Significance
The 5′ end of eukaryotic mRNA is capped and methylated to protect mRNA from degradation and enhance protein synthesis. However, the cap can be removed from mRNA by decapping. We identified a recapping enzyme with 5′-monophosphate RNA kinase activity from trypanosome and provide evidence that decapped transcripts can be recapped to regenerate translatable mRNA. The kinase activity is dependent on mRNA leader sequence and is stimulated by hypermethylation found in the trypanosome mRNA. We also identify a trypanosome decapping enzyme that removes cap structure from the mRNA, but is less active on hypermethylated capped mRNA. These results suggest that hypermethylated cap structure can influence certain transcripts to be preferentially decapped or recapped during the parasite life cycle.
Keywords: mRNA recapping, mRNA decapping, RNA repair, cap methylation, Trypanosoma brucei
Abstract
The 5′ terminus of trypanosome mRNA is protected by a hypermethylated cap 4 derived from spliced leader (SL) RNA. Trypanosoma brucei nuclear capping enzyme with cap guanylyltransferase and methyltransferase activities (TbCgm1) modifies the 5′-diphosphate RNA (ppRNA) end to generate an m7G SL RNA cap. Here we show that T. brucei cytoplasmic capping enzyme (TbCe1) is a bifunctional 5′-RNA kinase and guanylyltransferase that transfers a γ-phosphate from ATP to pRNA to form ppRNA, which is then capped by transfer of GMP from GTP to the RNA β-phosphate. A Walker A-box motif in the N-terminal domain is essential for the RNA kinase activity and is targeted preferentially to a SL RNA sequence with a 5′-terminal methylated nucleoside. Silencing of TbCe1 leads to accumulation of uncapped mRNAs, consistent with selective capping of mRNA that has undergone trans-splicing and decapping. We identify T. brucei mRNA decapping enzyme (TbDcp2) that cleaves m7GDP from capped RNA to generate pRNA, a substrate for TbCe1. TbDcp2 can also remove GDP from unmethylated capped RNA but is less active at a mature cap 4 end and thus may function in RNA cap quality surveillance. Our results establish the enzymology and relevant protein catalysts of a cytoplasmic recapping pathway that has broad implications for the functional reactivation of processed mRNA ends.
The earliest modification to the eukaryotic mRNA is the addition of a cap structure (m7GpppN; cap 0) at the 5′ end, to protect the mRNA from degradation and recruit factors that promote RNA splicing, export, and translation initiation (1). The cap is formed in the nucleus by three sequential enzymatic reactions: the 5′ triphosphate of the nascent mRNA is hydrolyzed to a diphosphate by RNA triphosphatase; the diphosphate end is capped with GMP by RNA guanylyltransferase; and the GpppRNA is methylated by (guanine N7) methyltransferase to form cap 0 (2). Nucleotides adjacent to the cap are typically methylated on the first and second nucleosides to form cap 1 and cap 2 structures, respectively. The most elaborate cap structure, called cap 4, is found in Trypanosoma brucei and other kinetoplastid parasites and consists of a standard cap 0 with 2′-O methylations on the first four ribose sugars (AmAmCmUm), and additional base methylations on the first adenine (m6,6A) and the fourth uracil (m3U) (3). Although additional methylations have been shown to enhance translation efficiency (4, 5), whether they affect the RNA decay process is unknown.
All mRNA is subject to degradation by either a 5′-to-3′ or a 3′-to-5′ exonucleolytic pathway, generally initiated by shortening of the poly(A) tail (6). In the 5′-to-3′ pathway, the cap is removed as m7Gpp by the RNA decapping enzyme Dcp2, a member of the Nudix hydrolase superfamily, leaving a 5′-monophosphorylated RNA (pRNA) (7, 8). The exposed pRNA is progressively degraded by a 5′-to-3′ exonuclease (Xrn1/Rat1). Incompletely capped RNAs that lack the N7 methyl moiety, as well as defective mRNAs with premature termination codons, are decapped by a cellular quality-control machinery (9–12).
Studies in yeast and mammals suggest that decapping is a regulated process, and the uncapped mRNAs can be sequestered from degradation in the P bodies and stress granules (6, 13). Several studies suggest that stable uncapped mRNAs accumulate in cells and that they can potentially acquire a 5′-cap structure to regenerate translatable mRNAs (14, 15). However, the mRNAs that are decapped or cleaved by endonucleases are produced as pRNAs and thus need to be converted to diphosphorylated RNAs (ppRNAs) to be capped by a guanylyltransferase. The existence of pRNA kinase in mammalian cells has been proposed (16, 17) and such an activity has been found in the cytosol, complexed with a guanylyltransferase (18). However, the responsible protein has not yet been identified.
In trypanosomes, cap 4 structure is derived from cap 0 and formed exclusively on a 39-nucleotide spliced leader (SL) RNA, which is transferred by trans-splicing to individual ORFs derived from a polycistronic transcript (19, 20). The cap 0 is formed by an RNA triphosphatase, TbCet1, and a bifunctional guanylyltransferase-methyltransferase, TbCgm1 (21–23). In addition, three 2′-O-nucleoside RNA methyltransferases implicated in cap 4 meth-ylation on the SL RNA have been identified and characterized (24–27), and a stand-alone guanylyltransferase (TbCe1) and a stand-alone cap methyltransferase (TbCmt1) have been identified (28, 29).
Here we show that TbCe1 is a cytoplasmic recapping enzyme with 5′-monophosphate RNA kinase and guanylyltransferase activities and that it can convert pRNA into GpppRNA via a ppRNA intermediate. We also identify TbDcp2 as the Nudix-family decapping enzyme that generates pRNA from a capped RNA. Our findings define a decapping/recapping pathway in trypanosoma and its responsiveness to cap methylation status.
Results
TbCe1 Is a Cytoplasmic Capping Enzyme with a 5′-Monophosphate RNA Kinase Activity.
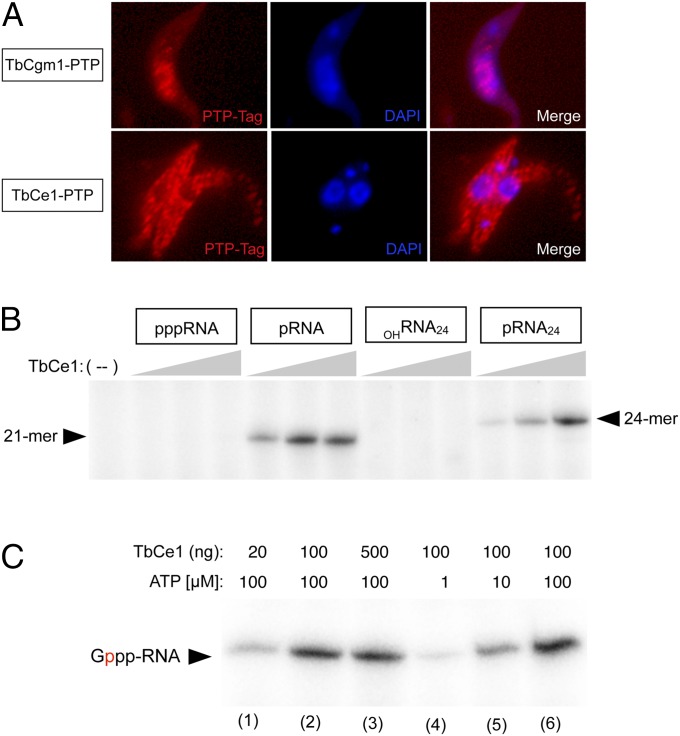
TbCgm1 is deemed responsible for cap 4 biosynthesis on the SL RNA, insofar as silencing of TbCgm1 increases the abundance of uncapped SL RNA and leads to accumulation of hypomethylated SL RNAs (22, 23). In contrast, silencing of TbCe1 does not prevent SL RNA capping (23). To assess the function of TbCe1, we examined the subcellular localization of both TbCgm1 and TbCe1 in T. brucei procyclic cells by indirect immunofluorescence with a protein A antibody that recognizes the PTP fusion protein epitope tag (Fig. 1A and Fig. S1A). As expected, TbCgm1–PTP localized to the nucleus, where SL RNAs are synthesized, consistent with the finding that TbCgm1 is recruited to the SL RNA promoter to cap the SL RNA (22). In contrast, TbCe1 localized to the cytoplasm. We conclude that TbCe1 is a cytosolic enzyme and likely functions in a different capping pathway from TbCgm1.
Fig. 1.
TbCe1 is a cytoplasmic capping enzyme that possesses 5′-monophosphate RNA kinase and guanylyltransferase activities. (A) Localization of PTP-tagged TbCgm1 and TbCe1. Procyclic trypanosomes expressing either TbCgm1–PTP (Top) or TbCe1–PTP (Bottom) were fixed and permeabilized, and the PTP–tag was detected by immunofluorescence. The kinetoplast and nuclear DNA were counterstained with DAPI. A typical image is shown. (B) TbCe1 specifically phosphorylates pRNA. Reaction mixtures (10 µL) containing 50 mM Tris⋅HCl (pH 8), 100 μM [γ-32P] ATP, 1 mM DTT, 0.5 mM MgCl2, and either 2 pmol of 21-mer pppRNA, 21-mer pRNA, 24-mer OHRNA24, or OHRNA24 treated with polynucleotide kinase (pRNA24), as indicated, were incubated with 20, 100, or 500 ng of TbCe1 (proceeding from Left to Right within each titration series). A control reaction lacking enzyme (−) is shown in the lane indicated. (C) RNA capping activity. Reaction mixtures (10 µL) containing 50 mM Tris⋅HCl (pH 8.5), 1 mM DTT, 0.5 mM MgCl2, 20 µM [α-32P] GTP, 100 nM of pRNA, with TbCe1 and ATP concentration as specified. Position of the capped-labeled 21-mer RNA is indicated. Radiolabeled phosphate is indicated in red.
To evaluate whether phosphotransferase activity is intrinsic to TbCe1, recombinant protein was produced in bacteria and purified from soluble lysates by nickel–agarose and DEAE chromatography (Fig. S1B). TbCe1 was incubated with either a pRNA, a 5′-triphosphate-terminated SL RNA (pppRNA), or a 5′-hydroxyl-terminated RNA (OHRNA), with a sequence that corresponds to the first 21 or 24 nt of SL RNA, in the presence of [γ-32P]ATP and magnesium. TbCe1 was capable of effectively transferring radiolabeled γ-phosphate from ATP to both the chemically phosphorylated 21-mer pRNA and enzymatically phosphorylated 24-mer pRNA but not to the pppRNA or OHRNA (Fig. 1B). Moreover, it converted the pRNA into a capped RNA in an ATP-dependent manner, as evidenced by radiolabel transfer of [α-32P]GMP from [α-32P]GTP in the presence of ATP (Fig. 1C).
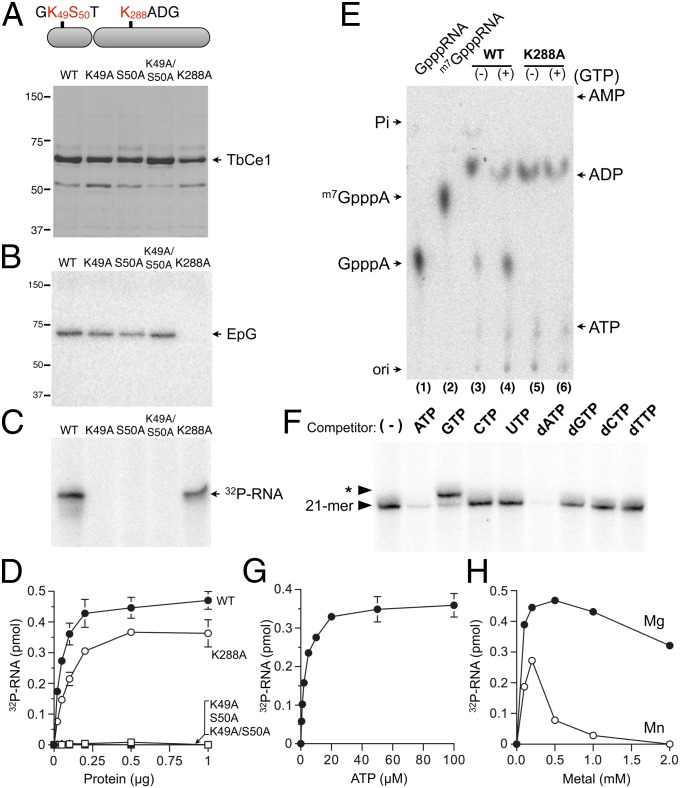
Alanine mutations within the conserved nucleotide-binding motif of TbCe1 (K49A, S50A, or K49A/S50A double mutations) abolished its phosphotransferase activity without affecting its guanylyltransferase activity (Fig. 2 A–D). We verified that the product generated by TbCe1 has a 5′-diphosphate end by isolating the radiolabeled RNA product from the gel and subjecting it to nuclease P1 digestion (Fig. 2E). A small percentage (∼15%) of the radiolabeled product migrating at the position of GpppA was observed, suggesting that the ppRNA formed by TbCe1 is subsequently converted to GpppRNA due to the presence of preguanylated enzyme in the recombinant protein preparation. Indeed, when GTP was included in the reaction, the ppRNA was converted to a capped RNA, as evident from an increase in the ratio of GpppA/ADP (Fig. 2E, lane 4). Notably, GpppA was not detected when the TbCe1–K288A mutant protein (lacking the lysine nucleophile for enzyme–GMP formation) was tested (Fig. 2E, lanes 5 and 6). We conclude that TbCe1 is a bifunctional capping enzyme with novel 5′-monophosphate RNA kinase and guanylyltransferase activities that can convert pRNA into GpppRNA. The nucleotide-binding motif in the N-terminal domain of TbCe1 is essential for the RNA kinase activity, and is likely to be the active site that coordinates the ATP.
Fig. 2.
Characterization of TbCe1 pRNA kinase activity. (A) Mutational analysis. Aliquots (4 µg) of wild-type (WT) TbCe1 and the indicated Ala mutants were subjected to SDS/PAGE and visualized with Coomassie blue. The positions of marker proteins (in kilodaltons) are indicated. (B) Guanylyltransferase activity. Reaction mixtures (20 µL) containing 50 mM Tris⋅HCl (pH 8), 1 mM DTT, 1 mM MgCl2, 25 μM [α-32P] GTP, and 100 ng of WT or mutant TbCe1 protein, as indicated in A, were incubated at 30 °C for 15 min. Enzyme–GMP complex (EpG) is indicated by an arrow. (C) RNA kinase activity. Reaction mixtures (20 µL) contained 50 mM Tris⋅HCl (pH 8), 20 μM [γ-32P] ATP, 1 mM DTT, 1 mM MgCl2, and 100 nM of pRNA, and were incubated at 30 °C for 15 min. (D) Protein titration. Standard RNA kinase assay (10 µL) with indicated amount of WT or mutant TbCe1 protein. The extent of 32P-labeled RNA formed is plotted as a function of input enzyme. (E) Analysis of a reaction product. The 32P-labeled RNA products generated by WT and K288A in C were excised from the gel, digested with nuclease P1, and analyzed by TLC developed by 1 M formic acid and 0.5 M LiCl. (F) Competition assay. A reaction mixture (20 µL) containing 50 mM Tris⋅HCl (pH 8), 20 μM [γ-32P] ATP, 1 mM DTT, 1 mM MgCl2, and 100 nM of the SL pRNA, 50 ng of TbCe1, and 200 μM of the indicated nucleotides was incubated at 30 °C for 15 min. A control reaction without added competitor nucleotide is indicated (−). Asterisk indicates position of capped 21-mer RNA. (G) ATP dependency. The standard RNA kinase assay (10 µL) contained 200 ng of TbCe1 and the indicated concentration of [γ-32P] ATP. The yield of 32P-labeled RNA was plotted as a function of ATP concentration. (H) Metal dependency. Standard RNA kinase assay contained 200 ng of TbCe1 and either MgCl2 or MnCl2. The yield of 32P-labeled RNA was plotted as a function of divalent cation concentration. The data shown represent the average of three separate experiments, with SE bars.
Characterization of pRNA Kinase Activity.
The pRNA kinase activity was proportional to the TbCe1 concentration (Fig. 2D). We calculated a specific activity of 11 fmol of 32P-labeled RNA formed per nanogram of TbCe1 during a 15-min reaction, which corresponds to a turnover number of 0.05 min−1. ATP and dATP were able to compete as phosphate donors, whereas other rNTP or dNTPs were not (Fig. 2F). Addition of GTP to the RNA kinase reaction shifted the radiolabeled RNA product by a single nucleotide, consistent with the formation of capped GpppRNA. The extent of pRNA phosphorylation was proportional to the ATP concentration and leveled off at 20 µM ATP (Fig. 2G). A Km of 2.7 µM ATP was calculated from nonlinear regression fitting of the data to the Michaelis–Menten equation using Prism. No RNA kinase activity was detectable in the absence of a divalent cation (Fig. 2H). Magnesium was a more effective cofactor than manganese, with an optimum of 0.2–0.5 mM MgCl2.
The properties of the TbCe1 guanylyltransferase are similar to those of the TbCgm1 and RNA guanylyltransferases found in other eukaryotes and viruses (Fig. S2). TbCe1 was unable to transfer GMP to a triphosphate-terminated poly(A) unless the 5′ end was converted to a diphosphate end by RNA triphosphatase (Fig. S2E). The N-terminal 5′-monophosphate kinase activity is not essential for the C-terminal guanylyltransferase activity, as neither mutation abolishing the kinase activity (Fig. 2B) nor a deletion of the entire N-terminal kinase domain (28) affected the enzyme–GMP complex formation.
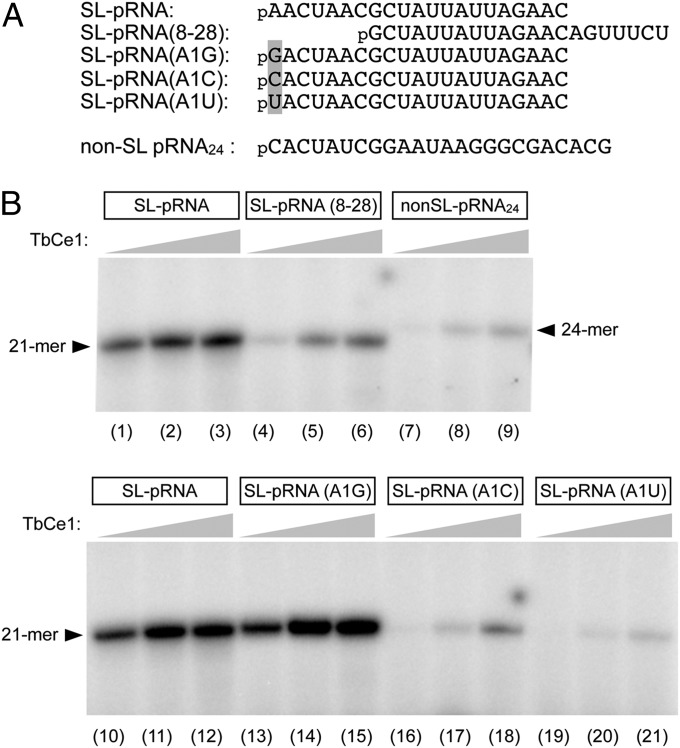
The pRNA Kinase Activity Is Specific to the SL RNA and Stimulated by Cap 4 Methylation.
In trypanosomes, mRNAs acquire an identical 39-nt SL RNA through trans-splicing. The sequence of the pRNA substrate used to characterize the pRNA kinase and capping activity in this study corresponds to the first 21 nucleotides of the SL RNA. To address whether this sequence influences the pRNA kinase activity, we prepared a pRNA that has a sequence corresponding to nucleotides +8 to +28 of the SL RNA [SL-pRNA(8–28)] and a 24-mer non-SL RNA (non-SL pRNA) (Fig. 3A). As shown in Fig. 3B, SL pRNA(8–28) and non-SL pRNA were less effective substrates than the SL pRNA, resulting in reductions to 9% and 0.8%, respectively, in specific activity compared with that when the SL pRNA was used. Substitution of A with G at position 1 did not affect the kinase activity, whereas a substitution with either C or U led to a drop to 1% and 0.3% of the activity relative to that for the wild-type SL pRNA (Fig. 3B). These results demonstrate that TbCe1 preferentially targets SL RNAs and that the presence of a purine at position 1 is critical for its pRNA kinase activity.
Fig. 3.
Phosphotransferase activity is stimulated by spliced leader RNA sequence. (A) The sequences of pRNA substrates are illustrated. (B) Specificity for the SL RNA. Standard RNA kinase assay (10 µL) contained either 1 pmol of SL-pRNA, SL-pRNA(8–28), non-SL pRNA24, SL-pRNA(A1G), SL-pRNA(A1C), or SL-pRNA(A1U), with either 20, 100, or 500 ng of TbCe1 (proceeding from Left to Right within each titration series). A phosphorimager scan of the gel is shown.
The findings that the TbCe1 RNA kinase activity is dependent on the SL RNA sequence prompted us to examine whether 2′-O methylations within the first 4 nucleosides can influence the activity. We evaluated two methylated SL pRNAs for their effectiveness as substrates for TbCe1: one has 2′-O methylation at position A1 (SL pRNAr1), and another has 2′-O methylations at positions A1, A2, C3, and U4 (SL pRNAr1234). The 2′-O methylation of the pRNA enhanced the TbCe1 kinase activity ∼3-fold over that for a pRNA lacking any modification (Fig. 4 A and B). Both SL pRNAr1 and SL pRNAr1234 were phosphorylated to a similar extent. The effect of 2′-O methylation on the TbCe1 kinase activity was further evaluated at various ATP concentrations (Fig. 4C). The stimulation was most pronounced at limiting ATP concentrations; in this context the increase in activity was 10-fold. The Km for ATP in the presence of the SL pRNAr1 and SL pRNAr1234 substrates was 0.26 µM and 0.36 µM, respectively; compared with 2.7 µM for the unmethylated SL pRNA. These results suggest that TbCe1 preferentially targets methylated RNAs derived from the mature cap 4 mRNA for recapping. Because cap 4 methylation occurs in the nucleus and depends on the cap 0 structure, the hypermethylated uncapped pRNA must be derived from a mature mRNA that has undergone decapping.
Fig. 4.

Effect of cap 4 methylation on TbCe1 phosphotransferase activity. (A) Protein titration. The standard RNA kinase assay (10 µL) contained 100 µM [γ-32P] ATP and 100 nM of either SL-pRNA, pRNAr1 (with 2′-O ribose methylation at position 1) or pRNAr1,2,3,4 (with 2′-O ribose methylation at position 1, 2, 3, and 4) and TbCe1, as indicated. The yield of 32P-labeled RNA was plotted as a function of input TbCe1. (B) Kinetics. Standard RNA kinase assay (80 µL) contained 160 ng of TbCe1 and 100 nM of either SL-pRNA, pRNAr1, or pRNAr1,2,3,4. An aliquot (10 μL) was withdrawn at the indicated time point, and the products were resolved by denaturing PAGE. The yield of 32P-labeled RNA product is plotted as a function of incubation time. (C) ATP titration. The standard RNA kinase assay (10 µL) contained 200 ng of TbCe1, 100 nM of either SL-pRNA, pRNAr1, or pRNAr1,2,3,4, and [γ-32P] ATP at the indicated concentration. The yield of 32P-labeled RNA was plotted as a function of ATP concentration. The data shown represent the average of three separate experiments. SE bars are included for each datum point.
TbCe1 Knockdown Accumulates Uncapped mRNAs.
Based on the above-described experiments, we predicted that silencing of TbCe1 would prevent the recapping of certain mRNA transcripts and lead to the accumulation of pRNAs in the cell. To test this prediction, we performed a ligation-mediated RT-PCR assay to detect pRNAs in T. brucei (Fig. S3). The anchor RNA oligonucleotide was ligated with poly(A) RNA isolated from TbCe1 RNAi-induced and uninduced cells, in the presence or absence of splint DNA, whose 3′ half is complementary to the anchor RNA sequence and whose 5′ half is complementary to the SL RNA sequence (Fig. 5 and Fig. S3). The RNA was converted to cDNA using oligo(dT) as a primer, and splint ligation products were detected by PCR using oligo(dT) and a DNA primer specific to the anchor sequence. A series of PCR products, in the range of 0.4–1.2 kb, was detected in TbCe1 RNAi cell lines (Fig. 5). The amounts of splint ligation products, as well as their heterogeneity, were significantly higher in TbCe1-silenced than the unsilenced control. Omission of splint DNA in the parallel reaction resulted in loss of the majority of the signal, although trace amounts of nonspecific ligation products were detected. These results suggest that decapped 5′-monophosphate-terminated mRNA is the substrate for TbCe1 in vivo. Although the trypanosome scavenger decapping enzyme DcpS, which hydrolyzes the m7GpppN cap dinucleotide, has been characterized (30), a decapping enzyme that releases m7Gpp and generates a pRNA has not yet been identified in this organism.
Fig. 5.
TbCe1 knockdown results in accumulation of 5′-monophosphate RNA. Poly(A) RNAs from TbCe1 RNAi uninduced (−) and induced with tetracycline for the number of days indicated were subjected to ligation-mediated RT-PCR (Fig. S3). Splint-ligation products were detected by PCR using an anchor forward primer and oligo(dT) as a reverse primer (Top). Products were resolved on agarose gel. Control reactions in which the splint DNA was omitted were carried out in parallel. Internal control reaction was performed using SL-specific forward primer and an α-tubulin-specific reverse primer (Bottom). The positions of marker DNA (in kilobases) are indicated to the Right.
Identification of the T. brucei Decapping Enzyme.
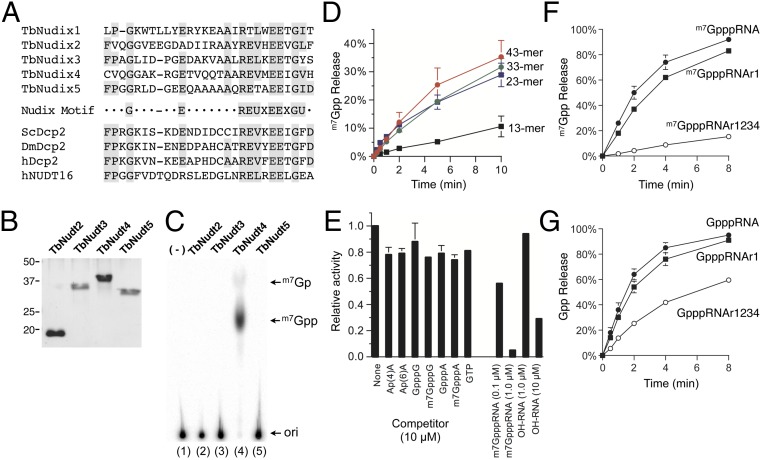
The cytosolic Dcp2 decapping enzymes from fungi and metazoans belong to the Nudix superfamily of hydrolases (31), which release m7Gpp from capped mRNA, generating pRNA. Five putative Nudix proteins have been identified by bioinformatics analysis of the T. brucei proteome: TbNudix1 (GenBank accession: EU711412.1), TbNudix2 (Tb927.5.4350), TbNudix3 (Tb11.01.1570), TbNudix4 (Tb927.6.2670), and TbNudix5 (Tb10.70.2530) (Fig. 6A). Among these proteins, TbNudix1 has been characterized as mitochondrial edited mRNA stability factor (32), and thus we did not test it for a decapping activity. TbNudix2 and TbNudix3 were found in the glycosome but their catalytic activities have not been defined (33). The functions of TbNudix4 and TbNudix5 are unknown.
Fig. 6.
Identification and characterization of T.brucei Nudix protein with RNA decapping activity. (A) Alignment of Nudix motif from five Trypanosoma brucei Nudix proteins (TbNudix), the yeast, fly, and human Dcp2 proteins, and the human Nud16 protein. (B) Protein purification. The TbNudix proteins were expressed and purified as a His10–tag fusions. A Coomassie blue-stained gel is shown. (C) Decapping activity. The standard decapping assay mixture (10 µL) contained 100 nM of 32P cap-labeled m7GpppRNA43 and 20 ng of indicated TbNudix proteins. A phosphorimager scan of the TLC plate is shown. (D) RNA dependency. The standard decapping assay mixture (20 µL) contained 2 ng of TbNudix4 and 100 nM of cap-labeled RNAs as indicated. Liberation of m7Gpp is plotted as a function of incubation time. (E) Competition assay. Standard decapping assay contained 100 nM of 32P cap-labeled m7GpppRNA43, 1 ng of TbNudix4 with either 10 µM of the indicated cap dinucleotide analog, 10 µM GTP, 0.1 µM, or 1 µM of m7GpppRNA43, and 1 µM or 10 µM of the OHRNA. A control reaction (none) was incubated in parallel without any competitor. Decapping activity was normalized to that of the control reaction without competitor (defined as 1.0). (F) Effect of 2′-O methylation on decapping activity. Standard decapping assay mixture (30 µL) containing 15 ng of TbNudix4, with 100 nM of 32P-labeled cap 0 (m7GpppRNA), cap 1 (m7GpppRNAr1), or cap 4 (m7GpppRNAr1234). Liberation of m7Gpp is plotted as a function of incubation time. (G) Identical to F except that the substrates for the assay were Gppp-RNA, Gppp-RNAr1, or Gppp-RNAr1,2,3,4. The data shown represent the average of three separate experiments. SE bars are included for each datum point.
To determine if TbNudix2, TbNudix3, TbNudix4, and TbNudix5 possess an RNA decapping activity, we produced His-tagged fusion proteins in bacteria (Fig. 6B) and assayed for the release of m7Gpp in vitro using a cap-labeled 43-mer RNA (m7GpppRNA43). Liberation of m7Gpp was detected by TLC analysis. TbNudix4 was the only enzyme that led to release m7Gpp from m7GpppRNA43 in the presence of magnesium (Fig. 6C and Fig. S4). We verified that the product generated is m7Gpp by subjecting the reaction product to alkaline phosphatase digestion (Fig. S4). We therefore rename TbNudix4 as TbDcp2.
The amino acid sequence of the 294 amino acid TbDcp2 is shown in Fig. S5A. TbDcp2 lacks a conserved amino-terminal Box A domain and a carboxy-domain present in other Dcp2 proteins. TbDcp2 sedimented as a single peak, between BSA and cytochrome C in a parallel gradient, which suggested that it is a monomer in solution (Fig. S5B). Its activity profile coincided with the distribution of the TbDcp2 polypeptide (Fig. S5C), and we calculated a specific activity of 60 fmol of m7Gpp released per nanogram in 1 min, which corresponded to a turnover rate of 2 min−1.
What distinguishes decapping enzyme from nucleotide pyrophosphatase is a dependence on RNA. TbDcp2 hydrolyzed m7Gpp from 33-mer and 23-mer RNA with an efficiency similar to that for the 43-mer substrate (Fig. 6D), but the activity was significantly reduced with the 13 mer (∼30% compared to m7GpppRNA43). The RNA dependence was also demonstrated by competition assay using cap analogs (Fig. 6E); none of the cap analogs or GTP effectively competed against the m7GpppRNA43 substrate. A 10-fold excess of hydroxyl-terminated RNA (1 µM) had a minimal effect on decapping activity, whereas a 100-fold excess of hydroxyl-terminated RNA (10 µM) resulted in 70% inhibition, suggesting that TbDcp2 recognizes both the cap and the RNA chain (Fig. 6E). In summary, our data demonstrate that TbDcp2 possesses canonical RNA decapping activity in vitro.
TbDcp2 Preferentially Hydrolyzes Prematurely Hypomethylated RNA.
To address whether cap 4 methylation can influence TbDcp2 activity, we prepared a cap-labeled SL RNA that resembles cap 1 (m7GpppRNAr1) and cap 4 (m7GpppRNAr1234) from the 21-mer SL pRNA, using recombinant TbCe1 (SI Materials and Methods). The rate of m7Gpp release from the cap 1 substrate was comparable to that of the cap 0 (Fig. 6F), indicating that 2′-O methylation at position 1 does not affect decapping. In contrast, the rate of m7Gpp release from cap 4 was reduced ∼10-fold compared with that of the cap 0, indicating that hypermethylated RNA was resistant to decapping by TbDcp2.
We also examined the effect of unmethylated capped SL RNA (GpppRNA) on decapping activity. TbDcp2 can efficiently hydrolyze Gpp from Gpp-terminated SL RNA (Fig. 6G). The rate of Gpp release was 40% faster than that of m7Gpp. Similarly, 2′-O methylation at position 1 did not have significant influence on Gpp hydrolysis. However, the rate of decapping from GpppRNAr1234 was reduced by 65% compared with that for GpppRNA, consistent with the notion that hypermethylated RNAs are less efficiently decapped by TbDcp2. The optimal substrates for decapping by TbDcp2 were: GpppRNA ≥ GpppRNAr1 = m7GpppRNA ≥ m7GpppRNAr1 > GpppRNAr1234 > m7GpppRNAr1234. These results demonstrate that cap 4 methylation can influence the decapping efficiency of TbDcp2 and raise the prospect that TbDcp2 may function as a surveillance enzyme to decap mRNAs lacking either N7 guanine methylation or ribose 2′O-methylation at nucleosides 2, 3, and 4.
Discussion
TbCe1 Kinase Activity Is Preferentially Targeted to Hypermethylated Uncapped SL RNAs.
In this report, we present the first evidence to our knowledge that a 5′-monophospate RNA kinase activity is directly involved in the recapping of pRNA. The kinase and the guanylyltransferase activities are physically linked as a single TbCe1 polypeptide, and TbCe1 depletion results in an accumulation of pRNA in T. brucei. The fact that the RNA kinase activity depended on the SL RNA sequence and was stimulated by methylation of cap 4 provides strong evidence that this enzyme preferentially acts on uncapped hypermethylated mRNAs that have undergone decapping. Because cap 2′-O ribose methyltransferases are in the nucleus but decapping likely takes place in the cytoplasm, uncapped hypermethylated pRNAs with a 5′ SL would most likely accumulate in the cytoplasm, where TbCe1 resides. This substrate specificity of TbCe1 would likely exclude capping of other RNA types (such as tRNAs, rRNAs, and noncoding RNAs) that are present at high levels in the cytoplasm. The endonucleolytically cleaved RNAs are also likely to be excluded for recapping by TbCe1, thereby preventing the generation of N-terminally truncated proteins, a scenario that could be potentially deleterious to the parasite.
The pRNA kinase activity of TbCe1 required the Walker A nucleotide binding motif within the N-terminal region. The phosphotransferase activity is likely to be mechanistically similar to the reaction carried out by adenylate kinase and 5′-OHRNA kinases, given that the following two residues were essential for this activity (34, 35): invariant Lys49, which in the other kinases stabilizes the phosphate groups on the NTP, and conserved Ser50, which likely coordinates the divalent cation for phosphoryl transfer. The fact that either ATP or dATP can serve as a phosphate donor, whereas other rNTPs and dNTPs did not complete with ATP, implied that TbCe1 recognizes the adenine base. The enzymatic properties of TbCe1 are similar to those of the 5′-RNA kinase purified from crude fractions prepared from vaccinia virions with respect to their magnesium and RNA dependence, as well as their nucleotide (ATP or dATP) specificity as a phosphate donor (17). However, TbCe1 does not appear to convert ppRNA to pppRNA. Further mutational and structural analysis is expected to provide insights into how TbCe1 interacts with the SL sequence and how its phosphotransferase activity is enhanced by cap 4 methylation.
In order for a translatable mRNA to be generated through recapping, the GpppRNA must be methylated at the N7 position. We speculate that TbCmt1, a second guanine-N7 methyltransferase encoded by trypanosomes, functions in the recapping pathway to convert the TbCe1-generated GpppRNA into a translatable m7GpppRNA (29). Indeed, silencing of TbCmt1 did not affect the growth of procyclic cells or capping of the SL RNA, similar to the phenotype observed in TbCe1 depletion (23).
TbDcp2 Is RNA Decapping and Surveillance Enzyme.
Of the four T. brucei Nudix proteins characterized, TbDcp2 (TbNudix4) is the only one capable of releasing m7Gpp from m7GpppRNA in a magnesium-dependent manner. Cap analogs were not effective competitors for TbDcp2, but uncapped RNA effectively inhibited Dcp2 activity, a property shared by other RNA decapping enzymes (8, 36, 37). TbDcp2 may also function as a surveillance enzyme that removes incompletely capped mRNA, consistent with the decapping activity detected in cytoplasmic extracts from Leptomonas seymouri (37). Our finding that TbDcp2 activity is influenced by cap 4 methylation suggests that the 5′-to-3′ turnover may be regulated by differential cap methylation.
Role of Cap 4 Modification in the Decapping and Recapping Pathway.
Hypermethylated cap 4 is a unique feature of the mRNA of kinetoplastids and is involved in trans-splicing and translational enhancement (5, 22). Although cap 4 methylation partially protects mRNA from decapping by TbDcp2, the decapped transcripts—which likely retain the methyl moieties on their 5′ end—may have an innate stability, inhibiting 5′-to-3′ degradation by exonuclease. If so, stable uncapped methylated pRNA may accumulate in the cytoplasm. Unless it is degraded by the 3′-to-5′ decay pathway, TbCe1 can preferentially recap the hypermethylated pRNA.
Model of mRNA Decapping and Recapping Pathway in Trypanosomes.
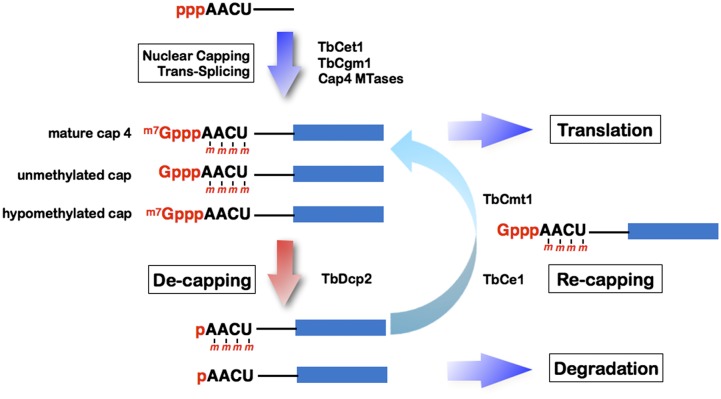
Fig. 7 summarizes a proposed model for mRNA decapping and recapping pathway in trypanosomes. In the nucleus, the SL RNA is capped by TbCet1 and TbCgm1 and then hypermethylated by a series of cap 4 methyltransferases. Trans-spliced and polyadenylated mature mRNAs are transported to the cytoplasm for protein synthesis. TbDcp2 may act as a conventional RNA decapping enzyme to generate uncapped pRNAs. It may also decap mRNA with unmethylated or hypomethylated cap structures. The decapped mRNAs are either digested by 5′-to-3′ exonuclease or sequestered from the degradation machinery and stored in a cytoplasmic compartment. Under certain conditions, the recapping apparatus may restore the uncapped pRNA to GpppRNA via TbCe1 and methylate the N7 moiety of the cap via the TbCmt1, to regenerate translatable mRNA.
Fig. 7.
Proposed model of mRNA decapping and recapping in T. brucei. Nascent SL RNA with 5′ triphosphate end is converted to diphosphate by RNA triphosphatase (TbCet1), capped and methylated by a nuclear capping enzyme (TbCgm1), and hypermethylated by a series of cap 4 methyltransferases (collectively indicated as cap 4 MTases). Trans-spliced and polyadenylated cap 4 mRNA can enter the translational pool, whereas unmethylated capped and hypomethylated capped mRNA are subject to either 3′-to-5′ degradation (not shown) or converted into pRNA by a decapping enzyme (TbDcp2). The decapped pRNA can either be degraded by a 5′-3′ exonuclease, or have its 5′ cap restored by TbCe1 and TbCmt1 and then return to the translational pool.
The mRNA recapping pathway is particularly advantageous for trypanosomes and other kinetoplastid organisms that rely on a trans-splicing mechanism for gene expression. In trypanosomes, the selective expression of a single gene of interest requires that (i) a long polycistronic pre-mRNA is transcribed; (ii) the transcript is resolved by trans-splicing and polyadenylation; (iii) the mature mRNA is transported to the cytoplasm; (iv) unwanted mRNAs are selectively degraded; and (v) the mRNA to be translated is protected. Overall, this requires more time and resources than the expression of a single gene in other eukaryotes. The decapping and recapping pathway may function to regulate the gene expression in response to stress or sudden environmental changes.
Materials and Methods
Two critical protocols are briefly described below and more details can be found in SI Materials and Methods.
RNA Kinase Assay.
The standard reaction contained 50 mM Tris⋅HCl (pH 9.0), 1 mM DTT, 0.5 mM MgCl2, 100 µM [γ-32P] ATP, 100 nM 5′-monophosphorylated RNA, and TbCe1 protein for 15 min at 30 °C, unless otherwise specified. Products were resolved by 18% (wt/vol) Urea-PAGE, and radiolabeled product was visualized and quantitated by phosphorimager.
RNA Decapping Assay.
The standard reaction, containing 50 mM of Tris⋅HCl (pH 7.5), 2 mM MgCl2, 100 nM of cap-labeled RNA substrate and protein as specified, was incubated for 10 min at 25 °C. EDTA was added to a final concentration of 16 mM to quench the reaction. An aliquot of the reaction mixture was spotted onto TLC PEI Cellulose F (Millipore EMD), and was developed with 0.75 M LiCl. Liberation of the radiolabeled 5′ terminus was visualized and quantified by phosphorimager analysis.
Supplementary Material
Acknowledgments
We thank Laurie Read (SUNY Buffalo) for T. brucei DNA and Chris Lima (Sloan-Kettering Institute) for the expression vector. We also thank Beate Schwer (Cornell University), Stewart Shuman (Sloan-Kettering Institute), and members of the Laboratory of Infection Biology (University of Tsukuba) for their helpful suggestions. This material is based upon work supported by the National Science Foundation under Grant 1050984.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1424909112/-/DCSupplemental.
References
- 1.Furuichi Y, Shatkin AJ. Viral and cellular mRNA capping: Past and prospects. Adv Virus Res. 2000;55:135–184. doi: 10.1016/S0065-3527(00)55003-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shuman S. Structure, mechanism, and evolution of the mRNA capping apparatus. Prog Nucleic Acid Res Mol Biol. 2001;66:1–40. doi: 10.1016/s0079-6603(00)66025-7. [DOI] [PubMed] [Google Scholar]
- 3.Bangs JD, Crain PF, Hashizume T, McCloskey JA, Boothroyd JC. Mass spectrometry of mRNA cap 4 from trypanosomatids reveals two novel nucleosides. J Biol Chem. 1992;267(14):9805–9815. [PubMed] [Google Scholar]
- 4.Kuge H, Richter JD. Cytoplasmic 3′ poly(A) addition induces 5′ cap ribose methylation: Implications for translational control of maternal mRNA. EMBO J. 1995;14(24):6301–6310. doi: 10.1002/j.1460-2075.1995.tb00320.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zamudio JR, Mittra B, Campbell DA, Sturm NR. Hypermethylated cap 4 maximizes Trypanosoma brucei translation. Mol Microbiol. 2009;72(5):1100–1110. doi: 10.1111/j.1365-2958.2009.06696.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parker R, Sheth U. P bodies and the control of mRNA translation and degradation. Mol Cell. 2007;25(5):635–646. doi: 10.1016/j.molcel.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 7.Dunckley T, Parker R. The DCP2 protein is required for mRNA decapping in Saccharomyces cerevisiae and contains a functional MutT motif. EMBO J. 1999;18(19):5411–5422. doi: 10.1093/emboj/18.19.5411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang Z, Jiao X, Carr-Schmid A, Kiledjian M. The hDcp2 protein is a mammalian mRNA decapping enzyme. Proc Natl Acad Sci USA. 2002;99(20):12663–12668. doi: 10.1073/pnas.192445599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jiao X, Chang JH, Kilic T, Tong L, Kiledjian M. A mammalian pre-mRNA 5′ end capping quality control mechanism and an unexpected link of capping to pre-mRNA processing. Mol Cell. 2013;50(1):104–115. doi: 10.1016/j.molcel.2013.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lykke-Andersen J. Identification of a human decapping complex associated with hUpf proteins in nonsense-mediated decay. Mol Cell Biol. 2002;22(23):8114–8121. doi: 10.1128/MCB.22.23.8114-8121.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cao D, Parker R. Computational modeling and experimental analysis of nonsense-mediated decay in yeast. Cell. 2003;113(4):533–545. doi: 10.1016/s0092-8674(03)00353-2. [DOI] [PubMed] [Google Scholar]
- 12.Muhlrad D, Parker R. Premature translational termination triggers mRNA decapping. Nature. 1994;370(6490):578–581. doi: 10.1038/370578a0. [DOI] [PubMed] [Google Scholar]
- 13.Eulalio A, Behm-Ansmant I, Izaurralde E. P bodies: At the crossroads of post-transcriptional pathways. Nat Rev Mol Cell Biol. 2007;8(1):9–22. doi: 10.1038/nrm2080. [DOI] [PubMed] [Google Scholar]
- 14.Mukherjee C, et al. Identification of cytoplasmic capping targets reveals a role for cap homeostasis in translation and mRNA stability. Cell Reports. 2012;2(3):674–684. doi: 10.1016/j.celrep.2012.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schoenberg DR, Maquat LE. Re-capping the message. Trends Biochem Sci. 2009;34(9):435–442. doi: 10.1016/j.tibs.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schibler U, Perry RP. Characterization of the 5′ termini of hn RNA in mouse L cells: Implications for processing and cap formation. Cell. 1976;9(1):121–130. doi: 10.1016/0092-8674(76)90058-1. [DOI] [PubMed] [Google Scholar]
- 17.Spencer E, Loring D, Hurwitz J, Monroy G. Enzymatic conversion of 5′-phosphate-terminated RNA to 5′-di- and triphosphate-terminated RNA. Proc Natl Acad Sci USA. 1978;75(10):4793–4797. doi: 10.1073/pnas.75.10.4793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Otsuka Y, Kedersha NL, Schoenberg DR. Identification of a cytoplasmic complex that adds a cap onto 5′-monophosphate RNA. Mol Cell Biol. 2009;29(8):2155–2167. doi: 10.1128/MCB.01325-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Agabian N. Trans splicing of nuclear pre-mRNAs. Cell. 1990;61(7):1157–1160. doi: 10.1016/0092-8674(90)90674-4. [DOI] [PubMed] [Google Scholar]
- 20.Liang XH, Haritan A, Uliel S, Michaeli S. trans and cis splicing in trypanosomatids: Mechanism, factors, and regulation. Eukaryot Cell. 2003;2(5):830–840. doi: 10.1128/EC.2.5.830-840.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ho CK, Shuman S. Trypanosoma brucei RNA triphosphatase. Antiprotozoal drug target and guide to eukaryotic phylogeny. J Biol Chem. 2001;276(49):46182–46186. doi: 10.1074/jbc.M108706200. [DOI] [PubMed] [Google Scholar]
- 22.Ruan J-P, Shen S, Ullu E, Tschudi C. Evidence for a capping enzyme with specificity for the trypanosome spliced leader RNA. Mol Biochem Parasitol. 2007;156(2):246–254. doi: 10.1016/j.molbiopara.2007.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Takagi Y, Sindkar S, Ekonomidis D, Hall MP, Ho CK. Trypanosoma brucei encodes a bifunctional capping enzyme essential for cap 4 formation on the spliced leader RNA. J Biol Chem. 2007;282(22):15995–16005. doi: 10.1074/jbc.M701569200. [DOI] [PubMed] [Google Scholar]
- 24.Arhin GK, Ullu E, Tschudi C. 2′-O-methylation of position 2 of the trypanosome spliced leader cap 4 is mediated by a 48 kDa protein related to vaccinia virus VP39. Mol Biochem Parasitol. 2006;147(1):137–139. doi: 10.1016/j.molbiopara.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 25.Arhin GK, Li H, Ullu E, Tschudi C. A protein related to the vaccinia virus cap-specific methyltransferase VP39 is involved in cap 4 modification in Trypanosoma brucei. RNA. 2006;12(1):53–62. doi: 10.1261/rna.2223406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hall MP, Ho CK. Functional characterization of a 48 kDa Trypanosoma brucei cap 2 RNA methyltransferase. Nucleic Acids Res. 2006;34(19):5594–5602. doi: 10.1093/nar/gkl573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zamudio JR, et al. Complete cap 4 formation is not required for viability in Trypanosoma brucei. Eukaryot Cell. 2006;5(6):905–915. doi: 10.1128/EC.00080-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Silva E, Ullu E, Kobayashi R, Tschudi C. Trypanosome capping enzymes display a novel two-domain structure. Mol Cell Biol. 1998;18(8):4612–4619. doi: 10.1128/mcb.18.8.4612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hall MP, Ho CK. Characterization of a Trypanosoma brucei RNA cap (guanine N-7) methyltransferase. RNA. 2006;12(3):488–497. doi: 10.1261/rna.2250606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Banerjee H, et al. 2009. Identification of the HIT-45 protein from Trypanosoma brucei as an FHIT protein/dinucleoside triphosphatase: Substrate specificity studies on the recombinant and endogenous proteins. RNA 15:1554–1564.
- 31.Mildvan AS, et al. Structures and mechanisms of Nudix hydrolases. Arch Biochem Biophys. 2005;433(1):129–143. doi: 10.1016/j.abb.2004.08.017. [DOI] [PubMed] [Google Scholar]
- 32.Weng J, et al. Guide RNA-binding complex from mitochondria of trypanosomatids. Mol Cell. 2008;32(2):198–209. doi: 10.1016/j.molcel.2008.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Güther MLS, Urbaniak MD, Tavendale A, Prescott A, Ferguson MAJ. High-confidence glycosome proteome for procyclic form Trypanosoma brucei by epitope-tag organelle enrichment and SILAC proteomics. J Proteome Res. 2014;13(6):2796–2806. doi: 10.1021/pr401209w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang LK, Shuman S. Mutational analysis defines the 5′-kinase and 3′-phosphatase active sites of T4 polynucleotide kinase. Nucleic Acids Res. 2002;30(4):1073–1080. doi: 10.1093/nar/30.4.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weitzer S, Martinez J. The human RNA kinase hClp1 is active on 3′ transfer RNA exons and short interfering RNAs. Nature. 2007;447(7141):222–226. doi: 10.1038/nature05777. [DOI] [PubMed] [Google Scholar]
- 36.Cohen LS, et al. Dcp2 Decaps m2,2,7GpppN-capped RNAs, and its activity is sequence and context dependent. Mol Cell Biol. 2005;25(20):8779–8791. doi: 10.1128/MCB.25.20.8779-8791.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Milone J, Wilusz J, Bellofatto V. Identification of mRNA decapping activities and an ARE-regulated 3′ to 5′ exonuclease activity in trypanosome extracts. Nucleic Acids Res. 2002;30(18):4040–4050. doi: 10.1093/nar/gkf521. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.