Significance
The dawn of sustainable life on earth is preserved in the form of fossil or chemical evidence in ancient rock sequences, such as the Barberton Greenstone Belt in South Africa. Studies of sedimentary rocks offered a glimpse at life at the earth’s surface, and trace fossils in pillow lavas offered evidence for a potential deep biosphere back in time to the Paleoarchean. Recent data cast doubt on the biogenicity of these putative trace fossils, rejecting their potential in exploring a deep biosphere. We discuss biogenicity of Cenozoic and Archean examples of such putative biocorrosion textures and conclude that microbial origin remains the best explanation for the textures described previously in these Paleoarchean rocks (e.g., >3.4 Ga).
Keywords: biogeosciences, early life, Paleoarchean, astrobiology, ichnofossil
Abstract
Microbial corrosion textures in volcanic glass from Cenozoic seafloor basalts and the corresponding titanite replacement microtextures in metamorphosed Paleoarchean pillow lavas have been interpreted as evidence for a deep biosphere dating back in time through the earliest periods of preserved life on earth. This interpretation has been recently challenged for Paleoarchean titanite replacement textures based on textural and geochronological data from pillow lavas in the Hooggenoeg Complex of the Barberton Greenstone Belt in South Africa. We use this controversy to explore the strengths and weaknesses of arguments made in support or rejection of the biogenicity interpretation of bioalteration trace fossils in Cenozoic basalt glasses and their putative equivalents in Paleoarchean greenstones. Our analysis suggests that biogenicity cannot be taken for granted for all titanite-based textures in metamorphosed basalt glass, but a cautious and critical evaluation of evidence suggests that biogenicity remains the most likely interpretation for previously described titanite microtextures in Paleoarchean pillow lavas.
Microbial corrosion textures in volcanic glass are well studied in Cenozoic seafloor basalts, they are distinct from the well-understood abiotic alteration textures, and they are considered an indication of an active deep oceanic biosphere down to at least 500 m below the seafloor (Fig. 1 A–C and Figs. S1–S4) (1, 2). Such corrosion textures were proposed to be preserved in the form of analogous titanite textures in chloritized volcanic glass in greenschist metamorphosed glass from pillow basalts of the ca. 3.5 Ga Barberton Greenstone Belt (BGB, South Africa) (3, 4) (Fig. 1 D–F and Fig. S5) and the Pilbara Craton (PC/Australia) (Fig. S6) (5), suggesting the presence of a deep oceanic biosphere at this early time in the evolution of life on earth. Recently, the biogenicity interpretation of such titanite textures was rejected based on a geochemical, textural, and geochronological study of a 180-m drilled core section through pillow lavas of the Hooggenoeg Complex of the BGB (6). This controversy affords an opportunity to critically evaluate biogenicity in terms of what is known about bioalteration of volcanic glass and how these trace fossils may be preserved and be recognized in greenschist metamorphosed volcanic glass.
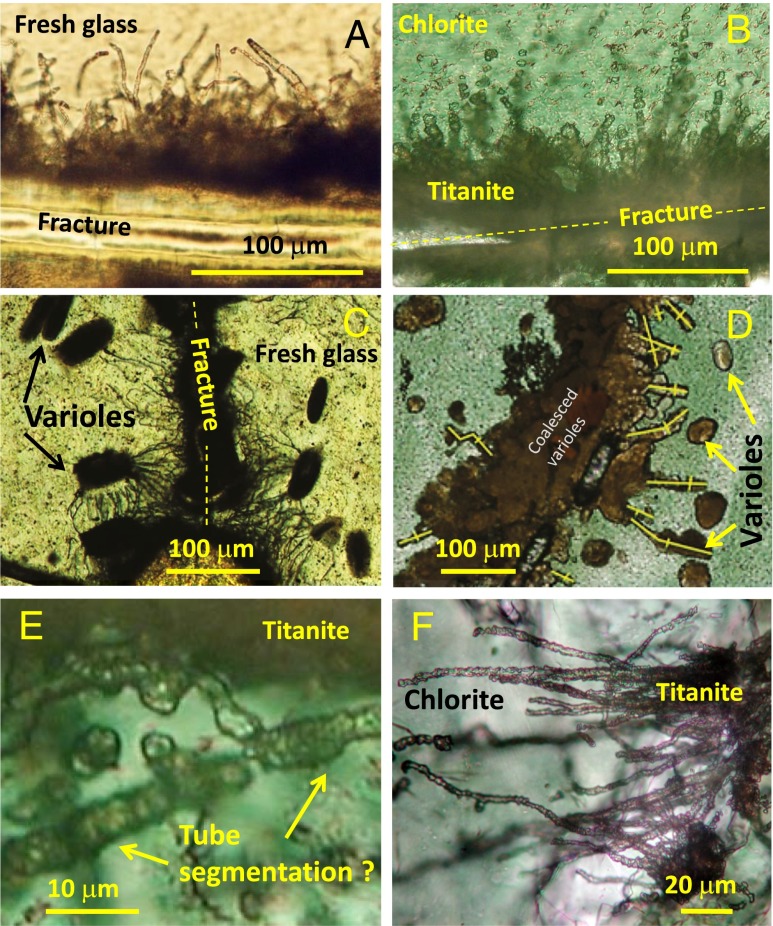
Fig. 1.
Biotextures in fresh Cenozoic basalt glass and titanite microtextures in Archean greenschist pillow lavas. Comparison between tubular biotextures in (A) the 110-Ma-old deep sea drilling project (DSDP) sample 418A, 56–2, 129–132 cm, and (B) similarly shaped and sized tubular textures of the interpillow hyaloclastite (sample 29-BG-03) of the 3,470-Ma-old Hooggenoeg Complex. (C) DSDP sample 418A, 62–4, 64–70 cm, showing several dark brown varioles within the fresh glass and biogenerated filaments rooted in a fracture. (D) Figure S2F from ref. 6 suggesting that some of these titanite microtextures may be varioles intermixed with tubular biotextures. (E) Details from sample 29-BG-03 from BGB (22) in which a 6-µm-wide tube, rooted in a titanite-filled fracture, may show a segmented nature. (F) Thin, 2–3-µm-wide segmented tubes extending from the edge of a former glass fragment (now mainly chlorite) from sample 74-PG-04 of the 3.35 Ga Euro Basalt of the PC, Western Australia (5).
Biotextures are widely found as dissolution features on the surfaces of volcanic glass in Cenozoic seafloor volcanics and may take on two major forms, micrometer-sized spherical cavities or tubules that extend into the glass up to about 100 µm (2, 7, 8). Such glass dissolution cavities, in particular their more complex signature expressions such as bifurcating, annulated, decorated, or coiled tubes (9), are considered impossible to have been caused by abiotic dissolution. Well-documented cases of microbial drilling into soil feldspars (10) and mollusk shells (11) offer analogs that give precedence for biogenicity of such dissolution features (2). Microbial dissolution of glass is likely to be caused or at least aided by the excretion of organic acids in the contact area of colonizing microbes or their cell extensions such as fungal hyphae (2). Reasons for microbial boring into glass remain uncertain, but there are several potentially chemical energy-producing weathering reactions of volcanic glass that would become available in an otherwise largely oligotrophic environment. Although many details of microbial drilling into volcanic glass remain to be explored (2), biogenicity appears to be the widely accepted explanation for the origin of granular and tubular alteration textures in Cenozoic volcanic glass (6).
Putative bioalteration textures were found in Paleoarchean greenstone belts, in particular the upper sequences of the Hooggenoeg Complex of the BGB in South Africa (3, 4) (Fig. 1 B and E and Fig. S5) and the Euro Basalts of the PC in Australia (5) (Fig. 1F and Fig. S6). However, these putative trace fossils in greenstones are preserved not as cavities but as mineral fillings, especially by titanite, and they are recognizable by their remarkable textural similarities with Cenozoic glass bioalteration, including their association with external glass surfaces and cracks. Such titanite textures have been the subject of a petrographic, geochemical, and geochronological study of a drill core in the Hooggenoeg Complex of the BGB, but they were interpreted as metamorphic features without a biogenic precursor (6).
In this paper, we will take the opportunity to explore weaknesses and strengths of various lines of evidence cited in support or rejection of biogenicity for purported biotextures in Cenozoic volcanic glass and in Paleoarchean greenstones. Our discussion will address but not be limited to the four main arguments extended recently by Grosch and McLoughlin (6): (i) the apparent lack of chemical biomarkers in their samples, (ii) the difficulties in finding the most unique signature fossils (spiraling or annulated tubes), (iii) the apparent differences in tube diameter size distributions between titanite replacement textures and precursor fossils, and (iv) the substantial age difference between crustal ages and apparent titanite replacement ages. We will show that many of Grosch and McLoughlin's arguments are flawed or ill-supported, and none of them carry much weight in ruling out biogenicity of the textures studied. However, we concur with Grosch and McLoughlin's (6) assessment that many of the textures in their study site are indeed abiotic or at least ambiguous in terms of a potential biotic precursor. In conclusion, we suggest that previous evidence for biogenicity for titanite-based textures remains the most likely interpretation, at least for the better preserved and more complex tubular textures found in the BGB and PC.
Glass Bioalteration Preservation by Titanite
Most, if not all, issues we address in this paper relate to the mechanisms of trace fossil replacement by titanite, a typical metamorphic mineral, while more typical fossil replacement minerals are of diagenetic origin. This is likely the biggest “stumbling block” in accepting the step, from the widely accepted bioalteration corrosion textures in volcanic glass to the putative, titanite-based trace fossil textures in chloritized glasses in Archean pillow basalts.
Titanite is an obvious candidate replacement phase, as it is a common accessory phase in greenschist facies metabasalts and the most probable host mineral for Ti that is mobilized as basaltic glass is transformed into greenschist minerals. The chemical reorganization of glass into chlorite and accessory phases can be readily observed in petrographic analysis of greenstones where titanite commonly forms as a newly formed metamorphic mineral during prograde metamorphism. Titanite is the only mineral that can accommodate the relatively large fraction of Ti in basaltic glass (TiO2 is around 1 wt% in the actual metabasalts) that is incompatible with other greenschist facies minerals. Titanite occurs mostly as metamorphic mineral growths in the chloritized glass, but it also commonly replaces varioles, a common quench texture in the pillow lavas of the Hooggenoeg Complex of the BGB (12). Clearly identifiable titanite-based biotextures are much rarer than the broadly occurring titanite in these metamorphic rocks.
Why would this common metamorphic mineral crystallize in and around bioalteration cavities in basaltic glass? It is well known that alteration of basaltic glass leads to the passive accumulation of Ti in its alteration products such as palagonite (13) and such accumulation has been found in the walls of microtubules as well (14, 15). These observations may be combined into a two-stage model for the origin of titanite bioalteration replacement textures (16). The first stage involves the Ti enrichment in the walls of the microbial excavations in volcanic glass, and the second stage is marked by the titanite crystallization stage during greenschist metamorphism in and around these zones of Ti preenrichment. A chemical mass balance requires that these titanites also have to draw additional Ti from the surrounding glass as it is transformed to chlorite.
Grosch and McLoughlin (6) offer a different concept for titanite crystallization, drawing from a retrograde metamorphic reaction that involves the formation of titanite (and actinolite) from the breakdown of clinopyroxene, ilmenite, and quartz. Grosch and McLoughlin argue that this process occurred during retrograde cooling from >600 °C to 350 °C subsequent to contact metamorphism associated with the intrusion of an 84-m-thick diorite dike 18–19 m away from the analyzed samples (figure S3B in ref. 6). Grosch and McLoughlin propose that this process (figure S3B in ref. 6) begins with a complexly shaped ilmenite texture that resembles the putative bioalteration textures and that titanite grows outward from these shapes to increase the length of apparent tubular textures. Although these processes are clearly possible from a phase equilibrium perspective, the Grosch and McLoughlin model (6) has a series of weaknesses: (i) it does not provide any observational evidence for the envisioned retrograde metamorphic path, in particular with respect to the peak temperature; (ii) there is no petrographic support for the presence of key reactants, in particular the precursor phases pyroxene, quartz, and ilmenite or the association of titanite with these phases (in fact, overwhelming evidence from greenschist metamorphosed basaltic glass suggests chloritized glass as the only precursor, specifically including varioles contained in the glass); (iii) although the elaborate precursor morphologies in figure S3B in ref. 6 plays a key role in explaining some tubular-like morphologies, the drawings are not based on documented observations; and (iv) this model cannot be extended to other sites that have not experienced any contact metamorphism. In aggregate, these arguments show that the Grosch and McLoughlin model (6) has little merit in the discussion of bioalteration features in Paleoarchean pillow lavas, as it appears ad hoc for its own samples, and it cannot be applied to previously described locations where such textures have been found (3, 4).
Biogenicity of Putative Cenozoic and Archean Biotextures
The biogenicity of putative biotextures in Paleoarchean chloritized glasses has to be explored in the context of biogenicity arguments for their unmetamorphosed precursors in Cenozoic volcanic glasses, as well as their metamorphic equivalents. In addition, when discussing putative titanite biotextures, it is important to be aware that titanite minerals in glass may be caused by a combination of biotic and/or abiotic processes.
The biogenicity of bioalteration textures in Cenozoic fresh glass is widely accepted (6), but it is important to understand the strengths and weaknesses of the two main arguments used, supporting biogenicity from the actual shape of the textures and from the presence of chemical biomarkers within them (Abiotic and Biotic Glass Alteration and Figs. S1–S4). When discussing these biogenicity issues, one has to be reminded that bioalteration textures are reported as trace fossils (9), with cavities excavated by microbial activity, and not as fossils where the actual organism is preserved, physically or chemically. Hence, arguments for proving biogenicity of these trace fossils should focus on explaining the textures and not the contents of these cavities. Biomarkers within them would be considered chemical fossils within these trace fossils, but they only prove association of life with these trace fossils and not causation of these cavities. In fact, they may have nothing to do with creating the cavity, but they are left behind only by the last microbe occupying the cavity to evade predators. Hence, although the study of such biomarkers clearly is an interesting research topic in glass bioalteration, they do not carry any weight in the biogenicity discussion until a causal relationship is established between the organism and the formation of the cavity.
Biogenicity of microborings has been well established in soil silicates (10) for carbonates (11) and in association with lichens on natural and manmade rock surfaces, including boring by fungi as well as prokaryotes (12, 17). Although much of the processes of microboring remain to be explored, it is reasonable to call on microbial boring by eukaryotes or prokaryotes as a good first-order explanation for biocorrosion features in volcanic glass (2). Biogenicity of these glass alteration textures is further supported by a number of observations and arguments: (i) Many (putative) biotextures in glass have complex “signature shapes,” such as coiled, ornamented, or annulated shapes (9), that are relatively common in biological systems but found lacking during abiotic dissolution and weathering of volcanic glass (Abiotic and Biotic Glass Alteration and Fig. S1); (ii) biotextures are never completely enclosed in volcanic glass, and they always originate on glass surfaces, assuring access to circulating water for any potential microbe that may be responsible for its excavation; (iii) volcanic glass contains many nutrients that are necessary for microbial function and chemolithotrophic energy sources that could be used by microbes, in particular from redox reactions involving the reduced forms of iron, manganese, or sulfur in the glass; and (iv) the presence of biomarkers in excavated cavities links textures to biological activity even though their relevance to the actual excavation process may not be clear.
Key biogenicity arguments, however, rely on textural observations that may be misinterpreted or misunderstood. Key issues include the following:
-
i)
Tubular textures may be confused with filamentous textures, whereby the former is a negative form and the latter is a positive form of the same shape. A positive form like a filament can readily grow from a solution mediated by chemical or biological processes, such as blade-like quench crystals from silicate melts or filaments produced by the production of extracellular polysaccharides by microbes. A negative form, such as a tunnel, cannot “accrete”; it has to be dissolved into glass in a targeted fashion to form up to 100-µm-long tunnels with a nearly constant or regularly annulated diameter of a few micrometers, often in elaborate shapes. Biotextures commonly have sharp edges (e.g., granular alteration in Fig. S2 A and B) that would be rounded off by an abiotic dissolution process. Formation of such features requires a directed excavation process, as it is commonly observed for microbial excavation of carbonates (11).
-
ii)
When using textural arguments, it is important to distinguish the goal of proving biogenicity for a type of cavity in general or as a specific feature that has been formed by a known organism. General biogenicity is relatively well established in silicates and carbonates, but there are currently no ideas about specific microbes involved in glass bioalteration.
-
iii)
“Signature textures” such as complex tunnels with annulation, bifurcation, or helical shapes (e.g., ref. 9) have to be distinguished from the more generic shapes, such as granular alteration. Signature textures offer stronger proof of biogenicity than simple shapes that one could envision of having been formed by abiotic surface pitting of volcanic glass. Simple shapes are commonly interpreted as biogenic, in part because the process itself has been validated by the more complex signature fossils in the same rock sample.
-
iv)
It is key to biogenicity interpretations that the textural context is known: Biotextures are not and cannot be completely enclosed in glass; all of them are related to surfaces that provide physical connectedness to circulating water. This connectedness is a key requirement for the biogenicity interpretation in Cenozoic textures (2) and in Archean titanite replacement textures (3, 4).
Expanding the biogenicity discussion to titanite replacement textures adds more complexities in terms of the probability of preservation and quality of preservation. The rarity of obvious biogenic titanite textures suggests that the probability of their preservation is very low. Conditions for focusing titanite crystallization along the inner surfaces of biotextures have to be just right to allow for preservation. In addition, the overall conditions of metamorphism have to be right so deformation and degree of recrystallization are minimal and do not completely mask micrometer-scale bioalteration textures. Furthermore, metamorphism may destroy the critical context of biotextures, in particular by healing cracks and obscuring grain boundaries by recrystallization. All of these issues may be compounded by complex metamorphic histories, one of which may be given in the case of Grosch and McLoughlin (6), where early regional metamorphism may have been overprinted by local contact metamorphism.
Grosch and McLoughlin (6) rejected biogenicity of titanite textures in their drill core partly because they did not observe signature textures. We suggest here that this is due to its original scarcity as precursor fossils and its low probability of preservation. Quantitative studies of bioalteration texture abundance in Cenozoic glasses have shown that by far the most abundant bioalteration features are granular textures, making up more than 90% of all textures observed (1, 18). The remaining 10% (or less) are made by tubular or tunnel-like cavities, whereby the most distinctive “signature” types of tunnels, spirals, annulated, or decorated tubes, or bifurcating tubes, are only a miniscule fraction of that 10%. In fact, some of the most spectacular signature textures are unique, found only (to our best knowledge) in one particular occurrence of a paleo-oceanic sequence of the Troodos ophiolite, Cyprus (figures 1, 4, and 5 in ref. 9). This shows that the chances of finding such features are extremely low for metamorphic rocks in general and in particular in a limited sampling effort in one 180-m drill core. Absence of titanite-based signature fossils in one site does not prove absence of biocorrosion everywhere else.
Lastly, it is also important to recognize that some of the more complex biotic–abiotic combinations of microtextures found in Cenozoic glasses may offer significant interpretative challenges after they have been transformed into titanite microtextures. As a case in point, we describe the textural relationships of two potential precursors to such titanite microtextures: tubular bioalteration textures and varioles in volcanic glass. Varioles are near-spherical or oval quench textures commonly formed in basaltic glass as rapid spherulitic growth of clinoyroxene (and/or feldspar) fibers and magnetite (Fig. 1C). Varioles commonly occur in the outermost 1–5 mm of pillow margins, where they range in size from ∼5–50 microns, and occur as isolated occurrences, in bands, or they may coalesce into opaque glass or tachylite (13, 18–21). Bioalteration textures may show an intimate textural relationship with such varioles (Fig. 1C), where tubular features enter the volcanic glass at a crack and apparently seek out and connect with some, but not all, of the varioles. Tubules may originate at a variety of angles from the crack and curve in a systematic way toward the spherules, at times coalescing into a bundle of individual tubes. The texture illustrated for biotextures in volcanic glass in Fig. 1C shows remarkable similarities to Fig. 1D, which is taken from figure S2f of Grosch and McLoughlin (6). Without the benefit of studying the thin section itself, we suggest that our interpretation is at least a possible explanation for these textures, and hence there is a potential complex titanite microtexture that could have been formed by biotic and by abiotic processes. This interpretation is further supported by our own observations from other rocks from the Hooggenoeg Complex, where titanite-replaced varioles are very common. Grosch and McLoughlin (6) describe globular titanite textures in many of their samples, but they do not consider nor rule out possible variolitic precursors. Inadvertently including varioles with biotextures into one “morphological continuum” (6) negates its value as a reasoning in a biogenicity discussion.
Tube Diameters in Cenozoic Biotextures and Their Titanite-Based Replacements
Grosch and McLoughlin (6) reject biogenicity of the Hooggenoeg pillow lavas because diameters of their tubular textures appear to be much larger than the diameters measured by Furnes et al. (22) in a small number of seafloor drill sites.
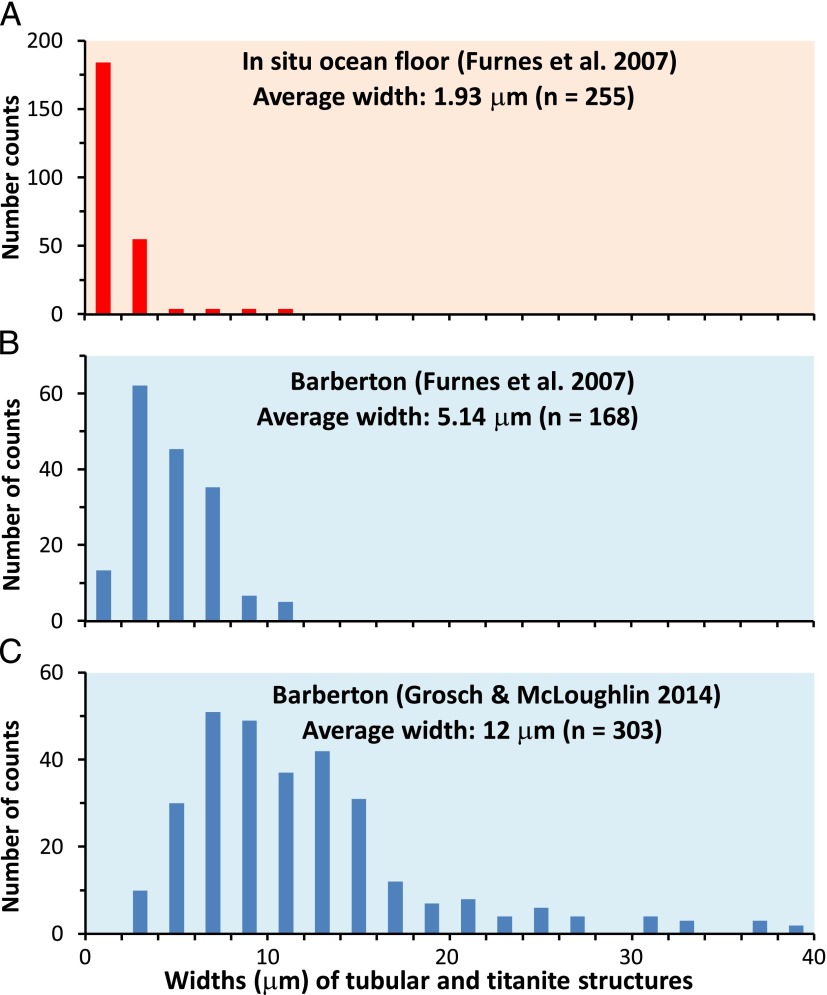
Before reanalyzing the size data in figure 2 of Grosch and McLoughlin (6), it is pertinent to note that earlier investigators cautioned that titanite replacements of tubes are likely to be bigger than the original ones (e.g., refs. 6, 16) simply due to their origin by titanite crystal growth in the walls of tubular textures rather than by simply filling the void. Furthermore, it is important to note that tube diameters in volcanic glass show a substantial range that goes beyond the measurements carried out in a few seafloor drill sites (22). For example, the first description of such microtunnels in subglacial hyaloclastites in Iceland suggested diameters of about 8 µm (7), and examples from the Troodos ophiolite reach up to 30 µm (23). Although the preponderance of tubular textures in basaltic glass cluster around 2 µm, natural occurrences have a much wider range than implied by Grosch and McLoughlin (6).
In Fig. 2 we have compared the tube diameter data from a selection of seafloor drill sites (Fig. 2A) and Paleoarchean titanite textures (Fig. 2B) (22) with the tube diameter data in the BGB drill hole (Fig. 2C) (6). We note several discrepancies between figure 1 in Grosch and McLoughlin (6) and the original published histograms (2): The average size of fresh seafloor tube diameters from ref. 22 is 1.93, whereas ref. 6 cites 1.3 µm for the ref. 22 dataset. Tube diameter data for biotextures in Cenozoic glass in five ocean floor drill sites (Fig. 2A) substantially overlap with titanite microtextures measured in BGB lavas of ref. 22 as well as the BGB drill hole (6). Hence, the size comparison provided by ref. 6 is somewhat misleading, as it underestimates the original size variation and overstates the difference to the titanite textures. For these reasons, we suggest that the differences in tube diameters, as they are reported to date, have very little, if any, bearing on biogenicity arguments. The observed size differences are well explained by the very large natural range in tubular textures in Cenozoic seafloor glasses, with a likely widening of tube diameters during replacement by titanite.
Fig. 2.
Comparison between widths of tubular structures from (A) glassy margin of Cenozoic pillow lavas from in situ oceanic crust and titanite structures from the Hooggenoeg Complex. (B and C) Size of tubular structures rooted in fracture (A and B data are from ref. 22) and (C) filamentous and globular structures (unrelated to fractures) shown in figure 1I of ref. 6.
Antiquity of Paleoarchean Trace Fossils
Recent U/Pb isotopic ages of titanite microstructures in the BGB pillow lavas (6) yielded much younger ages than an earlier study (24), and this discrepancy was used to reject biogenicity of putative biotextures in BGB pillow lavas (6). To explore these issues, we have to understand the duration of glass bioalteration in Cenozoic ocean crust, the age differences between BGB pillow lavas and their titanite biotextures, and how titanite ages may or may not be used to understand their biogenicity.
Seafloor bioalteration has never been dated directly, and its duration can only be inferred indirectly. Arguing by the intensity of fluid flow in newly emplaced oceanic crust, one might suggest that bioalteration occurs only within a few million years of formation of the oceanic crust. However, in reality, microbial corrosion of glass may take place any time as long as there is any fluid circulation through the oceanic crust and fresh glass is available. We know that fresh glass can be found in any age ocean crust, even the 165-Ma-old ocean floor at ocean drilling project site 801C (25). So the critical termination age is linked to the circulation of water through the crust, which is commonly assumed to terminate at 65 Ma, but slow circulation of water may occur much longer, throughout the life of the oceanic crust until it is subducted (26). Although no dates are available for bioalteration in Cenozoic volcanic glasses, their formation is likely to begin nearly synchronously with the formation of the oceanic crust and may continue for over 160 Ma after that.
Much of the current discussion revolves around the geological history of the BGB and how to interpret the recently emerging discrepancies in titanite U/Pb geochronological data (6, 24). The early geological history of Hooggenoeg pillow lavas is well known (12, 27): They erupted at >2 km ocean depth between 3.47 and 3.46 Ga and underwent in situ hydrothermal metamorphism. Then they were uplifted and eroded to within 70 m below a regional unconformity and then overlain by shallow water to subaerial terrestrial sediments dated at ca. 3.458 Ga. U/Pb titanite ages for biotexture replacements in samples in the BGB yielded an age of 3.342 ± 0.068 Ba (24), postdating the Hooggenoeg eruption age by a time period that is consistent with the duration of bioalteration of in situ oceanic crust. During this time, the BGB is likely to have been submerged based on global heat loss arguments (28) and the geology of overlying sediment (29). The BGB does not offer any evidence for any major geological events until the intrusion of some dolerite–diorite dikes at shallow crustal depth just before the deposition of the unconformably overlying marine deposits of the Transvaal Group (max. age 2.7–2.8 Ga). Grosch and McLoughlin (6) dated titanites from one of those dikes and the Hooggenoeg pillow lavas 18–19 m away from the intrusion where titanites potentially replace biotextures in chloritized glass. The 85-m-thick dolerite–diorite dike and the pillow lavas 18–19 m away from the dike yielded a nicely clustering U/Pb titanite age distribution at ca. 2.9 Ga, about 450 Ma later than earlier studies of biotexture-replacing titanites from a sample that was taken 55–60 m away from this dike (24). Grosch and McLoughlin (6) interpreted these data such that these titanites were formed as a retrograde mineral subsequent to contact metamorphism from the dike intrusion and rejected earlier interpretations that the more distant samples reflect biotexture replacements shortly after formation of these pillow lavas.
An alternate explanation of the discrepancies in titanite U/Pb ages of the samples close to the 85-m dike (6) suggests that they were thermally reset by contact metamorphism during dike intrusion and the more distant samples (24) retained their ages because they were not reheated as extensively. We explored the potential for such a scenario using numerical models for the contact aureole of a similar dike intrusion, a 100-m basaltic dike intruded into the Karoo shales in South Africa (29). These models suggest peak contact metamorphic temperatures of 570 °C at a 20-m distance to the dike margin, which is in general agreement with the >600 °C peak temperature envisioned by Grosch and McLoughlin for their samples 18–19 m from the dike contact (see figure S3B of ref. 6). At 60 m distance, the approximate distance of the previously dated titanite biotextures (24), the numerical models (29) suggest a temperature of about 350 °C, about 225 °C lower than the maximum temperature at the proximal location. The high temperatures near the contact and the steep thermal gradient away from the dike suggest that the sample closer to the dike margin may well have been heated above the closure temperature of titanite, whereas the more distant one stayed below it.
Closure temperatures of titanite are commonly cited as >600 °C (30), but Hooggenoeg titanites might have lower closure temperatures because they are smaller and skeletal compared with the larger euhedral crystals used in other studies. The closure temperature of a mineral is a function of crystal size relative to the diffusive radius of the element considered. The BGB titanites (6, 24) are generally smaller than 50 µm, which lowers the experimentally determined closure temperature to 550 °C and below for the smaller ones (30). Furthermore, BGB titanites never reached temperatures to anneal any radiation damage (>800 °C) (31), and an unknown extent of radiation damage may further lower their effective closure temperatures. Experimental data on highly radiation-damaged (metamict) titanites suggest closure temperatures as low as 200 °C for a 50-µm crystal size (30). Although the extent of radiation damage within BGB titanites remains unknown, it is clear that the closure temperatures of most BGB titanites are at, or lower than, 550 °C, which is below the likely contact metamorphic temperatures within 20 m of the dike but significantly higher than the maximum temperatures reached at a distance of 60 m of the margin.
The above discussion shows that thermal rejuvenation might explain the differences in titanite ages (6, 24). In this scenario, earlier titanite U/Pb age data (24) in the Hooggenoeg Complex pillow lavas correctly represented the age of titanites that replaced biotextures shortly after eruption of the Hooggenoeg lavas. The data in ref. 6, however, likely represent thermal reheating ages, even though this does not exclude that some of the titanites may have formed during intrusion of the dike. For this reason, the much younger ages of proximal samples (6) do not contradict conclusions of antiquity based on earlier age estimates of the more distant samples. However, we note that none of the titanite biotexture replacement ages (6, 24) have any bearing on the antiquity and biogenicity of their precursor fossils because they are unrelated processes.
Lessons Learned
Our discussion of the strengths and limitations of arguments used in supporting biogenicity of putative glass bioalteration trace fossils makes us caution that the biogenicity of trace fossils in fresh Cenozoic glasses and Archean titanite textures has to be carefully argued. Complexities in metamorphic reactions may have a profound impact on whether and how well precursor fossils are preserved in titanite microtextures. U/Pb ages of very small titanites may be thermally reset during later stages of metamorphism, but replacement ages may never be used to prove or disprove biogenicity of their precursors. We disagree with the categorical rejection of biogenicity for all previous descriptions of titanite-based replacement textures. At least some of the more delicate signature textures found in the Hooggenoeg Complex of the BGB (Fig. 1B) and those from the Euro Basalt in Pilbara (Fig. 1F) serve as examples for true biotextures that formed before the metamorphism of these complexes. Continued critical exploration of these textures and verification of their biogenicity remains a very promising line of research that might lead to a better understanding of the deep oceanic biosphere at a time when life began to survive on Earth.
Materials and Methods
Paleoarchean samples discussed in this paper were collected in outcrops of the Euro Basalts of the Pilbara Complex in Australia and the Hooggenoeg Complex along the Komati River, South Africa. Cenozoic in situ seafloor comparison samples come from the Atlantic Ocean, South of Bermuda Rise (sites 417 and 418) and from the Costa Rica Rift in the Easter Pacific Ocean (sites 504B and 896). Textural observations are based on optical and scanning electron microscopy using petrographic thin sections.
Supplementary Material
Acknowledgments
This paper benefitted substantially from the constructive reviews of two anonymous reviewers. We acknowledge funding from Scripps Institution of Oceanography and the Institute for Geophysics and Planetary Physics (to H.S.) and the Norwegian Research Council and the Meltzer Foundation at the University of Bergen (to H.F.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1421052112/-/DCSupplemental.
References
- 1.Furnes H, Staudigel H. Biological mediation in ocean crust alteration: How deep is the deep biosphere? Earth Planet Sci Lett. 1999;166(3-4):97–103. [Google Scholar]
- 2.Staudigel H, et al. 3.5 billion years of glass bioalteration: Volcanic rocks as a basis for microbial life? Earth Sci Rev. 2008;89:156–176. [Google Scholar]
- 3.Furnes H, Banerjee NR, Muehlenbachs K, Staudigel H, de Wit M. Early life recorded in Archean pillow lavas. Science. 2004;304(5670):578–581. doi: 10.1126/science.1095858. [DOI] [PubMed] [Google Scholar]
- 4.Banerjee NR, Furnes H, Muehlenbachs K, Staudigel H, de Wit M. Preservation of ∼3.4–3.5 Ga microbial biomarkers in pillow lavas and hyaloclastites from the Barberton Greenstone Belt, South Arica. Earth Planet Sci Lett. 2006;241(3-4):707–722. [Google Scholar]
- 5.Banerjee NR, et al. Direct dating of Archean microbial ichnofossils. Geology. 2007;35(6):487–490. [Google Scholar]
- 6.Grosch EG, McLoughlin N. Reassessing the biogenicity of Earth’s oldest trace fossils with implications for biosignatures in the search for early life. PNAS. 2014;111(23):8380–8385. doi: 10.1073/pnas.1402565111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thorseth IH, Furnes H, Heldal M. The importance of microbiological activity in the alteration of natural basaltic glass. Geochim Cosmochim Acta. 1992;56(2):845–850. [Google Scholar]
- 8.Furnes H, Thorseth IH, Tumyr O, Torsvik T, Fisk MR. Microbial activity in the alteration of glass from pillow lavas from Hole 896A. Proc Ocean Drill Prog Sci Results. 1996;148:191–206. [Google Scholar]
- 9.McLoughlin N, Furnes H, Banerjee NR, Muehlenbachs K, Staudigel H. Ichnotaxonomy of microbial trace fossils in volcanic glass. J Geol Soc London. 2009;166:159–169. [Google Scholar]
- 10.Jongmans AG, van Breemen N, Lundstrom U. Rock-eating fungi. Nature. 1997;389(6652):682–683. [Google Scholar]
- 11.Golubic S, Radtke G, Le Campion-Alsumard T. Endolithic fungi in marine ecosystems. Trends Microbiol. 2005;13(5):229–235. doi: 10.1016/j.tim.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 12.de Wit MJ, Furnes H, Robins B. Geology and tectonostratigraphy of the Onverwacht Suite, Barberton Greenstone Belt, South Africa. Precambrian Res. 2011;186(1-4):1–27. [Google Scholar]
- 13.Staudigel H, Hart SR. Alteration of basaltic glass: Mechanism and significance to the oceanic crust–seawater budget. Geochim Cosmochim Acta. 1983;47(3):337–350. [Google Scholar]
- 14.Izawa MRM, Banerjee NR, Flemming RL, Bridge NJ. Preservation of microbial ichnofossils in basaltic glass by titanite mineraliation. Can Min. 2010;48(5):1255–1265. [Google Scholar]
- 15.Knowles E, Staudigel H, Templeton A. Geochemical characterization of tubular alteration features in subseafloor basalt glass. Earth Planet Sci Lett. 2013;374:239–250. [Google Scholar]
- 16.Staudigel H, Furnes H, Smits M. Deep biosphere record of in situ oceanic lithosphere and ophiolites. Elements. 2014;10(2):121–126. [Google Scholar]
- 17.Banciu HL. Diversity of endolithic prokaryotes living in stone monuments. Studia UBB Biologia. 2013;LVIII(1):99–109. [Google Scholar]
- 18.Furnes H, et al. Bioalteration of basaltic glass in the oceanic crust. Geochem Geophys Geosyst. 2001;2(8):2000GC000150. [Google Scholar]
- 19.Furnes H. Variolitic structure in Ordovician pillow lava and its possible significance as an environmental indicator. Geology. 1973;1(1):27–30. [Google Scholar]
- 20.Phillips WJ. Interpretation of crystalline spheroidal structures in igneous rocks. Lithos. 1973;6(3):235–244. [Google Scholar]
- 21.Alt JC, et al. 1993. Proceedings of the Ocean Drilling Program Results (Ocean Drilling Program, College Station, TX), Vol 148.
- 22.Furnes H, et al. Comparing petrographic signatures of bioalteration in recent to Mesoarchean pillow lavas: Tracing subsurface life in oceanic igneous rocks. Precambrian Res. 2007;158(3-4):156–176. [Google Scholar]
- 23.Furnes H, Muehlenbachs K, Tumyr O, Torsvik T, Xenophontos C. Biogenic alteration of volcanic glass from the Troodos ophiolite, Cyprus. J Geol Soc London. 2001;158(2):75–84. [Google Scholar]
- 24.Fliegel D, et al. In-situ dating of the Earth’s oldest trace fossils at 3.34 Ga. Earth Planet Sci Lett. 2010;299(3-4):290–298. [Google Scholar]
- 25.Fisk M, Kelley KA. Probing the Pacific’s oldest MORB glass: Mantle chemistry and melting conditions during the birth of the Pacific plate. Earth Planet Sci Lett. 2002;202(3-4):741–752. [Google Scholar]
- 26.Staudigel H. Chemical fluxes from hydrothermal alteration of the oceanic crust. In: Turekian HK, editor. Treatise of Geochemistry, Holland. Vol 4. Amsterdam: Elsevier; 2013. pp. 583–606. [Google Scholar]
- 27.Grosch EG, et al. Paleoarchean detrital zircon ages from the earliest tectonic basin in the Barberton greenstone belt, Kaapvaal Craton, South Africa. Precam Res. 2011;191(1-2):85–99. [Google Scholar]
- 28.Korenaga J. Urey ratio and the structure and evolution of Earth’s mantle. Rev Geophys. 2008;46(2):RG2007. [Google Scholar]
- 29.Aarnes I, et al. Contact metamorphic devolatilization of shales in the Karoo Basin, South Africa, and the effects of multiple sill intrusions. Chem Geol. 2011;281(3-4):181–194. [Google Scholar]
- 30.Cherniak D. Lead diffusion in titanite and preliminary results on the effects of radiation damage on Pb transport. Chem Geol. 1993;110(1-3):177–194. [Google Scholar]
- 31.Lowe DR. Petrology and sedimentology of cherts and related silicified sedimentary rocks in the Swaziland Supergroup, in geologic evolution of the Barberton Greenstone Belt, South Africa. Geol Soc Am Spec Pap. 1999;329:83–114. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.