Significance
Enveloped viruses infect cells via fusion of viral and host cell membranes mediated by highly conserved fusion proteins. HIV-1 glycoprotein 41 (gp41) represents a family of fusion proteins with similar structures and fusion mechanisms. They couple their energetic folding to draw two membranes close for fusion, forming trimers of helical hairpins. Yet, the energy release, force generation, and kinetics associated with folding of these proteins are poorly quantified. We found that gp41 hairpins fold sequentially but in a kinetically coupled manner and that an anti-HIV drug blocked gp41 folding by a new mechanism. As major proteins on viral surfaces, fusion proteins are primary targets for vaccine development and fusion inhibitors to intervene in major infectious diseases such as AIDS, Ebola, and influenza.
Keywords: gp41 complex, optical tweezers, protein folding, fusion inhibitor, viral fusion
Abstract
HIV-1 glycoprotein 41 (gp41) mediates viral entry into host cells by coupling its folding energy to membrane fusion. Gp41 folding is blocked by fusion inhibitors, including the commercial drug T20, to treat HIV/AIDS. However, gp41 folding intermediates, energy, and kinetics are poorly understood. Here, we identified the folding intermediates of a single gp41 trimer-of-hairpins and measured their associated energy and kinetics using high-resolution optical tweezers. We found that folding of gp41 hairpins was energetically independent but kinetically coupled: Each hairpin contributed a folding energy of ∼−23 kBT, but folding of one hairpin successively accelerated the folding rate of the next one by ∼20-fold. Membrane-mimicking micelles slowed down gp41 folding and reduced the stability of the six-helix bundle. However, the stability was restored by cooperative folding of the membrane-proximal external region. Surprisingly, T20 strongly inhibited gp41 folding by actively displacing the C-terminal hairpin strand in a force-dependent manner. The inhibition was abolished by a T20-resistant gp41 mutation. The energetics and kinetics of gp41 folding established by us provides a basis to understand viral membrane fusion, infection, and therapeutic intervention.
The HIV-1 glycoprotein 41 (gp41) homotrimer constitutes the transmembrane stem of the envelope glycoprotein complex (Env) and plays key roles in viral entry, the first step of viral infection (1–3) (Fig. 1A). Comprising three gp120/gp41 heterodimers, the Env complex recognizes receptors on target cells and primes gp41 for membrane fusion (4, 5). Gp41 is initially held in a largely unfolded state and shielded by gp120 in the metastable Env complex like a loaded spring (1). During membrane fusion, gp41 inserts its fusion peptide into the host cell membrane with the help of gp120, forming an extended gp41 prehairpin conformation (6, 7) (Fig. 1B). Then the extended gp41 folds back, drawing the two membranes into proximity. In this process, gp41 uses its folding energy to lower the energy barrier of membrane fusion and thus increases the rate of fusion. After fusion, gp41 forms an extraordinarily stable trimer-of-hairpins in a six-helix bundle (6HB) conformation, in which three N-terminal heptad repeats (NHRs) form a central coiled coil and three C-terminal heptad repeats (CHRs) bind in the grooves of the NHR coiled coil in an antiparallel fashion (8, 9) (Fig. 1 B and C). The 6HB further extends to the membrane-proximal external region (MPER) and its complementary fusion peptide-proximal region (FPPR) (10). Although significant progress has been made by defining gp41 structures in the initial and final stages of membrane fusion, how gp41 transits between the two structures remains unclear. Addressing this question requires better determination of the intermediates, energy, and kinetics associated with gp41 folding.
Fig. 1.
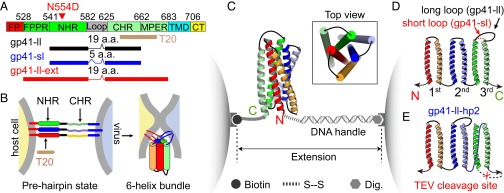
Sequences, conformations, and constructs of gp41 and the experimental setup. (A) Domain structure of HIV-1 gp41. The three truncated gp41 sequences used in this study, the sequence of T20, and T20-resistant mutation (N554D) are indicated. CHR, C-terminal heptad repeat; CT, cytoplasmic tail; FP, fusion peptide; FPPR, fusion peptide-proximal region; MPER, membrane-proximal external region; NHR, N-terminal heptad repeat; TMD, transmembrane domain. (B) Diagram showing gp41 folding and viral membrane fusion. In the prehairpin state, three gp41 monomers form a stable three-helix bundle in their NHRs with largely unfolded CHRs. We refer to the conformational transition from this prehairpin state to the six-bundle (6HB) state as gp41 folding, in contrast to de novo formation of the 6HB from free gp41 monomers as measured in previous ensemble experiments (18). (C) Experimental setup to pull a single gp41 complex using dual-trap optical tweezers. (D) Topology of the construct used to pull the third hairpin in the gp41 complex. Two constructs with different loop sizes were made, gp41-ll with a 19-aa loop and gp41-sl with a 5-aa loop. (E) Topology of the construct to specifically pull the second gp41 hairpin. The construct contained a cleavage site of the TEV protease (indicated by x). After TEV cleavage and purification, the C-terminal pulling site of gp41-ll-hp2 was generated.
Knowledge of gp41 folding is crucial to understanding the mechanisms of viral membrane fusion and infection as well as pharmaceutical intervention in AIDS (11, 12). Membrane fusion needs to overcome an energy barrier over ∼50 kBT (13). A commensurate energy is demanded from folding of one or multiple gp41 complexes to catalyze viral membrane fusion. However, a large gp41 folding energy may also destabilize the metastable Env complex (6, 14). Mutations that decrease or increase gp41 stability in turn stabilize or destabilize the Env complex (15). Thus, the folding energy of gp41 seems to be optimized to balance the rate of membrane fusion and the stability of the Env complex, thereby achieving highest infectivity. However, the folding energy of a single gp41 trimer has not been accurately measured owing to irreversibility and protein aggregation associated with gp41 folding in traditional ensemble-based experiments (16).
Once attached to the host cell, an HIV virion requires at least 15 min to fuse (6, 17). Several gp120-dependent gp41 folding intermediates have been implicated in the fusion process (6, 7). After gp120 is shed, folding intermediates of the gp41 complex alone have not been reported (18). However, intermediates may appear due to independent or cooperative folding of three gp41 monomers. All these intermediates, regardless of their dependence on gp120, are important, because they are essential for membrane fusion and primary targets of anti-HIV drugs, including various fusion inhibitors and broadly neutralizing antibodies (11, 12). In contrast, the conserved gp41 and gp120 regions are protected by gp120 glycan shield on the surface of the Env complex, and thus are not accessible for most drugs and antibodies (1, 2). T20 (enfuvirtide) is the first commercial antiretroviral fusion inhibitor (19, 20). It is a 36-aa synthetic polypeptide that shares its sequence with regions of CHR and MPER in gp41 (Fig. 1A). Thus, T20 competes with these regions to bind their cognate sequences on gp41 to inhibit gp41 folding (Fig. 1B). T20 tightly associates with the five-helix bundle (5HB) missing one CHR with a dissociation constant around 30 nM and efficiently inhibits HIV-1 viral infection (21). However, the efficacy of T20 is compromised by certain gp41 mutations. Gp41 mutation N554D (or N43D based on gp41 amino acid numbering) is a common T20-resistant mutation identified from AIDS patients treated with T20 (22). How T20 efficiently inhibits folding of the wild-type, but not the drug-resistant, gp41 is not well understood.
Gp41 folding is profoundly affected by membranes. Several studies showed that gp41 fails to fold into the 6HB conformation in the presence of membranes or detergent micelles but remains in largely unfolded monomer form (23, 24). However, other studies suggested that gp41 does form the 6HB structure that can even be strengthened by SDS (25). These different reported affinities between gp41 monomers and membranes or membrane mimics inversely correlate with the stability of the 6HB and lead to different models of membrane fusion. In principle, proteins that mediate membrane fusion, including viral fusion proteins and SNARE (soluble N-ethylmaleimide-sensitive factor attachment receptor) proteins, may promote membrane fusion either mechanically or chemically. In the mechanical model, fusion proteins, such as SNARE proteins, transduce their folding energy to mechanical force to draw two membranes into proximity for fusion (26). A single neuronal SNARE complex folds/assembles in multiple stages and outputs a total energy of −65 kBT (27), or −41 kcal/mol. In the chemical model, fusion proteins or chemical agents such as polyethylene glycol alter membrane properties (for example, by dehydration of membranes) to enhance membrane merging without large energy output from folding of fusion proteins, much like strewing salt on snow. However, these two extreme models may not be exclusive. It will be interesting to see whether gp41 fuses membranes similarly to SNARE proteins or by a different mechanism.
Finally, HIV-1 gp41 represents a family of structurally and mechanistically conserved class-I viral fusion proteins, including the glycoprotein GP2 of Ebola virus and hemagglutinin of the influenza virus (1, 4). To our knowledge, none of these proteins has been well studied in terms of their folding energy and kinetics. Therefore, detailed measurements of gp41 folding energy and kinetics will shed light on the working mechanism of these viral fusion proteins and help to develop antiretroviral fusion inhibitors against diseases caused by these viruses (11, 20).
We investigated folding of a single gp41 complex using high-resolution optical tweezers (28). Our single-molecule manipulation approach used mechanical force to unfold the gp41 complex under a physiological solution condition, to probe its folding energy and kinetics, and to mimic the membrane repulsive force during functional gp41 folding. In addition, our method overcame ensemble averaging and synchronization required by traditional protein folding studies that often obscure folding intermediates (27, 29). We found that each gp41 hairpin in the complex folded in a two-state manner and kinetically coupled with other hairpins. The total folding energy of a single gp41 complex was estimated to be −71 kBT. We discovered that T20 inhibited gp41 folding by a novel force-dependent strand-displacement mechanism. Although detergent micelles destabilize the gp41 complex, they do not abolish the complex formation.
Results
Constructs of gp41 Complexes and Experimental Setup.
The ectodomain of gp41 monomer and complex are insoluble in aqueous solutions (30). To facilitate protein preparation and manipulation, we constructed gp41 proteins containing truncated NHR, CHR, and loop (Fig. 1A) and joined the three gp41 monomers into one polypeptide as previously reported (31) (Fig. 1 C and D). We made two gp41 constructs that only differed in the size of the loop kept within the most C-terminal gp41 hairpin, designed as gp41-sl (short loop) and gp41-ll (long loop) with 5-aa and 19-aa loops, respectively (Fig. 1 A and D). Compared with the gp41 hairpin with a wild-type loop (42 aa), the gp41 hairpins with shortened loops were predicted to fold faster, but with similar folding energy (32). We measured the folding energies and rates of both gp41 constructs and extrapolated the loop size to derive the corresponding quantities of the gp41 hairpin with a wild-type loop, which will be described in the following sections. Both constructs were designed to specifically study folding back of the third gp41 hairpin in the presence of two other folded hairpins. We also made other constructs that allowed us to study folding of the second hairpin with the first one folded and the third one unfolded (Fig. 1E) as well as other features of the complex, which will be detailed in the following text.
All constructs contained a unique cysteine at the N terminus and an Avi-tag added to the C terminus. The recombinant gp41 proteins were purified from Escherichia coli, enzymatically biotinylated at the Avi-tag, and cross-linked to a 2,260-bp DNA handle (33) through the cysteine residue. A single gp41 complex was attached at one end to a streptavidin-coated bead and at the other end to an anti-digoxigenin-coated bead (Fig. 1C). The complex was pulled or relaxed by moving one optical trap relative to the other at a speed of 10 nm/s or held at a constant mean force by keeping a constant trap separation. The extension and force of the protein–DNA tether were recorded at 10 kHz and used to derive the conformation and energy of the gp41 complex in real time. Specifically, for a reversible two-state transition, the folding energy of the associated protein domain can be measured based on the mechanical work to unfold the domain, which is equal to the equilibrium force multiplied by the extension change accompanying the transition (33–35).
Folding Energy and Kinetics of a Single gp41 Hairpin.
We first investigated the folding and unfolding transitions of the gp41 construct with a long loop (gp41-ll). We pulled a single gp41 complex up to 50 pN and obtained the force-extension curve (FEC) shown in Fig. 2A and Fig. S1. The FEC contains continuous regions interrupted by discrete extension changes in specific force ranges. In the continuous regions, the monotonic extension increase upon force increase was caused by stretching of the semiflexible DNA handle and unfolded polypeptide while the gp41 complex remained in the same folding state. These regions of FEC were fit well by a worm-like chain model for the semiflexible polymer (36) (Fig. 2A). The fitting yielded contour lengths or numbers of amino acids of the unfolded polypeptides in different gp41 folding states. In contrast, discrete extension jumps or drops resulted from cooperative unfolding or refolding of proteins or protein domains. We found two distinct protein transitions: one reversible transition in a low-force range (8–10 pN) (Fig. 2 A and B) and the other irreversible unfolding in a high-force range (30–50 pN) (Fig. 2A). Based on the contour length of the unfolded polypeptide in each state, the topology of the gp41 construct, and results from the gp41 constructs with mutation N554D (Fig. 2C) and truncation (Table 1 and Fig. S2), we deduced that the first transition was caused by folding and unfolding of the third gp41 hairpin between the 6HB and the 5HB states (states 1 and 2 in Fig. 2A, Inset). In the 5HB state, only the CHR of the most C-terminal gp41 hairpin was unfolded. The transition equilibrated at an average force of 8.9 pN (±0.6 pN, SD throughout the text, n = 75) and an average extension change of 11.0 (±0.6) nm (Fig. 2C and Table 1). The irreversible transition originated from complete unfolding of the remaining 5HB (Fig. 2A, state 3). Accordingly, no additional discrete transitions were observed when the gp41 protein was pulled to even higher forces.
Fig. 2.
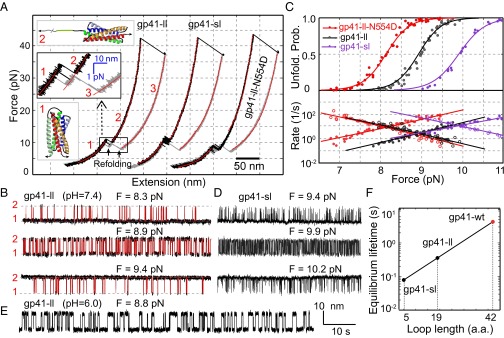
Comparison of folding energies and kinetics of different gp41 complexes. (A) Force-extension curves (FECs) of gp41-ll, gp41-sl, and gp41-ll-N554D. FECs were obtained by pulling (black) and then relaxing (gray) single gp41 complexes and fit by the worm-like chain model (red) in the continuous regions, revealing different gp41 folding states (insets). Data associated with all FECs shown in this work were mean-filtered using a 10-ms time window. A close-up view of the transition region is shown as an inset. (B) Extension-time trajectories of gp41-ll under three different constant mean forces revealing the reversible transition between the 6HB and the 5HB states. The idealized state transitions based on HMM are shown in red. (C) Force-dependent unfolding probabilities (Upper) and transition rates (Lower) for gp41-ll (black), gp41-sl (purple), and gp41-ll-N554D (red). Solid and hollow symbols indicate unfolding and folding rates, respectively. The measurements (symbols) were fit with a theoretical model (lines, see Materials and Methods). (D) Extension-time trajectories of gp41-sl under three constant forces. (E) Extension-time trajectory of gp41-ll at pH 6.0. (F) Loop size-dependent equilibrium lifetimes and the extrapolation to the wild-type loop size of the gp41 hairpin (red dot). The traces in B, D, and E shared the same scale bar.
Table 1.
Average equilibrium force, extension change, folding energy, folding energy barrier, and position and folding rate at the equilibrium force associated with folding of a single gp41 hairpin within the gp41 complex
| Gp41 complex | Equilibrium force, pN | Extension change, nm | Folding energy (kBT) | Transition state energy* (kBT) | Transition state position† (amino acids) | Rate at equilibrium force, s−1 |
| Gp41-ll | 8.9 (0.6) | 11.0 (0.6) | −23.5 (1.2) | 1.9 (1.6) | 26 (2) | 2.8 (1.6) |
| Gp41-ll-CΔ7 | 7.5 (0.7) | 7.8 (0.6) | −16.2 (1.0) | 2.1 (1.8) | 21 (1) | 22.8 (0.2) |
| Gp41-sl | 9.9 (0.8) | 10.2 (0.8) | −23.6 (1.3) | −1.2 (1.1) | 21 (1) | 12.7 (0.5) |
| Gp41-ll-hp2 (CHR2)ǂ | 9.7 (0.6) | 11.4 (1.0) | −24.1 (0.9) | NA | NA | 0.3 (0.1) |
| Gp41-ll-hp2 (CHR3) | 8.6 (0.5) | 13.2 (1.0) | −24.2 (2.0) | 3.4 (1) | 36 (2) | 0.2 (0.1) |
| Gp41-ll + DPC | 6.0 (0.6) | 6.5 (0.9) | −8.4 (3.2) | 10.2 (4.2) | 28 (6) | 0.5 (0.4) |
| Gp41-sl + DPC | 8.6 (0.9) | 8.3 (1.0) | −18.2 (4.2) | 6.2 (1.9) | 26 (2) | 1.3 (1.2) |
| Gp41-ll-ext + Triton | 8.9 (0.7) | 9.7 (1.2) | −22.0 (3.3) | 2.7 (2) | 38 (2) | 1.2 (0.1) |
| Gp41-ll-N554D | 8.1 (0.6) | 10.5 (0.9) | −20.3 (0.7) | 2.5 (2.2) | 25 (2) | 11.8 (1.8) |
The equilibrium force or rate is defined at an unfolding probability of 0.5. The SD of the average is shown in parentheses. NA, not assessed.
The number of amino acids from the C terminus of the CHR in the gp41 hairpin.
ǂThe transition of the second hairpin in this construct.
When relaxed, the gp41 protein remained unfolded until the force dropped to ∼8 pN, leading to a large hysteresis in the FECs (Fig. 2A). Complete refolding occurred in two sequential steps via the 5HB intermediate state (Fig. 2A, Inset). Folding from the fully unfolded protein to the 5HB was irreversible, indicating a great energy barrier for the unfolding/refolding process under our experimental conditions. In contrast, the transition between the 5HB and the 6HB in the relaxation phase was reversible, as seen in the pulling phase. Further relaxation to lower forces (<7 pN) led to the FEC’s overlapping the corresponding FEC in the pulling phase, suggesting complete gp41 refolding (Fig. S1A).
We then held a single gp41 complex under constant mean forces to observe its spontaneous transitions between the 5HB state and the 6HB state (Fig. 2B). Two-state transitions are clearly seen from extension-time trajectories, as were further confirmed by the two peaks in the corresponding extension histogram distributions (Fig. S1B). The equilibrium shifted to the 5HB state as the force increased. We analyzed the extension-time trajectories using a two-state hidden Markov model (HMM) (27, 35, 37), yielding the idealized state transitions that agreed with the measurements (Fig. 2B, red traces). The analyses also revealed the unfolding probabilities and transition rates of the gp41 hairpin (Fig. 2C). The sigmoidal force dependence of the unfolding probability is consistent with a two-state hairpin transition. Accordingly, the folding or unfolding rate decreases or increases approximately exponentially in the force range tested, indicative of a well-defined transition state (34). The unfolding probability and transition rate could be extrapolated to zero force based on a nonlinear force-dependent model previously reported (27, 38) (Table 1). Specifically, folding of a single gp41 hairpin released energy of 23.5 (±1.2) kBT, with a small energy barrier of ∼2 kBT (Fig. S3). The folding energy is consistent with the binding affinity between the 5HB and the CHR peptide (Kd = 0.6 pM, or binding energy ∼28 kBT) (39).
HIV-1 virions enter host cells through plasma membranes or endosomal membranes after endocytosis of the virions (40, 41). Correspondingly, gp41 complexes fold under either neutral (pH 7.4) or acidic (pH 6) conditions. To investigate the possible effect of pH on gp41 folding, we measured the folding energy and kinetics of gp41-ll under a pH value of 6. We found that gp41 folding was not significantly affected by pH under our experimental conditions (Fig. 2E), corroborating pH-independent HIV-1 entry (40, 41).
Folding Energies and Rates of gp41 Hairpins with Different Loop Sizes.
To investigate the effect of loop size on gp41 folding, we further reduced the loop size to 5 aa (gp41-sl). The FECs and the extension-time trajectories measured for this gp41 construct were qualitatively similar to those of gp41-ll (Fig. 2 A and D), suggesting similar folding and unfolding pathways for the two constructs. However, the reduction in loop size caused several quantitative changes in folding of gp41-sl compared with gp41-ll (Fig. 2C and Table 1). The biggest change was a 4.5-fold increase in equilibrium transition rate at the equilibrium force (compare the middle traces in Fig. 2 B and D). As a result, the curve of folding rate as a function of force is shifted toward higher force (Fig. 2C). In contrast, the unfolding rate curve remains approximately unchanged. This is because the loop size decrease did not significantly change the position and energy of the transition state relative to the folded state (Fig. S3). In addition, the average equilibrium force slightly increased to 9.9 (±0.8, n = 17) pN (Fig. 2C). Finally, the average extension change decreased from 11 nm to 10.2 nm, as expected. Accordingly, the folding energy remained approximately the same (Table 1).
Our observations quantitatively agree with previous results obtained by Woodside et al. (32). Using a DNA hairpin with a loop in variable length, they found that the lifetime at the equilibrium force exponentially decreases with the loop length, whereas the folding energy remains constant. Correspondingly, we derived that the lifetime of the gp41 hairpin with a wild-type loop at its equilibrium force was ∼4.3 (±0.6) s (Fig. 2F). In addition, the folding free energy of the hairpin could be calculated as the average of the measured folding energy of the two gp41 constructs (gp41-ll and gp41-sl), that is, −23.6 (±0.9) kBT.
Kinetic Coupling Between Folding of Different gp41 Hairpins.
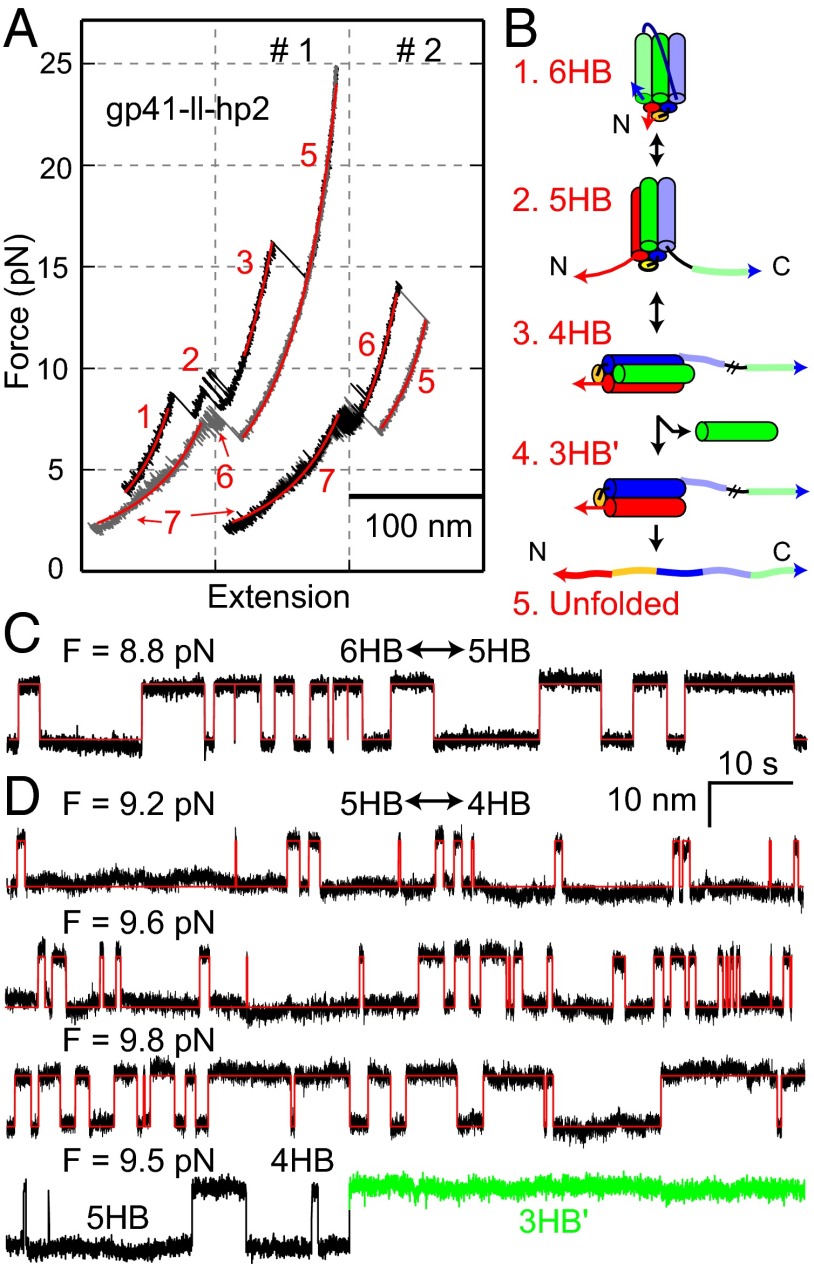
To examine possible coupled folding among three gp41 hairpins within a single gp41 complex, we measured the folding energy and kinetics of the second hairpin, when the third hairpin had not yet folded. To this end, we designed a new gp41 construct (gp41-ll-hp2) that joined the three gp41 hairpins in a topology different from our previous constructs (Fig. 1E). When pulled to a high force, the third hairpin and the second hairpin reversibly and sequentially unfolded in force ranges of 8–9.4 pN and 9–10 pN, respectively (Fig. 3 A and B and Fig. S4), suggesting that folding of the two hairpins was not completely cooperative. This finding allowed us to specifically measure the folding energies and kinetics of both hairpins. Based on the extension changes (Fig. 3 A, C, and D), we derived that unfolding of the third and the second hairpins generates the 5HB and 4HB structures, respectively, both similar to those in the 6HB structure (Fig. 3B). The 4HB state unfolded at a high force ∼17 pN (Fig. 3A), indicating its high stability. Complete unfolding of the 4HB state led to a large irreversible jump. When relaxed, stepwise refolding of the complex was observed at ∼8 pN. However, the gp41 complex did not completely refold into the 6HB conformation, as was seen by a shift of the relaxing FEC to a higher extension compared with the pulling FEC in the force range below 6 pN (Fig. 3A). This observation suggested that the NHR helix in the third hairpin dissociated from the tethered gp41 protein after the complex was completely disassembled, and that this helix was required for full assembly of the gp41 complex (Fig. 3B). The NHR helix dissociation was also infrequently observed under constant forces, which abolished the reversible hairpin transition (Fig. 3D).
Fig. 3.
Folding energy and kinetics of the second gp41 hairpin. (A) FECs obtained by pulling (black) and then relaxing (gray) a single gp41-ll-hp2 for two rounds (#1 and #2) and their best-fits by the worm-like chain model (red). The derived gp41 folding states are illustrated in B. (B) Diagrams of different gp41 folding states corresponding to the FECs in A. Note that the transition from states 3–4 or 5 led to dissociation of the unlinked NHR, and thus is irreversible. The completely unfolded polypeptide robustly refolded into at least two different states (6, 7) missing one NHR, with undetermined conformations. (C and D) Force-dependent extension-time trajectories of gp41-ll-hp2 showing reversible transitions of the third (C) and the second (D) gp41 hairpin under constant forces. The idealized transitions derived from HMM are shown in red. The traces in C and D share the same scale bars.
Our experiments at different forces revealed that both hairpins in gp41-ll-hp2 and the third hairpin in gp41-ll have approximately the same folding energies (Table 1 and Fig. S4), indicating energetically independent folding of the second and the third hairpins. The third and the second hairpins in gp41-ll-hp2 slowly folded and unfolded at the equilibrium rates of 0.2 (±0.1) s−1 and 0.3 (±0.1) s−1, respectively. However, the two hairpins have different loop lengths (19 aa vs. 60 aa) and orthogonal directions of pulling on the 5HBs in the unfolded states (compare state 2 in Figs. 2A and 3B). Therefore, we could not directly compare their folding kinetics. Instead, we compared the second hairpin in gp41-ll-hp2 with the third hairpin in gp41-ll to check possible coupling between hairpin folding, because they had the same loop size (19 aa) and pulling geometry. The second hairpin has an equilibrium transition force (9.7 ± 0.6 pN) and extension change (11.4 ± 1 nm) close to the corresponding parameters of the third hairpin (8.9 ± 0.6 pN and 11.0 ± 0.6 nm), supporting their similar folding energies (Table 1). As the second hairpin refolded, its CHR could bind either of two equivalent vacant grooves in the 4HB. Therefore, the folding rate to each groove is half of the observed rate, leading to an equilibrium rate of 0.15 s−1 for each groove. This transition rate is the lowest among all of the gp41 constructs tested and about 20-fold less than the equilibrium rate of the third hairpin in gp41-ll (Table 1). Therefore, folding of different hairpins is energetically independent, but kinetically coupled: Folding of the second hairpin significantly accelerates folding of the third hairpin.
Detergent Micelles Slow Down and Weaken gp41 Folding.
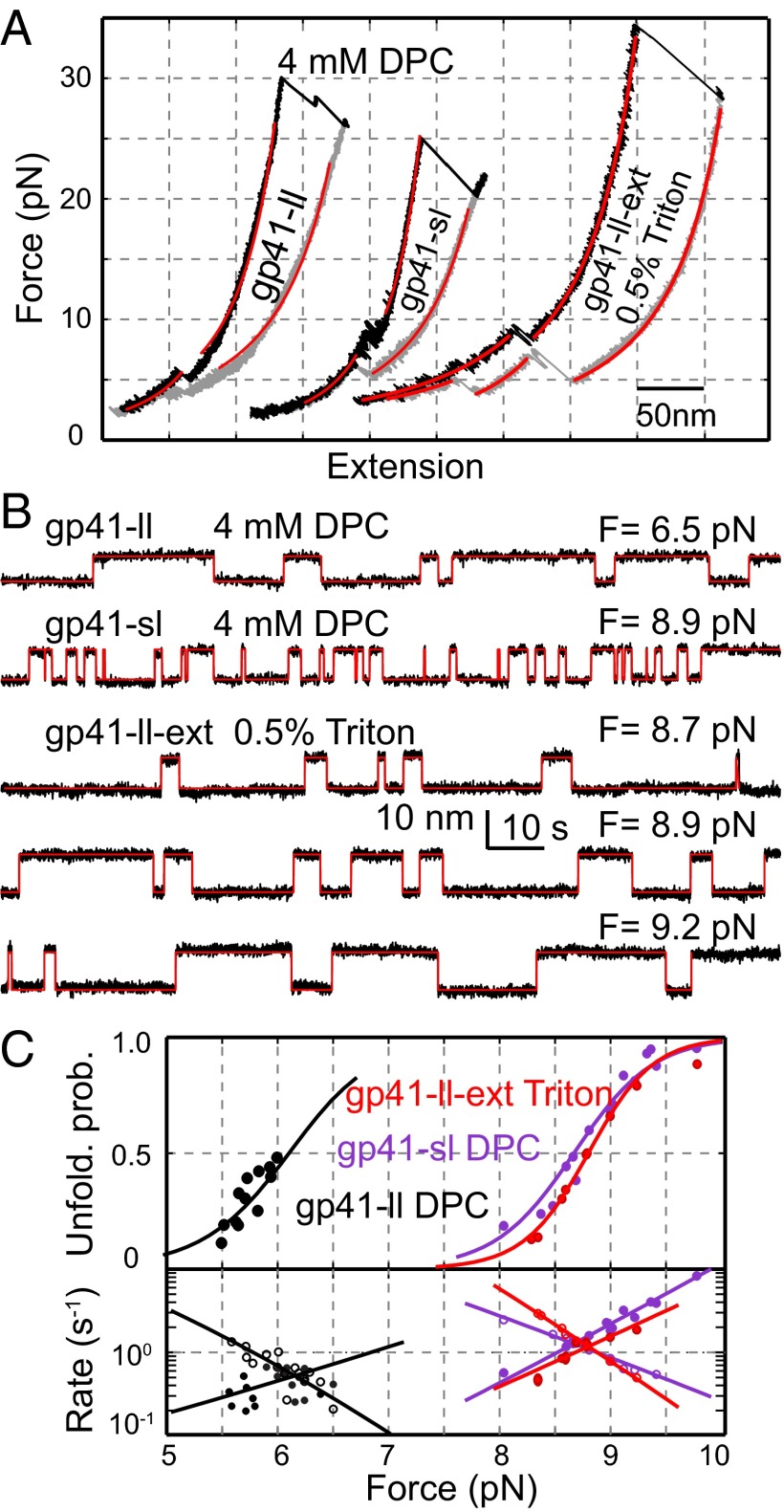
To investigate effects of membranes on gp41 folding, we pulled gp41-ll and gp41-sl in the presence of 4 mM dodecylphosphocholine (DPC) as in a recent study (24). As in the PBS, both complexes unfolded and refolded in two steps at distinct force ranges (Fig. 4A). This observation suggests that both gp41 complexes followed the same folding and unfolding pathways regardless of the presence of DPC micelles. In particular, both complexes readily refolded after they were completely unfolded, as indicated by the overlapping FECs at the low force range (<∼5 pN) (Fig. 4A and Fig. S5A). Detailed analyses demonstrated that the reversible transitions again represent folding and unfolding of the third gp41 hairpin between the 5HB state and the 6HB state.
Fig. 4.
Detergent micelles reduce the stability and folding rate of gp41. (A) FECs of gp41-ll and gp41-sl in 4 mM DPC and gp41-ll-ext in 0.5% Triton. The FECs were obtained by pulling (black) and then relaxing (gray) a single gp41 complex and fit by the worm-like chain model (red). (B) Extension-time trajectories of gp41-ll, gp41-sl, or gp41-ll-ext under the indicated forces showing transitions between the six-helix and the five-helix states in DPC or Triton. The idealized transitions derived from HMM are shown in red. (C) Force-dependent unfolding probabilities (Upper) and transition rates (Lower) of gp41-ll (black) and gp41-sl (purple) in DPC or gp41-ll-ext (red) in Triton. The measurements (symbols) were fit by the theoretical model (lines).
However, DPC quantitatively altered gp41 folding in a loop size-dependent manner. First, DPC slowed down gp41 transitions by ∼10-fold for gp41-sl and ∼6-fold for gp41-ll compared with their corresponding transitions in PBS. Furthermore, DPC weakened both gp41 complexes, as indicated by increases in gp41 folding energies and decreases in equilibrium forces (Table 1). Because detergents generally bind hydrophobic amino acids and protein folding requires displacing these bound detergent molecules, we hypothesized that such detergent displacement increased the folding energy barrier and the folding energy, accounting for our observations. Interestingly, the energy increase is greater for the gp41 construct with a longer loop, with 15.1 (±3.4) kBT for gp41-ll and 5.4 (±4.4) kBT for gp41-sl, suggesting a role of the loop in destabilizing the gp41 hairpin with DPC. In addition, gp41-ll has an even smaller extension change than gp41-sl (6.5 nm vs. 8.3 nm, Table 1). Our control experiment using the gp41 construct truncated at CHR C terminus showed that the extension decrease resulted from DPC-dependent extension reduction of the loop region in the 5HB state (Fig. S5B). Thus, we concluded that DPC strongly interacts with the 19-aa loop and/or the N-terminal CHR region in gp41-ll. This conclusion is justified, because the loop is rich in hydrophobic amino acids and tightly bound by gp120 to prevent gp41 folding in the Env complex (1). As a result, DPC association in the loop and the nearby region not only prevents full extension of the loop in the 5HB state, leading to the smaller extension change, but also significantly attenuates its folding into the 6HB state, resulting in lower folding energy. The DPC interactions deduced from our observations are consistent with the results of a recent structural study of gp41 by NMR (42).
Zippering Between MPER and FPPR Contributes to gp41 Folding Energy.
The MPER of gp41 is important, because it stabilizes the Env complex (43) and is the primary target of anti-HIV broadly neutralizing antibodies (11, 44) as well as T20. It forms an extended 6HB with its complementary FPPR, presumably contributing to additional gp41 folding energy (10). Both MPER and FPPR are highly hydrophobic and reported to bind membranes strongly (45). As a step to elucidate the role of MPER in gp41 folding, we made a longer version of gp41 6HB bundle including MPER and FPPR (10) and the 19-aa loop in the third hairpin (gp41-ll-ext, Fig. 1A). The gp41 protein was not soluble in aqueous solution. However, in 0.5% Triton X-100 we successfully purified, enzymatically biotinylated, and finally pulled the protein. The complex exhibited a reversible transition at 8–10 pN (Fig. 4 A and B), corresponding to the transition between the extended 6HB and 5HB. Therefore, the CHR and MPER folded and unfolded cooperatively. The transition had an average equilibrium force of 8.9 (±0.7, n = 8) pN and an extension change of 9.7 (±1.2) nm, both of which are greater than the corresponding measurements for gp41-ll complex in DPC (6.0 pN and 6.5 nm). The greater extension change indicates that gp41-ll-ext formed an extended 6HB structure similar to its crystal structure (10). In addition, the folding energy of gp41-ll-ext (−22 kBT) is also much less than that of gp41-ll (−8 kBT), indicating that the MPER and FPPR zippering contributes a net folding energy of ∼−14 kBT. Given the relatively small sizes of MPER and FPPR compared with CHR and NHR, zippering of MPER and FPPR greatly stabilized the gp41 complex in micelles. We hypothesize that viral membrane fusion requires continued zippering between MPER and FPPR and the accompanying changes in their interactions with membranes. As a result, mutations in both regions likely interfere with the gp41 zippering, thereby impairing viral membrane fusion and infectivity (46, 47).
T20 Actively Inhibits gp41 Folding by a Strand-Displacement Mechanism.
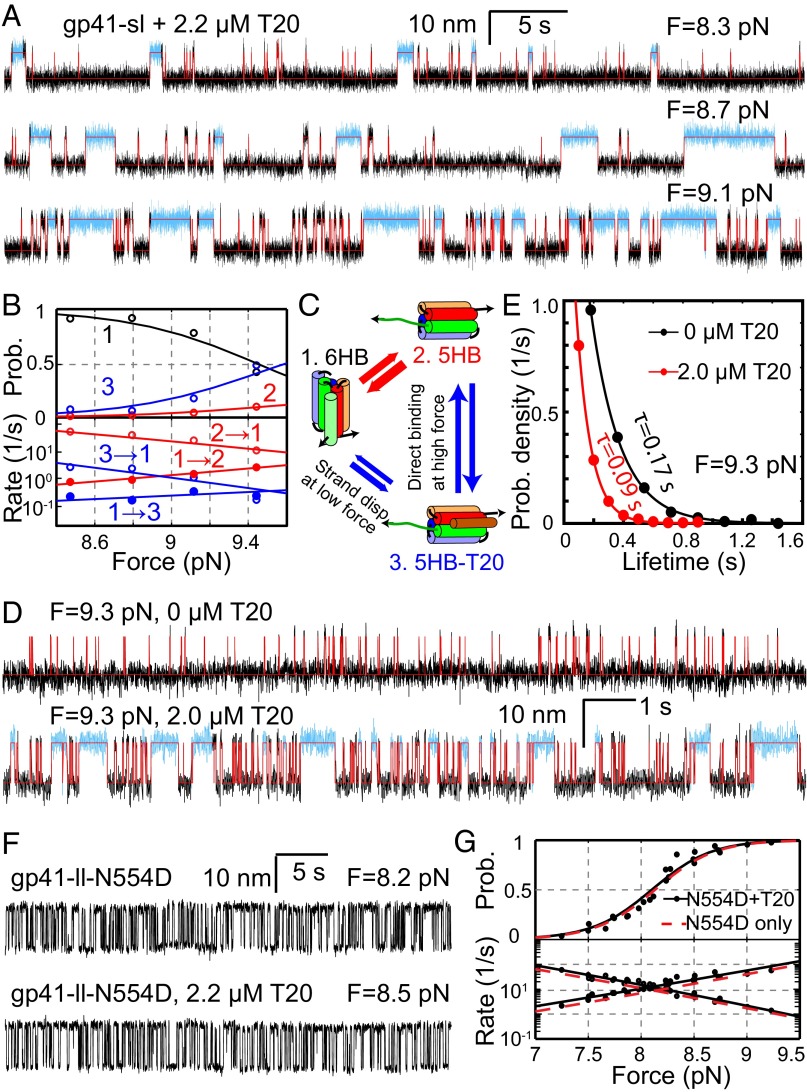
To test the effect of T20 on gp41 folding, we pulled gp41-sl in the presence of 2.2 µM T20 in the solution. A distinct state appeared in the extension-time trajectories obtained under constant forces (Fig. 5A and Fig. S6A), compared with the trajectories in the absence of T20 (Fig. 2D). This new state had approximately the same average extension as the 5HB state but lasted about 10-fold longer on average than the 5HB state (Fig. S6B). In addition, the new state occurred more frequently in the presence of higher T20 concentration (Fig. S6A) or force (Fig. 5 A and B). Taken together, we concluded that the new state represented the T20-bound 5HB state (designated as 5HB-T20).
Fig. 5.
T20 inhibits folding of wild-type gp41, but not T20-resistant N554D mutant. (A) Extension-time trajectories of gp41-sl under different constant forces in the presence of 2.2 µM T20. The measured extensions were analyzed by the three-state HMM (red traces), revealing the 6HB state and the free and T20-bound 5HB states, with the T20-bound 5HB state highlighted in cyan. (B) Force-dependent state probabilities (Upper) and transition rates (Lower) of the gp41-sl complex in the presence of T20. (C) Diagram showing three-state transitions of gp41-sl with significant force-dependent and T20-induced 6HB unfolding and binding. (D) Extension-time trajectories of the same gp41-sl molecule under 9.3 pN force in the absence (Upper) or presence (Lower) of 2.0 µM T20. (E) Dwell-time probability density distributions of the 6HB state of gp41-sl (dots) and their single-exponential fits (lines) corresponding to traces in D. For better comparison, each measured distribution is normalized by its maximum. (F) Extension-time trajectories of gp41-ll-N554D with the indicated forces and T20 concentrations. (G) Force-dependent unfolding probabilities (Upper) and transition rates (Lower) of gp41-ll-N554D in the presence (black) and absence (red) of 2.2 µM T20. The measurements (black symbols) were well fit by a theoretical model (black lines). For clarity, only the best-fit line (red, Fig. 2C) is shown here for gp41-ll-N554D in the absence of T20.
To unambiguously determine the different states and their transition rates, we analyzed the extension trajectories using a three-state HMM. The analyses revealed the best-fit state transitions, state populations, and transition rates (Fig. 5 A and B). Surprisingly, we found that the T20-bound 5HB state predominately formed from and dissociated to the 6HB state, instead of the free 5HB state at a low force range (<9.4 pN) (Fig. 5C and Fig. S6A). In other words, T20 mainly displaced the CHR to bind NHR in the 6HB or was displaced by CHR to dissociate from the 6HB under our experimental conditions. As a result, the T20-bound state was populated even when the free 5HB state had a probability less than ∼5% in most of the force range tested (Fig. 5B). Such an active or induced T20 binding mechanism may contribute to T20’s high potency in inhibiting gp41 folding and HIV-1 infection, because gp41 hairpins are generally in dynamic equilibrium between the folded and the unfolded states before a sufficient number of gp41 hairpins or complexes accumulate at the fusion site to complete fusion. However, at forces greater than 9.4 pN, the free 5HB state was more populated and became the major target of direct T20 binding (Fig. S7). The affinity between T20 and the 5HB state barely changes with force (Fig. S6C), with an average dissociation constant of 0.5 µM close to the previous measurement of ∼0.8 µM using the same truncated gp41 complex (21). Finally, we analyzed the lifetime distribution of the gp41 complex in the free and T20-bound H5B states and found that the distribution can be accounted for by the transition rates derived from HMM (Supporting Information and Fig. S7), supporting the induced T20 binding mechanism.
The induced binding mechanism (Fig. 5C) predicts that the induced binding rate (k13) is proportional to T20 concentration ([T20]), that is, k13 = kind[T20], where kind is the induced T20 binding rate constant. Therefore, the lifetime of the 6HB state (τ1) decreases as [T20] increases at a constant force, or τ1 = 1/(kind[T20] + k12), where both kind and the unfolding rate of 6HB alone (k12) are force-dependent. In contrast, if T20 only passively binds to the 5HB state, the lifetime τ1 = 1/k12 should be independent of [T20]. To further distinguish the active and passive T20 binding mechanisms, we measured the lifetimes of the 6HB state in the absence and presence of 2.0 µM T20. Owing to heterogeneity in folding kinetics of different single molecules and ∼10% systematic error in absolute force measurement by optical tweezers (35, 48), the measured unfolding probabilities and state lifetimes often differ among different single molecules even at the same force under our experimental conditions. To overcome these issues, we stretched a single gp41 complex gp41-sl to a constant force and then measured its transition kinetics in a microfluidic chamber that allowed us to add T20 during the measurement (49). We only compared the lifetimes of the same gp41 complex at the same force with and without T20 in the absence of flow (Fig. 5D). We found that addition of T20 decreased the lifetime of the 6HB state by 2- to 10-fold depending on the force (Fig. 5E). Similarly, the lifetime of the 5HB state decreased as the T20 concentration increased (Fig. S8). In contrast, the lifetime of the T20-bound state did not significantly change with T20 concentration. All these observations are consistent with the predictions of our proposed T20 binding mechanism (Fig. 5C). Therefore, our results support that T20 can bind to the 6HB via an induced binding mechanism.
The induced T20 binding rate k13 increases as force increases (Fig. 5B), indicating that force promotes the active T20 binding. We hypothesized that force facilitates CHR fraying in the 6HB, exposing a toehold for T20 to bind and unfold the 6HB, in a mechanism similar to strand displacement observed for DNA or RNA (50). To test this hypothesis, we examined T20 binding kinetics using the construct gp41-ll-CΔ7 and compared the kinetics to those of gp41-ll. Compared with gp41-ll, gp41-ll-CΔ7 had the seven C-terminal amino acids of CHR truncated (Fig. S2). The truncation significantly destabilized the gp41 hairpin (Fig. S2), making it impossible to measure T20 binding to the two gp41 constructs at the same force. Instead, we pulled the two constructs to different forces such that the two hairpins had approximately equal unfolding probabilities in the absence of T20 (Fig. S9). Then we added 2.0 µM T20 and measured the apparent rates of T20 binding to the two hairpins. Because the rate for direct T20 binding is equal for both constructs, any difference in the measured T20 binding rate should result from the difference in the induced binding rate. Indeed, we found that T20 bound to gp41-ll-CΔ7 much more frequently than to gp41-ll, indicating that the truncation enhances the induced binding rate (Fig. S9). This finding supports that T20 binds to 6HB via a toehold-dependent strand-displacement mechanism.
T20-Resistant Mutation Barely Changes gp41 Folding but Abolishes T20 Inhibition.
To determine the mechanism of T20 resistance, we first characterized the folding energy and kinetics of gp41-ll-N554D alone (Fig. 2A). The gp41-ll-N554D construct was identical to gp41-ll, except for the T20-resistant N554D mutation in all three gp41 monomers (Fig. 1A). The mutation reduced the equilibrium force between the 5HB and the 6HB states to 8.1 (±0.6, n = 42) pN (Fig. 2C) but did not significantly change the extension difference between the two states (Table 1). Accordingly, the folding energy of gp41-ll-N554D was measured to be −20.3 ± 0.7 kBT (Table 1), only slightly higher than that of gp41-ll. Because the mutation (corresponding to 15 aa from the C terminus of the gp41 hairpin) lies in the 6HB state relative to the transition state (at 25 ± 2 aa) (Table 1 and Fig. S3), the mutation caused a shift of the unfolding rate curve toward a higher rate, whereas the folding rate curve was unchanged (Fig. 2C). Owing to a decrease in the equilibrium force, the equilibrium rate of gp41-ll-N554D increased compared with that of the wild type. The modest change in folding energy and the minimal change in the folding rate (at the same force) are consistent with the fact that the mutant gp41 is capable of mediating HIV viral fusion and infection.
We then added T20 into the solution and repeated the above experiment. We found that the extension-time trajectories obtained in the presence of T20 were identical to those in the absence of T20 (Fig. 5F). Specifically, no T20-dependent long-lived states were detected. Accordingly, the extension-time trajectories could be fit by a two-state HMM well and revealed the force-dependent unfolding probability and transition rates overlapping those of gp41-ll (Fig. 5G). Thus, we concluded that gp41 mutation N554D reduced T20 inhibition of gp41 folding. Taken together, our results revealed that N554D mutation barely changed gp41 folding but reduced T20 inhibition, clarifying the mechanism of T20 resistance of the mutation.
Discussion
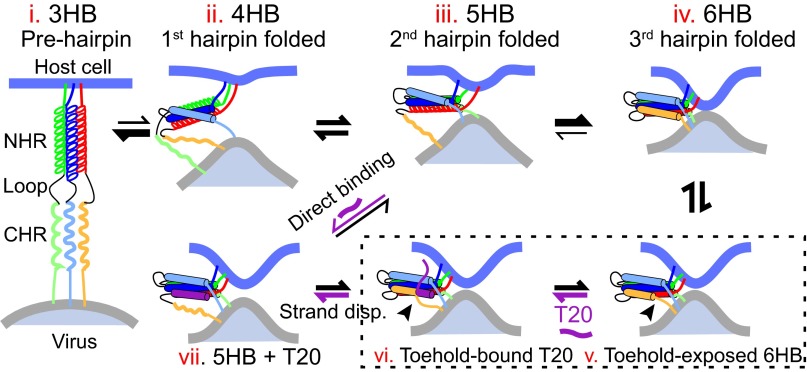
Crystal structures of gp41 6HB show that the three CHRs do not directly interact with each other. Thus, the kinetic coupling observed by us must arise from indirect interactions via conformational changes of the central three-helix bundle. Trimerization of NHRs is an obligate step to form the extended prehairpins for class-I viral fusion proteins (1, 4). Previous studies reveal that the NHR-helix bundle (3HB) and the folding intermediates of the gp41 complex, the 4HB and 5HB, are structurally similar to their corresponding structures in the 6HB and thermodynamically stable in solution over the time period of our typical experiments (1, 19, 51). Our single-molecule measurements are consistent with these findings. Taken together, these observations indicate that the gp41 constructs used in our studies do not significantly alter the structures of the different gp41 intermediates or folding of different hairpins. However, small and fast conformational changes in the three-to-five helical bundles around their favorable conformations during gp41 folding are possible (7). Based on these previous results and our experimental data, we propose a working model to account for gp41 folding in the absence and presence of T20 (Fig. 6). In this model, the conformation of the NHR trimer is successively adjusted by sequential folding of three gp41 hairpins. The altered NHR trimer in turn accelerates folding of the next hairpin. Thus, folding of the first gp41 hairpin may be the rate-limiting step. Gp41 folding becomes faster and faster as the second and the third hairpins fold. Therefore, we derived two intermediates of gp41 folding corresponding to the gp41 complex with only one and two folded gp41 hairpins, respectively.
Fig. 6.
Model of sequential gp41 folding during membrane fusion. The dynamic NHR three-helix bundle (state i) is progressively altered by folding of gp41 hairpins (states ii–iv), which in turn accelerates folding of the next hairpin (indicated by thicker arrows as more hairpins fold). The dynamic and altered helices are represented by solenoids and solid cylinders, respectively. The membrane repulsive force dynamically unravels the C-terminal CHR in the folded hairpin (state v), providing a toehold for T20 to bind (vi) and displace the CHR by a strand-displacement mechanism (from states vi to vii). The states v and vi in the dashed box serve as transition states for active T20 binding. Alternatively, T20 directly binds the unfolded gp41 hairpin (state iii). Note that in the presence of the common membrane force load different gp41 hairpins or complexes may fold more cooperatively than illustrated here (56).
Our current construct design prevents us from directly measuring the folding energy and kinetics of the first gp41 hairpin. Assuming an equal folding energy for three hairpins, we estimated the total folding energy of a gp41 complex to be −71 kBT, close to the folding energy of a single neuronal SNARE complex (27). Thus, like SNARE complexes (52), a single gp41 complex is capable of driving viral membrane fusion by mechanical forces, corroborating the previous observation (53).
It has been generally believed that T20 binds the prehairpin conformation of gp41 to block its folding. Surprisingly, we discovered that T20 directly interacts with the 6HB in a force-dependent manner. In this case, force promotes only partial C-terminal unfolding of the 6HB bundle, providing a toehold for T20 to bind and unfold the 6HB by strand displacement (50). During viral membrane fusion, a similar force is exerted on gp41 by the apposed membranes. The first gp41 hairpin slowly folds against the highest force, exposing the unfolded state long enough for T20 to directly bind the hairpin. As more hairpins fold and split the force load, the folded hairpins are stabilized and likely become the primary target of active T20 binding. Together, the two T20 binding modes may lead to efficient inhibition of gp41 folding (21). However, the toehold site required for active T20 binding is likely abolished by N554D mutation, causing its drug resistance.
We showed that the gp41 complex with a 19-aa loop is very stable in DPC and Triton X-100 and that the loop in the hairpin plays a major role in the detergent-mediated destabilization of the gp41 complex. These observations may have important implications in gp41-mediated membrane fusion. During viral membrane fusion, the loop is likely stretched away from the membranes by the opposed membranes upon formation of the prehairpins. Thus, gp41 folding into the 6HB is probably not compromised by the membranes.
In conclusion, our research clarifies the mechanisms of viral membrane fusion and inhibition and may help to develop better fusion inhibitors for therapeutic intervention of AIDS and diseases caused by other viruses.
Materials and Methods
Gp41 Constructs and Sequences.
Except in the construct containing the extended 6HB (gp41-ll-ext), all gp41 monomers contain 40-aa NHRs and 38-aa CHRs corresponding to gp160 amino acids 543–582 and 625–662, respectively. The NHRs and CHRs were linked by 5-aa flexible spacers (Gly/Ser) as previously designed (31) except for the gp41 hairpin to be investigated (Fig. 1 D and E), which contains either a 5-aa short linker (sl) or a 19-aa long linker (ll) corresponding to gp160 amino acids 597–601 and 583–601, respectively. The different gp41 monomers were joined by 6-aa flexible spacers. The natural cysteine in the loop region was mutated to serine. An N-terminal spacer containing a cysteine residue (CGGSGGSKGGSNG) and a C-terminal spacer containing a Flag tag and an Avi-tag (GGNSGDYKDDDDKGSGGSGNGGSGDSLEFIASKLAGGLNDIFEAQKIEWHE) were added to the tandem repeat sequences of gp41 monomers. The gp41-ll-hp2 construct is similar to gp41-ll, except that the second and the third hairpins were joined by the flexible spacer sequence GSGNSKSAGSGGSGSVSPSNKLKSSDAYGSKKAWGNNQDGVVASQSGANSGGLKGGQSSG and the NHR and CHR of the third hairpin were connected by the spacer GGNSGDYKDDDDKGSGGSGNGGSGDSLEFIASKLAGGLNDIFEAQKIEWHES GENLYFQSGSGGGSGSGNGG in which the Tobacco Etch Virus (TEV) cleavage site is underlined. The gp41-ll-ext construct is identical to gp41-ll, except that the FPPR (amino acids 528–542) the MPER (amino acids 663–683) were added to the N terminus of NHR and the C terminus of CHR (10), respectively (Fig. 1A).
Genes corresponding to these gp41 constructs were codon-optimized, synthesized, subcloned into the protein expression vector pET-SUMO, and induced to express in BL21 (DE3) E. coli cells using isopropyl β-d-1-thiogalactopyranoside as previously described (27). The proteins were purified using Ni-NTA resin (GE Healthcare Biosciences) and biotinylated using biotin ligase (Avidity). The gp41-ll-hp2 protein was cleaved by TEV protease at room temperature for over 2 h before further purification to remove the protease.
High-Resolution Dual-Trap Optical Tweezers.
The tweezers were home-built as previously described (48, 49, 54). The machine was operated remotely using a LabVIEW (National Instruments) interface. The force and displacement were calibrated by Brownian motion of the trapped polystyrene beads before each single-molecule experiment.
Single-Molecule Protein Folding Experiment.
The gp41 protein was reduced by Tris-(2-carboxyethyl) phosphine, mixed with the thiol-containing DNA handle treated with dithiodipyridine in a typical 50:1 protein:DNA molar ratio, and cross-linked to the DNA handle overnight. An aliquot of the protein–DNA conjugate was mixed with anti–digoxigenin-coated beads and injected into the microfluidic channel (49). One DNA-bound bead was caught by one optical trap, brought close to a streptavidin-coated bead held in the other optical trap, formed a single gp41-DNA tether, and pulled by optical tweezers. The single-molecule folding experiment was performed at room temperature (22 °C) in PBS supplemented with an oxygen scavenging system. In addition, 4 mM DPC, 0.5% Triton X-100, and/or T20 was added into the PBS buffer as indicated.
Data Analysis.
Methods of data analysis are described in detail elsewhere (27, 38). A new algorithm is used for the three-state HMM of the gp41 transition in the presence of T20. The algorithm maximizes the likelihood function based on the gradient descent method (55) with constraints for detailed balance.
Supplementary Material
Acknowledgments
We thank W. Mothes, M. Root, Y. Shai, N. Elizabeth, F. Tang, M. Poirier, and B. Chen for discussions and advice, and L. Oswald, Z. Xi, and X. M. Zhang for help. The following reagent was obtained through the NIH AIDS Research and Reference Reagent Program: Roche T-20, Fusion Inhibitor (N-acetylated derivative, catalog no. 9845). This work was supported by NIH Grant GM093341 and a seed grant from the Brain Research Foundation (to Y.Z.). We acknowledge support from the Raymond and Beverly Sackler Institute for Biological, Physical and Engineering Sciences.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1424995112/-/DCSupplemental.
References
- 1.Pancera M, et al. Structure and immune recognition of trimeric pre-fusion HIV-1 Env. Nature. 2014;514(7523):455–461. doi: 10.1038/nature13808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Julien JP, et al. Crystal structure of a soluble cleaved HIV-1 envelope trimer. Science. 2013;342(6165):1477–1483. doi: 10.1126/science.1245625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Munro JB, et al. Conformational dynamics of single HIV-1 envelope trimers on the surface of native virions. Science. 2014;346(6210):759–763. doi: 10.1126/science.1254426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harrison SC. Viral membrane fusion. Nat Struct Mol Biol. 2008;15(7):690–698. doi: 10.1038/nsmb.1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eckert DM, Kim PS. Mechanisms of viral membrane fusion and its inhibition. Annu Rev Biochem. 2001;70:777–810. doi: 10.1146/annurev.biochem.70.1.777. [DOI] [PubMed] [Google Scholar]
- 6.Haim H, et al. 2009. Soluble CD4 and CD4-mimetic compounds inhibit HIV-1 infection by induction of a short-lived activated state. PLoS Pathog 5(4):e1000360.
- 7.Tran EEH, Borgnia MJ, Kuybeda O, Schauder DM, Bartesaghi A, Frank GA, Sapiro G, Milne JLS, Subramaniam S. 2012. Structural mechanism of trimeric HIV-1 envelope glycoprotein activation. PLoS Pathog 8(7):e1002797. [DOI] [PMC free article] [PubMed]
- 8.Chan DC, Fass D, Berger JM, Kim PS. Core structure of gp41 from the HIV envelope glycoprotein. Cell. 1997;89(2):263–273. doi: 10.1016/s0092-8674(00)80205-6. [DOI] [PubMed] [Google Scholar]
- 9.Weissenhorn W, Dessen A, Harrison SC, Skehel JJ, Wiley DC. Atomic structure of the ectodomain from HIV-1 gp41. Nature. 1997;387(6631):426–430. doi: 10.1038/387426a0. [DOI] [PubMed] [Google Scholar]
- 10.Buzon V, et al. Crystal structure of HIV-1 gp41 including both fusion peptide and membrane proximal external regions. PLoS Pathog. 2010;6(5):e1000880. doi: 10.1371/journal.ppat.1000880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burton DR, et al. A blueprint for HIV vaccine discovery. Cell Host Microbe. 2012;12(4):396–407. doi: 10.1016/j.chom.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frey G, et al. A fusion-intermediate state of HIV-1 gp41 targeted by broadly neutralizing antibodies. Proc Natl Acad Sci USA. 2008;105(10):3739–3744. doi: 10.1073/pnas.0800255105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuzmin PI, Zimmerberg J, Chizmadzhev YA, Cohen FS. A quantitative model for membrane fusion based on low-energy intermediates. Proc Natl Acad Sci USA. 2001;98(13):7235–7240. doi: 10.1073/pnas.121191898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim JH, Song H, Austin JL, Cheng W. Optimized infectivity of the cell-free single-cycle human immunodeficiency viruses type 1 (HIV-1) and its restriction by host cells. PLoS ONE. 2013;8(6):e67170. doi: 10.1371/journal.pone.0067170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sanders RW, et al. Stabilization of the soluble, cleaved, trimeric form of the envelope glycoprotein complex of human immunodeficiency virus type 1. J Virol. 2002;76(17):8875–8889. doi: 10.1128/JVI.76.17.8875-8889.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jelesarov I, Lu M. Thermodynamics of trimer-of-hairpins formation by the SIV gp41 envelope protein. J Mol Biol. 2001;307(2):637–656. doi: 10.1006/jmbi.2001.4469. [DOI] [PubMed] [Google Scholar]
- 17.Muñoz-Barroso I, Durell S, Sakaguchi K, Appella E, Blumenthal R. Dilation of the human immunodeficiency virus-1 envelope glycoprotein fusion pore revealed by the inhibitory action of a synthetic peptide from gp41. J Cell Biol. 1998;140(2):315–323. doi: 10.1083/jcb.140.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marti DN, Bjelić S, Lu M, Bosshard HR, Jelesarov I. Fast folding of the HIV-1 and SIV gp41 six-helix bundles. J Mol Biol. 2004;336(1):1–8. doi: 10.1016/j.jmb.2003.11.058. [DOI] [PubMed] [Google Scholar]
- 19.Wild CT, Shugars DC, Greenwell TK, McDanal CB, Matthews TJ. Peptides corresponding to a predictive alpha-helical domain of human immunodeficiency virus type 1 gp41 are potent inhibitors of virus infection. Proc Natl Acad Sci USA. 1994;91(21):9770–9774. doi: 10.1073/pnas.91.21.9770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lalezari JP, et al. TORO 1 Study Group Enfuvirtide, an HIV-1 fusion inhibitor, for drug-resistant HIV infection in North and South America. N Engl J Med. 2003;348(22):2175–2185. doi: 10.1056/NEJMoa035026. [DOI] [PubMed] [Google Scholar]
- 21.Champagne K, Shishido A, Root MJ. Interactions of HIV-1 inhibitory peptide T20 with the gp41 N-HR coiled coil. J Biol Chem. 2009;284(6):3619–3627. doi: 10.1074/jbc.M809269200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mink M, et al. Impact of human immunodeficiency virus type 1 gp41 amino acid substitutions selected during enfuvirtide treatment on gp41 binding and antiviral potency of enfuvirtide in vitro. J Virol. 2005;79(19):12447–12454. doi: 10.1128/JVI.79.19.12447-12454.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kliger Y, Peisajovich SG, Blumenthal R, Shai Y. Membrane-induced conformational change during the activation of HIV-1 gp41. J Mol Biol. 2000;301(4):905–914. doi: 10.1006/jmbi.2000.4004. [DOI] [PubMed] [Google Scholar]
- 24.Roche J, Louis JM, Grishaev A, Ying J, Bax A. Dissociation of the trimeric gp41 ectodomain at the lipid-water interface suggests an active role in HIV-1 Env-mediated membrane fusion. Proc Natl Acad Sci USA. 2014;111(9):3425–3430. doi: 10.1073/pnas.1401397111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shu W, Ji H, Lu M. Interactions between HIV-1 gp41 core and detergents and their implications for membrane fusion. J Biol Chem. 2000;275(3):1839–1845. doi: 10.1074/jbc.275.3.1839. [DOI] [PubMed] [Google Scholar]
- 26.Südhof TC, Rothman JE. Membrane fusion: Grappling with SNARE and SM proteins. Science. 2009;323(5913):474–477. doi: 10.1126/science.1161748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gao Y, et al. Single reconstituted neuronal SNARE complexes zipper in three distinct stages. Science. 2012;337(6100):1340–1343. doi: 10.1126/science.1224492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang X, Ma L, Zhang Y. High-resolution optical tweezers for single-molecule manipulation. Yale J Biol Med. 2013;86(3):367–383. [PMC free article] [PubMed] [Google Scholar]
- 29.Stigler J, Ziegler F, Gieseke A, Gebhardt JCM, Rief M. The complex folding network of single calmodulin molecules. Science. 2011;334(6055):512–516. doi: 10.1126/science.1207598. [DOI] [PubMed] [Google Scholar]
- 30.Lu M, Blacklow SC, Kim PS. A trimeric structural domain of the HIV-1 transmembrane glycoprotein. Nat Struct Biol. 1995;2(12):1075–1082. doi: 10.1038/nsb1295-1075. [DOI] [PubMed] [Google Scholar]
- 31.Root MJ, Kay MS, Kim PS. Protein design of an HIV-1 entry inhibitor. Science. 2001;291(5505):884–888. doi: 10.1126/science.1057453. [DOI] [PubMed] [Google Scholar]
- 32.Woodside MT, et al. Nanomechanical measurements of the sequence-dependent folding landscapes of single nucleic acid hairpins. Proc Natl Acad Sci USA. 2006;103(16):6190–6195. doi: 10.1073/pnas.0511048103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cecconi C, Shank EA, Bustamante C, Marqusee S. Direct observation of the three-state folding of a single protein molecule. Science. 2005;309(5743):2057–2060. doi: 10.1126/science.1116702. [DOI] [PubMed] [Google Scholar]
- 34.Bustamante C, Chemla YR, Forde NR, Izhaky D. Mechanical processes in biochemistry. Annu Rev Biochem. 2004;73:705–748. doi: 10.1146/annurev.biochem.72.121801.161542. [DOI] [PubMed] [Google Scholar]
- 35.Gao Y, Sirinakis G, Zhang Y. Highly anisotropic stability and folding kinetics of a single coiled coil protein under mechanical tension. J Am Chem Soc. 2011;133(32):12749–12757. doi: 10.1021/ja204005r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marko JF, Siggia ED. Stretching DNA. Macromolecules. 1995;28(6):8759–8770. [Google Scholar]
- 37.McKinney SA, Joo C, Ha T. Analysis of single-molecule FRET trajectories using hidden Markov modeling. Biophys J. 2006;91(5):1941–1951. doi: 10.1529/biophysj.106.082487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xi Z, Gao Y, Sirinakis G, Guo H, Zhang Y. Single-molecule observation of helix staggering, sliding, and coiled coil misfolding. Proc Natl Acad Sci USA. 2012;109(15):5711–5716. doi: 10.1073/pnas.1116784109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Steger HK, Root MJ. Kinetic dependence to HIV-1 entry inhibition. J Biol Chem. 2006;281(35):25813–25821. doi: 10.1074/jbc.M601457200. [DOI] [PubMed] [Google Scholar]
- 40.Miyauchi K, Kim Y, Latinovic O, Morozov V, Melikyan GB. HIV enters cells via endocytosis and dynamin-dependent fusion with endosomes. Cell. 2009;137(3):433–444. doi: 10.1016/j.cell.2009.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Herold N, et al. HIV-1 entry in SupT1-R5, CEM-ss, and primary CD4+ T cells occurs at the plasma membrane and does not require endocytosis. J Virol. 2014;88(24):13956–13970. doi: 10.1128/JVI.01543-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lakomek NA, Kaufman JD, Stahl SJ, Wingfield PT. HIV-1 envelope protein gp41: An NMR study of dodecyl phosphocholine embedded gp41 reveals a dynamic prefusion intermediate conformation. Structure. 2014;22(9):1311–1321. doi: 10.1016/j.str.2014.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ringe RP, et al. Cleavage strongly influences whether soluble HIV-1 envelope glycoprotein trimers adopt a native-like conformation. Proc Natl Acad Sci USA. 2013;110(45):18256–18261. doi: 10.1073/pnas.1314351110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huang J, et al. Broad and potent neutralization of HIV-1 by a gp41-specific human antibody. Nature. 2012;491(7424):406–412. doi: 10.1038/nature11544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kyrychenko A, et al. Structural plasticity in the topology of the membrane-interacting domain of HIV-1 gp41. Biophys J. 2014;106(3):610–620. doi: 10.1016/j.bpj.2013.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bellamy-McIntyre AK, et al. Functional links between the fusion peptide-proximal polar segment and membrane-proximal region of human immunodeficiency virus gp41 in distinct phases of membrane fusion. J Biol Chem. 2007;282(32):23104–23116. doi: 10.1074/jbc.M703485200. [DOI] [PubMed] [Google Scholar]
- 47.Salzwedel K, West JT, Hunter E. A conserved tryptophan-rich motif in the membrane-proximal region of the human immunodeficiency virus type 1 gp41 ectodomain is important for Env-mediated fusion and virus infectivity. J Virol. 1999;73(3):2469–2480. doi: 10.1128/jvi.73.3.2469-2480.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Moffitt JR, Chemla YR, Izhaky D, Bustamante C. Differential detection of dual traps improves the spatial resolution of optical tweezers. Proc Natl Acad Sci USA. 2006;103(24):9006–9011. doi: 10.1073/pnas.0603342103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang Y, Sirinakis G, Gundersen G, Xi Z, Gao Y. DNA translocation of ATP-dependent chromatin remodeling factors revealed by high-resolution optical tweezers. Methods Enzymol. 2012;513:3–28. doi: 10.1016/B978-0-12-391938-0.00001-X. [DOI] [PubMed] [Google Scholar]
- 50.Zhang DY, Seelig G. Dynamic DNA nanotechnology using strand-displacement reactions. Nat Chem. 2011;3(2):103–113. doi: 10.1038/nchem.957. [DOI] [PubMed] [Google Scholar]
- 51.Bewley CA, Louis JM, Ghirlando R, Clore GM. Design of a novel peptide inhibitor of HIV fusion that disrupts the internal trimeric coiled-coil of gp41. J Biol Chem. 2002;277(16):14238–14245. doi: 10.1074/jbc.M201453200. [DOI] [PubMed] [Google Scholar]
- 52.van den Bogaart G, et al. One SNARE complex is sufficient for membrane fusion. Nat Struct Mol Biol. 2010;17(3):358–364. doi: 10.1038/nsmb.1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yang X, Kurteva S, Ren X, Lee S, Sodroski J. Stoichiometry of envelope glycoprotein trimers in the entry of human immunodeficiency virus type 1. J Virol. 2005;79(19):12132–12147. doi: 10.1128/JVI.79.19.12132-12147.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sirinakis G, et al. The RSC chromatin remodelling ATPase translocates DNA with high force and small step size. EMBO J. 2011;30(12):2364–2372. doi: 10.1038/emboj.2011.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Qin F, Auerbach A, Sachs F. A direct optimization approach to hidden Markov modeling for single channel kinetics. Biophys J. 2000;79(4):1915–1927. doi: 10.1016/S0006-3495(00)76441-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hernandez JM, Kreutzberger AJB, Kiessling V, Tamm LK, Jahn R. Variable cooperativity in SNARE-mediated membrane fusion. Proc Natl Acad Sci USA. 2014;111(33):12037–12042. doi: 10.1073/pnas.1407435111. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.