Abstract
p38, a member of the mitogen-activated protein kinase (MAPK) superfamily, is activated in response to a variety of cellular stresses and ligands. Since the genome of the nematode C. elegans has been sequenced, we sought to identify and characterize the nematode homolog of mammalian p38. By sequence analysis and RT-PCR, we isolated cDNAs encoding three kinases, PMK-1, PMK-2, and PMK-3, which we call p38 map kinases due to their high sequence identity with p38. The three genes are contiguous on chromosome IV and comprise an operon. By use of a GFP reporter, we found that the promoter of the pmks is active throughout the intestine. An active form of MAPK/ERK Kinase 6 (MEK6) phosphorylated and activated recombinant PMK-2 and PMK-3 in vitro. PMK-2 and PMK-3 phosphorylated Activating Transcription Factor-2 (ATF-2), indicating an activity similar to mammalian p38. When transfected into mammalian cells, these kinases, like p38, are stimulated by osmotic stresses.
Keywords: MAP kinase, MEK, stress response, MEKK
Introduction
The mitogen-activated protein kinases (MAPKs) comprise a family of serine/threonine kinases that function in a wide variety of biological processes. MAPKs are activated by tyrosine and threonine phosphorylation catalyzed by MAPK/ERK kinases (MEKs). The MEKs themselves are activated when they are phosphorylated by MEK kinases (MEKKs). These kinase cascades respond to an array of extracellular stimuli such as growth factors and environmental stressors.
Extracellular-signal Regulated Kinases (ERKs), Stress-Activated Protein Kinases/c-jun N-Terminal Kinases (SAPK/JNKs), and p38 are the three best characterized subfamilies of MAP kinases and have representatives in all eukaryotes. Genetic analysis of Drosophila and C. elegans has revealed that MAPK pathways are necessary for normal development. The ERK pathway in Drosophila, which includes D-Raf, the MEK1/2 ortholog D-sor1, and the ERK ortholog Rolled, mediates cell signals important for the differentiation of photoreceptor cells (1) among others. In C. elegans, the ERK1/2 pathway, consisting of LIN-45, MEK-2, and the ERK ortholog MPK-1, plays an important role in vulval development (2). More recently, components of the JNK pathway, jkk-1 and jnk-1, have been identified in C. elegans as part of a neuronal pathway controlling coordinated movement (3). Also, mek-1, a C. elegans homolog of a MEK in stress-sensitive pathways, plays a role in a nematode stress response (4).
The MAP kinase p38 is activated in response to various ligands and environmental conditions, such as hyperosmolarity, tumor necrosis factor (TNFα), and lipopolysaccharide (LPS) (5). Consistent with its functions in mammals, mammalian p38 complements a deletion of the yeast MAP kinase that mediates the response to glycerol (5). The kinase is inhibited by the pyridinyl imidazole SB203580 (6). Overexpression and in vitro experiments in mammals reveal that p38 can be activated by MEKs 3,4, and 6 (7). Here, we identify and characterize three p38 isoforms in C. elegans termed p38 Map Kinases 1-3 (PMK-1, PMK-2, and PMK-3). Interestingly, these three proteins appear to lie within an operon, allowing for the transcription of three highly related proteins from a single promoter. Their single promoter is active throughout the length of the intestine and the kinases are activated by osmotic stresses when expressed in mammalian cells. PMK-2 and PMK-3, which share the greatest identity to mammalian p38, are selectively phosphorylated and activated by only one of the three MEK family members that recognize mammalian p38s.
Materials and Methods
Cloning and Expression of p38 Homologs
Whole worm RNA was isolated using the Tri-reagent (Molecular Bioresources) and the RNA was subsequently used for RT-PCR with the cDNA cycle kit (Invitrogen) to isolate the cDNAs encoding PMK-1,2, and 3. Each of the cDNAs was amplified and inserted into the expression plasmids pRSET (Invitrogen) and either pCMV5-HA or pCMV5-(HA)3. All cDNAs were sequenced to ascertain that no errors occurred in the amplification. For splice leader 2 (SL2) primed RT-PCR, the sequence of the SL2 primer used was GGTTTTAACCCAGTTACTCAAG (8).
Expression in HEK 293 cells was achieved with calcium phosphate transfection (9). Cells were harvested 48 hours post transfection in lysis buffer (50 mM Tris, pH 8.0, 1% Triton X-100, 100 mM NaCl, 5 mM EDTA, 1 mM Na-orthovanadate, 50 mM β-glycerophosphate) containing a cocktail of protease inhibitors (100 μM PMSF, 50 μg/ml each aprotinin, leupeptin, pepstatin A). Cells were stimulated by incubation in medium containing either 0.5 M sorbitol or 0.7 M NaCl for 10 minutes prior to harvest.
Recombinant (His)6-PMK-1, (His)6-PMK-2, and (His)6-PMK-3 were purified from the BL21 strain of E. coli after four hours of induction with 100 μM isopropyl-β-D-thiogalactopyranoside (IPTG). Cells were lysed by sonication in 50 mM sodium phosphate, pH 8, supplemented with the protease inhibitors noted above. After centrifugation at 30,000 × g for 30 minutes, the supernatants were incubated with Ni2+-NTA agarose beads. The resin was washed in buffer containing 0.3 M NaCl, 50 mM NaH2PO4, and 20 mM imidazole. Proteins were then eluted in pH 7.0 buffer containing 200 mM imidazole, 0.3 M NaCl, and 50 mM NaH2PO4.
Kinase Assays
For immune-complex kinase assays, immunoprecipitation was performed by adding anti-HA antibody and protein A-Sepharose beads to cell lysates for 2 hours. The sedimented beads were washed 3 times in a buffer containing 0.75 M NaCl, 20 mM Tris, pH 8.0 and once in a buffer containing 20 mM Tris, pH 8.0, 10 mM MgCl2. Kinase assays contained 10 mM MgCl2, 30 mM Tris, pH 8.0, 100 μM ATP ([γ-32P ]ATP, 2-7 cpm/fmol), and 1 mM dithiothreitol (DTT). Reactions were stopped by the addition of electrophoresis sample buffer and analyzed by SDS/PAGE and autoradiography.
For linked kinase assays, 200 ng of recombinant (His)6-TAO1(1-416) was incubated with 50 ng of the indicated MEK proteins for 45 minutes at 30°C; 15% of this reaction was added to the second reaction containing the recombinant PMKs. 15% of this reaction was then transferred to a third reaction containing GST-ATF-2 at 10 μg/ml.
Recombinant DNA for GFP Expression
PCR from genomic DNA was employed to amplify a 3 kb fragment that included 1.5 kb upstream of the start codon of pmk-1 and extended into the third exon of this gene. This PCR product was inserted into pPD95.81 at its SphI and BamHI sites. A second construct that extended into the last exon of pmk-1 was also made and inserted into pPD95.75. Plasmids pPD95.81 and pPD95.75 were kindly provided by A. Fire (10). PCR was used to generate a fragment extending from 1.5 kb upstream of the start codon of pmk-1 to the last exon of pmk-2. This piece was fused to GFP by overlap extension PCR for expression analysis of pmk-2. Wild type (Bristol N2) worms were injected with the constructs at 75 ng/μl along with an equivalent amount of the rol-6(d)-containing plasmid, pRAK3 (10,11). Worms were maintained by usual methods and grown on a lawn of the HB101 strain of E. coli.
The strains used were DA1750 adEx1750 [pmk-1∷GFP rol-6(d)] and deletion strains DA1755 pmk-1(ok169) IV and VC36 unc-5(gk29) pmk-2(gk21) IV/nT1(IV); +/nT1(V)] The deletion strains were obtained from the gene knockout consortium.
Results
The C. elegans Genome Encodes Three Protein Kinases Highly Related to p38
Genes encoding four mammalian p38 family members (α, β, γ, δ) have been identified (7). Database searches with their sequences reveals three previously uncharacterized open reading frames (ORF) of C. elegans that have significant similarity to mammalian p38. They are contiguous on chromosome IV. RT-PCR was employed to clone cDNAs that encode these three kinases, termed p38 map kinases 1-3 (PMK-1, PMK-2, PMK-3). (Accession numbers are PMK-1, T32642; PMK-2, T32643; PMK-3, T29750.) PMK-3 shares the highest identity with p38 family members while PMK-1 has the least. PMK-3 is approximately 60% identical and 75% similar to p38β while PMK-2 is 50% identical and 65% similar and PMK-1 is 45% identical and 60% similar (12) (Figure 1). The cDNA sequences obtained for pmk-1 and pmk-2 are shorter than the sequences predicted from the C. elegans genome database (ACeDB) (13). For both pmk-1 and pmk-2, blocks of amino acids in the genefinder predictions not found in mammalian p38β are not encoded in the cDNAs cloned by RT-PCR.
Figure 1. Sequence alignment of p38β and PMK-1,2 and 3.

Sequences obtained from cloned cDNAs were aligned using multalin and boxshade [12]. PMK-3 shares the highest identity (60%) and similarity (75%) to p38β followed by PMK-2 and then PMK-1. The TXY motif is noted by asterisks.
A distinguishing feature of MAP kinases is the conserved TXY motif in the activation loop, which includes the two sites phosphorylated to activate the kinases. In the case of p38, the intervening residue X is glycine. This motif is conserved in pmk-2 and pmk-3. pmk-1 contains Q at this position. TQY does not match the motif for any characterized MAP kinase.
pmk-1, pmk-2, and pmk-3 Comprise an Operon
The C. elegans genome contains many genes that are predicted to fall within operons based on their distance from upstream and downstream genes. Like bacterial operons, these genes are transcribed from a promoter region that lies upstream of the first gene of the operon (14,15).
The proximity and direct orientation of pmk-1- pmk-3 suggest that they form an operon (figure 2A). Previous work has indicated that SL2 trans-splicing is a reliable marker of operon configuration (16,17). To find out whether pmk-1,2, and 3 form an operon, RT-PCR from whole worm RNA was performed with SL2 as the upstream primer. PCR products of pmk-2 and pmk-3 should be formed if SL2 trans-splicing occurs between pmk-1 and pmk-2, and between pmk-2 and pmk-3. As shown in figure 2B, these products are present, supporting the conclusion that the two downstream genes are trans-spliced and consistent with the hypothesis that the pmk genes form an operon. In some operons, the first gene is SL1 trans-spliced (16,18); however, we did not detect SL1 trans-splicing to any of the pmk genes by RT-PCR experiments (data not shown).
Figure 2. pmk-2 and pmk-3 are SL2 trans-spliced.
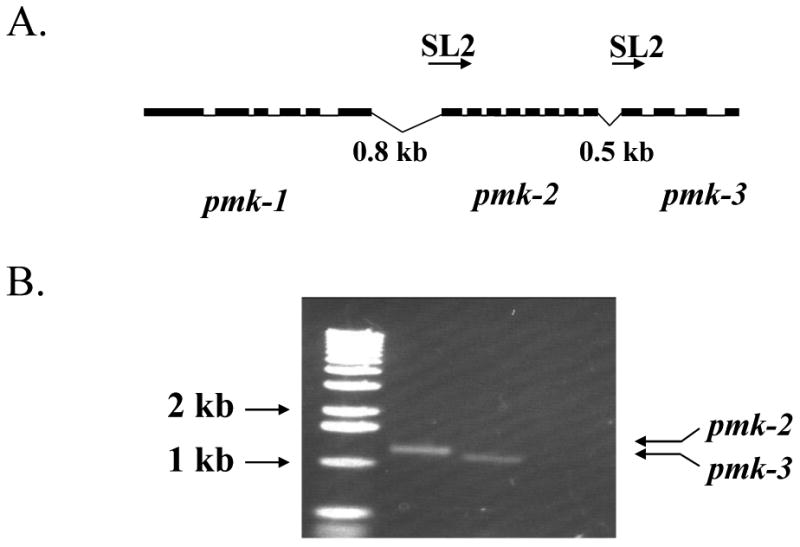
(A) A schematic of the location of the genes on chromosome IV. Boxed regions indicate exons. pmk-1 and pmk-2 are separated by 800 bases while 500 bases separate pmk-2 and pmk-3. (B) RT-PCR was performed on whole worm RNA using an oligonucleotide with the SL2 sequence as the upstream primer and the last 20 base pairs of either pmk-2 or pmk-3 as the downstream oligonucleotide. Amplified products were resolved on a 1% agarose gel and stained with ethidium bromide. Lane 1 is the size marker, lane 2 is pmk-2, and lane 3 is pmk-3.
Promoter of PMKs is Active in the Intestine
Using GFP as a reporter, we found that the pmk-1 promoter is active throughout the intestine. pmk-1∷GFP localizes to the nuclei of intestinal cells (figure 3). An identical expression pattern was found with two independent reporter constructs of pmk-1; one fusion construct had GFP linked to the first three exons of pmk-1 (DA1750) and the other fused GFP to the last exon and contained 90% of the coding region of pmk-1 (not shown). This nuclear localization was independent of growth conditions. A pmk-2∷ GFP fusion was expressed in the same cells but was not localized to the nuclei under any tested conditions (data not shown). Because mammalian p38 responds to cellular stress, attempts to affect cellular localization of the GFP reporter included growing the worms in the presence environmental stresses, such as heavy metals and high concentrations of NaCl (19,20). Because pmk-3 is the third gene of the operon, it is likely to have a similar expression pattern (15,21,22). We did not see any expression from fusion constructs for pmk-2 and pmk-3 in which the promoter region upstream of pmk-1 was excluded (data not shown). These data support the idea that pmk-2 and pmk-3 do not have separate promoter regions, but rather are expressed from the promoter region upstream of pmk-1.
Figure 3. GFP expression directed by the pmk-1 promoter.
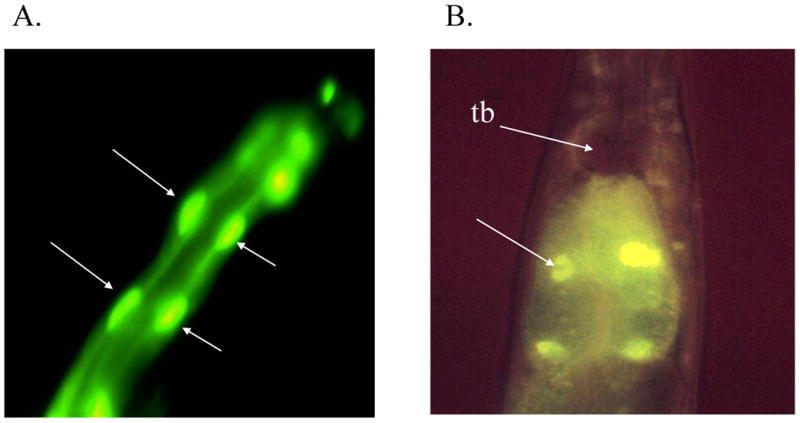
pmk-1∷GFP was injected into worms to determine its expression pattern. (A) Expression is seen throughout the intestine, with much of the GFP being localized to the nuclei of the intestinal cells (noted by arrows). (B) A higher magnification showing expression in the nuclei of the first four intestinal cells (one such nucleus in noted by an arrow). No promoter activity was detected in pharyngeal muscle, including the terminal bulb (tb).
PMKs Phosphorylate ATF-2, and PMK-2 and PMK-3 Are Selectively Activated by MEK6
In vitro, mammalian p38 is a substrate of MEKs 3,4, and 6 in stress-responsive MAPK pathways. In vivo, the best evidence exists for MEK3 and MEK6. The serine/threonine kinases TAO1 and TAO2 are capable of activating these MEKs toward p38 in vitro and in cells (23,24,25). Using recombinant proteins purified from bacteria, we performed linked kinase assays in order to test if the in vitro activity of PMK-1, PMK-2, and PMK-3 is comparable to mammalian p38α. We first activated MEKs 3,4, and 6 with TAO1. All these enzymes show enhanced phosphorylation of p38α, but none show enhanced phosphorylation of PMK-1 (not shown). On the other hand, MEK6 activated PMK-2 and PMK-3. We determined that TAO1 increases MEK6 activity toward PMK-3 five-fold (figure 4), and toward PMK-2 to a reduced extent (not shown). MEKs 3 and 4 do not phosphorylate PMK-2 (not shown) or PMK-3 (figure 4). The ability of PMK-3 to phosphorylate ATF-2 is increased eight-fold by phosphorylation by MEK6 but not by MEKs 3 or 4 (figure 4). MEK6 is able to recognize a more diverse group of structures than MEKs 3 and 4 (26); thus, it is not surprising that it is the only MEK with high activity toward PMK-3. The p38 inhibitor SB203580 inhibited the ability of both PMK-2 and PMK-3 to phosphorylate ATF-2 (figure 6). PMK-1 purified from bacteria was approximately 10 kDa smaller than predicted from its amino acid sequence when immunoblotted with anti-(His)6 antibody and was slightly smaller than both PMK-2 and PMK-3 as observed on immunoblots (data not shown). Because the (His)6 epitope was on the N-terminus of the fusion protein, this suggested that PMK-1 was cleaved on its C-terminus. This form of PMK-1 was not a substrate of MEKs 3, 4, or 6.
Figure 4. MEK6 phosphorylates and activates PMKs.
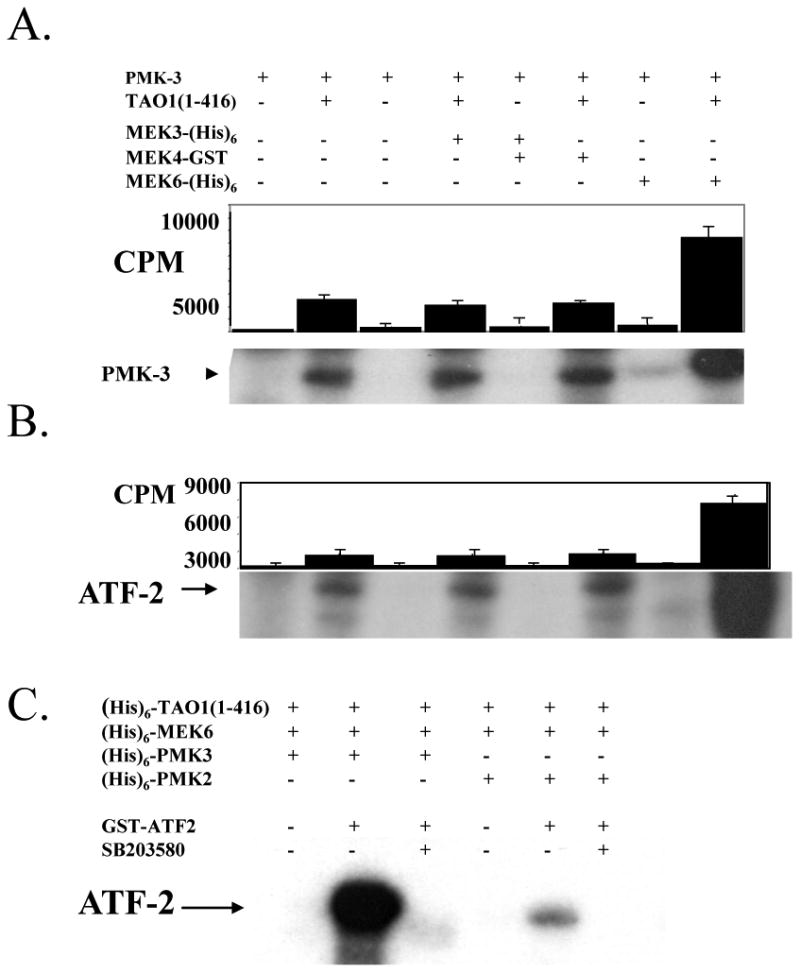
(A) Linked kinase reactions were performed using recombinant (His)6-TAO1(1-416), which was incubated alone or with recombinant MEK3, MEK4, or MEK6. A portion of this first reaction was incubated with recombinant (His)6-PMK-3 and analyzed by SDS/PAGE and autoradiography. Phosphorylation of PMK-3 was quantitated using a scintillation counter. (B) A portion of the linked reactions from A were incubated with ATF-2, analyzed by SDS/PAGE and ATF-2 phosphorylation was quantitated. (C) Activated PMK-2 and PMK-3 were incubated with ATF-2, resolved on SDS/PAGE and autoradiographed. Addition of SB203580 at 175 μM inhibited the ability of PMK-2 and PMK-3 to phosphorylate ATF-2. Note that PMK-3 has more activity than PMK-2 toward ATF-2.
PMK-1,2, and 3 are Stimulated by Osmotic Stresses in Mammalian Cells
Mammalian p38, like its yeast homolog Hog1, is activated by osmotic stresses such as sorbitol or high NaCl. We transfected epitope-tagged versions of PMK-1,2, and 3 into HEK-293 cells and stimulated the cells with 0.5 M sorbitol or 0.7 M NaCl for 15 minutes. The overexpressed proteins were immunoprecipitated and the immune-complexes were used for kinase assays (figure 5). All three PMK proteins phosphorylate ATF-2 in response to these stimuli, thus displaying the sensitivity of mammalian p38 to osmotic stress. In response to these stresses, PMK-3 exhibited much greater kinase activity than PMK-2 and PMK-1. This may be due to its higher expression level in mammalian cells and its greater recognition by mammalian kinases. Although PMK-1 and PMK-2 were not strongly activated in vitro by MEK6 as PMK-3 was, they do respond like p38 to a physiological stress.
Figure 5. PMK proteins are stimulated by osmotic stresses in mammalian cells.
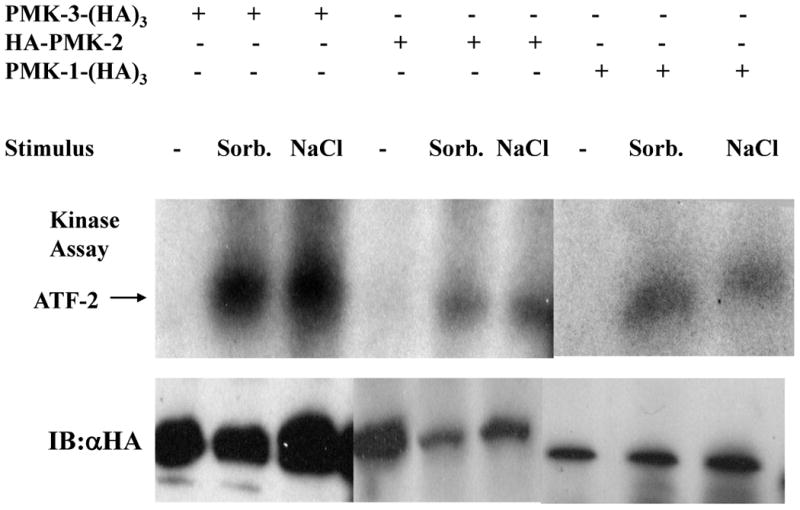
HEK-293 cells were transfected as indicated and incubated in medium containing either 0.5 M sorbitol or 0.7 M NaCl for fifteen minutes prior to harvest. Proteins were immunoprecipitated with a monoclonal anti-HA antibody and incubated with ATF-2 in kinase assays. Reactions were analyzed by SDS/PAGE and autoradiography.
Null Mutants of pmk-1, pmk-2
Recently, the C. elegans knockout consortium has generated deletion mutants pmk-1(ok169) and pmk-2(gk21). pmk-1(ok169) mutants have no observable growth defects or hypersensitivity to several known stresses. However, pmk-2 null mutants arrest and die in larval stage 1 (L1). (Although the pmk-2 strain also carries a deletion in unc-5, the phenotype of unc-5 is a locomotion defect that does not cause larval arrest or premature death.) The intestinal granule content of the L1 arrested worms deviates from normal, with fewer cells appearing to contain fat under microscopic evaluation (J McKay, personal communication, and data not shown). However, the intestine appears to be developing normally at the time of arrest. However, this larval lethality was not replicated and no abnormality of the worms was noted in dsRNAi-produced knockouts of PMK-2. Therefore, the larval lethality of the strain VC36 is most likely due to a distinct mutation of this strain unrelated to PMK-2. dsRNAi experiments aimed at producing a functional knockout of pmk-3 failed to yield worms with a discernible phenotype (not shown).
Discussion
We have identified and characterized three proteins in C. elegans highly similar to mammalian p38 isoforms. MAPK family members are phosphorylated by dual specificity kinases in a TXY motif in the phosphorylation lip. PMK-2 and PMK-3 contain the TGY motif characteristic of the p38 subfamily; the third homolog, PMK-1, has a TQY motif, which differs from p38, ERK (TEY), and JNK (TPY). Interestingly, the three pmk genes lie within an operon. Although 25% of the genome consist of operons (27), the function of operons in C. elegans is uncertain. In the case of the pmk genes, the only known mechanism for initiating transcription of pmk-2 and pmk-3 is the promoter upstream of pmk-1. This single promoter is thus responsible for the production of three proteins that share a high percentage of sequence identity. Operons may be a consequence of conservation in the relatively small genome of C. elegans (27). Because MEK6 did not activate all the PMKs, our in vitro data support the idea that PMKs are regulated by different MEK family members. If this is so, differential regulation of PMK-1,2, and 3 may allow them to have unique functions. Their linkage in an operon may allow for their coordinated and stoichiometric production in the same cells to mediate different reactions.
In unstimulated mammalian cells, many MAP kinases are found in the cytoplasm and upon stimulation, they accumulate in the nucleus. However, the subcellular localization of p38 is poorly understood. Experiments in myocytes indicate that inactive p38 localizes around the nucleus and diffusely throughout the cytoplasm and is translocated to the nucleus upon activation, after which it returns to the cytoplasm (28). On the other hand, other experiments suggest that activation does not cause nuclear redistribution (29). Other work showed that under resting conditions, p38 localizes to the nucleus where it is activated and subsequently translocates to the cytoplasm after phosphorylating its substrate (30). The constitutive localization of PMK-1 to the nucleus and PMK-2 to the cytoplasm suggests differential mechanisms of cellular localization that may be tied to their functions. Stresses such as heavy metals and high concentration of NaCl did not affect cellular localization. PMK-1 may contain a nuclear localization signal, although it doesn't contain a good match to any known consensus nuclear localization sequence.
Without identifying upstream activators of the PMKs, we can only speculate on the function of these kinases. Since p38 has been linked to stress responses in mammalian cells, PMK-1, 2, and 3 may form part of a stress-responsive pathway in the intestine of worms. Exposure of worms to heavy metals has been shown to elicit stress responses mainly in the pharynx but also in the intestine (21). pmk-1 mutants do not show hypersensitivity to heavy metals, such as copper and zinc.
The larval arrest/death phenotype and altered intestinal granules of the pmk-2 deletion mutants is a provocative phenotype but is not replicated by dsRNAi experiments. At this time, we cannot attribute this lethal phenotype to the lack of pmk-2. The observation of abnormal intestinal granules is interesting because p38 has been linked to adipogenesis in mammalian 3T3 cells (31). There are no fat cells in C. elegans; rather they store fat as granules in the intestinal cells (32).
Acknowledgments
We thank members of the Cobb and Avery laboratories for many helpful discussions and Dionne Ware for administrative assistance. This work was supported by grants from the National Institutes of Health (GM53032 to MHC and HL46154 to LA). KB was supported in part by an NIH Medical Scientist Training Program Grant and the Perot Family Foundation.
Abbreviations
- MAPK
mitogen activated protein kinase
- MEK
MAPK/ERK kinase
- MEKK
MEK kinase
- TAO
Thousand and One amino acid kinase
- GFP
green fluorescent protein
- ORF
open reading frame
- kDa
kilodalton
- PAGE
Polyacrylamide gel electrophoresis
- PMSF
phenylmethylsulfonyl fluoride
- UTR
untranslated region
- PCR
polymerase chain reaction
- DTT
dithiothreitol
- cDNA
DNA complementary to RNA
- HEK
human embryonic kidney
- IB
immunoblot
- IP
immunoprecipitation
- HSP
heat shock protein
- LPS
lipopolysaccharide
- TNF
tumor necrosis factor
- HOG
high osmolarity glycerol
- ATF-2
Activating Transcription Factor-2
References
- 1.Wassarman DA, Therrien M, Rubin GM. The Ras signaling pathway in Drosophila. Curr Opin Gen Dev. 1995;5:44–5. doi: 10.1016/s0959-437x(95)90052-7. [DOI] [PubMed] [Google Scholar]
- 2.Sundaram M, Han M. Control and integration of cell signaling pathways during C. elegans vulval development. Bioessays. 1996;18:473–80. doi: 10.1002/bies.950180609. [DOI] [PubMed] [Google Scholar]
- 3.Kawasaki M, Hisamoto N, Iino Y, Yamamoto M, Ninomiya-Tsuji J, Matsumoto K. A Caenorhabditis elegans JNK signal transduction pathway regulates coordinated movement via type-D GABAergic motor neurons. EMBO J. 1999;18:3604–3615. doi: 10.1093/emboj/18.13.3604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koga M, Zwaal R, Guan K, Avery L, Ohshima Y. A caenorhabditis elegans MAP kinase kinase, MEK-1, is involved in stress responses. EMBO J. 2000;19:5148–56. doi: 10.1093/emboj/19.19.5148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Han J, Lee JD, Tobias PS, Ulevitch RJ. Endotoxin induces rapid protein tyrosine phosphorylation in 70Z/3 cells expressing CD14. J Biol Chem. 1993;268:25009–14. [PubMed] [Google Scholar]
- 6.Lee JC, Laydon JT, McDonnell PC, Gallagher TF, Kumar S, Green D, McNulty D, Blumenthal MJ, Heys JR, Landvatter SW, et al. A protein kinase involved in the regulation of inflammatory cytokine biosynthesis. Nature. 1994;72:739–46. doi: 10.1038/372739a0. [DOI] [PubMed] [Google Scholar]
- 7.Ono K, Han J. The p38 signal transduction pathway: Activation and Function. Cell Signaling. 2000;12:1–13. doi: 10.1016/s0898-6568(99)00071-6. [DOI] [PubMed] [Google Scholar]
- 8.Huang X, Hirsh D. A second trans-spliced RNA leader sequence in the nematode C. elegans. Proc Natl Acad Sci USA. 1989;86:8640–44. doi: 10.1073/pnas.86.22.8640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 1989. [Google Scholar]
- 10.Mello C, Fire A. DNA Transformation. In: Epstein HF, Shakes DC, editors. Caenorhabditis elegans: Modern Biological Analysis of an Organism. Academic Press; NY: 1995. pp. 452–482. [Google Scholar]
- 11.Davis MW, Somerville D, Lee RY, Lockery S, Avery L, Fambrough DM. Mutations in the Caenorhabditis elegans Na,K-ATPase Alpha-subunit Gene, eat-6, Disrupt Excitable Cell Function. J Neurosci. 1995;15:8408–18. doi: 10.1523/JNEUROSCI.15-12-08408.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Corpet F. Multiple Sequence Alignment with Hierarchical clustering. Nucleic Acids Res. 1988;16:10881–90. doi: 10.1093/nar/16.22.10881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Durbin R, Thierry-Mieg J. The ACEDB genome database. In: Sandor Suhai., editor. Computational Methods in Genome Research. Plenum; New York: 1994. [Google Scholar]
- 14.Blumenthal T. Trans-splicing and polycistronic transcription in C. elegans. Trends in Genetics. 1995;11:132–36. doi: 10.1016/s0168-9525(00)89026-5. [DOI] [PubMed] [Google Scholar]
- 15.Huang LS, Tzou P, Sternberg PW. The lin-15 locus encodes two negative regulators of Caenorhabditis elegans vulval development. Mol Biol Cell. 1994;5:395–411. doi: 10.1091/mbc.5.4.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ross L, Freedman, Rubin C. Structure and Expression of Novel spliced leader sequence RNA genes in C. elegans. J Biol Chem. 1995;270:22066–75. doi: 10.1074/jbc.270.37.22066. [DOI] [PubMed] [Google Scholar]
- 17.Evans D, Zorio D, MacMorris M, Winter C, Lea K, Blumenthal T. Operons and SL2 transsplicing exist in nematodes outside the genus Caenorhabditis. Proc Natl Acad Sci USA. 1997;94:9751–56. doi: 10.1073/pnas.94.18.9751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Williams C, Xu L, Blumenthal T. SL1 trans-splicing and 3′-end formation in a novel class of C. elegans operon. Mol Cell Biol. 1999;19:376–83. doi: 10.1128/mcb.19.1.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jones D, Stringham EG, Babich SL, Candido EP. Transgenic strains of the nematode C. elegans in biomonitoring and toxicology: effects of captan and related compounds on the stress response. Toxicology. 1996;109:119–27. doi: 10.1016/0300-483x(96)03316-1. [DOI] [PubMed] [Google Scholar]
- 20.Stringham E, Candido P. Transgenic hsp16-lacZ Strains of the Soil Nematode C. elegans as Biological Monitors of Environmental Stress. Env Tox Chem. 1994;13:1211–1220. [Google Scholar]
- 21.Page AP. Cyclophilin and protein disulfide isomerase genes are co-transcribed in a functionally related manner in Caenorhabditis elegans. DNA Cell Biol. 1997;16:1335–43. doi: 10.1089/dna.1997.16.1335. [DOI] [PubMed] [Google Scholar]
- 22.Land M, Islas-Trejo A, Rubin CS. Origin, properties, and regulated expression of multiple mRNAs encoded by the protein kinase C1gene of Caenorhabditis elegans. J Biol Chem. 1994;269:14820–7. [PubMed] [Google Scholar]
- 23.Hutchison M, Berman KS, Cobb MH. Isolation of TAO1, a protein kinase that activates MEKs in stress-activated protein kinase cascades. J Biol Chem. 1998;273:28625–32. doi: 10.1074/jbc.273.44.28625. [DOI] [PubMed] [Google Scholar]
- 24.Chen Z, Hutchison M, Cobb MH. Isolation of the protein kinase TAO2 and identification of its mitogen-activated protein kinase/extracellular signal-regulated kinase kinase binding domain. J Biol Chem. 1999;274:28803–7. doi: 10.1074/jbc.274.40.28803. [DOI] [PubMed] [Google Scholar]
- 25.Chen Z, Cobb MH. Regulation of stress-responsive MAP kinase pathways by TAO2. J Biol Chem. 2001;276:16070–5. doi: 10.1074/jbc.M100681200. [DOI] [PubMed] [Google Scholar]
- 26.Wilsbacher J, Goldsmith E, Cobb MH. Phosphorylation of MAP kinases by MAPK/ERK kinases involved Multiple Regions of MAP kinases. J Biol Chem. 1999;274:16988–94. doi: 10.1074/jbc.274.24.16988. [DOI] [PubMed] [Google Scholar]
- 27.Zorio D, Cheng N, Blumenthal T, Spieth J. Operons as a common form of chromosomal organization in C. elegans. Nature. 1994;372:270–72. doi: 10.1038/372270a0. [DOI] [PubMed] [Google Scholar]
- 28.Maulik N, Yoshida T, Zu Y, Sato M, Banerjee A, Das D. Ischemic preconditioning triggers tyrosine kinase signaling: a potential role for MAPKAP kinase 2. Am J Phys. 1998;275:H1857–64. doi: 10.1152/ajpheart.1998.275.5.H1857. [DOI] [PubMed] [Google Scholar]
- 29.Raingeaud J, Gupta S, Rogers J, Dickens M, Han J, Ulevitch R, Davis R. Pro-inflammatory cytokines and environmental stress cause p38 mitogen-activated protein kinase activation by dual phosphorylation on tyrosine and threonine. J Biol Chem. 1995;270:7420–26. doi: 10.1074/jbc.270.13.7420. [DOI] [PubMed] [Google Scholar]
- 30.Ben-Levy R, Hooper S, Wilson R, Paterson H, Marshall C. Nuclear export of the stress-activated protein kinase p38 mediated by its substrate MAPKAP kinase-2. Curr Biol. 1998;8:1049–57. doi: 10.1016/s0960-9822(98)70442-7. [DOI] [PubMed] [Google Scholar]
- 31.Morooka T, Nishida E. Requirement of p38 mitogen activated protein kinase for neuronal differentiation in PC12 cells. J Biol Chem. 1998;273:24285–88. doi: 10.1074/jbc.273.38.24285. [DOI] [PubMed] [Google Scholar]
- 32.White J. Anatomy. In: Wood WB, editor. The Nematode Caenorhabditis elegans. Cold Spring Harbor; New York: 1988. pp. 81–122. [Google Scholar]


