Abstract
Background
The mitochondrial Na+/Ca2+ exchanger, NCLX, plays an important role in the balance between Ca2+ influx and efflux across the mitochondrial inner membrane in endothelial cells. Mitochondrial metabolism is likely to be affected by the activity of NCLX because Ca2+ activates several enzymes of the Krebs cycle. It is currently believed that mitochondria are not only centers of energy production but are also important sites of reactive oxygen species (ROS) generation and nucleotide-binding oligomerization domain receptor 3 (NLRP3) inflammasome activation.
Methods & Results
This study focused on NCLX function, in rat aortic endothelial cells (RAECs), induced by glucose. First, we detected an increase in NCLX expression in the endothelia of rats with diabetes mellitus, which was induced by an injection of streptozotocin. Next, colocalization of NCLX expression and mitochondria was detected using confocal analysis. Suppression of NCLX expression, using an siRNA construct (siNCLX), enhanced mitochondrial Ca2+ influx and blocked efflux induced by glucose. Unexpectedly, silencing of NCLX expression induced increased ROS generation and NLRP3 inflammasome activation.
Conclusions
These findings suggest that NCLX affects glucose-dependent mitochondrial Ca2+ signaling, thereby regulating ROS generation and NLRP3 inflammasome activation in high glucose conditions. In the early stages of high glucose stimulation, NCLX expression increases to compensate in order to self-protect mitochondrial maintenance, stability, and function in endothelial cells.
Keywords: Calcium ion, NCLX, Mitochondria, NLRP3 inflammasome, Reactive oxygen species
1. Introduction
Diabetes mellitus is a leading cause of death globally and a chronic disability, resulting in enormous social and economic burden. Angiopathy is a major contributor to the progression of diabetes and its associated complications.[1] While the pathophysiology of diabetic endothelial dysfunction is incompletely characterized, it seems to be multifactorial. In the past few decades, hyperglycemia-induced oxidative stress has been increasingly identified as a trait of diabetic vasculature.[2],[3] Mitochondria are well known as storehouses for intracellular calcium, a source of reactive oxygen species (ROS), and a sensor of oxidative stress.[4], [5] Furthermore, mitochondrial Ca2+([Ca2+]mito) can be dynamically changed upon stimulation by various ligands, for example, during high glucose (HG) conditions, [Ca2+]mito plays an important role in regulating mitochondrial as well as cellular functions, such as oxidative stress,[6] inflammasome activation[7], [8] and apoptosis.[9] Recent findings suggest that many stimuli engage Ca2+ signaling as an intermediate step to trigger mitochondrial instability, and result in generating mitochondrion-associated ligands to activate the NLRP3 inflammasome.[10] Thus, it is essential to fully understand how glucose-dependent [Ca2+]mito is regulated and how it affects mitochondrial and cellular functions in endothelial cells.
Ca2+ enters the mitochondria mainly via a Ca2+ selective channel and the Ca2+ uniporter, and this is driven by a large negative membrane potential.[11]–[13] [Ca2+]mito is extruded by the Na+-Ca2+exchanger and/or H+-Ca2+ exchanger.[14],[15] Although the activity of the mitochondrial Na+/Ca2+ exchanger was discovered more than 40 years ago, its molecular identity and mechanisms had remained unclear, until 2009 researchers found that NCLX protein (previously known as NCKX6) is a long-searched-for mitochondrial Na+/Ca2+ exchanger, which appears to be a gene product of mammalian SLC8B1.[16], [17]
The amplitude and duration of [Ca2+]mito elevations reflect the balance between uptake and release mechanisms.[18] Prolonged accumulation of [Ca2+]mito in the matrix space can lead to mitochondrial [Ca2+]mito overload, resulting in the outbreak of oxidative stress and the activation of cell death signals.[5],[19] To avoid this, mitochondria possess two membrane systems to extrude Ca2+: the Na+-Ca2+exchanger and the H+-Ca2+ exchanger. However, De Marchi, et al.[20] found that NCLX protein, but not the H+-Ca2+ exchanger, mainly mediated [Ca2+]mito extrusion. These new findings present novel opportunities to study the contributions of [Ca2+]mito to cellular and systemic activity in health and disease.
In this study, we found an increase in the amount of the mitochondrial Na+/Ca2+ exchanger, NCLX, in rats with endothelial diabetes mellitus, which is induced by injection of streptozotocin. Next, we examined whether NCLX expression modulates oxidative stress in HG conditions. Suppression of NCLX expression, using a siRNA construct (siNCLX), enhanced [Ca2+]mito influx and blocked efflux induced by glucose. Unexpectedly, silencing of NCLX expression induced ROS generation and increased activation of the NLRP3 inflammasome.
2. Methods
2.1. Reagents
Cell culture media, supplements, MitoTracker® and Lipofectamine™ RNAiMAX were obtained from Invitrogen. Primary antibodies were obtained from Sigma-Aldrich [anti-actin mouse monoclonal antibody (mAb)], Proteintech [anti-NCLX rabbit polyclonal antibody (pAb)], Abcam (anti-Caspase-1 rabbit pAb, anti-NLRP3 rabbit pAb) and Santa Cruz [anti-PECAM-1 (CD31) mouse mAb]. Secondary antibodies were obtained from Jackson ImmunoResearch Laboratories. Fluorescein isothiocyanate (FITC) and cyanine 3 (CY3) immunofluorescence secondary antibodies were obtained from Beyotime Institute of Biotechnology. SiGENOME SMARTpool Rat Slc24a6 (Lot#130809) was obtained from Thermo Scientific Dharmacon.
2.2. Animals
Male Wistar rats were intraperitoneally (i.p.) injected with streptozotocin (STZ,65 mg/kg) (Sigma-Aldrich, USA), which was freshly dissolved in 0.9% saline, to induce diabetes mellitus (DM). Rats were considered diabetic if their blood glucose was > 16.7 mmol/L. The rats were anesthetized with an injection of sodium pentobarbital (40 mg/kg, i.p.), and all efforts were made to minimize suffering, after maintaining the DM rat for one month. The descending thoracic aorta was isolated and cleaned from the surrounding adipose and connective tissues. The 0.3 to 0.5 cm long aorta was trapped using Tissue-Tek O.C.T Compound (Sakura, CA, USA) in liquid nitrogen. The tissue sample was then cryo-sectioned.
2.3. Isolation, culture, and transfection of rat aortic endothelial cells
Male Wistar rats were anesthetized with an injection of sodium pentobarbital (40 mg/kg, i.p.), and all efforts were made to minimize the suffering. Briefly, the aorta was isolated from the abdominal cavity of the rat. The aorta was cleaned, opened longitudinally, cut into 0.2 to 0.3 cm sections, and placed with the intimal side down onto Matrigel-coated plates, in growth medium RPMI-1640 containing 15% fetal bovine serum (FBS), 0.5 ng/mL of vascular endothelial growth factor (VEGF) (PeproTech Lot#1107436) and 100 U/mL of penicillin-streptomycin. Rat aortic endothelial cells (RAECs) were incubated in a humidified atmosphere containing 5% CO2, at 37°C. The growth medium RPMI-1640 was added every second day to prevent drying out. After 50−60 h, the pieces were removed and the cells were harvested. The cells were passaged when they reached 90% confluence. The identity of RAECs was confirmed by a positive immunofluorescence staining of platelet endothelial cell adhesion molecule (PECAM-1). RAECs were grown in RPMI-1640 medium supplemented with 10% FBS after a large number of RAECs were harvested. RAECs were transfected for 48 h with siNCLX or siControl using Lipofectamine™ RNAiMAX, according to the manufacturer's reverse transfection protocol. Transfection efficiency was determined using western blot analyses.
2.4. Western blotting analysis
The RAECs were lysed in radioimmunoprecipitation assay (RIPA) buffer [50 mmol/L Tris–HCl (pH 7.6), 5 mmol/L EDTA, 150 mmol/L NaCl, 0.5% NP-40, 0.5% Triton-X-100] containing 1 g/mL leupeptin, 1 g/mL aprotinin, and 1 g/mL antipain, and 0.5 mmol/L phenylmethanesulfonylfluoride (PMSF). Sample protein concentrations were measured using the BCA Protein assay. Next, 50 g of total protein was separated using 8% or 10% SDS–PAGE and then transferred onto a membrane. The membrane was blocked with 5% skimmed milk, probed with a primary antibody overnight at 4°C, and incubated with a horseradish peroxidase-conjugated secondary antibody. Proteins were detected by chemiluminescence using the enhanced chemiluminescence (ECL) reagent (Pierce, Rockford, IL, USA). Densitometry was performed using Quantity One (BIO-RED) analysis software. β-actin was used as the normalization control.
2.5. Measurement of interleukin-1β (IL-1β) concentration
The IL-1β concentration of the medium was measured using a colorimetric sandwich ELISA kit (IL-1β Quantikines ELISA kit) from R&D systems according to the manufacturer's instructions. The absorbance was measured at 490 nm using a microplate reader.
2.6. Measurement of [Ca2+]mito using laser confocal microscopy
Changes in [Ca2+]mito were detected through incubation for 30 min, at 37°C in the dark, with the Ca2+ probe Rhod-2 AM (Invitrogen) at a final concentration of 4 µmol/L. After incubation, the cells were washed twice with Hanks' Balanced Salt Solution (with Ca2+&Mg2+) (Beyotime Institute of Biotechnology) to remove the probes, and were analyzed using laser confocal microscopy. Fluorescence images were captured using a laser confocal system mounted on an inverted microscope equipped with an argon–krypton laser. Rhod-2 AM fluorescence (excitation at 559 nm) was detected at a wavelength of 577 nm.[21] In this glucose dependent experiment, the cells were pre-washed for 30 min with low glucose (5.56 mmol/L) containing HBSS, followed by HG (35 mmol/L) containing HBSS. The specific [Ca2+]mito line-scanning method was performed over a single horizontal position consisting of 160 lines at 5 s intervals. The Ca2+ level was expressed as a pseudo-ratio value (F/F0) of the actual fluorescence intensity (F) divided by the basal intensity of the [Ca2+]mito at rest (F0). F0 was calculated as the average value obtained during the 50–60 s prior to stimulus application.[22]
2.7. Measurement of total intracellular ROS levels
Changes in intracellular ROS levels were detected using the oxidant-sensing fluorescent probe: dichlorofluorescin diacetate (DCFDA), in the DCFDA Cellular ROS Detection Assay Kit (ab113851). This probe was loaded into previously subcultured RAECs, at a final concentration of 25 µmol/L, and the cells were then cultured for 45 min at 37°C, in the dark. After incubation, the culture medium was washed twice with a 1× buffer solution to remove the dyes, and analyzed using laser confocal microscopy. An excitation wavelength of 488 nm (argon laser) and an emission wavelength of 519 nm were used. The ROS line-scanning method was performed over a single horizontal position consisting of 50 lines at 10 s intervals. Changes in ROS levels were expressed as a pseudo-ratio value (F/F0) of the actual fluorescence intensity (F) divided by the basal intensity of the ROS at rest (F0). F0 was calculated as the average value obtained during the 50–60 s prior to stimulus application.[23],[24]
2.8. Statistical analysis
Traces from all the fluorescent imaging experiments were plotted using Olympus Fluoview (ver.2.1b FV1000). Influx of Ca2+ into mitochondria consistently began after stimulation of RAECs with HG. Similarly, Ca2+ efflux out of the mitochondria followed the influx phase, as previously described. Hence, the fluorescent ratio signals were normalized to the average signal obtained at the beginning of the measurements. The influx and efflux rates were calculated as the slope of the linear fit of the fluorescence change during the 30 s following the administered HG.[16]
Data were expressed as mean ± SD of at least three independent experiments, using 15–30 cells in each experiment. The statistical significance for all experiments was determined using one-way ANOVA test followed by Bonferroni post-hoc analysis. P < 0.05 was considered statistically significant.
3. Results
3.1. Increased NCLX expression in RAECs by high glucose stimulation
Recently, NCLX was identified as the mitochondrial Na+/Ca2+ exchanger in several cell types.[16], [25]
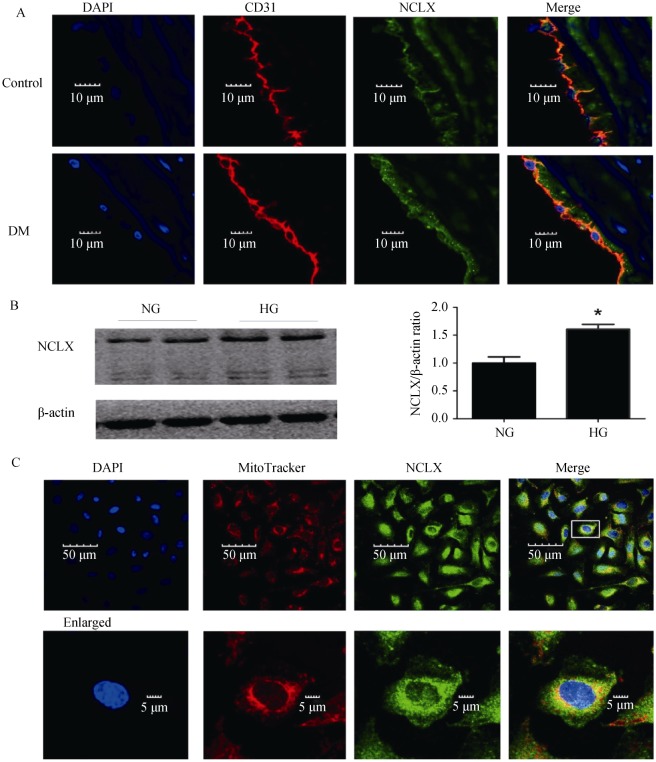
We first investigated whether NCLX was expressed in rat aortic endothelia, using double immunofluorescence labeling. Confocal microscopy analysis was performed on aorta cryosections. PECAM-1(CD31) was used to mark the endothelial level (red) (Figure 1A). Next, we found that NCLX expression was significantly increased in DM RAECs. This was consistent with the in vitro results for the RAECs, which were induced by HG (HG, 35 mmol/L) stimulation. Western blotting analysis (P < 0.05, Figure 1B) detected a major band of 100 kDa and a weaker band at 50 kDa, which correlated to the SDS-stable NCLX. Next, immunofluorescence double staining was performed using MitoTracker®, which indicated that NCLX was expressed in the mitochondria of RAECs[26] (Figure 1C, Pearson's colocalization coefficient = 0.68 ± 0.06, n = 15), a result consistent with previous studies.[27]
Figure 1. The expression and location of NCLX.
(A): NCLX is expressed in rat aortic endothelial and is further increased in DM rats; (B): Western blot analysis of NCLX expression in confluent RAECs which were cultured in medium containing normal glucose (5.5 mmol/L) or high glucose (35 mmol/L) levels; and (C): NCLX (green) and Mito Tracker (red) were co-localized. The nucleus was stained by DAPI (blue). DM: diabetes mellitus; HG: high glucose; NG: normal glucose; RAECs: rat aortic endothelial cells. *P < 0.05.
3.2. The role of NCLX in high glucose-induced [Ca2+]mito transport and ROS formation
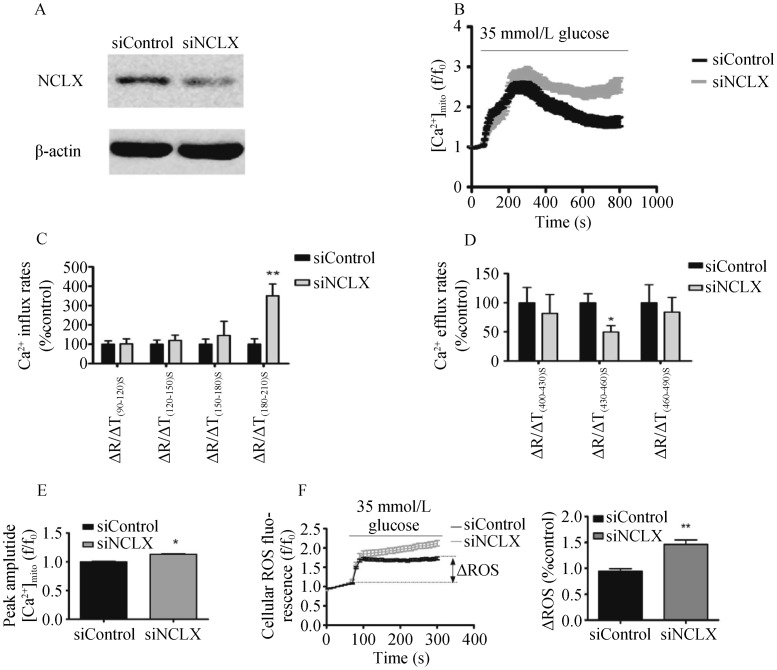
NCLX knockdown using siRNA caused a 47% ± 13.61% reduction in NCLX protein expression (Figure 2A). [Ca2+]mito was monitored in RAECs, which were initially superfused with low glucose (5.5 mmol/L), followed by application of HG (35 mmol/L) with HBSS (with Ca2+ and Mg2+). Application of HG triggered robust mitochondrial Ca2+ transients (Figure 2B). Knockdown of NCLX expression was followed by a time-dependent increase in Ca2+ influx (Figure 2C) and a time-dependent decrease in Ca2+ efflux (Figure 2D). Silencing of NCLX expression blocked glucose-dependent [Ca2+]mito efflux. Comparison of the peak amplitude of [Ca2+]mito signals of siNCLX vs. siControl, using HG stimulation, demonstrated an increase in cells transfected with siNCLX (Figure 2E).
Figure 2. The role of NCLX in high glucose-induced [Ca2+]mito transport and ROS formation.
(A): Western blotting analysis of NCLX expression in siNCLX vs. siControl (50 µg) transfected RAECs; (B): silencing of NCLX expression blocks the glucose dependent mitochondrial Ca2+ efflux; (C): time–dependent average rates of mitochondrial Ca2+ influx after high glucose conditions, n = 15 (PΔT180-210s < 0.01); (D): time–dependent average rates of mitochondrial Ca2+efflux, n = 15 (PΔT430-460s < 0.05); (E): average mitochondrial Ca2+ response peak amplitude, n = 15 (P < 0.05); (F): silencing of NCLX expression makes total intracellular ROS response more severe vs. siControl, by stimulation with high glucose levels (P < 0.01). Data are represented as the mean ± SD, of four independent experiments for each group. RAECs: rat aortic endothelial cells; ROS: reactive oxygen species; siNCLX: siRNA construct. *P < 0.05; **P < 0.01.
Mitochondria are important sites of ROS generation, and numerous studies have confirmed that HG levels generate ROS.[28],[29] Next, we determined the effect of NCLX expression on ROS production. ROS production was markedly increased by HG stimulation, in the siNCLX group (Figure 2F).
3.3. Knockdown of NCLX expression results in activation of the NLRP3 inflammasome
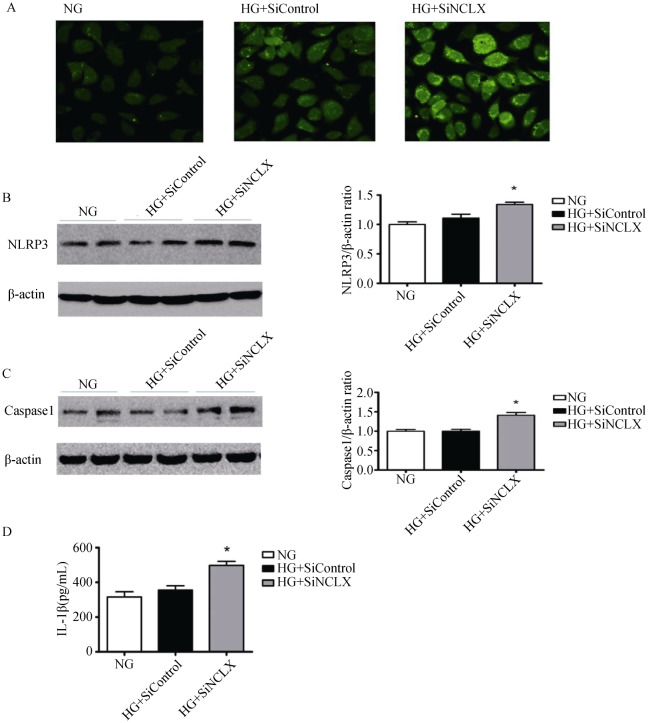
RAECs, which were cultured in medium containing normal glucose (NG), were used as the control group. We first compared the changes in total intracellular ROS production and NLRP3 expression in RAECs treated with siNCLX or siControl, which were cultured in HG (35 mmol/L) for 24 h. Total intracellular ROS production and NLRP3 expression were significantly increased in HG+siNCLX vs. HG+siControl cells (Figure 3 A&B). However, the change between the NG and the HG+siControl group was not notable. We next determined caspase-1 expression and IL-1β release, and there is the same result with NLRP3 expression among the three groups (Figure 3 C&D).
Figure 3. NLRP3 inflammasome activation.
(A): Representative images confocal microscopy for total intracellular ROS production; (B & C): Western blotting analysis of NLRP3 and caspase-1 expression (P < 0.05); (D): ELISA of IL-1β secretion (P < 0.05). HG: high glucose; NG: normal glucose; NLRP3: nucleotide-binding oligomerization domain receptor 3; siNCLX: siRNA construct; ROS: reactive oxygen species. *P < 0.05.
4. Discussion
The major findings of the present study include evidence that demonstrates that the mitochondrial Na+/Ca2+ exchanger, NCLX, is richly expressed in rat aortic endothelial cell mitochondria. NCLX expression in RAECs was increased in HG conditions. Unexpectedly, knockdown of NCLX induced ROS generation and increased activation of the NLRP3 inflammasome. The results of our study demonstrated that NCLX was linked to elevated glucose, ROS generation, and activation of the NLRP3 inflammasome by mediating [Ca2+]mito efflux. Our findings indicated that NCLX may protect against oxidative stress and inflammasome activation in HG conditions at an early stage.
NCLX is a member of the NCX family and is phylogenetically and functionally distinct from other known NCX or NCKX family members.[30],[31] The abbreviated term NCLX stands for Na/Li/Ca exchanger. Palty, et al.[16] found that NCLX can transport either lithium (Li+) or sodium (Na+), in exchange for Ca2+, while the plasma membrane exchangers NCX and NCKX do not transport Li+. Bongju Kim's experiments using EGFP-labeled NCLX in NIH/3T3 fibroblasts suggested that NCLX was exclusively expressed in mitochondria. Our experimental data were not only consistent with these previous studies, but also demonstrated for the first time that NCLX was expressed in endothelial cell mitochondria and was increased by HG stimulation. The balance between Ca2+ influx and efflux across the mitochondrial inner membrane is crucial for establishing Ca 2+ homeostasis within the cell, and therefore, specific Ca2+ transport mechanisms must exist, one of which is the newly identified NCLX. NCLX expression accelerates [Ca2+]mito efflux activity, whereas silencing of NCLX expression attenuates this process. Ca2+ efflux was fully rescued in NCLX-silenced cells by concomitant overexpression of NCLX.[16] The expression of NCLX is essential and sufficient to functionally complement mitochondrial Na+/Ca2+ exchange.[32] Despite the profound effect of NCLX expression on [Ca2+]mito efflux, it did not affect the steady-state resting [Ca2+]mito level.[16] Our results indicated that NCLX plays an important role in [Ca2+]mito responses triggered by HG conditions in endothelial cells. Importantly, knockdown of NCLX expression increased the Ca2+ influx rate, particularly in the late stages of absorption, increased mitochondrial peak Ca2+, and blocked the Ca2+ efflux rate. Therefore, the NCLX Ca2+ extrusion system can prevent long lasting [Ca2+]mito elevations. Our results were consistent with Nita's study using Min6 cells. He also found that knockdown of NCLX expression was followed by a minor increase in resting [Ca2+]mito levels, in the Min6 cells.[32] However, this finding was not detected in our test with RAECs. One possibility is that this may be dependent on cell type, as the tissue distribution of NCLX has a unique pattern (high in the pancreas, skeletal muscle and stomach, and low in the kidney and lung). Further, mitochondria from different tissues exhibit large differences in their permeability to Ca2+.[33]
Importantly, we found that knockdown of NCLX expression increased ROS generation induced by HG stimulation, in compared to the siControl. Mitochondrial metabolism is likely to be affected by the activity of NCLX because Ca2+ activates several enzymes of the Krebs cycle.[34] It is currently thought that mitochondria are not only centers of energy production but also important sites of ROS generation.[35],[36] Stimulation of the tricarboxylic acid cycle and oxidative phosphorylation by Ca2+ enhances ROS generation.[37] On the one hand, calcium overload in mitochondria was the major cause of ROS production.[36],[38] On the other hand, some studies have found ROS production prior to oxidative stress, and that ROS regulated Ca2+ release.[39] In conclusion, there is a complex and close link between [Ca2+]mito and ROS generation. Our results showed that blocking mitochondria Ca2+ efflux resulted in an increase in HG-induced ROS generation. HG-induced apoptosis is highly likely to be due to a time-dependent increase in Ca2+ overload and ROS production in mitochondria.[29] NCLX, by accelerating [Ca2+]mito shuttling, increases the duration of [Ca2+]mito transients and is thus likely to prevent calcium overload in HG conditions. Over-generation of ROS, via the mitochondrial electron transport chain, contributes to diabetic vascular injury.[40]–[42] Thus, in order to compensate for this and for self-protection, the mitochondria maintains its stability and function via an increase in NCLX expression in HG-induced early stages of action.
Ca2+ controls many aspects of mitochondrial biology. Ca2+ mobilization may modulate inflammasome activation by affecting other aspects of mitochondrial function. Several studies have identified that NLRP3 inflammasomes were activated in a manner dependent on Ca2+ signaling.[43] The NLRP3 inflammasome is a sensor of specific pathogen, host, and environmental danger molecules. Upon activation, NLRP3 recruits caspase-1, which cleaves and subsequently activates precursor interleukin-1β (IL-1β) and IL-18 to initiate immune responses. Zhou, et al.[7] first linked mitochondrial damage to activation of the NLRP3 inflammasome. They found that the accumulation of damaged mitochondria, by blocking mitophagy, increased activation of the NLRP3 inflammasome.[7] Mitochondria have been considered as central regulators of NLRP3 function by several recent studies.[7],[10],[44] The mechanisms of NLRP3 activation also included generation of ROS.[45]
Many stimuli engage Ca2+ signaling as an intermediate step to trigger mitochondrial destabilization and this destabilization plays a fundamental role in apoptosis of endothelial cells. Translocation of soluble adenylyl cyclase (sAC) to mitochondria can influence the development of apoptosis by cytochrome C release and caspase-9 cleavage in endothelial cells.[46] Overexpression of Bcl-2 and Bcl-XL prevents the decrease in mitochondrial membrane potential and protects endothelial cells against apoptosis.[47] The cellular mechanisms of the apoptotic and anti-apoptotic actions are complex. Our study demonstrated that silencing of NCLX expression blocked [Ca2+]mito efflux, resulting in an accumulation of damaged mitochondria, subsequent increased ROS generation and activation of the NLRP3 inflammasome, followed by caspase-1 cleavage in endothelial cells. Elevated ROS production was proposed to be a relevant signal of mitochondrial damage. Disruption of the electron transport chain, which resulted in an induction of ROS, was also sufficient to activate the NLRP3 inflammasome and caspase-1 cleavage. Therefore, NCLX localized to mitochondria seem to be an important target in anti-apoptosis in endothelial cells, by regulating [Ca2+]mito in the matrix influence over the NLRP3 inflammasome is conferred.
Overall, NCLX was linked to elevated glucose levels, ROS generation, and activation of the NLRP3 inflammasome. Thus, further studies characterizing the molecular basis and regulatory mechanisms of NCLX will enable a better understanding of the physiological role of this protein on the pathogenesis of diabetic vascular disease. In the future, development of drugs that modulate the activity of NCLX could represent a new strategy for the treatment of diabetes mellitus or its chronic complications.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Grant No. 81173625, 81373458). Thanks for the kind help of Dr. Wang (Pulmonary Division, Boston Children's Hospital, MA, USA), who was extremely helpful in the revision of the language.
References
- 1.Skyler JS, Bergenstal R, Bonow RO, et al. Intensive glycemic control and the prevention of cardiovascular events: implications of the ACCORD, ADVANCE, and VA diabetes trials: a position statement of the American Diabetes Association and a scientific statement of the American College of Cardiology Foundation and the American Heart Association. Circulation. 2009;119:351–357. doi: 10.1161/CIRCULATIONAHA.108.191305. [DOI] [PubMed] [Google Scholar]
- 2.Pennathur S, Heinecke JW. Oxidative stress and endothelial dysfunction in vascular disease. Curr Diab Rep. 2007;7:257–264. doi: 10.1007/s11892-007-0041-3. [DOI] [PubMed] [Google Scholar]
- 3.Choi SW, Benzie IF, Ma SW, et al. Acute hyperglycemia and oxidative stress: direct cause and effect? Free Radic Biol Med. 2008;44:1217–1231. doi: 10.1016/j.freeradbiomed.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 4.Hyun DH, Hunt ND, Emerson SS, et al. Up-regulation of plasma membrane-associated redox activities in neuronal cells lacking functional mitochondria. J Neurochem. 2007;100:1364–1374. doi: 10.1111/j.1471-4159.2006.04411.x. [DOI] [PubMed] [Google Scholar]
- 5.Melov S. Mitochondrial oxidative stress. Physiologic consequences and potential for a role in aging. Ann N Y Acad Sci. 2000;908:219–225. doi: 10.1111/j.1749-6632.2000.tb06649.x. [DOI] [PubMed] [Google Scholar]
- 6.Murphy MP. How mitochondria produce reactive oxygen species. Biochem J. 2009;417:1–13. doi: 10.1042/BJ20081386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou R, Yazdi AS, Menu P, et al. A role for mitochondria in NLRP3 inflammasome activation. Nature. 2011;469:221–225. doi: 10.1038/nature09663. [DOI] [PubMed] [Google Scholar]
- 8.Iyer SS, He Q, Janczy JR, et al. Mitochondrial cardiolipin is required for Nlrp3 inflammasome activation. Immunity. 2013;39:311–323. doi: 10.1016/j.immuni.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xie Y, Liu ZH, Li XY, et al. Protection effect of [Gly14]-Humanin from apoptosis induced by high glucose in human umbilical vein endothelial cells. Diabetes Res Clin Pract. 2014;106:560–566. doi: 10.1016/j.diabres.2014.09.020. [DOI] [PubMed] [Google Scholar]
- 10.Horng T. Calcium signaling and mitochondrial destabilization in the triggering of the NLRP3 inflammasome. Trends Immunol. 2014;35:253–261. doi: 10.1016/j.it.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kirichok Y, Krapivinsky G, Clapham DE. The mitochondrial calcium uniporter is a highly selective ion channel. Nature. 2004;427:360–364. doi: 10.1038/nature02246. [DOI] [PubMed] [Google Scholar]
- 12.Perocchi F, Gohil VM, Girgis HS, et al. MICU1 encodes a mitochondrial EF hand protein required for Ca(2+) uptake. Nature. 2010;467:291–296. doi: 10.1038/nature09358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baughman JM, Perocchi F, Girgis HS, et al. Integrative genomics identifies MCU as an essential component of the mitochondrial calcium uniporter. Nature. 2011;476:341–345. doi: 10.1038/nature10234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Castaldo P, Cataldi M, Magi S, et al. Role of the mitochondrial sodium/calcium exchanger in neuronal physiology and in the pathogenesis of neurological diseases. Prog Neurobiol. 2009;87:58–79. doi: 10.1016/j.pneurobio.2008.09.017. [DOI] [PubMed] [Google Scholar]
- 15.Celsi F, Pizzo P, Brini M, et al. Mitochondria, calcium and cell death: a deadly triad in neurodegeneration. Biochim Biophys Acta. 2009;1787:335–344. doi: 10.1016/j.bbabio.2009.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Palty R, Silverman WF, Hershfinkel M, et al. NCLX is an essential component of mitochondrial Na+/Ca2+ exchange. Proc Natl Acad Sci U S A. 2010;107:436–441. doi: 10.1073/pnas.0908099107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cai X, Lytton J. Molecular cloning of a sixth member of the K+-dependent Na+/Ca2+ exchanger gene family, NCKX6. J Biol Chem. 2004;279:5867–5876. doi: 10.1074/jbc.M310908200. [DOI] [PubMed] [Google Scholar]
- 18.Jean-Quartier C, Bondarenko AI, Alam MR, et al. Studying mitochondrial Ca(2+) uptake - a revisit. Mol Cell Endocrinol. 2012;353:114–127. doi: 10.1016/j.mce.2011.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rasola A, Bernardi P. Mitochondrial permeability transition in Ca(2+)-dependent apoptosis and necrosis. Cell Calcium. 2011;50:222–233. doi: 10.1016/j.ceca.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 20.De Marchi U, Santo-Domingo J, Castelbou C, et al. NCLX protein, but not LETM1, mediates mitochondrial Ca2+ extrusion, thereby limiting Ca2+-induced NAD(P)H production and modulating matrix redox state. J Biol Chem. 2014;289:20377–20385. doi: 10.1074/jbc.M113.540898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.MacGowan GA, Du C, Glonty V, et al. Rhod-2 based measurements of intracellular calcium in the perfused mouse heart: cellular and subcellular localization and response to positive inotropy. J Biomed Opt. 2001;6:23–30. doi: 10.1117/1.1316091. [DOI] [PubMed] [Google Scholar]
- 22.Feng Z, Wei C, Chen X, et al. Essential role of Ca2+ release channels in angiotensin II-induced Ca2+ oscillations and mesangial cell contraction. Kidney Int. 2006;70:130–138. doi: 10.1038/sj.ki.5000342. [DOI] [PubMed] [Google Scholar]
- 23.Puebla C, Farias M, Gonzalez M, et al. High D-glucose reduces SLC29A1 promoter activity and adenosine transport involving specific protein 1 in human umbilical vein endothelium. J Cell Physiol. 2008;215:645–656. doi: 10.1002/jcp.21347. [DOI] [PubMed] [Google Scholar]
- 24.Paauw A, Leverstein-van Hall MA, van Kessel KP, et al. Yersiniabactin reduces the respiratory oxidative stress response of innate immune cells. PLoS One. 2009;4:e8240. doi: 10.1371/journal.pone.0008240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim B, Takeuchi A, Koga O, et al. Pivotal role of mitochondrial Na(+)(-)Ca(2)(+) exchange in antigen receptor mediated Ca(2)(+) signalling in DT40 and A20 B lymphocytes. J Physiol. 2012;590:459–474. doi: 10.1113/jphysiol.2011.222927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kumar S, Flacke JP, Kostin S, et al. SLC4A7 sodium bicarbonate co-transporter controls mitochondrial apoptosis in ischaemic coronary endothelial cells. Cardiovasc Res. 2011;89:392–400. doi: 10.1093/cvr/cvq330. [DOI] [PubMed] [Google Scholar]
- 27.Takeuchi A, Kim B, Matsuoka S. The mitochondrial Na+-Ca2+ exchanger, NCLX, regulates automaticity of HL-1 cardiomyocytes. Sci Rep. 2013;3:2766. doi: 10.1038/srep02766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pelletier M, Lepow TS, Billingham LK, et al. New tricks from an old dog: mitochondrial redox signaling in cellular inflammation. Semin Immunol. 2012;24:384–392. doi: 10.1016/j.smim.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kumar S, Kain V, Sitasawad SL. High glucose-induced Ca2+ overload and oxidative stress contribute to apoptosis of cardiac cells through mitochondrial dependent and independent pathways. Biochim Biophys Acta. 2012;1820:907–920. doi: 10.1016/j.bbagen.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 30.Khananshvili D. The SLC8 gene family of sodium-calcium exchangers (NCX)-structure, function, and regulation in health and disease. Mol Aspects Med. 2013;34:220–235. doi: 10.1016/j.mam.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 31.Boyman L, Williams GS, Khananshvili D, et al. NCLX: the mitochondrial sodium calcium exchanger. J Mol Cell Cardiol. 2013;59:205–213. doi: 10.1016/j.yjmcc.2013.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nita II, Hershfinkel M, Fishman D, et al. The mitochondrial Na+/Ca2+ exchanger upregulates glucose dependent Ca2+ signalling linked to insulin secretion. PLoS One. 2012;7:e46649. doi: 10.1371/journal.pone.0046649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fieni F, Lee SB, Jan YN, et al. Activity of the mitochondrial calcium uniporter varies greatly between tissues. Nat Commun. 2012;3:1317. doi: 10.1038/ncomms2325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Szabadkai G, Duchen MR. Mitochondria: the hub of cellular Ca2+ signaling. Physiology (Bethesda) 2008;23:84–94. doi: 10.1152/physiol.00046.2007. [DOI] [PubMed] [Google Scholar]
- 35.Csordas G, Hajnoczky G. SR/ER-mitochondrial local communication: calcium and ROS. Biochim Biophys Acta. 2009;1787:1352–1362. doi: 10.1016/j.bbabio.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chan SH, Wu KL, Wang LL, et al. Nitric oxide- and superoxide-dependent mitochondrial signaling in endotoxin-induced apoptosis in the rostral ventrolateral medulla of rats. Free Radic Biol Med. 2005;39:603–618. doi: 10.1016/j.freeradbiomed.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 37.Brookes PS, Yoon Y, Robotham JL, et al. Calcium, ATP, and ROS: a mitochondrial love-hate triangle. Am J Physiol Cell Physiol. 2004;287:C817–C833. doi: 10.1152/ajpcell.00139.2004. [DOI] [PubMed] [Google Scholar]
- 38.Dedkova EN, Ji X, Lipsius SL, et al. Mitochondrial calcium uptake stimulates nitric oxide production in mitochondria of bovine vascular endothelial cells. Am J Physiol Cell Physiol. 2004;286:C406–C415. doi: 10.1152/ajpcell.00155.2003. [DOI] [PubMed] [Google Scholar]
- 39.Kim KY, Cho HJ, Yu SN, et al. Interplay of reactive oxygen species, intracellular Ca2+ and mitochondrial homeostasis in the apoptosis of prostate cancer cells by deoxypodophyllotoxin. J Cell Biochem. 2013;114:1124–1134. doi: 10.1002/jcb.24455. [DOI] [PubMed] [Google Scholar]
- 40.Abramov AY, Scorziello A, Duchen MR. Three distinct mechanisms generate oxygen free radicals in neurons and contribute to cell death during anoxia and reoxygenation. J Neurosci. 2007;27:1129–1138. doi: 10.1523/JNEUROSCI.4468-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Forbes JM, Coughlan MT, Cooper ME. Oxidative stress as a major culprit in kidney disease in diabetes. Diabetes. 2008;57:1446–1454. doi: 10.2337/db08-0057. [DOI] [PubMed] [Google Scholar]
- 42.Zheng Z, Chen H, Ke G, et al. Protective effect of perindopril on diabetic retinopathy is associated with decreased vascular endothelial growth factor-to-pigment epithelium-derived factor ratio: involvement of a mitochondria-reactive oxygen species pathway. Diabetes. 2009;58:954–964. doi: 10.2337/db07-1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Triantafilou K, Hughes TR, Triantafilou M, et al. The complement membrane attack complex triggers intracellular Ca2+ fluxes leading to NLRP3 inflammasome activation. J Cell Sci. 2013;126:2903–2913. doi: 10.1242/jcs.124388. [DOI] [PubMed] [Google Scholar]
- 44.Lawlor KE, Vince JE. Ambiguities in NLRP3 inflammasome regulation: is there a role for mitochondria? Biochim Biophys Acta. 2014;1840:1433–1440. doi: 10.1016/j.bbagen.2013.08.014. [DOI] [PubMed] [Google Scholar]
- 45.Dostert C, Petrilli V, Van Bruggen R, et al. Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica. Science. 2008;320:674–677. doi: 10.1126/science.1156995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kumar S, Kostin S, Flacke JP, et al. Soluble adenylyl cyclase controls mitochondria-dependent apoptosis in coronary endothelial cells. J Biol Chem. 2009;284:14760–14768. doi: 10.1074/jbc.M900925200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Flacke JP, Kumar S, Kostin S, et al. Acidic preconditioning protects endothelial cells against apoptosis through p38- and Akt-dependent Bcl-xL overexpression. Apoptosis. 2009;14:90–96. doi: 10.1007/s10495-008-0287-5. [DOI] [PMC free article] [PubMed] [Google Scholar]