Abstract
Acquired tracheoesophageal fistula (TEF) is a challenging, life threatening condition. It most commonly appears in critically ill patients requiring prolonged mechanical ventilation, who cannot withstand open neck or chest surgery. An endoscopic technique could be better tolerated by these patients. We present our experience using a cardiac Amplatzer ASD septal occluder for an endoscopic TEF repair in ventilation-dependent patients. Two high risk patients underwent the procedure under general anesthesia and close respiratory monitoring. In one patient the device was inserted through the trachea and in the other through the esophagus. In both cases fistula closure was achieved for different periods of time allowing the patients a temporary relief of symptoms. The procedure was well tolerated by the patients, and no significant adverse effect documented. The technique was successful as a temporary solution for unstable patients with TEFs and should be considered as a treatment modality for similar patients.
Keywords: Tracheoesophageal fistula, Amplatzer septal occluder, Mechanical ventilation
Introduction
Tracheoesophageal fistula (TEF) may be congenital or acquired, associated with either malignant or nonmalignant conditions. The most common etiology of acquired nonmalignant TEF is necrotizing tracheitis due to prolonged intubation or tracheostomy, usually with overinflated (pressure > 20 cm H2O) cuff, causing mucosal ischemia. Necrosis of the tracheal wall and neighboring esophagus may be substantial especially when nasogastric tube occupies the esophageal lumen [1]. Other factors associated with increased risk for TEF formation include excessive tube mobility, respiratory or esophageal infection, poor nutrition, advanced age, diabetes, anemia, hypotension and steroid treatment [2].
TEF is an uncommon life threatening condition, affecting less than 0.5 % of all patients undergoing mechanical ventilation [2]. Most of the fistulas are located at the posterior tracheal wall on the level of the mid tracheal third. Symptoms are mainly respiratory infections due to recurrent aspirations, sepsis, excessive secretions and cough, air leak around the cuffed tube and gastric distention. When one suspects TEF, contrast radiography is helpful in most cases. Computed tomography may be performed to identify the fistula, the surrounding soft tissue and essential structures. Bronchoscopy and esophagoscopy allow direct visualization yet small fistulas might not be noticed [1].
Spontaneous healing of the TEF rarely occurs, and often surgical closure is indicated. Timing of surgery is best scheduled after the patient is weaned of mechanical ventilation, when infections are controlled and dietary condition is optimal. Unfortunately most of the patients suffering from post intubation TEF do not meet the conditions required for successful surgical repair. Patient with severe comorbidities that require prolonged mechanical ventilation have low success rate and high mortality after surgery. Conservative treatment, including restraining oral feeding, antibiotics and dietary support may help stabilize the patients until surgery could be considered. Even then, saliva and gastric fluids may continuously leak into the respiratory tract and cause further inflammation.
Over the years some minimally invasive procedures have been attempted to treat TEF. Chemical cautery was found helpful in promoting local fibrosis and scar tissue formation and facilitated closure of small fistulas. There are reports on endoscopic repair using endoclips, metal or non-metal stents and suturing. The use of fibrin glue was found effective in occluding small fistulas. Injection of bioplastique, glutaraldehyde, crosslinked collagen and calcium hydroxyapatite directly into the fistula tract was tried with variable success [3]. Fogarty catheter and nasal septal button were suggested for temporary mechanical obliteration of the fistula.
Amplatzer septal occluder was previously used for the closure of tracheoesophageal, bronchopulmonary and broncho-neo-esophageal fistulas [4–10]. The present report describes the use of this device for endoscopic repair in mechanically ventilated patients.
Materials and Methods
Endoscopic fistula obliteration using an Amplatzer ASD occluder was practiced in two mechanically ventilated patients. Both participant patients were diagnosed with TEF following prolonged intubation (Fig. 1). The two suffered from severe illness and poor general condition and surgical intervention was prohibited. The procedure was performed at the operation room, under general anesthesia and under close respiratory monitoring. Prior to the procedure the patients’ legal guardians gave their informed consent.
Fig. 1.
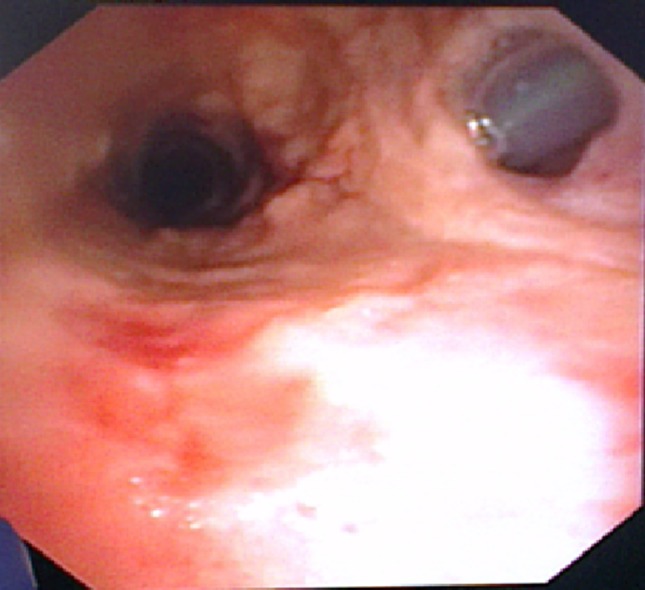
Case 1 Flexible bronchoscopy showing a fistula between the posterior tracheal wall and the esophagus. A nasogastric tube is seen inside the esophagus lumen
In the first patients, a Cobra 4F catheter with a glide wire was inserted via a rigid esophagoscope and passed through the fistula into the tracheal lumen with simultaneous observation by flexible endoscope. The wire was replaced with a long 300 cm rigid wire and the catheter replaced by a measuring balloon no. 18. The balloon was inflated under oblique contrast radiography and 4.5 mm fistula diameter was measured. A long sleeve delivery system was advanced over the wire and Amplatzer ASD occluder no. 6 inserted. After verifying the device’s location with direct endoscopic observation and fluoroscopy, the distal disc was opened in the trachea and the proximal disk in the esophagus across the fistula. At the end of the procedure, the device seemed well adhered to the margins, the fistula seemed to be occluded and there was no evident leak.
In the second patient, we used a flexible bronchoscope, which was introduced intranasally. Using a delivery system no. 7, an exchange wire no. 35 was passed through the tracheostomy and an Amplatzer ASD septal occluder no. 12 device was introduced. After verifying the correct location of the device, the distal disc was opened on the esophageal side, the proximal disc was opened on the tracheal side and stability across the fistula was verified before disconnection from the delivery system. The location of the device was verified using flexible bronchoscopy, serving also to view the esophageal side (Fig. 2). Surgical feeding gastrostomy was performed as well. At the end of the procedure the patient was well ventilated with no evidence of air or secretions leakage.
Fig. 2.
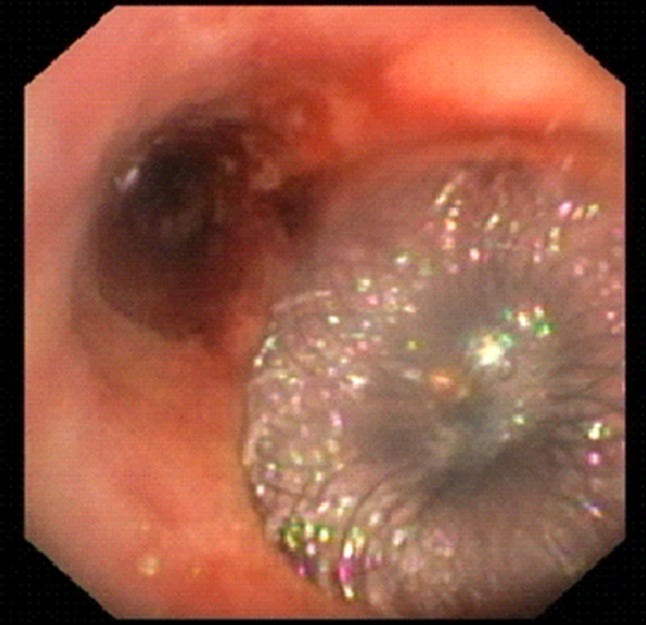
Case 2 Flexible bronchoscopy showing the Amplatzer ASD occluder seated within the fistula, with the proximal disc dilated in the tracheal lumen
Results
In the first patient, during the following 6 months, the patient was well ventilated through a tracheostomy tube and was orally fed, in a home care setting. Then, following aspiration pneumonia, feeding was diverted to gastrostomy (PEG), without further attempt to obliterate the fistula. Four years later, the patient’s general condition remains stable. She is continuously ventilated, is being fed through gastrostomy, and when oral feeding is attempted there is a leak through the tracheostomy tube.
In the second patient, 2 weeks following the procedure, signs reoccurred and flexible bronchoscopy demonstrated dislocation of the occluder. At that time the patient was critically ill with fungal septicemia and passed away a few days later.
Discussion
Acquired TEF is a challenging, life threatening condition. It most commonly appears in critically ill patients requiring prolonged mechanical ventilation, who cannot withstand open neck or chest surgery. An endoscopic minimally invasive approach offers fistula closure, albeit temporary, and allows some relief.
In both presented cases, the patients were high-risk, ventilation-dependent patients. During the intervention a close anesthesiologic monitoring was required, with several breaks throughout the procedure to allow improved oxygenation and stabilization.
The Amplatzer ASD occluder is designed for transcatheter cardiac atrial septal defect closure. The device is made of nitinol mesh with two discs connected with a smaller diameter waist. When used in cardiac catheterization, the waist is placed inside the defect and the discs anchored from both sides. A polyester fabric inside the mesh facilitates occlusion and endothelial growth over the device by 3 months.
The Amplatzer occluder, originally introduced intravenously and monitored by fluoroscopy, can be inserted either through the trachea or via the esophagus. Concomitant esophagoscopy and bronchoscopy allows direct visualization and good control of the devise insertion, thus fluoroscopy is not really necessary. In the reported cases both insertion approaches were used. In two out of three of the previously reported cases, an endotracheal approach was taken [4–6]. In the closure of a broncho-neo-esophageal fistula, a transesophageal approach was preferred [7]. We found the bronchoscopic approach safer and more accessible. The rigid trachea, opposed to the collapsed esophageal lumen, allows easier instrumentation and lower risk for perforation. By intermittent deflation of the tube balloon we could easily advance the wire and guide, and use bronchoscopy forceps, without compromising ventilation. Obviously careful respiratory monitoring is warranted, with assured cooperation with the anesthesiologist.
The occluder is available in different sizes. The central waist diameter ranges from 4 to 40 mm, and the respective distal and proximal disk diameters are 14 and 10 mm. Matching the correct diameter to the size of the fistula is important, as overclosure may enlarge the fistula. In our first patient a no. 6 devise was used to occlude a 4.5 mm diameter fistula. In the second patient, a no. 12 occluder was used for a 10 mm diameter fistula. After disc release on both sides of the defect, the device seemed well anchored to the wide fistula margins. It provided immediate effective closure of the defect, and a temporary relief, which for the first patient (6 months) was crucial to allow home care: proper ventilation, minimizing further bronchopulmonary injury and even oral feeding. This patient demonstrates that with proper care TEF may be a chronic condition, even in a ventilated patient.
While in the heart there is endothelial cover by 3 month, the reports on bronchopulmonary fistula occlusion describe granulation tissue and mucosa covering the surface of the disc after 1–6 months [4, 8–10]. In the few previous reports on TEF occlusion, on two occasions epithelization was evident 3–4 months after the procedure [4, 6] and in one case the device had migrated 2 months later into the bronchial tree and fistula was enlarged [5]. In our experience, no epithelization of the disc surface was evident after 6 months in patient 1, and follow up was too brief in the second case.
The technique described requires simple manipulations and was well tolerated by the patients. It may provide unstable patients a temporary relief of TEF symptoms and with experience gained with more patients it may offer a permanent solution.
Acknowledgments
The assistance and cooperation of Dr Sherman, Dr Zuckerman and Dr Rosen (Departments of Anesthesiology, Chest Surgery and Surgery A) is gratefully acknowledged. The excellent assistance of the Operating Room nurses enabled the efficient and safe implementation of the procedures.
Abbreviations
- TEF
Tracheoesophageal fistula
References
- 1.Mooty RC, Rath P, Self M, et al. Review of tracheo-esophageal fistula associated with endotracheal intubation. J Surg Educ. 2007;64:237–240. doi: 10.1016/j.jsurg.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 2.Payne DK, Anderson WM, Romero MD, et al. Tracheoesophageal fistula formation in intubated patients: risk factors and treatment with high-frequency jet ventilation. Chest. 1990;98:161–164. doi: 10.1378/chest.98.1.161. [DOI] [PubMed] [Google Scholar]
- 3.Kasbeker AV, Sherman IW. Closure of minor tracheoesophageal fistulae with calcium hydroxlapatite. Aurius Nasus Larynx. 2013;40:491–492. doi: 10.1016/j.anl.2013.01.008. [DOI] [PubMed] [Google Scholar]
- 4.Sxordamaglio PR, Tedde ML, Minamoto H, et al. Endoscopic treatment of tracheobronchial tree fistulas using atrial septal defect occluders: preliminary results. J Bras Pneumol. 2009;35:1156–1160. doi: 10.1590/S1806-37132009001100015. [DOI] [PubMed] [Google Scholar]
- 5.Coppola F, et al. Cardiac septal umbrella for closure of a tracheoesophageal fistula. Endoscopy. 2010;42:E318–E319. doi: 10.1055/s-0030-1255822. [DOI] [PubMed] [Google Scholar]
- 6.Repici A, Presbitero P, Carlino A, et al. First human case of esophagus-tracheal fistula closure by using a cardiac septal occluder. Gastrointest Endosc. 2010;71:867–869. doi: 10.1016/j.gie.2009.08.036. [DOI] [PubMed] [Google Scholar]
- 7.Green DA, Moskowitz WB, Shepherd RW. Closure of a broncho-to-neoesophageal fistula using an Amplatzer Septal Occluder device. Ann Thorac Surg. 2010;89:2010–2012. doi: 10.1016/j.athoracsur.2009.11.046. [DOI] [PubMed] [Google Scholar]
- 8.Boudoulas KD, Elinoff J, Resae JR. Bronchopulmonary fistula closure with an Amplatzer Multi-Fenestrated Septal Occluder. Catheter Cardiovasc Interv. 2010;75:455–458. doi: 10.1002/ccd.22258. [DOI] [PubMed] [Google Scholar]
- 9.Kramer MR, Peled N, Shitrit D, et al. Use of Amplatzer device for endobronchial closure of bronchopleural fistulas. Chest. 2008;133:1481–1484. doi: 10.1378/chest.07-1961. [DOI] [PubMed] [Google Scholar]
- 10.Tedde ML, Scordamaglio PR, Minamoto H, Figueiredo VR, Pedra CC, Jatene FB. Endobronchial closure of total bronchopleural fistula with Occlutech Figulla ASD N device. Ann Thorac Surg. 2009;88:e25–e26. doi: 10.1016/j.athoracsur.2009.06.069. [DOI] [PubMed] [Google Scholar]


