Significance
Using state-of-the-art micropatterning technology and single-molecule imaging, we revisited a prevalent paradigm in cell locomotion, the molecular clutch, which states that the intracellular actin motile machinery is dynamically coupled to transmembrane adhesion, allowing cells to move forward by applying forces on the substrate. We combined primary neuronal cultures on micropatterned substrates to trigger selective micron-scale N-cadherin adhesions, with single-molecule mapping of actin, N-cadherin, and α-catenin at the ventral surface of growth cones using photoconvertible fluorescent proteins. We demonstrate that actin filaments transit on a time scale of seconds between a flowing state and a state bound to immobilized N-cadherin/catenin complexes. These transient connections represent the first direct evidence for an intrinsic molecular clutch mechanism underlying growth cone locomotion.
Keywords: growth cone, actin flow, N-cadherin adhesion, micropatterned substrates, single-molecule tracking
Abstract
Neuronal growth cones move forward by dynamically connecting actin-based motility to substrate adhesion, but the mechanisms at the individual molecular level remain unclear. We cultured primary neurons on N-cadherin–coated micropatterned substrates, and imaged adhesion and cytoskeletal proteins at the ventral surface of growth cones using single particle tracking combined to photoactivated localization microscopy (sptPALM). We demonstrate transient interactions in the second time scale between flowing actin filaments and immobilized N-cadherin/catenin complexes, translating into a local reduction of the actin retrograde flow. Normal actin flow on micropatterns was rescued by expression of a dominant negative N-cadherin construct competing for the coupling between actin and endogenous N-cadherin. Fluorescence recovery after photobleaching (FRAP) experiments confirmed the differential kinetics of actin and N-cadherin, and further revealed a 20% actin population confined at N-cadherin micropatterns, contributing to local actin accumulation. Computer simulations with relevant kinetic parameters modeled N-cadherin and actin turnover well, validating this mechanism. Such a combination of short- and long-lived interactions between the motile actin network and spatially restricted adhesive complexes represents a two-tiered clutch mechanism likely to sustain dynamic environment sensing and provide the force necessary for growth cone migration.
Growth cones are motile structures at the extremity of axons responsible for path finding and neurite extension during nervous system development and repair. Growth cones translate extracellular signals into directional migration through a coordinated regulation of cytoskeleton, adhesion, and membrane processes (1). At the cytoskeletal level, motility is generated by polarized actin treadmilling, which, together with myosin contraction, generates a continuous retrograde actin flow from the periphery to the base of growth cones (2–7). At the membrane level, adhesion proteins form dynamic bonds with immobilized extracellular ligands, allowing step-by-step locomotion (8).
The molecular clutch model postulates that the mechanical coupling between ligand-bound transmembrane adhesion receptors and the actin flow allows traction forces to be transmitted to the substrate, resulting in local diminution of the retrograde flow and forward progression (9–11). Optical tweezers and flexible substrate experiments using microspheres coated with adhesion molecules revealed clutch-like mechanisms for integrins (12, 13), Ig cell adhesion molecules (14, 15), and cadherins (16, 17). However, the mechanism of clutch engagement at the individual molecular level remains elusive. For integrin-based adhesion, single-molecule tracking experiments suggested that talin and vinculin could switch between a state bound to flowing actin and a state bound to immobilized integrins (18, 19). For cadherin-based adhesion, biochemical experiments suggested that α-catenin could transit between being bound to actin or to the cadherin/β-catenin complex (20, 21), but a direct visualization of such behavior is lacking. In addition, vinculin, which can bind both actin and α-catenin, is recruited at cadherin-based intercellular junctions under mechanical force (22–24), but its dynamic behavior in growth cone migration is unknown.
By combining spatially controlled adhesion in growth cones with single-molecule tracking, fluorescence recovery after photobleaching (FRAP) experiments, and computer simulations, we report that N-cadherin/α-catenin complexes, but not vinculin, are selectively trapped at N-cadherin micropatterns. In addition, 80% of actin molecules flow rearward, making transient pauses on the order of seconds with immobilized N-cadherin/α-catenin complexes, whereas 20% of confined actin molecules contribute to local actin enrichment. This association of short- and long-lasting individual bonds underlies the differential coupling between the actin motile machinery and substrate adhesions supporting growth cone migration.
Results
N-Cadherin/Catenin Complex Is Diffusionally Trapped at N-Cadherin Micropatterns.
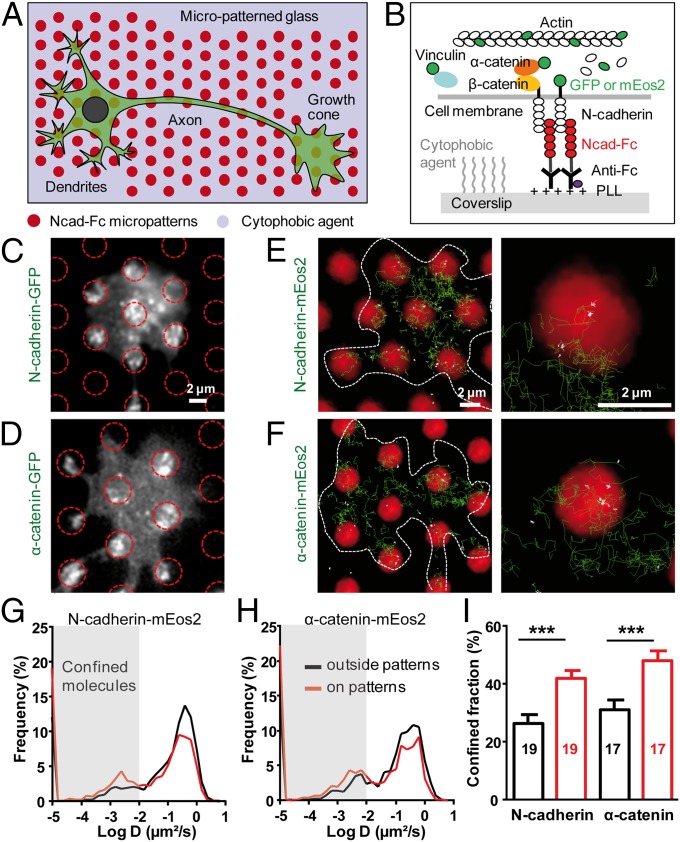
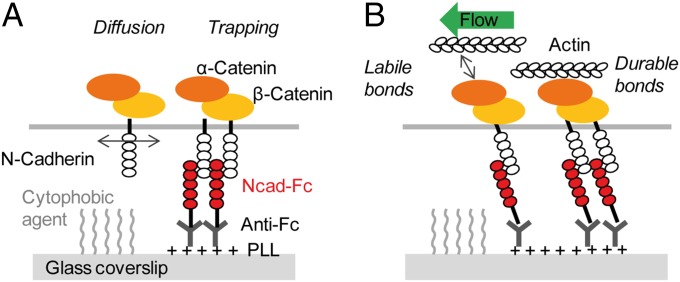
To induce N-cadherin adhesions at spatially controlled locations, we cultured primary hippocampal neurons on micropatterned substrates coated with recombinant N-cadherin–Fc (Ncad-Fc) as described (25) (Fig. 1A). Neurons developed faster on those substrates than on glass coverslips uniformly coated with polylysine (PLL) or micropatterned substrates coated with Fc (Fig. S1 A–D), in agreement with the growth-promoting activity of N-cadherin (26). Neurons extended numerous and long neurites as on substrates uniformly coated with Ncad-Fc (16), showing that the cytophobic layer between micropatterns did not alter axonal growth. A single growth cone typically spread over several Ncad-Fc–coated dots, allowing a paired comparison between N-cadherin adhesions and nonadhesive areas.
Fig. 1.
Membrane N-cadherin and α-catenin are selectively immobilized at N-cadherin micropatterns. (A) Neurons were cultured on micropatterned glass substrates comprising regularly spaced Ncad-Fc–coated dots (red) separated by a cytophobic agent (violet). (B) Side view of the molecular interactions at the N-cadherin/actin interface. (C and D) Epifluorescence images of growth cones expressing Ncad-GFP or α-catenin–GFP on Ncad-Fc patterns (red circles). (E and F, Left) Individual trajectories of N-cadherin–mEos2 (Ncad-mEos2) or α-catenin–mEos2 molecules (green) superimposed on Ncad-Fc patterns (red). (E and F, Right) In higher magnification images, confined trajectories with D < 0.01 μm2⋅s−1 are white, whereas Brownian trajectories with D > 0.01 μm2⋅s−1 are green. (Scale bars: C–F, 2 μm.) (G and H) Distributions of diffusion coefficients for Ncad-mEos2 and α-catenin–mEos2 molecules, respectively, at Ncad-Fc patterns or outside regions (between 1,222 and 4,062 trajectories analyzed). Gray-shaded areas represent confined molecules with D < 0.01 μm2⋅s−1. (I) Corresponding fraction of confined trajectories. Data represent the mean ± SEM of confined fractions (***P < 0.0001 by paired Student’s t test). The number of growth cones examined is given in each column.
To visualize molecules engaged in adhesions at the ventral surface of growth cones (Fig. 1B), we electroporated neurons with N-cadherin, actin, α-catenin, or vinculin constructs fused to either GFP for conventional epifluorescence illumination or to the photoconvertible protein mEos2 (27) for observation of individual molecule behavior by single-particle tracking combined to photoactivated localization microscopy (sptPALM) (19, 28). We checked by immunocytochemistry that recombinant proteins were expressed at relatively low levels and incorporated well into endogenous protein pools (Fig. S1 E–G). N-cadherin GFP (Ncad-GFP) was strongly accumulated at Ncad-Fc–coated micropatterns (Fig. 1C and Table 1), indicating that membrane N-cadherin was engaged in homophilic adhesion. At the individual level, N-cadherin–mEos2 molecules displayed a variety of behaviors, ranging from Brownian motion with a high diffusion coefficient (D ≥ 0.1 μm2⋅s−1), to mixed trajectories (0.01 < D < 0.1 μm2⋅s−1), to complete immobilization (D < 0.001 μm2⋅s−1) (Fig. 1 E and G, Fig. S2, and Movie S1). Trajectories with diffusion coefficients below 0.01 μm2⋅s−1 were called confined. The fraction of confined N-cadherin–mEos2 trajectories was significantly higher on Ncad-Fc micropatterns than on nonadhesive areas (42% vs. 25%, respectively) (Fig. 1 E and I), reflecting the formation of specific interactions with Ncad-Fc on the substrate. Similar to N-cadherin, α-catenin–GFP was strongly recruited at Ncad-Fc micropatterns (Fig. 1D and Table 1), and individual α-catenin–mEos2 molecules exhibited greater confinement at Ncad-Fc–coated micropatterns than on nonadhesive areas (Fig. 1 F, H, and I), revealing a preferential association with N-cadherin adhesions. The fact that we cannot resolve the motion of cytosolic molecules by sptPALM (19) and that the diffusion/trapping behaviors of N-cadherin–mEos2 and α-catenin–mEos2 were extremely similar strongly suggests that N-cadherin and α-catenin travel as a single complex in the membrane. In contrast, vinculin-GFP was not enriched at Ncad-Fc–coated micropatterns, and single vinculin-mEos2 molecules did not preferentially stop at these locations (Table 1 and Fig. S3 A–D), indicating a minor role of vinculin in the assembly of cadherin adhesions in hippocampal growth cones. Nevertheless, GFP- and mEos2-tagged vinculin, as well as endogenous vinculin, strongly accumulated at intercellular junctions between glial cells present in the cultures and at peripheral contacts between glial cells and Ncad-Fc–coated micropatterns (Fig. S3 F and G). These results demonstrate that tagged vinculin is functional and confirm that vinculin is selectively recruited at cadherin contacts under high mechanical constraint (22–24).
Table 1.
Enrichment of GFP tagged N-cadherin, α-catenin, actin, and vinculin at Ncad-Fc micropatterns
| Transfected protein | Ncad-GFP | α-Catenin–GFP | Actin-GFP | Vinculin-GFP |
| Epifluorescence | 1.35 ± 0.08 (19) | 1.27 ± 0.05 (10) | 1.04 ± 0.06 (15) | 1.04 ± 0.07 (11) |
| TIRF | 1.74 ± 0.09 (19) | 1.51 ± 0.08 (9) | 1.45 ± 0.05 (15) | 1.04 ± 0.04 (9) |
Fluorescence levels were normalized to control regions outside micropatterns, on the same growth cones. Values are presented as the mean ± SEM (number of growth cones examined).
Mapping and Regulation of the Actin Flow in Growth Cones.
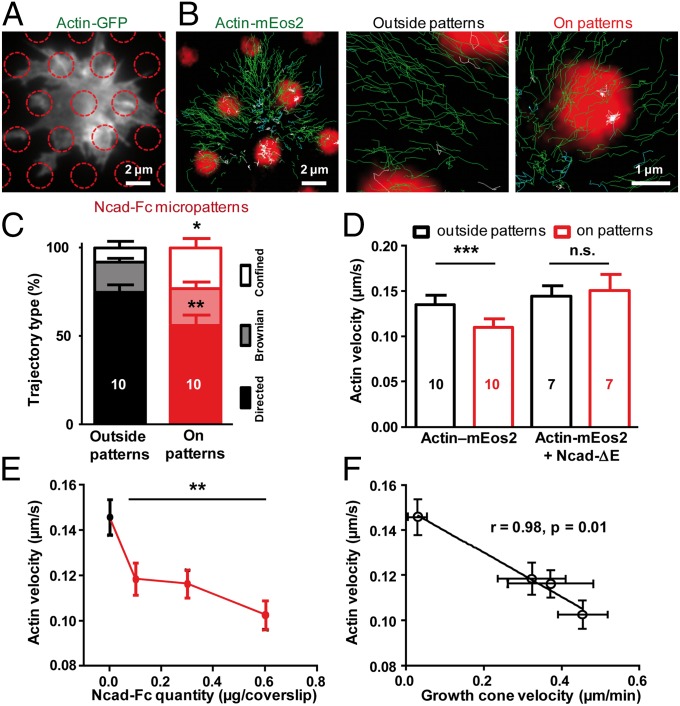
In contrast to the diffusion/trapping behavior of N-cadherin and α-catenin, individual actin-mEos2 molecules displayed directed rearward motion at the periphery of growth cones and then became diffusive or confined in the intermediary and central regions (Fig. 2B and Movie S2). Three types of trajectories were distinguished (directed, Brownian, or confined) according to the shape of the mean squared displacement over time (parabolic, linear, or anomalous, respectively) (Fig. S4). Directed trajectories yielded an average velocity of 0.14 μm⋅s−1, consistent with values obtained from actin-GFP kimographs in hippocampal neurons (11). The actin-capping drug cytochalasin D, the monomeric actin-sequestering drug latrunculin A, and the actin-stabilizing agent jasplakinolide all dramatically diminished the fraction of rearward-moving actin-mEos2 molecules (21%, 7%, and 2%, respectively) (Fig. S5), consistent with a major role of actin tread-milling in generating the flow (2, 3, 5). Myosin-light chain (MLC)-GFP was localized in the intermediary region of the growth cone, and MLC-Eos2 trajectories were mostly confined in this area (Fig. S6). Addition of the phosphatase inhibitor calyculin A to enhance myosin II contractility (29) induced a major retraction of the actin-GFP peripheral network in <5 min, an effect that was blocked by preincubation with the myosin II inhibitor blebbistatin. Finally, blebbistatin decreased the fraction of directed actin-mEos2 trajectories and reduced their velocity by 23%. Together, these results indicate that myosin II contributes to actin flow generation by pulling on actin filaments, consistent with previous studies (5, 30).
Fig. 2.
Actin flow is slowed down at N-cadherin micropatterns. (A) Growth cone expressing actin-GFP on Ncad-Fc–coated micropatterns (red circles) by epifluorescence. (B) Individual actin-mEos2 trajectories (green), with magnified views focusing on a micropattern or a neighboring nonadhesive area. Directed trajectories (exponent α > 1.4) are shown in green, diffusive trajectories (0.8 < α < 1.2) are shown in cyan, and confined trajectories (α < 0.8) are shown in white. (Scale bars: A and B, 2 μm; magnified views in B, 1 μm.) (C) Repartition of the three types of behaviors (directed, Brownian, and confined) for trajectories outside (black) and inside (red) Ncad-Fc patterns (479 and 535 trajectories, respectively). Fractions were compared by paired t tests (*P = 0.04; **P = 0.0045). (D) Velocity of actin-mEos2 molecules in growth cones from neurons electroporated with either actin-mEos2 alone or actin-mEos2 plus Ncad-ΔE and plated on Ncad-Fc–coated micropatterned substrates. Measurements were performed for trajectories exclusively inside or outside micropatterns. The mean ± SEM values of several growth cones are shown, and the number of growth cones examined is given in each column. Data were compared by paired t tests (***P = 0.0003). n.s., not significant. (E) Retrograde velocity of actin-mEos2 molecules in growth cones migrating on substrates uniformly coated with increasing concentrations of Ncad-Fc. Data were compared with the control PLL coating condition by the Kruskal–Wallis test (**P = 0.0062). (F) Negative correlation between actin-mEos2 velocity and growth cone forward velocity on substrates uniformly coated with Ncad-Fc (16). The numbers of growth cones examined are in the range of seven to 18 for actin-mEos2 velocity and 45–75 for growth cone migration speed.
Engagement of N-Cadherin Adhesions Reduces Actin Flow.
In contrast to N-cadherin and α-catenin, actin-GFP was not specifically enriched at Ncad-Fc–coated micropatterns under epifluorescence illumination (Fig. 2A and Table 1), suggesting that actin was weakly bound to the N-cadherin/α-catenin complex in growth cones. However, rearward actin velocity dropped with increasing Ncad-Fc coating concentration (Fig. 2E) and negatively correlated with forward growth cone migration speed (Fig. 2F), revealing partial clutch engagement between N-cadherin adhesion and actin (6). Furthermore, individual actin-mEos2 molecules often slowed down or stopped at Ncad-Fc–coated micropatterns (Fig. 2B), resulting in less directed (56% vs. 74%) and more confined (23% vs. 8%) trajectories at micropatterns compared with nonadhesive areas (Fig. 2C). The retrograde velocity of actin molecules displaying rearward motion was 20% lower on Ncad-Fc patterns than on cytophobic areas or on PLL-coated substrates (Fig. 2 D and E). To address the molecular mechanisms underlying this effect, neurons were coelectroporated with actin-mEos2 and an N-cadherin construct deleted of the whole extracellular domain (Ncad-ΔE), which was thus unable to form homophilic adhesions. This mutant acts as a dominant negative construct by sequestering α- and β-catenins, thereby interfering with the binding of actin to endogenous N-cadherin (16, 29). Upon Ncad-ΔE expression, the velocity of actin-mEos2 molecules on Ncad-Fc micropatterns was rescued to the high levels outside micropatterns (Fig. 2D), suggesting that the reduction of actin velocity on Ncad-Fc micropatterns involves a connection through catenins. This experiment also demonstrates that the slowing down of actin filaments on Ncad-Fc micropatterns is not due to a nonspecific frictional effect attributable to molecular crowding at adhesion sites.
Transient Pauses of Actin Molecules on N-Cadherin Micropatterns.
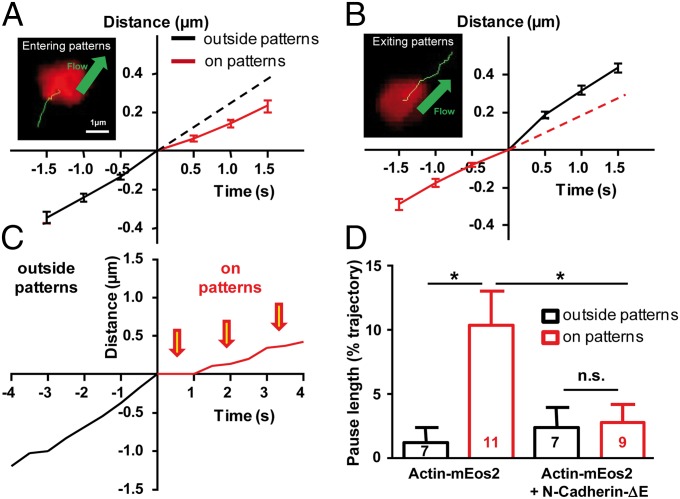
We then analyzed in detail actin-mEos2 trajectories, focusing on molecules spending part of their time inside a micropattern and the other part outside (Fig. 3 A and B). Actin-mEos2 molecules entering a pattern showed a significant reduction in velocity (Fig. 3A), whereas molecules exiting a pattern displayed acceleration (Fig. 3B). Thus, individual rearward-moving actin filaments can rapidly reduce their speed by contacting N-cadherin adhesions, and these interactions are spatially restricted to Ncad-Fc micropatterns, suggesting that the actin network is loosely interconnected. Actin molecules within Ncad-Fc micropatterns often exhibited pauses around 1–2 s, which appeared less frequently outside patterns (Fig. 3C). The percentage of the trajectory duration spent in pauses was approximately fivefold higher for actin trajectories localized within Ncad-Fc patterns than for those actin trajectories localized outside (Fig. 3D). This significant increase in pause proportion was blocked by the expression of Ncad-ΔE (Fig. 3D), indicating that these pauses reflect specific labile interactions between actin filaments and N-cadherin adhesions, mediated by catenins.
Fig. 3.
Transient pauses of actin filaments on N-cadherin micropatterns. (A and B) Examples of actin-mEos2 trajectories (green) entering and exiting Ncad-Fc patterns (red), respectively, and corresponding graphs showing displacement vs. time for the mean ± SEM of 23 entering and 31 exiting trajectories. Dashed lines that prolong the initial slopes are guides to the eye. (Scale bars: A and B, 1 μm.) (C) Individual example showing the displacement of a molecule entering a region coated with Ncad-Fc. Note that the trajectory was fairly linear before entering the region and showed multiple pauses thereafter (arrows). (D) Median of pause durations, normalized by the total duration of each trajectory, for neurons expressing actin-mEos2 alone or actin-mEos2 plus Ncad-ΔE. Data are presented as the mean ± SEM, with the number of growth cones indicated in the columns. Regions within and outside patterns were compared by unpaired t tests (*P < 0.05).
Different Turnover of N-Cadherin and Actin at N-Cadherin Micropatterns.
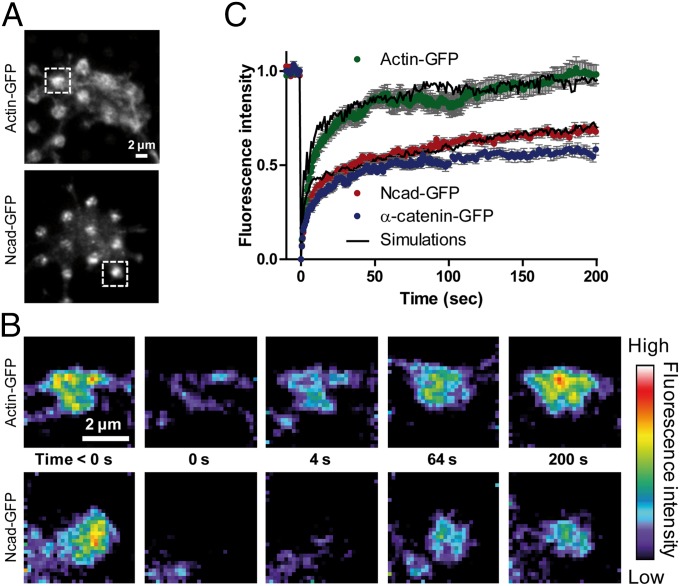
To assess whether N-cadherin and actin also exhibited differential dynamics at the ensemble level, we performed FRAP experiments on GFP-tagged N-cadherin, α-catenin, vinculin, and actin at Ncad-Fc micropatterns. We used total internal reflection fluorescence (TIRF) to isolate molecules close to the substrate (∼100 nm) (Fig. 4A). TIRF illumination confirmed the robust accumulation of Ncad-GFP at Ncad-Fc micropatterns (175% enrichment), but also revealed significant enrichment of actin-GFP (145%) organized in various ways at or around the micropatterns (Fig. S5C). Phalloidin staining was very strong at Ncad-Fc micropatterns in TIRF (Fig. S5D), demonstrating the recruitment of endogenous F-actin. Addition of latrunculin A to growth cones migrating on Ncad-Fc–coated substrates decreased the number of confined actin-mEos2 trajectories compared with untreated growth cones (Fig. S5 F and H), suggesting that G-actin was not selectively recruited at N-cadherin adhesions, consistent with assays using purified proteins (31). FRAP of actin-GFP revealed a rapid 80% recovery in about 50 s, followed by a slower recovery to 100% in the remaining 150 s (Fig. 4 B and C). These phases tightly correspond to the two populations of actin-mEos2 observed by sptPALM on Ncad-Fc–coated patterns (i.e., 80% of flowing molecules with directed or Brownian trajectories showing weak interactions with N-cadherin adhesions and 20% of confined molecules with long-lasting interactions) (Fig. 2 B and C). This second population likely contributes to actin enrichment at Ncad-Fc micropatterns. Ncad-GFP also recovered in two phases (Fig. 4 B and C): a rapid recovery within 20 s to ∼50%, roughly corresponding to the fraction of freely diffusing molecules identified in sptPALM, and a slower linear recovery reflecting molecules trapped by homophilic interactions at Ncad-Fc–coated micropatterns and slowly exchanging. The latter corresponds to the 45% fraction of confined N-cadherin molecules in sptPALM. α-Catenin–GFP behaved very similar to Ncad-GFP under FRAP (Fig. 4C), again indicating that N-cadherin and α-catenin were tightly associated. In contrast, vinculin-GFP recovered very fast (Fig. S3E), suggesting that it was not bound to Ncad-Fc patterns.
Fig. 4.
FRAP on actin-GFP, α-catenin–GFP, and Ncad-GFP accumulated at N-cadherin micropatterns. (A) Growth cones from neurons expressing Ncad-GFP or actin-GFP were observed by TIRF illumination, revealing significant enrichment of both proteins at Ncad-Fc patterns (circles). (Insets) Regions selected for photobleaching. (B) Ncad-GFP or actin-GFP signals were photobleached on two to three patterns per cone, with an ∼300-ms laser pulse, and the fluorescence recovery was recorded for 200 s (color coding). (Scale bars: A and B, 2 μm.) (C) Graphs of fluorescence recovery over time for Ncad-GFP, α-catenin–GFP, and actin-GFP. Solid dots represent the average of three independent experiments [10–20 growth cones, 18–50 patterns per condition ± SEM (gray)], and plain curves are the average of three independent simulations.
Model of Cadherin and Actin Dynamics.
To integrate these experimental data into a single consistent framework allowing comparison between single-molecule recordings and ensemble measurements, we performed Monte Carlo simulations describing the motion of single N-cadherin and actin molecules within growth cones containing localized adherent zones (Fig. S7 A–C). N-cadherin molecules were considered to move in the plasma membrane with D = 0.17 μm2⋅s−1, which was calculated from the mobile population in sptPALM, and were allowed to form transient interactions with Ncad-Fc immobilized at micropatterns, with a slow turnover rate (0.002 s−1) in agreement with values reported in the literature (32, 33) (Fig. 5A and Movie S3). Superimposition of thousands of individual simulations, mimicking a fluorescence image, revealed strong enrichment of N-cadherin on Ncad-Fc–coated micropatterns compared with nonadhesive areas (∼180%), as observed for Ncad-GFP with TIRF illumination (Fig. 5 C and F and Table 1). Furthermore, FRAP simulations showed recovery kinetics in close agreement with experimental data (Fig. 4C, Fig. S8A, and Movie S5), validating the coexistence of a rapidly diffusing (unbound) N-cadherin population and a fraction strongly bound to Ncad-Fc (Fig. S7D).
Fig. 5.
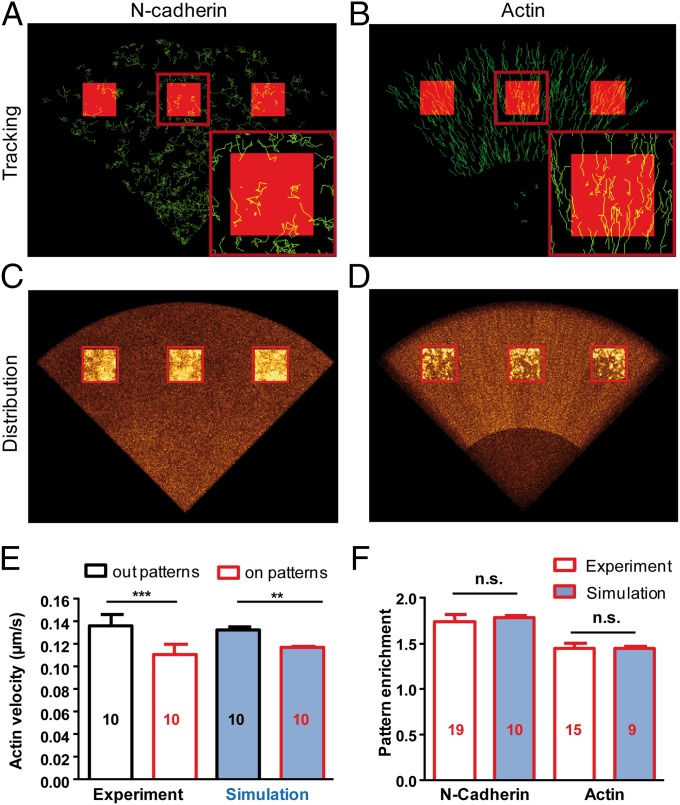
Simulations of N-cadherin and actin dynamics. (A) Computer-generated images showing trajectories of N-cadherin molecules in a model growth cone exhibiting Brownian diffusion outside adhesive patterns (dark) and binding to Ncad-Fc inside patterns (red squares). (B) Simulations showing the uniform rearward flow of actin molecules and transient trapping at Ncad-Fc patterns (red squares). (C and D) Projected images of N-cadherin and actin distribution (gold color coding), revealing differential enrichment at micropatterns. (E) Experimental and simulated actin retrograde velocity at patterns and control areas. **P < 0.01; ***P < 0.001. (F) Experimental and simulated enrichment of N-cadherin and actin at Ncad-Fc patterns. The number of independent simulations, each comprising 1,000 individual molecules, is given in the bars.
The actin model included fast free diffusion of monomers, slow rearward motion of actin filaments assembled at the tip, intermittent coupling to localized N-cadherin adhesions, and disassembly at the base (Fig. 5B and Movie S4). Association and dissociation rates between actin and the α-catenin/N-cadherin complex were taken as the frequency of the pauses (kon = 0.2 s−1) and the inverse of pause duration (koff = 0.8 s−1), respectively. The average actin velocity measured for trajectories localized within micropatterns was consistent with experimental values (0.11 μm⋅s−1), whereas normal velocity (0.14 μm⋅s−1) was restored when kon was set to zero, mimicking the effect of Ncad-ΔE (Fig. 5E). We further introduced a 20% actin population displaying durable interactions with N-cadherin adhesions (koff = 0.001 s−1), allowing us to mimic the 145% actin enrichment at Ncad-Fc micropatterns observed with TIRF (Fig. 5 D and F and Table 1) and to simulate the slower phase of actin-GFP recovery observed in FRAP experiments (Fig. 4C, Fig. S8B, and Movie S6). The model also predicted regulation of the balance between G-actin and F-actin by latrunculin A and jasplakinolide in both sptPALM and FRAP paradigms (Figs. S7E and S8C).
Discussion
This study exploring the dynamic coupling between actin motility and N-cadherin adhesions in growth cones can be summarized by a two-step model (Fig. 6): (i) The membrane-diffusing N-cadherin/catenin complex is trapped at Ncad-Fc–coated micropatterns, and (ii) flowing actin filaments interact with this adhesive complex through a combination of short- and long-lived interactions.
Fig. 6.
Two-step model of the interaction between flowing actin filaments and the immobilized N-cadherin/catenin complex. (A) Diffusional trapping of the N-cadherin/catenin complex at Ncad-Fc micropatterns. (B) Combination of labile and durable interactions between flowing actin filaments and immobilized N-cadherin/catenin complexes.
At the adhesion level, diffusion and trapping of N-cadherin and α-catenin are reminiscent of the behavior of integrins and talin at focal contacts in fibroblasts (19). FRAP experiments allowed us to calculate an average dwell time of ∼500 s, which is compatible with previous measurements on Ncad-GFP accumulated at Ncad-Fc beads in growth cones (32) or at focal adhesion-like structures formed by myogenic cells on substrates coated with Ncad-Fc (33). Introducing this parameter in the simulations, we mimicked the experimentally observed ∼180% enrichment of Ncad-GFP at micropatterns. This value is less than the fivefold enrichment observed for integrins at focal contacts with fibronectin (19), suggesting that Ncad-Fc binding sites are partially saturated with Ncad-GFP molecules, leaving out an ∼55% population of diffusive N-cadherin. α-Catenin exhibited a very similar behavior to N-cadherin in both sptPALM and FRAP, suggesting that observed α-catenin is mostly in the monomeric form, which associates strongly with N-cadherin and β-catenin, and not in the dimeric form, which preferentially binds actin (20, 21, 34). Vinculin also exhibited a mixture of diffusion and confinement but did not associate with N-cadherin adhesions in this system. It is noteworthy that we did not observe any directional movement of N-cadherin, α-catenin, or vinculin in growth cones in contrast to epithelial cell junctions, where both lateral and basal-to-apical flows of cadherins were documented (35, 36).
At the cytoskeletal level, actin molecules flowed rearward at a velocity very close to the speed of Ncad-Fc–coated microspheres placed on the growth cone surface (16), indicating that actin-mEos2 readily assembles into native actin filaments. Using pharmacological compounds, we showed that both actin polymerization forces and myosin II tension contributed to flow generation. Actin flow was high in the peripheral region, where actin monomers incorporate into new filaments, and slowed down toward the central region, where actin filaments disassemble, consistent with fluorescence speckle microscopy data (2, 18, 37) and the universal actin cycling model (38, 39). An important parameter is the time that a given actin monomer spends in the retrograde flow, which seems to be independent of the flow rate itself. For example, in growth cones, where the flow rate is high (∼0.14 μm⋅s−1), actin monomers travel a fairly long distance (∼10 μm) before disassembling, thus staying in the flow for around 1 min (2). In dendritic spines, where the actin flow rate is much slower (∼0.01 μm⋅s−1) (29, 40), the traveled distance is shorter (0.5 μm) but actin molecules also flow for a duration of about 1 min. Thus, this time scale seems to be imposed by an internal clock driven by actin assembly and disassembly reactions.
When studying growth cone locomotion on substrates uniformly coated with Ncad-Fc (16), the difference in actin-mEos2 velocities between the low and high Ncad-Fc concentrations (2.4 μm⋅min−1) remains larger than the respective gain in growth cone migration speed (0.6 μm⋅min−1). This result indicates that only a fraction of the flow energy is translated into forward motion, with the rest being dissipated or possibly converted into elastic energy (41). Consistent with this concept, hippocampal growth cones display an approximately threefold higher actin flow rate than growth cones from dorsal root ganglion neurons and exert comparatively less force on the substrate (11). The fact that Ncad-ΔE, which has previously been shown to decrease axonal length of neurons cultured on Ncad-Fc substrates (16), restored normal actin flow on Ncad-Fc–coated micropatterns confirms the inverse relationship between actin flow and growth cone migration speed.
Actin-mEos2 molecules made transient pauses on the order of 1–2 s at N-cadherin micropatterns, illustrating a slipping clutch mechanism (17). These short pauses are consistent with labile interactions between the ternary N-cadherin/β-catenin/α-catenin complex and actin (21, 31). Such rupture of individual molecular bonds between the flowing actin network and Ncad-Fc–coated beads was previously inferred from optical tweezers experiments (16). The duration of those 1-pN slip bonds was longer (∼5 s), owing to the more compliant optical trap compared with the rigid micropatterned substrate (42). Interestingly, those two experiments reflect the load-and-fail and frictional slippage clutch regimes predicted to occur at varying stiffness (13). The breakage forces (1 pN) remain lower than the 5–15 pN necessary to unfold single α-catenin molecules and recruit vinculin in magnetic tweezers experiments (43). This observation might explain why vinculin did not accumulate at Ncad-Fc–coated micropatterns in growth cones, whereas it was strongly recruited in glial cells adhering on the same substrates. Overall, growth cones exert very little stress on their environment (7, 11, 44) compared with the forces generated by epithelial cells at intercellular junctions or onto cadherin-coated beads or micropatterns, where vinculin is recruited and participates in contact strengthening (22–24).
Finally, sptPALM and FRAP experiments revealed an ∼20% near-immobile actin population at Ncad-Fc–coated micropatterns, corresponding to the accumulation of F-actin labeled with phalloidin under TIRF illumination. This process revealing full clutch engagement (17) resembles the dynamic assembly of a flexible actin network occurring at restrained beads coated with Aplysia neural cell adhesion molecule or N-cadherin (14, 16, 41). The confined fraction may correspond to flowing actin filaments being stalled by interactions with a high local density of N-cadherin/α-catenin complexes, as predicted theoretically (45). The various shapes of actin-GFP at Ncad-Fc patterns may reflect such a differential anchorage of flowing actin to N-cadherin adhesions. Alternatively, although G-actin sequestered by latrunculin A was not trapped at Ncad-Fc substrates, we cannot rule out a local nucleation of branched actin filaments (46, 47) at N-cadherin adhesions arising, for instance, from local depletion of homodimeric α-catenin competing with Arp2/3 for actin binding (20, 21). In any case, those strongly anchored actin filaments might bear the frictional force necessary for growth cones to move forward, whereas the more rapidly interacting filaments may allow dynamic sampling of the environment.
Methods
Experimental protocols are given in SI Methods. mEos2-tagged N-cadherin, α-catenin, vinculin, MLC, and actin were generated from GFP-tagged plasmids as described previously (16, 29, 32). Rat hippocampal neurons were dissected and electroporated with those plasmids before plating. Neurons were cultured on coverslips homogeneously coated with PLL or Ncad-Fc, or on Ncad-Fc–coated micropatterned glass substrates (16, 25). Immunostaining with antibodies to N-cadherin, α-catenin, and actin or with phalloidin was performed as described (29). SptPALM experiments using a 405-nm photoactivation laser and a 561-nm excitation laser were performed as reported (19), with high acquisition frequency for mEos2-tagged N-cadherin, α-catenin, and vinculin (50 Hz) and a slow frame rate for actin-mEos2 and MLC-Eos2 (2 Hz) (40). Image stacks were analyzed using custom analysis programs. FRAP experiments on GFP-tagged N-cadherin, α-catenin, vinculin, and actin were performed using a focused 491-nm laser, and recordings were made by TIRF illumination. Computer simulations of N-cadherin and actin dynamics were based on previous frameworks (48).
Supplementary Material
Acknowledgments
We thank R. M. Mège, P. O. Strale, M. P. Sheetz, C. Holt, A. Bresnick, and A. Matus for the gift of plasmids; M. Mondin, P. Legros, C. Poujol, and S. Marais for support in microscopy (Bordeaux Imaging Center, part of the FranceBioImaging national infrastructure); O. Rossier and G. Giannone for single-molecule tracking; K. Czöndör and A. Azioune for micropattern coating; M. Bornens for helpful discussions; A. Fuchs and M. L. Calvo-Munoz for chip production; and B. Tessier, D. Bouchet, C. Breillat, A. Frouin, N. Retailleau, Z. Karatas, and R. Sterling for cell culture and technical assistance. This work received funding from the Centre National de la Recherche Scientifique, Agence Nationale pour la Recherche (Grant Synapse-2Dt), Conseil Régional Aquitaine, Fondation pour la Recherche Médicale, LabEx BRAIN Cluster of Excellence and Ministère de l’Enseignement Supérieur et de la Recherche (ANR-10-INBS-04 FranceBioImaging).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1423455112/-/DCSupplemental.
References
- 1.Vitriol EA, Zheng JQ. Growth cone travel in space and time: The cellular ensemble of cytoskeleton, adhesion, and membrane. Neuron. 2012;73(6):1068–1081. doi: 10.1016/j.neuron.2012.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Van Goor D, Hyland C, Schaefer AW, Forscher P. The role of actin turnover in retrograde actin network flow in neuronal growth cones. PLoS ONE. 2012;7(2):e30959. doi: 10.1371/journal.pone.0030959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Craig EM, Van Goor D, Forscher P, Mogilner A. Membrane tension, myosin force, and actin turnover maintain actin treadmill in the nerve growth cone. Biophys J. 2012;102(7):1503–1513. doi: 10.1016/j.bpj.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Diefenbach TJ, et al. Myosin 1c and myosin IIB serve opposing roles in lamellipodial dynamics of the neuronal growth cone. J Cell Biol. 2002;158(7):1207–1217. doi: 10.1083/jcb.200202028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Medeiros NA, Burnette DT, Forscher P. Myosin II functions in actin-bundle turnover in neuronal growth cones. Nat Cell Biol. 2006;8(3):215–226. doi: 10.1038/ncb1367. [DOI] [PubMed] [Google Scholar]
- 6.Lin CH, Forscher P. Growth cone advance is inversely proportional to retrograde F-actin flow. Neuron. 1995;14(4):763–771. doi: 10.1016/0896-6273(95)90220-1. [DOI] [PubMed] [Google Scholar]
- 7.Bridgman PC, Dave S, Asnes CF, Tullio AN, Adelstein RS. Myosin IIB is required for growth cone motility. J Neurosci. 2001;21(16):6159–6169. doi: 10.1523/JNEUROSCI.21-16-06159.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thoumine O. Interplay between adhesion turnover and cytoskeleton dynamics in the control of growth cone migration. Cell Adhes Migr. 2008;2(4):263–267. doi: 10.4161/cam.2.4.7274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mitchison T, Kirschner M. Cytoskeletal dynamics and nerve growth. Neuron. 1988;1(9):761–772. doi: 10.1016/0896-6273(88)90124-9. [DOI] [PubMed] [Google Scholar]
- 10.Suter DM, Forscher P. Transmission of growth cone traction force through apCAM-cytoskeletal linkages is regulated by Src family tyrosine kinase activity. J Cell Biol. 2001;155(3):427–438. doi: 10.1083/jcb.200107063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koch D, Rosoff WJ, Jiang J, Geller HM, Urbach JS. Strength in the periphery: Growth cone biomechanics and substrate rigidity response in peripheral and central nervous system neurons. Biophys J. 2012;102(3):452–460. doi: 10.1016/j.bpj.2011.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schmidt CE, Dai J, Lauffenburger DA, Sheetz MP, Horwitz AF. Integrin-cytoskeletal interactions in neuronal growth cones. J Neurosci. 1995;15(5 Pt 1):3400–3407. doi: 10.1523/JNEUROSCI.15-05-03400.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chan CE, Odde DJ. Traction dynamics of filopodia on compliant substrates. Science. 2008;322(5908):1687–1691. doi: 10.1126/science.1163595. [DOI] [PubMed] [Google Scholar]
- 14.Suter DM, Errante LD, Belotserkovsky V, Forscher P. The Ig superfamily cell adhesion molecule, apCAM, mediates growth cone steering by substrate-cytoskeletal coupling. J Cell Biol. 1998;141(1):227–240. doi: 10.1083/jcb.141.1.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shimada T, et al. Shootin1 interacts with actin retrograde flow and L1-CAM to promote axon outgrowth. J Cell Biol. 2008;181(5):817–829. doi: 10.1083/jcb.200712138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bard L, et al. A molecular clutch between the actin flow and N-cadherin adhesions drives growth cone migration. J Neurosci. 2008;28(23):5879–5890. doi: 10.1523/JNEUROSCI.5331-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Giannone G, Mège R-M, Thoumine O. Multi-level molecular clutches in motile cell processes. Trends Cell Biol. 2009;19(9):475–486. doi: 10.1016/j.tcb.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 18.Hu K, Ji L, Applegate KT, Danuser G, Waterman-Storer CM. Differential transmission of actin motion within focal adhesions. Science. 2007;315(5808):111–115. doi: 10.1126/science.1135085. [DOI] [PubMed] [Google Scholar]
- 19.Rossier O, et al. Integrins β1 and β3 exhibit distinct dynamic nanoscale organizations inside focal adhesions. Nat Cell Biol. 2012;14(10):1057–1067. doi: 10.1038/ncb2588. [DOI] [PubMed] [Google Scholar]
- 20.Drees F, Pokutta S, Yamada S, Nelson WJ, Weis WI. Alpha-catenin is a molecular switch that binds E-cadherin-beta-catenin and regulates actin-filament assembly. Cell. 2005;123(5):903–915. doi: 10.1016/j.cell.2005.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Benjamin JM, et al. AlphaE-catenin regulates actin dynamics independently of cadherin-mediated cell-cell adhesion. J Cell Biol. 2010;189(2):339–352. doi: 10.1083/jcb.200910041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.le Duc Q, et al. Vinculin potentiates E-cadherin mechanosensing and is recruited to actin-anchored sites within adherens junctions in a myosin II-dependent manner. J Cell Biol. 2010;189(7):1107–1115. doi: 10.1083/jcb.201001149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yonemura S, Wada Y, Watanabe T, Nagafuchi A, Shibata M. alpha-Catenin as a tension transducer that induces adherens junction development. Nat Cell Biol. 2010;12(6):533–542. doi: 10.1038/ncb2055. [DOI] [PubMed] [Google Scholar]
- 24.Thomas WA, et al. α-Catenin and vinculin cooperate to promote high E-cadherin-based adhesion strength. J Biol Chem. 2013;288(7):4957–4969. doi: 10.1074/jbc.M112.403774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Czöndör K, et al. Micropatterned substrates coated with neuronal adhesion molecules for high-content study of synapse formation. Nat Commun. 2013;4:2252. doi: 10.1038/ncomms3252. [DOI] [PubMed] [Google Scholar]
- 26.Hirano S, Takeichi M. Cadherins in brain morphogenesis and wiring. Physiol Rev. 2012;92(2):597–634. doi: 10.1152/physrev.00014.2011. [DOI] [PubMed] [Google Scholar]
- 27.McKinney SA, Murphy CS, Hazelwood KL, Davidson MW, Looger LL. A bright and photostable photoconvertible fluorescent protein. Nat Methods. 2009;6(2):131–133. doi: 10.1038/nmeth.1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Manley S, et al. High-density mapping of single-molecule trajectories with photoactivated localization microscopy. Nat Methods. 2008;5(2):155–157. doi: 10.1038/nmeth.1176. [DOI] [PubMed] [Google Scholar]
- 29.Chazeau A, et al. Mechanical coupling between transsynaptic N-cadherin adhesions and actin flow stabilizes dendritic spines. Mol Biol Cell. 2015;26(5):859–873. doi: 10.1091/mbc.E14-06-1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang Q, Zhang X-F, Pollard TD, Forscher P. Arp2/3 complex-dependent actin networks constrain myosin II function in driving retrograde actin flow. J Cell Biol. 2012;197(7):939–956. doi: 10.1083/jcb.201111052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yamada S, Pokutta S, Drees F, Weis WI, Nelson WJ. Deconstructing the cadherin-catenin-actin complex. Cell. 2005;123(5):889–901. doi: 10.1016/j.cell.2005.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thoumine O, Lambert M, Mège R-M, Choquet D. Regulation of N-cadherin dynamics at neuronal contacts by ligand binding and cytoskeletal coupling. Mol Biol Cell. 2006;17(2):862–875. doi: 10.1091/mbc.E05-04-0335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lambert M, et al. Nucleation and growth of cadherin adhesions. Exp Cell Res. 2007;313(19):4025–4040. doi: 10.1016/j.yexcr.2007.07.035. [DOI] [PubMed] [Google Scholar]
- 34.Pokutta S, Choi H-J, Ahlsen G, Hansen SD, Weis WI. Structural and thermodynamic characterization of cadherin·β-catenin·α-catenin complex formation. J Biol Chem. 2014;289(19):13589–13601. doi: 10.1074/jbc.M114.554709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peglion F, Llense F, Etienne-Manneville S. Adherens junction treadmilling during collective migration. Nat Cell Biol. 2014;16(7):639–651. doi: 10.1038/ncb2985. [DOI] [PubMed] [Google Scholar]
- 36.Kametani Y, Takeichi M. Basal-to-apical cadherin flow at cell junctions. Nat Cell Biol. 2007;9(1):92–98. doi: 10.1038/ncb1520. [DOI] [PubMed] [Google Scholar]
- 37.Vallotton P, Gupton SL, Waterman-Storer CM, Danuser G. Simultaneous mapping of filamentous actin flow and turnover in migrating cells by quantitative fluorescent speckle microscopy. Proc Natl Acad Sci USA. 2004;101(26):9660–9665. doi: 10.1073/pnas.0300552101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pollard TD, Cooper JA. Actin, a central player in cell shape and movement. Science. 2009;326(5957):1208–1212. doi: 10.1126/science.1175862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bindschadler M, Osborn EA, Dewey CF, Jr, McGrath JL. A mechanistic model of the actin cycle. Biophys J. 2004;86(5):2720–2739. doi: 10.1016/S0006-3495(04)74326-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chazeau A, et al. Nanoscale segregation of actin nucleation and elongation factors determines dendritic spine protrusion. EMBO J. 2014;33(23):2745–2764. doi: 10.15252/embj.201488837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mejean CO, et al. Elastic coupling of nascent apCAM adhesions to flowing actin networks. PLoS ONE. 2013;8(9):e73389. doi: 10.1371/journal.pone.0073389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Evans EA, Calderwood DA. Forces and bond dynamics in cell adhesion. Science. 2007;316(5828):1148–1153. doi: 10.1126/science.1137592. [DOI] [PubMed] [Google Scholar]
- 43.Yao M, et al. Force-dependent conformational switch of α-catenin controls vinculin binding. Nat Commun. 2014;5:4525. doi: 10.1038/ncomms5525. [DOI] [PubMed] [Google Scholar]
- 44.Hyland C, Mertz AF, Forscher P, Dufresne E. Dynamic peripheral traction forces balance stable neurite tension in regenerating Aplysia bag cell neurons. Sci Rep. 2014;4:4961. doi: 10.1038/srep04961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bangasser BL, Rosenfeld SS, Odde DJ. Determinants of maximal force transmission in a motor-clutch model of cell traction in a compliant microenvironment. Biophys J. 2013;105(3):581–592. doi: 10.1016/j.bpj.2013.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Korobova F, Svitkina T. Arp2/3 complex is important for filopodia formation, growth cone motility, and neuritogenesis in neuronal cells. Mol Biol Cell. 2008;19(4):1561–1574. doi: 10.1091/mbc.E07-09-0964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.San Miguel-Ruiz JE, Letourneau PC. The role of Arp2/3 in growth cone actin dynamics and guidance is substrate dependent. J Neurosci. 2014;34(17):5895–5908. doi: 10.1523/JNEUROSCI.0672-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Czöndör K, et al. Unified quantitative model of AMPA receptor trafficking at synapses. Proc Natl Acad Sci USA. 2012;109(9):3522–3527. doi: 10.1073/pnas.1109818109. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.