Abstract
BACKGROUND
Hypertension (HTN) is the most prevalent non-infectious disease worldwide and can lead to mortality. This trial aimed to compare the effect of N-acetylcysteine (NAC) and angiotensin-converting enzyme inhibitors (ACEIs) on controlling blood pressure in hypertensive patients.
METHODS
This cross-sectional clinical trial was conducted in Hajar Hospital, Shahrekord, Iran, in 2009. A sample of 126 patients with HTN was selected and randomly divided into 2 groups (group A and group B). First, group A was treated with ACEI alone and group B with ACEI + NAC for 2 months. Blood pressure of all patients was evaluated each week. After a 2 week period of washout, the drugs were changed. In the second period of the trial, group A was treated with ACEI + NAC and group B with NAC alone and their blood pressure was evaluated in the same manner as the previous period. The data were analyzed using SPSS.
RESULTS
A significant reduction was observed in systolic and diastolic blood pressure of patients (P < 0.050). However, during both periods of the trial, the group receiving NAC + ACEI experienced a more significant reduction in blood pressure compared with the ACEI group (P < 0.050).
CONCLUSION
NAC accompanied with ACEI decreased the patients’ systolic and diastolic blood pressure significantly; however, ACEI alone did not have any significant effects on blood pressure. Systolic blood pressure decreased 7 mmHg on average and fluctuated during the trial.
Keywords: N-acetylcysteine, Angiotensin-Converting Enzyme Inhibitors, Hypertension
Introduction
Hypertension (HTN) (high blood pressure) is a risk factor which leads to renal failure, peripheral vascular disease, retinopathy, stroke, and heart attacks.1,2 Some research studies have indicated that oxidative stress and reactive oxygen species participate in the pathogenesis of cardiovascular diseases, including HTN and atherosclerosis.3,4
The relevant literature indicates the stability of or decrease in HTN prevalence in developed countries and increase in its prevalence in developing countries. In addition, no significant cross-sectional association was observed between developed and developing countries regarding the prevalence of awareness, treatment, and control of HTN. The mean level among men in developed countries was higher than that in developing countries. Prevalence of HTN varies worldwide, with the lowest prevalence in rural India (3.4% in men and 6.8% in women) and the highest prevalence in Poland (68.9% in men and 72.5% in women).5,6
The purpose of treating this disease is to regulate blood pressure; lower than 140/90 in healthy individuals and lower than 130/85 in patients suffering from diabetes or kidney disease. In most cases, HTN treatment has various side-effects, and thus, results in patients’ non-cooperation. Hence, administration of a drug that is effective in reducing cardiovascular disease risk factors (decrease in cholesterol, homocysteine, and plasma lipoprotein a, and increase in high-density lipoprotein) will lead to the progress of HTN treatments and increase of these patients’ prognosis. According to the investigations conducted on anti-HTN drugs, angiotensin-converting enzyme inhibitors (ACEIs) are the first choice in treating HTN. These drugs are useful especially in patients with kidney HTN, renovascular HTN, diabetes, as well as accelerated and malignant HTN. In mild and uncomplicated HTN, these drugs are as effective as beta-blockers and thiazides. Individuals afflicted with bilateral artery stenosis also suffer from acute renal failure.7,8 Several lines of evidence have shown the antihypertensive role of cysteine. Some reports using dietary supplementation of the cysteine analog N-acetylcysteine (NAC) have indicated that it prevents or attenuates increased blood pressure in animal models of HTN.9-20 It has also been demonstrated that the cysteine precursor methionine results in increasing of the cardiovascular risk factor and homocysteine results in increasing of blood pressure in normal rats.21-23 Homocysteine has been shown to lower blood pressure in hypertensive rats.24,25 In addition, in human studies, using NAC as an adjunct to other antihypertensive therapies resulted in a decrease in blood pressure.26,27
No research has been performed using NAC as a monotherapy in humans suffering from HTN. However, in a study including six hypertensive participants with good blood pressure control (mean: 139/93 mmHg) with the ACE inhibitor lisinopril, the increasing of NAC by 1.2 g/day for 1 week resulted in a significant decrease in both systolic and diastolic blood pressure.28 In another study, the participants consisted of 18 hypertensive smokers whose blood pressure was not controlled with ACE inhibitor monotherapy (enalapril or captopril). These participants received 1.8 g/day NAC for 21 days. This treatment resulted in a decrease in 24 hour ambulatory and daytime systolic and diastolic blood pressure.29 Another study assessed the influence of a combination of equal doses of NAC and arginine [the substrate of nitric oxide (NO) synthase], 1.2 g/day, in a group of 12 type 2 diabetic patients with HTN.30
One of the pathophysiologic mechanisms suggested in HTN is reduction in the vasodilating factor derived from endothelial cell (such as NO). Moreover, NAC, as an antioxidant, causes an increase in NO derived from endothelial cells due to its mechanism on NO. NAC can decrease homocysteine and lipoprotein and protect the heart against ischemic and perfusion damages on the myocytes through replenishing group of sulfhydryl. Its other effects are increasing the nitroglycerin activity, doubling the anti-platelet effect, dilating coronary veins, and reducing the ratio of tolerance to the hemodynamic effects of nitroglycerine.27,28,31 Considering the abovementioned effects, this drug can lead to blood pressure reduction. Many studies examining the effect of NAC (based on the mechanism dependent on NO) in combination with ACEI in patients with HTN have shown contradictory results.29,32 Due to the daily increase in the worldwide death toll due to HTN, and some damages due to this disease, which affect the whole society, and because no research similar to the present one existed in Iran or worldwide, this clinical trial was conducted to compare the effect of NAC and ACEIs on controlling blood pressure in hypertensive patients.
Materials and Methods
This clinical trial with ethics code 89-2-1 was conducted in Hajar Hospital in Shahrekord, Iran, in 2009-2010. The population studied included all patients of 18 years and older with HTN referring to this hospital. The inclusion criteria consisted of age above 18 years, systolic blood pressure of 140 mmHg or higher, diastolic blood pressure of 90 mmHg or higher in spite of taking ACEI, isolated systolic or diastolic HTNs, and diagnosis of HTN by a cardiologist. In addition, patients with HTN who took ACEI (enalapril or captopril) with doses determined by the respective physician and not changed during the trial, and did not take any other antihypertensive drugs, were also allowed to enter the study. Individuals with cystinuria, kidney stones, especially cysteine stones, and severe sensitivity to ACEI and NAC (such as coughing, digestive disorder, and etc.), and also patients who needed other antihypertensive drugs besides ACEI to control their blood pressure were excluded from the trial.
The sampling method was based on convenience sampling. The sample size was calculated based on a 95% confidence and a power of 80% to see a difference equal to 50% of standard deviation in the mean of blood pressure between two groups. The estimated sample size was 126 patients (63 patients in each group). At the beginning of the study, a consent form was filled by the patients and the ethical principles were taken into consideration.
Finally, 126 patients with HTN were entered into the study, and then, randomly divided into two groups (A and B). ACEI alone was administered to group A and NAC tablets with the doses of 600 mg/12 hours in combination with ACEI was prescribed for 2 months for group B. In addition, NAC treating dose did not change during the trial. During these 2 months, blood pressures of both groups were measured every 5-7 days in the clinic. After this period, none of the two groups received NAC for 2 weeks and only received ACEI (washout period). Then, the two groups’ programs were exchanged, and hence, for 2 months the group that received NAC+ACEI received ACEI alone and the group that received ACEI alone received NAC and ACEI. Subsequently, similar to the previous 2-month period, the blood pressure (systolic and diastolic) of all patients was measured by two observers every 5-7 days using the same manometer (Riester, Jungingen, Germany) and stethoscope (Welch Allyn, Tycos Instruments Inc., Skaneateles Falls, NY, USA) in the clinic. Finally, the results were reported as the mean decrease in blood pressure. Every equipment in this study was calibrated once a month.
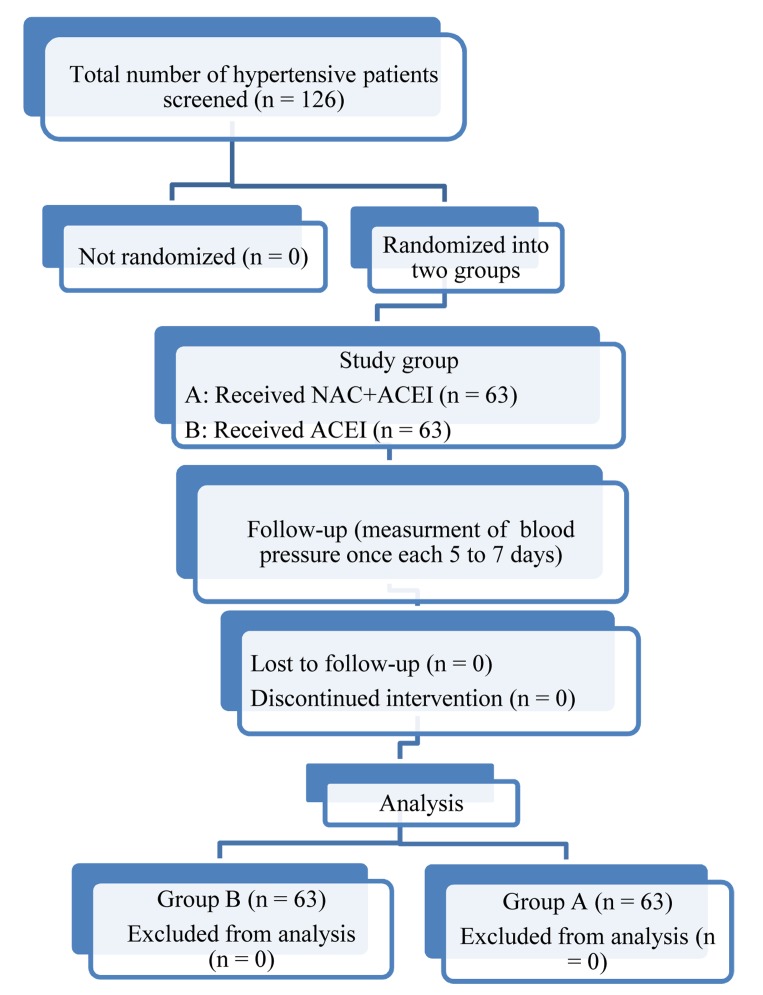
Data collection was conducted using the questionnaire and measuring the patients’ blood pressures. The questionnaire asked about the patients’ age, gender, place of residence, duration of HTN history, antihypertensive drugs (enalapril or captopril), and duration of drug taking, blood pressure, diabetes, and smoking (Figure 1).
Figure 1.
Number of subjects involved at each stage of the study (Flowchart)
For continuous variables, data were presented as means ± standard deviation (SD) and for categorical variables, as number with frequency. Because the sample size was moderately high in each group, the parametric test was used. A repeated measures analysis of variance (ANOVA) was used to compare the blood pressures of the two groups. The multivariate F-tests of Greenhouse-Geisser were used within the subject analysis because of the violation in sphericity assumptions. The comparisons of other variables of interest between the two groups were made using the chi-square or Fisher’s exact test for categorical variables and independent t-test for continuous variables. Statistical analysis was performed using SPSS (version 11.5, SPSS Inc., Chicago, IL, USA). All P values below 0.050 were considered statistically significant.
Results
There were 65 patients in each group at the beginning of the study, 4 of whom from group A were excluded due to not referring or following the treatment. Therefore, 61 and 65 individuals continued drug-taking until the completion of the study in the first and second groups, respectively. The mean (± SD) of age in groups A and B were 58.9 ± 12.4 and 57.1 ± 9 years, respectively, with no significant difference between the two groups (P = 0.340). Among all studied patients, 42 and 84 individuals (33.3 vs. 66.7%) were male and female, respectively. There were 17 and 25 men in groups A and B (27.9 vs. 38.5%), respectively. Furthermore, there was no significant difference between the two groups in terms of gender (P = 0.210). Out of the 126 patients studied, 22(17.5%) had diabetes, 9 and 13 (14.8 vs. 20%) of whom were in groups A and B, respectively. In addition, 6 patients smoked, 2 and 4 of whom were in groups A and B, respectively. Moreover, 25 and 32 participants (41 vs. 49.2%) of groups A and B, respectively, took Enalapril. There was no significant difference in the frequency of diabetes, smoking, and the type of drug taken between the two groups (P > 0.050).
The mean systolic blood pressure during the study is shown in table 1 and the mean diastolic blood pressure in table 2. Furthermore, the trend of systolic and diastolic blood pressures of the two groups for the two periods is illustrated in figures 2 and 3, respectively.
Table 1.
Mean and standard deviation (SD) of systolic blood pressure in the two groups
| Time | Group time | A |
B |
P |
|---|---|---|---|---|
| Mean ± SD | Mean ± SD | |||
| The first 2 month | Beginning of the study | 12.0 ± 156.1 | 12.1 ± 157.2 | 0.610 |
| At the end of 1st week | 10.0 ± 150.9 | 14.0 ± 147.9 | 0.170 | |
| At the end of 2 week | 10.2 ± 151.9 | 13.6 ± 145.3 | 0.003 | |
| At the end of 3 week | 10.6 ± 151.6 | 14.3 ± 142.1 | < 0.001 | |
| At the end of 4 week | 10.5 ± 149.0 | 12.1 ± 140.4 | < 0.001 | |
| At the end of 5 week | 8.7 ± 150.7 | 10.9 ± 138.8 | < 0.001 | |
| At the end of 6 week | 10.4 ± 151.6 | 13.3 ± 138.2 | < 0.001 | |
| At the end of 7 week | 9.0 ± 148.7 | 12.0 ± 137.4 | < 0.001 | |
| At the end of 8 week | 10.1 ± 152.0 | 11.5 ± 138.7 | < 0.001 | |
| The second 2 month | At the initiation to inter the second treatment month | 8.8 ± 149.2 | 9.9 ± 152.6 | 0.420 |
| At the end of 1st week | 10.1 ± 143.0 | 12.4 ± 151.7 | < 0.001 | |
| At the end of 2 week | 11.0 ± 141.0 | 12.5 ± 150.8 | < 0.001 | |
| At the end of 3 week | 10.0 ± 137.9 | 11 ± 150.3 | < 0.001 | |
| At the end of 4 week | 11.0 ± 138.7 | 11.5 ± 149.9 | < 0.001 | |
| At the end of 5 week | 9.6 ± 138.3 | 12.2 ± 151.1 | < 0.001 | |
| At the end of 6 week | 10.3 ± 138.5 | 12.3 ± 151.7 | < 0.001 | |
| At the end of 7 week | 8.7 ± 137.0 | 11.3 ± 149.5 | < 0.001 | |
| At the end of 8 week | 9.7 ± 4139.4 | 10.2 ± 151.3 | < 0.001 |
Group A: the first treatment 2 months with ACEI alone and the second with NAC + ACEI; Group B: the first treatment 2 months with NAC + ACEI and the second with ACEI alone; SD: Standard deviation; ACEI: Angiotensin converting enzyme inhibitor; NAC: N-acetylcysteine
Table 2.
Mean and standard deviation (SD) of diastolic blood pressure in the two groups
| Time | Group time | A |
B |
P |
|---|---|---|---|---|
| Mean ± SD | Mean ± SD | |||
| The first 2 month | Beginning of the study | 9.8 ± 100.5 | 18.3 ± 96.8 | 0.170 |
| At the end of 1st week | 8.6 ± 96.9 | 10.0 ± 92.2 | < 0.005 | |
| At the end of 2 week | 9.5 ± 96.4 | 10.6 ± 89.7 | < 0.001 | |
| At the end of 3 week | 9.0 ± 96.7 | 10.4 ± 86.8 | < 0.001 | |
| At the end of 4 week | 9.6 ± 94.9 | 8.8 ± 85.5 | < 0.001 | |
| At the end of 5 week | 7.7 ± 96.1 | 8.0 ± 84.5 | < 0.001 | |
| At the end of 6 week | 9.2 ± 97.5 | 10.2 ± 84.4 | < 0.001 | |
| At the end of 7 week | 8.2 ± 94.4 | 7.7 ± 82.6 | < 0.001 | |
| At the end of 8 week | 8.3 ± 97.4 | 8.0 ± 38.8 | < 0.001 | |
| The second 2 month | At the initiation to inter the second treatment month | 7.4 ± 95.4 | 8.3 ± 86.3 | 0.520 |
| At the end of 1st week | 9.4 ± 91.1 | 10.1 ± 95.3 | 0.016 | |
| At the end of 2 week | 10.0 ± 87.7 | 10.0 ± 94.5 | 0.001 | |
| At the end of 3 week | 8.7 ± 85.4 | 8.7 ± 94.3 | < 0.001 | |
| At the end of 4 week | 10.0 ± 86.1 | 13.8 ± 92.8 | < 0.001 | |
| At the end of 5 week | 8.8 ± 86.1 | 9.0 ± 94.7 | < 0.001 | |
| At the end of 6 week | 6.8 ± 85.4 | 9.9 ± 94.8 | < 0.001 | |
| At the end of 7 week | 7.8 ± 85.2 | 8.5 ± 93.6 | < 0.001 | |
| At the end of 8 week | 8.0 ± 84.5 | 7.3 ± 95.0 | < 0.001 |
Group A: the first treatment 2 months with ACEI alone and the second with NAC + ACEI; Group B: the first treatment 2 months with NAC + ACEI and the second with ACEI alone; SD: Standard deviation; ACEI: Angiotensin converting enzyme inhibitor; NAC: N-acetylcysteine
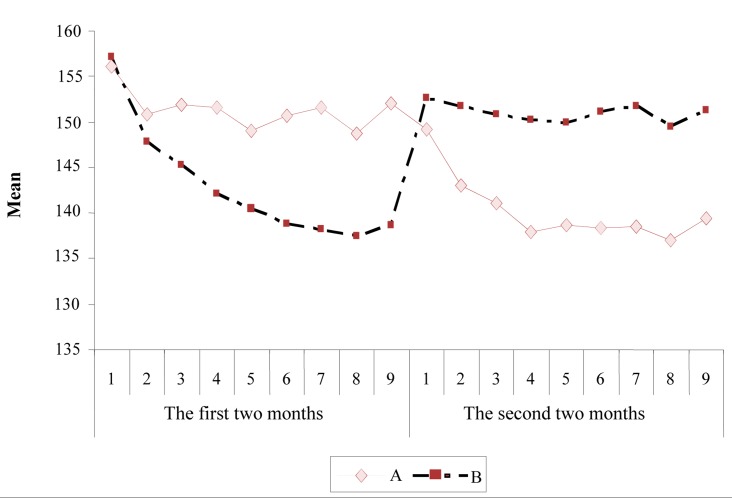
Figure 2.
The mean trend of systolic blood pressure in the two groups [Group A: the first 2 months of treatment with angiotensin converting enzyme inhibitors (ACEI) alone and the second with Nacetylcysteine (NAC) + ACEI; Group B: the first 2 months of treatment with NAC + ACEI and the second with ACEI alone]
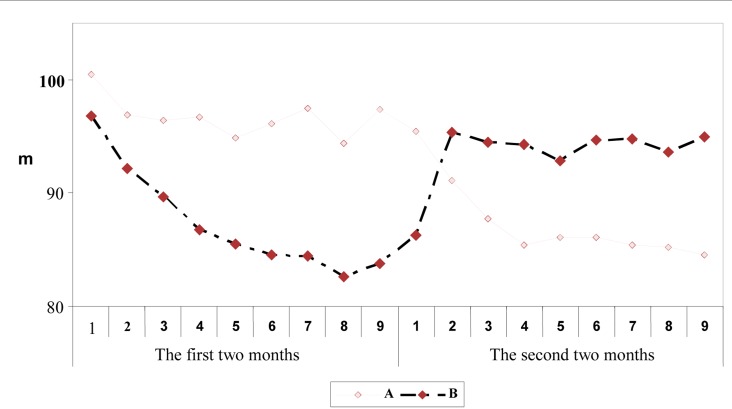
Figure 3.
The mean trend of diastolic blood pressure in the two groups [Group A: The first 2 months of treatment with angiotensin converting enzyme inhibitors (ACEI) alone and the second with Nacetylcysteine (NAC) + ACEI; Group B: The first 2 months of treatment with NAC + ACEI and the second with ACEI alone]
No difference was observed in the mean systolic and diastolic blood pressure at the beginning of the study. The comparison of mean systolic and diastolic blood pressures during the study showed that the blood pressure of the group receiving NAC + ACEI (group A) was lower than the group receiving ACEI (group B). Based on the repeated measure analysis of variance, a significant reduction trend exists in systolic and diastolic blood pressures of patients during the study in the two periods (P < 0.050). However, during the two periods, group A had a more significant reduction in systolic and diastolic blood pressures compared to group B (P < 0.050). Furthermore, the result of repeated measure ANOVA for the blood pressures is shown in table 3.
Table 3.
The result of repeated measure analysis of variance for the blood pressures factor
| Blood pressure | Period | Source of variation | df | F | P |
|---|---|---|---|---|---|
| Systolic | First | Time | 6.76 | 44.4 | < 0.001 |
| Time × group | 6.76 | 16.0 | < 0.001 | ||
| Error | 838 | ||||
| Second | Time | 6.88 | 14.2 | < 0.001 | |
| Time × group | 6.88 | 7.2 | < 0.001 | ||
| Error | 853 | ||||
| Diastolic | First | Time | 5.08 | 26.1 | < 0.001 |
| Time × group | 5.08 | 9.3 | < 0.001 | ||
| Error | 630 | ||||
| Second | Time | 6.06 | 14.6 | < 0.001 | |
| Time × group | 6.06 | 6.9 | < 0.001 | ||
| Error | 752.00 |
Df: Degree of freedom
Discussion
The purpose of this study was to compare the effects of NAC (group A) plus ACEIs with ACEIs alone (group B) in controlling the patients’ blood pressure. Based on the obtained results, NAC accompanied with ACEIs can decrease the patients' systolic and diastolic blood pressure (group A) significantly. However, ACEIs (group B) did not have any significant effects on their blood pressures. Systolic blood pressure decreased 7 mmHg on average, and fluctuated during the study. It is ACEIs accompanied with NAC that can have a quite significant effect on the reduction trend of the patients’ systolic blood pressure. Systolic blood pressure reduction in these patients was 18.5 mmHg on average; in other words, taking NAC could decrease the patients’ systolic blood pressures by more than two times. In addition, the rate of diastolic blood pressure reduction in patients who received ACEIs alone (group B) decreased 3.1 mmHg on average, while the group A patients’ blood pressures reduced by 13 mmHg on average. On the other hand, the fluctuations of diastolic blood pressure in these patients were less than the patients who used ACEI alone (group B).
Regarding the effect of NAC on blood pressure, there have so far been numerous studies on animals, most of which have obtained positive results regarding the reduction of blood pressure. In the study conducted by Barrios et al., the heart ward of Madrid Ramony Cajal Hospital, NAC was presented as a receptor of the sulfhydryl group which can strengthen the antihypertensive effect of the drugs acting through NO mechanism. The studied participants with HTN who smoked consisted of 15 men and 3 women with the mean age of 69 ± 5 years. A considerable reduction (about 7%) was observed in the daily and 24 hour systolic and diastolic blood pressure of patients who took NAC accompanied with ACEI, comparable with ACEI alone. According to the mentioned studies, adding NAC to ACEI strengthens their antihypertensive effects in smoking patients with HTN. This effect can be dependent on NO mechanism and NAC causes an increase in it through the protective effect from NO oxidation.28 In another study conducted by Bernatova et al., it was concluded that in the blood pressure dependent on NO shortage, the patients will improve more significantly through treatment by antioxidants due to the increase in NO production.31 In the study by Ruiz et al., NAC was presented as a sulfhydryl group giver which automatically strengthened the reaction to captopril and enalapril treatment in rabbits with hypertension, and this effect was implemented by the mechanism dependent on NO and NAC-increased level of NO.11 In the study by Martina et al. the decrease in systolic blood pressure was associated with type 2 diabetes in hypertensive patients.29 Pharmacological analysis of the underlying mechanisms indicated voltage-gated potassium channels engagement in vasodilatory effect of NAC.32 In another study, NAC was shown to prevent HTN, insulin resistance, and oxidative stress in rats chronically fed with glucose.33 Renke et al. reported that the effect of NAC on kidney function, kidney damages, and blood pressure in HTN sensitive to salt was examined. In this study, 44 rabbits of 7-8 weeks of age that received high doses of sodium for 5 weeks underwent treatment by NAC with the dose of 4 g/kg/day. While mean arterial pressure had gone up to 1183 mm Hg, NAC treatment caused the arterial pressure to decrease to 4121 mmHg. In addition, NAC caused a 91 and 83% reduction in glomerular necrosis and tubulo-interstitial nephritis, respectively. NAC strengthens the kidney system, decreases kidney function disorder and arterial blood pressure, and thus, leads to the improvement of kidney damage.34 In a study, NAC was not effective on blood pressure and surrogated markers of cardiovascular injury among non-diabetic patients suffering from a chronic kidney disease.14 Ozaydin et al. concluded that NAC decreased the incidence of postoperative atrial fibrillation.35 In the research conducted by Krug et al. in Australia, the effect of NAC on HTN resulting from adrenocorticoids was examined and the results were indicative of blood pressure reduction following taking NAC. In this study, the effect of NAC on HTN resulting from dexametazone usage was examined. In their study, 60 patients receiving dexametazone received 10 g/l NAC for 4-11 days and the patients’ blood pressure reduced. Since dexametazone decreases the level of NO in plasma, which is a blood vessels tone setter, and increases blood pressure, NAC structure decreases the level of free radicals due to its antioxidant property, elevates the level of NO, and thus, causes the relative reduction in blood pressure and in fact its setting.36 In their study, Girouard et al. concluded that increase in NO-mediated vasodilator tone and the possible decrease in adrenergic vasoconstriction induced by NAC treatment in SHR could explain the hypotensive effect of NAC in this model of HTN.14 Meanwhile, in the study by Martina et al.29 on patients with diabetic nephropathy and HTN, the effect of the combination of l-arginine and NAC was examined on the level of the patients’ blood pressures. Free radicals can decrease NO, so it is possible to keep the level of NO unchanged through administration of NAC as an antioxidant and also l-arginine as an NO setter. In these patients with HTN, 1200 mg l-arginine and 600 mg NAC in the case group and placebo in the control group were prescribed. At the end of the study, the mean diastolic and systolic blood pressure in the case group decreased.17 The results of several studies are consistent with our study results and show that NAC is effective in reducing blood pressure. Perhaps more noticeable and reliable results were obtained by our study than other studies due to our study time duration.
Conclusion
A patient with HTN with the accurate indications and no contradictions for drug usage, especially patients with resistant HTN, can be treated with NAC plus ACEIs.
Acknowledgments
We would like to thank the Research and Technology Deputy of Shahrekord University of Medical Sciences for giving a grant number 882 to the thesis and all those who helped us conduct this study. This article was registered as IRCT2014022616750N1 in the Iranian Registry of Clinical Trials.
Footnotes
Conflicts of Interest
Authors declare no conflict of interests.
REFERENCES
- 1.Fauci A, Braunwald E, Kasper D, Hauser S, Longo D, Jameson J, et al. Harrison's Principles of Internal Medicine. 17th. New York, NY: McGraw-Hill; 2008. pp. 1549–62. [Google Scholar]
- 2.Ong KL, Cheung BM, Man YB, Lau CP, Lam KS. Prevalence, awareness, treatment, and control of hypertension among United States adults 1999-2004. Hypertension. 2007;49(1):69–75. doi: 10.1161/01.HYP.0000252676.46043.18. [DOI] [PubMed] [Google Scholar]
- 3.Griendling KK, Sorescu D, Ushio-Fukai M. NAD(P)H oxidase: role in cardiovascular biology and disease. Circ Res. 2000;86(5):494–501. doi: 10.1161/01.res.86.5.494. [DOI] [PubMed] [Google Scholar]
- 4.Alexander RW. Theodore Cooper Memorial Lecture. Hypertension and the pathogenesis of atherosclerosis. Oxidative stress and the mediation of arterial inflammatory response: a new perspective. Hypertension. 1995;25(2):155–61. doi: 10.1161/01.hyp.25.2.155. [DOI] [PubMed] [Google Scholar]
- 5.Kearney PM, Whelton M, Reynolds K, Whelton PK, He J. Worldwide prevalence of hypertension: a systematic review. J Hypertens. 2004;22(1):11–9. doi: 10.1097/00004872-200401000-00003. [DOI] [PubMed] [Google Scholar]
- 6.Pereira M, Lunet N, Azevedo A, Barros H. Differences in prevalence, awareness, treatment and control of hypertension between developing and developed countries. J Hypertens. 2009;27(5):963–75. doi: 10.1097/hjh.0b013e3283282f65. [DOI] [PubMed] [Google Scholar]
- 7.Goldman L, Ausiello DA. Cecil Medicine. 23th. Philadelphia, PA: Saunders Elsevier; 2008. pp. 50–50. [Google Scholar]
- 8.O'Brien E, Barton J, Nussberger J, Mulcahy D, Jensen C, Dicker P, et al. Aliskiren reduces blood pressure and suppresses plasma renin activity in combination with a thiazide diuretic, an angiotensin-converting enzyme inhibitor, or an angiotensin receptor blocker. Hypertension. 2007;49(2):276–84. doi: 10.1161/01.HYP.0000253780.36691.4f. [DOI] [PubMed] [Google Scholar]
- 9.Boesgaard S, Aldershvile J, Pedersen F, Pietersen A, Madsen JK, Grande P. Continuous oral N-acetylcysteine treatment and development of nitrate tolerance in patients with stable angina pectoris. J Cardiovasc Pharmacol. 1991;17(6):889–93. doi: 10.1097/00005344-199106000-00005. [DOI] [PubMed] [Google Scholar]
- 10.Ardissino D, Merlini PA, Savonitto S, Demicheli G, Zanini P, Bertocchi F, et al. Effect of transdermal nitroglycerin or N-acetylcysteine, or both, in the long-term treatment of unstable angina pectoris. J Am Coll Cardiol. 1997;29(5):941–7. doi: 10.1016/s0735-1097(97)00005-3. [DOI] [PubMed] [Google Scholar]
- 11.Ruiz FJ, Salom MG, Ingles AC, Quesada T, Vicente E, Carbonell LF. N-acetyl-L-cysteine potentiates depressor response to captopril and enalaprilat in SHRs. Am J Physiol. 1994;267(3 Pt 2):R767–R772. doi: 10.1152/ajpregu.1994.267.3.R767. [DOI] [PubMed] [Google Scholar]
- 12.Vasdev S, Mian T, Ford CA, Longerich L, Parai S. Role of aldehydes in spontaneously hypertensive rats and disulfiram-induced hypertensive rats. Nutr Metab Cardiovasc Dis. 1996;6:130–40. [Google Scholar]
- 13.Cabassi A, Dumont EC, Girouard H, Bouchard JF, Le JM, Lamontagne D, et al. Effects of chronic N-acetylcysteine treatment on the actions of peroxynitrite on aortic vascular reactivity in hypertensive rats. J Hypertens. 2001;19(7):1233–44. doi: 10.1097/00004872-200107000-00008. [DOI] [PubMed] [Google Scholar]
- 14.Girouard H, Chulak C, Wu L, Lejossec M, de Champlain J. N-acetylcysteine improves nitric oxide and alpha-adrenergic pathways in mesenteric beds of spontaneously hypertensive rats. Am J Hypertens. 2003;16(7):577–84. doi: 10.1016/s0895-7061(03)00863-x. [DOI] [PubMed] [Google Scholar]
- 15.Pechanova O, Zicha J, Kojsova S, Dobesova Z, Jendekova L, Kunes J. Effect of chronic N-acetylcysteine treatment on the development of spontaneous hypertension. Clin Sci (Lond) 2006;110(2):235–42. doi: 10.1042/CS20050227. [DOI] [PubMed] [Google Scholar]
- 16.Zhang L, Fujii S, Igarashi J, Kosaka H. Effects of thiol antioxidant on reduced nicotinamide adenine dinucleotide phosphate oxidase in hypertensive Dahl salt-sensitive rats. Free Radic Biol Med. 2004;37(11):1813–20. doi: 10.1016/j.freeradbiomed.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 17.Tian N, Rose RA, Jordan S, Dwyer TM, Hughson MD, Manning RD. N-Acetylcysteine improves renal dysfunction, ameliorates kidney damage and decreases blood pressure in salt-sensitive hypertension. J Hypertens. 2006;24(11):2263–70. doi: 10.1097/01.hjh.0000249705.42230.73. [DOI] [PubMed] [Google Scholar]
- 18.Kunes J, Dobesova Z, Zicha J. Chronic N-acetylcysteine treatment prevents the development of salt hypertension in immature Dahl rats. J Hypertension Supp. 2004;22:153. [Google Scholar]
- 19.Vasdev S, Ford CA, Longerich L, Gadag V, Wadhawan S. Role of aldehydes in fructose induced hypertension. Mol Cell Biochem. 1998;181(1-2):1–9. doi: 10.1023/a:1006844222963. [DOI] [PubMed] [Google Scholar]
- 20.Song D, Hutchings S, Pang CC. Chronic N-acetylcysteine prevents fructose-induced insulin resistance and hypertension in rats. Eur J Pharmacol. 2005;508(1-3):205–10. doi: 10.1016/j.ejphar.2004.12.018. [DOI] [PubMed] [Google Scholar]
- 21.Rauchova H, Pechanova O, Kunes J, Vokurkova M, Dobesova Z, Zicha J. Chronic N-acetylcysteine administration prevents development of hypertension in N(omega)-nitro-L-arginine methyl ester-treated rats: the role of reactive oxygen species. Hypertens Res. 2005;28(5):475–82. doi: 10.1291/hypres.28.475. [DOI] [PubMed] [Google Scholar]
- 22.Zicha J, Dobesova Z, Kunes J. Antihypertensive mechanisms of chronic captopril or N-acetylcysteine treatment in L-NAME hypertensive rats. Hypertens Res. 2006;29(12):1021–7. doi: 10.1291/hypres.29.1021. [DOI] [PubMed] [Google Scholar]
- 23.Ciaccio M, Bivona G, Bellia C. Therapeutical approach to plasma homocysteine and cardiovascular risk reduction. Ther Clin Risk Manag. 2008;4(1):219–24. doi: 10.2147/tcrm.s1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Robin S, Maupoil V, Groubatch F, Laurant P, Jacqueson A, Berthelot A. Effect of a methionine-supplemented diet on the blood pressure of Wistar-Kyoto and spontaneously hypertensive rats. Br J Nutr. 2003;89(4):539–48. doi: 10.1079/BJN2002810. [DOI] [PubMed] [Google Scholar]
- 25.Mariotti F, Hammiche A, Blouet C, Dare S, Tome D, Huneau JF. Medium-term methionine supplementation increases plasma homocysteine but not ADMA and improves blood pressure control in rats fed a diet rich in protein and adequate in folate and choline. Eur J Nutr. 2006;45(7):383–90. doi: 10.1007/s00394-006-0611-1. [DOI] [PubMed] [Google Scholar]
- 26.Robin S, Maupoil V, Laurant P, Jacqueson A, Berthelot A. Effect of a methionine-supplemented diet on the blood pressure of Sprague-Dawley and deoxycorticosterone acetate-salt hypertensive rats. Br J Nutr. 2004;91(6):857–65. doi: 10.1079/BJN20041116. [DOI] [PubMed] [Google Scholar]
- 27.Suarez C, del Arco C, Lahera V, Ruilope LM. N-acetylcysteine potentiates the antihypertensive effect of angiotensin converting enzyme inhibitors. Am J Hypertens. 1995;8(8):859. doi: 10.1016/0895-7061(95)00153-G. [DOI] [PubMed] [Google Scholar]
- 28.Barrios V, Calderon A, Navarro-Cid J, Lahera V, Ruilope LM. N-acetylcysteine potentiates the antihypertensive effect of ACE inhibitors in hypertensive patients. Blood Press. 2002;11(4):235–9. doi: 10.1080/08037050213760. [DOI] [PubMed] [Google Scholar]
- 29.Martina V, Masha A, Gigliardi VR, Brocato L, Manzato E, Berchio A, et al. Long-term N-acetylcysteine and L-arginine administration reduces endothelial activation and systolic blood pressure in hypertensive patients with type 2 diabetes. Diabetes Care. 2008;31(5):940–4. doi: 10.2337/dc07-2251. [DOI] [PubMed] [Google Scholar]
- 30.Hultberg B, Andersson A, Isaksson A. The effects of homocysteine and copper ions on the concentration and redox status of thiols in cell line cultures. Clin Chim Acta. 1997;262(1-2):39–51. doi: 10.1016/s0009-8981(97)06531-5. [DOI] [PubMed] [Google Scholar]
- 31.Bernatova I, Pechanova O, Babal P, Kysela S, Stvrtina S, Andriantsitohaina R. Wine polyphenols improve cardiovascular remodeling and vascular function in NO-deficient hypertension. Am J Physiol Heart Circ Physiol. 2002;282(3):H942–H948. doi: 10.1152/ajpheart.00724.2001. [DOI] [PubMed] [Google Scholar]
- 32.Han WQ, Zhu DL, Wu LY, Chen QZ, Guo SJ, Gao PJ. N-acetylcysteine-induced vasodilation involves voltage-gated potassium channels in rat aorta. Life Sci. 2009;84(21-22):732–7. doi: 10.1016/j.lfs.2009.02.023. [DOI] [PubMed] [Google Scholar]
- 33.El MA, Ismael MA, Lu H, Fantus IG, de CJ, Couture R. Comparative effects of N-acetyl-L-cysteine and ramipril on arterial hypertension, insulin resistance, and oxidative stress in chronically glucose-fed rats. Can J Physiol Pharmacol. 2008;86(11):752–60. doi: 10.1139/Y08-090. [DOI] [PubMed] [Google Scholar]
- 34.Renke M, Tylicki L, Rutkowski P, Larczynski W, Neuwelt A, Aleksandrowicz E, et al. The effect of N-acetylcysteine on blood pressure and markers of cardiovascular risk in non-diabetic patients with chronic kidney disease: a placebo-controlled, randomized, cross-over study. Med Sci Monit. 2010;16(7):I13–I18. [PubMed] [Google Scholar]
- 35.Ozaydin M, Peker O, Erdogan D, Kapan S, Turker Y, Varol E, et al. N-acetylcysteine for the prevention of postoperative atrial fibrillation: a prospective, randomized, placebo-controlled pilot study. Eur Heart J. 2008;29(5):625–31. doi: 10.1093/eurheartj/ehn011. [DOI] [PubMed] [Google Scholar]
- 36.Krug S, Zhang Y, Mori TA, Croft KD, Vickers JJ, Langton LK, et al. N-Acetylcysteine prevents but does not reverse dexamethasone-induced hypertension. Clin Exp Pharmacol Physiol. 2008;35(8):979–81. doi: 10.1111/j.1440-1681.2008.04947.x. [DOI] [PubMed] [Google Scholar]