Abstract
Heart failure (HF) with preserved ejection fraction (HFpEF) is the most common form of HF in older adults, and is increasing in prevalence as the population ages. Furthermore, HFpEF is increasing out of proportion to HF with reduced EF (HFrEF), and its prognosis is worsening while that of HFrEF is improving. Despite the importance of HFpEF, our understanding of its pathophysiology is incomplete, and optimal treatment remains largely undefined. A cardinal feature of HFpEF is reduced exercise tolerance, which correlates with symptoms as well as reduced quality of life. The traditional concepts of exercise limitations have focused on central dysfunction related to poor cardiac pump function. However, the mechanisms are not exclusive to the heart and lungs, and the understanding of the pathophysiology of this disease has evolved. Substantial attention has focused on defining the central versus peripheral mechanisms underlying the reduced functional capacity and exercise tolerance among patients with HF. In fact, physical training can improve exercise tolerance via peripheral adaptive mechanisms even in the absence of favorable central hemodynamic function. In addition, the drug trials performed to date in HFpEF that have focused on influencing cardiovascular function have not improved exercise capacity. This suggests that peripheral limitations may play a significant role in HF limiting exercise tolerance, a hallmark feature of HFpEF.
Keywords: Exercise intolerance, Heart failure, Peripheral limitations, Skeletal muscle
1. Introduction
Heart failure (HF) with preserved ejection fraction (HFpEF) is the predominant form of HF in older adults, and is increasing in prevalence as the overall population ages.[1] Although the long-term mortality in HFpEF is similar to HF with reduced EF (HFrEF), guideline based medications that improve survival in HFrEF have not been successful in reducing mortality in HFpEF patients.[2]–[7]
This syndrome was historically considered to be caused exclusively by left ventricular (LV) diastolic dysfunction. However, recent data from multiple sources indicating that even in well-characterized, symptomatic HFpEF, many patients do not have echo-Doppler indexes of diastolic dysfunction that differ greatly from that expected based on age and comorbidities.[8],[9] These findings suggested that abnormalities of intrinsic diastolic function may not always be present during or completely explain the occurrence of HFpEF.[10] In acknowledgement of these considerations, as well as data supporting a broader paradigm for HFpEF pathophysiology and outcomes, the 2013 American College of Cardiology/American Heart Association (ACC/AHA) HF management guideline takes a practical, approach to HFpEF. It states that the diagnosis of HFpEF is based on: (1) typical symptoms and signs of HF; (2) normal or near normal LVEF; and (3) no other obvious factors to account for the apparent HF symptoms, including significant valvular abnormalities.[11]
Substantial attention has focused on defining the central versus peripheral mechanisms underlying the reduced functional capacity and symptoms among patients with HF. Numerous prior studies have investigated the physiological mechanisms underlining the reduced exercise intolerance in patients with HFrEF,[12]–[14] however much less is known regarding its mechanisms in patients with HFpEF. In this review, we will summarize the current understanding of the pathophysiology of exercise intolerance and how peripheral limitations, including skeletal muscle, contribute to exercise intolerance in HFpEF patients.
2. Epidemiology of HFpEF
HFpEF is the most common form of HF in older adults. The annual incidence of HF in both men and women doubles with every decade increase in age after age 65, and the prevalence of HF increases from less than 0.5% in the age group of 20–39 years to more than 10% in those 80 years of age and older.[1] Elderly persons have a substantial risk for death after a diagnosis of HF, and a normal LVEF does not ensure a favorable outcome, (Figure 1).[15] Although the adjusted mortality risk was greatest in participants with HFrEF, only a minority of community-based elderly persons were in this category.[15] Outcomes following hospitalization for decompensated HFpEF are quite poor, with over 1/3 of patients dead or rehospitalized within 60–90 days of discharge.[16]
Figure 1. Survival of patients in the cardiovascular health study.
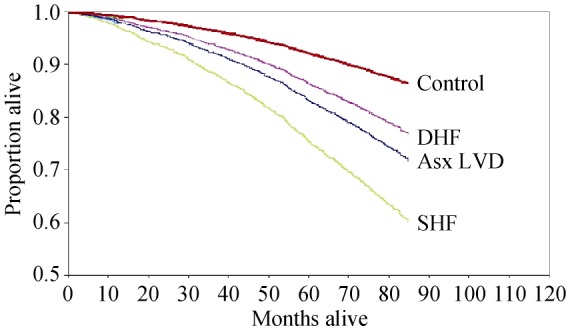
Control: nested case controls without heart failure; DHF: heart failure with a normal ejection fraction; Asx LVD: reduced ejection fraction with no symptoms of heart failure; SHF: systolic heart failure. Modified from: Gottdiener, et al.[15]
3. Pathophysiology of exercise intolerance
The primary chronic symptom in patients with HFpEF, even when well compensated, is severe exercise intolerance, which can be measured objectively as decreased peak oxygen consumed during maximal effort exercise (peak VO2), and is a strong determinant of prognosis and reduced quality of life.[17],[18]
According to the Fick equation, VO2 is equal to the product of cardiac output (CO) and arterial–venous oxygen content difference (A-VO2 Diff); therefore, the reduced peak VO2 in patients with HFpEF may be caused by decreased CO or by decreased oxygen delivery to or impaired oxygen utilization by the exercising skeletal muscles. Early studies suggested that the reduced peak VO2 in HFpEF patients was primarily due to reduced CO secondary to an inability to increase end-diastolic volume and stroke volume via the Frank-Starling mechanism.[19] However, this study had a very small number of patients, only four of whom would be considered typical HFpEF by current criteria. Further, in that study, there was a trend toward reduced “calculated” A-VO2 Diff in HFpEF. Later, other investigators found that the blunted CO was secondary to chronotropic incompetence,[20],[21] impaired systolic reserve function and vasodilator reserve,[21] or abnormal ventricular -vascular coupling.[22] In contrast, others have found that the reduced peak VO2 is due to reductions in both peak CO and “calculated” A-VO2 Diff [19],[23],[24] or primarily due to reduced peak A-VO2 Diff secondary to impaired skeletal muscle oxidative metabolism.[25] Although peak VO2 has been observed to correlate with both changes in CO and A-VO2 Diff with exercise in patients with HFpEF, recent studies have reported that peak A-VO2 Diff or the change in A-VO2 Diff from rest to peak exercise is the strongest independent predictor of peak VO2.[20],[23],[26] Reduced peak heart rate (chronotropic incompetence) was present in the HFpEF patients and contributed to reduced CO, however there was no difference in stroke volume response compared to healthy age-matched controls. Moreover, Haykowsky, et al.[27] found that improved peak “calculated” A-VO2 Diff accounted for the nearly all of the improvement in peak VO2 following exercise training with no significant improvement in CO. Similarly, a full year of training in 12 invasively studied HFpEF patients failed to alter cardiac compliance or improve ventricular-arterial coupling.[28] In a recent updated and more comprehensive meta-analysis of six randomized controlled trials of exercise training in patients with HFpEF revealed exercise training improved peak VO2 and quality of life without any significant change in resting diastolic or systolic function.[29] Accordingly, impaired skeletal muscle O2 extraction may be an important factor limiting exercise tolerance in HFpEF.
Importantly, the finding of increased peak A-VO2 Diff indicates that after exercise training there was an improvement in either diffusive oxygen transport via improved peripheral vascular, microvascular function and/or skeletal muscle adaptations that increase diffusive oxygen transport and/or improvements in oxygen extraction by skeletal muscle.[28],[30],[31]
4. Impaired arterial function
In healthy older adults, the 11-fold increase in blood flow to the active muscles during peak cycle exercise is caused by sympathetic-mediated redistribution of blood from non-exercising regions to the working muscles coupled with metabolic-mediated vasodilation in the exercising muscles.[32],[33] Normal aging is associated with significant alterations in peripheral arterial blood flow responses at rest and after a variety of stressors, including exercise.[34]–[37] Changes in central and peripheral arterial function may result in inefficient distribution of CO to the active muscles and contribute to exercise intolerance in patients with HFpEF.[17]
Conduit artery (aorta and large artery) stiffening occurs as part of the normal aging process which can be accentuated by many of the diseases associated with HFpEF. Both aortic distensibility[38] and carotid artery distensibility[39] are severely reduced in elderly HFpEF patients and correlate with their degree of exercise intolerance and objectively measured peak exercise VO2. Puntawangkoon, et al.[40] found that post-exercise submaximal exercise leg blood flow was reduced in older HF patients versus healthy controls. They also indicated that older HF patients have reduced leg blood flow with exercise beyond that which is associated with normal aging.
Impaired peripheral arterial endothelial function may result in impaired exercise blood flow reserve in patients with HFpEF. Using phase-contrast magnetic resonance imaging (superficial femoral artery), Hundley, et al.[41] showed that resting and flow-mediated increases in leg blood flow in elderly HFpEF patients are not significantly impaired and are similar to those of age-matched healthy subjects. Haykowsky, et al[42] using high resolution brachial artery ultrasound to assess flow-mediated dilation and healthy age matched controls, found no reduction in endothelial function in HFpEF patients who were free of clinically significant coronary, cerebrovascular, and peripheral arterial disease. Similarly, in elderly HFpEF patients, 16 weeks of endurance exercise training improved peak VO2 without altering endothelial function or arterial stiffness.[43] In a recent pilot study, four weeks of exercise training in HFpEF patients significantly improved VO2 without affecting endothelial function assessed by brachial artery flow-mediated dilation.[44] This suggests that large vessel endothelial dysfunction may not be an inherent feature of HFpEF. An important feature of these studies was exclusion of patients with any evidence of clinical atherosclerosis, which is known to independently reduce endothelial function.
However, flow-mediated vasodilation in large conduit arteries (e.g., femoral) may differ from that observed in the microvasculature. Microvascular endothelial dysfunction as measured by digital artery tonometry was impaired in HFpEF compared with controls and correlated with reduced exercise capacity and greater symptoms.[21] Similarly in another study microvascular endothelial dysfunction was an independent predictor of poorer prognosis, mainly readmission, in patients with HFpEF.[45] A consequence of the blunted microvascular reserve is that it may be associated with decreased diffusive oxygen transport to the active muscle, which would reduce exercise tolerance. Recently in an autopsy-based study, Mohammed et al.[8] reported reduced microvascular density in HFpEF patients which was independent of coronary artery disease and hypertension and in adjusted analyses appeared to account for the increased fibrosis. Their findings suggest that co morbidities other than hypertension may perpetuate microvascular rarefaction.[8] Advanced age and common HFpEF comorbidities such as obesity, systemic hypertension and diabetes mellitus have been shown to be associated with coronary microvascular dysfunction.[46],[47] This supports an over-arching hypothesis for HFpEF pathogenesis as originally proposed by Paulus: a systemic pro-inflammatory state that results in systemic arterial and microvascular dysfunction.[48] Indeed peripheral endothelial dysfunction might impair matching of perfusion to regional demand in skeletal muscle microcirculation.[49]
5. Role of skeletal muscle in exercise intolerance
After delivery of O2 to skeletal muscle, O2 utilization is dependent on the pathway consisting of skeletal muscle tissue microcirculatory O2 exchange vessels and muscle units. Decreased AVO2D diff may suggests a potential role of impaired skeletal muscle vasodilatory capacity in small resistance vessels. Moreover In healthy individuals, there is a net increase in level of O2 extraction relative to O2 delivery during exercise.[50] This is indicated by an exercise-related fall in O2 levels in venous blood, consistent with increased utilization of O2 by respiring mitochondria relative to the rate of increase in O2 delivery.[50] It is known that in conditions in which there is a defect in oxygen utilization, such as mitochondrial myopathies, the peak VO2 is depressed despite normal cardiac function.[51]
Esposito and colleagues have demonstrated that HFrEF severely reduces muscle oxygen diffusion conductance and this may also account for poor muscle function and exercise intolerance.[49],[52] It is well known that in HFrEF every facet of the O2 transport pathway is compromised, which can explain the premature fatigue in this condition.[49] In addition, morphologic and histochemical changes in skeletal muscle have been described in HFrEF, including marked abnormalities in skeletal muscle mass, density, fiber type, oxidative metabolism, mitochondrial mass, and mitochondrial function.[53]–[57] The multinational SICA-HF study found that muscle wasting is a frequent co-morbidity among patients with chronic HFrEF and associated with worse exercise capacity.[58]
As most of these studies have been performed in patients with HFrEF, the specific changes of skeletal muscle in patients with HFpEF were limited.
6. Skeletal muscle mass, oxygen utilization and exercise intolerance in HFpEF
Using dual energy X-ray absorptiometry, Haykowsky and colleagues found percent body fat and percent leg fat were significantly increased, whereas percent body lean and leg lean mass were significantly reduced, in older HFpEF patients versus healthy controls.[59] Moreover, the slope of the relation of peak VO2 with percent leg lean mass was markedly reduced in the HFpEF versus healthy control group. These investigators extended these results by directly characterizing thigh muscle composition using phase-contrast MRI, which showed abnormal fat infiltration into the thigh skeletal muscle and that this was associated with reduced peak exercise VO2 in HFpEF (Figure 2).[60]
Figure 2. Magnetic resonance imaging axial image of the mid-thigh in a patient with HFpEF and HC.
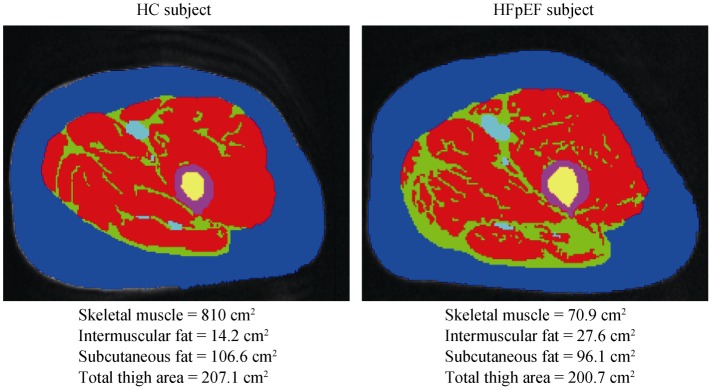
Red = skeletal muscle; green = IMF; blue = subcutaneous fat; purple = femoral cortex; yellow = femoral medulla. IMF (green) is substantially increased in the patient with HFpEF compared with the HC despite similar subcutaneous fat. HC: healthy controls; HFpEF: heart failure with preserved ejection fraction; IMF: intermuscular fat.
In adipose tissue, either adipocytes directly or infiltrating macrophages produces pro-inflammatory cytokines,[61] and these cytokines have direct catabolic effects on skeletal muscle. Thus, a pro-inflammatory state may be one of the key factors in creating a vicious cycle of decreased muscle strength among older adults. Moreover, it has been hypothesized that muscle fat infiltration causes insulin resistance in obese individuals.[62],[63] Insulin resistance promotes muscle catabolism, mitochondrial dysfunction, and impairs protein synthesis in skeletal muscle. Heinonen, et al,[64] using positron emission tomography, found that adipose tissue blood flow adjacent to the active muscles increased sevenfold during continuous isometric knee-extension exercise in non-obese younger healthy sedentary women. Thus, increased thigh intermuscular fat in older patients with HFpEF may “steal” blood that would normally be delivered to the active muscles during exercise thereby reducing perfusive oxygen delivery to the thigh muscle. Thus, fatty infiltration of skeletal muscle is associated with reduced strength[65],[66] and functional status,[67] muscle dysfunction,[66] decreased contractility,[66] and reduced mitochondrial mass, biogenesis, oxidative metabolism.[68] Indeed, using phosphate-31 magnetic resonance spectroscopy during and after performing static leg lifts, they revealed impaired skeletal muscle oxidative metabolism in patients with HFpEF.[25]
Together, these findings support the concept that altered skeletal muscle composition (remodeling) and poor “quality” of skeletal muscle may contribute to the reduced peak VO2 found in older HFpEF patients.
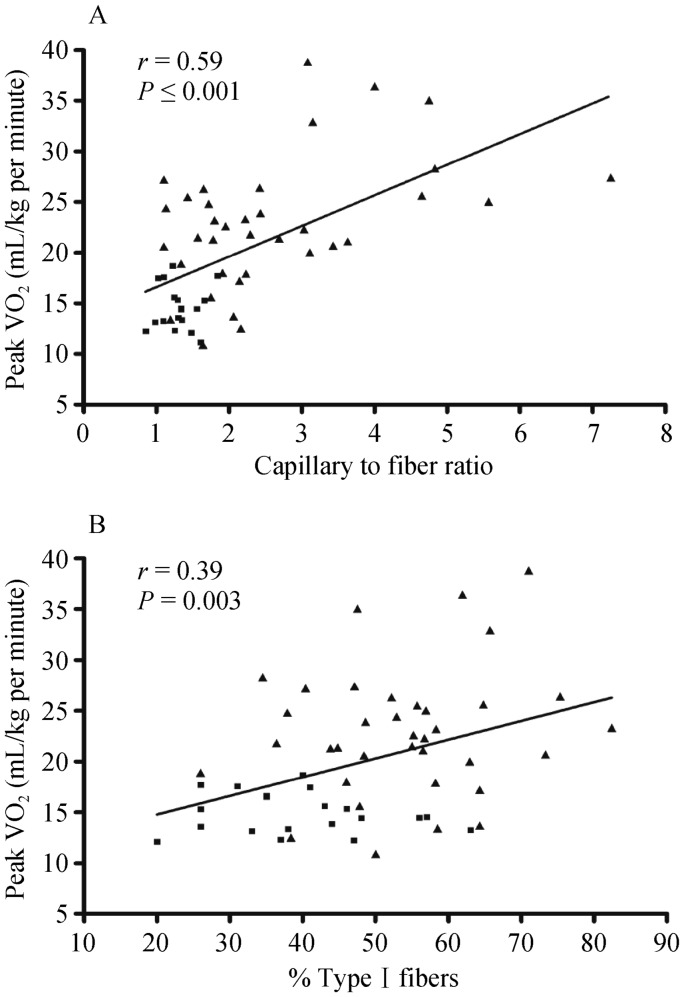
Kitzman, et al.[69] further showed compared with HC subjects, older HFpEF patients had a shift in skeletal muscle fiber type distribution with a reduced percentage of slow twitch type I fibers and reduced type I-to-type-II fiber ratio and reduced capillary-to-fiber ratio. Furthermore, both the capillary-to-fiber ratio and percentage of type I fibers were significant, independent predictors of peak VO2 (Figure 3). A reduction in the percentage of type I fibers could be associated with reduced oxidative capacity and mitochondrial density and thereby contribute to the reduced peak VO2 in HFpEF. The reduction in blood flow to exercising muscle may lead to greater reliance on anaerobic glycolysis, predisposing to earlier exhaustion. The pattern of altered skeletal muscle fiber type and capillary-to-fiber ratio that observed in elderly HFpEF patients is strikingly similar to that reported by others in HFrEF patients,[54],[70]–[72] and the fiber type alteration is dissimilar to that seen with aging alone.[73] This parallels with a recent systematic autopsy-based study, that showed HFpEF patients had reduced microvascular density in cardiac muscle.[8] Therefore, the reduced capillary-to-fiber ratio in HFpEF patients would be expected to result in a decreased diffusive capacity for O2 transport to active skeletal muscle during exercise and limit exercise capacity.[49]
Figure 3. Relationship of capillary-to-fiber ratio (A) and percentage of type I muscle fibers (B) with peak O2 uptake (VO2) in older patients with heart failure with preserved ejection fraction (▪) and age-matched healthy control subjects (▴).
Potential causes for the skeletal muscle abnormalities in HFpEF patients might include neuroendocrine activation, sympathetic overdrive, oxidative stress, inflammation, abnormal Ca2+ cycling and excitation-contraction coupling, and deconditioning[74] (though skeletal muscle dysfunction has been shown to occur in HFrEF in the absence of deconditioning).[75]
7. Impact of aging, frailty and comorbidities
Aging is associated with a progressive decline in exercise capacity and decreased physiological reserve in cardiovascular function as well as in most other organ systems. Aging is associated with a decline in a variety of neural, hormonal and environmental trophic signals to muscle that can result in loss of muscle mass and mass-specific strength.[76]–[78] This can also contribute to aging associated characteristic changes in body composition, including decreases in lean body mass and muscle strength, and increases in adiposity.[79]–[81] In addition, aging is associated with a systemic pro inflammatory state, and associated with increased levels of cytokines,[82]–[85] that may lead to a functional decline in multiple organs even in absence of a specific disease.[86]
The majority of older HFpEF patients have multiple comorbidities and high proportions are frail.[87],[88] The adverse impacts of aging, frailty and comorbidities on functional capacity and clinical outcomes are cumulative and synergistic.[88] This synergy may be mediated in large part by the reduction in physical activity that accompanies each condition. Muscle atrophy leads to reduction in metabolic rate both at rest and during physical activity, thus further aggravating the sedentary state, all of which can cause obesity. Approximately 85% of elderly HFpEF patients are overweight or obese, and the HFpEF epidemic has largely paralleled the obesity epidemic.[89] Obesity has a similar pathophysiological burden on skeletal muscle with aging, including inflammation, oxidative stress, and insulin resistance.[62],[90]
Furthermore, aging and obesity, which are well established, risk factors for both HFpEF and several common respiratory diseases [like chronic obstructive lung disease (COPD)]. In addition, COPD occurs in approximately one-third of HF patients, with a slightly higher prevalence in HFpEF patients compared with HFrEF patients.[91] Moreover, patients with preserved EF do not have the alternative diagnosis of low EF; they may be more likely to receive a COPD diagnosis as an explanation for dyspnea.[92],[93] Interestingly, in a recent pilot study, lung function abnormalities are seen among 94% in patients with HFpEF, in that cohort, 93% of patients with a restrictive ventilatory abnormality were overweight (BMI > 25 kg/m2).[94] Thus, these lung functional abnormalities can be due to either HFpEF itself and/or to the presence of concomitant comorbid respiratory diseases.
It is noteworthy that patients with multiple comorbidities have often been actively excluded from clinical HF studies, thereby producing results that may not be applicable to typical older HFpEF patients.[95] Mounting evidence indicates that in the elderly HFpEF population, non-HF hospitalizations dominate and non-cardiac reasons account for a large proportion of overall deaths.[87] Given such a multi-factorial, complex milieu, it's not surprising that drugs and interventions aimed primarily at a central hemodynamics repeatedly failed to strongly impact overall outcomes in HFpEF.[2]–[7] Given these considerations, what kinds of novel interventions are promising?
8. Therapeutic options and clinical outcomes
Pharmacological trials in HFpEF to improve outcomes and symptoms have been particularly disappointing.[2]–[7] Of the three large randomized trials of angiotensin-converting enzyme inhibitor (ACE-I)/angiotensin II type I receptor blocker (ARB) performed to date in HFpEF, only the CHARM-preserved study found nominal benefit for candesartan in reducing HF hospitalizations over three years of follow-up.[6],[7] I-PRESERVE was a very large, multi-center trial of HFpEF and enrolled 4,128 patients and randomly assigned them to the ARB, irbesartan or placebo. Mortality or rates of hospitalizations for cardiovascular causes were not improved by treatment with an ARB.[2] The Aldo-DHF trial of 12 months treatment of spironolactone aldosterone inhibitor improved some measures of diastolic function, though maximal exercise capacity, clinical symptoms, and quality of life were not changed.[4] The large TOPCAT trial of spironolactone failed to show statistically significant benefit for the clinical composite primary end -point. Similarly, the role of β-blockers remains uncertain and data to date have not been encouraging. Both carvedilol (the J-DHF study) and nebivolol (ELANDD study) had neutral effects on their primary outcomes in HFpEF patients.[96],[97] In the Digitalis Interaction Group trial (DIG), there was a trend noted towards decreased hospitalization and improved exercise tolerance in a subgroup of 988 patients with EF > 45% who were randomized to placebo or to digoxin.[98]
9. Novel pharmacological agents
In a recent RELAX trial, sildenafil did not improve 6-min walk distance or quality of life, and was associated with modest worsening of renal function.[99] The DILATE-1 study showed that riociguat, a soluble guanylate cyclase stimulator, did not have any impact on the primary end -point of peak change in mean pulmonary artery pressure in patients with HFpEF and pulmonary hypertension.[100] Even though observational data in HFpEF patients suggest a mortality benefit with use of HMG-Co-A reductase inhibitors, definitive trials have not been performed yet.[101],[102] In a seven-day study, ivabradine, a selective sinus node If sodium channel inhibitor increased peak VO2 in 61 patients with HFpEF.[103] Compared to valsartan alone, the LCZ696 (Neprilysin, the zinc-dependent metalloprotease that degrades biologically active natriuretic peptides) group had significantly lower NT-pro BNP levels and at 36 weeks, decreased LA size and showed a trend toward improved functional class in PARAMOUNT study.[104] The findings of this phase-2 study are promising and a large, multi-center trial, PARAGON, is underway comparing LCZ696 to valsartan in patients with HFpEF. Serelaxin, a recombinant form of human relaxin-2, administered to acute HF patients, caused in improvement of symptoms with a reduction in 180-day mortality, compared with placebo.[105],[106] In HFpEF patients, treatment with a sitaxsentan sodium selective endothelin type A receptor antagonist appeared to increase exercise time on the treadmill. This agent (as were other endothlelin type A antogonsits) was not beneficial in multiple outcomes trials of HFrEF; it had hepatotoxicity, and has been removed from development. Thus, novel agents tested for HFpEF to date have fared only a little better than the standard agents adapted from treatment of HFrEF.
The most evidenced-based promising way strategy at present to improve exercise intolerance in HFpEF patients appears to be exercise training, but the optimal approach is still unknown. Four months of endurance exercise training increased peak VO2, ventilatory anaerobic threshold, 6-min walk distance, and physical quality-of-life scores in patients with HFpEF.[107] These results were confirmed in a subsequent multicenter study of 64 HFpEF patients randomized to three months of combined exercise training and strength training.[108] In four months of upper and lower extremity endurance exercise training, Kitzman, et al.[43] found a significant increase in peak VO2 without altering carotid arterial stiffness or brachial artery flow mediated dilation in HFpEF patients. Taken together, the few studies performed to date indicate that endurance exercise training is an effective nonpharmacologic therapy that improves clinically stable patients with HFpEF exercise tolerance. In a recent meta-analysis, exercise training improves physical function and quality of life in patients with HFpEF. This improvement appears to occur primarily through non-cardiac mechanisms, such as improved arterial and skeletal muscle function.[29]
Traditional exercise training programs for patients with HFpEF have primarily focused on moderate intensity endurance exercise training. Despite favorable anti-remodeling and quality of life benefits, moderate-intensity training is associated with relatively moderate improvements in peak VO2.[109],[110] A meta-analysis of seven small trials showed that high-intensity aerobic interval training in HFrEF patients was more effective than traditional continuous moderate-intensity exercise in increasing peak VO2 whereas changes in LVEF were not significant.[111] Recently, Angadi, et al.[44] showed that in HFpEF patients four weeks of high intensity interval training significantly improved peak VO2 compared to moderate-intensity aerobic continuous training. Even though this study had a small sample size, it suggests that high intensity interval training might provide a more robust stimulus than moderate-intensity aerobic continuous training for early exercise training adaptations in HFpEF. A randomized multi-center study comparing three months supervised moderate intensity continuous training versus high intensity interval training versus a control group followed by nine months of telemedically monitored home-based training is under way.[112]
Furthermore, the effects of aging, multiple comorbidities, and frailty on the use of exercise training in older HF patients are profound. The marked impairment of aerobic capacity, ambulatory function, strength, and balance often seen in this population presents major challenges to effectively and safely implement exercise training. Progress will likely require innovative multidisciplinary team approaches that recognize the importance of non-cardiac factors.
10. New avenues for HFpEF
In addition, emerging evidence suggests that enhancing nitric oxide bioavailability by beetroot juice or inorganic nitrate supplementation can effectively lower the mitochondrial O2 cost of ATP production, thereby lowering the exercising VO2 requirement.[113] Recently, Zamani, et al.[114] found that a single dose of inorganic nitrate administered before exercise significantly improves peak VO2 in subjects with HFpEF by improving the peripheral response to exercise and by providing greater O2 delivery to exercising muscles. Several current clinical trials are testing novel agents to regenerate skeletal muscle in elderly with multiple comorbidities and sarcopenia; if successful, these could inform new approaches to HFpEF. If HFpEF is triggered by systemic inflammation, then a promising signal is the novel agent LCZ696, an angiotensin receptor neprilysin inhibitor, which is currently being tested in a large clinical trial. This agent appears to reduce tumor necrosis factor-α levels and this correlates with improvements in cardiac features of HFpEF.[115] Another potential signal is that statins may modify systemic inflammation and stabilize endothelium.[116]
Intentional weight loss via caloric restriction has the potential to reduce excess adiposity. However, weight loss is controversial in patients with HF. More recently, a U-shaped curve relating survival to body weight has shown excess mortality at the extremes, morbid obesity and cachexia. These trends are seen in HFpEF as well.[117] Therapeutically, injection of a myostatin-blocking antibody in mice with preexisting HF preserved muscle mass.[118] Thus, myostatin inhibition might be a medically relevant avenue for the treatment of muscle wasting in HF. In a recent randomized trial in patients with HFrEF, growth hormone replacement increased peak VO2 and exercise duration, and improved quality of life.[119] A meta-analysis of modestly sized randomized, placebo-controlled trials showed that testosterone supplementation in patients with HFrEF is associated with an increase of about 54 m on the 6 min walk test, as well as improvements in peak VO2 and NYHA class.[120] However, these hormones also have the potential to increase LV mass, which is already abnormally increased in some HFPEF patients. Thus, these hormones administration requires formal testing specifically in older HFpEF patients.
11. Conclusions
In summary, recent work indicates that peripheral abnormalities contribute significantly to symptoms of exercise intolerance in elderly HFpEF patients. Future therapeutic strategies are needed to improve exercise tolerance by targeting the integrated functions of these systems. This is particularly relevant since skeletal muscle and microvascular function often have greater capacity for regeneration than cardiac muscle. A paradigm shift in our understanding of the mechanisms that may be targeted in HFpEF, and the patients most likely to benefit from these targeted approaches, is needed.
Acknowledgments
This review supported in part by NIH grant R01AG18915, P30AG021332 (Dr. Kitzman). Dr. Kitzman reports the following potential financial conflicts of interest: consulting for GSK, Relypsa, Abbvie, Regeneron; DC Devices; grant support from Novartis; stock ownership in Gilead Sciences and Relypsa. Dr. Upadhya reports the following potential financial conflicts of interest: consulting for Novartis and DC devices. No other members of the writing group have conflicts of interest to declare.
References
- 1.Roger VL, Go AS, Lloyd-Jones DM, et al. Heart disease and stroke statistics—2011 update: a report from the American Heart Association. Circulation. 2011;123:e18–e209. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Massie BM, Carson PE, McMurray JJ, et al. Irbesartan in patients with heart failure and preserved ejection fraction. N Engl J Med. 2008;359:2456–2467. doi: 10.1056/NEJMoa0805450. [DOI] [PubMed] [Google Scholar]
- 3.Kitzman DW, Hundley WG, Brubaker P, et al. A randomized, controlled, double-blinded trial of enalapril in older patients with heart failure and preserved ejection fraction; effects on exercise tolerance, and arterial distensibility. Circ Heart Fail. 2010;3:477–485. doi: 10.1161/CIRCHEARTFAILURE.109.898916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Edelmann F, Aldo-DHF investigators Effect of spironolactone on diastolic function and exercise capacity in patients with heart failure with preserved ejection fraction: the Aldo-DHF randomized controlled trial. JAMA. 2013;309:781–791. doi: 10.1001/jama.2013.905. [DOI] [PubMed] [Google Scholar]
- 5.Pitt B, Pfeffer M, Assmann S, et al. Spironolactone for heart failure with preserved ejection fraction. N Engl J Med. 2014;370:1383–1392. doi: 10.1056/NEJMoa1313731. [DOI] [PubMed] [Google Scholar]
- 6.Cleland JGF, Tendera M, Adamus J, et al. The perindopril in elderly people with chronic heart failure (PEP-CHF) study. Eur Heart J. 2006;27:2338–2345. doi: 10.1093/eurheartj/ehl250. [DOI] [PubMed] [Google Scholar]
- 7.Yusuf S, Pfeffer MA, Swedberg K, et al. Effects of candesartan in patients with chronic heart failure and preserved left-ventricular ejection fraction: the CHARM-Preserved Trial. Lancet. 2003;362:777–781. doi: 10.1016/S0140-6736(03)14285-7. [DOI] [PubMed] [Google Scholar]
- 8.Mohammed SF, Hussain S, Mirzoyev SA, et al. Coronary microvascular rarefaction and myocardial fibrosis in heart failure with preserved ejection fraction. Circulation. 2015;131:550–559. doi: 10.1161/CIRCULATIONAHA.114.009625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kitzman DW, Upadhya B, Vasu S. What the dead can teach the living: the systemic nature of heart failure with preserved ejection fraction. Circulation. 2015;131:522–524. doi: 10.1161/CIRCULATIONAHA.114.014420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burkhoff D, Maurer MS, Packer M. Heart failure with a normal ejection fraction: is it really a disorder of diastolic function? Circulation. 2003;107:656–658. doi: 10.1161/01.cir.0000053947.82595.03. [DOI] [PubMed] [Google Scholar]
- 11.Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA Guideline for the management of heart-failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62:e147–e239. doi: 10.1016/j.jacc.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 12.Wilson JR, Mancini DM, Dunkman WB. Exertional fatigue due to skeletal muscle dysfunction in patients with heart failure. Circulation. 1993;87:470–475. doi: 10.1161/01.cir.87.2.470. [DOI] [PubMed] [Google Scholar]
- 13.Sato A, Hayashi M, Saruta T. Relative long-term effects of spironolactone in conjunction with an angiotensin-converting enzyme inhibitor on left ventricular mass and diastolic function in patients with essential hypertension. Hypertens Res. 2002;25:837–842. doi: 10.1291/hypres.25.837. [DOI] [PubMed] [Google Scholar]
- 14.Esposito F, Mathieu-Costello O, Shabetai R, et al. Limited maximal exercise capacity in patients with chronic heart failure: partitioning the contributors. J Am Coll Cardiol. 2010;55:1945–1954. doi: 10.1016/j.jacc.2009.11.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gottdiener JS, McClelland R, Marshall RJ, et al. Outcome of congestive heart failure in elderly persons: Influence of left ventricular systolic function. The Cardiovascular Health Study. Ann Intern Med. 2002;137:631–639. doi: 10.7326/0003-4819-137-8-200210150-00006. [DOI] [PubMed] [Google Scholar]
- 16.Fonarow GC, Stough WG, Abraham WT, et al. Characteristics, treatments, and outcomes of patients with preserved systolic function hospitalized for heart failure: A report from the OPTIMIZE-HF Registry. J Am Coll Cardiol. 2007;50:768–777. doi: 10.1016/j.jacc.2007.04.064. [DOI] [PubMed] [Google Scholar]
- 17.Haykowsky M, Brubaker P, Kitzman D. Role of physical training in heart failure with preserved ejection fraction. Curr Heart Fail Rep. 2012;9:101–106. doi: 10.1007/s11897-012-0087-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guazzi M, Adams V, Conraads V, et al. Clinical recommendations for cardiopulmonary exercise testing data assessment in specific patient populations. Circulation. 2012;126:2261–2274. doi: 10.1161/CIR.0b013e31826fb946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kitzman DW, Higginbotham MB, Cobb FR, et al. Exercise intolerance in patients with heart failure and preserved left ventricular systolic function: failure of the Frank-Starling mechanism. J Am Coll Cardiol. 1991;17:1065–1072. doi: 10.1016/0735-1097(91)90832-t. [DOI] [PubMed] [Google Scholar]
- 20.Borlaug BA, Melenovsky V, Russell SD, et al. Impaired chronotropic and vasodilator reserves limit exercise capacity in patients with heart failure and a preserved ejection fraction. Circulation. 2006;114:2138–2147. doi: 10.1161/CIRCULATIONAHA.106.632745. [DOI] [PubMed] [Google Scholar]
- 21.Borlaug BA, Olson TP, Lam CSP, et al. Global cardiovascular reserve dysfunction in heart failure with preserved ejection fraction. J Am Coll Cardiol. 2010;56:845–854. doi: 10.1016/j.jacc.2010.03.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kawaguchi M, Hay I, Fetics B, et al. Combined ventricular systolic and arterial stiffening in patients with heart failure and preserved ejection fraction. Circulation. 2003;107:714–720. doi: 10.1161/01.cir.0000048123.22359.a0. [DOI] [PubMed] [Google Scholar]
- 23.Haykowsky MJ, Brubaker PH, John JM, et al. Determinants of exercise intolerance in elderly heart failure patients with preserved ejection fraction. J Am Coll Cardiol. 2011;58:265–274. doi: 10.1016/j.jacc.2011.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maeder MT, Thompson BR, Brunner-La Rocca H-P, et al. Hemodynamic basis of exercise limitation in patients with heart failure and normal ejection fraction. J Am Coll Cardiol. 2010;56:855–863. doi: 10.1016/j.jacc.2010.04.040. [DOI] [PubMed] [Google Scholar]
- 25.Bhella PS, Prasad A, Heinicke K, et al. Abnormal haemodynamic response to exercise in heart failure with preserved ejection fraction. Eur J Heart Fail. 2011;13:1296–1304. doi: 10.1093/eurjhf/hfr133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dhakal BP, Malhotra R, Murphy RM, et al. Mechanisms of exercise intolerance in heart hailure with preserved ejection fraction: the role of abnormal peripheral pxygen extraction. Circ Heart Fail. 2015;8:286–294. doi: 10.1161/CIRCHEARTFAILURE.114.001825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haykowsky MJ, Brubaker PH, Stewart KP, et al. Effect of endurance training on the determinants of peak exercise oxygen consumption in elderly patients with stable compensated heart failure and preserved ejection fraction. J Am Coll Cardiol. 2012;60:120–128. doi: 10.1016/j.jacc.2012.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fujimoto N, Prasad A, Hastings JL, et al. Cardiovascular effects of 1 year of progressive endurance exercise training in patients with heart failure with preserved ejection fraction. Am Heart J. 2012;164:869–877. doi: 10.1016/j.ahj.2012.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pandey A, Parashar A, Kumbhani DJ, et al. Exercise training in patients with heart failure and preserved ejection fraction: meta-analysis of randomized control trials. Circ Heart Fail. 2015;8:33–40. doi: 10.1161/CIRCHEARTFAILURE.114.001615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Erbs S, Hollriegel R, Linke A, et al. Exercise training in patients with advanced chronic heart failure (NYHA IIIb) promotes restoration of peripheral vasomotor function, induction of endogenous regeneration, and improvement of left ventricular function. Circ Heart Fail. 2010;3:486–494. doi: 10.1161/CIRCHEARTFAILURE.109.868992. [DOI] [PubMed] [Google Scholar]
- 31.Tyni-Lenne R, Gordon A, Europe E, et al. Exercise-based rehabilitation improves skeletal muscle capacity, exercise tolerance, and quality of life in both women and men with chronic heart failure. J Card Fail. 1998;4:9–17. doi: 10.1016/s1071-9164(98)90503-6. [DOI] [PubMed] [Google Scholar]
- 32.Beere PA, Russell SD, Morey MC, et al. Aerobic exercise training can reverse age-related peripheral circulatory changes in healthy older men. Circulation. 1999;100:1085–1094. doi: 10.1161/01.cir.100.10.1085. [DOI] [PubMed] [Google Scholar]
- 33.Katz SD, Zheng H. Peripheral limitations of maximal aerobic capacity in patients with chronic heart failure. J Nucl Cardiol. 2002;9:215–225. doi: 10.1067/mnc.2002.123183. [DOI] [PubMed] [Google Scholar]
- 34.Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part I: aging arteries: a “set up” for vascular disease. Circulation. 2003;107:139–146. doi: 10.1161/01.cir.0000048892.83521.58. [DOI] [PubMed] [Google Scholar]
- 35.Leithe ME, Hermiller JB, Magorien RD, et al. The effect of age on central and regional hemodynamics. Gerontology. 1984;30:240–246. doi: 10.1159/000212638. [DOI] [PubMed] [Google Scholar]
- 36.Dinenno FA, Jones PP, Seals DR, et al. Limb blood flow and vascular conductance are reduced with age in healthy humans: relation to elevations in sympathetic nerve activity and declines in oxygen demand. Circulation. 1999;100:164–170. doi: 10.1161/01.cir.100.2.164. [DOI] [PubMed] [Google Scholar]
- 37.Julius S, Amery A, Whitlock LS, et al. Influence of age on the hemodynamic response to exercise. Circulation. 1967;36:222. doi: 10.1161/01.cir.36.2.222. [DOI] [PubMed] [Google Scholar]
- 38.Hundley W, Kitzman D, Morgan T, et al. Cardiac cycle-dependent changes in aortic area and aortic distensibility are reduced in older patients with isolated diastolic heart failure and correlate with exercise intolerance. J Am Coll Cardiol. 2001;38:796–802. doi: 10.1016/s0735-1097(01)01447-4. [DOI] [PubMed] [Google Scholar]
- 39.Kitzman DW, Herrington DM, Brubaker P, et al. Carotid arterial stiffness and its relationship to exercise intolerance in older patients with heart failure and preserved ejection fraction. Hypertension. 2013;61:112–119. doi: 10.1161/HYPERTENSIONAHA.111.00163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Puntawangkoon C, Kitzman D, Kritchevsky S, et al. Reduced peripheral arterial blood flow with preserved cardiac output during submaximal bicycle exercise in elderly heart failure. J Cardiovasc Magn Reson. 2009;11:48. doi: 10.1186/1532-429X-11-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hundley WG, Bayram E, Hamilton CA, et al. Leg flow-mediated arterial dilation in elderly patients with heart failure and normal left ventricular ejection fraction. Am J Physiol Heart Circ Physiol. 2007;292:H1427–H1434. doi: 10.1152/ajpheart.00567.2006. [DOI] [PubMed] [Google Scholar]
- 42.Haykowsky MJ, Herrington DM, Brubaker PH, et al. Relationship of flow mediated arterial dilation and exercise capacity in older patients with heart failure and preserved ejection fraction. J Gerontol A Biol Sci Med Sci. 2013;68:161–167. doi: 10.1093/gerona/gls099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kitzman D, Brubaker P, Herrington D, et al. Effect of endurance exercise training on endothelial function and arterial stiffness in older patients with heart failure and preserved ejection fraction: a randomized, controlled, single-blind trial. J Am Coll Cardiol. 2013;62:584–592. doi: 10.1016/j.jacc.2013.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Angadi SS, Mookadam F, Lee CD, et al. High-intensity interval training vs. moderate-intensity continuous exercise training in heart failure with preserved ejection fraction: a pilot study. J Appl Physiol. doi: 10.1152/japplphysiol.00518.2014. Published Online First: 2014 Sep 4. [DOI] [PubMed] [Google Scholar]
- 45.Matsue Y, Suzuki M, Nagahori W, et al. Endothelial dysfunction measured by peripheral arterial tonometry predicts prognosis in patients with heart failure with preserved ejection fraction. Int J Cardiol. 2013;168:36–40. doi: 10.1016/j.ijcard.2012.09.021. [DOI] [PubMed] [Google Scholar]
- 46.Feihl F, Liaudet L, Waeber B, et al. Hypertension: a disease of the microcirculation? Hypertension. 2006;48:1012–1017. doi: 10.1161/01.HYP.0000249510.20326.72. [DOI] [PubMed] [Google Scholar]
- 47.Boodhwani M, Sodha NR, Mieno S, et al. Functional, cellular, and molecular characterization of the angiogenic response to chronic myocardial ischemia in diabetes. Circulation. 2007;116:I31–I37. doi: 10.1161/CIRCULATIONAHA.106.680157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Paulus W, Tschope C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol. 2013;62:263–271. doi: 10.1016/j.jacc.2013.02.092. [DOI] [PubMed] [Google Scholar]
- 49.Poole DC, Hirai DM, Copp SW, et al. Muscle oxygen transport and utilization in heart failure: implications for exercise (in)tolerance. Am J Physiol Heart Circ Physiol. 2012;302:H1050–H1063. doi: 10.1152/ajpheart.00943.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Taivassalo T, Abbott A, Wyrick P, et al. Venous oxygen levels during aerobic forearm exercise: an index of impaired oxidative metabolism in mitochondrial myopathy. Ann Neurol. 2002;51:38–44. doi: 10.1002/ana.10027. [DOI] [PubMed] [Google Scholar]
- 51.Taivassalo T, Jensen TD, Kennaway N, et al. The spectrum of exercise intolerance in mitochondrial myopathies: a study of 40 patients. Brain. 2013;126:413–423. doi: 10.1093/brain/awg028. [DOI] [PubMed] [Google Scholar]
- 52.Esposito F, Reese V, Shabetai R, et al. Isolated quadriceps training increases maximal exercise capacity in chronic heart failure: the role of skeletal muscle convective and diffusive oxygen transport. J Am Coll Cardiol. 2011;58:1353–1362. doi: 10.1016/j.jacc.2011.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Georgiadou P, Adamopoulos S. Skeletal muscle abnormalities in chronic heart failure. Curr Heart Fail Rep. 2012;9:128–132. doi: 10.1007/s11897-012-0090-z. [DOI] [PubMed] [Google Scholar]
- 54.Sullivan MJ, Green HJ, Cobb FR. Skeletal muscle biochemistry and histology in ambulatory patients with long-term heart failure. Circulation. 1990;81:518–527. doi: 10.1161/01.cir.81.2.518. [DOI] [PubMed] [Google Scholar]
- 55.Drexler H, Riede U, Schaefer HE. Reduced oxidative capacity of skeletal muscle in patients with severe heart failure [abstract] Circulation. 1998;76(Suppl 4) [Google Scholar]
- 56.Drexler H, Riede J, Munzel T, et al. Alterations of skeletal muscle in chronic heart failure. Circulation. 1992;85:1751–1759. doi: 10.1161/01.cir.85.5.1751. [DOI] [PubMed] [Google Scholar]
- 57.Clark AL, Poole-Wilson PA, Coats A. Exercise limitation in chronic heart failure: central role of the periphery. J Am Coll Cardiol. 1996;28:1092–1102. doi: 10.1016/S0735-1097(96)00323-3. [DOI] [PubMed] [Google Scholar]
- 58.Fulster S, Tacke M, Sandek A, et al. Muscle wasting in patients with chronic heart failure: results from the studies investigating co-morbidities aggravating heart failure (SICA-HF) Eur Heart J. 2013;34:512–519. doi: 10.1093/eurheartj/ehs381. [DOI] [PubMed] [Google Scholar]
- 59.Haykowsky M, Brubaker P, Morgan T, et al. Impaired aerobic capacity and physical functional performance in older heart failure patients with preserved ejection fraction: role of lean body mass. J Gerontol A Biol Sci Med Sci. 2013;68:968–975. doi: 10.1093/gerona/glt011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Haykowsky M, Kouba EJ, Brubaker PH, et al. Skeletal muscle composition and its relation to exercise intolerance in older patients with heart failure and preserved ejection fraction. Am J Cardiol. 2014;113:1211–1216. doi: 10.1016/j.amjcard.2013.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fantuzzi G. Adipose tissue, adipokines, and inflammation. J Allergy Clin Immunol. 2005;115:911–919. doi: 10.1016/j.jaci.2005.02.023. [DOI] [PubMed] [Google Scholar]
- 62.Goodpaster FH, Brown FF. Skeletal muscle lipid and its association with insulin resistance: what is the role for exercise? Exerc Sport Sci Rev. 2005;33:150–154. doi: 10.1097/00003677-200507000-00008. [DOI] [PubMed] [Google Scholar]
- 63.Kelley DE, Slasky BS, Janosky J. Skeletal muscle density: effects of obesity and non-insulin-dependent diabetes mellitus. Am J Clin Nutr. 1991;54:509–515. doi: 10.1093/ajcn/54.3.509. [DOI] [PubMed] [Google Scholar]
- 64.Heinonen I, Bucci M, Kemppainen J, et al. Regulation of subcutaneous adipose tissue blood flow during exercise in humans. J Appl Physiol. 2012;112:1059–1063. doi: 10.1152/japplphysiol.00732.2011. [DOI] [PubMed] [Google Scholar]
- 65.Goodpaster BH, Carlson CL, Visser M, et al. Attenuation of skeletal muscle and strength in the elderly: The Health ABC Study. J Appl Physiol. 2001;90:2157–2165. doi: 10.1152/jappl.2001.90.6.2157. [DOI] [PubMed] [Google Scholar]
- 66.Visser M, Kritchevsky SB, Goodpaster BH, et al. Leg muscle mass and composition in relation to lower extremity performance in men and women aged 70 to 79: the health, aging and body composition study. J Am Geriatr Soc. 2002;50:897–904. doi: 10.1046/j.1532-5415.2002.50217.x. [DOI] [PubMed] [Google Scholar]
- 67.Sipila S, Suominen H. Knee extension strength and walking speed in relation to quadriceps muscle composition and training in elderly women. Clin Physiol. 1994;14:433–442. doi: 10.1111/j.1475-097x.1994.tb00402.x. [DOI] [PubMed] [Google Scholar]
- 68.Civitarese AE, Carling S, Heilbronn LK, et al. Calorie restriction increases muscle mitochondrial biogenesis in healthy humans. PLoS Med. 2007;4:e76. doi: 10.1371/journal.pmed.0040076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kitzman DW, Nicklas B, Kraus WE, et al. Skeletal muscle abnormalities and exercise intolerance in older patients with heart failure and preserved ejection fraction. Am J Physiol Heart Circ Physiol. 2014;306:H1364–H1370. doi: 10.1152/ajpheart.00004.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sullivan MJ, Green HJ, Cobb FR. Altered skeletal muscle metabolic response to exercise in chronic heart failure. Relation to skeletal muscle aerobic enzyme activity. Circulation. 1991;84:1597–1607. doi: 10.1161/01.cir.84.4.1597. [DOI] [PubMed] [Google Scholar]
- 71.Sullivan MJ, Duscha BD, Klitgaard H, et al. Altered expression of myosin heavy chain in human skeletal muscle in chronic heart failure. Med Sci Sports Exerc. 1997;29:860–866. doi: 10.1097/00005768-199707000-00004. [DOI] [PubMed] [Google Scholar]
- 72.Toth M, Matthews DE, Ades PA, et al. Skeletal muscle myofibrillar protein metabolism in heart failure: relationship to immune activation and functional capacity. Am J Physiol Endocrinol Metab. 2005;288:E685–E692. doi: 10.1152/ajpendo.00444.2004. [DOI] [PubMed] [Google Scholar]
- 73.Larsson L, Sjodin B, Karlsson J. Histochemical and biochemical changes in human skeletal muscle with age in sedentary males, age 22–65 years. Acta Physiol Scand. 1978;103:31–39. doi: 10.1111/j.1748-1716.1978.tb06187.x. [DOI] [PubMed] [Google Scholar]
- 74.Middlekauff HR. Making the case for skeletal myopathy as the major limitation of exercise capacity in heart failure. Circ Heart Fail. 2010;3:537–546. doi: 10.1161/CIRCHEARTFAILURE.109.903773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.National Heart, Lung, and Blood Institute Report of the Task Force on Research in Heart Failure; Bethesda, USA, National Institutes of Health. 1994.
- 76.Marcell TJ. Sarcopenia: causes, consequences, and preventions. J Gerontol A Biol Sci Med Sci. 2003;58:M911–M916. doi: 10.1093/gerona/58.10.m911. [DOI] [PubMed] [Google Scholar]
- 77.Morley JE, Baumgartner RN, Roubenoff R, et al. Sarcopenia. J Lab Clin Med. 2001;137:231–243. doi: 10.1067/mlc.2001.113504. [DOI] [PubMed] [Google Scholar]
- 78.Roubenoff R. Sarcopenia: effects on body composition and function. J Gerontol A Biol Sci Med Sci. 2003;58:1012–1017. doi: 10.1093/gerona/58.11.m1012. [DOI] [PubMed] [Google Scholar]
- 79.Mazess RB. On aging bone loss. Clin Orthop Relat Res. 1982;165:239–252. [PubMed] [Google Scholar]
- 80.Forbes GB, Halloran E. The adult decline in lean body mass. Hum Biol. 1976;48:161–173. [PubMed] [Google Scholar]
- 81.Larsson L, Karlsson J. Isometric and dynamic endurance as a function of age and skeletal muscle characteristics. Acta Physiol Scand. 1978;104:129–136. doi: 10.1111/j.1748-1716.1978.tb06259.x. [DOI] [PubMed] [Google Scholar]
- 82.Matsubara J, Sugiyama S, Nozaki T, et al. Pentraxin 3 is a new inflammatory marker correlated with left ventricular diastolic dysfunction and heart failure with normal ejection fraction. J Am Coll Cardiol. 2011;57:861–869. doi: 10.1016/j.jacc.2010.10.018. [DOI] [PubMed] [Google Scholar]
- 83.Kalogeropoulos A, Georgiopoulou V, Psaty B, et al. Inflammatory markers and incident heart failure risk in older adults: the Health ABC (Health, Aging, and Body Composition) study. J Am Coll Cardiol. 2010;55:2129–2137. doi: 10.1016/j.jacc.2009.12.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Collier P, Watson C, Voon V, et al. Can emerging biomarkers of myocardial remodelling identify asymptomatic hypertensive patients at risk for diastolic dysfunction and diastolic heart failure? Eur J Heart Fail. 2011;13:1087–1095. doi: 10.1093/eurjhf/hfr079. [DOI] [PubMed] [Google Scholar]
- 85.Shah K, Kop W, Christenson R, et al. Prognostic utility of ST2 in patients with acute dyspnea and preserved left ventricular ejection fraction. Clin Chem. 2011;57:874–882. doi: 10.1373/clinchem.2010.159277. [DOI] [PubMed] [Google Scholar]
- 86.Franceschi C, Bonafe M, Valensin S, et al. Inflamm-aging: An evolutionary perspective on immunosenescence. Ann N Y Acad Sci. 2000;908:244–254. doi: 10.1111/j.1749-6632.2000.tb06651.x. [DOI] [PubMed] [Google Scholar]
- 87.Ather S, Chan W, Bozkurt B, et al. Impact of noncardiac comorbidities on morbidity and mortality in a predominantly male population with heart failure and preserved versus reduced ejection fraction. J Am Coll Cardiol. 2012;59:998–1005. doi: 10.1016/j.jacc.2011.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Murad K, Kitzman D. Frailty and multiple comorbidities in the elderly patient with heart failure: implications for management. Heart Fail Rev. 2011;17:581–588. doi: 10.1007/s10741-011-9258-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ndumele CE, Coresh J, Lazo M, et al. Obesity, subclinical myocardial injury, and incident heart failure. JACC Heart Fail. 2014;2:600–607. doi: 10.1016/j.jchf.2014.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Fontana L, Eagon JC, Trujillo ME, et al. Visceral fat adipokine secretion is associated with systemic inflammation in obese humans. Diabetes. 2007;56:1010–1013. doi: 10.2337/db06-1656. [DOI] [PubMed] [Google Scholar]
- 91.Hawkins NM, Petrie MC, Jhund PS, et al. Heart failure and chronic obstructive pulmonary disease: diagnostic pitfalls and epidemiology. Eur J Heart Fail. 2009;11:130–139. doi: 10.1093/eurjhf/hfn013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mentz RJ, Fiuzat M, Kraft M, et al. Bronchodilators in heart failure patients with COPD: is it time for a clinical trial? J Card Fail. 2012;18:413–422. doi: 10.1016/j.cardfail.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 93.Caruana L, Petrie MC, Davie AP, et al. Do patients with suspected heart failure and preserved left ventricular systolic function suffer from “diastolic heart failure” or from misdiagnosis? A prospective descriptive study. BMJ. 2000;321:215–218. doi: 10.1136/bmj.321.7255.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Andrea R, Lopez-Giraldo A, Falces C, et al. Lung function abnormalities are highly frequent in patients with heart failure and preserved ejection fraction. Heart Lung Circ. 2014;23:273–279. doi: 10.1016/j.hlc.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 95.Braunstein JB, Anderson GF, Gerstenblith G, et al. Noncardiac comorbidity increases preventable hospitalizations and mortality among medicare beneficiaries with chronic heart failure. J Am Coll Cardiol. 2003;42:1226–1233. doi: 10.1016/s0735-1097(03)00947-1. [DOI] [PubMed] [Google Scholar]
- 96.Hummel S, Seymour E, Brook R, et al. Low-sodium dietary approaches to stop hypertension diet reduces blood pressure, arterial stiffness, and oxidative stress in hypertensive heart failure with preserved ejection fraction. Hypertension. 2012;60:1200–1206. doi: 10.1161/HYPERTENSIONAHA.112.202705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Conraads V, Metra M, Kamp O, et al. Effects of the long-term administration of nebivolol on the clinical symptoms, exercise capacity, and left ventricular function of patients with diastolic dysfunction: results of the ELANDD study. Eur J Heart Fail. 2012;14:219–225. doi: 10.1093/eurjhf/hfr161. [DOI] [PubMed] [Google Scholar]
- 98.Ahmed A, Rich MW, Fleg JL, et al. Effects of digoxin on morbidity and mortality in diastolic heart failure: the ancillary digitalis investigation group trial. Circulation. 2006;114:397–403. doi: 10.1161/CIRCULATIONAHA.106.628347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Redfield M, Chen H, Borlaug B, et al. Effect of phosphodiesterase-5 inhibition on exercise capacity and clinical status in heart failure with preserved ejection fraction: a randomized clinical trial. JAMA. 2013;309:1268–1277. doi: 10.1001/jama.2013.2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bonderman D, Pretsch I, Steringer-Mascherbauer R, et al. Acute hemodynamic effects of riociguat in patients with pulmonary hypertension associated with diastolic heart failure (dilate-1): A randomized, double-blind, placebo-controlled, single-dose study. Chest. 2014;146:1274–1285. doi: 10.1378/chest.14-0106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Fukuta H, Sane DC, Brucks S, et al. Statin therapy may be associated with lower mortality in patients with diastolic heart failure: A Preliminary Report. Circulation. 2005;112:357–363. doi: 10.1161/CIRCULATIONAHA.104.519876. [DOI] [PubMed] [Google Scholar]
- 102.Fukuta H, Little W. Observational studies of statins in heart failure with preserved systolic function. Heart Fail Clin. 2008;4:209–216. doi: 10.1016/j.hfc.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 103.Kosmala W, Holland DJ, Rojek A, et al. Effect of If-channel inhibition on hemodynamic status and exercise tolerance in heart failure with preserved ejection fraction: a randomized trial. J Am Coll Cardiol. 2013;62:1330–1338. doi: 10.1016/j.jacc.2013.06.043. [DOI] [PubMed] [Google Scholar]
- 104.Solomon S, Zile M, Pieske B, et al. The angiotensin receptor neprilysin inhibitor LCZ696 in heart failure with preserved ejection fraction: a phase 2 double-blind randomised controlled trial. The Lancet. 2012;380:1387–1395. doi: 10.1016/S0140-6736(12)61227-6. [DOI] [PubMed] [Google Scholar]
- 105.Teerlink JR, Cotter G, Davison BA, et al. Serelaxin, recombinant human relaxin-2, for treatment of acute heart failure (RELAX-AHF): a randomised, placebo-controlled trial. Lancet. 2012;381:29–39. doi: 10.1016/S0140-6736(12)61855-8. [DOI] [PubMed] [Google Scholar]
- 106.Metra M, Cotter G, Davison BA, et al. Effect of serelaxin on cardiac, renal, and hepatic biomarkers in the relaxin in acute heart failure (RELAX-AHF) development program: correlation with outcomes. J Am Coll Cardiol. 2013;61:196–206. doi: 10.1016/j.jacc.2012.11.005. [DOI] [PubMed] [Google Scholar]
- 107.Kitzman D, Brubaker P, Morgan T, et al. Exercise training in older patients with heart failure and preserved ejection fraction. Circ Heart Fail. 2010;3:659–667. doi: 10.1161/CIRCHEARTFAILURE.110.958785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Edelmann F, Gelbrich G, Dungen H, et al. Exercise training improves exercise capacity and diastolic function in patients with heart failure with preserved ejection fraction: results of the Ex-DHF (Exercise training in Diastolic Heart Failure) pilot study. J Am Coll Cardiol. 2011;58:1780–1791. doi: 10.1016/j.jacc.2011.06.054. [DOI] [PubMed] [Google Scholar]
- 109.Haykowsky M, Liang Y, Pechter D, et al. A meta-analysis of the effect of exercise training on left ventricular remodeling in heart failure patients: the benefit depends on the type of training performed. J Am Coll Cardiol. 2007;49:2329–2336. doi: 10.1016/j.jacc.2007.02.055. [DOI] [PubMed] [Google Scholar]
- 110.Taylor RS, Davies EJ, Dalal HM, et al. Effects of exercise training for heart failure with preserved ejection fraction: a systematic review and meta-analysis of comparative studies. Int J Cardiol. 2012;162:6–13. doi: 10.1016/j.ijcard.2012.05.070. [DOI] [PubMed] [Google Scholar]
- 111.Haykowsky MJ, Timmons MP, Kruger C, et al. Meta-analysis of aerobic interval training on exercise capacity and systolic function in patients with heart failure and reduced ejection fractions. Am J Cardiol. 2013;111:1466–1469. doi: 10.1016/j.amjcard.2013.01.303. [DOI] [PubMed] [Google Scholar]
- 112.Suchy C, Massen L, Rognmo O, et al. Optimising exercise training in prevention and treatment of diastolic heart failure (OptimEx-CLIN): rationale and design of a prospective, randomised, controlled trial. Eur J Prev Cardiol. 2014;21:18–25. doi: 10.1177/2047487314552764. [DOI] [PubMed] [Google Scholar]
- 113.Vanhatalo A, Fulford J, Bailey SJ, et al. Dietary nitrate reduces muscle metabolic perturbation and improves exercise tolerance in hypoxia. J Physiol. 2011;589:5517–5528. doi: 10.1113/jphysiol.2011.216341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zamani P, Rawat D, Shiva-Kumar P, et al. Effect of inorganic nitrate on exercise capacity in heart failure with preserved ejection fraction. Circulation. 2015;131:371–380. doi: 10.1161/CIRCULATIONAHA.114.012957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Jhund PS, Claggett BL, Voors AA, et al. Elevation in high-sensitivity troponin T in heart failure and preserved ejection fraction and influence of treatment with the angiotensin receptor neprilysin inhibitor LCZ696. Circ Heart Fail. 2014;7:953–959. doi: 10.1161/CIRCHEARTFAILURE.114.001427. [DOI] [PubMed] [Google Scholar]
- 116.Oikonomou E, Siasos G, Zaromitidou M, et al. Atorvastatin treatment improves endothelial function through endothelial progenitor cells mobilization in ischemic heart failure patients. Atherosclerosis. 2014;238:159–164. doi: 10.1016/j.atherosclerosis.2014.12.014. [DOI] [PubMed] [Google Scholar]
- 117.Haass M, Kitzman DW, Anand IS, et al. Body mass index and adverse cardiovascular outcomes in heart failure patients with preserved ejection fraction/clinical perspective. Circ Heart Fail. 2011;4:324–331. doi: 10.1161/CIRCHEARTFAILURE.110.959890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Heineke J, Auger-Messier M, Xu J, et al. Genetic deletion of myostatin from the heart prevents skeletal muscle atrophy in heart failure. Circulation. 2010;121:419–425. doi: 10.1161/CIRCULATIONAHA.109.882068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Cittadini A, Marra AM, Arcopinto M, et al. Growth hormone replacement delays the progression of chronic heart failure combined with growth hormone deficiency: an extension of a randomized controlled single-blind study. JACC Heart Fail. 2013;1:325–330. doi: 10.1016/j.jchf.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 120.Toma M, McAlister FA, Coglianese EE, et al. Testosterone supplementation in heart failure: a meta-analysis. Circ Heart Fail. 2012;5:315–321. doi: 10.1161/CIRCHEARTFAILURE.111.965632. [DOI] [PubMed] [Google Scholar]