Abstract
Information on the clinicopathologic characteristics of invasive carcinomas arising from mucinous cystic neoplasms (MCNs) is limited, because in many early studies they were lumped and analyzed together with noninvasive MCNs. Even more importantly, many of the largest prior studies did not require ovarian-type stroma (OTS) for diagnosis. We analyzed 178 MCNs, all strictly defined by the presence of OTS, 98% of which occurred in perimenopausal women (mean age, 47 y) and arose in the distal pancreas. Twenty-nine (16%) patients had associated invasive carcinoma, and all were female with a mean age of 53. Invasion was far more common in tumors with grossly visible intracystic papillary nodule formation ≥ 1.0 cm (79.3% vs. 8.7%, P = 0.000) as well as in larger tumors (mean cyst size: 9.4 vs. 5.4 cm, P = 0.006); only 4/29 (14%) invasive carcinomas occurred in tumors that were < 5 cm; however, none were < 3 cm. Increased serum CA19-9 level (> 37 U/L) was also more common in the invasive tumors (64% vs. 23%, P = 0.011). Most invasive carcinomas (79%) were of tubular type, and the remainder (5 cases) were mostly undifferentiated carcinoma (2, with osteoclast-like giant cells), except for 1 with papillary features. Interestingly, there were no colloid carcinomas; 2 patients had nodal metastasis at the time of diagnosis, and both died of disease at 10 and 35 months, respectively. While noninvasive MCNs had an excellent prognosis (100% at 5 y), tumors with invasion often had an aggressive clinical course with 3- and 5-year survival rates of 44% and 26%, respectively (P = 0.000). The pT2 (> 2 cm) invasive tumors had a worse prognosis than pTl (≤ 2 cm) tumors (P = 0.000), albeit 3 patients with T1a (< 0.5 cm) disease also died of disease. In conclusion, invasive carcinomas are seen in 16% of MCNs and are mostly of tubular (pancreatobiliary) type; colloid carcinoma is not seen in MCNs. Serum CA19-9 is often higher in invasive carcinomas, and invasion is typically seen in OTS-depleted areas with lower progesterone receptor expression. Invasion is not seen in small tumors (< 3 cm) and those lacking intracystic papillary (mural) nodules of ≥ 1 cm, thus making the current branch-duct intraductal papillary mucinous neoplasm management protocols also applicable to MCNs.
Keywords: pancreas, mucinous cystic neoplasm, ovarian-type stroma, invasive carcinoma
For many years the clinicopathologic characteristics and biological behavior of pancreatic mucinous cystic neoplasms (MCN) with carcinomatous transformation have been a highly controversial area of pancreatic pathology. In 1979, Compagno and Oertel1 of the Armed Forces Institute of Pathology first described MCNs with “overt and latent malignancy” and concluded that latent tumors (cystadenomas) could behave in a malignant manner, whereas overtly malignant tumors (cystadenocarcinomas) frequently demonstrated indolent behavior. In 1999, a larger series from the same institution confirmed similar findings.2 However, these conclusions were challenged by subsequent studies, which found that if an invasive carcinoma was excluded by extensive sampling, noninvasive MCNs behaved in a uniformly benign manner.3–8 Meanwhile, the nature and behavior of MCNs with associated invasive carcinoma has remained largely elusive. This is due in part to the wide variety of terms used to describe these tumors (mucinous cystadenoma, mucinous cystadenocarcinoma, and MCN). Until recently, most major studies did not mandate ovarian-type stroma (OTS) for diagnosis,1,3,9–16 hence some of these studies were likely “corrupted” by intraductal papillary mucinous neoplasms (IPMNs), thus prohibiting the proper characterization of MCNs and, more importantly, the invasive carcinomas that arise from them.17 Another factor that has contributed greatly to the conflicting reports in the literature is the fact that many previous studies lumped MCN-related invasive carcinoma and noninvasive MCNs with high-grade dysplasia (HGD) into a single “malignant MCN” group.9–16,18–22
Thus, the incidence, histology, extent, and biological behavior of the invasive carcinomas arising in MCNs, relative to ordinary pancreatic ductal adenocarcinoma, had remained a controversial enigma. Meta-analysis of the literature has shown markedly disparate rates of prevalence of MCN-related invasive carcinoma with some as low as 2.9% and others as high as 33%.23 Whereas some studies have claimed that these tumors behave in a predominantly indolent manner,24 others (Crippa et al6 and Yamao et al7) suggest otherwise, with 5-year survival rates for MCN-related invasive carcinoma of 57% and 63%, respectively. A recent analysis of 16 “minimally invasive” MCN-associated carcinomas reported benign behavior in all but 1 case,24 a finding that gives the impression that these tumors behave in a relatively “benign” or indolent manner.
The objective therefore of this study is to present one of the largest clinicopathologic studies of invasive carcinoma (29 cases) arising from a well-characterized cohort of 178 MCNs, all defined by the current diagnostic criteria of OTS. This analysis serves to update the current literature on this rare and poorly understood entity.
MATERIALS AND METHODS
Appropriate institutional approvals were obtained for this study.
Case Selection
The surgical pathology databases of Samsung Medical Center (Seoul, Korea), Emory University Hospital (Atlanta, GA), Wayne State University Detroit Medical Center (Detroit, MI), and University of Virginia Hospital (Charlottesville, VA) as well as the consultation files of the authors’ were searched for pancreatic cystic tumors with a diagnosis of mucinous cystadenoma, mucinous cystadenocarcinoma, and MCN. Separately, 1380 consecutive primary invasive pancreatic carcinomas identified in the files of Wayne State University and Emory University were evaluated by 1 of the authors (V.A.) for the presence of an MCN component.
For each identified case, information regarding overall cyst (tumor) size and total number of submitted blocks was obtained from the surgical pathology reports, and cases in which <1 block/cm of cyst (tumor) was submitted for evaluation were excluded from the study. All slides of the remaining cases were reassessed histologically. Only cases with definite evidence of OTS—defined as densely packed spindle-shaped cells with sparse cytoplasm and round or elongated nuclei, undermining the epithelium17—were included. If OTS was focal or ill-defined, progesterone receptor (PR) immunohistochemical staining was performed to confirm its existence.
Classification and Clinicopathologic Parameters Investigated
Medical records were reviewed to obtain clinical and demographic data, including age, sex, preoperative serum CEA and CA19-9 levels, tumor recurrence, and follow-up period. Survival data were also obtained from medical records or from the United States Social Security Death Index.
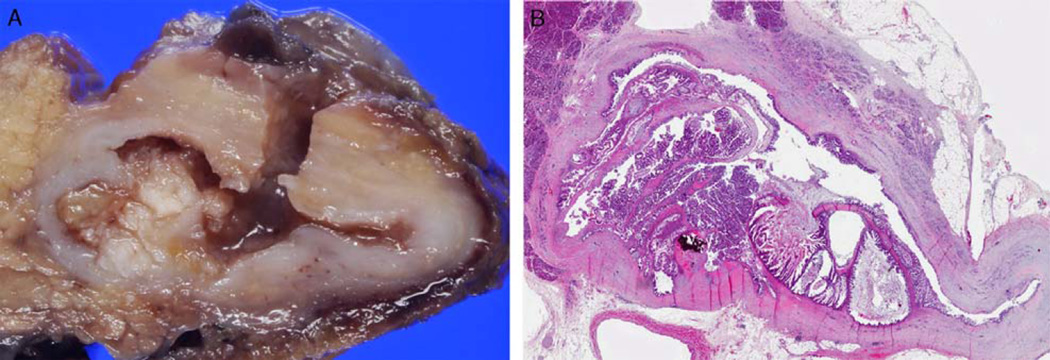
Size of the entire cyst (tumor) was extracted from the pathology reports. The presence of intracystic papillary excrescences was determined on the basis of the correlation of gross and microscopic findings, and those that formed nodules ≥ 1 cm were classified as intracystic papillary nodules (Fig. 1).
FIGURE 1.
A, Gross photograph of MCN showing intracystic papillary nodule within the cyst lumen. B, Whole mount image of the same intracystic papillary nodule (hematoxylin and eosin stain).
Epithelial dysplasia was graded on the basis of the most severe focus identified, using the grading criteria put forth in the current (2010) World Health Organization (WHO) classification of MCNs, as low-grade (LGD), intermediate-grade (IGD), and HGD/carcinoma in situ (HGD/CIS).
In MCNs with invasive carcinoma, the histologic type of the invasive component was also determined according to WHO guidelines, on the basis of the predominant histologic tumor type in the invasive component. Depth of invasion was microscopically measured from the base of the cyst wall to the leading edge of the invasive component. The overall size of invasive carcinoma was also recorded. On the basis of this measurement, the invasive carcinomas were categorized as early (≤ 2 cm, pT1) and advanced (> 2 cm, pT2 and beyond). pT1 tumors were further subcategorized into pT1a (< 0.5 cm), pT1b (0.5 to 1 cm), and pT1c (>1 cm) as per the recently proposed scheme,25 which is more objective than the “minimally invasive” terminology.
Statistical Analysis
Statistical analysis was performed using SPSS statistical software package (SPSS, Chicago, IL). Results were analyzed using the Student t test. Cumulative survival rates were calculated using the Kaplan-Meier method and then compared using the log-rank test. A P value of <0.05 was considered statistically significant.
RESULTS
General Characteristics
We identified a total of 178 MCNs with OTS meeting the inclusion criteria outlined above.
The mean age of patients was 48±13 (range, 23 to 81 y). There were only 2 tumors in male patients, both of which also had characteristic zones of OTS. In addition, both male MCNs showed only LGD. Most MCNs (168/171, 98.2%) were located in the distal body/tail except for 3 MCNs (1.8%) located in the head of pancreas (2 with LGD and 1 with HGD/CIS).
Twenty-nine (16.3%) MCNs had an associated invasive carcinoma (21.1% [19/90] of cases from the United States and 11.4% [10/88] of cases from Korea). Of the remaining 149 noninvasive cases, 109 (61.2%) revealed LGD, 27 (15.2%) showed IGD, and 13 (7.3%) had HGD/CIS.
The prevalence of invasive carcinoma was higher in MCNs with intracystic papillary nodules (≥ 1 cm) (79.3% [23/29] vs. 8.7% [13/149], P = 0.000), and the nodules were larger in the invasive MCNs (2.3 vs. 1.6 cm, P = 0.05).
The mean number of submitted blocks per case was 19 (range, 6 to 65). The mean number of blocks submitted per centimeter of cyst (tumor) size was 3.6 (1 to 13) for MCNs with LGD, 3.8 (1.8 to 10) for MCNs with IGD, 4.5 (1.5 to 11.3) for MCNs with HGD/CIS, and 3.4 (1.0 to 13.7) for MCNs with invasive carcinoma (Table 1). All slides were then reevaluated microscopically.
TABLE 1.
General Characteristics of 178 MCNs
| LGD | IGD | HGD/CIS | Invasive Carcinoma | |
|---|---|---|---|---|
| No. of cases (%) | 109 (61.2) | 27 (15.2) | 13 (7.3) | 29 (16.3) |
| Mean # of blocks/cm of cyst (tumor) | 3.6 (1–13) | 3.8 (1.8–10) | 4.5 (1.5–11.3) | 3.4 (1.0–13.7) |
| Sex (M:F) | 2:107 | 0:27 | 0:13 | 0:29 |
| Location (head vs. body/tail) | 2:107 | 0:27 | 1:12 | 0:29 |
F indicates female; M, male.
All demographic data are summarized in Table 1.
Comparative Analysis of MCNs With and Without Invasion
MCNs with invasive carcinoma were more common in older female individuals (mean age 53 vs. 46 y, P = 0.214). Although this was not statistically significant, it showed a trend toward progressively increasing risk for carcinoma with age. Sex ratio and cyst location were similar (predominantly female patients and involvement of the distal body/tail) in MCNs both with and without invasive carcinoma.
Preoperative serum CA19-9 level information was available in 54 cases. Elevated CA19-9 level (>37U/L) was significantly more common in MCNs with invasive carcinoma (63.6% vs. 23.3%, P= 0.011). Preoperative serum CEA level information was also available in 39 MCNs; however, the elevated serum CEA (> 7 ng/mL) level was only detected in 4 MCNs, all with LGD (Table 2).
TABLE 2.
Comparative Analysis of MCNs With and Without Invasive Carcinoma
| Clinicopathologic Findings | MCN With Invasive Carcinoma (n=29) | MCN Without Invasive Carcinoma (n=149)* | P |
|---|---|---|---|
| Mean age (range) | 53 (23–81) | 46 (29–80) | 0.214 |
| High CA19-9 (> 37U/L) (n [%]) | 7/11 (63.6) | 10/43 (23.3) | 0.011 |
| High CEA (> 7ng/mL) | 0/8 | 4/31 (12.9%) | 0.284 |
| Mean cyst (tumor) size (cm) | 9.4 | 5.4 | 0.006 |
| Intracystic papillary nodule (≥ 1 cm) | |||
| Incidence (n [%]) | 23/29 (79.3) | 13/149 (8.7) | 0.000 |
| Median size (cm) | 2.3 | 1.6 | 0.05 |
MCN with LGD, IGD, and HGD/CIS.
CA19-9 indicates carbohydrate antigen 19-9; CEA, carcinoembryonic antigen.
The mean cyst size was significantly larger for MCNs with invasive carcinoma (9.4 vs. 5.4 cm, P = 0.006) than those without (Table 2). Only 4 invasive carcinomas arose from cysts <5 cm, and these ranged in size from 3.5 to 4.8 cm, but none were <3 cm. Intracystic nodules/papillae (≥ 1 cm) were also more common in MCNs with invasive carcinoma (79.3% vs. 8.7%, P=0.000), and only 6 MCN-associated invasive carcinomas did not contain intracystic papillary nodules. Although occasional smaller nodules/papillary excrescences (<1 cm) were present in MCNs with LGD and IGD, most of these were not true epithelial papillae but instead corresponded to thickened septae of small cysts or nodular growth of stromal tissue.
On microscopic examination, within these intra-cystic papillae, there was frequently an inflammatory infiltrate (composed mainly of neutrophils), akin to the “cryptitis” seen in inflammatory bowel disease. In some tumors the epithelial cells within these areas showed variable degrees of reactive atypia that could potentially lead to overestimation of (grade of) dysplasia. In addition, in cases with marked inflammation, especially those associated with denudation, the detached epithelial strips formed papillae that mimicked the microscopic appearance of invasive micropapillary carcinoma; these detached papillae were not considered invasive carcinoma in our cohort.
Characteristics of Invasive Carcinoma
In 23 (79.3%) MCNs with invasive carcinoma, the invasive component was of tubular (ductal)-type adenocarcinoma and was morphologically indistinguishable from conventional pancreatic ductal (pancreatobiliary-type) adenocarcinoma. Of the remaining 6 cases, 1 was an adenocarcinoma with prominent papillary growth, and 5 were undifferentiated carcinomas, 2 with osteoclast-like giant cells (Table 3). All 5 undifferentiated carcinomas occurred in MCNs with papillary nodules and had variable amounts of a conventional ductal adenocarcinoma component, supportive of their epithelial/ductal nature. The adenocarcinoma with prominent in situ–like papillary growth was associated with metastatic deposits (to the ovaries and colon) that were identical to the papillary nodules in the primary MCN. This case did not have a conventional (tubular-type) invasive adenocarcinoma despite sampling in 68 blocks. Interestingly, none of the invasive carcinomas were of colloid (mucinous) type.
TABLE 3.
Comparative Analysis of MCNs With Early Versus Advanced Invasion
| Early Invasion (≤ 2 cm; n = 17) |
Advanced Invasion (> 2 cm; n = 12) |
P | |
|---|---|---|---|
| Mean size of invasion | 0.5 cm | 2.7 cm | 0.001 |
| pT1 (n) | 17 | 0 | |
| pT2 (n) | 0 | 4 | |
| pT3 (n) | 0 | 8 | |
| pNl (n) | 0 | 2 | |
| Subsequent distal metastasis | 0 | 4 | |
| Local recurrence | 1 | 2 | |
| Death | 3 | 8 | |
| Histologic type of invasive carcinoma (n = 29) | |||
| Conventional tubular-type adenocarcinoma | 23 | ||
| Undifferentiated carcinoma | 5 | ||
| Adenocarcinoma with prominent papillary growth pattern | 1 | ||
| Colloid carcinoma | 0 | ||
The invasive component was ≤ 2 cm (early invasion) in 17 cases (all pT1) and >2 cm in 12 (4 pT2 and 8 pT3) (Table 3). When MCNs with early invasion were sub-categorized according to the proposed scheme,25 which we also advocate, 13 cases fell into pT1a, 3 into pT1b, and 1 into pT1c category. Three patients with pT1a invasion had multiple invasive foci, and all died of disease at 13, 29, and 64 months (mean: 35.3 mo). This raises the question of whether these tumors were understaged due to undersampling, as none of the 3 was submitted entirely for microscopic evaluation. However, these tumors were 7.0, 6.5, and 15 cm in diameter, and the total number of submitted blocks per case was 16, 18, and 50, respectively. Thus, the mean number of submitted blocks per centimeter tumor size was 2.3, 2.8, and 3.3, respectively. More importantly, all intracystic papillae and adjacent cyst walls were entirely submitted for microscopic evaluation. Therefore, the possibility of undersampling seems unlikely in these 3 cases.
Lymph node metastasis was identified at the time of diagnosis in 2 MCNs with invasive carcinoma, and both patients died of disease at 10 and 35 months, respectively. During the follow-up period, 3 cases had local recurrence (with peritoneal spread in 2 and direct invasion of colon and duodenum in 1), and 4 cases developed liver metastasis (1 synchronous and 3 metachronous).
Comparative analysis of MCNs with, and without, invasive carcinoma is summarized in Table 2. Comparative analysis of MCN with early and advanced invasive carcinoma is summarized in Table 3.
Distribution and PR Expression in OTS
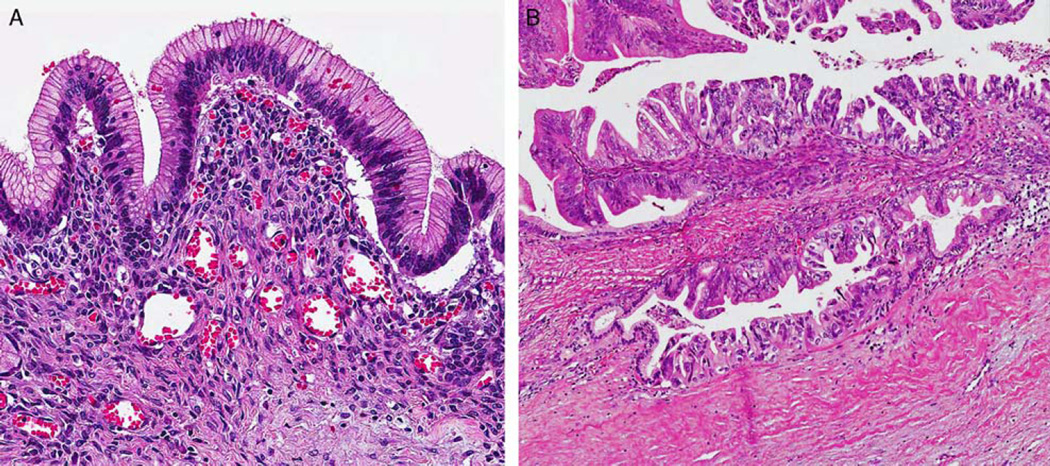
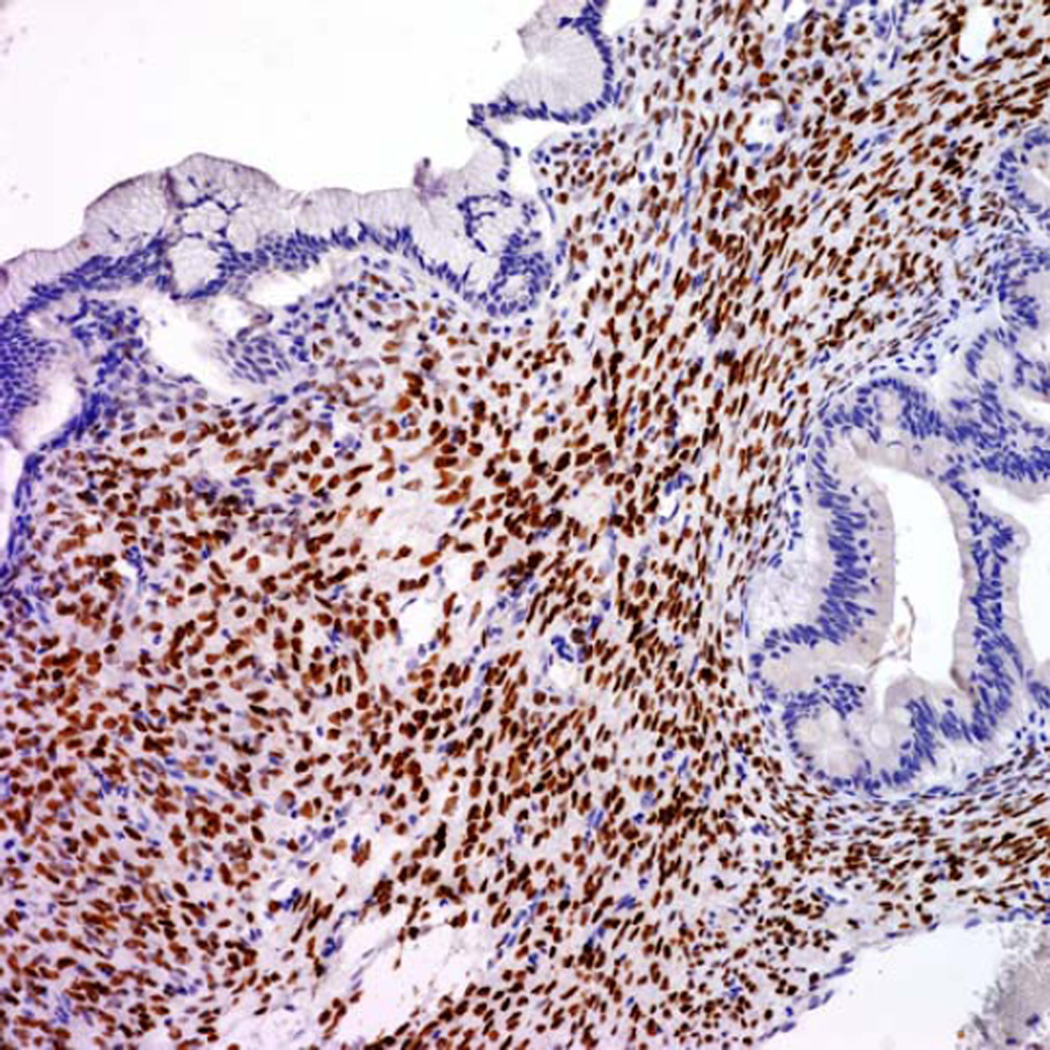
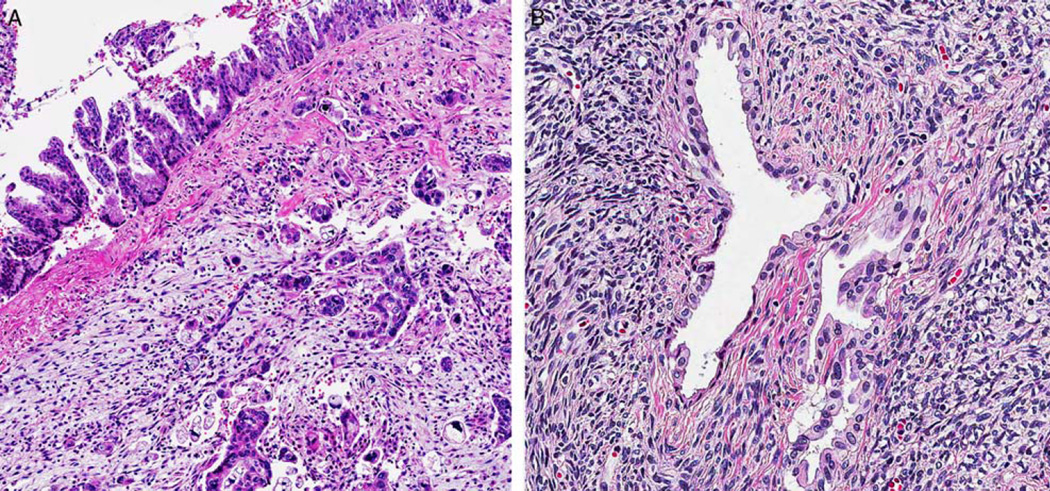
Although OTS was typically present in the sub-epithelial regions of MCNs, there was marked variation in its distribution and visibility. In some areas, the OTS was either atrophic or invisible on cursory examination, especially in large cystic tumors with thin-walled cystic areas, but it was commonly present and more prominent in septa. Interestingly the MCNs with LGD and IGD revealed relatively well-preserved typical OTS; however, the amount of OTS was sometimes decreased around areas of HGD/CIS and/or invasive carcinoma component (Fig. 2). PR immunohistochemical staining was performed in 109 MCNs (68 with LGD, 17 with IGD, 9 with HGD/CIS, and 15 with invasive carcinoma) and confirmed this observation. PR immunoreactivity was diffuse/strong in OTS around LGD and IGD (Fig. 3) but was weak in the OTS around areas of HGD/CIS. PR expression was frequently lost in areas with true invasion even when the OTS surrounding the LGD component of the same case exhibited relatively preserved PR expression. In fact, in 8 MCNs (3 with HGD/CIS and 5 with invasive carcinoma), the presence of OTS could only be confirmed after PR staining.
FIGURE 2.
A, OTS was commonly found in the subepithelial regions of MCNs with LGD (hematoxylin and eosin). B, The volume of OTS was decreased or lost around areas of HGD and invasive carcinoma (hematoxylin and eosin).
FIGURE 3.
MCN with OTS showing diffuse positivity for PR in an area of LGD.
Survival and Prognosis
Follow-up information was available in 162 (91%) patients. Of the remaining 16 patients, 7 had LGD, 3 had IGD, 2 had HGD/CIS, and 4 had invasive carcinoma. The follow-up period ranged from 1 to 167 months (median: 18 mo). At last follow-up, 11 patients were dead. Ten of them had invasive carcinoma and died of their disease; 1 had HGD/CIS and died of other causes at age 63, 118 months after surgery. Of note, her tumor measured 3.5 cm in greatest dimension, and the total number of blocks submitted was 18 (5.1 blocks/cm tumor size). Of the 5 patients with undifferentiated carcinoma component, 3 died of disease at 2, 15, and 16 months. No follow-up information was available for the remaining 2 patients. None of the patients whose MCNs showed LGD or IGD died of disease, but 3 patients with LGD died of other conditions.
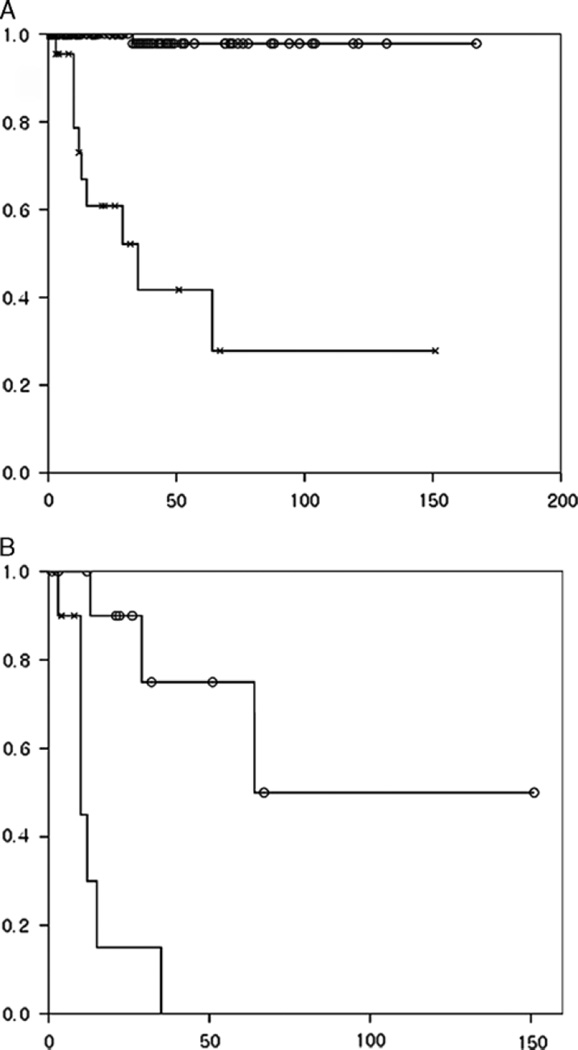
The 3-, 5-, and 10-year survival rates of all MCNs were 88%, 84%, and 84%, respectively. However, the survival rates of those with invasive carcinoma were significantly lower than that of tumors without invasion. The 3- and 5-year survival rates of MCN with and without invasive carcinoma were 44% and 26% versus 100% and 100%, respectively (P= 0.000) (Fig. 4A). MCNs with early (≤ 2 cm, pT1) invasion had better prognosis than MCNs with advanced (>2 cm, pT2 and beyond) invasion (P = 0.000) (Fig. 4B; Table 3). The number of the cases in the pT1 substaging categories was too small to determine their prognostic correlation.
FIGURE 4.
A, MCNs with invasive carcinoma (line with “x”) had significantly worse prognosis than MCNs without invasive carcinoma (line with “o”) (P=0.000). B, Similarly, MCNs with advanced invasion (line with “x”: ≥ 2 cm) had worse prognosis than MCNs with early invasion (line with “o”: <2 cm) (P =0.000).
DISCUSSION
This study constitutes one of the largest series of histologically confirmed MCN-associated invasive carcinomas, defined by the refined WHO-2010 diagnostic criteria of OTS.17 Our findings confirm that MCNs occur almost exclusively in perimenopausal women (mean age, 47y and are >98% female) and are located in the pancreatic tail in >98% of cases. This is similar to the findings in more recent studies that also utilized the OTS criteria for diagnosis of MCN5–7,24,26–28; however, our results differ somewhat from those of some earlier studies that did not mandate OTS criteria, had a larger number of male patients, and occurred more frequently in the pancreatic head.3,11,16,20
The frequency of invasive carcinoma in our MCN cohort (16.3%) is similar to 2 other recent studies that reported frequencies of 12% (Crippa et al,6 Italian and American cohort) and 13% (Baker et al,8 Italian, American, and German cohort), respectively. Of note, Baker’s cohort included the 19 MCN-associated invasive cancers that were previously reported by Crippa et al6 in 2008, thus making Baker’s prevalence figures somewhat skewed. However, a multi-institutional Japanese study reported a much lower incidence of 3.9%.7 It is unclear whether this reflects populational differences or the exclusion of smaller invasive carcinomas and (more importantly) undifferentiated carcinomas. In our study, 21% of the cases from the United States had invasion, whereas only 11% of the cases from Korea had invasive carcinoma. The higher incidence in the US cohort could be partly attributable to the fact that they were identified through systematic analysis of invasive carcinomas in the cohort, which was not the case in the Korean group. This may have skewed the frequency results somewhat. Invasive carcinoma (16.3%) was also twice as frequent as HGD/CIS (7.3%) in our American cohort, which is somewhat similar to other major studies (Table 4).2,4–6,27 This suggests that carcinomatous transformation in MCNs rapidly progresses to invasion.
TABLE 4.
Summary of Previous MCN Studies With OTS Criterion
| References | All Cases (n) |
LGD (n) |
IGD (n) |
HGD (n) |
Invasive (n [%]) |
Undiff | Survival Data |
|---|---|---|---|---|---|---|---|
| 1. Zamboni et al4 | 56 | 22 | 12 | 6 | 16 (28.6) | 3 | 8/16 Dead (median FU: 11 mo) |
| 2. Thompson et al2 | 130 | 60 | 23 | 47 (36.2) | 1 | 58/70 Alive (mean FU: 9.9 y) | |
| 3. Izumo et al26 | 34 | 28 | 2 | 3 | 1 (2.9) | 0 | 2 DOD & 2 Recurrence (median FU: 73 mos) |
| 4. Kosmahl et al27 | 32 | 10 | 8 | 3 | 11 (34.4) | 1 | NA |
| 5. Reddy et al5 | 56 | 50 | 2 | 4 (7.1) | 0 | 3 DOD & 3 Recurrence | |
| 6. Crippa et al6 | 163 | 118 | 17 | 9 | 19 (11.7) | 0 | 5 YSR: 57% |
| 7. Yamao et al7 | 156 | 129 | 21 | 6 (3.8) | 0 | 5 YSR: 63% | |
| 8. Baker et al8 | 291 | NA | 38 (13.4) | 3 | NA | ||
| This study | 178 | 109 | 27 | 13 | 29 (16.3) | 5 | 5 YSR: 26% |
| n | Benign (LGD & IGD) | Malignant (HGD & Invasive) (n [%]) |
Malignant (Invasive Only) (%) |
Studies | |||
| All previous MCN studies | |||||||
| MCN (> 30) | 627 | 456 | 171 (37.5) | 16.6 | 1+2+3+4+5+6+7 | ||
| MCN (> 50) | 561 | 408 | 153 (37.5) | 16.4 | 1+2+5+6+7 | ||
DOD indicates died of disease; FU, follow-up; NA, not available; undiff, undifferentiated carcinoma; YSR, year survival rate.
Several key pathologic factors were noted in association with invasion. One of these was mean cyst size, which was significantly larger in MCNs with invasive carcinoma (mean 9.4 cm) versus those without invasion (mean 5.4 cm) (P= 0.006), and all but 4 invasive carcinomas occurred in MCNs >5 cm. None of the invasive carcinomas occurred in MCNs that were <3 cm, but 4 occurred in cases between 3 and 5 cm. This concurs with other recent studies that also used the OTS diagnostic criterion and reported that tumor size ranged from 5.5 to 10.5 cm.5–7,26,27 Although others have stated that invasive carcinoma is not seen in MCNs <5 cm, our study showed that it can be seen in tumors <5 cm (albeit rarely). Therefore, the (branch-duct) IPMN-related criterion of watchful waiting based on small cyst size is potentially applicable to MCNs as the 2 appear to behave in a similar manner.
In addition, the presence of intracystic papillary nodules had a significant association with invasive carcinoma, a finding also noted by others.2,4,6,7 When we used the cutoff size of 1 cm, intracystic papillary nodules that were ≥ 1 cm were strongly associated with invasive carcinoma. In fact, 23 of the 29 invasive cases had nodules ≥ 1 cm. These findings further suggest that MCNs <3 cm and lacking mural nodules are less likely to harbor invasive carcinoma. Accordingly, the protocols used for branch-duct IPMNs may also be applicable to MCN,25 and depending on a patient’s surgical fitness, watchful waiting may be a valid consideration in smaller tumors.
The fact that serum CA19-9 was significantly higher in the invasive tumors versus the noninvasive ones was not surprising. Elevated CA19-9 has a known association with pancreatic ductal adenocarcinoma.29 This marker could potentially also be used to monitor patients with established MCNs, with elevation indicating a need for resection. Serum CEA had no specific association with invasion.
Another noteworthy finding in our cohort is that the vast majority of MCN-associated invasive carcinomas are tubular (ductal)-type adenocarcinomas, with a much smaller subset (~20%) of undifferentiated/sarcomatoid carcinoma with and without osteoclast-like giant cells. Intriguingly, one seemingly noninvasive MCN (which was sampled in 68 blocks) had metastatic deposits in the ovary and gastrointestinal tract that on resection showed a well-differentiated “in situ–like” “papillary adenocarcinoma” pattern that was indistinguishable from the papillary nodules in the pancreatic MCN. Of note, this pancreatic MCN had purulent inflammation and had ruptured at the time of presentation. Therefore this patient’s so-called intra-abdominal tumor deposits could conceivably represent “implants” rather than true metastasis. Alternatively, some of the papillary nodules seen in the primary pancreatic tumor may in fact represent an unconventional, pushing border–type invasion that was not easily recognizable as invasion by current criteria. This patient died of disease 1.5 years after diagnosis. Other than this peculiar case, all invasive carcinomas were either ordinary ductal adenocarcinomas by morphology or high-grade undifferentiated/sarcomatoid cancers.
Interestingly, there were no cases of colloid carcinoma in the invasive carcinomas examined. Colloid (mucinous) carcinoma occurs in a significant subset of IPMNs (a kindred of MCNs) and has a more protracted clinical course than ordinary tubular-type pancreatic ductal adenocarcinoma.30 Although there are some reports of colloid carcinoma arising in MCNs,2,30,31 on the basis of current diagnostic criteria most of those cases would now be classified as IPMN-associated, including a case previously published by the current authors before the OTS criteria was established.30,32 The current study shows that MCNs strictly defined by OTS are not associated with colloid-type invasive carcinoma, a finding that was also noted in another recent study.8 Our findings also confirm that the intestinal pathway of carcinogenesis characteristic of a subset of IPMNs33 is not valid for MCNs, which may have management implications for the future treatment of these tumors as more specific targeted therapies are developed.
This is the largest study of MCN-associated invasive carcinomas showing that these tumors have a significantly worse prognosis than previously reported in the literature. The 5-year survival of noninvasive cases was 100%, in accordance with the recent major studies using OTS diagnostic criteria4,5,26,28 (and extensive tumor sampling); and there were no disease-related deaths in our non-invasive MCNs, which confirms that noninvasive tumors are cured by complete removal. However, for invasive cases, the 5-year survival rate was a mere 26%. This is significantly lower than that reported by Crippa et al6 in their analysis of 19 invasive carcinomas (which showed 57% 5-y survival),6 and Yamao et al7 (who reported a 63% 5-y survival rate). The difference in their and our results is partly attributable to the fact that we had several cases with larger invasive carcinomas. In fact, 12 cases of invasion were >2 cm.
The size (stage) of invasive carcinoma was an important prognostic parameter in MCNs in our study, with pT1 (<2 cm) cases having a better prognosis than pT2 (>2 cm) tumors. Two of our cases also had lymph node metastasis in the resection specimen, an occurrence that was not recorded in other major studies. Considering that these invasive carcinomas are of tubular (pancreatobiliary) type, with all the morphologic features of ordinary ductal adenocarcinomas, it is not surprising that the prognosis is so dismal in this group, especially in those with a larger invasive component. What is surprising perhaps are the 3 T1a carcinomas (< 0.5 cm invasion) that were also associated with demise of the patients, a finding similar to the unusual clinical course (of liver metastasis) of minimally invasive carcinoma of MCN reported by Yamao et al.7 The aggressive clinical behavior seen in these 3 pT1 tumors is also comparable to a recent study by Lewis et al24 who noted that only 1/16 “minimally invasive” MCNs had tumor recurrence and death (at 42 mo) after surgery.24 Although our 3 cases were examined in 16, 18, and 50 block sections, respectively, none of them were entirely submitted for evaluation, and it is therefore possible that their invasive component may have been undersampled and thus under-staged. Another explanation for this discrepancy may be that, when we reviewed the 3 pT1a MCNs with local recurrence or metastasis, all 3 proved to have multifocal invasive carcinoma. In Lewis et al’s24 study, the recurrent, minimally invasive MCN also had multifocal invasion. Although supported by only 3 cases, we believe that multifocal invasive carcinoma may be a sign of more aggressive behavior in these small invasive carcinomas, and such cases may have to be staged differently and reported as such in pathology reports, to ensure that patients receive the appropriate (closer) follow-up after resection.
This study documents the clinical behavior of a spectrum of MCN-associated invasive carcinomas including undifferentiated and larger examples. On the one hand, the prognosis of MCN-associated invasive carcinomas appears to be fairly aggressive but conversely, they are comparatively better than ordinary ductal adenocarcinomas arising from pancreatic intraepithelial neoplasia, which show only 10% to 15% 5-year survival. This parallels the observations of other tumoral intraepithelial neoplasm– associated invasive carcinomas of the pancreatobiliary tract such as IPMN-associated,34,35 intraductal tubulopapillary neoplasm–associated,36 intraductal papillary neoplasm of the bile duct–associated,37,38 intra-ampullary papillary tubular neoplasm–associated,39 and intracholecystic papillary tubular neoplasm–associated 40 invasive carcinomas. All of these invasive carcinomas have uniformly aggressive but, nonetheless, better outcome than the ordinary invasive carcinomas of their respective organs, even when they are stage-matched, indicating that tumoral intraepithelial neoplasm-associated invasive carcinomas may have distinctive biological properties. It is possible that the invasive tubular-type carcinomas arising in MCNs have different characteristics than ordinary ductal adenocarcinomas arising from pancreatic intraepithelial neoplasia, although they are morphologically indistinguishable.
We also observed a decrease in OTS volume in MCNs with HGD/CIS and/or invasive carcinoma. Zamboni et al4 and Lam et al41 also noted that OTS was sometimes lost after malignant transformation of MCN. Although this may be an age-related phenomenon, it is also possible that it may be a surrogate factor of carcinogenesis (progression from LGD to HGD/CIS) by as yet unknown mechanisms, as suggested by Shimizu et al.28 In our study, although some minimally invasive carcinomas were confined to the OTS, most others were surrounded by desmoplastic stroma or proliferating fibroblasts and not by typical OTS (Fig. 5A). In addition, we observed that even PR immunoreactivity was weak in the OTS surrounding HGD/CIS areas and was often lost in areas with true invasion. Interestingly, as far back as 15 years ago Thompson et al2 speculated that the loss of PR expression might be associated with worse prognosis. Regardless of the mechanism, this may be important both diagnostically and for cancer researchers to investigate the potential role and mechanism of OTS in MCN carcinogenesis.
FIGURE 5.
A, An invasive carcinoma component of MCN surrounded by proliferating fibroblasts, and not by OTS, is depicted here. B, Some MCNs revealed regional overgrowth of OTS with entrapped atrophic glands that were lined by epithelium that was not overtly mucinous (hematoxylin and eosin stain).
In conclusion, invasive carcinoma should be considered in MCNs with large cyst size and intracystic nodule/papillae (≥1 cm). Watchful waiting may be a viable consideration for these tumors. Invasive carcinomas arising from MCNs can be fairly aggressive even when they are small. Thus, if minimal or focal invasion is noted in an MCN, thorough histopathologic examination is warranted so as to accurately document the extent of tumor, depth of invasion, and presence of multifocality in the pathology report.
Footnotes
Conflicts of Interest and Source of Funding: The authors have disclosed that they have no significant relationships with, or financial interest in, any commercial companies pertaining to this article.
REFERENCES
- 1.Compagno J, Oertel JE. Mucinous cystic neoplasms of the pancreas with overt and latent malignancy (cystadenocarcinoma, cystadenoma). A clinicopathologic study of 41 cases. Am J Clin Pathol. 1978;69:573–580. doi: 10.1093/ajcp/69.6.573. [DOI] [PubMed] [Google Scholar]
- 2.Thompson LD, Becker RC, Przygodzki RM, et al. Mucinous cystic neoplasm (mucinous cystadenocarcinoma of low-grade malignant potential) of the pancreas: a clinicopathologic study of 130 cases. Am J Surg Pathol. 1999;23:1–16. doi: 10.1097/00000478-199901000-00001. [DOI] [PubMed] [Google Scholar]
- 3.Wilentz RE, Albores-Saavedra J, Zahurak M, et al. Pathologic examination accurately predicts prognosis in mucinous cystic neoplasms of the pancreas. Am J Surg Pathol. 1999;23:1320–1327. doi: 10.1097/00000478-199911000-00002. [DOI] [PubMed] [Google Scholar]
- 4.Zamboni G, Scarpa A, Bogina G, et al. Mucinous cystic tumors of the pancreas: clinicopathological features, prognosis, and relationship to other mucinous cystic tumors. Am J Surg Pathol. 1999;23:410–422. doi: 10.1097/00000478-199904000-00005. [DOI] [PubMed] [Google Scholar]
- 5.Reddy RP, Smyrk TC, Zapiach M, et al. Pancreatic mucinous cystic neoplasm defined by ovarian stroma: demographics, clinical features, and prevalence of cancer. Clin Gastroenterol Hepatol. 2004;2:1026–1031. doi: 10.1016/s1542-3565(04)00450-1. [DOI] [PubMed] [Google Scholar]
- 6.Crippa S, Salvia R, Warshaw AL, et al. Mucinous cystic neoplasm of the pancreas is not an aggressive entity: lessons from 163 resected patients. Ann Surg. 2008;247:571–579. doi: 10.1097/SLA.0b013e31811f4449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yamao K, Yanagisawa A, Takahashi K, et al. Clinicopathological features and prognosis of mucinous cystic neoplasm with ovarian-type stroma: a multi-institutional study of the Japan pancreas society. Pancreas. 2011;40:67–71. doi: 10.1097/MPA.0b013e3181f749d3. [DOI] [PubMed] [Google Scholar]
- 8.Baker ML, Seeley ES, Pai R, et al. Invasive mucinous cystic neoplasms of the pancreas. Exp Mol Pathol. 2012;93:345–349. doi: 10.1016/j.yexmp.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 9.Le Borgne J, de Calan L, Partensky C. Cystadenomas and cystadenocarcinomas of the pancreas: a multiinstitutional retrospective study of 398 cases. French Surgical Association. Ann Surg. 1999;230:152–161. doi: 10.1097/00000658-199908000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sarr MG, Carpenter HA, Prabhakar LP, et al. Clinical and pathologic correlation of 84 mucinous cystic neoplasms of the pancreas: can one reliably differentiate benign from malignant (or premalignant) neoplasms? Ann Surg. 2000;231:205–212. doi: 10.1097/00000658-200002000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Warshaw AL, Compton CC, Lewandrowski K, et al. Cystic tumors of the pancreas. New clinical, radiologic, and pathologic observations in 67 patients. Ann Surg. 1990;212:432–443. doi: 10.1097/00000658-199010000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim SG, Wu TT, Lee JH, et al. Comparison of epigenetic and genetic alterations in mucinous cystic neoplasm and serous micro-cystic adenoma of pancreas. Mod Pathol. 2003;16:1086–1094. doi: 10.1097/01.MP.0000094088.37888.A6. [DOI] [PubMed] [Google Scholar]
- 13.Scott J, Martin I, Redhead D, et al. Mucinous cystic neoplasms of the pancreas: imaging features and diagnostic difficulties. Clin Radiol. 2000;55:187–192. doi: 10.1053/crad.1999.0341. [DOI] [PubMed] [Google Scholar]
- 14.Shyr YM, Su CH, Tsay SH, et al. Mucin-producing neoplasms of the pancreas. Intraductal papillary and mucinous cystic neoplasms. Ann Surg. 1996;223:141–146. doi: 10.1097/00000658-199602000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Suzuki Y, Atomi Y, Sugiyama M, et al. Cystic neoplasm of the pancreas: a Japanese multiinstitutional study of intraductal papillary mucinous tumor and mucinous cystic tumor. Pancreas. 2004;28:241–246. doi: 10.1097/00006676-200404000-00005. [DOI] [PubMed] [Google Scholar]
- 16.Yeh TS, Jan YY, Chiu CT, et al. Characterisation of oestrogen receptor, progesterone receptor, trefoil factor 1, and epidermal growth factor and its receptor in pancreatic cystic neoplasms and pancreatic ductal adenocarcinoma. Gut. 2002;51:712–716. doi: 10.1136/gut.51.5.712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bosman FT, Carneiro F, Hruban RH, et al. WHO Classification of Tumours of the Digestive System. Lyon, France: IARC. 2010 [Google Scholar]
- 18.Fujino Y, Suzuki Y, Ajiki T, et al. Surgical treatment for mucin-producing tumors of the pancreas. Hepatogastroenterology. 2001;48:1157–1161. [PubMed] [Google Scholar]
- 19.Shima Y, Mori M, Takakura N, et al. Diagnosis and management of cystic pancreatic tumours with mucin production. Br J Surg. 2000;87:1041–1047. doi: 10.1046/j.1365-2168.2000.01496.x. [DOI] [PubMed] [Google Scholar]
- 20.Sugiyama M, Atomi Y, Kuroda A. Two types of mucin-producing cystic tumors of the pancreas: diagnosis and treatment. Surgery. 1997;122:617–625. doi: 10.1016/s0039-6060(97)90136-7. [DOI] [PubMed] [Google Scholar]
- 21.Wilentz RE, Albores-Saavedra J, Hruban RH. Mucinous cystic neoplasms of the pancreas. Semin Diagn Pathol. 2000;17:31–42. [PubMed] [Google Scholar]
- 22.Yamaguchi K, Yokohata K, Noshiro H, et al. Mucinous cystic neoplasm of the pancreas or intraductal papillary-mucinous tumour of the pancreas. Eur J Surg. 2000;166:141–148. doi: 10.1080/110241500750009492. [DOI] [PubMed] [Google Scholar]
- 23.Goh BK, Tan YM, Chung YF, et al. A review of mucinous cystic neoplasms of the pancreas defined by ovarian-type stroma: clinicopathological features of 344 patients. World J Surg. 2006;30:2236–2245. doi: 10.1007/s00268-006-0126-1. [DOI] [PubMed] [Google Scholar]
- 24.Lewis GH, Wang H, Bellizzi AM, et al. Prognosis of minimally invasive carcinoma arising in mucinous cystic neoplasms of the pancreas. Am J Surg Pathol. 2013;37:601–605. doi: 10.1097/PAS.0b013e318273f3b0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tanaka M, Fernandez-del Castillo C, Adsay V, et al. International consensus guidelines 2012 for the management of IPMN and MCN of the pancreas. Pancreatology. 2012;12:183–197. doi: 10.1016/j.pan.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 26.Izumo A, Yamaguchi K, Eguchi T, et al. Mucinous cystic tumor of the pancreas: immunohistochemical assessment of “ovarian-type stroma”. Oncol Rep. 2003;10:515–525. [PubMed] [Google Scholar]
- 27.Kosmahl M, Pauser U, Peters K, et al. Cystic neoplasms of the pancreas and tumor-like lesions with cystic features: a review of 418 cases and a classification proposal. Virchows Arch. 2004;445:168–178. doi: 10.1007/s00428-004-1043-z. [DOI] [PubMed] [Google Scholar]
- 28.Shimizu Y, Yasui K, Yamao K, et al. Possible oncogenesis of mucinous cystic tumors of the pancreas lacking ovarian-like stroma. Pancreatology. 2002;2:413–420. doi: 10.1159/000065090. [DOI] [PubMed] [Google Scholar]
- 29.O’Brien DP, Sandanayake NS, Jenkinson C, et al. Serum CA19-9 is significantly up-regulated up to 2 years prior to diagnosis with pancreatic cancer: implications for early disease detection. Clin Cancer Res. 2014 doi: 10.1158/1078-0432.CCR-14-0365. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Adsay NV, Pierson C, Sarkar F, et al. Colloid (mucinous noncystic) carcinoma of the pancreas. Am J Surg Pathol. 2001;25:26–42. doi: 10.1097/00000478-200101000-00003. [DOI] [PubMed] [Google Scholar]
- 31.Klimstra DS. Cystic, mucin-producing neoplasms of the pancreas: the distinguishing features of mucinous cystic neoplasms and intra-ductal papillary mucinous neoplasms. Semin Diagn Pathol. 2005;22:318–329. doi: 10.1053/j.semdp.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 32.Adsay NV, Merati K, Basturk O, et al. Pathologically and biologically distinct types of epithelium in intraductal papillary mucinous neoplasms: delineation of an “intestinal” pathway of carcinogenesis in the pancreas. Am J Surg Pathol. 2004;28:839–848. doi: 10.1097/00000478-200407000-00001. [DOI] [PubMed] [Google Scholar]
- 33.Seidel G, Zahurak M, Iacobuzio-Donahue C, et al. Almost all infiltrating colloid carcinomas of the pancreas and periampullary region arise from in situ papillary neoplasms: a study of 39 cases. Am J Surg Pathol. 2002;26:56–63. doi: 10.1097/00000478-200201000-00006. [DOI] [PubMed] [Google Scholar]
- 34.Koh YX, Chok AY, Zheng HL, et al. Systematic review and meta-analysis comparing the surgical outcomes of invasive intraductal papillary mucinous neoplasms and conventional pancreatic ductal adenocarcinoma. Ann Surg Oncol. 2014;21:2782–2800. doi: 10.1245/s10434-014-3639-0. [DOI] [PubMed] [Google Scholar]
- 35.Waters JA, Schnelldorfer T, Aguilar-Saavedra JR, et al. Survival after resection for invasive intraductal papillary mucinous neoplasm and for pancreatic adenocarcinoma: a multi-institutional comparison according to American Joint Committee on Cancer Stage. J Am Coll Surg. 2011;213:275–283. doi: 10.1016/j.jamcollsurg.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 36.Schlitter A, Jang K, Saka B, et al. Intraductal tubulo-papillary neoplasms of the bile ducts: clinicopathologic and immunohistochemical analysis of 19 cases (Abstract) Mod Pathol. 2014;27:428A. [Google Scholar]
- 37.Rocha FG, Lee H, Katabi N, et al. Intraductal papillary neoplasm of the bile duct: a biliary equivalent to intraductal papillary mucinous neoplasm of the pancreas? Hepatology. 2012;56:1352–1360. doi: 10.1002/hep.25786. [DOI] [PubMed] [Google Scholar]
- 38.Jang KT. Hepatobiliary/Pancreas Pathology: SY11-2 Intracholecystic papillary-tubular neoplasm of the gallbladder. Pathology. 2014;46(suppl 2):S24. [Google Scholar]
- 39.Ohike N, Kim GE, Tajiri T, et al. Intra-ampullary papillary-tubular neoplasm (IAPN): characterization of tumoral intraepithelial neoplasia occurring within the ampulla: a clinicopathologic analysis of 82 cases. Am J Surg Pathol. 2010;34:1731–1748. doi: 10.1097/PAS.0b013e3181f8ff05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Adsay V, Jang KT, Roa JC, et al. Intracholecystic papillary-tubular neoplasms (ICPN) of the gallbladder (neoplastic polyps, adenomas, and papillary neoplasms that are >/ =1.0 cm): clinicopathologic and immunohistochemical analysis of 123 cases. Am J Surg Pathol. 2012;36:1279–1301. doi: 10.1097/PAS.0b013e318262787c. [DOI] [PubMed] [Google Scholar]
- 41.Lam MM, Swanson PE, Upton MP, et al. Ovarian-type stroma in hepatobiliary cystadenomas and pancreatic mucinous cystic neoplasms: an immunohistochemical study. Am J Clin Pathol. 2008;129:211–218. doi: 10.1309/U2BBP4EMBAHCM6E6. [DOI] [PubMed] [Google Scholar]