Abstract
Typical configurations of extracorporeal membrane oxygenation (ECMO) include venovenous (VV) and venoarterial (VA) configurations; however, other configurations of ECMO may be necessary in certain situations. We performed VA ECMO for a 71-year-old man who experienced refractory hypoxaemia associated with a brief cardiac arrest after resection of the small intestine showing necrosis. As the cardiac function improved, the patient showed a complication of poor oxygenation in the upper body due to insufficient respiratory function. Therefore, we performed VA-venous ECMO, which further improved his cardiac function and allowed him to be converted to VV ECMO. It is very important to consider different configuration strategies of ECMO by adjusting the patient's cardiopulmonary conditions appropriately.
Background
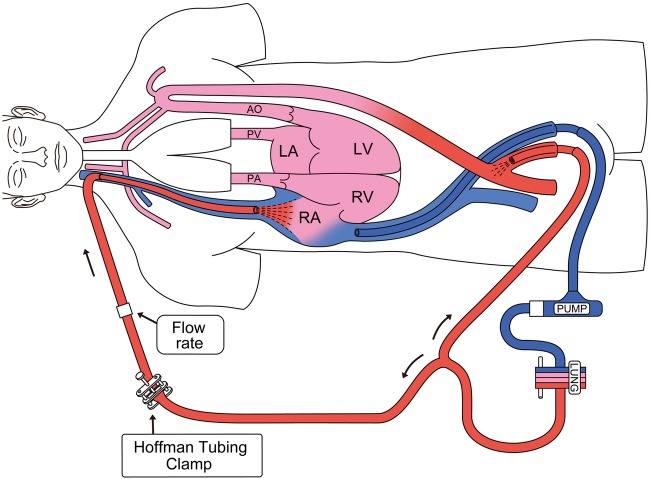
Extracorporeal membrane oxygenation (ECMO) has been rapidly developed and is widely used. Venoarterial (VA) and venovenous (VV) are the most common ECMO configurations for circulatory and respiratory support, respectively.1 In VA ECMO, less-oxygenated blood is delivered from the native lungs, which results in cardiac and cerebral hypoxia if the patient has concomitant respiratory failure with some residual cardiac function. In such cases, VA-venous (VAV) ECMO configuration is necessary. VAV ECMO can improve oxygen delivery to the cerebral and coronary circulation by adding an oxygenated reinfusion limb to the internal jugular vein (figure 1); however, to the best of our knowledge, reports regarding VAV ECMO are limited.2
Figure 1.
Venoarterial-venous extracorporeal membrane oxygenation (LV, left ventricle; LA, left atrium; RV, right ventricle; RA, right atrium; PA, pulmonary artery; PV, pulmonary vein; AO, aorta).
In this study, we report a patient with severe cardiopulmonary failure who was unresponsive to maximum medical treatment and was successfully managed after applying different ECMO configuration strategies, including VA, VAV and VV ECMO.
Case presentation
A 71-year-old man was admitted to our emergency department because of severe septic shock. On admission, his Glasgow Coma Scale was 3, respiratory rate was 18 breaths/min, heart rate was 109 bpm and mean blood pressure (mBP) was 45 mm Hg. The following laboratory findings were observed: white cell count 5830/μL; C reactive protein level 14.11 mg/dL; procalcitonin level 4.17 ng/mL and lactate concentration 9.1 mmol/L. Small intestine necrosis was observed during abdominal CT imaging, and resection of the necrotic small intestine was performed.
After the operation, the patients mBP was 60 mm Hg and heart rate was 108 bpm with vasopressin (2.4 U/h) and norepinephrine (0.15 µg/kg/min). Moreover, he developed severe hypoxia; his arterial oxygen tension was 52.7 at fraction of inspired oxygen 1.0 and the positive end-expiratory pressure (PEEP) was 12 cm H2O. The patient developed cardiac arrest on the third day after surgery, and was placed on VA ECMO (Rotaflow, Maquet, Germany, Biocube 6000, Nipro, Japan) with a left femoral 21 Fr drainage cannula (Bio-Medicus, Medtronic, USA) and a left femoral 16 Fr return cannula (Optisite, Edwards LifeSciences Corporation). ECMO was initiated at a blood flow of 2.9 L/min and gas flow at 2.4 L/min of 100% oxygen. After VA ECMO was initiated, the patient was quickly weaned off vasopressors. However, severe pulmonary infiltrations and bilateral pleural effusions were observed on the chest radiograph. On day 3 of VA ECMO, the left ventricular ejection fraction was 22%, according to the echocardiogram. We then administered dobutamine (2 µg/kg/min). After initiating dobutamine, we observed that the oxygen saturation (SpO2) from the right finger decreased from 91% to 87%, and left regional cerebral tissue oxygen saturation (rStO2) measured by using INVOS 5100C (Covidien) decreased from 71% to 60%. According to the follow-up chest radiograph, severe pulmonary oedema was still present. On day 5 of VA ECMO, the SpO2 level from the right radial artery and rStO2 decreased to 81% and 56%, respectively (figure 2). During the trialling off ECMO, the flow decreased to 1 L/min when evaluating cardiac function, and the patient's mBP was 65 mm Hg, with 2 µg/kg/min of dobutamine, and the ejection function was 35% on echocardiography (table 1).
Figure 2.
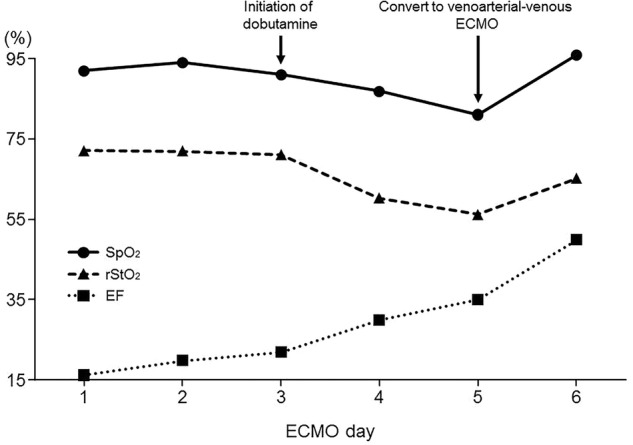
Changes in oxygen saturation, regional cerebral tissue oxygen saturation and ejection fraction during extracorporeal membrane oxygenation.
Table 1.
Effects of VAV ECMO
| ECMO | Mode | Before VAV-ECMO | After VAV-ECMO |
|---|---|---|---|
| VA | VAV | ||
| Pump flow (L/min) | 1 | Venous flow 1.87 Arterial flow 0.50 |
|
| Mechanical ventilation | FIO2 | 1.0 | 1.0 |
| Catecholamines (µg/kg/min) | Dobutamine | 2 | 2 |
| Cardiorespiratory | Mean blood pressure (mm Hg) | 65 | 95 |
| Heart rate (bpm) | 80 | 86 | |
| SpO2 (%) | 81 | 97 | |
| Ejection fraction (%) | 35 | 50 | |
| Arterial blood gas analysis | pH | 7.372 | 7.323 |
| PaCO2 (mm Hg) | 40.5 | 49.4 | |
| PaO2 (mm Hg) | 38.5 | 71.7 | |
| HCO3− (mmol/L) | 22.7 | 25.1 | |
| Lactate (mmol/L) | 2.5 | 1.4 |
ECMO, extracorporeal membrane oxygenation; FIO2, fraction of inspired oxygen; PaCO2, arterial carbon dioxide tension; PaO2, arterial oxygen tension; SpO2, peripheral capillary oxygen saturation; VA, venoarterial; VAV, VA-venous.
We considered that the direct conversion of the patient to VV ECMO was a high risk for cardiac arrest. Therefore, we decided to perform VAV ECMO with the intention of improving oxygen delivery to the cerebral and coronary circulation on day 6 of VA ECMO. An additional 16 Fr return cannula was inserted into the right internal jugular vein, which was connected to the circuit branch from the original returning limb (figure 1). Venous blood from the left femoral vein was drained and oxygenated by performing ECMO, and then perfused into the left femoral artery and the right internal jugular vein. We titrated the arterial and venous reinfusion flow by applying a Hoffman tubing clamp on the venous return limb.
Outcome and follow-up
After VAV ECMO, the oxygenated blood entered the right atrium and bypassed the non-functioning lungs. Finally, the oxygenated blood reached the ascending aorta, and the right radial SpO2 increased from 81% to 95%, and rStO2 increased from 56% to 65%; the patient's ejection fraction dramatically improved from 35% to 50%. On day 7 of ECMO, VAV ECMO was weaned off again. We decreased arterial reinfusion flow to 0.5 L/min, and observed that the patient's mBP was 95 mm Hg with 2 µg/kg/min of dobutamine (table 1). Thereafter, we successfully converted the patient to VV ECMO. On day 12 of ECMO, pulmonary infiltrations improved, and the patient was subsequently weaned off ECMO.
Discussion
We successfully treated a patient with severe cardiorespiratory failure due to intestinal necrosis by using appropriate ECMO configurations, including VA, VAV and VV ECMO. During VA ECMO, if the blood is infused into the femoral artery, fully saturated blood from the ECMO circuit mixes with blood ejected from the native ventricle in the middle of the aorta. The location of this mixing point depends on the amount of ECMO support provided and the degree of left ventricular ejection. In severe cardiac dysfunction cases, the mixing point is in the proximal ascending aorta. As cardiac function improves, the mixing point may migrate more distally into the aortic arch. Therefore, less-oxygenated blood is delivered to the aortic arch and coronary arteries in patients undergoing VA ECMO who develop respiratory failure with some residual cardiac function.3 In this situation, VAV ECMO can improve oxygenation by adding an oxygenated reinfusion limb to the internal jugular vein (figure 1).2
Biscotti et al4 reported their experiences with VAV ECMO for two patients who were initially placed on VA ECMO. One patient underwent VAV ECMO and transitioned to VV ECMO; the other patient underwent VAV ECMO to treat differential oxygen saturation.4 Choi et al5 successfully managed differential hypoxia by performing VAV ECMO. Zhao et al6 tested VAV ECMO in eight adult sheep. They found that femoral artery saturation was 100%, but the saturation from the left ventricle (LVSO2) was only 70.3±7.8% in VA ECMO.6 Only 20% and 50% of the hybrid flow significantly increased LVSO2 to 88% and 96%, respectively.6
Our patient's cardiac function dramatically improved without increasing catecholamine after conducting VAV ECMO, which may be due to the improved oxygen delivery to the coronary arteries. We encountered a clinical dilemma after performing VA ECMO; by using inotropic agents to improve our patient's cardiac function, inadequately oxygenated blood would be delivered to the cerebral and coronary circulation due to poor circulation in the lung. Furthermore, cardiac function was not sufficient to convert the patient to VV ECMO. Therefore, we considered VAV ECMO as the best strategy during that time. We believe that we were able to successfully treat this patient because we adapted different ECMO configuration strategies by adjusting the patient's cardiopulmonary condition. However, it might have been possible to shorten the duration of VA ECMO if VAV ECMO had been performed earlier.
Studies regarding the VAV ECMO configuration are limited. Further research should be undertaken to confirm the effectiveness of VAV ECMO on the mortality and duration of ECMO. Clear criteria for the initiation of VAV ECMO should also be investigated in future studies.
A patient with septic shock concomitant severe acute respiratory failure who was unresponsive to maximum medical treatment was successfully treated by performing VA, VAV and VV ECMO. ECMO configuration should be managed appropriately according to the patient's cardiopulmonary conditions.
Learning points.
Although typical configurations of extracorporeal membrane oxygenation (ECMO) include venoarterial (VA) and venovenous, venoarterial-venous (VAV) ECMO may be necessary for certain conditions.
During VA ECMO, less-oxygenated blood is delivered to the aortic arch and coronary arteries if the patient develops respiratory failure with some residual cardiac function. In this situation, VAV ECMO can improve oxygenation by adding an oxygenated reinfusion limb to the internal jugular vein.
VAV ECMO should be performed when patients have concomitant respiratory failure with some residual cardiac function.
Acknowledgments
The authors would like to thank all employees of the intensive care unit who contributed to this study.
Footnotes
Contributors: NU wrote the first draft of the manuscript and rewrote new drafts based on input from the coauthors. NU and KT performed the literature search. SI and YU controlled the decision to publish. All authors read and approved the final manuscript.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Lindstrom SJ, Pellegrino VA, Butt WW. Extracorporeal membrane oxygenation. Med J Aust 2009;191:178–82. [DOI] [PubMed] [Google Scholar]
- 2.Allen S, Holena D, McCunn M et al. A review of the fundamental principles and evidence base in the use of extracorporeal membrane oxygenation (ECMO) in critically ill adult patients. J Intensive Care Med 2011;26:13–26. 10.1177/0885066610384061 [DOI] [PubMed] [Google Scholar]
- 3.Bartlett RH, Zwischenberger JB. Management of blood flow and gas exchange during ECLS. In: Gail M. Annich, William R. Lynch, Graeme MacLaren, Jay M. Wilson, Robert H. Bartlett, eds. Extracorporeal cardiopulmonary support in critical care. 4th edn USA: Extracorporeal Life Supporting Organization, 2012:149–52. [Google Scholar]
- 4.Biscotti M, Lee A, Basner RC et al. Hybrid configurations via percutaneous access for extracorporeal membrane oxygenation: a single-center experience. ASAIO J 2014;60:635–42. 10.1097/MAT.0000000000000139 [DOI] [PubMed] [Google Scholar]
- 5.Choi JH, Kim SW, Kim YU et al. Application of veno-arterial-venous extracorporeal membrane oxygenation in differential hypoxia. Multidiscip Respir Med 2014;9:55 10.1186/2049-6958-9-55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhao J, Wang D, Zhou X et al. Hybrid ECMO using AvalonElite DLC for circulatory support guarantees adequate heart/brain oxygen supply. J Heart Lung Transplant 2013;32:S117–18. 10.1016/j.healun.2013.01.249 [DOI] [Google Scholar]