Abstract
Here, the use of emulsomes as a drug delivery system is reviewed and compared with other similar lipidic nanoformulations. In particular, we look at surface modification of emulsomes using S-layer proteins, which are self-assembling proteins that cover the surface of many prokaryotic organisms. It has been shown that covering emulsomes with a crystalline S-layer lattice can protect cells from oxidative stress and membrane damage. In the future, the capability to recrystallize S-layer fusion proteins on lipidic nanoformulations may allow the presentation of binding functions or homing protein domains to achieve highly specific targeted delivery of drug-loaded emulsomes. Besides the discussion on several designs and advantages of composite emulsomes, the success of emulsomes for the delivery of drugs to fight against viral and fungal infections, dermal therapy, cancer, and autoimmunity is summarized. Further research might lead to smart, biocompatible emulsomes, which are able to protect and reduce the side effects caused by the drug, but at the same time are equipped with specific targeting molecules to find the desired site of action.
Keywords: Drug delivery system, emulsome, lipid nanoparticles, S-layer proteins
1. INTRODUCTION
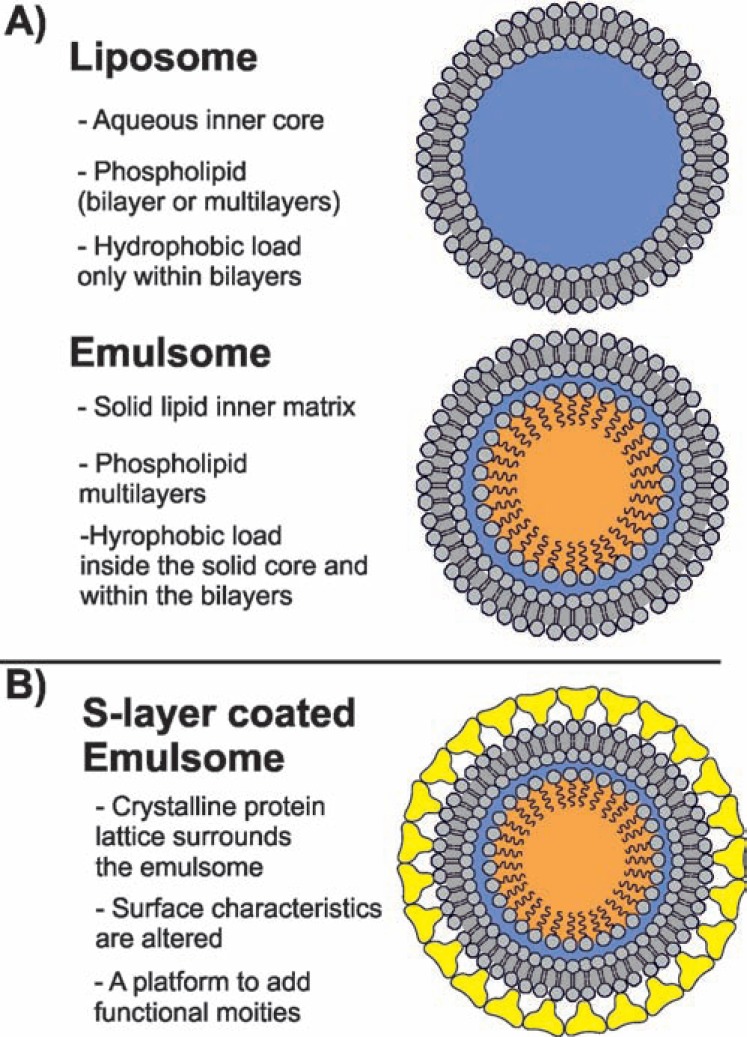
As a lipoidal vesicular system, emulsomes are composed of an internal solid fat core surrounded by phospholipid (PL) multilayers (Fig. 1A) [1-4]. The lipophilic load can be localized in the inner fat core, and the PL layers. The key feature of these nanoformulations is that the fat core is in the bulk in a solid or liquid crystalline phase rather than existing as oil in a fluid phase [4, 5]. This feature discriminates emulsomes from emulsions and allows the encapsulation of higher amounts of lipophilic compounds [2].
Fig. (1).
Schematic drawings of (A) a liposome and an emulsome; (B) S layer coated emulsome. The PLs surrounding liposomes and emulsomes, as well as the S-layer are not drawn up-to-scale.
Similar formulations have been studied for their lipophilic drug delivery capacity and are known in the literature as solid lipid nanoparticles (SLNs) [6, 7] and lipospheres [8-10]. The SLN is defined as a crystalline solid lipid core matrix with a mean diameter of 50-1000 nm, which is stabilized by surfactants (emulsifiers) [6, 7, 11]. Therefore, it differs from emulsomes in the outermost shell composition. Nonetheless, when formulated with PL and triglyceride (TG), SLNs have exactly the same composition as emulsomes [12-14]. On the other hand, lipospheres consist of water-dispersible solid “microparticles” composed of a solid hydrophobic fat core which are stabilized by one monolayer ofPL molecules that form their surface [15, 16]. Thus, lipospheres are distinguished from emulsomes by the larger size, which are in the micrometers scale, i.e. 0.2-500 µm [16, 17].
Emulsomes, similarly to SLNs and lipospheres, increase the solubility and bioavailability of poorly water-soluble bioactive agents, which are entrapped inside the solid lipid matrix of the nanocarrier. Solid lipid matrices have been proven to overcome certain stability problems, such as drug leakage or coalescence, which are often observed in drug-loaded lipid dispersions such as emulsions and liposomes [18]. Similar to (solid) polymeric nanoparticles, the drug release from the solid matrix of emulsomes is degradation-controlled and, hence, slower than the diffusioncontrolled release from emulsions [12, 18]. This ensures sustained drug release over a prolonged period of time [3, 12, 19]. In addition, this formulation is safe and nontoxic [5].
Having partially the characteristics of both liposomes and emulsions, emulsomes possess certain advantages. Firstly, stabilized by PL layers (Fig. 1A) as the outermost structure, emulsomes require no surfactant as a stabilizing agent. This feature endows emulsomes with a high degree of biocompatibility for therapeutic applications. Secondly, unlike the lipid emulsion, which has a fluid core, the emulsome with its solid fat core can prolong the release of incorporated drugs significantly [3, 12, 13, 19-21]. Lastly, having a similar shell structure to liposomes (Fig. 1A), the surface of emulsomes can be further tailored to fulfill specific requirements such as longer blood circulation or to enable cell targeting and active drug delivery (Table 1) [20, 22-24].
Table 1.
Summary of the main advantages of emulsomal for-mulations.
| High load capacity for poorly water‑soluble drugs |
| Improvements in both, drug absorption and drug bioavailability |
| High stability |
| Slow drug release profile and prolonged drug efficacy |
| Safe cytotoxicity profile |
| Further possible surface modifications,e.g., for cellular targeting |
| Low production cost and simplicity for large‑scale production |
A deep understanding of lipidbased drug delivery systems (DDSs) is essential to figure out the most suitable application of each emulsomal formulation. For instance, lipidbased DDSs may positively influence drug absorption in a number of ways, including bioavailability enhancement, enhanced membrane permeability, inhibiting efflux transporters, targeted lymphatic transport and modulated enterocyte-based drug transport [25, 26]. The lymphatic route is known to play a crucial role in the formation of metastases for a wide spectrum of cancers to enter systemic circulation and develop secondary tumors [27]. Consequently, the lymphatic route becomes an option for the delivery of therapeutic agents including bioactive molecules treating cancer and human immunodeficiency virus (HIV) by using these lipid formulations [28]. The entry of lipidbased DDSs to the lymphatic system is therefore important to highlight the potential of emulsomes for targeting drugs to cancer tissues and viral infections. As a consequence, the literature addressing emulsomes is focused on these two latter issues.
In this review, we shed light on the characteristic features of this novel formulation, summarize and analyze the recent progresses in emulsomal drug formulations, and finally highlight the potential of nanotechnological bottomup (self-assembly) techniques such as bacterial crystalline surface (S) layer proteins [29, 30] to improve the intrinsic characteristics of emulsomes (Fig. 1B). As discussed in detail, S-layer technology may benefit to emulsomes in different ways, including in i) improved antifouling characteristics, which are particularly important in the biological environment; ii) the possibility to expose functional domains in a well-ordered fashion; and iii) the opportunity to self-assemble more than one S-layer (fusion) protein on emulsomes for the synthesis of multi-functional DDSs [31].
2. BUILDING BLOCKS FOR BIOMIMETIC NANOFORMULATIONS
In nature, many types of virus particles exist either as naked nucleocapsids or as nucleocapsids surrounded by viral envelopes composed of one, or in rare cases, even more lipid bilayers [32]. An interesting biomimetic approach in nanomedicine is the use of this building principle for the synthesis of novel drug nanoformulations with increased efficacy and application potential. In the following, the characteristic features of lipids and proteins as building blocks to create DDSs will be discussed:
2.1. Lipids
Lipids show poor solubility in water but are soluble in non-polar organic solvents [33]. Their intermolecular interactions are largely regulated by van der Waals interactions [34] and the hydrophobic effect [35]. One prominent example for this class of lipids are TGs (also named triacylglycerol), colloquially known as fats. TGs are composed of one molecule of glycerol, which is joined via ester bonds with three molecules of long-chain fatty acids. Generally saturated fats have a higher melting point and, hence, are solid at room temperature. TGs serve as energy storage molecules, particularly in adipose tissue, and are also part of lipoproteins, but are not considered as membrane lipids because of their entirely non-polar characteristics [33, 36]. Additional functions of TGs are to protect organs; constitute isolation against cold; carry the fat-soluble vitamins A, D, E, and K into the body and help in the adsorption of these vitamins in the intestines; and supply essential fatty acids [36]. In the present context TGs are used to solubilize lipophilic drugs and other bioactive substances for diagnostic or therapeutic applications or hydrophobic nanoparticles or dyes for imaging purposes.
Membrane lipids are, however, amphiphilic molecules, which associate with other molecules (such as membrane proteins) and with aqueous solvents through electrostatic interactions and hydrogen bonding [37]. The most prominent lipid within this class is the PL (also named glycerophospholipid). As with TGs, PLs are composed of a glycerol backbone forming ester linkages to two fatty acids [38]. These alkyl chains constitute the hydrophobic part of the PLs. The third position is occupied by a polar head group joined through a phosphodiester, rather than a fatty acyl ester [37]. As a consequence, the PL molecule is considerably more polar, in particular as the phosphate group is negatively charged under physiological conditions [38]. Moreover, each head alcohol residue contributes to the net charge differently. For instance, choline and ethanolamine have a positive charge, serine and glycerol are neutral, and inositol is negatively charged [37].
PLs are the most important lipids for bacterial and mammalian cells as they form the lamellar core structure of these biological membranes [33]. Thus, PLs are the components that give cellular membranes the grass morphology of a closed structure and constitute an important barrier function. In most cases, PLs are necessary for the stabilization and function of native membrane-bound proteins, but may also be regarded as important intracellular signaling molecules [39].
2.2. S-layer Proteins
One of the most common cell surface structures in bacteria and archaea are two-dimensional crystalline arrays composed of identical species of protein or glycoprotein subunits, called S-layers [30, 40]. In general, S-layers are monomolecular assemblies of single subunit species with a molecular weight ranging between 40 and 200 kDa [41]. S-layer lattices generally exhibit oblique (p1, p2), square (p4), or hexagonal (p3, p6) space group symmetry with a center-to-center spacing of the morphological units of 3.5-35 nm [29, 40, 41]. High-resolution transmission electron microscopy (TEM) and atomic force microscopy (AFM) studies on the 5 to 10-nm thick bacterial S-layers showed a rather smooth outer and a more corrugated inner surface [29, 30, 42]. Moreover, S-layers form lattices with a porosity of 30-70% and pores uniform in size and morphology in the 2-8 nm range [29, 43]. However, two or even more distinct classes of pores may be observed in many S-layer lattices [29, 40].
Upon removal of the disrupting agent (e.g. urea or guanidine hydrochloride) used in the dissociation procedure, isolated S-layer proteins reassemble into regular arrays [44-46]. Most important for application purposes, S-layer proteins are able to reassemble into large coherent monolayers or intercalating bilayers on solid supports such as silicon, semiconductors, metals, and polymers, and can assemble on planar and spherical lipid films such as liposomes and emulsomes [40-42, 46].
With respect to practical applications it is interesting to note that an S-layer on a solid support shows either cell adhesive (cytophilic) or cell repulsive (cytophobic) surface properties against cells in tissue culture depending on whether the inner or outer side is exposed to the aqueous environment [41]. For example, the orientation and, thus, function of the S-layer protein SbpA from Lysinibacillus sphaericus CCM 2177 can be regulated by simply changing the conditions from a basic (pH 9) to an acidic (pH 4) which further leads to formation of an outer smooth cytophobic or an inner rough cytophilic surface pattern, respectively [47].
An outstanding antifouling function of the S-layer lattice SbpA in the presence of highly concentrated human serum albumin solutions, plasma and whole blood samples was also recently reported [41, 48]. This result was attributed to the inherently (zwitterionic) neutral surface charge of SbpA [48] and might be a very important observation in the context of DDSs.
2.3. S-layer Fusion Proteins
To expand the great potential of native S-layer proteins as patterning elements and nanoscale building blocks, genetic techniques were applied for modifying and changing the natural properties of S-layer proteins [30, 41, 46, 49]. Recrystallization studies and surface accessibility screens with various genetically produced N-terminally and/or C-terminally truncated S-layer proteins have been performed in order to figure out which parts of the S-layer protein are necessary for the recrystallization process and which protein domains are freely accessible on the recrystallized lattice [46]. In this way, truncated recombinant S-layer proteins (with their amino acids given as subscripted character; e.g. rSbpA31-1068) were figured out as optimal candidates to carry fused functional proteins or protein domains for the construction of chimeric S-layer proteins with the retained capability to assemble into geometrically well-defined layers [46]. Three different mutants of the enhanced green fluorescent protein (EGFP) and the red fluorescent protein mRFP1 have been fused to S-layer proteins to design novel fluorescent building blocks suitable for the generation of, e.g. pH indicators both in vivo and in vitro or as fluorescent markers for DDSs [46, 50-52]. A further promising application is the coating of liposome-DNA complexes with fluorescent S-layer fusion proteins as novel DDSs for the transfection of eukaryotic cell lines. One representative example for the uptake of S-layer coated-liposomes into eukaryotic cells is the S-layer fusion protein rSbpA31-1068/EGFP which was recrystallized as a one-molecule thick layer on the positively charged surface of liposomes [53].
The peptide mimotope F1 mimicking an immunodominant epitope of Epstein–Barr virus (EBV) was also fused to an S-layer protein [54, 55]. The resulting SbpA/F1 fusion proteins fully retained intrinsic recrystallization capability, but, most importantly, the F1 mimotope was well presented on the outer surface and, hence, accessible for antibody binding. The assessed diagnostic values of SbpA/F1 resulted in a remarkable specificity. Furthermore, no cross-reactivity with related viral disease states including rheumatoid factor was observed in solid-phase immunoassays using EBV diagnostics as a model system [54]. Moreover, the S-layer preserved the structure of the peptide to a higher extent than peptides immobilized on common solid supports.
S-layer enzyme fusion proteins have been generated to provide a platform onto which enzymes are immobilized in a well-defined and oriented manner [46, 56-58]. The recrystallized S-layer/enzyme fusion proteins may preserve the activities up to 100% and may also be retained for a longer period of time [54]. Moreover, recently S-layer lattices could even be used as patterning element for multimeric extremozymes [59]. Hence, it may be conceivable that emulsomes armed with a coat composed of S-layer fusion proteins presenting enzymes in a well-oriented and easily accessible fashion will have advantages in attacking cells that have an outer polymeric surface. This idea is derived from, for example, the influenza virus neuraminidase, which cleaves off sialic acid from glycans on cells allowing release of progeny viruses from the host cell [60].
Chimeric antibody-binding S-layer proteins utilized as functional coating for any nanoformulation used as drug delivery and targeting system are of special interest. For this purpose, the chimeric rSbpA31–1068/ZZ protein carrying two copies of the 58 amino acids long FC-binding Z-domain (a synthetic analogue of the immunoglobulin G (IgG)-binding domain of protein A from Staphylococcus aureus) was constructed and its binding capacity for human IgG was investigated [46, 61]. Moreover, a biocompatible matrix for the microsphere-based detoxification system used for extracorporeal blood purification of patients suffering from autoimmune disease has been generated by recrystallization of rSbpA31–1068/ZZ on microbeads [46, 61].
Recently, two protein G domains having a specific affinity to IgG were genetically coupled to SbpA [62]. In this study, Protein G was chosen as the IgG-receptor not only because human IgG antibodies and animal IgG equivalents strongly bind to protein G rather than to its alternative, i.e., protein A, but also because protein G possesses a higher specificity for IgG, whereas protein A reacts also with immunoglobulin classes other than IgG [63]. Both fusion proteins carrying either ZZ or GG domains constitute promising functions acting as homing proteins for targeted DDSs.
An alternative to the above approach is an S-layer fusion protein presenting the sequence of an antibody. This has already been achieved with the variable domain of a heavy chain camel antibody for recognizing lysozyme as antigen [64] or directed against prostate-specific antigen (PSA) [65]. Finally, for vaccine development it has been demonstrated that recombinant S-layer/allergen fusion proteins can even be produced pyrogen-free [66]. Endotoxin-free S-layer fusion protein preparations showed excellent recrystallization properties and immune reactivity, and hence, may attain high importance for medical and pharmaceutical applications.
3. STRUCTURE AND FEATURES OF EMULSOMES
3.1. Composition
Emulsomes are composed of two structural divisions: i) the internal core, and ii) the surrounding shell. The internal core is composed of a fat or lipid that exhibits a solid or liquid crystal physical condition, or has mixed solid and liquid crystal phases at room temperature [2]. TGs, monoesters, waxes, cholesterol, and cholesteryl esters can be used pure or as a mixture. However, TGs composed of natural, even-numbered and unbranched fatty acids such as trilaurin, tripalmitin, and tristearin are widely preferred [2]. Incorporation of cholesterol and cholesteryl esters into the core alters its packaging structure and induces the formation of a liquid crystal phase [2]. The internal core is stabilized by a multilamellar shell which can be composed of natural or (partially) hydrogenated lecithin or of pure, synthetic, hydrogenated, or partially hydrogenated forms of PLs [1, 2]. Charged PLs can also be used to confer a higher stability to the formulation because aggregation is prevented mainly by repulsive forces between the emulsomes [2]. In addition, charged surfaces may influence the biodistribution of the formulation in vivo. For instance, cationic emulsomes were found to be cleared faster from the circulation by macrophages resulting in an increased drug concentration in the liver which is desired for treatment of intracellular liver infections [3]. Therefore, as the outermost layer modulates the contact of the emulsome with the environment, the PL shell determines the in vivo behavior of the nanoformulation including circulation half-life time, toxicity, and cellular targeting. Surface modifications such as ligand and antibody binding [5, 20, 22, 23] or protein coating [31] have been considered to improve the efficacy of the drug formulation and to minimize the possible side effects of the formulation as well as the drug. In the near future, more surface modifications will be reported for emulsomes because their outermost PL surface is analogous to liposomes.
3.2. Multilamellarity
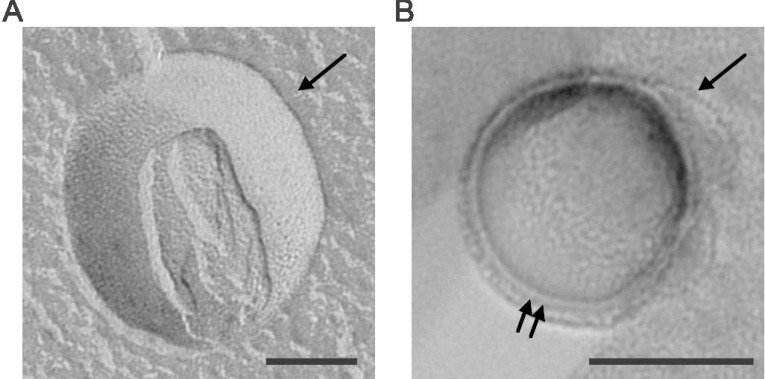
The multilamellar character of emulsomes was first indirectly concluded through estimation of the amount of water-soluble markers trapped in the aqueous phase between the PL bilayers [2, 12]. Later on, the layers surrounding the internal solid core were visualized by freeze-fracture electron microscopy [13, 31, 67]. Figure 2 shows a replica of a tripalmitin-based emulsome that remained intact in a convex shape during freeze fracture. The multilamellar PL shell surrounding the core of the emulsome revealed a thickness of approximately 26 nm (Fig. 2B). The degree of multilamellarity, in other words the number of PL bilayers or the thickness of the PL shell, varies largely depending on the PL:TL (total lipid) ratio as well as the physical characteristics of the PL and lipid mixture. As high amounts of PL favor the formation of bilayers [3, 12], care must be taken to avoid by-product formation, for instance liposomes [31, 68]. According to Amselem et al. (1996), PL:TL molar ratios between 0.1 and 0.5 will provide formation of stable emulsomes with high purity [2]. In agreement with this, Heiati et al. (1996) corroborated that multiple bilayer formation begins at PL:TL ratio of 0.13 (or PL:TG ratio of 0.15 as originally reported) [12].
Figure 2.
TEM micrograph of replicas of freeze fractured emulsomes. The direction of Pt/C shadowing is indicated by an arrow in the upper right-hand corner. (A) Concave fracture face: Most part of the solid tripalmitin core was removed during the fracture process. Remnants of the multilayered PLs can be seen; (B) Convex fracture face exhibiting the cross-fractured multilayered PLs (double arrows). Each bar represents 100 nm (Adapted from [31]. Copyright ©2013 with permission from WILEY-VCH).
3.3. Size
The PL:TL ratio plays an essential role for both particle size and stability of emulsomes. Below and above the PL:TL ratio interval of 0.1 to 0.5 either the particle stability could not be maintained or by-product formation was observed [1]. It was demonstrated that each increase in the PL:TL ratio between 0.14 and 0.4 yields emulsomes larger in size and thus an enhanced entrapment efficiency for the loaded drug [3] (PL:TL values are calculated from the PL:TG ratios in the original paper, i.e. 0.25-1). The increase in size was attributed to an increased number of formed PL bilayers [3]. However, a distinct correlation between the relative amount of PL and the particle size could not be traced.
PLs are known for their emulsification properties to form nanoparticles in a concentration-dependent manner [69]. Accordingly, when the amount of other constituents is kept constant, the increase in PL concentration results in formation of more emulsomes, i.e. particles with smaller diameter. In agreement with this hypothesis the smallest particle sizes were achieved when TG concentration was decreased and/or PL concentration was increased [12, 31, 70].
Beside the PL:TL ratio, the properties of the solid lipids forming the internal core may become important in regulating the particle size, as tristearin-based emulsomes were observed to be larger in diameter than trilaurin-based emulsomes, while keeping the sonication time as well as all other parameters constant [3]. The larger size was attributed to the longer alkyl chain of the tristearin molecule.
Furthermore, the size of emulsomes can be tuned mechanically by the homogenization process. As previously reported, longer sonication time [5, 20, 68] or extrusion of the preparation through filters with smaller pore size [31] allow to some extent the formation of smaller particles. Interestingly, the diameter of the formulation may determine the route of clearance in the body. For instance, particles with a larger diameter than 200 nm were reported to be rapidly cleared from the circulation via macrophages. Hence, the former nanoparticles are highly suitable for macrophage targeting, whereas smaller nanoparticles are more suitable for systems targeting inflammatory sites such as a tumor [71].
3.4. Stability
Insofar as nanoparticle-mediated DDSs are concerned, the term stability refers to the ability of the nanocarrier to maintain its biophysical characteristics such as size, zeta potential and drug retention for a long period of time. Compared to other lipid-based formulations such as liposomes, emulsomes have a higher stability in suspensions, a finding which could be of considerable advantage in clinical practice [21]. The high stability of emulsomes is generally attributed to the internal solid lipid core and the multilamellar character of the shell. Beside the biophysical characteristics of the used lipids, the storage temperature is also an important parameter influencing the stability of the nanocarrier. For instance, tripalmitin-based emulsomes were shown to be stable at 4oC for at least 1 month [31], whereas in another study amphotericin B-loaded emulsomes were found to be stable for at least 3 months at the same storage temperature [21]. Ucisik et al. (2013) demonstrated that tripalmitin-based emulsomes preserve their initial particle size and zeta potential values for the longest period of time when stored at refrigerated temperatures (≈4oC), whereas in contrast storage at 20oC and 37oC resulted in loss of surface charge and particle aggregation [31]. Supporting these findings, Raza et al. (2013) reported the lowest drug leakage out of emulsomes at 6oC compared to higher temperatures (30oC and 45oC) [70], and Paliwal et al. (2009) observed that size and drug entrapment rate of emulsomes remain relatively constant when stored at 4oC for 3 months, whereas storage at room temperature led to leakage of the incorporated drug and growth in size [68]. As an exceptional case, trilaurin-based emulsomes were reported to be stable for a period of 10 weeks at 20oC, whereas an increase in size and expulsion of the incorporated drug were observed at 4oC and 37oC [14]. To achieve even longer storage times, emulsomal formulations may be lyophilized in the presence of cryoprotectants such as sucrose [68] or trehalose [14], and stored at -20oC.
3.5. Drug Encapsulation
Drug loading of emulsomes occurs during the production procedure of emulsomes, i.e. when the lipid components assemble. Owing to their nature, the lipophilic drugs dissolve and are entrapped in the internal core and become intercalated inside the PL bilayers. As previously demonstrated, the high degree of multilamellarity enhances the drug encapsulation rates [3, 68]. Hydrophilic molecules can only be entrapped in the aqueous space between the PL bilayers. In addition, amphiphilic molecules become preferentially integrated in the PL layers.
The high loading capacity of emulsomes for lipophilic compounds was recently proven by a curcumin-emulsome formulation study, where the solubility of the compound in water, which was ≤11 ng/ml [72], was enhanced by up to 10,000 fold corresponding to 0.11 mg/ml, a concentration which facilitated the biomedical use of the lipophilic compound tremendously [19].
Although emulsomes are distinguished in particular for their high load capacity for lipophilic compounds, they may also be used to deliver water-soluble components because multilamellar PLs capture a reasonably large aqueous volume between the bilayers. One may estimate that emulsomes are composed of 1-3 bilayers on average, and therefore have a trapped aqueous volume of approximately 1.1±0.6 µl per µmol PL, which is significantly higher than the aqueous volume of single small unilamellar vesicles (SUV), i.e. 0.3±0.2 µl per µmol PL [2]. In the light of these findings, emulsomes should be considered as a nanocarrier applicable for the simultaneous delivery of both hydrophilic and hydrophobic compounds rather than a DDS for only hydrophobic molecules.
3.6. Surface Modifications
Having an analogous surface to liposomes, emulsomes can further be tailored to fulfill specific surface requirements such as longer blood circulation or to enable cell targeting and active drug delivery. For instance, to develop macrophage-targeted systems Gill et al. (2011) coated emulsomes with O-palmitoyl amylopectin (OPA) [23], whereas Gupta et al. (2007), Pal et al. (2012), and Vyas et al. (2009) coated them with O-palmitoyl mannan (OPM) [5, 20, 22, 24]. In addition, Vyas et al. (2009) coated emulsomes with monoclonal antibody EBA-2 for the targeting of fungal infections [24]. Most recently, the study of Ucisik et al. (2013) provided evidence for the capability of crystalline bacterial cell surface layer (S-layer) proteins to be recrystallized on the surface of emulsomes and to entirely modify the surface characteristics of the nanocarrier with their unique lattice features [31].
3.7. Cellular Uptake
Both, cationic as well as anionic emulsomes are essentially internalized either through endocytosis or by direct diffusion [3, 31]. Before undergoing lysosomal degradation, emulsomes destabilize endosomal membranes by means of charge–charge interaction. Subsequently, the emulsomes may enter the cell cytoplasm where the delivered drug is slowly released from the internal solid lipid matrix, thereby maintaining an effective intracellular drug concentration over a prolonged period of time [3, 19-21].
3.8. Administration Routes
Because particulate DDSs have the natural tendency to localize to the mononuclear phagocyte system, in particularly to the spleen and liver macrophages, the intravenous administration of emulsomes is frequently performed [73]. For instance, emulsomes were intensively studied for the delivery of antileishmanial drugs to macrophages and to the liver [5, 20-23]. In another study that aimed to deliver a drug to alveolar macrophages, aerosolized emulsomal formulations were applied through pulmonary administration [24].
On the other hand, advantages of the delivery to the intestinal lymphatic system include avoidance of hepatic first pass metabolism and the potential to target diseases spreading via the lymphatics, e.g. certain lymphomas and HIV [74]. Although oral administration is generally preferred, in systems lacking the stability for gastric fluid, lymphatic drug delivery was achieved through the intraduodenal route [68]. Gel formulations of emulsomes were utilized for topical dermal delivery of drugs [70].
4. GENERATION OF EMULSOMES AND S-LAYER COATED EMULSOMES
The thin-film hydration technique was proposed by Amselem et al. (1994) and is now widely accepted for the production of emulsomes [1]. Accordingly, all the lipid compounds (i.e. solid lipids, cholesterol, phosphatidylcholine etc.) and the lipophilic drug are — together or separately - dissolved in a volatile solvent such as dichloromethane, chloroform or methanol. The organic solutions are mixed in a round-bottomed flask and the solvent is removed under reduced pressure in a rotary evaporator to form a thin lipid film on the wall of the flask [2, 3, 19, 20, 23, 31]. After complete removal of the solvent, the resulting dry lipid film is hydrated by an aqueous solution, whereupon the self-assembly of emulsomes is initiated. Mechanical shaking may be optionally applied to yield a better dispersion [2, 21]. During this procedure, micron-sized droplets are formed which can be sized-down to the nano scale by i) high-pressure homogenization [2, 12, 13, 21], ii) ultrasonication [3, 5, 20, 22, 23, 70], or iii) high-pressure extrusion through polycarbonate membranes [19, 31]. Free, unentrapped drug is removed by passing the dispersion through size-exclusion chromatography [3, 5, 13, 20, 22, 23, 68] or by applying ultracentrifugation where emulsomes (dispersed in water) remain in the supernatant [19].
Emulsomes are coated by an S-layer lattice through the self-assembly of protein monomers on the surface of the nanocarrier [31]. Accordingly in a first step, the native S-layer proteins are isolated from the surface of the bacterial cell, or, alternatively, recombinant S-layer proteins are isolated from the cytoplasm of host bacterial cells. Then, incubated with emulsomes, the isolated proteins reassemble or recrystallize on the surface of the emulsome, thereby preserving their characteristic crystalline S-layer lattice symmetry. This approach has been successfully applied on different colloidal nanoparticles including liposomes [75, 76], silica oxide particles [51, 77], polyelectrolyte nanocapsules [78], and very recently on emulsomes [31]. Moreover, it has been demonstrated that wild-type SbsB S-layer protein of Geobacillus stearothermophilus PV72/p2 and its recombinant counterpart (rSbsB) expressed in E. coli are able to entirely coat the surface of positively charged emulsomes [31].
5. APPLICATIONS
The solid lipid core and PL multilayer surrounding the core of emulsomes allow encapsulation of lipophilic drugs whose medical implementation could be limited owing to the lack of solubility. In this section, we highlight the most recent applications of emulsomes in clinical medicine, including viral and fungal infections, dermal therapy, cancer, and autoimmunity (Table 2).
Table 2.
Summary of recent applications of emulsomes in the literature.
| Category | Drug Loaded/Integrated | Significance | References |
|---|---|---|---|
| Viral Infections | Azidothymidine palmitate (AZT-P) | Development of a drug formulation against HIV | [13, 14] |
| Azidothymidine (AZT) | Treatment of intracellular liver infections | [3] | |
| Fungal Infections | Amphotericin B (AmB) | Treatment of systemic infections with Candida albicans | [21] |
| Macrophage-targeted delivery of AmB against visceral leishmaniasis (VL) | [5, 20, 22, 23] | ||
| Treatment of fungal (Aspergillus) infections in the lung | [24] | ||
| Dermal Therapy | Dithranol | Treatment of psoriasis | [70] |
| Cancer Therapy | Methotrexate (MTX) | Treatment of sarcoma of intestinal lymphatic region | [68] |
| Curcumin | Delivery of curcumin into the human liver carcinoma HepG2 cells in vitro | [19] | |
| Autoimmunity | Mucosal Vaccine | Enhanced immunogenicity | [113, 114] |
| Anti-CD3 monoclonal antibody (mAb) | Enhanced Th2 responses | [115] |
5.1. Viral Infections
Azidothymidine (AZT), also called Zidovudine, was the first drug reported to be efficacious in inhibiting human immunodeficiency virus (HIV) replication leading to clinical and immunologic improvements [79, 80]. In addition, the therapeutic potency of AZT against hepatitis and some other viral infections is also well recognized. However, apart from the clinical benefits, side-effects like bone marrow toxicity or hematological changes and relatively low plasma half-life time of AZT minimize the ability for a consequent anti-viral therapy [81, 82]. The adverse side effects are mainly due to the exposure of AZT to the entire body at high doses. Hence, an urgent demand for targeted delivery systems for an efficient treatment is given. For instance, enabling the application of the drug in reduced doses with macrophage targeting seems to be a promising strategy for AIDS therapy [84, 85].
For the delivery of AZT, Heiati et al. (1997) used trilaurin-based emulsomes, or, as they refer to them, SLNs stabilized with dipalmitoyl phosphatidylcholine (DPPC) or a mixture of DPPC and dimyristoyl phosphatidylglycerol (DMPG) [13, 14]. DPPC gave the emulsomes a neutral surface charge, whereas DMPG resulted in a net negative charge. An ester prodrug of Zidovudine, AZT palmitate (AZT-P), was synthesized and incorporated in the emulsomes. AZT-P is an amphiphilic molecule and preferentially integrates within the PL bilayers of the nanoformulation rather than the internal solid core [13]. Negatively charged PL bilayers, i.e. DMPG, enhanced the incorporation of AZT-P compared with emulsomes of neutral charge. This incidence was attributed to the repulsion of DMPG molecules between the bilayers resulting in a further facilitated packaging of AZT-P within the bilayers. On the other hand, differences in release profile of AZT-P from different PL layers was accredited to the main phase transition of the used PL and provided evidence that control over the drug release profile is achievable by selecting the PL composition accordingly. For instance, the loss of AZT-P from emulsomes formulated with DMPG (phase transition temperature; Tm=23oC) was at a rate of 11% in 4 days, and was due to the fluid state of DMPG at 37oC, whereas those formulated with DPPC (Tm=41oC) were more stable, with a drug release of less than 4% in 4 days [13].
Emulsomal formulations for the treatment of intracellular liver infections by sustained and targeted delivery of AZT were also developed [3]. Trilaurin- and tristearin-based emulsomes with a positive charge were expected to protect the system from lysosomal degradation and ensure the internalization of the drug. Indeed, the emulsomal formulations were expeditiously cleared from the blood and were accumulated in the liver leading to higher concentrations of AZT; 54-61% was recovered after 1 hour, whereas only 15% of the drug was recovered from the liver at the same time when AZT was applied in its free form. In contrast to the in vitro studies disclosing a slow release profile for each emulsome preparation, in in vivo studies only cationic emulsomes were successful in maintaining their high drug concentrations in liver for a long period time (at least 6 hours). Accordingly, approximately 47% and 51% of the drug were recovered in the liver 6 hours after the cationic trilaurin- and tristearin-based emulsomes were applied, respectively. Interestingly, the drug recovery for neutral emulsomes was as low as 18% and 20%, in the same order. The higher performance of cationic preparations was attributed to the ability of charge-charge interactions, which destabilize endosomal membranes during cellular internalization. This causes the relief of the emulsome into the cell cytoplasm before any lysosomal degradation could have occurred. Subsequently, slow release of AZT from the internal solid core permits sustained delivery of the drug into the cell, and, hence, the concentrations of AZT in the liver may remain at high levels for a longer period of time.
5.2. Fungal Infections
Amphotericin B (AmB), a polyene macrolide anti-fungal antibiotic has been used for more than 50 years, mainly for the treatment of invasive fungal infections [86-88]. AmB offers a broad spectrum of activity against fungal and protozoan pathogens combined with a relatively rare resistance [89]. Since the bioavailability of AmB by oral route is poor, parenteral route is rather preferred, but then the anti-fungal therapy with AmB is compromised by a high incidence of adverse reactions including fever, chills, nausea, vomiting, headache and renal dysfunction with associated anemia, hypokalemia and hypomagnesaemia [20, 90]. Recent studies have shown that lipid-based AmB formulations have advantages over conventional AmB in terms of reduced renal toxicity [91-93]. The principle behind this is the altered pharmacokinetic properties of AmB, which accumulates as AmB-lipid formulations in the organs of the reticulo-endothelial system instead of the kidney, which largely reduces its toxicity [20, 94, 95].
Kretschmar et al. (2001) introduced the AmB-emulsome formulation (EAmB) specifically against systemic infections with Candida albicans, demonstrating competent drug efficacies compared to commercial AmB preparations such as the AmB-deoxycholate free drug formulation Fungizone (Bristol-Myers Squibb, Munich, Germany) and the AmB-lipid complex AmBisome (Nexstar, San Dimas, CA, USA) [21]. All these preparations were able to reduce the fungal burden in liver and kidney [21]. AmBisome was more effective against fungal burden in the liver, whereas EAmB and Fungizone were slightly more effective in the kidney. However, owing to the characteristic feature of emulsomes (i.e. the solid fat core), a delay in the killing of C. albicans with EAmB was observed in vitro in comparison to Fungizone despite the similar minimal inhibitory concentration (MIC) values in both preparations. This result suggested that AmB was released completely but with certain delay from the emulsomes [21]. Related to the altered pharmacokinetic properties as well as the slow release of the drug, the EAmB formulation was shown to reduce the toxicity by 30- to 50-fold compared with Fungizone. Moreover, EAmB preparations were stable in suspensions (at 4oC) for more than 3 months, constituting a considerable advantage over the liposomal formulations in clinical practice.
Gupta et al. (2007) designed trilaurin-based emulsome formulations for macrophage-targeted delivery of AmB against visceral leishmaniasis (VL) [22]. It is known that mannose receptors are present on the surface of macrophages. Various ligands with mannose-free endings are able to interact with the surface of macrophages and can be used for specific targeting of the emulsomal formulations by macrophages [23, 96]. For this purpose, AmB-bearing emulsomes were coated with macrophage-specific ligand O-palmitoyl mannan (OPM) [22]. Modified with OPM, EAmB showed higher in vivo antileishmanial efficacy (73.7±6.7%) than both the plain EAmB (51.7±5.4%) and the conventional AmB deoxycholate formulation (30.4±4.8%). This provided evidence that OPM-grafted EAmB has the potential to further increase the efficacy of AmB against VL. Later on, the studied biodistribution pattern demonstrated that both modified and plain emulsomes are superior in accumulating AmB in macrophage rich organs, i.e. the liver and spleen, which is attributed to the improved antileishmanial activity of these emulsomal formulations [20].
In another study, trilaurin-based emulsomes were modified with O-palmitoyl amylopectin (OPA), again for macrophage targeting [23]. The lipophilic palmitoyl chain was incorporated in the PL bilayer, and, hence, enabled the anchoring of amylopectin on the surface of the emulsome. Using stearylamine, cationic EAmBs were prepared in parallel to study their synergic antileishmanial activity. Although the ligand-receptor (i.e. amylopectin-mannose) interaction slightly improved the in vitro antileishmanial activity of the EAmB, cationic EAmB yielded the highest antileishmanial activity. The superior efficacy of cationic EAmB was attributed to the selective membrane disruption of the parasite as an effect of charge neutralization of stearylamine-bearing emulsomes with polyanionic phosphatidylserine (PS), one of the PL constituents of the Leishmania promastigote plasma membrane [97, 98]. As PS is not expressed on normal mammalian cells, cationic emulsomes are supposed to interact more effectively with infected macrophages. Together with the loaded drug, this leads to a dual action against the parasite resulting in a higher antileishmanial activity than OPA-modified emulsomes [23].
By changing the inner composition of the EAmB, tripalmitin-based OPM-grafted EAmBs for the treatment of VL were introduced [5]. Tripalmitin-based EAmB displayed better antileishmanial activity compared to intraperitoneal injections of AmB-deoxycholate (Mycol, VHB Life Sciences Inc., Gujarat, India). The efficacy can further be enhanced by applying the drug in the OPM-grafted form. For instance, 62.76±3.54% parasite inhibition could be achieved at a dose of 1 mg/kg. The empty formulations were proven to be safe and nontoxic.
In addition to emulsomes in suspension, aerosolized emulsome preparations for treatment of fungal (Aspergillus) infections in the lung have been developed [24]. Accordingly, trilaurin-based EAmBs were generated and modified either with the alveolar macrophage-specific OPM or the monoclonal antibody EBA-2 to benefit from ligand-mediated active targeting and to increase the rate and extent of macrophage accumulation of the drug, as mannose receptors are expressed abundantly in alveolar macrophages [24]. Indeed, OPM and mAB-coated EAmB displayed enhanced therapeutic efficacies with final survival rates of 83.3% and 100%, respectively. The final survival rate for unmodified EAmB remained at 66.7%. Studies on the biodistribution revealed that surface modifications enabled site-specific localization of the drug and prevented fast systemic absorption and subsequent redistribution in other tissues such as liver. Consequently, the OPM- and monoclonal antibody (mAb) coatings exhibited great potential for targeted pulmonary anti-fungal chemotherapy.
In brief, enhanced efficacy in combination with reduced cytotoxicity profiles of AmB, formulated with emulsomes and targeting moieties, highlight the potential of emulsomal formulations to replace conventional AmB applications in medicine.
5.3. Dermal Therapy
Dithranol, also known as anthralin, has been a mainstay for the treatment of psoriasis (i.e. a non-contagious autoimmune skin disorder) for over 80 years. Yet its use has fallen steadily owing to unwanted side effects such as skin irritation, erythema, peeking and staining [99]. However, encapsulation of dithranol within the lipidic core of emulsomes enhanced significantly their permeation across the skin and improved drug retention in skin tissues [70]. Various compritol (glyceryl behenate)-based emulsomes were prepared by using the formulation by design (FbD) approach. FbD defines analytically the emulsome composition yielding the best entrapment rate and drug efficacy. Accordingly, the highest entrapment efficacy was achieved for emulsomal formulations composed of 63-75% compritol and 25-37% PL. An increase in the amount of PLs decreased the permeation flux through the skin due to the assembly of multilamellar barriers [100]. Nonetheless, the effect of compritol was profound as indicated by highest flux values (37.5-60%) achieved by compritol-rich preparations. The skin retention was found to be directly related to the amount of PL, as increasing amounts of PL increased skin retention as previously reported [101]. The antipsoriatic activity of the preparations was assessed on mouse-tail models, where emulsomes displayed a better pharmacodynamics activity (52.4%) than commercial products (40.6%). Moreover, no erythema and wrinkles were produced on the mice skin, providing evidence that emulsomes can diminish potential side effects of dithranol while improving its medical efficacy.
5.4. Cancer
Emulsomal formulations encapsulating two anticancer agents, methotrexate (MTX) [68] and curcumin [19], have recently been reported in the literature:
MTX is an anti-folate drug synthesized to fight against a broad spectrum of human cancers including osteosarcoma, lymphoma, choriocarcinoma, and leukemia [102]. As an anti-metabolite chemotherapeutic agent, MTX is also used for psoriasis, rheumatoid arthritis, systemic sclerosis, placenta accreta, and ectopic pregnancy [103]. Unfortunately, MTX as a low molecular and poorly water-soluble anticancer agent shows a very short half-life in plasma [104]. Moreover, the high efflux rate of MTX causes drug resistance and potential side effects in particular in the marrow, liver, kidney and gastrointestinal tract [104].
For lymphatic delivery of MTX, Paliwal et al. (2009) mimicked the structural design of the chylomicron, an endogenously synthesized lipoprotein, and synthesized emulsome formulations composed of compritol® 888 ATO as the lipid core and soya lecithin as a stabilizing shell [68]. Various MTX-loaded emulsomes were characterized according to their entrapment efficacy, in vitro release, lymphatic uptake, and plasma profiles. In vitro drug release analysis demonstrated that emulsomes slowly release MTX at pH 7.4 (simulated intestinal fluid), whereas at pH 1.2 (simulated gastric fluid) a burst release of MTX occurs, indicating that in gastrointestinal tract the PL bilayers and compritol® 888 ATO did not provide stability to the formulation [105]. Therefore, intraduodenal administration of the formulation was preferred in the rat model. In vivo, emulsome formulations improved the bioavailability of MTX up to 5.7-fold [68]. The maximum plasma concentration of MTX could be increased from 2.4 µg/ml up to 7.1 µg/ml, whereas the time to reach the peak concentration was shifted from 1 hour to 4 hours for MTX-bearing emulsome formulations. One can conclude that emulsome formulation not only achieved the lymphatic delivery of MTX through the intestinal milieu but also prolonged its plasma residence time by sustained delivery of the drug.
Curcumin, a common name for diferuloylmethane, is a hydrophobic polyphenol derived from the rhizome of the herb Curcuma longa (turmeric) of the Zingiberaceae family [106]. In recent years, particular interest has been shown in curcumin owing to its therapeutic effect against a broad variety of different cancers including gastrointestinal, genitourinary, breast, ovarian, lung, and neurological cancer; leukemia and lymphoma; head and neck squamous cell carcinoma; melanoma; and sarcoma [107-109]. However despite the promising therapeutic activity as well as safety profile, the clinical advancement of curcumin has been affected by its short biological half-life, poor water solubility, and low bioavailability after oral administration [106, 110].
Addressing these limitations, Ucisik et al. (2013) introduced curcumin-emulsome nanoformulations, the socalled CurcuEmulsomes, to improve the solubility of curcumin and facilitate its delivery into cells and tissues [19]. Encapsulation increased the solubility of curcumin by up to 10,000-fold corresponding to a concentration of 0.11 mg/ml. CurcuEmulsomes successfully facilitated the delivery of curcumin into human liver carcinoma HepG2 cells in vitro, and the release of the drug at the target site was prolonged. The latter was assessed by cell viability, apoptosis and cell cycle analysis. The slow release profile endowed the drug to sustain its therapeutic effect for longer period of time (i.e. >48 hours) [19].
5.5. Autoimmunity
When a vaccine composition is combined with i) protein/peptide antigens, ii) optionally added hydrophobic material and iii) an immune-potentiating membranous carrier such as an emulsome, the antigenic integrity of the protein or peptide epitopes is preserved and the immunogenicity of the vaccine is enhanced [111, 112]. For the first time, Lowell et al. (1997) applied emulsomes as adjuvants for mucosal vaccines [113]. Intranasal immunization of mice with baculovirus-expressed gp160 formulated in proteasomes (i.e. multimolecular protein complexes with diameter of 60-100 nm [114]) with emulsomes elicited strong serum, lung, intestinal, and vaginal IgG and IgA responses [113, 114]. A bioadhesive polymer, carbocol 934, was anchored to emulsomes to confer the lipid nanoformulation with mucoadhesive properties and to further improve the delivery and attachment of vaccine antigens to target mucosal surfaces [113]. In a subsequent study, local production of HIV-1 specific Ig was demonstrated in the genital tract, intestinal tract and lungs of mice immunized with proteasome vaccine plus emulsome [114]. These studies were particularly important due to the observation that emulsomes enhance immunogenicity by promoting stickiness to the mucosal vaccine, thereby retarding rapid clearance from the respiratory tract and facilitating antigen uptake by mucosal cells [113].
To cite another example of the use of emulsomes for autoimmunity, it was demonstrated that a combination of anti-CD3 mAb and emulsome (as adjuvant) suppressed antibody production against type II collagen and improved the severity of joint pathology as a result of reduced inflammatory cytokines in joints [115]. Emulsomes enhanced the Th2 response resulting in the induction of LAP+ (i.e., latency-associated peptide) regulatory T cells and suppression of ongoing arthritis by both nasal and oral anti-CD3 administration [115]. The approach was found to be safe and applicable for mucosal, noninvasive therapy for rheumatoid arthritis.
6. CONCLUSION AND PERSPECTIVES
In emulsomes, the internal core composed of fats (e.g., TGs) is stabilized in the form of an oil/water emulsion by PL molecules. The solidified internal fatty core provides better opportunity to load lipophilic drugs in high concentration into these versatile DDSs. Moreover, there is also the ability to encapsulate water-soluble drugs in the aqueous compartments surrounding the PL layers [2]. Emulsomes, having the characteristics of both liposomes and emulsions are designed to overcome problems associated with drugs such as a poor bioavailability, protection from harsh gastric environment, and enzyme hydrolysis, as the TGs are protecting the drug. Emulsomes provide an efficient way for the controlled release of drugs and, hence, decrease the toxicity and further adverse effects associated with it.
Recently this technology has been investigated in great detail to improve both the efficacy of the drug release and the selectivity of the drugs. Emulsomes have a wide range of therapeutic application, e.g., in parental drug delivery, gene therapy and also oral formulations. Moreover, in the future emulsomes composed of, for example, antisense oligonucleotides or plasmids for gene therapy as genetic drugs will be increasingly available. Important for future health care, emulsomes are a clear economical alternative to current commercial lipid formulations because functioning as sustained release systems, the dosing frequency of drugs can be reduced. Moreover, as best carrier for intravenous as well as oral delivery of drugs, these new emerging DDSs could play an essential function in the effective treatment of life-threatening viral and fungal infections such as hepatitis, HIV, EBV, leishmaniasis, etc. [3, 5, 13, 20-22, 24, 70, 114], but also for various kinds of cancer [19, 68] (Table 2).
Curcumin, a very potent drug against a variety of different cancer diseases [107], has recently been entrapped in emulsomes. These so-called CurcuEmulsomes were investigated for the in vitro delivery of curcumin in the HepG2 model cell line. Most important, it could be demonstrated that incorporation in emulsomes enhances the poor water solubility of curcumin. Moreover, the biological activity as well as fluorescence integrity of curcumin was preserved. Finally, curcumin was gradually released into the cells, thereby resulting in prolonged cytotoxicity and cell cycle arrest on HepG2, and, over the long term, the incorporated curcumin acted as efficiently as free curcumin dissolved in organic solvent. Consequently, by enabling curcumin to reach its effective concentration inside the cell, this approach may allow therapeutic applications of curcumin, where medical use is otherwise limited [19].
As shown for the first time in a recent study [31], a nanopatterned S-layer lattice could be recrystallized on the surface of emulsomes. In this way, a biomimetic viral envelope comprised of a proteinaceous shell on a lipidic core was successfully assembled [31]. The proteinaceous lattice did not only envelope and modify the surface of the emulsome but additionally improved its biocompatibility, showing a significantly reduced toxicity even at a high concentration compared with positively charged, bare emulsomes. Consequently, the S-layer lattice protected the cell from oxidative stress and cell membrane damage and decreased the cytotoxicity initiated by the outermost positively charged PL multilayer [31].
Equal to liposomes [53], emulsomes can also be coated for imaging purposes with S-layer fusion proteins carrying EGFP. Because of its fluorescence ability, emulsomes coated with, for example, rSbpA31-1068/EGFP will represent a useful tool to visualize the uptake of emulsomes by eukaryotic cells [53, 116]. With regard to future aspects, the most interesting advantage of fusion proteins can be seen in co-recrystallization of, for example, rSbpA31-1068/EGFP in combination with other S-layer fusion proteins (e.g., rSbpA/streptavidin [117]) on the same emulsomal surface. The uptake of these specially coated emulsomes by target cells and the functionality of delivered drugs could be investigated simultaneously without using any additional labelling [41].
The efficiency of emulsomes could be improved by the attachment of freely accessible homing functions (e.g. protein domains) on the surface of the DDSs to achieve active drug targeting. One elegant way could be to anchor or fuse functional molecules on S-layer proteins covering the surface of emulsomes. Indeed, recently emulsomes have successfully been coated not only by S-layer proteins [31], but also by S-layer fusion proteins carrying two protein G domains (Fig. 3) having a specific affinity to IgG [62]. In principle, having an S-layer cover results not only in an overall stabilization of the spherical nanoformulation but will also allow the functionalization of emulsomes by immobilization of functional molecules (e.g. human IgG) either by direct binding or by immobilization via the Fc-specific ligand protein A [41]. Emulsomes could also be coated with genetically engineered S-layer proteins incorporating binding functions where, for example, biotinylated ligands could be bound to the S-layer-coated emulsomes via the biotin–streptavidin system as previously described for liposomes [117]. The most elegant method is, however, the coating with S-layer fusion proteins incorporating functional protein domains (e.g. ZZ or GG domains) [61, 62]. If desired, through co-recrystallization of S-layer fusion proteins presenting different functions a multifunctional DDS with targeting, recognition, binding, and active drug release capabilities can be designed.
Figure 3.
(A) TEM images of emulsomes coated with rSbpA-GG (i.e., S-layer proteins fused with double protein G domains); (B) Closer view to one rSbpA-GG coated emulsome. The rSbpA-GG layer assembles on the surface with square lattice symmetry as the wild type, and covers the emulsome entirely. The bar corresponds to 200 and 100 nm, respectively.
Although this review is focused highly on medical applications of emulsomes, the diverse use of emulsomes in other fields such as food and agriculture is conceivable. For instance, emulsomal DDSs might be used for the delivery of pesticides in agriculture to minimize the potential hazards of the pesticides to the environment.
Consequently, further research might lead to smart and biocompatible emulsomes of natural building blocks protecting encapsulated drugs against degradation, besides reducing unwanted side effects of the drugs. Combining this with specific targeting systems allow the emulsomes to be targeted to the desired site of action.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflicts of interest.
ACKNOWLEDGEMENTS
The work was supported by Air Force Office of Scientific Research (AFOSR), Agreement Award Nr.: FA9550-09-0342 and Agreement Award Nr.: FA9550-10-1-0223, and the Austrian Science Fund (FWF), project P20256-B11. The authors wish to thank Dr. Gary Dorken from Institute for Biologically Inspired Materials (Department of Nanobiotechnology, University of Natural Resources and Life Sciences (BOKU) Vienna) for assistance in the proof-reading of the manuscript.
REFERENCES
- 1.Amselem AS, Yogev A, Zawoznik E, Friedman D. In Emulsomes, A Novel Drug Delivery Technology. International Symposium on the Control and Release of Bioactive Materials. 1994:1369. [Google Scholar]
- 2.Amselem A S, Zawoznik E, Yogev A, Friedman D. Emulsomes, a new type of lipid assembly Handbook of Nonmedical Applications of Liposomes. In: Barenholz Y, Lasic D D, editors; III. Florida: CRC Press:; 1996. pp. 209–223. [Google Scholar]
- 3.Vyas SP, Subhedar R, Jain S. Development and characterization of emulsomes for sustained and targeted delivery of an anti-viral agent to liver. J. Pharm. Pharmacol. 2006;58(3 ):321–326. doi: 10.1211/jpp.58.3.0005. [DOI] [PubMed] [Google Scholar]
- 4.Amselem AS, Friedman D. Solid fat nanoemulsions. United States Patent No. 5662932 02.09. 1997 2.09
- 5.Pal A, Gupta S, Jaiswal A, Dube A, Vyas SP. Development and evaluation of tripalmitin emulsomes for the treatment of experimental visceral leishmaniasis. J. Liposome Res. 2012;22(1 ):62–71. doi: 10.3109/08982104.2011.592495. [DOI] [PubMed] [Google Scholar]
- 6.Mehnert W, Mäder K. Solid lipid nanoparticles: Production, characterization and applications. Adv. Drug Deliv. Rev. 2001;47(2-3 ):165–196. doi: 10.1016/s0169-409x(01)00105-3. [DOI] [PubMed] [Google Scholar]
- 7.Müller RH, Mäder K, Gohla S. Solid lipid nanoparticles (SLN) for controlled drug delivery – a review of the state of the art. Europ. J. Pharmaceut. Biopharmaceut. 2000;50(1 ):161–177. doi: 10.1016/s0939-6411(00)00087-4. [DOI] [PubMed] [Google Scholar]
- 8.Domb AJ, Maniar M. Liposphere delivery systems for local anesthetics. United States Patent No. 5227165 13.07.1993. 1993 [Google Scholar]
- 9.Domb AJ, Bergelson L, Amselem AS. Liposheres for controlled delivery of substances Microencapsulation. In: Benita S, editor; Methods and Industrial Applications. Second Edition. Vol. 158. Boca Raton FL: CRC Press, Taylor & Francis Group; 1996. pp. 297–316. [Google Scholar]
- 10.Rawat M, Saraf S. Lipospheres: Emerging carriers in the delivery of proteins and peptides. Int. J. Pharmaceut. Sci. Nanotechnol. 2008;1(3 ):207–214. [Google Scholar]
- 11.zur Mühlen A, Schwarz C, Mehnert W. Solid lipid nanoparticles (SLN) for controlled drug delivery – Drug release and release mechanism. Europ. J. Pharmaceut. Biopharmaceut. 1998;45(2 ):149–155. doi: 10.1016/s0939-6411(97)00150-1. [DOI] [PubMed] [Google Scholar]
- 12.Heiati H, Phillips NC, Tawashi R. Evidence for phospholipid bilayer formation in solid lipid nanoparticles formulated with phospholipid and triglyceride. Pharm. Res. 1996;13(9 ):1406–1410. doi: 10.1023/a:1016090420759. [DOI] [PubMed] [Google Scholar]
- 13.Heiati H, Tawashi R, Shivers RR, Phillips NC. Solid lipid nanoparticles as drug carriers. I. Incorporation and retention of the lipophilic prodrug 3'-azido-3'-deoxythymidine palmitate. Int. J. Pharmaceut. 1997;146(1 ):123–131. [Google Scholar]
- 14.Heiati H, Tawashi R, Phillips NC. Drug retention and stability of solid lipid nanoparticles containing azidothymidine palmitate after autoclaving, storage and lyophilization. J. Microencapsulat. 1998;15(2 ):173–184. doi: 10.3109/02652049809006847. [DOI] [PubMed] [Google Scholar]
- 15.Domb AJ. Long-acting injectable oxytetracycline-liposphere formulations. Int. J. Pharmaceut. 1995;124(2 ):271–278. [Google Scholar]
- 16.Nastruzzi C. Lipospheres in drug targets and delivery: Approaches, methods, and applications. Taylor & Francis: 2004 [Google Scholar]
- 17.Patra CN. Liposphere: A versatile controlled release carrier for hydrophobic drugs. J. Pharmaceut. Drug Develop. 2013;1(2 ) [Google Scholar]
- 18.Westesen K, Bunjes H. Do nanoparticles prepared from lipids solid at room temperature always possess a solid lipid matrix?. Int. J. Pharmaceut. 1995;115(1 ):129–131. [Google Scholar]
- 19.Ucisik MH, Kupcu S, Schuster B, Sleytr UB. Characterization of CurcuEmulsomes: nanoformulation for enhanced solubility and delivery of curcumin. J. Nanobiotechnol. 2013;11(1 ):37–0. doi: 10.1186/1477-3155-11-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gupta S, Vyas SP. Development and characterization of amphotericin B bearing emulsomes for passive and active macrophage targeting. J. Drug Target. 2007;15(3 ):206–217. doi: 10.1080/10611860701195395. [DOI] [PubMed] [Google Scholar]
- 21.Kretschmar M, Amselem S, Zawoznik E, Mosbach K, Dietz A, Hof H, Nichterlein T. Efficient treatment of murine systemic infection with Candida albicans using amphotericin B incorporated in nanosize range particles (emulsomes). Mycoses . 2001;44(7-8 ):281–286. [PubMed] [Google Scholar]
- 22.Gupta S, Dube A, Vyas SP. Antileishmanial efficacy of amphotericin B bearing emulsomes against experimental visceral leishmaniasis. J. Drug Target. 2007;15(6 ):437–444. doi: 10.1080/10611860701453836. [DOI] [PubMed] [Google Scholar]
- 23.Gill V, Kumar MS, Khurana B, Arora D, Mahadevan N. Development of Amphotericin B Loaded Modified Emulsomes for Visceral Leishmaniasis: In vitro Int. J. Recent Adv. Pharm. Res. 2011;1(2 ):14–20. [Google Scholar]
- 24.Vyas SP, Khatri K, Goyal AK. Functionalized nanocarrier(s) to image and target fungi infected immune cells. Medical Mycol. 2009;47(Suppl 1):S362–368. doi: 10.1080/13693780802464430. [DOI] [PubMed] [Google Scholar]
- 25.Jannin V, Musakhanian J, Marchaud D. Approaches for the development of solid and semi-solid lipid-based formulations. Adv. Drug Deliv. Rev. 2008;60(6 ):734–746. doi: 10.1016/j.addr.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 26.O'Driscoll CM, Griffin BT. Biopharmaceutical challenges associated with drugs with low aqueous solubility--the potential impact of lipid-based formulations. Adv. Drug Deliv. Rev. 2008;60(6 ):617–624. doi: 10.1016/j.addr.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 27.Cai S, Yang Q, Bagby TR, Forrest ML. Lymphatic drug delivery using engineered liposomes and solid lipid nanoparticles. Adv. Drug Deliv. Rev. 2011;63(10-11 ):901–908. doi: 10.1016/j.addr.2011.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khan AA, Mudassir J, Mohtar N, Darwis Y. Advanced drug delivery to the lymphatic system: Lipid-based nanoformulations. Int. J. Nanomed. 2013;8(1 ):2733 – 2744. doi: 10.2147/IJN.S41521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sleytr UB, Beveridge TJ. Bacterial S-layers. Trends Microbiol. 1999;7(6 ):253–260. doi: 10.1016/s0966-842x(99)01513-9. [DOI] [PubMed] [Google Scholar]
- 30.Sleytr UB, Schuster B, Egelseer EM, Pum D. S-layers: Principles and applications. FEMS Microbiol. Rev. 2014;38(5 ):823–864. doi: 10.1111/1574-6976.12063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ucisik MH, Küpcü S, Debreczeny M, Schuster B, Sleytr UB. S-layer coated emulsomes as potential nanocarriers. Small . 2013;9(17 ):2895–2904. doi: 10.1002/smll.201203116. [DOI] [PubMed] [Google Scholar]
- 32.Lucas W, Knipe DM. Viral capsids and envelopes: Structure and function. Encyclopedia Life Sci. 2002:1–7. [Google Scholar]
- 33.Van Meer G, Voelker DR, Feigenson GW. Membrane lipids: Where they are and how they behave. Nature Rev. Molecul. Cell Biol. 2008;9(2 ):112–124. doi: 10.1038/nrm2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Israelachvili JN. Van der waals forces in biological systems. Quart. Rev. Biophys. 1973;6(4 ):341–387. doi: 10.1017/s0033583500001566. [DOI] [PubMed] [Google Scholar]
- 35.Tanford C. The hydrophobic effect and the organization of living matter. Science . 1978;200(4345 ):1012–1018. doi: 10.1126/science.653353. [DOI] [PubMed] [Google Scholar]
- 36.Krahmer N, Farese Jr RV, Walther TC. Balancing the fat: Lipid droplets and human disease. EMBO Molecul. Med. 2013;5(7 ):905–915. doi: 10.1002/emmm.201100671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lehninger AL, Nelson DL, Cox MM. Lehninger Principles of Biochemistry. W. H. Freeman: 2005 [Google Scholar]
- 38.Seddon AM, Casey D, Law RV, Gee A, Templer RH, Ces O. Drug interactions with lipid membranes. Chem. Soc. Rev. 2009;38(9 ):2509–2519. doi: 10.1039/b813853m. [DOI] [PubMed] [Google Scholar]
- 39.Sandermann Jr H. Regulation of membrane enzymes by lipids. Biochim. et Biophys. Acta . 1978;513(3 ):209–237. doi: 10.1016/0304-4157(78)90015-1. [DOI] [PubMed] [Google Scholar]
- 40.Sleytr UB, Messner P, Pum D, Sara M. Crystalline bacterial cell surface layers (S layers): From supramolecular cell structure to biomimetics and nanotechnology. Angewandte Chemie-Int. 1999;38(8 ):1035–1054. doi: 10.1002/(SICI)1521-3773(19990419)38:8<1034::AID-ANIE1034>3.0.CO;2-#. Edit. [DOI] [PubMed] [Google Scholar]
- 41.Schuster B, Sleytr UB. Biomimetic interfaces based on S-layer proteins, lipid membranes and functional biomolecules. J. Royal Soc. Interface . 2014;11(96 ) doi: 10.1098/rsif.2014.0232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pum D, Toca-Herrera J L, Sleytr UB. S-layer protein self-assembly. Int. J. Molecul. Sci. 2013;14(2 ):2484–501. doi: 10.3390/ijms14022484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schuster B. Biomimetic design of nanopatterned membranes. NanoBiotechnology . 2005;1(2 ):153–164. [Google Scholar]
- 44.Sleytr UB, Egelseer EM, Ilk N, Pum D, Schuster B. S-Layers as a basic building block in a molecular construction kit. Febs. J. 2007;274(2 ):323–334. doi: 10.1111/j.1742-4658.2006.05606.x. [DOI] [PubMed] [Google Scholar]
- 45.Sleytr UB, Sára M, Pum D, Schuster B. Crystalline bacterial cell surface layers (S layers) In: Ciferri A, editor; A versatile self-assembly system Supra-Molecular Polymers. 2nd ed. Boca Raton Florida: Taylor & Francis Group; 2005. pp. 583–616. [Google Scholar]
- 46.Ilk N, Egelseer EM, Sleytr UB. S-layer fusion proteins - construction principles and applications. Curr. Opin. Biotech. 2011;22(6 ):824–831. doi: 10.1016/j.copbio.2011.05.510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rothbauer M, Küpcü S, Sticker D, Sleytr UB, Ertl P. Exploitation of S-layer anisotropy: pH-Dependent nanolayer orientation for cellular micropatterning. ACS Nano . 2013;7(9 ):8020–8030. doi: 10.1021/nn403198a. [DOI] [PubMed] [Google Scholar]
- 48.Picher MM, Küpcü S, Huang C-J, Dostalek J, Pum D, Sleytr UB, Ertl P. Nanobiotechnology advanced antifouling surfaces for the continuous electrochemical monitoring of glucose in whole blood using a lab-on-a-chip. Lab on a Chip . 2013;13(9 ):1780–1789. doi: 10.1039/c3lc41308j. [DOI] [PubMed] [Google Scholar]
- 49.Sleytr UB, Bayley H, Sara M, Breitwieser A, Kupcu S, Mader C, Weigert S, Unger FM, Messner P, JahnSchmid B, Schuster B, Pum D, Douglas K, Clark NA, Moore JT, Winningham TA, Levy S, Frithsen I, Pankovc J, Beale P, Gillis HP, Choutov DA, Martin KP. Applications of S-layers. FEMS Microbiol. Rev. 1997;20((1-2) ):151–175. doi: 10.1111/j.1574-6976.1997.tb00306.x. [DOI] [PubMed] [Google Scholar]
- 50.Kainz B, Küpcü S, Steiner K, Sleytr UB, Toca-Herrera JL, Pum D. Nanostructured fluorescent S-layer coatings on silica particles. Europ. Cells Mat. 2010;20(Suppl. 3):130–0. [Google Scholar]
- 51.Kainz B, Steiner K, Sleytr UB, Pum D, Toca-Herrera JL. Fluorescent S-layer protein colloids. Soft Matter. 2010;6(16 ):3809–3814. [Google Scholar]
- 52.Kainz B, Steiner K, Moller M, Pum D, Schaffer C, Sleytr U B, Toca-Herrera JL. Absorption, steady-state fluorescence, fluorescence lifetime, and 2D self-assembly properties of engineered fluorescent S-layer fusion proteins of Geobacillus stearothermophilus nrs 2004/3a. Biomacromolecules . 2010;11(1 ):207–214. doi: 10.1021/bm901071b. [DOI] [PubMed] [Google Scholar]
- 53.Ilk N, Kupcu S, Moncayo G, Klimt S, Ecker RC, Hofer-Warbinek R, Egelseer EM, Sleytr UB, Sara M. A functional chimaeric S-layer-enhanced green fluorescent protein to follow the uptake of S-layer-coated liposomes into eukaryotic cells. Biochem. J. 2004;379:441–448. doi: 10.1042/BJ20031900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tschiggerl H, Casey JL, Parisi K, Foley M, Sleytr UB. Display of a peptide mimotope on a crystalline bacterial cell surface layer (S-layer) lattice for diagnosis of Epstein-Barr virus infection. Bioconjugate Chem. 2008;19(4 ):860–865. doi: 10.1021/bc7003523. [DOI] [PubMed] [Google Scholar]
- 55.Sleytr UB, Egelseer EM, Ilk N, Messner P, Schäffer C, Pum D, Schuster B. In Prokaryotic Cell Wall Components: Structure and Biochemistry. In: König H, Claus H, Varma A, editors; Springer-Verlag: B. Berlin: Springer-Verlag; 2010. pp. 459–481. [DOI] [Google Scholar]
- 56.Schäffer C, Novotny R, Küpcü S, Zayni S, Scheberl A, Friedmann J, Sleytr UB, Messner P. Novel biocatatysts based on S-layer self-assembly of Geobacillus stearothermophilus NRS 2004/3a: A nanobiotechnological approach. Small. 2007;3(9 ):1549–1559. doi: 10.1002/smll.200700200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ilk N, Egelseer E M, Ferner-Ortner J, Küpcü S, Pum D, Schuster B, Sleytr U B. Surfaces functionalized with self-assembling S-layer fusion proteins for nanobiotechnological applications. Colloids Surf. A: Physicochemic. Engin. Aspects. 2008;321(1-3 ):163–167. [Google Scholar]
- 58.Ferner-Ortner-Bleckmann J, Schrems A, Ilk N, Egelseer EM, Sleytr UB, Schuster B. Multitechnique study on a recombinantly produced Bacillus halodurans laccase and an S-layer/laccase fusion protein. Biointerphases. 2011;6(2 ):63–72. doi: 10.1116/1.3589284. [DOI] [PubMed] [Google Scholar]
- 59.Ferner-Ortner-Bleckmann J, Gelbmann N, Tesarz M, Egelseer EM, Sleytr UB. Surface-layer lattices as patterning element for multimeric extremozymes. Small . 2013;9(22 ):3887–3894. doi: 10.1002/smll.201201014. [DOI] [PubMed] [Google Scholar]
- 60.Zhu X, McBride R, Nycholat C M, Yu W, Paulson JC, Wilson IA. Influenza virus neuraminidases with reduced enzymatic activity that avidly bind sialic acid receptors. J. Virol. 2012;86(24 ):13371–13383. doi: 10.1128/JVI.01426-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Völlenkle C, Weigert S, Ilk N, Egelseer E, Weber V, Loth F, Falkenhagen D, Sleytr UB, Sara M. Construction of a functional S-layer fusion protein comprising an immunoglobulin G-binding domain for development of specific adsorbents for extracorporeal blood purification. Appl. Environment. Microbiol. 2004;70(3 ):1514–1521. doi: 10.1128/AEM.70.3.1514-1521.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ucisik MH, Küpcü S, Breitwieser A, Gelbmann N, Schuster B, Sleytr UB. S-layer fusion protein as a tool functionalizing emulsomes and Curcu Emulsomes for antibody binding and targeting. Colloids Surfac. B: Biointerfaces. 2015 doi: 10.1016/j.colsurfb.2015.01.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Björck L, Kronvall G. Purification and some properties of streptococcal protein G, a novel IgG-binding reagent. J. Immunol. 1984;133(2 ):969–974. [PubMed] [Google Scholar]
- 64.Pleschberger M, Neubauer A, Egelseer EM, Weigert S, Lindner B, Sleytr UB, Muyldermans S, Sara M. Generation of a functional monomolecular protein lattice consisting of an S-layer fusion protein comprising the variable domain of a camel heavy chain antibody. Bioconjugate Chem. 2003;14(2 ):440–8. doi: 10.1021/bc025603+. [DOI] [PubMed] [Google Scholar]
- 65.Pleschberger M, Saerens D, Weigert S, Sleytr UB, Muyldermans S, Sára M, Egelseer EM. An S-layer heavy chain camel antibody fusion protein for generation of a nanopatterned sensing layer to detect the prostate-specific antigen by surface plasmon resonance technology. Bioconjug. Chem. 2004;15(3 ):664–671. doi: 10.1021/bc049964w. [DOI] [PubMed] [Google Scholar]
- 66.Ilk N, Schumi CT, Bohle B, Egelseer EM, Sleytr UB. Expression of an endotoxin-free S-layer/allergen fusion protein in gram-positive Bacillus subtilis 1012 for the potential application as vaccines for immunotherapy of atopic allergy. Microbial Cell Factories. 2011;10 doi: 10.1186/1475-2859-10-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sleytr UB. Fracture faces in intact cells and protoplasts of Bacillus stearothermophilus. A study by conventional freeze-etching and freeze-etching of corresponding fracture moieties. Protoplasma . 1970;71(3 ):295–312. doi: 10.1007/BF01279638. [DOI] [PubMed] [Google Scholar]
- 68.Paliwal R, Paliwal SR, Mishra N, Mehta A, Vyas SP. Engineered chylomicron mimicking carrier emulsome for lymph targeted oral delivery of methotrexate. Int. J. Pharmaceut. 2009;380(1-2 ):181–188. doi: 10.1016/j.ijpharm.2009.06.026. [DOI] [PubMed] [Google Scholar]
- 69.Ishii F, Nii T. Properties of various phospholipid mixtures as emulsifiers or dispersing agents in nanoparticle drug carrier preparations. Colloids Surfac. B: Biointerfaces. 2005;41(4 ):257–262. doi: 10.1016/j.colsurfb.2004.12.018. [DOI] [PubMed] [Google Scholar]
- 70.Raza K, Katare OP, Setia A, Bhatia A, Singh B. Improved therapeutic performance of dithranol against psoriasis employing systematically optimized nanoemulsomes. J. Microencapsulation. 2013;30(3 ):225–236. doi: 10.3109/02652048.2012.717115. [DOI] [PubMed] [Google Scholar]
- 71.Khar R, Vyas S, Khar R. Drug delivery to bone marrow Targeted and Controlled Drug Delivery Novel Carrier Systems. In: Vyas S, editor; New Delhi India: CBS Publisher and Distributor:; 2002. pp. 562–85. [Google Scholar]
- 72.Kaminaga Y, Nagatsu A, Akiyama T, Sugimoto N, Yamazaki T, Maitani T, Mizukami H. Production of unnatural glucosides of curcumin with drastically enhanced water solubility by cell suspension cultures of Catharanthus roseus. FEBS Lett. 2003;555(2 ):311–6. doi: 10.1016/s0014-5793(03)01265-1. [DOI] [PubMed] [Google Scholar]
- 73.Allen TM, Cullis PR. Drug delivery systems: Entering the mainstream. Science . 2004;303(5665 ):1818–1822. doi: 10.1126/science.1095833. [DOI] [PubMed] [Google Scholar]
- 74.O'Driscoll CM. Lipid-based formulations for intestinal lymphatic delivery. Europ. J. Pharmaceut. Sci. 2002;15(5 ):405–415. doi: 10.1016/s0928-0987(02)00051-9. [DOI] [PubMed] [Google Scholar]
- 75.Küpcü S, Sara M, Sleytr UB. Liposomes coated with crystalline bacterial cells surface protein (S-layer) as immobilization structures for macromolecules. Biochim. et Biophysic. Acta . 1995;1235(2 ):263–269. doi: 10.1016/0005-2736(95)80013-6. [DOI] [PubMed] [Google Scholar]
- 76.Mader C, Küpcü S, Sleytr UB, Sara M. S-layer-coated liposomes as a versatile system for entrapping and binding target molecules. Biochim. et Biophysic. Acta – Biomembranes. 2000;1463(1 ):142–150. doi: 10.1016/s0005-2736(99)00190-x. [DOI] [PubMed] [Google Scholar]
- 77.Kainz B, Steiner K, Sleytr UB, Pum D, Toca-Herrera JL. Fluorescence energy transfer in the bi-fluorescent S-layer tandem fusion protein ECFP-SgsE-YFP. J. Structural Biol. 2010;172(3 ):276–283. doi: 10.1016/j.jsb.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 78.Toca-Herrera JL, Krastev R, Bosio V, Küpcü S, Pum D, Fery A, Sára M, Sleytr UB. Recrystallization of bacterial S-layers on flat polyelectrolyte surfaces and hollow polyelectrolyte capsules. Small . 2005;1(3 ):339–348. doi: 10.1002/smll.200400035. [DOI] [PubMed] [Google Scholar]
- 79.Mitsuya H, Weinhold KJ, Furman PA, St Clair MH, Lehrman SN, Gallo RC, Bolognesi D, Barry DW, Broder S. 3'-azido-3'-deoxythymidine (BW A509U): An anti-viral agent that inhibits the infectivity and cytopathic effect of human T-lymphotropic virus type III/lymphadenopathy-associated virus in vitro. Proc. Natl. Acad. Sci. USA . 1985;82(20 ):7096–7100. doi: 10.1073/pnas.82.20.7096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wright K. Aids therapy: First tentative signs of therapeutic promise. Nature . 1986;323(6086 ):283–283. doi: 10.1038/323283a0. [DOI] [PubMed] [Google Scholar]
- 81.Richman DD, Fischl MA, Grieco MH, Gottlieb MS, Volberding PA, Laskin OL, Leedom JM, Groopman JE, Mildvan D, Hirsch MS, Jackson GG, Durack DT, Nusinoff-Lehrman S. The toxicity of azidothymidine (AZT) in the treatment of patients with AIDS and AIDS-related complex. New Engl. J. Med . 1987;317(4 ):192–197. doi: 10.1056/NEJM198707233170402. [DOI] [PubMed] [Google Scholar]
- 82.Löbenberg R, Araujo L, Kreuter J. Body distribution of azidothymidine bound to nanoparticles after oral administration. Europ. J. Pharmaceut. Biopharmaceut. 1997;44(2 ):127–132. [Google Scholar]
- 83.Löbenberg R, Araujo L, von Briesen H, Rodgers E, Kreuter J. Body distribution of azidothymidine bound to hexyl-cyanoacrylate nanoparticles after . injection to rats. J. Control. Release . 1998;50(1-3 ):21–30. doi: 10.1016/s0168-3659(97)00105-3. [DOI] [PubMed] [Google Scholar]
- 84.Löbenberg R, Kreuter J. Macrophage targeting of azidothymidine: A promising strategy for AIDS therapy. AIDS Res. Human Retroviruses . 1996;12(18 ):1709–1715. doi: 10.1089/aid.1996.12.1709. [DOI] [PubMed] [Google Scholar]
- 85.Mahajan SD, Aalinkeel R, Law WC, Reynolds JL, Nair BB, Sykes DE, Yong KT, Roy I, Prasad PN, Schwartz S A. Anti-HIV-1 nanotherapeutics: Promises and challenges for the future. Int. J. Nanomed. 2012;7:5301–5314. doi: 10.2147/IJN.S25871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kleinberg M. What is the current and future status of conventional amphotericin B?. International Journal of Antimicrobial Agents. 2006;27(Supplement 1 ):12–16. doi: 10.1016/j.ijantimicag.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 87.Gubbins PO, Anaissie EJ. Chapter 7 - anti-fungal therapy. In: Anaissie E J, McGinnis M R, Pfaller M A, editors; In Clinical Mycology (Second Edition) Edinburgh: Churchill Livingstone; 2009. pp. 161–195. [Google Scholar]
- 88.Chudzik B, Tracz IB, Czernel G, Fiolka MJ, Borsuk G, Gagos M. Amphotericin B–copper(II) complex as a potential agent with higher anti-fungal activity against Candida albicans. Europ. J. Pharmaceut. Sci . 2013;49(5 ):850–857. doi: 10.1016/j.ejps.2013.06.007. [DOI] [PubMed] [Google Scholar]
- 89.Ellis D. Amphotericin B: Spectrum and resistance. J. Antimicrob. Chemotherap. 2002;49(Suppl 1):7–10. doi: 10.1093/jac/49.suppl_1.7. [DOI] [PubMed] [Google Scholar]
- 90.Butani D, Yewale C, Misra A. Amphotericin B topical microemulsion: Formulation, characterization and evaluation. Colloids Surfac. B: Biointerfaces . 2014;116:351–358. doi: 10.1016/j.colsurfb.2014.01.014. [DOI] [PubMed] [Google Scholar]
- 91.Wong-Beringer A, Jacobs RA, Guglielmo BJ. Lipid formulations of amphotericin B: Clinical efficacy and toxicities. Clin. Infect. Dis. 1998;27(3 ):603–618. doi: 10.1086/514704. [DOI] [PubMed] [Google Scholar]
- 92.Barrett JP, Vardulaki KA, Conlon C, Cooke J, Daza-Ramirez P, Evans EGV, Hawkey PM, Herbrecht R, Marks DI, Moraleda JM, Park GR, Senn SJ, Viscoli C. A systematic review of the anti-fungal effectiveness and tolerability of amphotericin B formulations. Clin. Therapeut. 2003;25(5 ):1295–1320. doi: 10.1016/s0149-2918(03)80125-x. [DOI] [PubMed] [Google Scholar]
- 93.Dupont B. Overview of the lipid formulations of amphotericin B. J. Antimicrob. Chemotherap. 2002;49(Suppl 1):31–36. doi: 10.1093/jac/49.suppl_1.31. [DOI] [PubMed] [Google Scholar]
- 94.Hiemenz JW, Walsh TJ. Lipid formulations of amphotericin B: Recent progress and future directions. Clin. Infect. Dis. 1996;22 (Suppl. 2)(Suppl 2):S133–S144. doi: 10.1093/clinids/22.supplement_2.s133. [DOI] [PubMed] [Google Scholar]
- 95.Torrado JJ, Espada R, Ballesteros MP, Torrado-Santiago S. Amphotericin B formulations and drug targeting. J. Pharm. Sci. 2008;97(7 ):2405–2425. doi: 10.1002/jps.21179. [DOI] [PubMed] [Google Scholar]
- 96.Vyas SP, Katare YK, Mishra V, Sihorkar V. Ligand directed macrophage targeting of amphotericin B loaded liposomes. Int. J. Pharmaceut. 2000;210(1-2 ):1–14. doi: 10.1016/s0378-5173(00)00522-6. [DOI] [PubMed] [Google Scholar]
- 97.Glew RH, Saha AK, Das S, Remaley AT. Biochemistry of the Leishmania species. Microbiologic. Rev . 1988;52(4 ):412–432. doi: 10.1128/mr.52.4.412-432.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tripathi A, Gupta CM. Transbilayer translocation of membrane phosphatidylserine and its role in macrophage invasion in Leishmania promastigotes. Molecul. Biochemic. Parasitol. 2003;128(1 ):1–9. doi: 10.1016/s0166-6851(03)00024-0. [DOI] [PubMed] [Google Scholar]
- 99.Menter A, Griffiths CEM. Current and future management of psoriasis. Lancet . 2007;370(9583 ):272–284. doi: 10.1016/S0140-6736(07)61129-5. [DOI] [PubMed] [Google Scholar]
- 100.Kato A, Ishibashi Y, Miyake Y. Effect of egg yolk lecithin on transdermal delivery of bunazosin hydrochloride. J. Pharm. Pharmacol. 1987;39(5 ):399–400. doi: 10.1111/j.2042-7158.1987.tb03407.x. [DOI] [PubMed] [Google Scholar]
- 101.Seth AK, Misra A, Umrigar D. Topical liposomal gel of idoxuridine for the treatment of herpes simplex: Pharmaceutical and clinical implications. Pharmaceut. Developm. Technol. 2005;9(3 ):277–289. doi: 10.1081/pdt-200031432. [DOI] [PubMed] [Google Scholar]
- 102.Longo-Sorbello G, Bertino JR. Current understanding of methotrexate pharmacology and efficacy in acute leukemias. Use of newer anti-folates in clinical trials. Haematologica . 2001;86(2 ):121–127. [PubMed] [Google Scholar]
- 103.Olson K, Jiranantakan T. sixth edition Poisoning and Drug Overdose. Sixth ed. United States of America: McGraw-Hill Education; 2011. Poisoning and drug overdose. [Google Scholar]
- 104.Liu Y, Xie M, Wang S, Zheng Q, Chen A, Deng Q. Facile fabrication of high performances MTX nanocomposites with natural biomembrane bacterial nanoparticles using GP. Materials Lett. 2013;100:248–251. [Google Scholar]
- 105.Paliwal R, Rai S, Vaidya B, Khatri K, Goyal AK, Mishra N, Mehta A, Vyas SP. Effect of lipid core material on characteristics of solid lipid nanoparticles designed for oral lymphatic delivery. Nanomed.: Nanotechnol. Biol. Med. 2009;5(2 ):184–191. doi: 10.1016/j.nano.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 106.Anand P, Kunnumakkara AB, Newman RA, Aggarwal BB. Bioavailability of curcumin: Problems and promises. Molecul. Pharmaceut. 2007;4(6 ):807–18. doi: 10.1021/mp700113r. [DOI] [PubMed] [Google Scholar]
- 107.Anand P, Sundaram C, Jhurani S, Kunnumakkara AB, Aggarwal BB. Curcumin and cancer: An "old-age" disease with an "age-old" solution. Cancer Lett. 2008;267(1 ):133–164. doi: 10.1016/j.canlet.2008.03.025. [DOI] [PubMed] [Google Scholar]
- 108.Aggarwal B B, Sundaram C, Malani N, Ichikawa H. Curcumin: The indian solid gold. Adv. Experiment. Med. Biol. 2007;595:1–75. doi: 10.1007/978-0-387-46401-5_1. [DOI] [PubMed] [Google Scholar]
- 109.Ravindran J, Prasad S, Aggarwal BB. Curcumin and cancer cells: How many ways can curry kill tumor cells selectively?. AAPS J. 2009;11(3 ):495–510. doi: 10.1208/s12248-009-9128-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bisht S, Maitra A. Systemic delivery of curcumin: 21st century solutions for an ancient conundrum. Curr. Drug Discov. Technologies . 2009;6(3 ):192–199. doi: 10.2174/157016309789054933. [DOI] [PubMed] [Google Scholar]
- 111.Lowell G, Vancott T, Birx D. Protein and peptide vaccines for inducing mucosal immunity. U.S. Patent No. 0155120 A1. 2002 Oct 10;
- 112.Lowell GH, Vancott TC, Birx DL. Protein and peptide vaccines for inducing mucosal immunity. WO Patent No. 9801558. 1998 Janaury;
- 113.Lowell GH, Kaminski RW, VanCott TC, Slike B, Kersey K, Zawoznik E, Loomis-Price L, Smith G, Redfield RR, Amselem S, Birx DL. Proteosomes, emulsomes, and cholera toxin B improve nasal immunogenicity of human immunodeficiency virus gp160 in mice: Induction of serum, intestinal, vaginal, and lung IgA and IgG. J. Infect. Dis. 1997;175(2 ):292–301. doi: 10.1093/infdis/175.2.292. [DOI] [PubMed] [Google Scholar]
- 114.VanCott TC, Kaminski RW, Mascola JR, Kalyanaraman VS, Wassef NM, Alving CR, Ulrich JT, Lowell GH, Birx DL. HIV-1 neutralizing antibodies in the genital and respiratory tracts of mice intranasally immunized with oligomeric gp160. J. Immunol. 1998;160(4 ):2000–2012. [PubMed] [Google Scholar]
- 115.Wu HY, Maron R, Tukpah AM, Weiner HL. Mucosal anti-CD3 monoclonal antibody attenuates collagen-induced arthritis that is associated with induction of LAP+ regulatory T cells and is enhanced by administration of an emulsome-based Th2-skewing adjuvant. J. Immunol. 2010;185(6 ):3401–3417. doi: 10.4049/jimmunol.1000836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Schuster B, Pum D, Sára M, Sleytr UB. S-layer proteins as key components of a versatile molecular construction kit for biomedical nanotechnology. Mini-Rev. Medicin. Chem. 2006;6(8 ):909–920. doi: 10.2174/138955706777935026. [DOI] [PubMed] [Google Scholar]
- 117.Moll D, Huber C, Schlegel B, Pum D, Sleytr UB, Sara M. S-layer-streptavidin fusion proteins as template for nanopatterned molecular arrays. Proc. Natl. Acad. Sci. USA . 2002;99(23 ):14646–14651. doi: 10.1073/pnas.232299399. [DOI] [PMC free article] [PubMed] [Google Scholar]