P2X7R leads to increased susceptibility to ileitis in mice infected orally with T. gondii associated with an over-exuberant inflammatory response rather than an inability to control parasite numbers. The increased levels of pro-inflammatory cytokines detected may be the result of impaired regulation of the NFκB and p38 MAPK pathways but whether this implies the existence of a P2X7R-dependent negative feedback mechanism for the inhibition of pro-inflammatory cytokine production or indicates more indirect effects, such as alterations in Treg populations and/or the intestinal microbiome, remains to be demonstrated conclusively.
Materials and Methods
Mice and Infections
Pathogen-free, 6–8 week old C57BL/6J mice were obtained from the Animal Resource Centre (Perth, WA, Australia) or from Jackson Laboratories (Bar Harbor, Maine, USA). p2rx7 gene-deleted mice (P2X7R-/-) on a C57BL/6J background (back-crossed for at least seven generations]) were originally provided by Pfizer, Inc. (Ann Arbor, MI, USA) and were subsequently bred at the Ernst Facility (University of Technology, Sydney, NSW, Australia) or were purchased from Jackson Laboratories and bred at the Immunogenetics Research Facility (James Cook University, Townsville, Queensland, Australia) or the National Institutes of Health, in Bethesda, USA. Lack of the p2rx7 gene in the knockout mice was confirmed routinely by PCR using the following primers: Forward 5’-CTATCTCTCCACGACTCACCCCC-3’ and Reverse 5’-TATAATCCCGGGAGGGATACTTGAAGCCACTGTAC-3’ [59].
All animal research was performed in strict accordance and with the approval of: the University of Technology Sydney/Royal North Shore Hospital Animal Care & Ethics Committee (Protocols UTS/ RNSH 0611-042A and UTS ACEC 2008–03); the James Cook University Animal Care & Ethics Committee (Approval Number A1698); and the National Institutes of Health, USA, and Animal Welfare Act (protocol LPD-22E), with mice housed and maintained in an animal facility accredited by the American Association for the Accreditation of Laboratory Animal Care.
Mouse infections were initiated by oral inoculation of cysts of the Type II T. gondii ME49 or 76KGFP-Luc strains. Cysts were isolated from the brains of chronically infected mice by homogenisation of the whole brain in sterile PBS and centrifugation through a discontinuous Percoll gradient. Suspensions were prepared at the concentrations indicated.
To assess relative susceptibility to T. gondii infection, male mice of each strain were infected orally with 10 or 20 cysts of T. gondii ME49, weighed daily and monitored for clinical signs of infection–weight loss, ruffled fur, hunched posture, lethargy and morbidity as per Animal Care & Ethics Protocol stipulations. This experiment was conducted six times; thrice at the University of Technology, Sydney, twice at James Cook University in Cairns, Australia, and once at the National Institutes of Health, USA, in Bethesda, with similar results each time. To assess intestinal pathology and identify aspects of the innate immune response that may be implicated in the increased susceptibility of P2X7R-/- mice to T. gondii infection, mice were euthanased on day 8 post-infection (p.i.) as per Animal Care & Ethics Protocol stipulations.
Ileal pathology and histopathology
The ileum was collected from each mouse and scored for gross pathology using a scoring system based on the following five observations: consistency of the intestinal contents; absence/presence of blood; absence/presence of pus; degree of swelling; and amount of angiogenesis. This system was adapted from Melgar et al. [60] and was based on an ascending scale of severity, for each parameter, as follows: 0 (no abnormality); 1 (minimal); 2 (moderate); or 3 (severe). The score for each parameter was added to give a total out of a maximum possible score of 15. A 2cm section of the ileum was cut away at the caecum and embedded in paraffin for histology. Sections (5μm) were stained with haematoxylin and eosin (H&E stain) and scored for histopathology by two individuals blinded as to sample identity. Histopathology was assessed using five parameters: epithelial cell damage; goblet cell loss; crypt dropout; neutrophil and mononuclear cell infiltration in the submucosa and neutrophil and mononuclear cell infiltration in the muscular layers. Three random fields of view at a 40x magnification were graded on an ascending scale of severity: 0 (no abnormality); 0.25 (minimal); 0.5 (mild); 0.75 (moderate); or 1 (severe). The grade assigned to each parameter was added to yield a total histopathology score, out of a possible 5, for the tissue within the field of view. An average was determined based on three fields of view from the one tissue. Scores from both investigators were then averaged to reduce any bias or discrepancy in the scoring system. Uninfected mice were included in the experiments as baseline references. The experiment was repeated four times.
Parasite burden
Ilea and spleens were examined for parasites in tissue sections using immunohistology with a rabbit anti-T. gondii polyclonal antibody (RayBiotech, Inc., Norcross, GA, USA) as described previously [20]; this preliminary examination confirmed that parasites were infecting similar sites within the intestines and spleens of infected mice in both strains examined (data not shown), often observed in foci in the villi and lamina propria, as recently described [39]. Parasite burden was then measured in the whole spleen and whole intestine of individual mice using a microtitre dilution method adapted from Buffet et al. [61], that we have described previously [13]. Briefly, on the day prior to the experiment, 96 well plates were seeded with 5x105 Vero cells/well and allowed to settle overnight. One row was allocated per mouse. Spleens were removed and single-cell suspensions were made by passing through a 70-mm sieve. Cells were pelleted at 1500g, and then resuspended in RPMI 1640 containing 5% FCS at a concentration of 1x107cells/ml. The entire small intestine was removed and processed to ensure consistency between mice strains when measuring parasite burden. Intestines were flushed with Hank’s Buffered Salt Solution (HBSS), chopped into small pieces and incubated in 1640 RPMI/10% Fetal Bovine Serum (FBS) containing 24U Dispase II, 200U collagenase, 100U DNAse at 37°C/5% CO2 for 2 hrs. Following incubation the intestines were reduced to single cell suspensions, washed three times by centrifugation then resuspended in 2 ml of fresh 1640 RPMI/10% FBS. Two hundred microliters of spleen or intestinal cell suspension was added to the first well of a 96-well plate and then serially diluted 1/2 across the plate. Plates were incubated at 37°C in 5% CO2 for 7 days before wells were examined for the presence of parasites. Relative parasite burden was estimated from the highest dilution in which parasites were visible. An arbitrary score–the “Parasite Burden score”–was allocated based on the last column in which the monolayer was fully lysed. Parasite burden was also determined by using a modified version of the plaque-forming assay described by Pfefferkorn and Pfefferkorn [62]. Five hundred μl aliquots of each intestinal suspension prepared as described above were transferred to flasks containing a confluent monolayer of Vero cells and incubated overnight at 37°C/5% CO2. The numbers of plaques formed per flask were then counted and recorded as Plaque Forming Units/500μl (PFU/500μl). These experiments were replicated thrice. In a separate experiment, mice were infected perorally with 5 cysts of luciferase-expressing 76KGFP-Luc T. gondii and parasite burden was quantified on days 6, 8, and 10 p.i. by firefly luciferase activity using an IVIS BLI system from Caliper Life Sciences as described previously [33].
Inflammatory mediator analysis
Inflammatory mediator levels were measured in ileal homogenates and in serum in three experiments. An 8 cm section of ileum was removed and flushed with sterile PBS using a syringe fitted with an 18G needle. Once clean, ileal sections were placed in 3 ml of sterile PBS in a 15 ml tube, kept on ice and homogenised using a hand-held glass homogeniser. Samples were then centrifuged at 400g for 10 min. Blood was obtained via terminal cardiac puncture, while mice were under anaesthetic, using a 1mL syringe fitted with a 26G needle. The samples were allowed to clot at room temperature before centrifugation at 500g for 10 mins. Serum was stored at -20°C until assayed. Ileal supernatants and sera were analysed for cytokine levels using either the BD Cytometric Bead Array Mouse Inflammation Kit, as per the manufacturer’s instructions, or by an Enyzme-Linked Immunosorbent Assay (ELISA).
Levels of IL-6, IL-10, IL-12, monocyte chemoattractant protein-1 (MCP-1 = CCL2) and TNF were measured using a BD Cytometric Bead Array kit (San Diego, CA, USA) according to the manufacturer’s instructions. Briefly, the serum samples and test standards supplied were mixed with cytokine capture beads and phycoerythrin (PE)-conjugated antibodies, and incubated for 2 h in the dark. After washing away unbound sample and reagent, fluorescence was measured in individual standards and samples on a BD LSR-II flow cytometer. Following data acquisition, standard curves were generated and cytokine amounts quantified using FCAP Array, version 1.0.1 (BD, San Diego, CA, USA). Levels of IL-1β, IL-18 and TGF-β were determined using OptEIA kits as per the manufacturer’s instructions (BD, San Diego, CA, USA). Reactive nitrogen intermediate levels were measured in serum and ileal homogenates using the Griess assay as described previously [20].
The endogenous levels of nuclear factor κB (NFκB), phosphorylated NFκB (Ser536), phosphorylated p38 mitogen-activated protein kinase (MAPK (Thr180/Try182)) and phosphorylated inhibitor of κBα (IκB-α (Ser32)) were measured in the intestinal homogenate of infected and uninfected mice using a Cell Signalling Technologies PathScan Inflammation Multi-Target Sandwich ELISA kit as per the manufacturer’s instructions. The endogenous levels of cAMP response elements (CRE) binding protein (CREB) and phosphorylated-CREB (Ser133) were measured in the intestinal homogenate of infected and uninfected mice using Cell Signalling Technologies PathScan Sandwich ELISA kits as per the manufacturer’s instructions.
Statistical analyses
The statistical significance of differences between groups was determined using a one-way ANOVA coupled to Tukey’s post-hoc test for the pathology scores and the intestinal and splenic parasite burdens. Changes in body mass were assessed by multivariate analysis of variance (MANOVA) with days assigned as the within-subjects variable and mouse strain/infection status assigned to the between subjects variable, followed by the assessment of significant interactions within each time point using planned comparisons (two-way ANOVA at each day post infection). A two-way ANOVA was used to assess luciferase activity in the bioluminescence parasite burden assay with day post infection and mouse strain as the independent variables. The inflammatory mediator and phosphorylated protein measurements were assessed by two-way ANOVA with infection status and mouse strain as the independent variables. In all two-way ANOVA assessments, the post-hoc analyses of pairwise multiple comparisons were performed using Tukey’s post-hoc test. A P value of <0.05 was considered significant.
Data Availability
All relevant data are within the paper.
Funding Statement
Professors Smith and Wiley gratefully acknowledge the support of the Australian Research Council for funding through its Discovery Project Scheme (Project number DP0666515). This work was also supported, in part, by the Intramural 24 Research Program of the National Institutes of Health, NIAID (Dr. Michael Grigg). Dr. Grigg is a Scholar of the Canadian Institute for Advanced Research (CIFAR) Integrated Microbial Biodiversity program. These funding bodies played no part in the experimental design, collection of data, interpretation, preparation of the manuscript or the decision to publish this research.
References
- 1. Miller CMD, Boulter NR, Ikin RJ, Smith NC. The immunobiology of the innate response to Toxoplasma gondii . Int. J. Parasitol. 2009; 39: 23–39. 10.1016/j.ijpara.2008.08.002 [DOI] [PubMed] [Google Scholar]
- 2. Egan CE, Cohen SB, Denkers EY. Insights into inflammatory bowel disease using Toxoplasma gondii as an infectious trigger. Immunol. Cell. Biol. 2012; 90: 668–675. 10.1038/icb.2011.93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Combe CL, Moretto MM, Schwartzman JD, Gigley JP, Bzik DJ, Khan IA.Lack of IL-15 results in the suboptimal priming of CD4+ T cell response against an intracellular parasite. Proc. Natl. Acad. Sci. USA 2006; 103: 6635–6640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kelly MN, Kolls JK, Happel K, Schwartzman JD, Schwarzenburger P, Combe C, et al. Interleukin-17/interleukin-17 receptor-mediated signaling is important for generation of an optimal polymorphonuclear response against Toxoplasma gondii infection. Infect. Immun. 2005; 73: 617–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Liesenfeld O, Kang OH, Park D, Nguyen TA, Parkhe CV, Watanabe H, et al. TNF-α, nitric oxide and IFN-γ are all critical for development of necrosis in the small intestine and early mortality in genetically susceptible mice infected perorally with Toxoplasma gondii . Parasite Immunol. 1999; 21: 365–376. [DOI] [PubMed] [Google Scholar]
- 6. Vossenkämper A, Struck D, Alvarado-Esquivel C, Went T, Takeda K, Akira S, et al. Both IL-12 and IL-18 contribute to small intestinal Th1-type immunopathology following oral infection with Toxoplasma gondii, but IL-12 is dominant over IL-18 in parasite control. Eur. J. Immunol. 2004; 34: 3197–3207. [DOI] [PubMed] [Google Scholar]
- 7. Muñoz M, Heimesaat MM, Danker K, Struck D, Lohmann U, Plickert R, et al. Interleukin 23 mediates Toxoplasma gondii-induced immunopathology in the gut via matrixmetalloproteinase-2 and IL-22 but independent of IL-17. J. Exp. Med. 2009; 206: 3047–3059. 10.1084/jem.20090900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Khan IA, Schwartzman JD, Matsuura T, Kasper LH.A dichotomous role for nitric oxide during acute Toxoplasma gondii infection in mice. Proc. Natl. Acad. Sci. USA 1997; 94: 13955–13960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Buzoni-Gatel D, Debbabi H, Mennechet FJ, Martin V, Lepage AC, Schwartzman JD, et al. Murine ileitis after intracellular parasite infection is controlled by TGF-β producing intraepithelia lymphocytes. Gastroenterology 2001; 120: 914–924. [DOI] [PubMed] [Google Scholar]
- 10. Mennechet FJ, Kasper LH, Rachinel N, Minns LA, Luangsay S, Vandewalle A, et al. Intestinal intraepithelial lymphocytes prevent pathogen-driven inflammation and regulate the Smad/T-bet pathway of lamina propria CD4+ T cells. Eur. J. Immunol. 2004; 34: 1059–1067. [DOI] [PubMed] [Google Scholar]
- 11. Suzuki Y, Sher A, Yap G, Park D, Neyer LE, Liesenfeld O, et al. IL-10 is required for prevention of necrosis in the small intestine and mortality in both genetically resistant BALB/c and susceptible C57BL/6 mice following peroral infection with Toxoplasma . J. Immunol. 2000; 164: 5375–5382. [DOI] [PubMed] [Google Scholar]
- 12. Miller CM, Boulter NR, Fuller SJ, Zakrzewski AM, Lees MP, Saunders B, et al. The role of P2X7 receptors in infectious diseases. PLoS Pathogens 2011; 7: e1002212 10.1371/journal.ppat.1002212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lees MP, Fuller S, McLeod R, Boulter NR, Miller CM, Zakrzewski AM, et al. P2X7 receptor-mediated killing of an intracellular parasite, Toxoplasma gondii by human and mouse macrophages. J. Immunol. 2010; 184: 7040–7046. 10.4049/jimmunol.1000012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cesaro A, Brest P, Hofman V, Hébuterne X, Wildman S, Ferrua B, et al. Amplification loop of the inflammatory process is induced by P2X7R activation in intestinal epithelial cells in response to neutrophil transepithelial migration. Am. J. Physiol. Gastrointest.Liver Physiol. 2010; 299: G32–G42. 10.1152/ajpgi.00282.2009 [DOI] [PubMed] [Google Scholar]
- 15. de Campos NE, Marques-da-Silva C, Corrêa G, Castelo-Branco MT, de Souza HS, Countinho-Silva R.Characterizing the presence and sensitivity of the P2X7 receptor in different compartments of the gut. J. Innate Immun. 2012; 4: 529–541. 10.1159/000336628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gulbransen BD, Bashashati M, Hirota SA, Gui X, Roberts JA, MacDonald JA, et al. Activation of neuronal P2X7 receptor-pannexin-1 mediates death of enteric neurons during colitis. Nature Med. 2012; 4: 600–605. 10.1038/nm.2679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kurashima Y, Amiya T, Nochi T, Fujisawa K, Haraguchi T, Iba H, et al. Extracellular ATP mediates mast cell-dependent intestinal inflammation through P2X7 purinoreceptors. Nature Comm. 2012; 3: 1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chessell IP, Hatcher JP, Bountra C, Michel AD, Hughes JP, Green P, et al. Disruption of the P2X7 purinoreceptor gene abolishes chronic inflammatory and neuropathic pain. Pain 2005; 114: 386–396. [DOI] [PubMed] [Google Scholar]
- 19. Friedle SA, Brautigam VM, Nikodemova M, Wright ML, Watters JJ.The P2X7-Egr pathway regulates nucleotide-dependent inflammatory gene expression in microglia. GLIA 2011; 59: 1–13. 10.1002/glia.21071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Miller CM, Zakrzewski AM, Ikin RJ, Boulter NR, Katrib M, Lees MP, et al. Dysregulation of the inflammatory response to the parasite, Toxoplasma gondii, in P2X7 receptor-deficient mice. Int. J. Parasitol. 2011; 41: 301–308. 10.1016/j.ijpara.2010.10.001 [DOI] [PubMed] [Google Scholar]
- 21. Jamieson SE, Peixoto-Rangel AL, Hargrave AC, Roubaix LA, Mui EJ, Boulter NR, et al. Evidence for associations between the purinergic receptor P2X7 [P2RX7] and toxoplasmosis. Genes and Immunity 2010; 11: 374–383. 10.1038/gene.2010.31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Heiss K, Jänner N, Mähnss B, Schumacher V, Koch-Nolte F, Haag F, et al. High sensitivity of intestinal CD8+ T cells to nucleotides indicates P2X7 as a regulator for intestinal T cell responses. J. Immunol. 2008; 181: 3861–3869. [DOI] [PubMed] [Google Scholar]
- 23. Atarashi K, Nishimura J, Shima T, Umesaki Y, Yamamoto M, Onoue M, et al. ATP drives lamina propria TH17 cell differentiation. Nature 2008; 455: 808–812. 10.1038/nature07240 [DOI] [PubMed] [Google Scholar]
- 24. Hofman P, Cherfils-Vicini J, Bazin M, Ilie M, Hébuterne X, Gilson E, et al. Genetic and pharmacological inactivation of the purinergic P2RX7 receptor dampens inflammation but increases tumor incidence in a mouse model of colitis-associated cancer. Cancer Res. 2015; 75:835–45. 10.1158/0008-5472.CAN-14-1778 [DOI] [PubMed] [Google Scholar]
- 25. Haas SL, Ruether A, Singer MV, Schreiber S, Böcker U. Functional P2X7 receptor polymorphisms (His155Tyr, Arg307Gln, Glu496Ala) in patients with Crohn’s Disease. Scand. J. Immunol. 2006; 65: 166–170. [DOI] [PubMed] [Google Scholar]
- 26. Sibley LD, Adams LB, Fukutomi Y, Krahenbuhl JL. Tumor necrosis factor-alpha triggers antitoxoplasmal activity of IFN-gamma primed macrophages. J. Immunol, 1991; 147: 2340–5. [PubMed] [Google Scholar]
- 27. Gazzinelli RT, Brezin A, Li Q, Nussenblatt RB, Chan CC. Toxoplasma gondii: acquired ocular toxoplasmosis in the murine model, protective role of TNF-alpha and IFN-gamma. Exp. Parasitol, 1994; 78: 217–29. [DOI] [PubMed] [Google Scholar]
- 28. Vossenkämper A, Struck D, Alvarado-Esquivel C, Went T, Takeda K, Akira S, et al. Both IL-12 and IL-18 contribute to small intestinal Th1-type immunopathology following oral infection with Toxoplasma gondii, but IL-12 is dominant over IL-18 in parasite control. Eur. J. Immunol, 2004; 34: 3197–207. [DOI] [PubMed] [Google Scholar]
- 29. Gaddi PJ, Yap GS.Cytokine regulation of immunopathology in toxoplasmosis. Immunol Cell Biol, 2007; 85: 155–9. [DOI] [PubMed] [Google Scholar]
- 30. Ewald SE, Chavarria-Smith J, Boothroyd JC.NLRP1 is an inflammasome sensor for Toxoplasma gondii . Infect. Immun. 2014; 82: 460–468. 10.1128/IAI.01170-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chardes T, Velge-Roussel F, Mevelec P, Mevelec MN, Buzoni-Gatel D, Bout D.Mucosal and systemic cellular immune responses induced by Toxoplasma gondii antigens in cyst orally infected mice. Immunology 1993; 78: 421–429. [PMC free article] [PubMed] [Google Scholar]
- 32. Brenier-Pinchart MP, Pelloux H, Simon J, Ricard J, Bosson JL, Ambroise-Thomas P. Toxoplasma gondii induces the secretion of monocyte chemotactic protein-1 in human fibroblasts, in vitro. Mol Cell Biochem, 2000; 209: 79–87. [DOI] [PubMed] [Google Scholar]
- 33. Gorfu G, Cirelli KM, Melo MB, Mayer-Narber K, Crown D, Koller BH, et al. Dual role for inflammasome sensors NLRP1 and NLRP3 in murine resistance to Toxoplasma gondii . mBio 2014; 5: e01117–13. 10.1128/mBio.01117-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Johnson RM, Kerr MS, Slaven JE.Plac8-dependent and inducible NO synthase-dependent mechanisms clear Chlamydia muridarum infections from the genital tract. J. Immunol. 2012; 188: 1896–1904. 10.4049/jimmunol.1102764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Amrouche-Mekkioui I, Djerdjouri B.N-acetylcysteine improves redox status, mitochondrial dysfunction, mucin-depleted crypts and epithelial hyperplasia in dextran sulfate sodium-induced oxidative colitis in mice. Eur. J. Pharmacol. 2012; 691: 2019–217. [DOI] [PubMed] [Google Scholar]
- 36. Zhong H, May MJ, Jimi E, Ghosh S.The phosphorylation status of nuclear NFκB determines its association with CBP/p300 or HDAC-1. Molecular Cell 2002; 9: 625–636. [DOI] [PubMed] [Google Scholar]
- 37. Viatour P, Merville M-P, Bours V, Chariot A.Phosphorylation of NF-kappaB and IkappaB proteins: implications in cancer and inflammation. Trends in Biochemical Sciences 2005; 30: 43–52 [DOI] [PubMed] [Google Scholar]
- 38. Lenertz LY, Gavala ML, Hill LM, Bertics PJ.Cell signaling via the P2X[7] nucleotide receptor: linkage to ROS production, gene transcription, and receptor trafficking. Purinergic Signal 2009; 5: 175–87. 10.1007/s11302-009-9133-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gregg B, Taylor BC, Beena J, Tait-Wojno ED, Girgis NM, Miller N, et al. Replication and distribution of Toxoplasma gondii in the small intestine after oral infection with tissue cysts. Infect. Immun. 2013; 81: 1635–1643. 10.1128/IAI.01126-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Boyle JP, Saeij JPJ, Boothroyd JC. Toxoplasma gondii: inconsistent dissemination patterns following oral infection in mice. Exp. Parasitol. 2007; 116: 302–305. [DOI] [PubMed] [Google Scholar]
- 41. Correa G, Marques da Silva C, De Abreu Moreira-Souza AC, Vommaro RC, Coutinho-Silva R.Activation of the P2X(7) receptor triggers the elimination of Toxoplasma gondii tachyzoites from infected macrophages. Microbes Infect. 2010; 12: 497–504. 10.1016/j.micinf.2010.03.004 [DOI] [PubMed] [Google Scholar]
- 42. Craven M, Egan CE, Dowd SE, McDonough SP, Dogan B, Denkers EY, et al. Inflammation drives dysbiosis and bacterial invasion in murine models of ileal Crohn’s disease. PLoS ONE 2012. 7: e41594 10.1371/journal.pone.0041594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Heimesaat MM, Bereswill S, Fischer A, Fuchs D, Struck D, Niebergall J, et al. Gram-negative bacteria aggravate murine small intestinal Th1-type immunopathology. J. Immunol. 2006; 177: 8785–8795. [DOI] [PubMed] [Google Scholar]
- 44. Hand TW, Dos Santos LM, Bouladoux N, Molloy MJ, Pagán AJ, Pepper M, et al. Acute gastrointestinal infection induces long-lived microbiota-specific T cell responses. Science 2012; 337: 1553–1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Egan CE, Sukhumavasi W, Butcher BA, and Denkers EY. Functional aspects of TLR-MyD88 signaling during protozoan infection: focus on Toxoplasma gondii . Clin. Exp. Immunol. 2009; 156: 17–24. 10.1111/j.1365-2249.2009.03876.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ferrari D, Chiozzi P, Falzoni S, Dal Susino M, Melchiorri L, Baricordi OR, et al. Extracellular ATP triggers IL-1 beta release by activating the purinergic P2Z receptor of human macrophages. J. Immunol. 1997; 159: 1451–1458. [PubMed] [Google Scholar]
- 47. Solle M, Labasi L, Perregaux DG, Stam E, Petrushova N, Koller BH, et al. Altered cytokine production in mice lacking P2X(7) receptors. J. Biol. Chem. 2001; 276: 125–132. [DOI] [PubMed] [Google Scholar]
- 48. Sluyter R, Shemon AN, Wiley JS. Glu496 to Ala polymorphism in the P2X7 receptor impairs ATP-induced IL-1 beta release from human monocytes. J. Immunol. 2004; 172: 3399–3405. [DOI] [PubMed] [Google Scholar]
- 49. Sluyter R, Dalitz JG, Wiley JS. P2X7 receptor polymorphism impairs extracellular adenosine 5’-triphosphate-induced interleukin-18 release from human monocytes. Genes Immun. 2004; 5: 588–591. [DOI] [PubMed] [Google Scholar]
- 50. Wiley JS, Sluyter R, Gu BJ, Stokes L, Fuller SJ. The human P2X7 receptor and its role in innate immunity. Tissue Antigens 2011; 78: 321–332. 10.1111/j.1399-0039.2011.01780.x [DOI] [PubMed] [Google Scholar]
- 51. Trabanelli S, Ocadlikova D, Gulinelli S, Curti A, Salvestrini V, Vieira RP, et al. Extracellular ATP exerts opposite effects on activated and regulatory CD4+ T cells via purinergic P2 receptor activation. J. Immunol. 2012; 189: 1303–1310. 10.4049/jimmunol.1103800 [DOI] [PubMed] [Google Scholar]
- 52. Oldenhove G, Bouladoux N, Wohlfert EA, Hall JA, Chou D, Dos Santos L, et al. Decrease of Foxp3+ Treg cell number and acquisition of effector cell phenotype during lethal infection. Immunity 2009; 31: 772–786. 10.1016/j.immuni.2009.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Brautigam VM, Frasier C, Nikodemova M, Watters JJ. Purinergic receptor modulation of BV-2 microglial cell activity: potential involvement of p38 MAP kinase and CREB. J. Neuroimmunol, 2005; 166: 113–25. [DOI] [PubMed] [Google Scholar]
- 54. Gavala ML, Pfeiffer ZA, Bertics PJ. The nucleotide receptor P2RX7 mediates ATP-induced CREB activation in human and murine monocytic cells. J. Leukoc. Biol, 2008; 84: 1159–71. 10.1189/jlb.0907612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Wen AY, Sakamoto KM, Miller LS. The role of the transcription factor CREB in immune function. J. Immunol, 2010; 185: 6413–6419. 10.4049/jimmunol.1001829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Bachstetter AD, Van Eldik LJ. The p38 MAP kinase family as regulators of proinflammatory cytokine production in degenerative diseases of the CNS. Aging Dis. 2010; 1: 199–211. [PMC free article] [PubMed] [Google Scholar]
- 57. Gorska MM, Liang Q, Stafford SJ, Goplen N, Dharajiya N, Guo L, et al. MK2 controls the level of negative feedback in the NF-kappaB pathway and is essential for vascular permeability and airway inflammation. J. Exp. Med. 2007; 204: 1637–1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Ananieva O, Darragh J, Johansen C, Carr M, McIlrath J, Park JM, et al. The kinases MSK1 and MSK2 act as negative regulators of Toll-like receptor signalling. Nat. Immunol. 2008; 9: 1028–1036. 10.1038/ni.1644 [DOI] [PubMed] [Google Scholar]
- 59. Adriouch S, Dox C, Welge V, Seman M, Koch-Nolte F, Haag F. Cutting Edge: A Natural P451L Mutation in the Cytoplasmic Domain Impairs the Function of the Mouse P2X7 Receptor. J. Immunol. 2002; 169: 4108–4112. [DOI] [PubMed] [Google Scholar]
- 60. Melgar S, Karlsson A, Michaelsson E. Acute colitis induced by dextran sulfate sodium progresses to chronicity in C57BL/6 but not in BALB/c mice: correlation between symptoms and inflammation. Am. J. Physiol. Gastrointest. Liver Physiol, 2005; 288: G1328–38. [DOI] [PubMed] [Google Scholar]
- 61. Buffet PA, Sulahian A, Garin YJF, Nassar N, Derouin F. Culture microtitration: a sensitive method for quantifying Leishmania infantum in tissues of infected mice. Antimicrob. Agents Chemother. 1995; 39: 2167–2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Pfefferkorn ER, Pfefferkorn LC. Toxoplasma gondii: isolation and preliminary characterization of temperature-sensitive mutants. Exp. Parasitol, 1976; 39: 365–76. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.