Abstract
The efficacy of combination therapy (antiretroviral therapy - ARV) is demonstrated by the high rates of viral suppression achieved in most treated HIV patients. Whereas contemporary treatments may continuously suppress HIV replication, they do not eliminate the latent reservoir, which can reactivate HIV infection if ARV is discontinued. The persistence of HIV proviral DNA and infectious viruses in CD4+ T cells and others cells has long been considered a major obstacle in eradicating the HIV virus in treated patients. Moreover, recent studies have demonstrated the persistence of HIV replication at low copies in most patients on suppressive ARV. The source of this ‘residual viraemia’ and whether it declines over years of therapy remain unknown. Similarly, little is known regarding the biological relationships between the HIV reservoir and viral replication at low copies. The question of whether this ‘residual viraemia’ represents active replication or the release of non-productive virus from the reservoir has not been adequately resolved.
From a clinical perspective, both the quantification of the HIV reservoir and the detection of low levels of replication in full-responder patients on prolonged ARV may provide important information regarding the effectiveness of treatment and the eradication of HIV. To date, the monitoring of these two parameters has been conducted only for research purposes; the routine use of standardised tests procedure is lacking. This review aims to assess the current data regarding the correlation between HIV replication at low copies and the HIV reservoir and to provide useful information for clinicians.
Keywords: HIV drug resistance, HIV reservoir, HIV-DNA, residual viraemia, virological failure
INTRODUCTION
For more than two decades, the CD4 + cell number and HIV viraemia levels have been considered the most reliable prognostic parameters of HIV infection [1]. The use of these two parameters primarily stems from the observations made on the natural history of the infection in different populations of HIV patients; in untreated subjects, there was a direct correlation between CD4 counts, viral load levels and the clinical progression of infection [2, 3]. Subsequent studies on patients who were on mono and dual antiretroviral therapy have confirmed that the combined plasma HIV-1 RNA level and the CD4 + lymphocyte count are also valid predictors of the clinical progression of HIV infection in treated patients [4-6]. In 1997, the introduction of new drugs and the modification of the strategic approach for the treatment of HIV-positive patients stimulated a review of the treatment-response parameters; it was found that the viraemia level was an important element in the clinical management of patients who could be used for assessing the prognosis and therapeutic efficacy [7]. In 1998, Raboud and co-workers showed how patients on triple therapy with zidovudine, didanosine, and nevirapine who had achieved a viral load < 20 copies/ml had a significantly lower risk of virological failure when compared with individuals of the same cohort with a nadir viraemia between 21 and 400 copies/ml [8], anticipating for many years current observations demonstrating that an increased risk of failure remains, even in responder patients with very low copies of viral replication during therapy [9-3]. This finding then raises the question of the optimal viraemia target while attempting to maintain tight control of viral replication during ARV.
One of the main features of HIV infection is its ability to infect, in the early stages of viral replication, resting CD4 + cells and other susceptible cells in the body, which may then become reservoirs for persistent infection [14]. The large viral burden in the different anatomical reservoir sites occurs during the acute phase of infection and during the clinical latency period in the untreated patient. Moreover, it has been shown that patients who have good control of viral replication, secondary to efficient immunological response (i.e., elite controllers) or antiretroviral therapy, had lower levels of HIV reservoirs compared with chronic untreated patients [15]. The peripheral blood mononuclear cell HIV proviral DNA (HIV-DNA) load correlates directly with the number of latently HIV-infected cells that constitute the viral reservoir and is considered an independent marker of disease progression. Studies have demonstrated an association among the low level of HIV replication (<20 copies/ml), T cell activation and increased levels of HIV DNA in subjects with a full response to treatment [16, 17]. This demonstrates a correlation between a low level of HIV replication and HIV reservoirs, which contradicts previous studies that have shown a slow decay of the reservoir in treated patients. Additionally, this former correlation highlights the fact that antiretroviral therapy may not be able to interrupt the supply of the reservoir.
The main purpose of this review is to provide an overview of the recent published data on the clinical correlates of HIV viraemia (below the limits of detection) and reservoirs to provide additional tools for the clinical assessment of ARV full-responder patients.
CLINICAL CORRELATES OF HIV REPLICATION AT LOW COPIES
Although the mechanisms underlying HIV low-level viraemia remain incompletely understood, two proposals are the most accredited alternative explanations of the issue. One supports the hypothesis that on-going viral replication contributes to the continuous filling of the reservoir and is the main cause of the phenomenon. The other considers replication at low copies as the result of the progressive emptying of the viral reservoirs during effective ARV. Whereas it is likely that both mechanisms contribute to maintaining viral replication, conflicting data about the stability and/or evolution of the virus in the reservoir, the emergence of drug resistance and the results of ARV intensification therapy have not aided in resolving the pathogenesis of low-level viraemia, which remains controversial [18].
To date, a shared definition of low-level viraemia (LLV) does not exist. Several previous studies have generally defined LLV as viral load values between 50-1000 copies/ml, whereas others have defined it as a viral load above the assay cut-off value. More recently, authors who have used the term LLV have referred to viraemia value levels above 1, 10, 20, or 40 copies/ml [10, 12]. In a recent review, Ryscavage and colleagues [19] defined LLV as values of plasma HIV-RNA between 50 and 500 copies/ml; very low-level viraemia as the presence of detectable plasma HIV-RNA values below 50 (20-40) copies/ml; and residual viraemia as plasma HIV-RNA values between 1 and 10 copies/ml.
The purpose of this review is to provide an evaluation of the recent data regarding HIV replication at low copies from a clinical perspective. With this in mind, we will consider an HIV replication at low copies as any detectable HIV replication above the limits of assay detection.
Considering the previous definitions, we will use the term LLV for viraemia values above 50-400 copies/ml and the terms very low-level and residual viraemia according to the provisions of Ryscavage [19].
Low-Level Viraemia, Risk of Virological Failure and Emergence of Drug Resistance
Since 2010, the WHO has recommended the use of viral load monitoring to assess the response to ARV. To date, only approximately 50% of countries (those generally recognised as high-income) have adopted a policy of routine viral load monitoring in managing patients on ARV [20]. Although general agreement exists regarding achieving undetectable HIV viraemia as the goal of ARV and a shared definition of virological failure as ‘the inability to achieve or maintain HIV viral replication below the low level of assay detection’, there is no agreement regarding the test detection limits that define virological failure. The latest U.S. guidelines for antiretroviral therapy [21] define virological failure as an HIV RNA level above 200 copies/ml, whereas the European AIDS Clinical Society defines virological failure as the lack of HIV viraemia suppression to values below 50 copies/ml [22]. The British AIDS guidelines [23] do not consider a single viral load of 50–400 copies/ml preceded by and followed by an undetectable viraemia as a cause for clinical concern; they recommend a single viraemia > 400 copies/ml as an indicator of virological failure.
This variability in the viral failure definition and the possibility of using validated tests with a detection limit of 50 copies/ml or less has generated many concerns regarding the clinical significance and management of patients with LLV. From a clinical standpoint, the most relevant issue remains the correlation between LLV, virological failure, and the emergence of drug resistance.
Although the number and characteristics of the populations studied are not comparable, many studies have demonstrated correlation between LLV and virologic rebound [8, 10-13, 24-31]. Many studies agree in reporting a correlation between higher levels of LLV (>200-500 copies/ml) and an increased risk of virologic rebound [8, 27-29]. A recent study demonstrated a greater risk of virological failure in patients with LLV who were from high-income countries compared with low-income countries [30]. A very recent study in a Swiss cohort [29] reported the beneficial effect of ARV modification on the recovery of viral load in patients with LLV to below the values of detection.
Very low-level viraemia is less correlated with virological failure and is associated with a longer virological response following the initiation of ARV. In particular, a study on 1,214 HIV-positive patients on ARV performed by Maggiolo and colleagues [10] demonstrated a higher rate of virological rebound in patients with a prolonged viral load of 3 to 50 copies/ml compared with those with fewer than 3 copies/ml (2% vs 0.4%, respectively). A study in a similarly sized population by Doyle et al. [11] demonstrated a higher risk of virological rebound in suppressed patients with a viraemia of 40-49 copies/ml compared with patients with fewer than 40 copies/ml. The risk was increased in both cases if RNA was detectable.
The emergence of drug resistance is one of the main phenomena associated with virologic rebound during ARV. However, studies on the emergence of resistance mutations in patients with LLV have been limited by the lower limit of 1000 copies required for the majority of genotypic tests. In recent years, in-house genotypic resistance assays, effective at low copies of HIV RNA, were developed by a number of laboratories. The results of these tests in populations of patients with LLV have been reported in the literature. The presence of drug resistance-associated mutations has been reported at variable percentages among the different studies. An overall strong correlation between the persistence of LLV and the detection of drug resistance has been demonstrated in approximately 50% and 70% of subjects with LLV of 50-200 and more than 1000 copies/ml, respectively [32-38]. The possibility of the development of new drug resistance during LLV has also been explored in some studies demonstrating that between 7% and 46% of new drug resistance [32-35, 37] occurred in patients with LLV. Correlations between the emergence of drug resistance mutations and subsequent virological failure have also been observed [36].
Low-Level Viraemia and Activation of the Immune System
Recently, the indefinite persistence of inflammation during the course of HIV infection (while on ARV, which suppresses viraemia) has been confirmed [39]. The chronic activation of the immune system induced by continuous HIV replication and the presence of stable reservoirs may contribute to the morbidity and mortality in HIV-1-infected patients [40]. Cardiovascular disease, kidney disease, cancer, neurocognitive impairment, and other diseases are more frequently found in HIV infected patients compared with age-matched individuals in the general population. This may be attributed to accelerated ageing induced by the inflammation [41]. In two randomised clinical trials in which raltegravir was added to standard therapy in patients with sustained suppressed viraemia, a correlation between the persistence of low levels of HIV replication and inflammation was found. This suggests the trigger role of the inflammatory response on HIV-1 replication [42, 43].
The correlation between LLV and immune activation has been explored by a number of studies [44-52]. With few exceptions [44, 45], there is a general consensus that HIV replication at low copies is correlated with the persistence of immune activation, and, in addition, recent data show a correlation between CD4 + T cell activation and diseases progression [53]. Higher levels of activated CD8+CD38+ and CD8+HLADR+ lymphocytes were frequently detected in patients with a viraemia > 20 copies/ml compared with those with <20 copies/ml [47]. The presence of detectable HIV viraemia below 20 copies/ml was correlated with soluble markers of immune activation [49]. Furthermore, persistent levels of CD4+ and CD8+ T-cell activation were demonstrated in patients with viraemia <50 copies/ml who had poor immunological reconstitution [50]. Recently, Zheng and colleagues [51] analysed a group of 833 patients on fully active ARV for more than 96 months. The study demonstrated that residual low-level viraemia between 51 and 200 copies/ml is associated with greater CD8 T-cell activation; in patients with viraemia <50 copies/ml, a greater CD8 T-cell activation was associated with older age, hepatitis C virus antibody positivity, higher pre-ARV CD8 T-cell activation, lower concurrent CD4/CD8 ratios, and lower CD4 T-cell counts. The persistence of HIV replication in the cerebrospinal fluid in patients who responded to ARV has been correlated with elevated levels of neopterin [52]. This suggests that the HIV replication at low copies, even in biological sanctuaries, correlates with inflammatory persistence.
Low-Level Viraemia and Progression of the Infection (Appearance of Clinical Symptoms and Death)
Very little is known regarding the persistence of LLV and the clinical evolution of HIV infection with respect to the emergence of AIDS-related diseases or survival. To date, the only study that has directly investigated the impact of LLV on the clinical outcomes of HIV patients was presented at the recent CROI 2014 [54]. The authors analysed a cohort of more than 17,000 European and American virologically suppressed patients on ARV for 3-9 months. Patients with LLV (approximately 3.5% of the total, with 50-200 copies/ml of HIV-RNA) had a 4.5-fold increased risk of virological failure but only a 1.2-fold increased risk of developing AIDS or death.
Interventional Strategies for Managing Low-Level Viraemia
Low-level viraemia may arise from on-going, new cycles of viral replication resulting from incomplete inhibitory activity or penetration of ARV and somehow represents a treatment failure. A number of possible actions as treatment-intensification, improve drug levels (therapeutic drug monitoring - TDM) and patient adherence (i.e., the simplification and/or modification of ARV composition) were considered to contain the viral replication to below the limits of assay detection.
A number of published studies have addressed the issue of ARV intensification [42 , 43, 55-58] and raltegravir, maraviroc or a combination of these two drugs were the most common additional treatment used to strengthen ARV. Taken together, the results of these studies failed to show a further decrease in viral load or immune activation in the majority of patients during treatment intensification. Otherwise, some possible effect in terms of a significant reduction in reservoir size and partial immune restoration was observed in patients treated with a five-drug regimen during acute HIV infection [57].
As the level of drug exposure is a major determinant of the virologic response to therapy, TDM could be considered when a defect in drug absorption or when drug interactions are suspected. The results of published studies demonstrated that the decrease in viral load was greater in patients in whom optimal protease inhibitors (PI) concentrations were maintained with respect to those who had suboptimal drug concentrations [59]. Thus, even lacking a recommendation for routine use, TDM is strongly suggested in conditions of liver or kidney failure, or in selecting better drug concentrations in the course of resistance [21]. Recently, Calcagno and colleagues [60] demonstrated that TDM could be used to evaluate drug penetration in the central nervous system and that optimal drug concentrations in central nervous fluid was protective for viral escape in a group of patients responsive to ARV. In another recent cohort study, the use of TDM was related to adherence improvement and reduced hospitalisation cost [61]. No published data have directly correlated the use of TDM in the management of LLV; however, considering LLV the expression of possible on-going replication during ARV, TDM should be the first step in evaluating the presence of any non-therapeutic drug levels due to poor adherence and/or to individual defects in drug absorption.
A lack of ARV adherence is associated with higher levels of residual HIV-1 viraemia [62] and is an important predictive factor in treatment outcome [63]; therefore, adherence to treatment should be assessed as a possible cause of LLV in treated patients. Adherence to therapy has a multifactorial origin and may be influenced by social, personal and treatment-linked conditions, some of which may be difficult to correct [64]. However, ARV complexity is one of the possible causes of medication non-adherence [65] and once-daily, low-pill-burden and single-tablet regimens (STR) have been shown to be associated with better adherence [66] and virological suppression, even if both these effects tend to be reduced in longer-term follow up.
Different classes of drugs are associated with the possibility of achieving virus undetectability in blood. The use of non-nucleoside reverse-transcriptase inhibitors (NNRTI) has been considered as an independent factor associated with lower levels of residual viraemia in many studies [10, 67, 68]. In a retrospective study of 994 German patients, LLV was less often observed in NNRTI-based regimens with respect to PI-based first-line HIV therapies [69]. The long half-life pharmacokinetic property, the best penetration into anatomical sanctuaries and the simplified dosing schedule of this class of drugs most likely contribute to resulting in better viraemic control. Conversely, recently Titanji and co-workers, who investigated the contribution of the cell-to-cell mechanism of HIV dissemination in residual viraemia pathogenesis, demonstrated that whereas different classes of antiretroviral drugs display variable efficacy against different modes of HIV-1 dissemination, PIs appear be more potent inhibitors of cell-to-cell spread than nucleoside reverse transcriptase inhibitors and NNRTI [70].
In the clinical approach to the patient with persistent LLV, multiple aspects must be considered. If drug resistance to on-going treatment is present, a modification of in the treatment composition that selects an effective association is necessary. A TDM evaluation would be useful in suspected faulty adherence or in suboptimal drug dosing secondary to drug interaction. A re-evaluation of the pharmacological classes used and strategies to improve adherence (i.e., reducing daily pill burden or choosing a STR) should be in place. Due to the poor results of intensification treatment obtained thus far, adding drugs to classic triple therapy does not appear useful. Otherwise, the modification of the current ARV composition may be addressed to improve its pharmacokinetic characteristics with the ultimate aim of obtaining complete virological control.
CLINICAL CORRELATES OF HIV RESERVOIR QUANTIFICATION
ARV is able to block new infections; however, the reservoir established during acute infection persists throughout life [71]. Although the mechanisms of persistence are not clear, the viral reservoir is maintained (even during ARV) by the long half-life of the resting cells and, albeit a small fraction of these cells contain integrated HIV-DNA capable of producing an HIV virus upon stimulation [72, 73], several studies have reported that the persistence of replication-competent virus or the presence of unintegrated HIV-DNA (linear and circularised) together with cryptic replication may contribute to the continuous replenishment of the reservoirs [74-76]. Although HIV-1 replicates poorly in CD4 T-activated cells with low expression of the CCR5 coreceptor and low levels of deoxyribonucleoside triphosphate [77, 78], the activation of the cells by several stimuli contribute to the release of new viral particles. This causes an increase in the HIV reservoir size and represents a major obstacle for HIV eradication in patients receiving ARV [79].
The quantification of total HIV-1 DNA in peripheral blood mononuclear cells provides a reliable and easy means of measuring the size of the cellular reservoirs of HIV. Indeed, it has been shown that the amount of HIV-DNA in PBMCs reflects the number of latently infected cells in other compartments [80]. The principal source for HIV-1 persistence are resting CD4 + T cells of the lymphoid compartment and peripheral blood lymphocytes. Additionally, macrophages may be productively infected by HIV and may function as a major reservoir for the virus as these cells are relatively resistant to viral cytopathic effects. Moreover, HIV-DNA is also quantified in CD4 + T cells from the gut-associated lymphoid tissue (GALT), where the frequency of HIV-1 infection is generally higher than in the blood [81, 82]. To date, there are no standardised assays for HIV-DNA detection; however, different methods aimed at the evaluation of the total HIV-DNA or integrated and non-integrated (episomal) forms have been used by numerous studies. Total HIV-DNA detection does not allow for distinguishing the replication-competent from the replication-defective forms; otherwise, it is more sensitive, less labour-intensive, less expensive, and also the most frequently used technique in studies aimed at determining a clinical evaluation [83].
A number of factors influence the size, distribution and stability of the viral reservoir. During the natural course of HIV infection, HIV-DNA levels directly correlate with HIV infection progression in terms of the number of decreasing CD4 + lymphocytes and increasing viral load [84]. Moreover, the amount of HIV-DNA is an independent predictor of disease progression in the course of primary infection [85]. The timing of ARV initiation is an important factor in reducing the size of the reservoir. The results of the Visconti cohort study showed that early and prolonged ARV treatment during the primary phase of HIV infection might allow the achievement of long-term infection control even at its suspension, suggesting the important impact of early treatment on the magnitude of the reservoir [86].
The reduction of HIV-DNA is quite slow; most of the decrease occurs in the first 3 years from the start of ARV and stabilises thereafter [87]. The effect of ARV in reducing HIV-DNA values is more relevant in early-treated chronic patients or in patients treated during the acute stage of infection [88, 89]. Lower levels of HIV-DNA in chronic patients on effective ARV correlate with pre-therapy low levels of HIV-DNA and HIV-RNA, high CD4+ T lymphocytes, the duration of ARV, and the use of nevirapine [66, 67, 90].
CORRELATION BETWEEN LLV AND HIV RESERVOIRS
There is a paucity of studies regarding the correlation between LLV and HIV-DNA; moreover, the data are limited regarding long-term viraemia and HIV DNA monitoring in treated patients [91-95]. It is not known whether the correlations between HIV DNA levels and viral load are continuous or may change over the course of the effective treatment (Fig. 1).
Fig. (1).
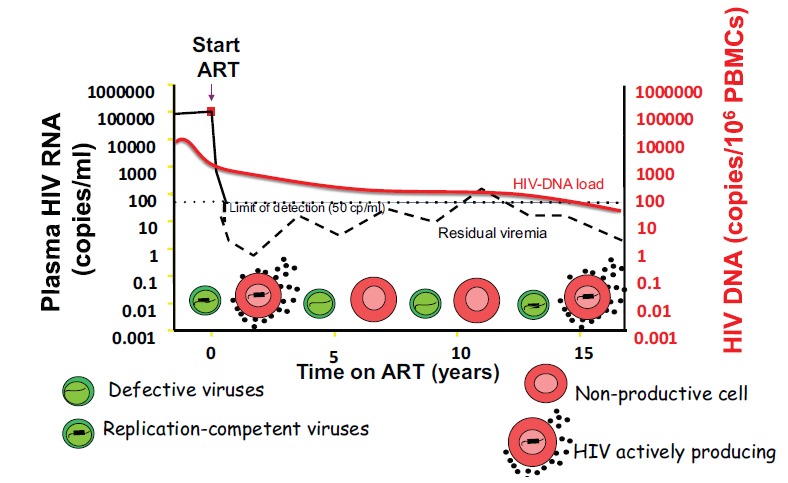
In ideal conditions, ARV ceases on-going viral replication, preventing the release of infectious particles from viral reservoirs. However, although ARV is able to limit the infection of healthy cells, the activation of long-lived cells (i.e., memory T-lymphocytes), infected before the initiation of therapy, contribute to the release of free virus in the blood (residual viraemia) and to maintaining the continuous filling of the HIV biological reservoirs.
Lower levels of HIV–DNA have been demonstrated in patients with undetectable viraemia compared with those with viraemia of 1-49 copies/ml [91, 92, 94]. In this regard, it may be possible, in patients with very low-level viraemia, that the amount of virus released from the viral reservoir is so low that it cannot be detected, even by the most sophisticated detection methods. A lower viral release into the plasma may facilitate the emptying and prevent the filling of the peripheral blood reservoir.
As reported in a study of 181 treated subjects who were followed for more than 4 years [93], a progressive decline in HIV-DNA has been correlated with low levels of HIV-DNA and residual viraemia (<2.5 copies). More recently, no correlation was found between the decline of HIV-DNA and HIV-RNA greater than or less than 1 copy/ml in a population of 30 patients who were followed for 4 years [95].
In a number of studies, HIV-DNA and LLV were monitored to evaluate clinical responsiveness in patients in whom alternative treatments were needed (ARV intensification or simplification). Conflicting results have been reported regarding the HIV-DNA trends in patients receiving intensification with raltegravir [96, 97]; the amount of integrated HIV-DNA seems to correlate with viraemia of less than 1 copy in patients receiving interferon alpha intensification therapy [98]. Alternatively, maraviroc intensification of ARV in chronically infected patients resulted in a reduction of HIV-DNA in monocytes with improvements in neurocognitive test performance [99].
In patients on darunavir/ritonavir monotherapy, the amount of HIV-DNA did not appear to be affected by the occasional virological rebound, although low levels of HIV-DNA have been associated with a lower risk of virological failure [100].
CONCLUDING REMARKS
A large majority of patients fully responding to ARV have detectable LLV; up to 77% of those patients with viral loads permanently below 50 copies were found to have residual viraemia (> 1 copy/ml) [101]. The presence of LLV has been correlated with the occurrence of virological failure and with the emergence of drug resistance; moreover, evidence exists that the modification of therapy is associated with the recovery of undetectable viral load.
The current method of detecting the presence of resistance mutations, even at low copies of viral replication, supports the careful evaluation of patients with LLV to exclude the presence of new drug-resistance mutations.
Selected categories of patients, such as those with a history of multiple virological failure or those who are evaluated for alternative therapeutic strategies (e.g., ARV simplification), who exhibit persistent levels of LLV, may benefit from further studies; these may include genotypic assay evaluation and the quantification of HIV-DNA, with the ultimate goal of selecting the best alternative regimen.
Due to the recent results regarding the role of ARV adherence in patients with LLV [62], all patients with LLV on prolonged ARV should be carefully evaluated to determine the correct drug dosing, the proper timing of drug intake and the possible absorption reductions of medications.
The high percentage of patients on ARV with suppressed viraemia and the possibility of future eradication treatments have stimulated the identification of new laboratory parameters in monitoring aviraemic patients. Although there are data correlating the trend of viral reservoir with LLV, routine HIV-DNA quantification in patients with LLV is limited by the lack of standardised methods. Higher HIV-DNA values have been suggested to be useful in tailoring more aggressive therapeutic choices [16, 102], whereas lower HIV-DNA levels may support strategies for simplifying the treatment in aviraemic patients [67, 91]. Combined monitoring of these two parameters may provide useful information in the evaluation of responder and non-responder patients when therapeutic alternatives are needed.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
ACKNOWLEDGEMENTS
Declared none.
REFERENCES
- 1.Mellors JW, Rinaldo CR Jr, Gupta P, et al. Prognosis in HIV-1 infection predicted by the quantity of virus in plasma. Science. 996;272(5265 ):1167–70. doi: 10.1126/science.272.5265.1167. [DOI] [PubMed] [Google Scholar]
- 2.Mellors JW, Muñoz A, Giorgi JV , et al. Plasma viral load and CD4+ lymphocytes as prognostic markers of HIV-1 infection. Ann Intern Med. 1997;126(12 ):946–54. doi: 10.7326/0003-4819-126-12-199706150-00003. [DOI] [PubMed] [Google Scholar]
- 3.Vlahov D, Graham N, Hoover D , et al. Prognostic indicators for AIDS and infectious disease death in HIV-infected injection drug users plasma viral load and CD4+ cell count. JAMA. Jan 7. 1998;279(1 ):35–40. doi: 10.1001/jama.279.1.35. [DOI] [PubMed] [Google Scholar]
- 4.O'Brien WA, Hartigan PM, Martin D, et al. Changes in plasma HIV-1 RNA and CD4+ lymphocyte counts and the risk of progression to AIDS. N Engl J Med. 1996;15; 334( 7):426–31. doi: 10.1056/NEJM199602153340703. [DOI] [PubMed] [Google Scholar]
- 5.O'Brien WA, Hartigan PM, Daar ES, et al. Changes in plasma HIV RNA levels and CD4+ lymphocyte counts predict both response to antiretroviral therapy and therapeutic failure VA Cooperative Study Group on AIDS. Ann Intern Med . 1997 Jun 15;126(12 ):939–45. doi: 10.7326/0003-4819-126-12-199706150-00002. [DOI] [PubMed] [Google Scholar]
- 6.Hughes MD, Johnson VA, Hirsch MS, et al. Monitoring plasma HIV-1 RNA levels in addition to CD4+ lymphocyte count improves assessment of antiretroviral therapeutic response ACTG 241 Protocol Virology Substudy Team. Ann Intern Med . 1997 Jun 15;126(12 ):929–38. doi: 10.7326/0003-4819-126-12-199706150-00001. [DOI] [PubMed] [Google Scholar]
- 7.Carpenter CCJ, Fischl MA, Scott M. Antiretroviral Therapy for HIV Infection in 1997 Updated Recommendations of the International AIDS Society—USA Panel. JAMA. 1997;277(24):1962–1969. [PubMed] [Google Scholar]
- 8.Raboud J, Montaner J, Conway B, et al. Suppression of plasma viral load below 20 copies/ml is required to achieve a long - term response to therapy. AIDS. 1998;12:1619–24. doi: 10.1097/00002030-199813000-00008. [DOI] [PubMed] [Google Scholar]
- 9.Bonora S, Nicastri E, Calcagno A, et al. Ultrasensitive assessment of residual HIV viremia in HAART-treated patients with persistently undetectable plasma HIV-RNA: a cross-sectional study. J Med Virol. 2009;81:400–405. doi: 10.1002/jmv.21405. [DOI] [PubMed] [Google Scholar]
- 10.Maggiolo F, Callegaro A, Cologni G , et al. Ultrasensitive assessment of residual low-level HIV viremia in HAART-treated patients and risk of virological failure. J Acquir Immune Defic Syndr. 2012;60:473–82. doi: 10.1097/QAI.0b013e3182567a57. [DOI] [PubMed] [Google Scholar]
- 11.Doyle T, Smith C, Vitiello P , et al. Plasma HIV-1 RNA detection below 50 copies/ml and risk of virologic rebound in patients receiving highly active antiretroviral therapy. Clin Infect Dis. 2012;54:724–32. doi: 10.1093/cid/cir936. [DOI] [PubMed] [Google Scholar]
- 12.Charpentier C, Landman R, Laouénan C, et al. Persistent low-level HIV-1 RNA between 20 and 50 copies/mL in antiretroviral-treated patients associated factors and virological outcome. J Antimicrob Chemother. 2012;67:2231–5. doi: 10.1093/jac/dks191. [DOI] [PubMed] [Google Scholar]
- 13.Álvarez Estévez M, Chueca Porcuna N, Guillot Suay V, et al. Quantification of viral loads lower than 50 copies per milliliter by use of the Cobas AmpliPrep/Cobas TaqMan HIV-1 test, version 2.0, can predict the likelihood of subsequent virological rebound to > 50 copies per milliliter. J Clin Microbio. 2013;51:1555–7. doi: 10.1128/JCM.00100-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chun TW, Finzi D, Margolick J, et al. In vivo fate of HIV-1-infected T cells quantitative analysis of the transition to stable latency. Nat Med. 1995;1:1284–1290. doi: 10.1038/nm1295-1284. [DOI] [PubMed] [Google Scholar]
- 15.Lewin SR, Rouzioux C. HIV cure and eradication how will we get from the laboratory to effective clinical trials? AIDS. AIDS. 2011 Apr; 24;25(7):885–97. doi: 10.1097/QAD.0b013e3283467041. [DOI] [PubMed] [Google Scholar]
- 16.Ramratnam B, Mittler JE, Zhang L, et al. The decay of the latent reservoir of replication-competent HIV-1 is inversely correlated with the extent of residual viral replication during prolonged anti-retroviral therapy. Nat Med. 2000;6:82–5. doi: 10.1038/71577. [DOI] [PubMed] [Google Scholar]
- 17.Ostrowski SR, Katzenstein TL, Thim PT, et al. Low-level viremia and proviral DNA impede immune reconstitution in HIV-1-infected patients receiving highly active antiretroviral therapy. J Infect Dis. 2005;1; 191(3):348–57. doi: 10.1086/427340. [DOI] [PubMed] [Google Scholar]
- 18.Shen L, Siliciano RF. Viral reservoirs, residual viremia, and the potential of highly active antiretroviral therapy to eradicate HIV infection. J Allergy Clin Immunol. 2008 Jul;122(1 ):22–8. doi: 10.1016/j.jaci.2008.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ryscavage P, Kelly S, Li JZ, et al. Significance and Clinical Management of Persistent Low-Level Viremia and Very-Low-Level Viremia in HIV-1-Infected Patients. Antimicrob Agents Chemother. 2014 Jul;58(7 ):3585–3598. doi: 10.1128/AAC.00076-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nelson LJ, Beusenberg M, Habiyambere V , et al. Adoption of national recommendations related to use of antiretroviral therapy before and shortly following the launch of the 2013 WHO consolidated guidelines. AIDS. 2014 Mar;28( Suppl 2):S217–24. doi: 10.1097/QAD.0000000000000239. [DOI] [PubMed] [Google Scholar]
- 21.Guidelines for the Use of Antiretroviral Agents in HIV-1-Infected Adults and Adolescents. http://aidsinfo.nih.gov/guidelines. on . [8/6/2014].
- 22.European AIDS Clinical Society GuidlinesVersion 7.02. http://eacsociety.org/Guidelines.aspx . [June 2014].
- 23.Wilkins E, Nelson M, Agarwal K, et al. British HIV Association guidelines for the management of hepatitis viruses in adults infected with HIV 2013. HIV Med. . 2013 Nov;14(Suppl 4):1–71. doi: 10.1111/hiv.12106. [DOI] [PubMed] [Google Scholar]
- 24.Laprise C, de Pokomandy A, Baril JG, et al. Virologic failure following persistent low-level viremia in a cohort of HIV-positive patients results from 12 years of observation. Clin Infect Dis. 2013 Nov;57(10):1489–96. doi: 10.1093/cid/cit529. [DOI] [PubMed] [Google Scholar]
- 25.Martin-Blondel G, Sauné K, Vu Hai V, et al. Factors associated with a strictly undetectable viral load in HIV-1-infected patients. HIV Med. 2012 Oct;13(9 ):568–73. doi: 10.1111/j.1468-1293.2012.01012.x. [DOI] [PubMed] [Google Scholar]
- 26.Murri R, Cozzi-Lepri A, Cicconi P. ICoNA Study Group. Is moderate HIV viremia associated with a higher risk of clinical progression in HIV- infected people treated with highly active antiretroviral therapy: evidence from the Italian cohort of antiretroviral-naive patients study. J Acquir Immune Defic Syndr. 2006 Jan 1;41(1):23–30. doi: 10.1097/01.qai.0000188337.76164.7a. [DOI] [PubMed] [Google Scholar]
- 27.Raboud JM, Rae S, Hogg RS, et al. Suppression of plasma virus load below the detection limit of a human immunodeficiency virus kit is associated with longer virologic responsethan suppression below the limit of quantitation. J Infect Dis. . 1999 Oct;180(4 ):1347–50. doi: 10.1086/314998. [DOI] [PubMed] [Google Scholar]
- 28.Sungkanuparph S, Groger RK, Overton ET, et al. Persistent low-level viraemia and virological failure in HIV-1-infected patients treated with highly active antiretroviral therapy. HIV Med. 2006 Oct;7(7):437–41. doi: 10.1111/j.1468-1293.2006.00403.x. [DOI] [PubMed] [Google Scholar]
- 29.Boillat-Blanco N, Darling KE, Schoni-Affolter F, et al. the Swiss HIV Cohort Study. Virological outcome and management of persistent low-level viraemia in HIV-1-infected patients 11 years of the Swiss HIV Cohort Study. Antivir Ther. 2014 Jun 25; doi: 10.3851/IMP2815. [DOI] [PubMed] [Google Scholar]
- 30.Kanapathipillai R, McManus H, Cuong D , et al. The significance of low-level viraemia in diverse settings analysis of the Treat Asia HIV Observational Database (TAHOD) and the Australian HIV Observational Database (AHOD). HIV Med. . 2014 Aug;15(7 ):406–16. doi: 10.1111/hiv.12124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Henrich TJ, Wood BR, Kuritzkes DR. Increased risk of virologic rebound in patients on antiviral therapy with a detectable HIV load <48 copies/mL. PLoS One. 2012;7(11 ):e50065–0. doi: 10.1371/journal.pone.0050065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Delaugerre C, Gallien S, Flandre P, et al. Impact of low-level-viremia on HIV-1 drug-resistance evolution among antiretroviral treated-patients. PLoS One. 2012;7(5 ):e36673. doi: 10.1371/journal.pone.0036673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li JZ, Gallien S, Do TD, et al. Prevalence and significance of HIV-1 drug resistance mutations among patients on antiretroviral therapy with detectable low-level viremia. Antimicrob Agents Chemother. 2012 Nov;56(11 ):5998–6000. doi: 10.1128/AAC.01217-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gallien S, Delaugerre C, Charreau I, et al. Emerging integrase inhibitor resistance mutations in raltegravir-treated HIV-1-infected patients with low-level viremia. AIDS. 2011 . Mar 13;25(5):665–9] Taiwo, B. doi: 10.1097/QAD.0b013e3283445834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Taiwo B, Gallien S, Aga E, et al. Antiretroviral drug resistance in HIV-1-infected patients experiencing persistent low-level viremia during first-line therapy. J Infect Dis. . 2011 Aug 15;204(4 ):515–20. doi: 10.1093/infdis/jir353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Swenson LC, Min JE, Woods CK , et al. HIV drug resistance detected during low-level viraemia is associated with subsequent virologic failure. AIDS. 2014 May 15;28(8):1125–34. doi: 10.1097/QAD.0000000000000203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gonzalez-Serna A, Min JE, Woods C , et al. Performance of HIV-1 drug resistance testing at low-level viremia and its ability to predict future virologic outcomes and viral evolution in treatment-naive individuals. Clin Infect Dis. 2014 Apr;58(8):1165–73. doi: 10.1093/cid/ciu019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Santoro MM, Fabeni L, Armenia D , et al. Reliability and clinical relevance of the HIV-1 drug resistance test in patients with low viremia levels. Clin Infect Dis. 2014 Apr;58(8):1156–64. doi: 10.1093/cid/ciu020. [DOI] [PubMed] [Google Scholar]
- 39.d'Ettorre G, Ceccarelli G, Giustini N , et al. aming HIV-related inflammation with physical activity a matter of timing. AIDS Res Hum Retroviruses. 2014;30(10 ):936–944. doi: 10.1089/aid.2014.0069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.d'Ettorre G, Paiardini M, Ceccarelli G, et al. HIV-associated immune activation from bench to bedside. AIDS Res Hum Retroviruses. 2011 Apr;27(4):355–64. doi: 10.1089/aid.2010.0342. [DOI] [PubMed] [Google Scholar]
- 41.Freiberg MS, Chang CC, Kuller LH , et al. HIV infection and the risk of acute myocardial infarction. JAMA Intern Med. 2013 Apr 22;173(8 ):614–22. doi: 10.1001/jamainternmed.2013.3728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hatano H1, Strain MC, Scherzer R, et al. Increase in 2-long terminal repeat circles and decrease in D-dimer after raltegravir intensification in patients with treated HIV infection a randomized, placebo-controlled trial. J Infect Dis. 2013 Nov;208(9):1436–42. doi: 10.1093/infdis/jit453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Buzón MJ, Massanella M, Llibre JM, et al. HIV-1 replication and immune dynamics are affected by raltegravir intensification of HAART-suppressed subjects. Nat Med. 2010;16(4):460–5. doi: 10.1038/nm.2111. [DOI] [PubMed] [Google Scholar]
- 44.Poizot-Martin I, Faucher O, Obry-Roguet V, et al. Lack of correlation between the size of HIV proviral DNA reservoir and the level of immune activation in HIV-infected patients with a sustained undetectable HIV viral load for 10 years. J Clin Virol. 2013;57(4):351–5. doi: 10.1016/j.jcv.2013.04.007. [DOI] [PubMed] [Google Scholar]
- 45.Schuler PJ, Macatangay BJ, Saze Z, et al. CD4+CD73+ T cells are associated with lower T-cell activation and C reactive protein levels and are depleted in HIV-1 infection regardless of viral suppression. AIDS. 2013;19; 27(10):1545–55. doi: 10.1097/QAD.0b013e328360c7f3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Karlsson AC, Younger SR, Martin JN, et al. Immunologic and virologic evolution during periods of intermittent and persistent low-level viremia. AIDS. 2004;18(7):981–989. doi: 10.1097/00002030-200404300-00005. [DOI] [PubMed] [Google Scholar]
- 47.Deeks SG, Martin JN, Sinclair E , et al. Strong cell-mediated immune responses are associated with the maintenance of low-level viremia in antiretroviral-treated individuals with drug-resistant human immunodeficiency virus type 1. J Infect Dis. 2004;189(2):312–321. doi: 10.1086/380098. [DOI] [PubMed] [Google Scholar]
- 48.Ostrowski SR, Katzenstein TL, Thim PT, et al. Low-level viremia and proviral DNA impede immune reconstitution in HIV-1-infected patients receiving highly active antiretroviral therapy. J Infect Dis. 2005;191(3 ):348–357. doi: 10.1086/427340. [DOI] [PubMed] [Google Scholar]
- 49.Ostrowski SR, Katzenstein TL, Pedersen BK, et al. Residual viraemia in HIV-1-infected patients with plasma viral load < or=20 copies/ml is associated with increased blood levels of soluble immune activation markers. Scand J Immunol. 2008 Dec;68(6):652–60. doi: 10.1111/j.1365-3083.2008.02184.x. [DOI] [PubMed] [Google Scholar]
- 50.Mavigner M, Delobel P, Cazabat M, et al. HIV-1 residual viremia correlates with persistent T-cell activation in poor immunological responders to combination antiretroviral therapy. PLoS One. 2009 Oct 30;4(10):e7658–0. doi: 10.1371/journal.pone.0007658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zheng L, Taiwo B, Gandhi RT , et al. Factors Associated With CD8+ T-Cell Activation in HIV-1-Infected Patients on Long-term Antiretroviral Therapy. J Acquir Immune Defic Syndr. 2014 Oct 1;67(2):153–60. doi: 10.1097/QAI.0000000000000286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dahl V, Peterson J, Fuchs D, et al. Low levels of HIV-1 RNA detected in the cerebrospinal fluid after up to 10 years of suppressive therapy are associated with local immune activation. AIDS. 2014 Jul 14; doi: 10.1097/QAD.0000000000000400. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Groves KC, Bibby DF, Clark DA, et al. Disease Progression in HIV-1-Infected Viremic Controllers. J Acquir Immune Defic Syndr. 2012 Dec 1;61(4):407–16. doi: 10.1097/QAI.0b013e318269c414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vandenhende MA, Ingle S, May M, et al. on behalf of the Antiretroviral Therapy Cohort Collaboration (ART-CC). Impact of Low-Level Viremia On Clinical and Virological Outcomes in Treated HIV Infected Patients. 21st Conference on Retrovirus and Opportunistic Infection (CROI 2014).; march 3-6 2014; Boston. Abstract 2014. [Google Scholar]
- 55.Dinoso JB, Kim SY, Wiegand AM, et al. Treatment intensification does not reduce residual HIV-1 viremia in patients on highly active antiretroviral therapy. Proc Natl Acad Sci U S A. 2009;9; 106(23):9403–8. doi: 10.1073/pnas.0903107106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.McMahon D, Jones J, Wiegand A, et al. Short-course raltegravir intensification does not reduce persistent low-level viremia in receipt of combination antiretroviral therapy. Clin Infect Dis. 2010;50:912–9. doi: 10.1086/650749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Puertas MC, Massanella M, Llibre JM, et al. MaraviBoost Collaborative Group Intensification of a raltegravir-based regimen with maraviroc in early HIV-1 infection. AIDS. 2014 Jan 28;28(3):325–34. doi: 10.1097/QAD.0000000000000066. [DOI] [PubMed] [Google Scholar]
- 58.Lafeuillade A, Assi A, Poggi C, et al. Failure of combined antiretroviral therapy intensification with maraviroc and raltegravir in chronically HIV-1 infected patients to reduce the viral reservoir the Intens HIV randomized trial. AIDS Res Ther. Oct. 2014;7; 11(1 ):33. doi: 10.1186/1742-6405-11-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Justesen US. Therapeutic drug monitoring and human immunodeficiency virus (HIV) antiretroviral therapy. Basic Clin Pharmacol Toxicol. 2006;98(1):20–31. doi: 10.1111/j.1742-7843.2006.pto_246.x. [DOI] [PubMed] [Google Scholar]
- 60.Calcagno A, Simiele M, Alberione MC, et al. Cerebrospinal Fluid Inhibitory Quotients of Antiretroviral Drugs in HIV-Infected Patients Are Associated With Compartmental Viral Control. Clin Infect Dis. 2015;15; 60(2):311–7. doi: 10.1093/cid/ciu773. [DOI] [PubMed] [Google Scholar]
- 61.Perrone V, Cattaneo D, Radice S, et al. Impact of therapeutic drug monitoring of antiretroviral drugs in routine clinical management of patients infected with human immunodeficiency virus and related health care costs a real-life study in a large cohort of patients. Clinicoecon Outcomes Res. 2014 Jul;14(6):341–8. doi: 10.2147/CEOR.S58036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Li JZ, Gallien S, Ribaudo H, et al. Incomplete adherence to antiretroviral therapy is associated with higher levels of residual HIV-1 viremia. AIDS. . 2014 Jan 14;28(2):181–6. doi: 10.1097/QAD.0000000000000123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bangsberg DR, Perry S, Charlebois ED, et al. Non-adherence to highly active antiretroviral therapy predicts progression to AIDS. AIDS. 2001;15(9):1181–83. doi: 10.1097/00002030-200106150-00015. [DOI] [PubMed] [Google Scholar]
- 64.Mathes T, Jaschinski T, Pieper D. Adherence influencing factors - a systematic review of systematic reviews. Arch. Public Health. 2014;72(1):37. doi: 10.1186/2049-3258-72-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Stone VE, Jordan J, Tolson J, et al. Perspectives on adherence and simplicity for HIV-infected patients on antiretroviral therapy self-report of the relative importance of multiple attributes of highly active antiretroviral therapy (HAART) regimens in predicting adherence. J Acquir Immune Defic Syndr. 2004;36(3):808–16. doi: 10.1097/00126334-200407010-00007. [DOI] [PubMed] [Google Scholar]
- 66.Nachega JB, Parienti JJ, Uthman OA , et al. Lower pill burden and once-daily antiretroviral treatment regimens for HIV infection A meta-analysis of randomized controlled trials. Clin Infect Dis. May. 2014;58(9):1297–307. doi: 10.1093/cid/ciu046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nicastri E, Palmisano L, Sarmati L, et al. HIV-1 residual viremia and proviral DNA in patients with suppressed plasma viral load (<400 HIV-RNA cp/ml) during different antiretroviral regimens. Curr HIV Res. May. 2008;6(3):261–6. doi: 10.2174/157016208784325010. [DOI] [PubMed] [Google Scholar]
- 68.Sarmati L, Parisi SG, Montano M, et al. Nevirapine use, prolonged antiretroviral therapy and high CD4 nadir values are strongly correlated with undetectable HIV-DNA and -RNA levels and CD4 cell gain. J Antimicrob Chemother. Dec. 2012;67(12):2932–8. doi: 10.1093/jac/dks331. [DOI] [PubMed] [Google Scholar]
- 69.Wiesmann F, Braun P, Knickmann M, Knechten H. Low level HIV viremia is more frequent under protease-inhibitor containing firstline therapy than under NNRTI-regimens. J Int AIDS Soc. Nov 2; 2014;17(4 Suppl 3):19828. doi: 10.7448/IAS.17.4.19828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Titanji BK, Aasa-Chapman M, Pillay D, Jolly C. Protease inhibitors effectively block cell-to-cell spread of HIV-1 between T cells. Retrovirology. 2013;24(10):161–0. doi: 10.1186/1742-4690-10-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Marsden MD, Zack JA. Neutralizing the HIV Reservoir. Cell. Aug 28; 2014;158(5):971–2. doi: 10.1016/j.cell.2014.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Finzi D, Hermankova M, Pierson T, et al. Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy. Science. Nov 14. 1997;278(5341):1295–300. doi: 10.1126/science.278.5341.1295. [DOI] [PubMed] [Google Scholar]
- 73.Chun TW, Justement JS, Moir S, et al. Decay of the HIV reservoir in patients receiving antiretroviral therapy for extended periods implications for eradication of virus. Infect Dis. Jun 15. 2007;195(12 ):1762–4. doi: 10.1086/518250. [DOI] [PubMed] [Google Scholar]
- 74.Chomont N, El-Far M, Ancuta P, et al. HIV reservoir size and persistence are driven by T cell survival and homeostatic proliferation. Nat Med. Aug. 2009;15(8):893–900. doi: 10.1038/nm.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Vandergeeten C, Fromentin R, DaFonseca S, et al. Interleukin-7 promotes HIV persistence during antiretroviral therapy. Blood. May 23; 2013;121(21 ):4321–9. doi: 10.1182/blood-2012-11-465625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Descours B, Cribier A, Chable-Bessia C, et al. SAMHD1 restricts HIV-1 reverse transcription in quiescent CD4(+) T-cells. etrovirology. Oct. 2012;23(9):8. doi: 10.1186/1742-4690-9-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Baldauf HM, Pan X, Erikson E, et al. SAMHD1 restricts HIV-1 infection in resting CD4(+) T cells. Nat Med. Nov. 2012;18(11):1682–7. doi: 10.1038/nm.2964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Douek DC. Immune activation, HIV persistence, and the cure. Top Antivir Med Sep-Oct. 2013;21(4):128–3211. [PMC free article] [PubMed] [Google Scholar]
- 79.Buzon MJ, Martin-Gayo E, Pereyra F, et al. Long-Term Antiretroviral Treatment Initiated at Primary HIV-1 Infection Affects the Size, Composition, and Decay Kinetics of the Reservoir of HIV-1-Infected CD4 T Cells. J Virol Sep 1. 2014;88(17):10056–65. doi: 10.1128/JVI.01046-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wong JK, Gunthard HF, Havlir DV, et al. Reduction of HIV-1-1 in blood and lymph nodes following potent antiretroviral therapy and the virologic correlates of treatment failure. Proc Natl Acad Sci USA. 1997;94:12574–9. doi: 10.1073/pnas.94.23.12574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chun TW, Carruth L, Finzi D, Shen X, DiGiuseppe JA. Quantification of latent tissue reservoirs and total body viral load in HIV-1 infection. Nature. 1997;387:183–188. doi: 10.1038/387183a0. [DOI] [PubMed] [Google Scholar]
- 82.Chun TW, Nickle DC, Justement JS, Meyers JH, Roby G, et al. Persistence of HIV in gut-associated lymphoid tissue despite long-term antiretroviral therapy. J Infect Dis. 2008;197:714–720. doi: 10.1086/527324. [DOI] [PubMed] [Google Scholar]
- 83.Strain MC, Richman DD. New assays for monitoring residual HIV burden in effectively treated individuals. Curr Opin HIV AIDS. Mar. 2013;8(2):106–10. doi: 10.1097/COH.0b013e32835d811b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Avettand-Fènoël V, Boufassa F, Galimand J, et al. ANRS SEROCO Cohort Study Group. HIV-1 DNA for the measurement of the HIV reservoir is predictive of disease progression in seroconverters whatever the mode of result expression is. J Clin Virol. Aug. 2008;42(4):399–404. doi: 10.1016/j.jcv.2008.03.013. [DOI] [PubMed] [Google Scholar]
- 85.Goujard C, Bonarek M, Meyer L. Agence Nationale de Recherche sur le Sida PRIMO Study Group. CD4 cell count and HIV DNA level are independent predictors of disease progression after primary HIV type 1 infection in untreated patients. Clin Infect Dis. Mar 1; 2006;42(5):709–15. doi: 10.1086/500213. [DOI] [PubMed] [Google Scholar]
- 86.Sáez-Cirión A, Bacchus C, Hocqueloux L, et al. ANRS VISCONTI Study Group. Post-treatment HIV-1 controllers with a long-term virological remission after the interruption of early initiated antiretroviral therapy ANRS VISCONTI Study. PLoS Pathog. Mar; 2013;9(3):e1003211. doi: 10.1371/journal.ppat.1003211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Viard JP, Burgard M, Hubert JB, et al. Impact of 5 years of maximally successful highly active antiretroviral therapy on CD4 cell count and HIV-1 DNA level. AIDS. 2004;2; 18(1 ):45–9. doi: 10.1097/00002030-200401020-00005. [DOI] [PubMed] [Google Scholar]
- 88.Strain MC, Little SJ, Daar ES, et al. Effect of treatment, during primary infection, on establishment and clearance of cellular reservoirs of HIV-1. J Infect Dis. 2005;1; 191(9):1410–8. doi: 10.1086/428777. [DOI] [PubMed] [Google Scholar]
- 89.Andreoni M, Parisi SG, Sarmati L, et al. Cellular proviral HIV-DNA decline and viral isolation in naïve subjects with <5000 copies/ml of HIV-RNA and >500 x 10(6)/l CD4 cells treated with highly active antiretroviral therapy. AIDS. Jan 7; 2000;14(1):23–9. doi: 10.1097/00002030-200001070-00003. [DOI] [PubMed] [Google Scholar]
- 90.Sarmati L, Parisi SG, Nicastri E, et al. Cellular HIV-1 DNA quantitation in patients during simplification therapy with protease inhibitor-sparing regimens. J Med Virol. Jul. 2007;79(7):880–6. doi: 10.1002/jmv.20914. [DOI] [PubMed] [Google Scholar]
- 91.Fourati S, Flandre P, Calin R, et al. Factors associated with a low HIV reservoir in patients with prolonged suppressive antiretroviral therapy. J Antimicrob Chemother. Mar. 2014;69(3):753–6. doi: 10.1093/jac/dkt428. [DOI] [PubMed] [Google Scholar]
- 92.Parisi SG, Andreis S, Mengoli C, et al. Baseline cellular HIV DNA load predicts HIV DNA decline and residual HIV plasma levels during effective antiretroviral therapy. J Clin Microbiol. 2012;50:258–63. doi: 10.1128/JCM.06022-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Parisi SG, Sarmati L, Andreis S, et al. Strong and persistent correlation between baseline and follow-up HIV-DNA levels and residual viremia in a population of naïve patients with more than 4 years of effective antiretroviral therapy. Clin Microbiol Infect. Mar; 2120;15(3):288.e5–7. doi: 10.1016/j.cmi.2014.10.009. [DOI] [PubMed] [Google Scholar]
- 94.Chun TW, Justement JS, Murray D, et al. Rebound of plasma viremia following cessation of antiretroviral therapy despite profoundly low levels of HIV reservoir implications for eradication. AIDS Nov 27. 2010;24(18):2803–8. doi: 10.1097/QAD.0b013e328340a239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Besson GJ, Lalama CM, Bosch RJ, et al. HIV-1 DNA Decay Dynamics in Blood During More Than a Decade of Suppressive Antiretroviral Therapy. Clin Infect Dis. Jul 29. 2014;pii ciu585 doi: 10.1093/cid/ciu585. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Llibre JM, Buzón MJ, Massanella M, et al. Treatment intensification with raltegravir in subjects with sustained HIV-1 viraemia suppression a randomized 48-week study. Antivir Ther. 2012;17(2):355–64. doi: 10.3851/IMP1917. [DOI] [PubMed] [Google Scholar]
- 97.Koelsch KK, Boesecke C, McBride K, et al. PINT study team. Impact of treatment with raltegravir during primary or chronic HIV infection on RNA decay characteristics and the HIV viral reservoir. AIDS. 2011;13; 25(17):2069–78. doi: 10.1097/QAD.0b013e32834b9658. [DOI] [PubMed] [Google Scholar]
- 98.Mexas AM, Graf EH, Pace MJ, et al. Concurrent measures of total and integrated HIV DNA monitor reservoirs and ongoing replication in eradication trials. . AIDS. Nov 28. 2012;26(18):2295–306. doi: 10.1097/QAD.0b013e32835a5c2f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ndhlovu LC, Umaki T, Chew GM, et al. Treatment intensification with maraviroc (CCR5 antagonist) leads to declines in CD16-expressing monocytes in cART-suppressed chronic HIV-infected subjects and is associated with improvements in neurocognitive test performance implications for HIV-associated neurocognitive disease (HAND). J Neurovirol. 2014;20(6):571–82. doi: 10.1007/s13365-014-0279-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Torres-Cornejo A, Benmarzouk-Hidalgo OJ, Gutiérrez-Valencia A, et al. Cellular HIV reservoir replenishment is not affected by blip or intermittent viremia episodes during darunavir/ritonavir monotherapy. AIDS. Jan 14. 2014;28(2):201–8. doi: 10.1097/QAD.0000000000000060. [DOI] [PubMed] [Google Scholar]
- 101.Palmer S, Maldarelli F, Wiegand A, et al. Low-level viremia persists for at least 7 years in patients on suppressive antiretroviral therapy. Proc Natl Acad Sci U S A. 2008;105(10):3879–84. doi: 10.1073/pnas.0800050105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Charpentier C, Fagard C, Colin C, et al. Role of baseline HIV-1 DNA level in highlyexperienced patients receiving raltegravir, etravirine and darunavir/ritonavir regimen (ANRS139 TRIO trial) PLoS One. 2013;8(1):e53621. doi: 10.1371/journal.pone.0053621. [DOI] [PMC free article] [PubMed] [Google Scholar]


