Summary
The pharynx of C. elegans, a model system for neural networks and for membrane excitability, has been chiefly studied by observing its behavior in normal worms, in mutant worms, and in worms lacking pharyngeal neurons. To complement this behavioral approach, we devised a method for recording currents produced by changes in pharyngeal muscle membrane potential. The electrical records, called electropharyngeograms, contain transients caused by pharyngeal muscle action potentials and by inhibitory synaptic transmission between pharyngeal neuron M3 and the muscle. Using the electropharyngeograms, we show that γ-aminobutyric acid is not likely to be the M3 neurotransmitter, that synaptic transmission is present but abnormal in mutants lacking synaptotagmin, and that worms mutant in the eat-4 gene are defective for M3 function or transmission.
Introduction
A major goal of neurobiology is to understand the cellular and molecular bases of behavior. The ideal system for approaching this goal should offer several experimental advantages. First, it should have a simple and well-described behavior. Second, the cells that orchestrate this behavior should be few in number and identifiable. Third, there should be a means for studying the physiological properties of these cells and their interconnections. And fourth, there should be a way to identify and to study in a biological context the molecules that are responsible for these physiological properties.
The pharynx of the nematode Caenorhabditis elegans has several features that make it suitable for cellular and molecular studies of behavior. The behavior of the pharynx is simple and well described. It consists of two motions, pumps and isthmus peristalses, which bring food into the pharyngeal lumen, grind it up, and pass it to the intestine (Doncaster, 1962; Seymour et al., 1983; Avery and Horvitz, 1989). There is a small number of cells in the pharynx. Of the 60 cells in the pharynx, 20are muscle cells of 8 anatomical types, 20 are neurons of 14 anatomical types, and the rest are structural and glandular cells (Albertson and Thomson, 1976). Serial section electron micrographs have been used to reconstruct the anatomy of each of these cells and to identify connections between them (Albertson and Thomson, 1976). The pharyngeal neurons constitute a nearly autonomous nervous system since they make a connection with only one bilaterally symmetric pair of extrapharyngeal neurons (Albertson and Thomson, 1976).
Roles of the pharyngeal nervous system in the behavior of the organ have been studied by laser ablating identified neurons (Avery and Horvitz, 1987, 1989; Avery, 1993b). The surprising result of these studies is that, even in the absence of all pharyngeal neurons, pharyngeal pumping continues (Avery and Horvitz, 1989). In fact, only one pharyngeal neuron, M4, is essential for feeding and hence for life (Avery and Horvitz, 1987, 1989). Animals lacking the other 19 pharyngeal neurons grow slowly and have abnormal pumping motions, yet they reach adulthood and are fertile. Therefore, mutants that affect the function of these 19 neurons can be readily isolated and propagated (Avery, 1993a). By comparing the pumping behavior of mutants to the behavior produced by killing identified neurons, it is possible to make educated guesses for the site of action of the mutation. The use of the laser ablation method to assign behavioral roles to neurons and thus provide a context for characterizing mutations has proven extremely powerful in the genetic dissection of other behaviors in C. elegans (for review see Bargmann, 1993). By the molecular cloning of genes altered by some of these mutations, molecules that function in specific behaviors have been identified and studied (e.g., Driscoll and Chalfie, 1991).
Though powerful for the initial analysis of nervous system function, the behavioral approach has limitations. First, unless the behavioral effect of a neuronal kill is obvious, one may not notice the behavior affected by the neuron. Second, the temporal resolution of behavioral observations is limited by camera sampling rate and by the ability of the experimenter to see the motion. Neuronal signaling events are typically much faster than the behavioral output perceived by the experimenter. Third and most important, the behavior represents only the final output of the neuronal network acting on the muscle. The cellular and network properties that function in the pharynx cannot be inferred from the behavior alone. To overcome these limitations, we developed a simple extracellular recording method to study the electrical properties of the pharynx. We combined this method with laser ablations of identified neurons to provide an electrical explanation for a behavioral role of a pharyngeal neuron type (Avery, 1993b). Our results suggest that the pharyngeal neuron M3 is an inhibitory spiking motor neuron which can trigger relaxation of pharyngeal muscle by causing inhibitory postsynaptic potentials. We used the M3-electrical phenotype to determine whether genes with known roles are necessary for M3 neurotransmission and to show that the gene eat-4 is necessary for M3 function or neurotransmission.
Results
Anatomy and Behavior of the Pharynx
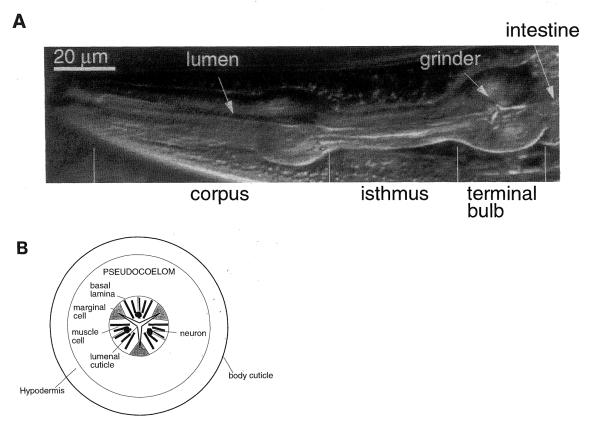
The C.elegans pharynx, a tubular organ between the mouth and the intestine, is suspended in the pseudocoelom, the body cavity of the worm. (Anatomical descriptions are based on Albertson and Thomson, 1976.) It is divided into three functional parts (Figure 1A). The corpus filters bacteria from worm’s surroundings and transports them to theisthmus (Seymour et al., 1983), which then delivers these bacteria to the terminal bulb (Avery and HorvitL,1987). The terminal bulb grinds up the bacteria and passes the debris to the intestine (Doncaster, 1962). Two motions carry out these pharyngeal functions (Avery and Horvitz, 1989). The first is a pump, which consists of a near-simultaneous contraction of the corpus, anterior isthmus, and terminal bulb, followed some 200 ms later by a near-simultaneous relaxation of these parts. During a pump, bacteria suspended in liquid are sucked into the lumen of the corpus and trapped there, whereas the liquid is expelled. At the same time, bacteria already in the corpus are moved posteriorly, and bacteria in the terminal bulb are ground up and moved to the intestine. The second is an isthmus peristalsis, a posteriorly directed wave of contraction of the posterior isthmus. This motion transports food trapped in the anterior isthmus to the terminal bulb.
Figure 1. Anatomy oi the Pharynx.
(A) lateral view of a pharynx (anterior is to the left). This shows the functional subdivisions of the pharynx: the corpus, isthmus, and terminal bulb. In the terminal bulb, acuticular specialization called the grinder mechanically disrupts bacteria before they pass to the intestine.
(B) Schematic of a transverse view based on reconstruction from serial section electron micrographs by Albertson and Thomson (1976) and anatomical drawings by White (1988). Two single cell layer epithelia, the pharyngeal muscle and the hypodermis, delimit the body cavity of the worm called the pseudocoelom. Three pharyngeal muscle cells situated with triradiate symmetry surround the pharyngeal lumen. Their contractile fibers are radial so that when they contract, the pharyngeal lumen opens. Their apical surface is lined by cuticle, whereas their basal surface is lined by a basal lamina. Between the pharyngeal muscle cells at the apkes of the lumen are three marginalcells. Pharyngeal neurons are embedded in grooves of the basal membranes of pharyngeal musdc in eachof tho three sectors. The boundary of the worm’s body is formed by a syncylial epithelium called the hypodermis. The hypodermis secretes a cuticle to line the body of the worm. Structures within the pseudocoelom such as the extrapharyngeal neurons and the body muscles are not shown.
In addition to their structural and contractile functions, pharyngeal muscle cells serve an epithelial function (Figure 1B). They separate the internal milieu of the worm, the pseudocoelom, from the external world, the pharyngeallumen. They are radially oriented polarized cells: their basalsurfaces are lined bya basal lamina and face the pseudocoelomic compartment, whereas their apical surfaces are Iined by a cuticle and face the lumenal compartment (Figure 1B).
Extracellular Signals Correlate with Pharyngeal Motions
We recorded pharyngeal electrical activity by sucking the head of a worm into a pipette to create two electrical compartments separated by the seal between the pipette and the cuticle of the worm’s head. We used a high input impedance voltage-following amplifier to record voltage transients produced by currents flowing out the worm’s mouth and across the resistance (5-20 MΩ) formed at the junction between the worm and the pipette. Alternatively, we measured currents flowing out the mouth and into the pipette by using a low input impedance current-following amplifier. These two methods gave similar results a regular pattern of electrical signals, seen only when the pharynx is pumping (Figure 2). We call the electrical record an electropharyngeogram or EPG.
Figure 2. EPG from Wild-Type Worms.
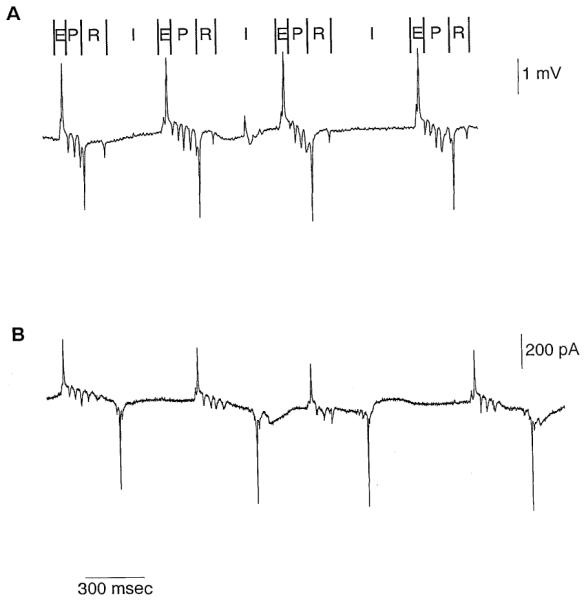
(A) Voltage recording made from a wild-type worm. The magnitude of the voltage signals is variable (compare with other figures) since it depends on the tightness uf the seal between the pipette and the worm’s body and on the size of the worm. Above the voltage trace, we define the phases of the EPG. The E, P, and R phases correspond with pharyngeal pu rn ps, whereas the I phases are the interpurnp intervals. The E phase consists of one or more (typically two) positive transients. The P phase contains a variable num lier oi negalive transients. The R phase typically rnnsists uf two negative transients, the iirsl larger than the second. Rarely, the electrical records have only one R phase transient (data not shown). The I phase contains occasional positive transients. Serotonin (10 mM) was included in the hath to stimula le pharyngeal pumping.
(B) Current recording made from a wild-type worm. We define positive current as current flowing out of the mouth. The magnitude of the current transients is less variable than the magnitude: of the voltage transients her.ause it docs not depend on the tightness of the seal formed between the pi pctte and the body of the worm. It docs, however, depend on the size of the worm (for example, compare with Figure 4B). Serotonin (10 mM) was induced in the bath to stimulate pumping.
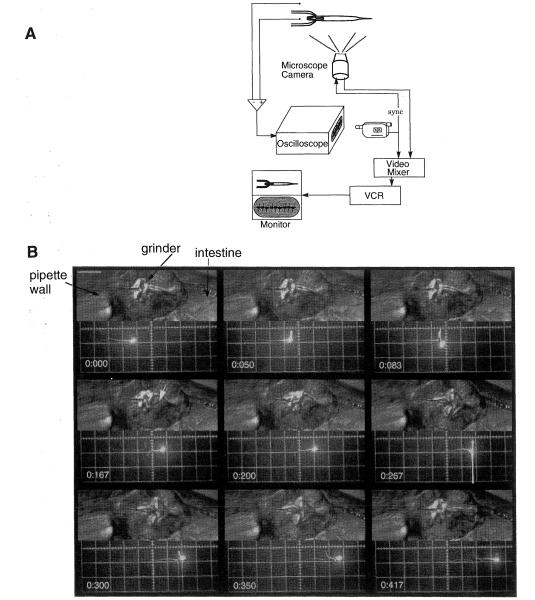
To find out whether these signals correlated precisely with pumping, we superimposed a display of the electrical signals on a synchronized video display of the behavior of the worm (Figure 3). This analysis showed that the electrical signals are indeed tightly correlated with pharyngeal motions. Furthermore, particular subsets of the electrical transients correspond to particular pharyngeal motions.
Figure 3. Video Correlation of the Electrical Signals and Pharyngeal Motions.
(A) Method: The worm’s head is sucked into a pipette. The potential difference between the pipette and the bath is amplified and sent to an oscilloscope. The oscilloscope display is filmed with a camcorder camera synchronized vith a microscope camera recording the worm’s behavior. The two video signals are combined into a split screen image by a video mixer.This image is stored on a videotape for subsequent analysis.
(B) Nine video fields corresponding to one pharyngeal pump. The unc-54 mutation, which encodes a body muscle myosin heavy chain (Macleod et al., 1977), was used to minimize body motion. The top half of each image shows a Nomarski image of the head oi the worm in the pipette (anterior is to the left). Only the terminal bulb is shown, but corpus motions arc tightly coupled to those of the terminal bulb (Avery and Horvitz, 1989; Avery, 1993a, 1993b). The bottom shows the simultaneously recorded electrical signals displayed on an oscilloscope. Relevant landmarks arc shown in the top left video picture. Bar, 10 μm. On the oscilloscope display, a vertical division is 1 mV, and a horizontal division is 100 ms. Time in seconds is shown in the bottom left of each video field. The voltage signal was high pass filtered before display to minimize baseline drift.
At time 0, the grinder is in its resting, anterior position while the oscilloscope beam is at O mV. The next two fields shown, of time 50 and 83 ms, show that the two electrical transients corresponding to excitation of the pharynx occur before any perceptible motion in the terminal bulb. Contraction of the terminal bulb is obvious at 167 ms and 200 ms. (By analyzing the video in slow motion, we can perceive a slight terminal bulb motion as early as 100 ms.) The grinder moves posteriorly, and the lumen of the terminal bulb opens (white arrow points to the open lumen in the 167 ms field). At 267 ms, a large negative transient occurs while the terminal bulb is in its maximally contracted position. Relaxation motion of the terminal bulb can be seen at 350 ms following the second, smaller R phase transient. The grinder moves forward, and the lumen closes. In the last video field shown, the grinder has returned to its resting, forward position.
We divided the typical EPG into four phases: E, P, R, and I (Figure 2A). The E, P, and R phases correlate with pharyngeal pumps. The E (for excitation) phase usually consists of two positive transients, the first smaller than the second, that correlate with contraction of the pharynx. The R (for relaxation) phase usually consists of two negative transients, the first larger than the second, that correlate with relaxation of the pharynx. Between the E and R phases is the P (for plateau) phase. The P phase contains a variable number of negative transients. These transients are present insome but not all P phases. They have no perceptible behavioral correlate. The I(for interpumpl phase has occasional solitary positive transients that do not correlate with any perceptible pharyngeal motion.
The Electrical Transients Represent Electrical Events in Pharyngeal Muscle Membranes
Although the transients in the EPG correlated with motions of the pharynx, it is unlikely that they are artifacts of the motion itself for three reasons. First, if the motion is causing the electrical signals, then it should occur at the same time or before the electrical event but never after. In fact, we perceived the beginningof contraction 17-50ms (one to three video fields) after the E phase transients, but never simultaneous with or before them (Figure 3B; data not shown). Likewise, we perceived the beginning of relaxation within 33 ms after the first relaxation transient but never before. Second, if motion of the pharynx in the pipette is causing the electrical signals, then a recording with the pharynx outside the pipette should eliminate or change the signals. To test this, we sucked the worm’s tail into the pipette instead of the head, such that the pharynx was outside the pipette. Recordings made from the tail consist of the same signals as those in recordings made from the head, but of opposite polarity (Figure 4A). The polarity is reversed because the electrodes are reversed: the bath electrode is now electrically continuous with the pharyngeal lumen. Third, if motion is causing the electrical transients, then decreasing the motion without changing the electrical properties of pharyngeal membranes should decrease the signals in the EPG. We recorded from act-2, an actin mutant with severely attenuated pharyngeal motions (Avery, 1993a). The magnitude of currents recorded from act-2 is about the same as that from wild-type worms of the same age and nutritional state (Figure 4B). Therefore, we conclude that the electrical signals represent underlying electrical events in the pharyngeal muscle membranes.
Figure 4. Controls for Motion Artifacts.
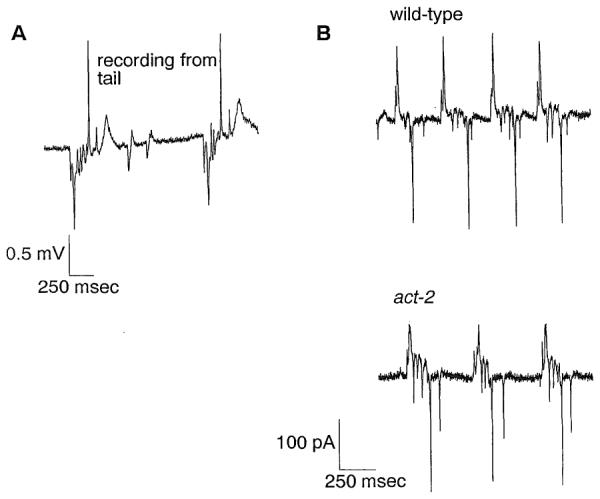
(A) Voltage record obtained by sucking the tail of a wild-type worm into the pipette. The whole body of the worm exrnpt the anterior part, which contains the pharynx, was in the pipette. Because in this arrangement the leads of the differential amplifier are electrically reversed from the arrangement in which the head is sucked into the pipelle, the polarity of the record is reversed. Exdtation current flows across the resistance between the negative and positive leads to produce negative voltage transients, whereas repolarization current flows in the opposite diredion to produce positive transients. A slow wave of unknown origin following the R phase in this record is also occasionally seen in the EPG recorded irom the head.
(B) Current records of wild-type (top) and act-2 mutants (bottom). For both recordings, the worm was sucked up to the terminal bulb such that only the corpus and isthmus were in the pipette. The wild-type worm was starved before the recording to normalize for the size and nutritional state of act-2 (which is severely eating defective and hence starved), thus making the comparison more meaningful. The magnitude of the current transients is approximately the same for both worms.
How do electrical events in the pharynx cause the EPGtransients? Depolarization of pharyngeal muscles results in positive transients, whereas repolarization results in negative transients. Conventional extracellular signals recorded directly from the external surface of active membranes reflect the direction of current flow. Current flow into the membrane, which depolarizes the membrane, is recorded as a negative potential by an external pipette, whereas current flow out of the membrane, which hyperpolarizes the membrane, is recorded as a positive potential (for examples see Brooks and Eccles, 1947; Fatt and Katz, 1952). Hence, the polarity of the transients in the EPG is opposite of that expected for a recording from the extracellular surface of an active membrane. However, it is consistent with an indired recording from the intracellular surface of such a membrane.
This polarity can be explained if one assumes that the action potential currents occur in the basal membranes of pharyngeal muscle cells and that the apical membranes are passive followers of the potential changes at the basal membranes (Figure 5). This assumption makes biological sense. Only the basal surface of pharyngeal muscle faces a regulated ionic environment, that of the pseudocoelom. The apical surface faces a lumenal solution that is influenced by the variable ionic environment of the worm (normally, soil water) and hence not conducive to the generation of consistent action potentials. Experimental support for the idea of a passive lumenal membrane in nematodes comes from the work of Byerly and Masuda (1979), who showed that, in the parasitic nematode Ascaris, muscle action potentials recorded from the pharyngeal lumen are of the same polarity as those recorded from pharyngeal muscle cells. With passive apical membranes, potential changes in the basal membranes generate capacitive currents in the lumen of the same polarity as the transmembrane potential changes.
Figure 5. Transepithelial Origin of Electrical Transients in the EPG.
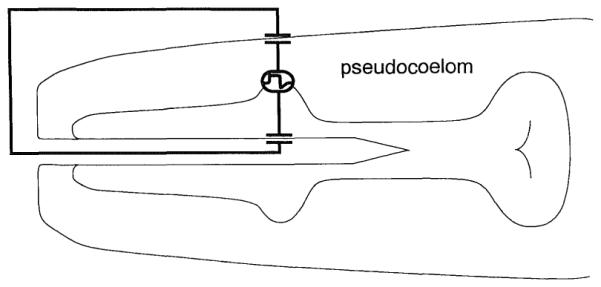
A schematic of the pharynx is drawn with a proposedconduclion path ol pharyngeal current. The basal side of pharyngeal membranes is the site of the action potential currents. These currents are coupled passively to the pharyngeal lumen, flow out the mouth, and then back across the hypodermis to complete the circuit. Pharyngeal lumcnal membranes and the hypodermal membranes are each represented by a capacitor to reflect their passive nature. The hypodcrmal membranes act as two capacitors in series that are equivalent to one smaller capacitor. Likewise, capadtance of the body and luminal cuticles, if any, is in series to the capacitance oi the body and luminal membranes. Each passive membrane may have a resistive component that acts in parallel to the capacitor, but, since we have no direct evidence that these resistors are small, they are not represented in this drawing. In voltage recordings, current transients flow across the resistance formed between the pipette and the body cuticle (5-20 MΩ). The resultant voltage drops are measured with a high input impedance amplifier. In current recordings, the current transients flow through the amplifier to the bath and are measured with the current-to-voltage converter of the amplifier.
Nervous System-Independent Transients
To see whether any of the electrical transients are caused !ly the pharyngeal nervous system, we made use of the fact that the pharyngeal musde cells are capable of myogenic activity and hence can pump in the absence of the pharyngeal nervous system (Avery and Horvitz, 1989). We killed all pharyngeal.neurons except M4. (M4, which controls isthmus peristalsis, is essential for survival and growth. We recently succeeded in recording from a worm lacking only the M4 neuron and found that the EPG contains E, P, and R phase transients [data not shown].) A record from such a worm contains two transients both during the E phase and during the R phase, but lacks the P phase transients (Figure 6A). The EPGs of pharynxes lacking most of their nervous system, together with the video analysis, indicate that the E and R phase transients represent depolarization and repolarization events, respectively, in pharyngeal muscle. In contrast, 1or more neurons are necessary for the P phase transients.
Figure 6. Origin of Nervous System-Independent Transients.
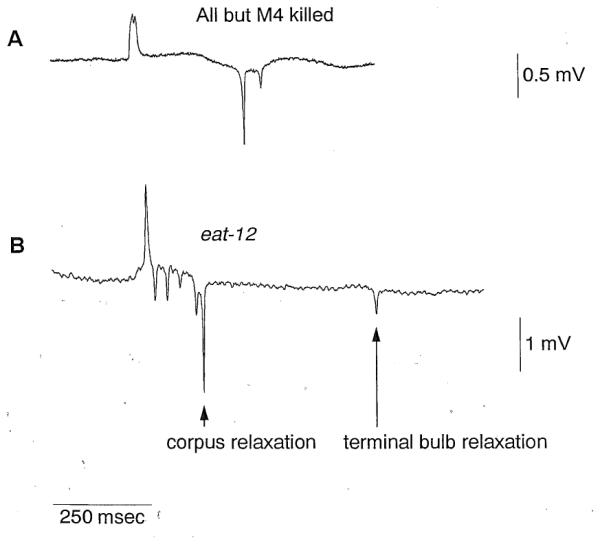
(A) EPG of a worm in which all pharyngeal neurons except M4 were killed. Two E phase and two R phase transients are present but the P phase transients are absent. This worm was an unc-29 mutant (see Experimental Procedures for rationale for using this mutant). Serotonin (10 mM) was included in the bath to stimulate pumping.
(B) EPC of an eat-12 mutant. The time between the first and second relaxation transients is variably prolonged in comparison with records from wild-type worms. Using the simultaneous video and electrical recordings described in Figure 3, we analyzed 79 pumps of 4 worms mutant for eat-12. As indicated in this figure, we found a perfect correlation between corpus relaxation and the first R phase transient and between terminal bulb relaxation and the second R phase transient.
Why is there more than one E and R phase transient? If the 20 pharyngeal muscle cells behaved as an electrical syncytium, one would expect a single capacitive transient during each potential change in the pharynx, a positive transient for depolarization, and a negative transient ior repolarization. We therefore hypothesized that multiple excitation and relaxation transients represent a slight asynchrony in the electrical activity of muscle cells in different regions of the pharynx. Indeed, previous video analysis had shown that motions of the corpus and terminal bulb, though highly correlated, are not perfectly synchronous (Avery, 1993a). Contraction of the two appears to begin at the same time, but relaxation of the corpus often begins before the start of terminal bulb relaxation. To see whether separate relaxations of the corpus and terminal bu Ib can account for the presence of two R phase transients, we needed to separate these events. To do so, we used a mutant, eat-12, in which terminal bulb relaxation is variably delayed with resped to that of the wrpus (Avery, 1993a). The EPG of the eat-12 mutant supported our hypothesis (Figure 6B). The time separating the first and second R phase transients was prolonged. This time was variable between pumps, but the relaxation motions of the corpus and of the terminal bulb remained tightly correlated with the first and second R phase transients, respectively (data not shown). We perceived the beginning of corpus relaxation within 33 ms aiter the first relaxation transient, and we perceived the beginning of terminal bulb relaxation within 33 ms after the second relaxation transient. Therefore, we conclude that the first R phase transient represents the synchronous repolarization of the corpus muscle cells and that the second R phase transient represents the synchronous repolarization of the terminal bulb muscle cells. Multiple E phase transients may also represent asynchronous potential changes in pharyngeal membranes, but we have not yet tested this proposal.
Nervous System-Dependent Transients
Since the polarity of the P phase transients is the same as that of the R phase transients, in the hyperpolarizing direction, they might influence the timing of pharyngeal repolarization. Because they were eliminated when the pharyngeal nervous system is killed, a plausible hypothesis is that they represent the activity of an inhibitory pharyngeal motor neuron active during the pump.
The records of worms mutant for eat-12 were informative with regard to the source of the P phase transients (Figure 6B). These transients occur only when the corpus and terminal bulb are contracted. There are no negative transients after the corpus has relaxed and while the terminal bulb is still contracted. This observation suggested that the neuron responsible for the P phase transients is effective only when the corpus is contracted. A neuron that is active when the corpus is contracted should have either sensory endings, motor output, or both in the corpus. Therefore, we centered our efforts on neurons whose anatomy suggests a function in the corpus and whose behavioral functions involve the control of the timing of pharyngeal relaxation.
One pharyngeal neuron type, M3, fits both criteria. The two M3 neurons, located at the corpus-isthmus boundary, have ultrastructurally defined sensory endings in the posterior corpus and motor output to the corpus and anterior isthmus (Albertson and Thomson, 1976). Two behaviors affected by M3, trapping of bacteria in the corpus and timing of pharyngeal relaxation,suggested that M3 is an inhibitory motor neuron with output to the corpus.
We tested the hypothesis that M3 is necessary for the P phase transients by killing the M3 neurons. The EPG of worms lacking the M3 neurons confirmed our hypothesis (Figure 7A; Table 1). Whereas the EPG of every normalworm has some P phase transients,the EPGs of worms with th M3 neurons killed never have P phase transients (Table 1).
Figure 7. The M3 Pharyngeal Neurons Are Necessary and Sufficient for the P Phase Transients.
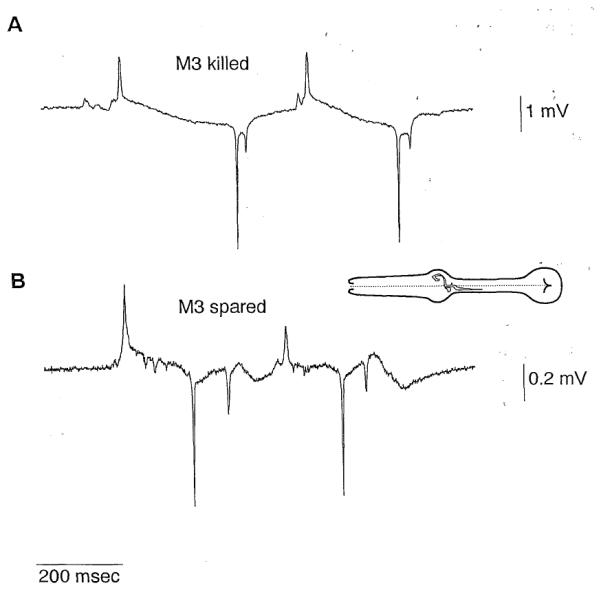
Inset is a drawing of a pharynx with an M3 neuron (redrawn from a drawing by Albertson and Thomson, 1976).
(A) Representative EPG of a worm in which the M3 neurons were killed. P phase transients are absent.
(B) EPG of a worm in which all pharyngeal neuron types except M3 and M4 were killed. These records contain P phasetransients. The genotype of this worm was unc-29. Serotonin (10 mM) was included in the bath to stimulate pumping.
Table 1.
The Effect of Neuron Kills on P Phase Transients
| Neuron Types Killed | Fraction of Worms with P Phase Transients |
|---|---|
| None | 27/27 |
| MC | 4/4 |
| NSM, MC, 12 | 1/1 |
| 13, MI | 2/2 |
| 15 | 3/3 |
| 11, 12, 13, 14, 15, 16, M1, M2, M5, NSM, MI | 6/6 |
| 11, 12, 13, 14, 15, 16, M1, M2, M5, NSM, MI, MMCa | 5/5 |
| M3 | 0/23b |
| 15, M3 | 0/3 |
| 11, 12, 13, 14, 15, 16, M1, M2, M3, M5, NSM, MI, MCa | 0/4c |
The presence of P phase transients was assessed as described in Experimental Procedures. The denominator in the right column is the number of worms tested.
These kills were done in worms mutant for unc-29. All other kills were done in wild-type worms. The EPG of intact unc-29 worms is indistinguishable from the EPG of intact wild-type worms (data not shown).
The EPGs of 16 of these worms were distinguished from those of 16 mock-operated N2 worms in a blind test based on the presence or absence of P phase transients (significant at p < 10−8; Fisher’s exact test).
Includes one male.
The demonstration of necessity of M3 function for the P phase transients indicated that M3 either acts directly onthe muscle or is necessary for another neuron to act on the muscle. If M3 is a motor neuron, then killing other pharyngeal neurons should not perturb its synapse onto the muscle. We killed all pharyngealneurons except M3 and M4. (M4 was also spared so that the worm would reach adulthood. There are no ultrastructurally defined synapses between M3 and M4.) The EPGs of these worms have P phase transients (Figure 7B; Table 1). Other abnormalities in this EPG, such as altered E phase and P phase transients (Figure 7B), might be the effect of killing other pharyngeal neurons (for example, preliminary results indicate that the pharyngeal neuron MCaffects the I phase and E phase transients). Together with previous behavioral data which Showed a role for M3 in controlling pump duration (Avery, 1993b), these electrical results suggest that M3 is an inhibitory motor neuron which can act the corpus during the pump trigger muscle repolarization.
Use of the EPG to Identify Mutations That Affect M3 Function
With the identification of theM3 neurons as necessary for the P phase transients, we have a sensitive means for identifying mutations that interfepe with M3 function. The EPG can be used in two ways. First, it can be used to assess whether genes with known roles affect M3 function. For example, we can test whether genes with a proposed role in neurotransmission are necessary for the P phase transients. One such gene, snt-I, encodes the C. elegans homolog of vertebrate synaptotagmin (Nonet et al., 1993). Synaptotagmin is a protein found in synaptic vesicles and is thought to play a key role in the regulated release of neurotransmitter (Bommert et al., 1993; DiAntonio et al., 1993; Elferink et al., 1993; Nonet et al., 1993). However, the behavioral phenotypes of C. elegans mutants with a complete loss of snt-I function suggest that some regulated synaptic function persists in the absence of synaptotagmin (Nonet et al., 1993). To see whether synaptic transmission does indeed persist, we recorded from a snt-I null mutant. The snt-1 EPG has P phase transients in most pumps (Figure 8A) that are mostly or entirely dependent on the presence of M3 (Table2). However, they are different from the P phase transients in the EPG from wild-type worms. They appear smaller and more numerous. These data support the conclusion drawn from the behavioral phenotypes of snt-1, that synaptic function persists but is abnormal in the absence of synaptotagmin (Nonet et al., 1993).
Figure 8. The Useof the EPG to Identify Mutations That Interfere with M3 Function.
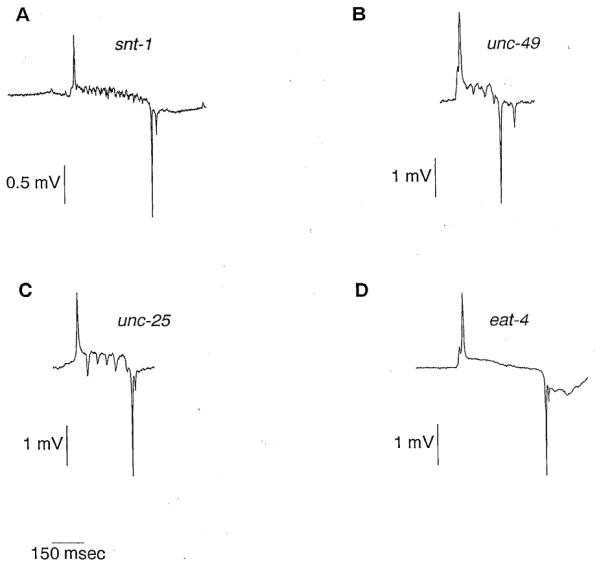
(A) EPG of aworm mutant for snt-1. Though the P phase transients are present, they appear different from those in EPGs from wildtype worms. They are smaller and more numerous. The greatly increased duration of the P phase may be partially a consequence of the abnormal M3 activity, but is probably mostly a consequence of the fact that snt-1 mutants are extremely starved and have infrequent pumps. Both starvation and aslow pumping rate appear to increase the pump duration (unpublished data). Serotonin (10 mM) was included in the bath to stimulate pumping. (B) EPG of a worm mutant for unc-49, a gene necessary for the inhibitory effect of the GABA agonist muscimol on pharyngeal pumping (McIntire et al., 1993a). P phase transients are present. (C) EPG of a worm mutant for unc-25, a gene necessary for the synthesis of GABA (McIntire et al., 1993a). P phase transients are present.
(D) Representative EPG of a worm mutant for eat-4 P phase transients are missing.
Other genes with an established role in neurotransmission are those involved with synthesis and response to γ-aminobutyric acid (GABA), one of the principal inhibitory neurotransmitters in C. elegans (Mcintire et al., 1993a). GABA immunoreactivity is not seen in the pharynx (McIntire et al., 1993b), and mutants defective in GABAergic transmission have no obvious detect in pumping (unpublished data). Nevertheless, it remains possible that some inhibitory pharyngeal neuron uses GABA since there are GABA immunoreactive neurons in the pharynx of the parasitic nematode Ascaris (Guastella et al., 1991) and since the GABA agonist muscimol has an inhibitory effect on pharyngeal pumping which is dependent on the unc-49 gene product, a likely component of a GABA postsynaptic receptor (Mcintire et al., 1993a). To test whether GABA is the M3 neurotransmitter, we recorded from worms mutant for unc-49 or for unc-25, a gene necessary for the synthesis of GABA (Mcintire et al., 1993a). The EPGs of both mutants have P phase transients (Figures 8B and BC; Table 2). Hence, we conclude that GABA is not the sole M3 neurotransmitter. The effect of muscimol on pumping could be explained as a direct effect on pharyngeal muscle that mimics the effect of an unidentified GABAergic pharyngeal neuron or of a GABAergic extrapharyngeal neuron, as an indirect effect on the pharynx (for example, by causing a decrease in the humoral concentration of a chemical that is excitatory to the pharynx), or as an effect with no physiological relevance.
Table 2.
The Effect of Mutations on P Phase Transients
| Genotype | Neuron Types Killed |
Fraction of Worms with P Phase Transients |
|---|---|---|
| eat-12 | None | 2/2 |
| M3 | 0/1 | |
| snt-1 | None | 5/5a |
| M3 | 0/8b | |
| unc-25 | None | 3/3 |
| unc-49 | None | 3/3 |
| eat-4 | None | 0/8c |
The presence of P phase transients was assessed as described in Experimental Procedures. The denominator in the right column is the number of worms tested.
Through P phase activity was present in the EPGs from all worms tested, it appeared abnormal. The transients were smaller and more numerous than in the EPGs from wild-type worms (see Figure 8A).
Two of these worms had one to two small P phase transients during a small fraction of the pumps.
Data are shown only for ad572, one of the strongest eat-4 alleles (unpublished data; Raymond Lee, personal communication). Some pumps for these worms had one or two small P phase transients but most pumps lacked P phase transients. All 8 were distinguished from 8 wild-type worms in a blind test based on their P phase activity (significantly at p < 0.001; Fisher’s exact test).
A second use of the EPG is the identification of new genes that affect M3 function. The gene eat-4 is a strong candidate tor affecting M3 function. Two pharyngeal phenotypes of worms with mutations at the eat-4 locus, defective trapping of bacteria in the corpus and prolonged pump durations, are similar to phenotypes seen in worms in which the M3 neurons had been killed alone or in combination with other neurons (Avery 1993a, 1993b; Raymond Lee, personal communication). The EPG of eat-4 worms confirms that some aspect of M3 function is defective in eat-4 mutants: P phase transients are greatly reduced or absent (Figure 8D; Table 2). Hence, eat-4 may be necessary for the development or function of M3, or it may be necessary in the muscle cells for their response to M3.
Discussion
The EPG Is Composed of Electrical Transients Caused by Potential Changes in Pharyngeal Membranes
Using an extracellular configuration, we record reproducible sets of electrical transients during pharyngeal pumping. We propose a simple electrical explanation that can account for these electrical transients. As shown in Figure 5, the transients are caused by transmembrane potential changes across the pharyngeal muscle basal membrane, the membrane that separates the muscle cell cytoplasm from the pseudocoelom. The total voltage difference between the muscle cytoplasm and the pseudocoelom must be the same along any path that connects them, including the long path which goes across the apical membrane of the muscle cell, into the pharyngeal lumen, out the mouth, through the surrounding bath solution, across the cuticle and hypodermis, and back into the pseudocoelom. Most of the voltage drop along this path should occur across membranes since the cytoplasm, pseudocoelom, and bath solution have low resistance. Furthermore, since the surface area of the pharyngeal apical membranes is much smaller than that of the hypodermis, they present the lowest capacitance in this path, and hence most of the transient voltage drop should occur across them. When the voltage across these membranes changes, a capacitive current flows into the lumen.
We can estimate an upper bound for the total charge movement expected during such a capacitive transient by making two assumptions: that all the charging current flows out the mouth of the worm and that all the voltage drop in the circuit occurs across the apical membranes. From the dimensions of the pharyngeal lumen and a specific capacitance typical for biological membranes (Cole, 1968), we estimate corpus lumenal capacitance to be 20 pF (see Experimental Procedures). Del Castillo and Morales (1967) measured potential changes as large as 100 mV on repolarization of Ascaris pharyngeal muscle. Thus, we estimate that, at most, 100 mV × 20 pF = 2 pC is needed to charge lumenal membranes during corpus repolarization. The actual charge that flows out the mouth of the worm during corpus repolarization (measured by integrating the first R phase transient; see Experimental Procedures) averaged 1.2 pC in normal adults (SD, 0.2 pC; 12 worms; 3059 pumps). The closeness of this measurement to the estimated upper bound suggests that much or all of the current does indeed arise as proposed.
M3 Is a Motor Neuron That Causes Rapid Membrane Potential Changes in Corpus Muscles during the Pump
M3 is necessary and sufficient for the negative transients that occur during the pharyngeal pump, the P phase transients. One interpretation of this result is that these transients represent action potential currents in the cell body of M3 neurons. Although this possibility cannot be excluded, it is unlikelythat these currents could be sensed in our recording configuration. Pharyngeal neurons, embedded in grooves of pharyngeal muscle (Figure 1B), are topologically within the pseudocoelomic compartment. Hence, action potential currents in the neuronal cell body would flow only locally and not out the lumen of the pharynx. Furthermore, P phase transients in the EPG of worms mutant for the synaptotagmin gene snt-1 appear abnormal. Since synaptotagmin is expressed in neurons at synapses (Nonet et al., 1993) and affects synaptic transmission (for review see Popov and Poo, 1993), this result suggests that the P phase transients are synaptic in origin.
Another interpretation is that the P phase transients reflect a mechanism internal to the muscle, and M3 neurotransmission just influences the probability that these transients will occur. That is, M3 releases a modulatory substance which increases the probability that rapid negative changes in membrane potential will occur. If this were true, one might expect a decrease in the probability of occurrence of P phase transients in the absence of M3 but not a complete abolition. The fact that, in recordings from 23 worms in which the M3 neurons had been killed, not one of the P phases has negative transients argues against the possibility of a modulatory role for M3 neurotransmission.
The most plausible interpretation is that the P phase transients represent inhibitory postsynaptic potentials in the muscle commanded by synaptic transmission from M3. That is, action potentials in the soma of M3 cause discrete release of transmitter, which opens inhibitory channels in the muscle. The transients may be a direct indication of the inhibitory postsynaptic potentials or may represent some amplification by the muscle of the synaptic currents.
The suggestion of a spiking neuron in a nematode is unusual but not unprecedented. Though most of the motor nervous system in the parasitic nematode Ascaris appears tofu nction passively (Davis and Stretton, 1989), the observation of discrete postsynaptic potentials in these motor neurons (Davis and Stretton, 1989) and of extracellularly recorded spikes in the ventral nerve cord (Davis and Stretton, 1992) suggests that a small subset of Ascaris interneurons has classical fast action potentials. One possible purpose for action potentials in these presumed interneurons is to carry information over distances of several centimeters. For these distances, even the high space constants of some Ascaris neurons (up to 10 mm; Davis and Stretton, 1989) do not suffice, and a regenerative mechanism is needed.
This teleological argument, however, does not explain the need for action potentials in M3. The radius of a published commissure of an Ascaris motor neuron is about 5 μm (from Davis and Stretton, 1989), whereas process radii of C. elegans pharyngeal neurons are about 0.5 μm (from Albertson and Thomson, 1976). The membrane space constant is proportional to the square root of the process radius (Hodgkin and Rushton, 1946).Therefore, using measurements made in Ascaris, we calculate that M3 should have a membrane space constant of about 1 mm. The M3 neuron reaches a maximal length of only about 50 μm; so unless our calculated space constant is a gross overestimate, M3 can transmit information purely by passive properties.
What, then, is the need for an action potential in a neuron of these dimensions? One possibility is that a postsynaptic mechanism which responds to the neuronal signal requires fast transmission. An example of such a requirement is found at the vertebrate neuromuscular junction where sodium channels mediat ing the regenerative upstroke of the action potential are both activated and' inactivated by depolarization (Gilman et al., 1990). If depolarization is slow, they inactivate and are unable to participate in an action potential until inactivation is removed by hyperpolarization. A similar mechanism may exist for relaxation of the C.elegans pharynx. The channel mediating repolarizatioh of pharyngeal muscle may require abrupt negative steps from depolarized potentials. Slower negative changes in potential may inactivate the channel responsible for repolarization. In Ascaris, a current with these properties is likely to be responsible for the repolarization phase of pharyngeal muscle action potentials (Del Castillo and Morales, 1967; Byerly and Masuda, 1979). This current, termed the negative spike current by Byerly and Masuda (1979), is carried by a potassium-selective channel (Del Castillo and Morales, 1967; Byerly and Masuda, 1979) that has kinetic properties opposite in sign from those of the sodium channel. That is, it is both activated and inactivated by hyperpolarizing voltage steps from depolarized potentials. Once inactivated, depolarization is required to remove the inactivation. Byerly and Masuda also observed that repolarization of pharyngeal action potentials appeared to be triggered by discrete postsynaptic potential-like events which were dependent on external calcium and hence likely caused by synaptic transmission (Byerly and Masuda, 1979). These postsynaptic potentials are functionally analogous to the P phase transients caused by M3 in C.elegans.
Although there is no direct evidence that a current similar to the negative spike current exists in the C. elegans pharynx, it is tempting to speculate that the R phase transients corresponding to pharyngeal repolarization are mediated by such a current.
M3: A Sensorimotor Neuron?
It is interesting that M3 is sufficient in the absence of most neuron types for the P phase transients. In addition to demonstrating a direct effect of M3 on the muscle, this result suggests two possibilities. It may be that M3 is active both during the pump and between pumps, but the reversal potential for the M3 synaptic current is near the resting potential of the muscle, so that inhibitory postsynaptic potentials are only observed when the muscle is depolarized during the pump. Alternatively, M3 may be active only during the pump. If M3 is active only du ring the pump, then it must be able to sense the contraction of the pharynx. Albertson and Thomson (1976) suggested that M3 has a sensory function because it has a process which ends just under the cuticle of the lumen and is connected to neighboring cells with desmosomes. This neuronal process may have a mechanosensory function that is able to sense the contraction. Recently, physiological demonstrations of bifunctional neu'rons have been made in annelids (Wenning et al., 1993), arthropods (Pasztor and Bush, 1989), and vertebrates (for review see Gonzalez et al., 1992). In each of these cases, the neuron is a neurosecretory cell with a sensory function. M3, in contrast, is a motor neuron with a possible sensory function. Such a dual function may provide a single-cell reflex loop: M3 may sense contraction of the pharynx and then command its relaxation. In addition to M3, several other pharyngeal neuron types (Albertson and Thomson, 1976) and at least one extrapharyngeal neuron type (Ward et al., 1975; Ware et al., 1975) have been proposed to have both sensory and motor roles based on anatomical data, so it is likely that other C.elegans neurons have dual physiological functions as proposed.
Directly testing the possibility that M3 has a sensory function will require recording from M3. Alternatively, genetic and molecular analysis may provide some answers regarding M3 function. For example, protein sequences predicted from the DNA sequence of eat-4 and other genes that affect the P phase transients may be homologous to proteins with known roles in sensory transduction in other systems.
In summary, the EPG reflects the electrical activity of both pharyngeal muscle cells and of synaptic transmission between pharyngeal neurons and muscle cells. As we have shown, the P phase of the EPG can be used to assess the effect of mutations on M3 function. The E and R phases reflect the depolarization and repolarization phases, respectively, of pharyngeal muscle action potentials. Furthermore, it is likely that the charge which moves during a current transient in the EPG is proportional to the potential change across pharyngeal muscle membranes. Hence, mutations that affect the excitable properties of the muscle can be identified and characterized using the EPG. Indeed, preliminary analysis of mutants that affect pharyngeal muscle has proven the usefulness of this approach (unpublished data). The combination of genetic, molecular, laser ablation, and electro-physiological methods to the C. elegans pharynx provides a powerful set of tools to dissect the function of this simple organ.
Experimental Procedures
General Methods and Strains
Worms were cultured and handled as described by Sulston and Hodgkin (1988) and were grown at 20°C. The wild type was C. elegans variety Bristol, strain N2. Mutant strains used were DA857 act-2(ad468sd) act-3(ad767) V (Avery, 1993a), DA572 eat-4(ad572) III (Avery, 1993a), DA695 eat-12(ad695) IV (Avery, 1993a), RM1613 snt-1(md290) II (Nonet et al., 1993), CB156 unc-25(e156) III (Brenner, 1974), CB1072 unc-29(e1072am) I (Brenner, 1974), CB382 unc-49(e382) III (Brenner, 1974), and CB190 unc-54(e190) I (Brenner, 1974).
RM1613 was kindly given to us by Jim Rand. All other strains came from our laboratory's collection. Most strains were grown on an Escherichia coli strain DA837 derived from the strain OPSO (Brenner, 1974). DA857 and RM1613 were grown on the E. coli strain HB101 (Boyer and Roulland-Dussoix, 1969). HB101 was used for strains with impaired pharyngeal function since worms can ingest it more easily than DA837 and hence grow faster (unpublished data).
Laser Killing of Pharyngeal Neurons
Neurons were killed by the laser ablation technique of White (Sulston and White, 1980). Details of our laser setup have been described (Avery and Horvitz, 1987; Avery, 1993a). Operations were done less than 3 hr after hatching. Operations were verified 1 day later by the assignment of a subjective confidence score to the operation. Only worms for which we were 95% confident or greater of their success are reported. Before we had established this strict criterion for defining a successful operation, 1 worm in which the M3 neurons had apparently been killed nevertheless had P phase transients. Since this operation was done in the early stages of these experiments before one of us had much experience using the laser, and since we have been unable to repeat this result, we suspect that verification of this operation as successful was wrong.
For operations that required killing most of the pharyngeal nervous system, we used the uncoordinated mutant unc-29 since wild-type worms, when extremely starved, tend to crawl off the aga."r plate and die. unc-29, which encodes a component of the levamisole-sensitive nicotinic acetylcholine receptor (Fleming et al., 1993), does not appear to affect the pharynx (Avery and Horvitz, 1990). Worms in which 12 or 13 neuron types were killed or in which M4 was killed were grown on HB101. (Methods describing how we grow M4-worms to adulthood will be described elsewhere.).
As indicated in Table 1, some worms were "mock operated." That is, they were treated exactly as the operated worms were except that the laser was not fired (Avery and Horvitz, 1989).
Saline and Electrophysiology
In all experiments, Ascaris saline was used for the bath and suction pipette solution. Ascaris saline is essentially the same as that described by Davis and Stretton (Davis and Stretton, 1989a) with a different buffer. It contains 4 mM sodium chloride, 125 mM sodium acetate, 24.5 mM potassium chloride, 5.9 mM calcium chloride, 4.9 mM magnesium chloride, and 5 mM HEPES (pH 7.4). The ingredients of this saline were not likely to be essential for these records since recordings made in M9 buffer (Sulston and Hodgkin, 1988)were qualitatively the same(data not shown). As indicated in the figure legends, in some recordings 10 mM serotonin was included in the saline to stimulate pumping (Horvitz et al., 1982; Avery and Horvitz, 1990).
Recording chambers were made by streaking vacuum grease in a circle of circa 2 cm diameter on a 35 × 50 mm rectangular glass coverslip 0.15 mm thick (Carolina Biological). These chambers held 200-400 μL of bath solution. Worms were transferred without bacteria into this chamber. Recordings were made at ambient temperatures (21°C-25°C) within 5-15 min of placing the worms in the bath. Unless noted otherwise, all recordings were made from gravid hermaphrodites.
Suction pipettes were pulled on a Sutter P87 puller and used without fire polishing. The pipettes were held with a pipette holder (World Precision Instruments) coupled to a suction syringe similar to the design described by Delcomyn (1974). Resistance of the pipettes filled with the bath saline was 0.1-1.5 MΩ. For measurement and amplification of voltage signals, a Grass Instruments P15 amplifier with 100 MΩ input impedance was used. The positive lead of the amplifier was connected to a chlorided silver electrode in the pipette. The negative lead was connected to a chlorided silver electrode in the bath. Voltage traces shown were filtered by ihe amplifier with a bandwidth of 0.1-1000 Hz (1/2 amplitude): For many of the results summarized in the tables, the filter was set at 10-1000 Hz to minimize low frequency drift. All transients present in the larger bandwidth and described in Results are also present in this smaller recording bandwidth.
Current measurements were done using an Axon Instruments Axopatch-1D amplifier without series resistance compensation. The current needed to hold the pipette solution and the bath at the same potential was measured. To remove spurious junction potentials, holding current was set to zero before sucking the worm into the pipette and occasionally during the recordings when the junction potential drifted excessively. Current was low pass filtered at 1000 Hz by a 4 pole Bessel filter in the amplifier.
Data Acquisition and Analysis
In early experiments, analog signals were digitized with an MCDAS board from Scientific Solutions and browsed with pCLAMP software (Axon Instruments). In later experiments, data were acquired with an AT-MI0-16 digitization board from National Instruments and with software written by Wayne Davis (personal communication) using the Labview software development environment from National Instruments. All data were digitized at a frequency of 2 kHz. Browsing and analysis of these records were done on a Sun Microsystems SPARCstation 2 computer running the MIT X window system and software we developed. Source code is available by anonymous FTP from eatworms. swmed.edu.
Microscopy and Video
Electrophysiological recordings were guided by a Zeiss Axiovert inverted microscope equipped with Nomarski differential interference contrast optics.
To correlate the behavior of the worms with the electrical signals, we adapted a two camera design similar to one used by others (e.g., Baader and Kristan, 1992) to our system. A Hitachi KP160 black-and-white CCD microscope camera was used to film the behavior of the worm. At the same time, an oscilloscope record was filmed using a Sony Handyman CCD-FX310 camcorder camera. The signals from the two cameras were combined by a Panasonic Digital AV-mixer, and the image was stored on Super-VHS videotape with a Panasonic AG1960 VCR. Computer analysis of the videotapes was done as described previously (Avery, 1993a). Images in Figure 3 were digitized on the same hardware running xm grabber and the Xwindows Xv extension (available by anonymous FTP from blue.rasterops.com).
Measurement of Charge Movement during Repolarization of the Corpus
Charge movement during corpus repolarization was measured in 12 current recordings, 4 made in the presence of 10 mM serotonin, and 8 without serotonin. A peak (during E phase, for example) was identified as a rise in current of at least 80 pA within 12 ms, followed by a fall of at least 80 pA within 12 ms. A trough (during R phase, for example) was identified as a drop in current of at least 80 pA within 8 ms, followed by a rise of at least 80 pA within 8 ms. Peaks and troughs were then parsed into pumps by a simple pattern recognition program that looked for a peak followed 100-500 ms later by a trough. The corpus repolarization spike was identified as the deepest trough. Finally, the pumps identified by this procedure were examined and corrected where necessary. (The error rate was about 1%.) The charge moved during the transient was computed as the area enclosed by the current trace and a line connecting the maxima within 8 ms on the left and 8 ms on the right.
In 1 recording, the area of the first R phase transient was bimodal, with modes at about 1.2 and 1.9 pC. Examination of this recording showed that the troughs of smaller area were almost always followed by a second R phase transient, whereas those of larger area almost never were. (The exceptions were pumps in which two R phase transients were visible, but overlapped.) We believe that in this worm, some of the pumps had a single R phase transient due to corpus and terminal bulb repolarization occurring within 5 ms of each other. Therefore, our estimate of charge moved during corpus repolarization is probably slightly higher than the true value. Histograms of the area of the first R phase transient in the remaining recordings were unimodal and centered near 1.2 pC, so the error in the average due to this source is probably small.
Calculation of Corpus Luminal Membrane Capacitance
Luminal area of the corpus was estimated using measurements from published electron micrographs of adult hermaphrodites (Albertson and Thomson, 1976). At the level of the anterior corpus (Figure 5; Albertson and Thomson, 1976), we measured the perimeter of a cross-section of the lumen to be 2 × 10−5 m. We assumed that this cross-sectional perimeter is constant along the length of the corpus (about 100 μm) and calculated a total area of 2 × 10−9 m2 Ultrastructural data indicate that there are no invaginations of the apical membranes. By assuming a specific capacitance of 10 mF/m2 (Cole, 1968), we calculated the capacitance of the corpus lumenal membrane to be 2 × 10−5 F. Most of the membrane lining the lumen is part of pharyngeal muscle cells. A small fraction of this membrane (about one-tenth) belongs to the marginal cells.
Scoring for the Presence or Absence of P Phase Transients
Though every wild-type worm had some pumps with P phase transients, not all pumps had these transients. Furthermore, the EPG from some worms had slow negative drifts during the pumps. (We do not know the origin of this drift but suspect that it may be related to motion in the isthmus and/or to the opening of the pharyngeal-intestinal valve.) For these reasons, the presence of the P phase transients was scored in a blind test comparing experimental with control worms. For example, worms lacking the M3 neurons were distinguished from mock-operated controls in a blind test based solely on the lack of P phase transients in their. EPGs. In these tests, we made 4 min recordings from each of the experimental and control worms without knowing which worms were experimental. We then browsed each record to decide whether it contained P phase transients. Records with excessive drift were high pass filtered either electronically or digitally before browsing. The presence of the transients appeared to be influenced by the rate of pumping: they were more likely to be present in records from worms that were pumping slowly than in those from worms that were pumping rapidly. During early phases of these studies, all recordings were made with 10 mM Serotonin in the bath to promote pumping (Horvitz et al., 1982; Avery and Horvitz, 1990), but all conclusions in this paper regarding the P phase transients were later confirmed in the absence of serotonin.
Acknowledgments
We are grateful to Ken Breedlove, who was the first to make electrophysiological recordings from C. elegans neurons and thus provided the impetus for this work. We thank Joe Dent, Dennis Liu, Shawn Lockery, Dean Smith, and Jim Thomas for helpful comments on the manuscript; Wayne Davis for computer programming; and Jim Rand for providing the snt-1 strain RM1613. This research was supported by research grant HL46154 from the Public Health Service. D. M. R. is partially supported by Medical Scientists Training Grant GM08014.
References
- Albertson DG, Thomson JN. The pharynx of Caenorhabditis elegans. Phil. Trans. Roy. Soc. (Lond.) B. 1976;275:299–325. doi: 10.1098/rstb.1976.0085. [DOI] [PubMed] [Google Scholar]
- Avery L. The genetics of feeding in Caenorhabditis elegans. Genetics. 1993a;133:897–917. doi: 10.1093/genetics/133.4.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avery L. Motor neuron M3 controls pharyngeal muscle relaxation timing in Caenorhabditis elegans. J. Exp. Biol. 1993b;175:283–297. doi: 10.1242/jeb.175.1.283. [DOI] [PubMed] [Google Scholar]
- Avery L, Horvitz HR. A cell that dies during wildtype C. elegans development can function as a neuron in a ced-3 mutant. Cell. 1987;51:1071–1078. doi: 10.1016/0092-8674(87)90593-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avery L, Horvitz HR. Pharyngeal pumping continues after laser killing of the pharyngeal nervous system of C. elegans. Neuron. 1989;3:473–485. doi: 10.1016/0896-6273(89)90206-7. [DOI] [PubMed] [Google Scholar]
- Avery L, Horvitz HR. Effects of starvation and neuroactive drugs on feeding in Caenorhabditis elegans. J. Exp. Zool. 1990;253:263–270. doi: 10.1002/jez.1402530305. [DOI] [PubMed] [Google Scholar]
- Baader AP, Kristan WB. Monitoring neuronal - activity during discrete behaviors: a crawling, swimming and shortening device for tethered leeches. J. Neurosci. Meth. 1992;43:215–223. doi: 10.1016/0165-0270(92)90031-8. [DOI] [PubMed] [Google Scholar]
- Bargmann CI. Genetic and cellular analysis of behavior in C. elegans. Annu. Rev. Neurosci. 1993;16:47–71. doi: 10.1146/annurev.ne.16.030193.000403. [DOI] [PubMed] [Google Scholar]
- Bommert K, Charlton MP, DeBello WM, Chin GJ, Betz H, Augustine GJ. lnihibition of neurotransmitter release by C2-domain peptides implicates synaptotagmin in exocytosis. Nature. 1993;363:163–165. doi: 10.1038/363163a0. [DOI] [PubMed] [Google Scholar]
- Boyer HW, Roulland-Dussoix D. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J. Mol. Biol. 1969;41:459–472. doi: 10.1016/0022-2836(69)90288-5. [DOI] [PubMed] [Google Scholar]
- Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks CM, Eccles JC. Electrical investigation of monosynaptic pathway through the spinal cord. Neurophysiol. 1947;10:251–273. doi: 10.1152/jn.1947.10.4.251. [DOI] [PubMed] [Google Scholar]
- Byerly L, Masuda MO. Voltage-clamp analysis of the potassium current that produces a negative-going action potential in Ascaris muscle. J. Physiol. 1979;288:263–284. [PMC free article] [PubMed] [Google Scholar]
- Cole KS. Membranes, Ions and Impulses: A Chapter of Classical Biophysics. University of California Press; Berkeley, California: 1968. [Google Scholar]
- Davis RE, Stretton A0W. Passive membrane properties of motorneurons and their role in long-distance signalling in the nematode Ascaris. J. Neurosci. 1989;9:403–414. doi: 10.1523/JNEUROSCI.09-02-00403.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis RE, Stretton AOW. Extracellular recordings from the motor nervous system of the nematode, Ascaris suum. J. Comp. Physiol. 1992;171:17–28. doi: 10.1007/BF00195957. [DOI] [PubMed] [Google Scholar]
- Del Castillo J, Morales T. The electrical and mechanical activity of the esophageal cell of Ascaris lumbricoides. J. Physiol. 1967;50:603–629. doi: 10.1085/jgp.50.3.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delcomyn F. A simple system for suction electrodes. J. Electrophysiol. Tech. 1974;3:22–25. [Google Scholar]
- DiAntonio A, Parfitt KD, Schwarz TL. Synaptic transmission persists in synaptotagmin mutants of Drosophila. Cell. 1993;73:1281–1290. doi: 10.1016/0092-8674(93)90356-u. [DOI] [PubMed] [Google Scholar]
- Doncaster CC. Nematode feeding mechanisms. I. Observations on Rhabditis and Pelodera. Nematologica. 1962;8:313–320. [Google Scholar]
- Driscoll M, Chalfie M. The mec-4 gene is a member of a family of Caenorhabditis elegans genes that can mutate to induce neuronal degeneration. Nature. 1991;349:588–593. doi: 10.1038/349588a0. [DOI] [PubMed] [Google Scholar]
- Elferink LA, Peterson MR, Scheller RH. A role for synaptotagmin (p65) in regulated exocytosis. Cell. 1993;72:153–159. doi: 10.1016/0092-8674(93)90059-y. [DOI] [PubMed] [Google Scholar]
- Fatt P, Katz B. Spontaneous subthreshold activity at motor nerve endings. J. Physiol. 1952;117:109–128. [PMC free article] [PubMed] [Google Scholar]
- Fleming JT, Tornoe C, Riina HA, Coadwell J, Lewis JA, Sattele DB. Acetylcholine receptor molecules of the nematode Caenorhabditis elegans. EXS. 1993;63:65–80. doi: 10.1007/978-3-0348-7265-2_4. [DOI] [PubMed] [Google Scholar]
- Gilman AG, Rall RW, Nies AS, Taylor R. The Pharmacological Basis of Therapeutics. Pergamon Press; New York: 1990. [Google Scholar]
- Gonzalez C, Almaraz L, Obeso A, Rigual R. Oxygen and acid chemoreception in the carotid body chemoreceptors. Trends Neurosci. 1992;15:146–153. doi: 10.1016/0166-2236(92)90357-e. [DOI] [PubMed] [Google Scholar]
- Guastella J, Johnson CD, Stretton A0W. GABAimmunoreactive neurons in the nematode Ascaris. J. Comp. Neural. 1991;307:584–597. doi: 10.1002/cne.903070406. [DOI] [PubMed] [Google Scholar]
- Hodgkin AL, Rushton WAH. The electrical constants of a crustracean nerve fibre. Proc. R. Soc. Lond. (B) 1946:133–444. doi: 10.1098/rspb.1946.0024. [DOI] [PubMed] [Google Scholar]
- Horvitz HR, Chalfie M, Trent C, Sulston J, Evans PD. Serotonin and octopamine in the nematode Caenorhabditis elegans. Science. 1982;216:1012–1014. doi: 10.1126/science.6805073. [DOI] [PubMed] [Google Scholar]
- MacLeod AR, Waterston RH, Fishpool RM, Brenner S. Identification of the structural genes for a myosin heavy chain in Caenorhabditis elegans. J. Mol. Biol. 1977;114:133–140. doi: 10.1016/0022-2836(77)90287-x. [DOI] [PubMed] [Google Scholar]
- McIntire SL, Jorgenson E, Horvitz HR. Genes required for GABA function in Caenorhabditis elegans. Nature. 1993a;364:334–337. doi: 10.1038/364334a0. [DOI] [PubMed] [Google Scholar]
- Mcintire SL, Jorgenson E, Kaplan J, Horvitz HR. The GABAergic nervous system of Caenorhabditis elegans. Nature. 1993b;364:337–341. doi: 10.1038/364337a0. [DOI] [PubMed] [Google Scholar]
- Nonet ML, Grundahi K, Meyer BJ, Rand JB. Synaptic function is impaired but not eliminated in C. elegans mutants lacking synaptotagmin. Cell. 1993;73:1291–1305. doi: 10.1016/0092-8674(93)90357-v. [DOI] [PubMed] [Google Scholar]
- Pasztor VM, Bush BM. Primary afferent responses of a crustacean mechanoreceptor are modulated by proctolin, octopamine and serotonin. J. Neurobiol. 1989;20:234–254. doi: 10.1002/neu.480200406. [DOI] [PubMed] [Google Scholar]
- Popov SV, Poo M.-m. Synaptotagmin: a calciumsensitive inhibitor of exocytosis? Cell. 1993;73:1247–1249. doi: 10.1016/0092-8674(93)90352-q. [DOI] [PubMed] [Google Scholar]
- Seymour MK, Wright KA, Doncaster CC. The action of the anterior feeding apparatus of Caenorhabditis elegans (Nematoda: Rhabditida) J. Zool. 1983;201:527–539. [Google Scholar]
- Sulston JE, Hodgkin JA. In: Methods. In The Nematode Caenorhabditis elegans. Wood WB, editor. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, New York: 1988. pp. 587–606. [Google Scholar]
- Sulston JE, White JG. Regulation and cell autonomy during post-embryonic development of Caenorhabditis elegans. Dev. Biol. 1980;78:577–597. doi: 10.1016/0012-1606(80)90353-x. [DOI] [PubMed] [Google Scholar]
- Ward S, Thomson N, White JG, Brenner S. Electron microscopical reconstruction of the anterior sensory anatomy of the nematode Caenorhabditis elegans. J. Comp. Neural. 1975;160:313–338. doi: 10.1002/cne.901600305. [DOI] [PubMed] [Google Scholar]
- Ware RW, Clark D, Crossland K, Russell RL. The nerve ring of the nematode Caenorhabditis elegans: sensory input and motor output. J. Comp. Neurol. 1975;162:71–110. [Google Scholar]
- Wenning A, Cahill MA, Hoeger U, Calabrese RL. Sensory and neurosecretory innervation of leech nephridia is accomplished by a single neurone containing FMRFamide. J. Exp. Biol. 1993;182:81–96. doi: 10.1242/jeb.182.1.81. [DOI] [PubMed] [Google Scholar]
- White JG. The anatomy. In: Wood WB, editor. The Nematode Caenorhabditis elegans. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, New York: 1988. pp. 81–122. [Google Scholar]