Significance
Nearly all mammals have tactile hairs. Distinct modular representations of the vibrissae within the central nervous system have been found in five mammalian orders (Marsupialia, Carnivora, Eulipotyphla, Lagomorpha, Rodentia). This work documents vibrissae-related modules in a sixth order: Primata. The presence of subcortical barrel-like modules in the prosimian galagos suggests broad similarities in the processing of sensory information among rodents and primates. Additionally, the thalamic “galagounculus” appears to show the representation of the entire body surface with unusual clarity.
Keywords: vibrissa, barrel cortex, trigeminal, somatosensory maps, primate
Abstract
Galagos are prosimian primates that resemble ancestral primates more than most other extant primates. As in many other mammals, the facial vibrissae of galagos are distributed across the upper and lower jaws and above the eye. In rats and mice, the mystacial macrovibrissae are represented throughout the ascending trigeminal pathways as arrays of cytoarchitecturally distinct modules, with each module having a nearly one-to-one relationship with a specific facial whisker. The macrovibrissal representations are termed barrelettes in the trigeminal somatosensory brainstem, barreloids in the ventroposterior medial subnucleus of the thalamus, and barrels in primary somatosensory cortex. Despite the presence of facial whiskers in all nonhuman primates, barrel-like structures have not been reported in primates. By staining for cytochrome oxidase, Nissl, and vesicular glutamate transporter proteins, we show a distinct array of barrelette-like and barreloid-like modules in the principal sensory nucleus, the spinal trigeminal nucleus, and the ventroposterior medial subnucleus of the galago, Otolemur garnetti. Labeled terminals of primary sensory neurons in the brainstem and cell bodies of thalamocortically projecting neurons demonstrate that barrelette-like and barreloid-like modules are located in areas of these somatosensory nuclei that are topographically consistent with their role in facial touch. Serendipitously, the plane of section that best displays the barreloid-like modules reveals a remarkably distinct homunculus-like patterning which, we believe, is one of the clearest somatotopic maps of an entire body surface yet found.
Nearly all mammals use facial vibrissae as a sensory organ to transduce distant touch. In some species, but most famously in rats and mice, individual whiskers have been shown to be represented as distinct modules in the ascending lemniscal and paralemniscal somatosensory pathways (1). These modules have been termed barrelettes, barreloids, and barrels in the brainstem, thalamus, and cortex, respectively (2–5). The discovery of the barrel pathway has allowed researchers to visualize how somatosensory inputs are anatomically organized in the brain. The barrel pattern provides clear anatomical landmarks that enable further research on the development, connection, functional organization, and plasticity of the somatosensory system. Many investigators have embraced this system in their research programs (for review, see refs. 6 and 7). Beyond the experimental convenience of the system, the nearly perfect correspondence of one whisker to one barrel invokes questions about the development and evolution of nervous systems in general, such as whether development of the organization of sensory systems is largely controlled by properties intrinsic to the central nervous system or largely dictated by the arrangement of peripheral receptors (8, 9). In addition, do whisker modules have a function, or are they just a spandrel (10, 11) that tells us more about the morphology of the periphery and brain development than sensory processing (12)?
Despite the plethora of work examining the function and organization of barrel structures, mostly in mice and rats, similar structures have yet to be identified in primates. Evidence for barrel-like structures in primates would provide a concrete anatomical link between primates and the body of work on rodent somatosensory systems.
Prosimian galagos (Fig. 1A) were selected because galagos have an array of whiskers on the face with muscular and nerve attachments at the base of the whisker follicles (13), suggesting they have some whisking function. In addition, galagos are a member of the most basal clade of primates, one that retained the nocturnal and arboreal lifestyle of early primates (14). We hypothesized the somatosensory system of galagos could resemble that of the common ancestor of primates.
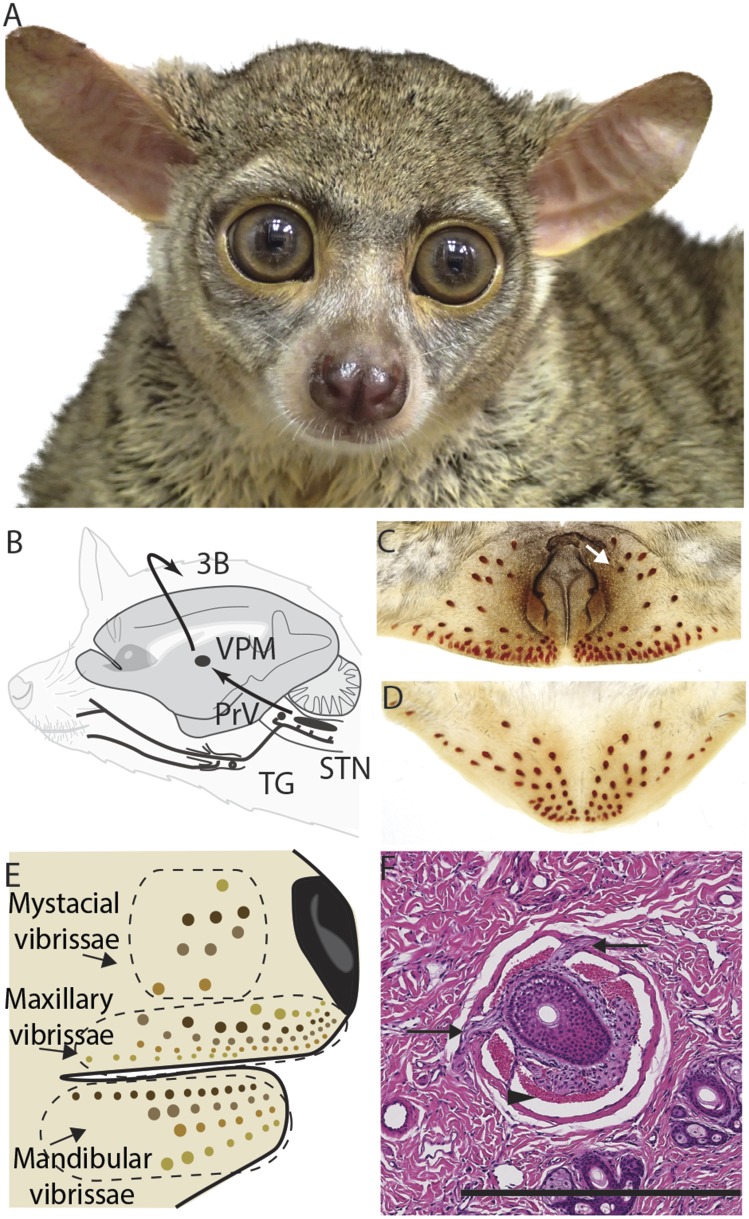
Fig. 1.
(A) An adult galago (O. garnetti). (B) A simplified diagram of the trigeminal somatosensory lemniscal pathway. (C and D) Cleared skin samples of the upper (C) and lower (D) face reveal the blood sinuses of vibrissae. The white arrow in C points to an asymmetrically present blood sinus. (E) Schematic of one side of a galago face indicating the mystacial, mandibular, and maxillary vibrissae. (F) A transverse section of the flattened skin of the face stained for hematoxylin and eosin showing the blood sinuses and innervation of a vibrissae follicle. The arrowhead indicates a blood sinus; arrows indicate nerves innervating the follicle. (Scale bar: 500 µm.) 3b, primate primary somatosensory cortex; STN, spinal trigeminal nucleus; TG, trigeminal ganglion; VPM, ventroposterior medial subnucleus of the thalamus.
The trigeminal lemniscal pathway in galagos, the main pathway leading to the barrels in the mouse and rat cortex, is illustrated in Fig. 1B. Our research systematically examined central nervous system nuclei within this pathway. First, we examined the whisker pattern on the face of galagos (Fig. 1 C and D). Then we used histology and neural tracers to investigate the anatomy and connections of the principal sensory nucleus (PrV) and the spinal trigeminal nucleus subnucelus interpolaris (SpVi), two divisions of the somatosensory brainstem that have distinct barrelette patterns in rodents. We then used the same techniques to investigate the anatomy of the ventroposterior medial subnucleus (VPM), the site of barreloids in the rodent thalamus. Additionally, we related the cortical histology to dense electrophysiolgical multiunit recording in the face representation in contralateral primary somatosensory area 3b, which receives projections from barreloid structures in VPM.
Methods
The PrV, SpVi, VPM, and cortex were examined in nine prosimian galagos (Otolemur garnetti). Four were used for neural tracers and histology (two male; all adult) and five were used for histology only (two male; one juvenile, four adults). All research was conducted in compliance with the guidelines established by the National Institutes of Health and were approved by the Animal Care and Use Committee of Vanderbilt University.
Thalamic tracer experiments were completed in five hemispheres from four animals. Animals were anesthetized with a mixture of ketamine hydrochloride (10–25 mg/kg) and isoflurane [1–2% (vol/vol) isoflurane in oxygen]. Area 3b of cortex was exposed and microelectrode multiunit recordings (1 MΩ at 1 kHz) were used to map receptive fields under ketamine and xylazine anesthesia. Small amounts (0.02 μL) of the neural tracer cholera toxin subunit B (1% CTB; Sigma) in sterile distilled water were injected into selected cortical regions at depths of 600–1,000 μm. Additionally, 10 µL of CTB-HRP (cholera toxin B subunit linked to horseradish peroxidase) was injected into the whisker follicles on the face of one animal. The opening was closed and the animal was monitored in recovery. Prophylactic antibiotics and analgesics were administered postoperatively. After 3 to 5 d, the animals were anesthetized as described above, and cortical mapping was resumed until a dense map of the face region of area 3B was obtained. All galagos were euthanized with sodium pentobarbital and perfused transcardially with phosphate-buffered (PB) saline followed by 2–3% (wt/vol) paraformaldehyde (in PB; pH 7.5). The brain from each animal was removed, blocked, and cryoprotected in 30% (wt/vol) sucrose overnight. Each block was cut on a freezing sliding microtome into 40-μm sections. Cortical blocks were flattened and cut tangential to the pial surface. Brainstem blocks were cut coronally, and thalamic blocks were cut 15° off of horizontal (see Fig. 3H). All brains were processed so that alternate sections highlighted different features. Tetramethylbenzidine immunohistochemistry was used to visualize CTB-HRP. Architectural boundaries were defined with stains for cytochrome oxidase (CO), Nissl substance, or for vesicular glutamate transporter 1 or 2 protein (vGluT1, vGluT2). The specificity of the vGluT antibodies has been confirmed in primates (15, 16). Details of the reagents used for the immunohistocemisty can be found in Table S1. The skin of the face was removed from four animals, of which three were cleared with xylene to remove skin pigmentation while leaving blood sinuses visible (17). One was stored in 10% (wt/vol) formaldehyde and then processed for hematoxylin and eosin.
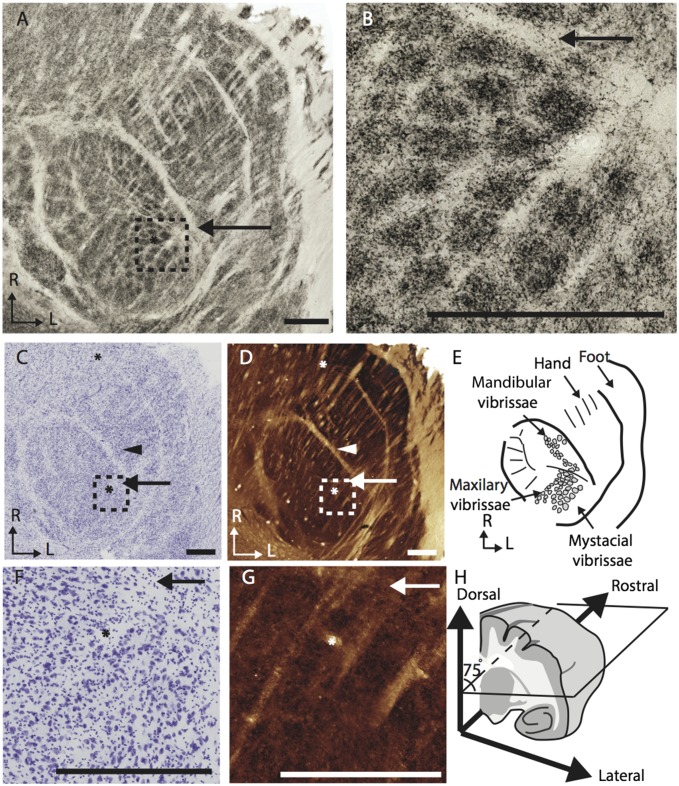
Fig. 3.
(A) Photomicrographs of a section of the ventroposterior nucleus in a galago stained for vGluT2 showing distinct septa between representations of different body regions, including the barreloid-like structures on the face representation and the septa for the digits of the hand. The dotted line in A displays the perimeter of B. (B) Enlarged selections of A showing barreloid-like clumping of vGluT2 positive terminals. (C and D) Adjacent sections stained for Nissl (C) and CO (D). (E) Diagram of the septa seen in A, C, and D showing the “galagounculus”. (F and G) Enlarged selections of C and D showing patchy neuron distribution, and subtle areas of CO dense staining. (H) Schematic of the angle of cut for the sections in A and B, C and D, and F and G. (Scale bars: 500 μm.)
High-resolution images of the processed tissue were obtained by using a SCN400 Slide Scanner (Leica) or a Nikon DXM1200 camera mounted on the microscope Nikon E800 microscope (Nikon). The images were manipulated only for brightness and contrast by using Adobe Photoshop (Adobe Systems). Distributions of labeled cells and terminals were plotted on the images, which were then overlaid on to adjacent sections stained for architectural features.
Results
Vibrissae.
In galagos, the mysticial vibrissae are as thin as the other facial vibrissae, similar in length to some of the caudal vibrissae just above the mouth and on the chin, and are not obviously whisked. Because the prefix “macro” and “micro” is ambiguous in this situation, we refer to galago vibrissae as mystacial, mandibular, and maxillary vibrissae (Fig. 1E).
Galagos have eight or nine mystacial vibrissae just caudal to the nose and numerous vibrissae near the mouth on both the upper and lower jaws (Fig. 1 C–E). The number and distribution of the mystacial vibrissae varied between animals and sometimes between sides of the face on the same animal (Fig. 1C). In the three animals investigated, the four mystacial vibrissae rows, from dorsal to ventral, contained one, three, two to three, and two vibrissae each. The vibrissae above and below the mouth were abundant. The number of maxillary vibrissae ranged from 39 to 51, and the number of mandibular vibrissae ranged from 35 to 37. All tactile hairs were thin hairs, with hair shafts measuring approximately 60 μm in diameter at the base. Mystacial vibrissae were 0.5–1.1 mm long; both the maxillary and mandibular vibrissae were mostly under 0.2 mm in length, although in both sets the most caudal vibrissae measured 0.7–0.8 mm.
Brainstem.
Barrelette-like modules are present in the PrV and caudal SpVi (Fig. 2). In SpVi, the modules are apparent as densely stained circles in sections processed for CO and vGluT1, but were not observed in Nissl stained sections (Fig. 2 E–H). In the PrV, the modules were less clear but still visible as densely stained areas in CO and vGluT1 stained sections. Similar PrV structures appeared as disorganized groups of cells separated by lightly stained septa in Nissl preparations (Fig. 2 A–D). The vGluT2 and CO dense modules were 50–60 µm in diameter in SpVi and 50–90 µm in the PrV.
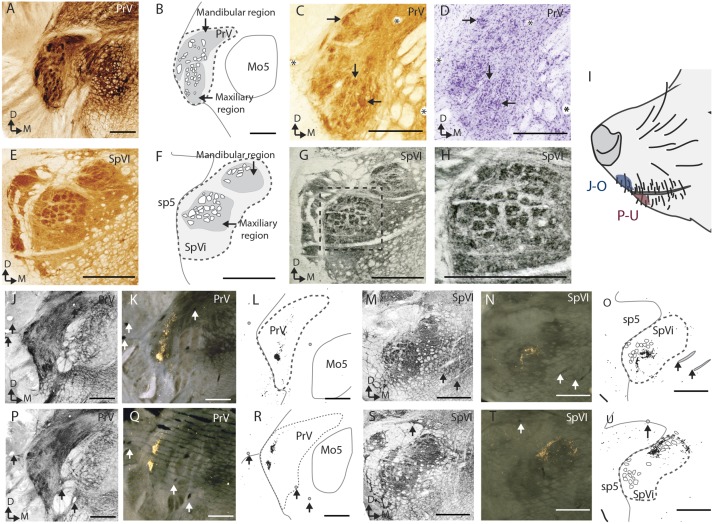
Fig. 2.
(A–D) Histology of the PrV. (A) A coronal section stained for CO showing densely stained modules. (B) A schematic of the anatomy in A. (C and D) Adjacent sections stained for CO and Nissl substance showing the CO dense modules and disorganized clumps of cell bodies. Asterisks mark the same blood vessels and arrows mark barrelette-like modules. (E–H) Histology of SpVi. (E) A coronal section stained for CO showing densely stained modules. (F) A schematic of the anatomy in E. (G and H) Two magnifications of a section adjacent to E, stained for vGluT1 in which modules are visible. (I) The location of the injections of the tracer (cholera toxin subunit B conjugated to horseradish peroxidase). (J–O) The results from the tracer injection into the tip of the upper jaw; a section stained for vGluT1 (SpVi, G; PrV, M), an adjacent section stained for the HRP conjugate of the tracer (SpVi, H; PrV, N), and an overlay schematic of G and H (SpVi, I; PrVm, O). (P–U) The results from the tracer injection into the tip of the upper jaw organized the same way as J–O. Mo5, motor nucleus of the fifth nerve; sp5, spinal tract of the fifth nerve. (Scale bars: 500 μm.)
In both the PrV and the SpVi, there are two separate clusters of the barrelette-like modules, one dorsomedial in the nucleus and one ventrolateral in the nucleus. In both nuclei, an injection of CTB-HRP in the vibrissae follicles of the upper jaw labeled terminals in the ventrolateral region (Fig. 2 J–O) and an injection in the lower jaw labeled terminals in the dorsomedial region (Fig. 2 P–U). The two distinct patches of label in the PrV following the upper-chin injection (Fig. 2Q) may be indicative of label filling two distinct barreloid-like modules. In both the SpVi and the PrV, there are too few barrelette-like structures in any one section for a one-to-one relationship between the vibrissae and the brainstem modules.
Thalamus.
The best plane for visualizing barreloid-like modules in the VPM is 15° off the horizontal plane as illustrated in Fig. 3H. This angle was found based on extrapolating from the rod-like anatomy visible in coronal sections of the VPM and with limited trial and error. In that favorable plane, there are distinct barreloid-like structures that are most apparent in vGlut2-stained sections (Fig. 3 A and B). These darkly stained areas reflect the density of vGluT2 protein in the presynaptic terminals. The barreloid-like structures are also visible in the patchy distribution of cell bodies in sections stained for Nissl substance, yet are barely detectable as darkly stained CO modules (Fig. 3 C, D, F, and G). The vGluT2 dense patches were 65–140 µm in diameter and were visible over multiple adjacent sections, spanning approximately 200 µm. The densely stained modules organized into two distinct groups, with one more caudal and lateral than the other. As in PrV and SpVi, there are not enough barrelette-like structures for a one-to-one relationship between vibrissae and thalamic modules.
In addition to the barreloid-like modules, there was an area with five larger segments in the posteromedial region of the VPM. These measured between 50 and 100 μm in diameter. Cell bodies in this area were labeled after tracer was injected into the tooth representation in area 3b.
Serendipitously, this best plane of cut for the barreloid-like modules also resulted in a galago-specific somatotopic pattern in the ventral posterior lateral subnucleus (Fig. 3E). The likely hand/face border, septa between digits, and a border between the palm of the forelimb and hindlimb were readily visible in all stained sections.
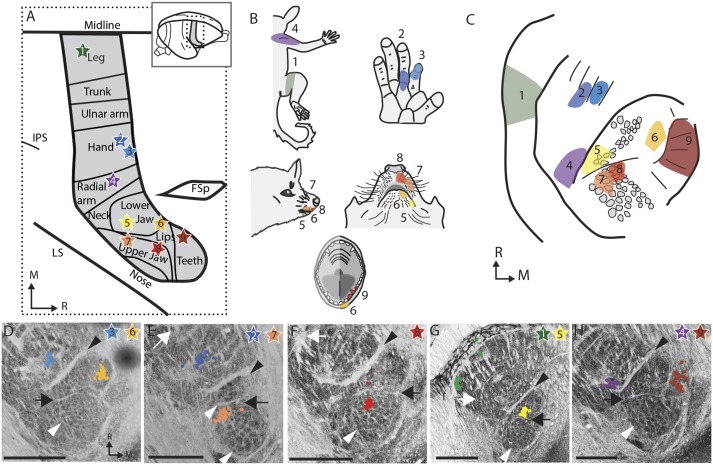
Microelectrode recordings were used to identify injection sites in S1 (area 3b), whose neurons responded to touch on various locations of the face and body. At selected locations, CTB tracer was injected to examine the thalamo-cortical connections. The results from the injection of tracers in area 3b indicate that thalamo-cortical projections are somatotopically arranged in the VPM (Fig. 4). The somatotopy following tracer injections matches the somatotopy suggested by the pattern of septa. Injections into the face representation in cortex (two in the lower jaw, two in the upper jaw, and one in the teeth representations) resulted in labeled cell bodies overlapping the barreloid-like structures in the thalamus. Locations of labeled neurons resulting from the separate injections in the arm, digits of the forelimb, and hindlimb representations confirmed the orientation of the thalamic representation and were consistent with the interpretation of the other major septa between the hand and face, the digits, and the hand and hind limb within VPM.
Fig. 4.
Somatotopy of the connections from the ventroposterior nucleus of the thalamus to cortical area 3b. (A) Summary schematic of the nine cortical injection sites (stars) for the tracer CTB in the five hemispheres investigated (four animals). The placement of the injection sites were defined experimentally with 100–300 points in each cortex as well as reference to ref. 42. The stars are numbered and colored to match the rest of the figure. FSp, posterior frontal suclus; IPS, Intraparietal sulcus; LS, lateral sulcus. (B) The multiunit receptive field recorded at the tracer injection sites. (C) A summary schematic of the locations of CTB-stained cell bodies. (D–H) The location of CTB-stained cell bodies (colored dots) mapped onto the adjacent section stained for vesicular glutamate transporter 2 (vGluT2). D–H are all from different hemispheres. Black arrowheads, hand/face border; black arrow, oral fissure border; white arrowhead, barreloid-like vGlut2 clusters; white arrows, hand/foot border. (Scale bars: 1 mm.)
Cortex.
Dense mapping of the face region of 3b revealed that most of the area devoted to the face representation was responsive to the vibrissae of the upper and lower jaw, whereas only a small region had receptive fields corresponding to the mystacial vibrissae. In the five hemispheres mapped, 387 electrode penetrations had tactile receptive fields on the head, 152 had receptive fields on the vibrissae of the upper lip, 76 on the vibrissae of the lower lip, and only 16 on the mystacial region. We were unable to find barrel-like structures in our histology of sections of the cortex in cytochrome oxidase, Nissl, vGluT2, parvalbumin, and myelin stains of adult and juvenile tissue (both coronal sections and sections cut tangenital to the surface of area 3b).
Discussion
Using histology and labeled connections, we found barrelette- and barreloid-like modules in the brainstem and thalamus of prosimian galagos. As anatomically distinct segments of the central nervous system that represent tactile hairs, they are similar in appearance to the barrel structures seen in rodents. However, there are differences between the modules found in this study and those seen in other animal groups. Regardless, the overarching theme of modular representation of discrete sensory organs is consistent. To our knowledge, such clear whisker-related modules have not been previously reported in primates, making these modules an important link between research on the rodent barrel system and research on primate sensory processing.
Vibrissae.
Our findings on the morphology of the mystacial vibrissae are in agreement with Muchlinski et al.’s recent comparative study (13), in which galagos were found to have discrete rows of mystacial vibrissae with well-developed intrinsic musculature. Our study differed from theirs in that we examined the vibrissae distribution on both sides of the face in three galagos, as opposed to one animal, and also examined the maxillary and mandibular vibrissae. It is unclear whether our unique finding of supernumerary whiskers is biologically significant. If variation in vibrissal arrays applies broadly to this species, then it could be indicative of relaxed selection on a nonvital sensory array. However, animals investigated here all originated in the same research-breeding colony, so the trait could be specific to this population. Vibrissae number is known to vary in between different strains of mice (18).
Another extensive comparative study counted whiskers on the upper jaw of 527 species, including O. garnetti (19). They found that galagos had 22 macrovibrissa and 12 mandibular vibrissae. Even allowing for different definitions of a macrovibrissa and a microvibrissa, this number is less than we counted. The difference is likely due to a difference in methodology; although Muchlinski (19) counted hairs and rete ridge collars visible by external examination of the snout with a dissection microscope, we used a pigment-clearing method to visualize and count blood sinuses innervating individual vibrissae. It is possible that there are more hair follicles with blood sinuses than externally visible rete ridge collars.
Brainstem.
Barrelettes were first defined as a cell-free hollow surrounded by a cell-dense perimeter separated from other barrelettes by a cell-sparse septa, all features that were visible in Nissl-stained sections of mouse brainstem (2). These barrelettes also stain darkly for CO, succinic dehydrogenase, and vGluT1 (5, 20) in rats and mice. Such clear nuclear organization was not observed in galagos, but densely stained CO and vGluT1 fields were identified. As in rats, the barrelette pattern was more easily delineated in SpVi than in the PrV (5). It is possible that the PrV barrelette pattern could be better visualized in sections cut at a slightly different angle, which facilitated the visualization of the PrV somatotopic map in other species (21).
In many species, the individual barrels are larger in the SpVi than in the PrV (5, 22). In mice, barrelettes in the SpVi have a cross-sectional diameter of approximately 140–170 μm and 75–105 μm in the PrV (2). The comparatively large SpVi follicle representations are intriguing, given that the smaller representations in the PrV are the main conduit for driving impulses to the large cortical representation. However, it has been hypothesized that the SpVi is important for monitoring sensations from self-generated movement—something that would be important for whisking species (23). In galagos, the cross-sectional diameter of the modules was much more similar in the SpVi and PrV. It is possible that similar-sized modules in the SpVi compared with the PrV are related to the minor role of vibrissal movement in galago tactile behavior.
The densely stained CO region in SpVi of galagos has distinct dorsal and ventral regions separated by a lightly stained septum. This lima-bean shape differs from the SpVi in mice and rats but looks remarkably similar to the SpVi in wallabies (24). Based on the location of the tracer-stained terminals in the tracer study, we hypothesize that the dorsal region corresponds to the mandibular region and the ventral portion to the maxillary region. The lima-bean shape probably comes from devoting a similar-sized volume of tissue to the representation of both the upper and the lower jaws, instead of the nucleus being dominated by the representation of the upper jaw. The inverted representation is consistent with that found in other animals, including rodents (25, 26), cats (27), moles (28), and pigeons (29).
Previously, macaques and squirrel monkeys have been shown to have a parcellated organization in the PrV and an unparcellated SpVi in CO-stained sections (30). Our results differ from those in that in galagos, the most distinct organization was seen within the SpVi and both nuclei had more distinct modules overall.
Thalamus.
The VP nucleus of the thalamus in primates has architectural features that reflect its somatotopic organization (31–33). For example, a distinct cell-poor septum, the arcuate lamella, marks the border between the hand and face representations. Other septa delineate the representations of the digits, and a more lateral septum separates the hand representation from the foot (34). In the VPM of macaques, there are rod-like segmentations that correspond to facial and oral regions such as the hard palate and regions of the posterior oral cavity (35). Our study adds to those findings by locating barreloid-like modules for facial vibrissae in the VPM of prosimian galagos.
Van der Loos defined mouse barreloids as rod-shaped domains roughly 70 μm in diameter (3), similar to the size of the structures in galagos. In mice, the barreloids have a cell-poor core surrounded by a cell dense perimeter three to four cells thick (3). Later investigations have been able to visualize the barreloids as areas of dense staining for a variety of other reaction products, such as succinic dehydrogenase, CO, and vGluT2 (5, 36). Our procedures did not reveal clear cell-hollow cylinders and only faintly labeled CO clusters; however, we found clear vGluT2 dense clusters. It is interesting that these modules were delineated so strongly by the localization of vGlut2 protein but not by more traditional stains. In rats, vGluT2 is expressed in the synaptic terminations from the PrV (37, 38), and an intact PrV is necessary for barreloid formation (39, 40). The patterning of the vGluT2 clusters in the VPM of galagos suggests that PrV also sends vGluT2-expressing projections to the VPM in primates.
In addition to the barreloid-like segmentation, we located another region within the VPM with larger segments. Based on the location of these segments between the hypothesized lower and upper jaw representations, and considering the location of the label resulting from an injection into the cortical representation of a tooth (Fig. 4), we believe these segments are relevant to the representation of the teeth and mouth. It is possible that these segments are similar to the VPM rods found in macaques (41).
The serendipitous finding of a plane of section resulting in a visible representation of the thalamic galago somatosensory map is, we believe, one of the clearest somatotopic maps of an entire skin surface of a mammal yet reported. Although the presence of a neural representation of the body surface in the somatosensory thalamus is of no great surprise, such a clear visualization reveals the somatosensory order of the representation with notable precision.
Cortex.
The topographic representation of the galago body surface in S1 (area 3b) has been established (42), but the details of the face representation had not been thoroughly investigated. In doing so, we found that the cortical area devoted to the mystacial vibrissae was small compared with the area devoted to the maxillary and mandibular vibrissae. The relative size of the representations of body areas in primary somatosensory cortex (S1) is loosely related to how important that body area is to the animal’s sensory biology. For example, the large representations of lips and hands in humans illustrate how important these regions are to us (43). More specifically, the size of each representation is often related to the sensitivity and acuity of touch for that body area (44, 45). The small size of the mystacial vibrissae representation suggests that these vibrissae are not highly important sensory organs to galagos. Based on the cortical representations, galagos are more invested in the tactile information from the small vibrissae on its chin and lips than the mystacial vibrissae.
Experiments in rats and mice suggest that microvibrissae of the chin and lips are important in object recognition, whereas the longer macrovibrissae are more important for gauging distances of near objects (46). Visual observations of galagos during regular feedings revealed no distinct whisking behavior related to the mystacial vibrissae. Actively foraging galagos were most often observed to reach for food items with their hands and then bring the grasped item to their faces; they infrequently grabbed for food directly with their mouth. It is possible that having grasping hands and improved visual acuity reduces the importance of macrovibrissae.
Our failure to find barrels in the cortex does not guarantee they are not present because the use of other histological markers or sections from an earlier timepoint in galago development could also reveal them. However, our group has performed research on galagos for decades and we have access to a collection of brain sections from these studies. Despite surveying cortex sections from more than 20 cases from previous studies that were processed for various stains (cytochrome oxidase, Nissl, vGluT2, parvalbumin, and myelin stains), cut at various angles (flattened preparations, coronal, and horizontal sections) and in animals of ages 1 d to mature adults, we found no evidence of barrel-like structures. These additional negative results support the conclusion that barrel-like structures are not present in area 3b of galagos.
The presence of subcortical whisker modules in species ranging from marsupial wallabies to primates suggests that mammals share many features of subcortical somatosensory processing. The absence of cortical whisker modules in many species suggests that the cortex may be the most likely site for species-specific changes.
Conclusion
Sensory vibrissae are a part of the tactile sensory system that is present in some form in nearly all species of mammals (excluding monotremes, anteaters, and humans) (19, 47). The phylogenetic distribution of barrel-like modules in the central nervous system is more disparate but is still well distributed among mammals. Here, we demonstrate these modules in the trigeminal somatosensory nuclei of the thalamus and brainstem in a primate. The wide distribution of discrete units in the trigeminal lemniscal pathway suggests that the units form because of a basal and shared trait related to the sensory possessing of the whisker array. The answer to “what is a barrel for?” needs to account for their presence in such a wide range of animals. Progress on this question might be made by investigating differences in sensory processing in an animal with prominent whiskers but without whisker-related modules. Although only mammals possess whiskers, some nonmammals have sensory arrays that, like whiskers, have punctate distributions [e.g., the integumentary sensory organs on crocodilians (48) and barbels on fish and turtles (49)]. It would be interesting to see whether these too are represented by modular units in the brainstem, because such a result would suggest broad commonalities in how the brain organizes sensory input across an even wider range of life.
Supplementary Material
Acknowledgments
We thank Laura Trice for help with histological procedures and Mary Feurtado, Daniel Miller, Jamie Reed, Emily Rockoff, and Iwona Stepniewaska for surgical assistance. This work was funded by NIH Grants NS016446 (to J.H.K.) and NS084706-02 (to E.K.S.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1506646112/-/DCSupplemental.
References
- 1.Bosman LW, et al. Anatomical pathways involved in generating and sensing rhythmic whisker movements. Front Integr Neurosci. 2011;5:53. doi: 10.3389/fnint.2011.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ma PM. The barrelettes—architectonic vibrissal representations in the brainstem trigeminal complex of the mouse. I. Normal structural organization. J Comp Neurol. 1991;309(2):161–199. doi: 10.1002/cne.903090202. [DOI] [PubMed] [Google Scholar]
- 3.Van Der Loos H. Barreloids in mouse somatosensory thalamus. Neurosci Lett. 1976;2(1):1–6. doi: 10.1016/0304-3940(76)90036-7. [DOI] [PubMed] [Google Scholar]
- 4.Woolsey TA, Van der Loos H. The structural organization of layer IV in the somatosensory region (SI) of mouse cerebral cortex. The description of a cortical field composed of discrete cytoarchitectonic units. Brain Res. 1970;17(2):205–242. doi: 10.1016/0006-8993(70)90079-x. [DOI] [PubMed] [Google Scholar]
- 5.Belford GR, Killackey HP. The development of vibrissae representation in subcortical trigeminal centers of the neonatal rat. J Comp Neurol. 1979;188(1):63–74. doi: 10.1002/cne.901880106. [DOI] [PubMed] [Google Scholar]
- 6.Fox K. Barrel Cortex. Cambridge Univ Press; New York: 2008. [Google Scholar]
- 7.Ebner FT, Kaas JH. 2015. Somatosensory system. The Rat Nervous System (Academic, San Diego), 4th Ed, pp 675–701.
- 8.van der Loos H, Dörfl J. Does the skin tell the somatosensory cortex how to construct a map of the periphery? Neurosci Lett. 1978;7(1):23–30. doi: 10.1016/0304-3940(78)90107-6. [DOI] [PubMed] [Google Scholar]
- 9.Kaas JH, Catania KC. How do features of sensory representations develop? BioEssays. 2002;24(4):334–343. doi: 10.1002/bies.10076. [DOI] [PubMed] [Google Scholar]
- 10.Gould SJ, Lewontin RC. The spandrels of San Marco and the Panglossian paradigm: A critique of the adaptationist programme. Proc R Soc Lond B Biol Sci. 1979;205(1161):581–598. doi: 10.1098/rspb.1979.0086. [DOI] [PubMed] [Google Scholar]
- 11.Purves D, Riddle DR, LaMantia AS. Iterated patterns of brain circuitry (or how the cortex gets its spots) Trends Neurosci. 1992;15(10):362–368. doi: 10.1016/0166-2236(92)90180-g. [DOI] [PubMed] [Google Scholar]
- 12.Horton JC, Adams DL. The cortical column: A structure without a function. Philos Trans R Soc Lond B Biol Sci. 2005;360(1456):837–862. doi: 10.1098/rstb.2005.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Muchlinski MN, Durham EL, Smith TD, Burrows AM. Comparative histomorphology of intrinsic vibrissa musculature among primates: Implications for the evolution of sensory ecology and “face touch”. Am J Phys Anthropol. 2013;150(2):301–312. doi: 10.1002/ajpa.22206. [DOI] [PubMed] [Google Scholar]
- 14.Martin RD. Primate Origins and Evolution. Chapman and Hall; London: 1990. [Google Scholar]
- 15.Balaram P, Hackett TA, Kaas JH. Differential expression of vesicular glutamate transporters 1 and 2 may identify distinct modes of glutamatergic transmission in the macaque visual system. J Chem Neuroanat. 2013;50-51:21–38. doi: 10.1016/j.jchemneu.2013.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baldwin MK, Balaram P, Kaas JH. Projections of the superior colliculus to the pulvinar in prosimian galagos (Otolemur garnettii) and VGLUT2 staining of the visual pulvinar. J Comp Neurol. 2013;521(7):1664–1682. doi: 10.1002/cne.23252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haidarliu S, Ahissar E. Spatial organization of facial vibrissae and cortical barrels in the guinea pig and golden hamster. J Comp Neurol. 1997;385(4):515–527. doi: 10.1002/(sici)1096-9861(19970908)385:4<515::aid-cne3>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 18.Van der Loos H, Dörfl J, Welker E. Variation in pattern of mystacial vibrissae in mice. A quantitative study of ICR stock and several inbred strains. J Hered. 1984;75(5):326–336. [PubMed] [Google Scholar]
- 19.Muchlinski MN. A comparative analysis of vibrissa count and infraorbital foramen area in primates and other mammals. J Hum Evol. 2010;58(6):447–473. doi: 10.1016/j.jhevol.2010.01.012. [DOI] [PubMed] [Google Scholar]
- 20.Sakurai K, et al. The organization of submodality-specific touch afferent inputs in the vibrissa column. Cell Reports. 2013;5(1):87–98. doi: 10.1016/j.celrep.2013.08.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Catania KC, Leitch DB, Gauthier D. A star in the brainstem reveals the first step of cortical magnification. PLoS ONE. 2011;6(7):e22406. doi: 10.1371/journal.pone.0022406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Catania KC, Catania EH, Sawyer EK, Leitch DB. Barrelettes without barrels in the American water shrew. PLoS ONE. 2013;8(6):e65975. doi: 10.1371/journal.pone.0065975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Diamond ME, von Heimendahl M, Knutsen PM, Kleinfeld D, Ahissar E. ‘Where’ and ‘what’ in the whisker sensorimotor system. Nat Rev Neurosci. 2008;9(8):601–612. doi: 10.1038/nrn2411. [DOI] [PubMed] [Google Scholar]
- 24.Waite PM, Marotte LR, Leamey CA. Timecourse of development of the wallaby trigeminal pathway. I. Periphery to brainstem. J Comp Neurol. 1994;350(1):75–95. doi: 10.1002/cne.903500106. [DOI] [PubMed] [Google Scholar]
- 25.Arvidsson J. Somatotopic organization of vibrissae afferents in the trigeminal sensory nuclei of the rat studied by transganglionic transport of HRP. J Comp Neurol. 1982;211(1):84–92. doi: 10.1002/cne.902110108. [DOI] [PubMed] [Google Scholar]
- 26.Erzurumlu RS, Murakami Y, Rijli FM. Mapping the face in the somatosensory brainstem. Nat Rev Neurosci. 2010;11(4):252–263. doi: 10.1038/nrn2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hayashi H, Sumino R, Sessle BJ. Functional organization of trigeminal subnucleus interpolaris: Nociceptive and innocuous afferent inputs, projections to thalamus, cerebellum, and spinal cord, and descending modulation from periaqueductal gray. J Neurophysiol. 1984;51(5):890–905. doi: 10.1152/jn.1984.51.5.890. [DOI] [PubMed] [Google Scholar]
- 28.Sawyer EK, Leitch DB, Catania KC. Organization of the spinal trigeminal nucleus in star-nosed moles. J Comp Neurol. 2014;522(14):3335–3350. doi: 10.1002/cne.23605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Silver R, Witkovsky P. Functional characteristics of single units in the spinal trigeminal nucleus of the pigeon. Brain Behav Evol. 1973;8(4):287–303. doi: 10.1159/000124359. [DOI] [PubMed] [Google Scholar]
- 30.Noriega AL, Wall JT. Parcellated organization in the trigeminal and dorsal column nuclei of primates. Brain Res. 1991;565(2):188–194. doi: 10.1016/0006-8993(91)91649-l. [DOI] [PubMed] [Google Scholar]
- 31.Welker WI. Principles of organization of the ventrobasal complex in mammals. Brain Behav Evol. 1973;7(4):253–336. doi: 10.1159/000124417. [DOI] [PubMed] [Google Scholar]
- 32.Kaas JH. 2008. The somatosensory thalamus and associated pathways. The Senses: A Comprehensive Reference, eds Basebaum AI, Kaneko A, Shepherd GM, Westheimer G, Gardner E, Kaas JH (Academic, San Diego), pp 117–141.
- 33.Jones EG. The Thalamus. 2nd Ed Cambridge Univ Press; New York: 2007. [Google Scholar]
- 34.Qi HX, Gharbawie OA, Wong P, Kaas JH. Cell-poor septa separate representations of digits in the ventroposterior nucleus of the thalamus in monkeys and prosimian galagos. J Comp Neurol. 2011;519(4):738–758. doi: 10.1002/cne.22545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rausell E, Jones EG. Histochemical and immunocytochemical compartments of the thalamic VPM nucleus in monkeys and their relationship to the representational map. J Neurosci. 1991;11(1):210–225. doi: 10.1523/JNEUROSCI.11-01-00210.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Louderback KM, Glass CS, Shamalla-Hannah L, Erickson SL, Land PW. Subbarrel patterns of thalamocortical innervation in rat somatosensory cortical barrels: Organization and postnatal development. J Comp Neurol. 2006;497(1):32–41. doi: 10.1002/cne.20969. [DOI] [PubMed] [Google Scholar]
- 37.Ge SN, et al. Coexpression of VGLUT1 and VGLUT2 in trigeminothalamic projection neurons in the principal sensory trigeminal nucleus of the rat. J Comp Neurol. 2010;518(15):3149–3168. doi: 10.1002/cne.22389. [DOI] [PubMed] [Google Scholar]
- 38.Graziano A, Liu XB, Murray KD, Jones EG. Vesicular glutamate transporters define two sets of glutamatergic afferents to the somatosensory thalamus and two thalamocortical projections in the mouse. J Comp Neurol. 2008;507(2):1258–1276. doi: 10.1002/cne.21592. [DOI] [PubMed] [Google Scholar]
- 39.Ding YQ, Yin J, Xu HM, Jacquin MF, Chen ZF. Formation of whisker-related principal sensory nucleus-based lemniscal pathway requires a paired homeodomain transcription factor, Drg11. J Neurosci. 2003;23(19):7246–7254. doi: 10.1523/JNEUROSCI.23-19-07246.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Killackey HP, Fleming K. The role of the principal sensory nucleus in central trigeminal pattern formation. Brain Res. 1985;354(1):141–145. doi: 10.1016/0165-3806(85)90077-x. [DOI] [PubMed] [Google Scholar]
- 41.Jones EG, Hendry SH, Brandon C. Cytochrome oxidase staining reveals functional organization of monkey somatosensory thalamus. Exp Brain Res. 1986;62(2):438–442. doi: 10.1007/BF00238863. [DOI] [PubMed] [Google Scholar]
- 42.Wu CW, Kaas JH. Somatosensory cortex of prosimian Galagos: Physiological recording, cytoarchitecture, and corticocortical connections of anterior parietal cortex and cortex of the lateral sulcus. J Comp Neurol. 2003;457(3):263–292. doi: 10.1002/cne.10542. [DOI] [PubMed] [Google Scholar]
- 43. Penfield WB, E. (1937) Somatic motor and sensorv representation in the cerebral cortex of man as studied by electrical stimulation. Brain 60:389–443.
- 44.Sur M, Merzenich MM, Kaas JH. Magnification, receptive-field area, and “hypercolumn” size in areas 3b and 1 of somatosensory cortex in owl monkeys. J Neurophysiol. 1980;44(2):295–311. doi: 10.1152/jn.1980.44.2.295. [DOI] [PubMed] [Google Scholar]
- 45.Catania KC, Remple FE. Tactile foveation in the star-nosed mole. Brain Behav Evol. 2004;63(1):1–12. doi: 10.1159/000073755. [DOI] [PubMed] [Google Scholar]
- 46.Brecht M, Preilowski B, Merzenich MM. Functional architecture of the mystacial vibrissae. Behav Brain Res. 1997;84(1-2):81–97. doi: 10.1016/s0166-4328(97)83328-1. [DOI] [PubMed] [Google Scholar]
- 47.Pocock RI. On the Facial Vibrissæ of Mammalia. Proc Zool Soc Lond. 1914;84:889–912. [Google Scholar]
- 48.Leitch DB, Catania KC. Structure, innervation and response properties of integumentary sensory organs in crocodilians. J Exp Biol. 2012;215(Pt 23):4217–4230. doi: 10.1242/jeb.076836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fox H. Barbels and barbel-like tentacular structures in sub-mammalian vertebrates: A review. Hydrobiologia. 1999;403(0):153–193. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.