Significance
The land carbon–climate feedback is incorporated into the earth system models that inform current Intergovernmental Panel on Climate Change projections. This feedback is driven by increases in soil microbial decomposition and carbon loss from soils under global change scenarios. The present study shows how trophic interactions in soil can mediate microbial responses to combined global change factors. As soil nitrogen deposition increases, the limitations on fungal growth are alleviated, stimulating total enzyme activity and decomposition rates. However, this process also affects the grazing activity of soil invertebrates. In the absence of nutrient limitation, top-down control by grazing isopods emerges as a dominant control, limiting any increases in fungal activity and carbon cycling.
Keywords: global change, soil feedback, biotic interaction, top-down control, bottom-up control
Abstract
Decomposition of organic material by soil microbes generates an annual global release of 50–75 Pg carbon to the atmosphere, ∼7.5–9 times that of anthropogenic emissions worldwide. This process is sensitive to global change factors, which can drive carbon cycle–climate feedbacks with the potential to enhance atmospheric warming. Although the effects of interacting global change factors on soil microbial activity have been a widespread ecological focus, the regulatory effects of interspecific interactions are rarely considered in climate feedback studies. We explore the potential of soil animals to mediate microbial responses to warming and nitrogen enrichment within a long-term, field-based global change study. The combination of global change factors alleviated the bottom-up limitations on fungal growth, stimulating enzyme production and decomposition rates in the absence of soil animals. However, increased fungal biomass also stimulated consumption rates by soil invertebrates, restoring microbial process rates to levels observed under ambient conditions. Our results support the contemporary theory that top-down control in soil food webs is apparent only in the absence of bottom-up limitation. As such, when global change factors alleviate the bottom-up limitations on microbial activity, top-down control becomes an increasingly important regulatory force with the capacity to dampen the strength of positive carbon cycle–climate feedbacks.
The Earth system models that inform the climate projections of the International Panel on Climate Change incorporate two feedbacks with terrestrial carbon exchanges (1, 2). The CO2 fertilization effect has the potential to partially offset anthropogenic emissions by the stimulation of primary productivity (1). In contrast, climate-induced increases in soil microbial decomposition are expected to enhance atmospheric warming via the land carbon–climate feedback, although the strength of this positive feedback remains a major uncertainty (1). Soil carbon dynamics are particularly sensitive to climate change in forest ecosystems (3), which contain ∼40% (787 Pg carbon) of the global soil carbon pool (2). These soils are generally dominated by basidiomycete fungi (4), which govern the rate-limiting steps in organic matter decomposition via the production of various hydrolytic and oxidative enzymes (5, 6). Elevated temperature is expected to stimulate growth and enzyme production of large, cord-forming basidiomycetes (7, 8), driving functional shifts in soil decomposer communities (9) and enhancing carbon losses from forest sinks (10). Simultaneously, global nitrogen deposition is predicted to double by 2050 (11), and the potential for interactive effects with warming on the activity of belowground communities is well recognized (3, 12, 13). Although the direct effects of these abiotic processes have been studied exhaustively in recent decades (3, 13), the stabilizing effects of biotic interactions are rarely considered in global studies. Indeed, recent advances in our mechanistic understanding of microbial feedbacks to climate change have been generated predominantly from ex situ studies, which use sieved soil that excludes fungal cords and interacting soil biota (10, 14, 15).
In recent years, a growing body of work highlights the potential for biotic interactions to override the initial effects of global change factors on aboveground plant communities (16, 17). Grazing of shrubs by musk oxen and caribou, for example, has been shown to mitigate the warming-induced increases in plant biomass within arctic ecosystems (18). In belowground systems, the grazing of fungal mycelia by soil invertebrates is an analogous trophic interaction, but the functional consequences of this process are poorly understood. Contemporary food web theory asserts that the complexity of the detrital food web will dampen the effects of any top-down interactions in soil, relative to aboveground systems (19), but laboratory-based studies suggest that the selective removal of dominant microbial groups by large invertebrates (isopods in particular) can be an important regulatory force (20). Indeed, simplified (two-species) model systems highlight the potential for interactive effects of warming and invertebrate grazing on fungal cord growth (7, 9), but it remains unclear whether these effects are relevant within natural, complex soil food webs. Despite their potential relevance for the land carbon–climate feedback, the capacity for animal–microbial interactions to mediate decomposition responses to global change remains untested. The failure to incorporate animals and their interactions with microbial communities into global decomposition models has been highlighted as a critical limitation in our understanding of carbon cycling under current and future climate scenarios (21, 22).
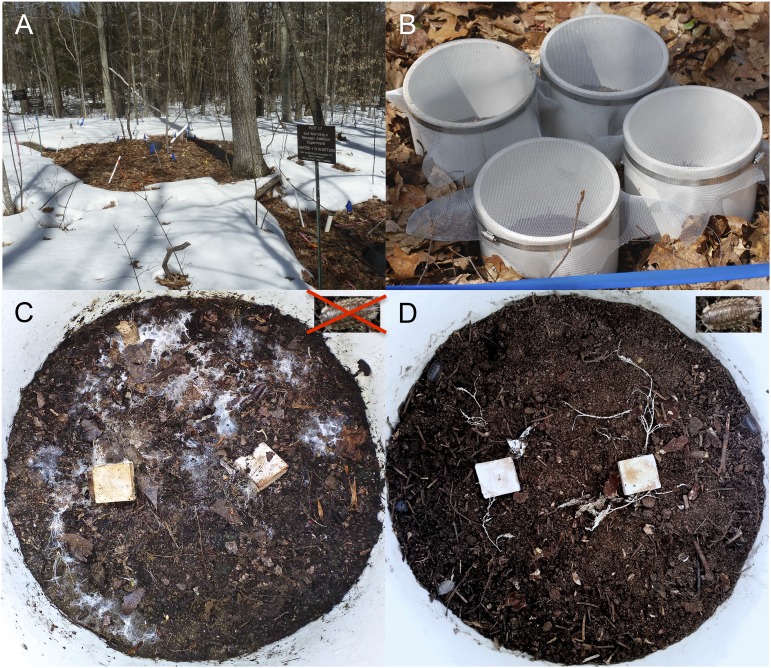
In this study, we explore the potential of grazing soil invertebrates to mediate the interactive effects of soil warming and nitrogen enrichment on microbial (fungal and bacterial) biomass and functioning (extracellular enzyme production and wood decomposition rates) in temperate forest soil. The study was conducted in a multifactor global change experiment (Fig. 1A) established 8 y ago at the Harvard Forest Long-Term Ecological Research (LTER) site. Experimental treatments include two levels of soil warming and nitrogen addition (ambient and elevated), each replicated six times across a fully factorial design, that reflect values predicted by worst-case climate scenarios for the year 2100 (2). We then manipulated soil communities, establishing four biotic treatment chambers within each plot: −fungal cords,−isopods, with isopods and fungal cords removed; +fungal cords,−isopods; −fungal cords,+isopods; and +fungal cords,+isopods (Fig. 1B) to explore whether top-down control of cord-forming fungi by isopod grazers can mediate the direct effects of the abiotic global change factors. We tested the specific hypotheses that (i) the combined global change factors will stimulate soil microbial biomass, enzyme production, and wood decomposition by enhancing the growth of cord-forming basidiomycete fungi, but (ii) by grazing on fungal cords, soil animals will limit increases in fungal growth, thus dampening the direct effects of these abiotic global change factors on the rates of soil nutrient processes.
Fig. 1.
Digital images showing (A) the Harvard Forest warming plots (although the study was conducted in the fall, this image was captured in winter to delineate the warming plots clearly); (B) the arrangement of biotic treatment mesocosms within each abiotic plot; (C) the surface of the soil (under the litter layer) within a warming+nitrogen addition plot in the absence of isopods; and (D) the surface of the soil within a warming+nitrogen addition plot in the presence of grazing isopods.
Results
Effects of Global Change Factors on Microbial Communities (Absent Macroinvertebrates).
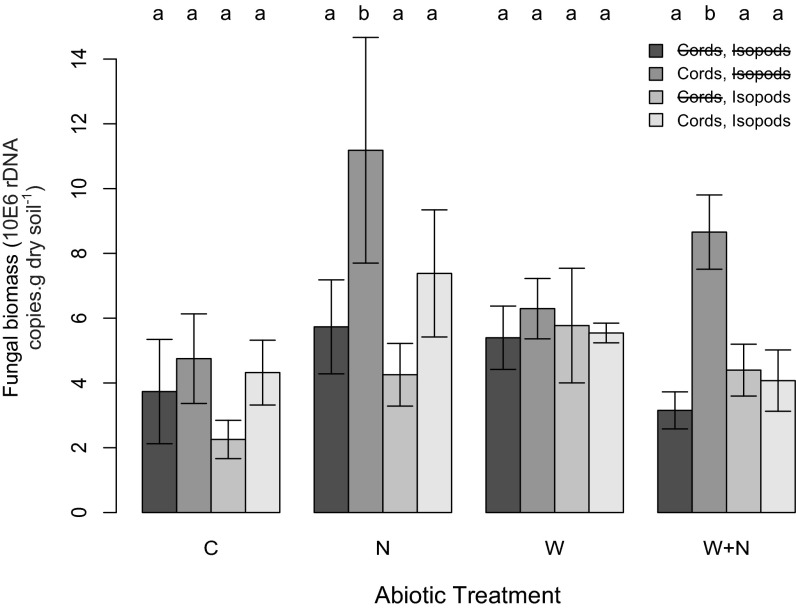
After one growing season (90 d), we harvested soil and wood from all chambers. The biomasses of fungi and bacteria in soil were estimated by quantifying the abundance of microbial gene copy numbers. Bacterial biomass was marginally reduced by soil warming (F1,16 = 4.89, P = 0.042)—an effect commonly observed in long-term soil warming studies (3)—but was unaffected by any other experimental manipulation. In contrast, total fungal biomass in soil was governed by the presence of fungal cords (F1,16 = 17.032, P < 0.001), and both global change factors had effects that were mediated by the presence of fungal cords (Fig. 2). Nitrogen enrichment increased fungal biomass but did so only in plots containing cord-forming fungi (N*Fungal cords; F1,53 = 5.56, P = 0.022) (Figs. 1 and 2). Also analogous to previous work (12), the stimulatory effect of nitrogen addition was marginally dampened by soil warming (N*Warming; F 1, 53 = 3.93, P = 0.049). Nevertheless, because of the overriding positive effect of soil nitrogen addition, fungal biomass in plots containing fungal cords was 1.8 times higher in future conditions (i.e., warming+nitrogen addition plots) than in ambient controls (Fig. 2).
Fig. 2.
Relative fungal sequence copies (mean ± SE) in treatment chambers, estimated using quantitative PCR. Fungal copy numbers were estimated by amplifying the ITS1/qITS2* universal primers. Bar color represents the biotic manipulation (darkest = −fungal cords,−isopods; second darkest = +fungal cords,−isopods; third darkest = −fungal cords,+isopods; lightest = +fungal cords,+isopods) within abiotic treatments (C, control; N, nitrogen addition; W, warmed; W+N, warming+nitrogen addition), showing how the presence of fungal cords enhanced fungal biomass in soil with nitrogen amendment, an effect that was mitigated by isopod grazing. Letters above the bars indicate significant (P < 0.05) differences between treatments. Note that error bars are included, but they cannot accurately represent the variation explained by the random effects of plot and soil moisture in the mixed-effects model.
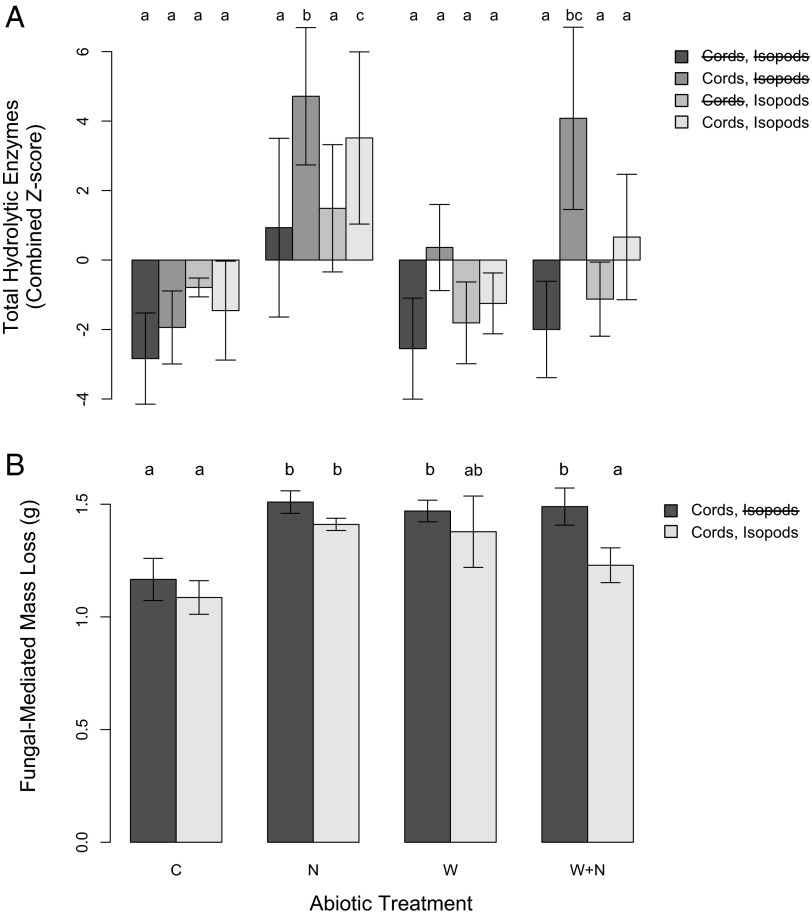
We explored the functional consequences of the microbial biomass changes by estimating the activity of extracellular enzymes [five hydrolytic and three oxidative enzymes associated with the mineralization of carbon, nitrogen, phosphorus, and sulfur-containing molecules (8)] and wood decomposition rates in plots. No treatments influenced the composition (relative abundance) of enzymes in soil. However, as with fungal biomass, the total abundance of enzymes was governed primarily by the presence of fungal cords, with greater hydrolytic (F1,53 = 13.780, P < 0.001) and oxidative (F1,53 = 17.741, P < 0.001) enzyme activity in plots containing cords. In addition, nitrogen addition and soil warming influenced the total activity of hydrolytic enzymes and wood decomposition rates, both following the patterns seen in soil fungal biomass (Fig. 3). Specifically, nitrogen addition enhanced total hydrolytic enzyme production (F1,53 = 4.65, P = 0.036) and the decomposition rates of fungal-colonized wood (F1,16 = 4.74, P = 0.045) but did so only in the presence of cord-forming fungi (Fig. 3). As with fungal biomass, soil warming partially dampened the positive effects of nitrogen enrichment (hydrolytic enzyme activity: N*Warming, F1,53 = 4.64, P = 0.03) and wood decomposition (N*Warming, F1,16 = 10.36, P = 0.005), but the combination of global change factors still enhanced hydrolytic enzyme production and wood decomposition relative to ambient-condition controls (Fig. 3). Although these process responses are highly indicative of increases in organic matter decomposition rates under anticipated global change (5, 8), these effects were all observed in the absence of soil macroinvertebrates.
Fig. 3.
Total standardized hydrolytic enzyme production (A) and wood decomposition (B) rates across all treatments. Z-scores were calculated for all enzymes to minimize the overriding effects of the most active enzymes detected. This value represents the number of SDs from the mean value for that enzyme across all 96 treatment chambers. Standardized values for each enzyme were summed to get an estimate of relative changes in total enzyme production. Values represent means across biotic and abiotic treatments (C, control; N, nitrogen addition; W, warmed; W+N, warming+nitrogen addition). Letters above bars indicate significant (P < 0.05) differences between treatments. Error bars cannot accurately represent the variation explained by the random effects of plot and soil moisture in our mixed effects model. Despite the effects of the global change factors on hydrolytic enzymes, no abiotic treatments significantly influenced the abundance of oxidative enzymes in soil, which were affected only by the presence of cord-forming fungi (F1,53 = 17.740891, P = 0.0001).
Top-Down Control.
The inclusion of isopods in the +fungal cords,+isopods chambers prevented the climate-induced increases in fungal biomass (Fig. 2). The regulatory effect of grazing was apparent only in chambers containing cord-forming fungi (Cords*Isopods; F1,53 = 3.76, P = 0.018), where isopods restored fungal biomass to values observed in cord-removal plots (Figs. 1 and 2). As observed in laboratory-based studies (20), the selective grazing of fungal cords also reduced total hydrolytic enzyme production (F1,48 = 4.28, P = 0.04) and fungal-mediated wood decomposition rates (F1,16 = 5.21, P = 0.036) (Fig. 3). However, these effects of grazing were not apparent under all abiotic scenarios. Although the three-way interaction between isopods, fungal cords, and nitrogen addition was only marginally significant (F1,53 = 3.84, P = 0.055), planned contrasts revealed that isopods affected fungal biomass or functioning only in the plots with added nitrogen (nitrogen addition and warming+nitrogen addition plots) (Figs. 2 and 3). These effects were independent of isopod survival rates, which did not differ significantly between treatments.
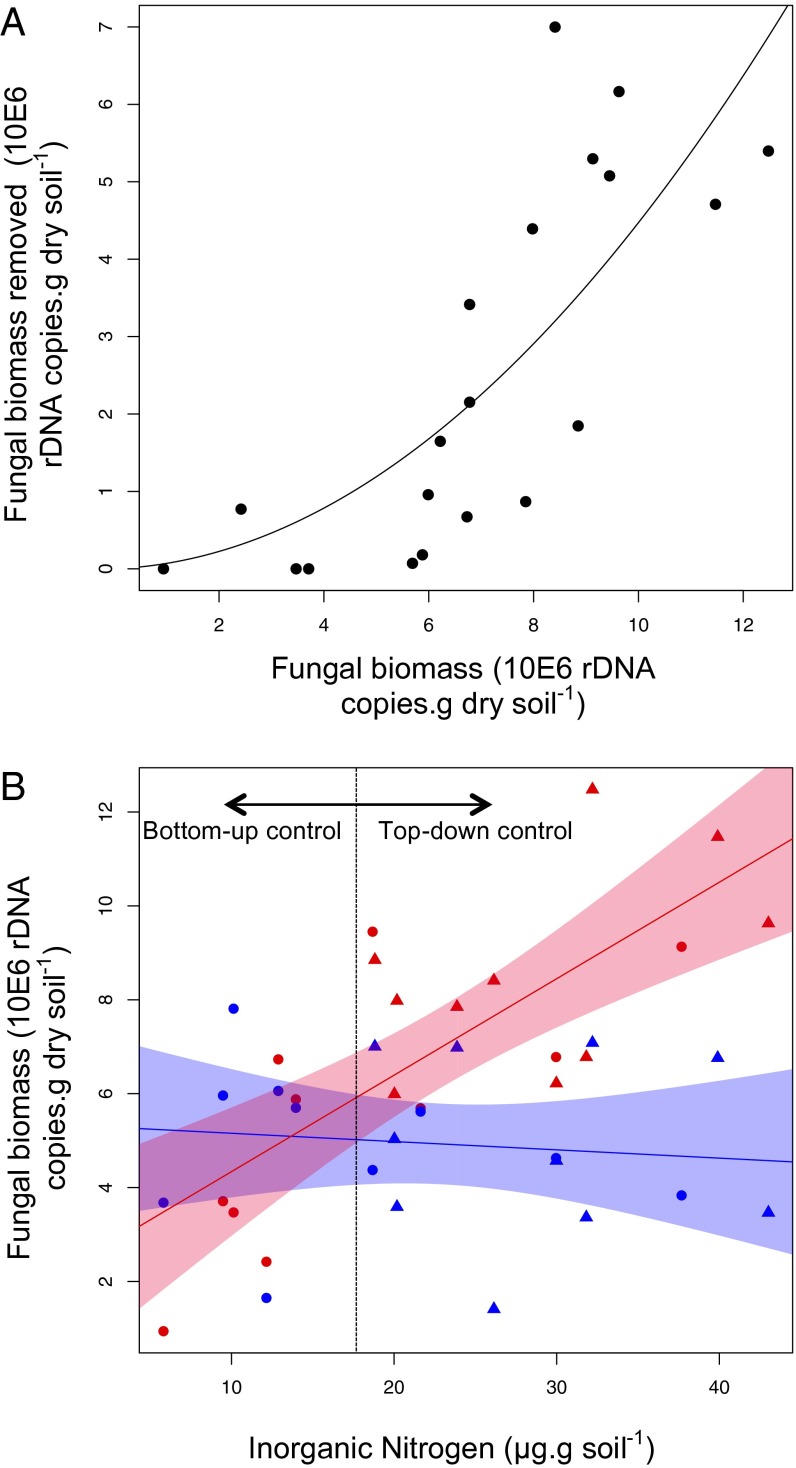
To examine the context-dependent nature of this top-down interaction, we estimated how grazing damage varied with fungal biomass on a continuous scale. The magnitude of grazing damage in each plot was estimated by subtracting the fungal biomass in +fungal cords,−isopods chambers from that in the +fungal cords,+isopods plots. Isopods displayed a distinctly nonlinear consumption pattern, indicative of a type III functional response (Fig. 4). There were no effects of grazing below a fungal biomass threshold of ∼6 μg/g soil, but the proportion of fungus removed by grazers increased with fungal biomass (Fig. 4). This pattern allowed us to identify the conditions under which top-down control emerges as a regulatory force across a gradient of soil nitrogen availability. Under ambient values of inorganic nitrogen availability (0–20 μg/g soil), fungal growth was limited by soil nutrient availability and remained below the threshold for isopod grazing effects. However, as increasing inorganic nitrogen availability alleviated the nutrient limitation on fungal cord growth, increasing rates of grazing damage allowed grazing to emerge as the dominant control on decomposer fungal activity.
Fig. 4.
Functional response of isopods along a gradient of fungal resource availability (A) and the consequences for fungal growth along a gradient of soil inorganic nitrogen availability (B). A shows how isopod grazing damage (estimated by subtracting fungal biomass in +fungal cords,−isopods chambers from that in the +fungal cords,+isopods plots), varies along a continuous gradient of resource availability. The initial lag in consumption rates is indicative of a type III functional response, as proportional grazing rates increase beyond a certain threshold of fungal availability. B shows changes in fungal biomass along an inorganic nitrogen gradient in the absence (red) and presence (blue) of grazing isopods. There were negligible effects of isopods on fungal biomass at ambient levels of nitrogen availability, but increasing fungal availability stimulated the proportional effects of grazing, mitigating the effect of nitrogen enrichment. Dots and triangles represent fungal biomass values in ambient and nitrogen-addition plots, respectively.
Discussion
The present study highlights the potential for trophic interactions to mediate the land carbon–climate feedback. The capacity for grazing animals to regulate climate-induced changes in aboveground plant communities is recognized (18). In contrast, despite the predicted strength of the feedback between global change and soil carbon turnover (10, 23), the regulatory effects of soil interactions have been confined to simplified model systems (7, 9) that cannot address the magnitude, scale, or context dependence of grazing effects. The dearth of studies incorporating soil food web interactions into global change studies likely results from the complex nature of soil communities and their opaque environment. However, by manipulating dominant components of the forest floor community within a multifactor field study, we generate a more nuanced understanding of how soil communities respond to global change, showing that trophic interactions can buffer microbes against the direct effects of abiotic drivers.
The enhanced fungal cord growth and nutrient cycling rates observed in our experiment under future (warming+nitrogen addition) global change scenarios were driven predominantly by nitrogen enrichment. Previous work highlights the idiosyncratic nature of microbial responses to soil nitrogen deposition (13, 24). Although nitrogen enrichment can inhibit fungal activity via the reduction of soil pH and the suppression of lignin-degrading (primarily oxidative) enzymes (13, 25), positive effects often are detected in nitrogen-limited environments (12, 13). By alleviating the nutrient limitation in forest soil, nitrogen enrichment stimulated the growth and dominance of cord-forming basidiomycete fungi. In contrast, soil warming marginally dampened the stimulatory effects of nitrogen. This negative effect of soil warming, recorded previously at this site (14), is likely to be a result of reduced labile carbon substrate availability (14) and physiological adjustment of heterotrophic microbes in warmed soil (26). Nevertheless, because of the overriding positive effect of soil nitrogen addition on fungal cord growth, enzyme activity and wood decomposition rates were consistently higher in future (warming+nitrogen addition) conditions than in ambient controls. These process responses are highly indicative of increases in soil carbon cycling recorded previously under global change scenarios (5, 8). However, the inclusion of isopods within the grazing treatment (+fungal cords,+isopods) restricted the increases in fungal biomass and restored process rates to those recorded under ambient conditions (Fig. 2).
The top-down control of fungal communities was contingent upon optimal growing conditions. Given the regulatory effects of isopods observed under simplified laboratory conditions (20, 27), we expected the top-down process to limit fungal biomass across all abiotic scenarios. However, the present study highlights that regulatory effects of grazers are apparent only above the ambient range of soil inorganic nitrogen concentrations (0–20 μg/g soil). This observation lends support to theoretical assumptions in soil food web models that fungal communities are generally controlled by bottom-up rather than by top-down forces (28, 29). Indeed, the nutrient-limited nature of forest soil is likely to explain the negligible effects of isopods recorded recently under ambient field conditions (30); even high-intensity grazing of mycelial cords cannot affect microbial communities if fungal activity already is limited by the availability of nutrients. However, we show that, as global change factors alleviate abiotic limitations on microbial growth, grazing invertebrates emerge as a dominant control on soil microbial communities and nutrient cycling rates.
Previous work suggests that isopods show a type II functional response when feeding on leaf litter (31). That is, isopod consumption rates increase proportionately with litter availability and plateau beyond a point of satiation. In contrast, when grazing on fungi, isopod consumption rates increased only beyond a threshold of fungal biomass, and the proportion of grazing increased following an initial handling time. This classic type III functional response likely results from the omnivorous nature of these detritivores and the formation of a new search image as fungal cords become more apparent in the upper soil horizons. Although leaf litter is a highly abundant resource for soil invertebrates in forest soil, it represents a relatively low-quality resource with high carbon:nitrogen ratios and lignin concentrations (32). Increasing availability of fungi, with relatively low carbon:nitrogen ratios, is likely to drive a switch in isopod feeding, from leaf litter to fungal cords. A switch in feeding is likely to have direct functional consequences for the turnover rates of organic material in soil: The grazing and shredding of leaf litter increases microbial activity and decomposition rates (28, 31, 32), whereas the direct grazing of fungal cords generally limits enzyme activity and decomposition in forest soil (7, 20, 27). Such a functional switch would reinforce the capacity of soil invertebrates to limit microbial responses to global change as nitrogen enrichment converts their feeding habits from litter consumption (stimulating decomposition) into fungal grazing (limiting decomposition).
We stress that our biotic manipulation focused only on one trophic interaction. A variety of other fauna, including meso- or microinvertebrates, also will contribute to the grazing effects recorded here (7, 33). Indeed, the exclusion of other common macroinvertebrates, such as diplopods, from experimental chambers means that the invertebrate treatments plots are likely to underrepresent the potential for grazers to regulate the fungal community response to global change (34). However, the unique capacity of isopods to sever and ingest thick fungal cords makes this interaction a particularly strong one within forest soil (20). The selective removal of fungal cords by isopods has the capacity to govern the outcomes of fungal interactions (33) and prevent the competitive exclusion of microscopic fungi (including Ascomycota and Zycomycota), thus maintaining microbial diversity as well as biomass in European forest soils (20). Increasing proportional consumption rates recorded along the gradient of soil inorganic nitrogen availability (Fig. 4) indicate that this interaction is likely to become a more important regulatory force under projected global change scenarios. Forest management practices therefore might harness this understanding to regulate soil organic matter responses to global change. For example, the effective management of coarse woody debris, which governs the abundance and activity of soil macroinvertebrates (35), may represent a viable approach for conserving the functioning of forest soils under global change scenarios.
Conclusions
We show that trophic interactions in soil can regulate ecosystem-level responses to global change, even within a highly complex natural food web. Contemporary theory posits that top-down control of soil communities is apparent only when microbial activity is not constrained by other limiting factors (20, 28, 29). We show that, when global change factors alleviate the bottom-up constraints on microbial activity, top-down control is likely to emerge as an increasingly important ecological force. The stabilizing effects of such biotic interactions may contribute to the eventual subsidence of carbon cycling rates observed following initial increases in microbial activity in long-term climate change experiments (10, 15). Global change studies that alleviate the temperature, moisture, or nutrient limitations on microbial activity without incorporating top-down interactions are likely to inflate the projected strengths of carbon cycle–climate feedbacks.
Methods
Study Design.
Soil communities were manipulated within the Soil Warming × Nitrogen Addition study located at the Harvard Forest LTER site in Massachusetts at the beginning of September 2013. Soils in this area consisted of a sandy loam (pH: 5.5; % carbon: 11.57; % nitrogen: 0.63; % sand: 87.4; % silt: 5.0; % clay: 7.6%). The site consisted of a fully factorial design including 3 × 3 m control (C), nitrogen addition (N), soil warming (W), and warming+nitrogen addition (W+N) plots, each replicated six times in a completely randomized plot design. These abiotic treatments were established in 2006. Soil warming was replicated by the use of underground cables that continuously warmed the soil by ∼5 °C above the ambient temperature. Although ambient plots did not have cables inserted, results from an adjacent soil warming experiment showed that the soil disturbance caused by cable installation was minimal and short-lived (36). Nitrogen was added to the nitrogen-amendment plots at a rate of 5 g nitrogen⋅m−2⋅y−1 in the form of an aqueous solution of NH4NO3. The levels of warming and nitrogen deposition were chosen to reflect those predicted by worst-case climate scenarios for the year 2100 (8); see SI Methods for further details of study site and experimental design.
For the biotic treatments, four chambers (PVC tubes; diameter: 20 cm; height: 20 cm) were established within each abiotic treatment plot. Tubes were submerged 10 cm into the ground and fitted with a steel mesh across the top to prevent the migration of fungi or macroinvertebrates (Fig. 1). Soil communities then were manipulated following Crowther et al. (20). Because fungal cords and isopods are highly heterogeneous in woodland soil, chambers were standardized to achieve mean field densities of both groups, in keeping with established approaches in aboveground food web studies (37). Each chamber was standardized initially via the removal of leaf litter, macroinvertebrates, and visible fungal cords from the soil surface. The relatively small size of the chambers and large size of these organisms allowed us to do the extractions by hand, minimizing disturbance to roots, mesofauna, microfauna, and other microbes in the soil (20). The first chamber was left in this state as the −fungal cords,−isopods treatment. Mycelial cords were restored into the second mesocosm by the addition of two 2 × 2 × 1 cm wood blocks precolonized by the highly cosmopolitan basidiomycetes Resinicium bicolor and Phanerochaete velutina. The inclusion of fungal cords increased the total fungal biomass in plots (Fig. 2), but these values were well within the natural range observed at this site (4, 38). This plot allowed the formation of a complete microbial community in the absence of macroinvertebrates (+fungal cords,−isopods). Eight isopods (Porcellio scaber, Latreille 1804) were added to the third chamber to account for the effects of grazers in the absence of fungal cords (i.e., to test whether grazing effects are specific to fungal cords or affect all soil fungi). The experimental density used (254/m2), representing the mean isopod density recorded within our plots, was within the range of field densities reported in the literature for these temperate woodlands (20, 35). The fourth chamber included both isopods and cord-forming fungi (added following the previous treatments) to represent the complete +fungal cords,+isopods treatment. Although this final treatment could not represent an unmanipulated control, it represented a standardized community containing mean field densities of both isopods and fungal cords, along with natural abundances of plant roots, mesofauna, microfauna, and other microbes. Thus we had one −fungal cords,−isopods chamber, one +fungal cords,−isopods chamber, one −fungal cords,+isopods chamber, and one +fungal cords,+isopods chamber within each abiotic treatment plot (4 abiotic treatments × 4 biotic treatments × 6 replicates = 96 plots). No wood blocks were added to the −fungal cords,−isopods or −fungal cords,+isopods chambers to minimize the colonization of other cord-forming, wood-decomposer fungi. The small size of added wood inocula ensured negligible effects of wood block presence on soil in these mesocosms (20). The original leaf litter then was replaced onto the soil surface within chambers, and plots were left for one growing season throughout the fall (90 d). At the end of November 2013, three soil cores (diameter: 2.5 cm; depth: 5 cm) were extracted from the center of each chamber, homogenized, and used to estimate fungal and bacterial biomass (using quantitative PCR), extracellular enzyme activity (five hydrolytic and four oxidative enzymes, using fluorescence and absorbance measurements), and inorganic nitrogen concentrations (using a KCl extraction and flow analyzer) in soil. Wood blocks also were removed from +fungal cords,−isopods and +fungal cords,+isopods treatments to estimate differences in fungal-mediated wood decay (see SI Methods for further information regarding biotic treatments and enzyme and molecular analyses).
Statistical Analyses.
Effects of biotic and abiotic manipulations on fungal biomass, bacterial biomass, fungal:bacterial ratios, soil enzyme activity (oxidative and hydrolytic), and fungal-mediated wood mass loss were assessed using linear mixed effects models and were carried out using the lme function in R's nlme package (version 3.1.108). We standardized enzyme measurements for differences in variance by calculating Z-scores (the number of SDs for the total mean) for each enzyme. Standardized activities of each enzyme then were summed to provide an estimate of total hydrolytic and total oxidative activity for each chamber. Overall models included the categorical treatments (warming, nitrogen, isopods, and fungal cords) along with all possible interactions as fixed effects. Plot ID and soil moisture were included as random terms to account for the nonindependence of chambers located within the same plot. Residuals from all models were checked for normality and homogeneity of variance. Model selection was performed using the dredge function within R’s MuMIm package (version 1.9.13) to identify the most plausible subset of models, ranked by Akaike Information Criterion with a correction for finite sample sizes (AICc) values. Planned contrasts then were performed to test for pairwise comparisons between selected treatments.
The shape of the isopod functional response curve was first assessed via a likelihood ratio comparing models with and without a squared fungal-density term (testing for nonlinearity); second, the frair test function in R’s frair package was used to test statistically for a type II versus a type III functional response curve.
Differences in soil enzyme composition between treatments were visualized using nonmetric multidimensional scaling (NMDS) based on Euclidean distance. NMDS was carried out via the metaMDS function within R's vegan package (39). Each ordination ran for 500 iterations or until the lowest global stress score was found; scores were sufficiently low (<0.05) in all runs for data to be interpreted reliably in two dimensions. Permutational multivariate analysis of variance (PERMANOVA) was used to assess whether treatment groupings apparent in NMDS plots were significantly different. All PERMANOVA tests were carried out via the adonis function in vegan and were based on 4,999 permutations. To assess the extent of unequal dispersion in our datasets, PERMANOVAs were followed by betadisper tests, a multivariate analog of Levene’s test for homogeneity of variances.
Supplementary Material
Acknowledgments
We thank Clara Rowe and Oswald Schmitz for comments on the manuscript, Mel Knorr for help and advice with practical work, and Peter and Peggy Barrett for help and support during practical work. This work was funded by grants from the Yale Climate and Energy Institute and the British Ecological Society (to T.W.C.) and by National Science Foundation (NSF) Grant DEB-1021098 (to M.A.B.). The Soil Warming × Nitrogen Addition study was initiated by NSF Grant DEB-0447967 (to S.D.F.) and is maintained with funding from the NSF Long-Term Ecological Research program.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1502956112/-/DCSupplemental.
References
- 1.Arora VK, et al. Carbon–concentration and carbon–climate feedbacks in cmip5 earth system models. J Clim. 2013;26(15):5289–5314. [Google Scholar]
- 2.Solomon S, et al. IPCC, 2007: Climate Change 2007: The Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge Univ Press; Cambridge, UK: 2007. [Google Scholar]
- 3.Lu M, et al. Responses of ecosystem carbon cycle to experimental warming: A meta-analysis. Ecology. 2013;94(3):726–738. doi: 10.1890/12-0279.1. [DOI] [PubMed] [Google Scholar]
- 4.Crowther TW, et al. Predicting the responsiveness of soil biodiversity to deforestation: A cross-biome study. Glob Change Biol. 2014;20(9):2983–2994. doi: 10.1111/gcb.12565. [DOI] [PubMed] [Google Scholar]
- 5.Bradford MA, et al. Climate fails to predict wood decomposition at regional scales. Nat Clim Chang. 2014;4(7):625–630. [Google Scholar]
- 6.Crowther TW, et al. Untangling the fungal niche: The trait-based approach. Front Microbiol. 2014;5:579. doi: 10.3389/fmicb.2014.00579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.A’Bear D, Boddy L, Hefin Jones T. Impacts of elevated temperature on the growth and functioning of decomposer fungi are influenced by grazing collembola. Glob Change Biol. 2012;18(6):1823–1832. [Google Scholar]
- 8.Baldrian P, et al. Responses of the extracellular enzyme activities in hardwood forest to soil temperature and seasonality and the potential effects of climate change. Soil Biol Biochem. 2013;56(2):60–68. [Google Scholar]
- 9.Crowther TW, Littleboy A, Jones TH, Boddy L. Interactive effects of warming and invertebrate grazing on the outcomes of competitive fungal interactions. FEMS Microbiol Ecol. 2012;81(2):419–426. doi: 10.1111/j.1574-6941.2012.01364.x. [DOI] [PubMed] [Google Scholar]
- 10.Karhu K, et al. Temperature sensitivity of soil respiration rates enhanced by microbial community response. Nature. 2014;513(7516):81–84. doi: 10.1038/nature13604. [DOI] [PubMed] [Google Scholar]
- 11.Galloway JN, et al. Transformation of the nitrogen cycle: Recent trends, questions, and potential solutions. Science. 2008;320(5878):889–892. doi: 10.1126/science.1136674. [DOI] [PubMed] [Google Scholar]
- 12.Xia J, Niu S, Wan S. Response of ecosystem carbon exchange to warming and nitrogen addition during two hydrologically contrasting growing seasons in a temperate steppe. Glob Change Biol. 2009;15(6):1544–1556. [Google Scholar]
- 13.Treseder KK. Nitrogen additions and microbial biomass: A meta-analysis of ecosystem studies. Ecol Lett. 2008;11(10):1111–1120. doi: 10.1111/j.1461-0248.2008.01230.x. [DOI] [PubMed] [Google Scholar]
- 14.Bradford MA, et al. Thermal adaptation of soil microbial respiration to elevated temperature. Ecol Lett. 2008;11(12):1316–1327. doi: 10.1111/j.1461-0248.2008.01251.x. [DOI] [PubMed] [Google Scholar]
- 15.Hartley IP, Hopkins DW, Garnett MH, Sommerkorn M, Wookey PA. Soil microbial respiration in arctic soil does not acclimate to temperature. Ecol Lett. 2008;11(10):1092–1100. doi: 10.1111/j.1461-0248.2008.01223.x. [DOI] [PubMed] [Google Scholar]
- 16.Manning P, et al. Decoupling the direct and indirect effects of nitrogen deposition on ecosystem function. Ecol Lett. 2006;9(9):1015–1024. doi: 10.1111/j.1461-0248.2006.00959.x. [DOI] [PubMed] [Google Scholar]
- 17.Suttle KB, Thomsen MA, Power ME. Species interactions reverse grassland responses to changing climate. Science. 2007;315(5812):640–642. doi: 10.1126/science.1136401. [DOI] [PubMed] [Google Scholar]
- 18.Post E, Pedersen C. Opposing plant community responses to warming with and without herbivores. Proc Natl Acad Sci USA. 2008;105(34):12353–12358. doi: 10.1073/pnas.0802421105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dyer LA, Letourneau D. Top-down and bottom-up diversity cascades in detrital vs. living food webs. Ecol Lett. 2003;6(1):60–68. [Google Scholar]
- 20.Crowther TW, et al. Top-down control of soil fungal community composition by a globally distributed keystone consumer. Ecology. 2013;94(11):2518–2528. doi: 10.1890/13-0197.1. [DOI] [PubMed] [Google Scholar]
- 21.Schmitz OJ, et al. Animating the Carbon Cycle. Ecosystems (N Y) 2013;17:344–359. [Google Scholar]
- 22.Wall DH, et al. Global decomposition experiment shows soil animal impacts on decomposition are climate-dependent. Glob Change Biol. 2008;14(11):2661–2677. [Google Scholar]
- 23.Melillo JM, et al. Soil warming and carbon-cycle feedbacks to the climate system. Science. 2002;298(5601):2173–2176. doi: 10.1126/science.1074153. [DOI] [PubMed] [Google Scholar]
- 24.Janssens IA, et al. Reduction of forest soil respiration in response to nitrogen deposition. Nat Geosci. 2010;3(5):315–322. [Google Scholar]
- 25.Allison SD, Czimczik CI, Treseder KK. Microbial activity and soil respiration under nitrogen addition in Alaskan boreal forest. Glob Change Biol. 2008;14(5):1156–1168. [Google Scholar]
- 26.Crowther TW, Bradford MA. Thermal acclimation in widespread heterotrophic soil microbes. Ecol Lett. 2013;16(4):469–477. doi: 10.1111/ele.12069. [DOI] [PubMed] [Google Scholar]
- 27.Crowther TW, Boddy L, Hefin Jones T. Functional and ecological consequences of saprotrophic fungus-grazer interactions. ISME J. 2012;6(11):1992–2001. doi: 10.1038/ismej.2012.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moore JC, Mccann K, Setälä H, De Ruiter PC. Top-down is bottom-up : Does predation in the rhizosphere regulate aboveground dynamics? Ecology. 2003;84(4):846–857. [Google Scholar]
- 29.Mikola J, Setala H. Productivity and trophic-level biomasses in a microbial-based soil food web. Oikos. 1998;82:158–168. doi: 10.1007/s004420050673. [DOI] [PubMed] [Google Scholar]
- 30.A’Bear D, Boddy L, Kandeler E, Ruess L, Jones TH. Effects of isopod population density on woodland decomposer microbial community function. Soil Biol Biochem. 2014;77:112–120. [Google Scholar]
- 31.Ott D, Rall BC, Brose U. Climate change effects on macrofaunal litter decomposition: The interplay of temperature, body masses and stoichiometry. Philos Trans R Soc Lond B Biol Sci. 2012;367(1605):3025–3032. doi: 10.1098/rstb.2012.0240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dray MW, et al. Effects of elevated CO2 on litter chemistry and subsequent invertebrate detritivore feeding responses. PLoS ONE. 2014;9(1):e86246. doi: 10.1371/journal.pone.0086246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Crowther TW, Boddy L, Jones TH. Outcomes of fungal interactions are determined by soil invertebrate grazers. Ecol Lett. 2011;14(11):1134–1142. doi: 10.1111/j.1461-0248.2011.01682.x. [DOI] [PubMed] [Google Scholar]
- 34.Bradford M, et al. Impacts of soil faunal community composition on model grassland ecosystems. Science. 2002;298(5593):615–618. doi: 10.1126/science.1075805. [DOI] [PubMed] [Google Scholar]
- 35.Topp W, Kappes H, Kulfan J, Zach P. Distribution pattern of woodlice (Isopoda) and millipedes (Diplopoda) in four primeval forests of the Western Carpathians (Central Slovakia) Soil Biol Biochem. 2006;38:43–50. [Google Scholar]
- 36.Melillo JM, et al. Soil warming, carbon-nitrogen interactions, and forest carbon budgets. Proc Natl Acad Sci USA. 2011;108(23):9508–9512. doi: 10.1073/pnas.1018189108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schmitz OJ. 2004. in Insects and Ecosystem Function, eds Weisser WW, Siemann E E (Springer, Berlin), pp 277–302.
- 38.DeAngelis KM, et al. Long-term forest soil warming alters microbial communities in temperate forest soils. Front Microbiol. 2015;6:104. doi: 10.3389/fmicb.2015.00104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Oksanen et al. (2012) Oksanen J, et al. (2013) vegan: Community ecology package. R package version 2.0-10. Available at CRAN.R-project.org/package=vegan.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.