Abstract
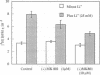
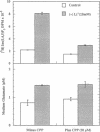
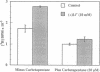
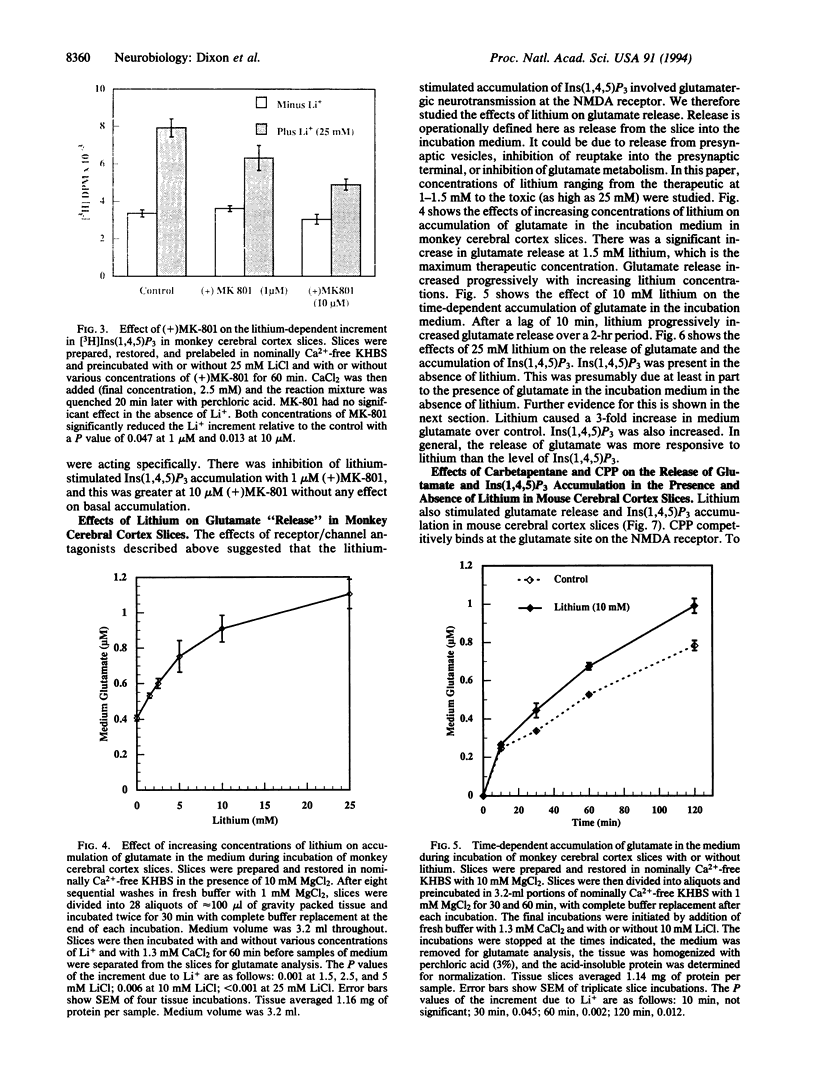
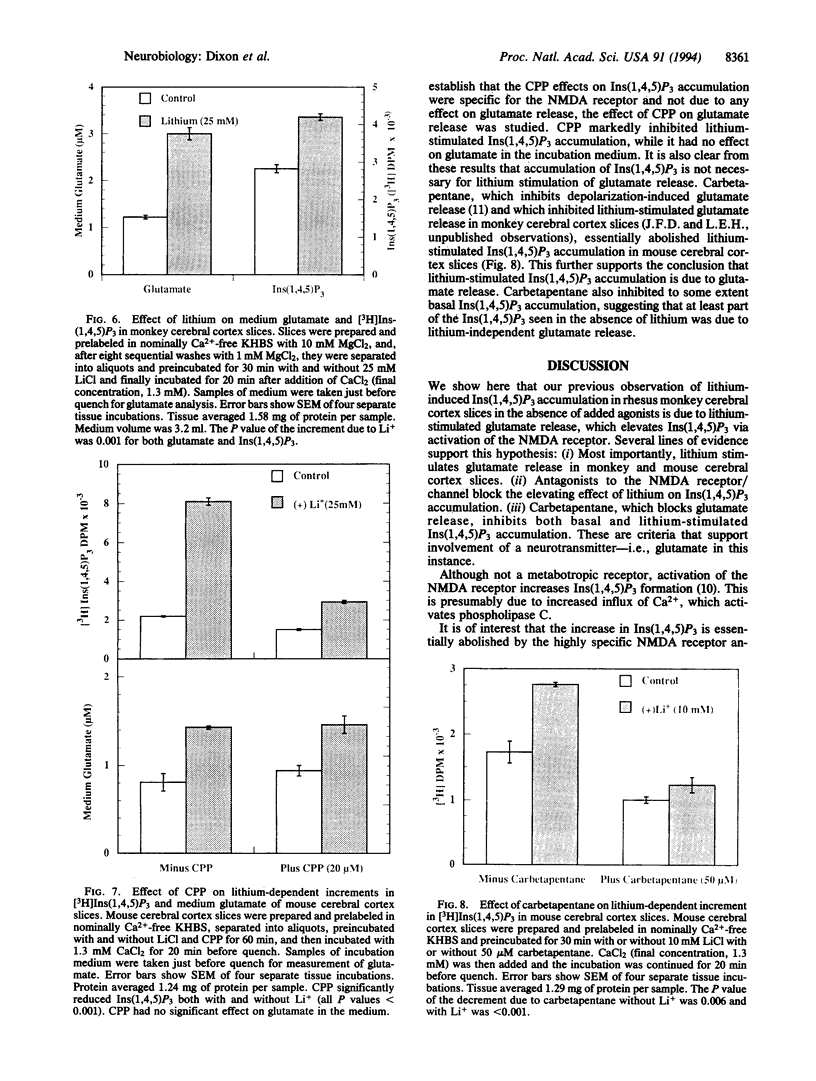
Beginning at therapeutic concentrations (1-1.5 mM), the anti-manic-depressive drug lithium stimulated the release of glutamate, a major excitatory neurotransmitter in the brain, in monkey cerebral cortex slices in a time- and concentration-dependent manner, and this was associated with increased inositol 1,4,5-trisphosphate [Ins(1,4,5)P3] accumulation. (+/-)-3-(2-Carboxypiperazin-4-yl)propyl-1-phosphoric acid (CPP), dizocilpine (MK-801), ketamine, and Mg(2+)-antagonists to the N-methyl-D-aspartate (NMDA) receptor/channel complex selectively inhibited lithium-stimulated Ins(1,4,5)P3 accumulation. Antagonists to cholinergic-muscarinic, alpha 1-adrenergic, 5-hydroxytryptamine2 (serotoninergic), and H1 histaminergic receptors had no effect. Antagonists to non-NMDA glutamate receptors had no effect on lithium-stimulated Ins(1,4,5)P3 accumulation. Possible reasons for this are discussed. Similar results were obtained in mouse cerebral cortex slices. Carbetapentane, which inhibits glutamate release, inhibited lithium-induced Ins(1,4,5)P3 accumulation in this model. It is concluded that the primary effect of lithium in the cerebral cortex slice model is stimulation of glutamate release, which, presumably via activation of the NMDA receptor, leads to Ca2+ entry. Ins(1,4,5)P3 accumulation increases due to the presumed increased influx of intracellular Ca2+, which activates phospholipase C. These effects may have relevance to the therapeutic action of lithium in the treatment of manic depression as well as its toxic effects, especially at lithium blood levels above 1.5 mM.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison J. H., Stewart M. A. Reduced brain inositol in lithium-treated rats. Nat New Biol. 1971 Oct 27;233(43):267–268. doi: 10.1038/newbio233267a0. [DOI] [PubMed] [Google Scholar]
- Annels S. J., Ellis Y., Davies J. A. Non-opioid antitussives inhibit endogenous glutamate release from rabbit hippocampal slices. Brain Res. 1991 Nov 15;564(2):341–343. doi: 10.1016/0006-8993(91)91474-f. [DOI] [PubMed] [Google Scholar]
- Batty I. H., Michie A., Fennel M., Downes C. P. The characteristics, capacity and receptor regulation of inositol uptake in 1321N1 astrocytoma cells. Biochem J. 1993 Aug 15;294(Pt 1):49–55. doi: 10.1042/bj2940049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon J. F., Lee C. H., Los G. V., Hokin L. E. Lithium enhances accumulation of [3H]inositol radioactivity and mass of second messenger inositol 1,4,5-trisphosphate in monkey cerebral cortex slices. J Neurochem. 1992 Dec;59(6):2332–2335. doi: 10.1111/j.1471-4159.1992.tb10129.x. [DOI] [PubMed] [Google Scholar]
- Feig S., Lipton P. N-methyl-D-aspartate receptor activation and Ca2+ account for poor pyramidal cell structure in hippocampal slices. J Neurochem. 1990 Aug;55(2):473–483. doi: 10.1111/j.1471-4159.1990.tb04160.x. [DOI] [PubMed] [Google Scholar]
- Hokin L. E., Dixon J. F. The phosphoinositide signalling system. I. Historical background. II. Effects of lithium on the accumulation of second messenger inositol 1,4,5-trisphosphate in brain cortex slices. Prog Brain Res. 1993;98:309–315. doi: 10.1016/s0079-6123(08)62413-9. [DOI] [PubMed] [Google Scholar]
- Jope R. S., Williams M. B. Lithium and brain signal transduction systems. Biochem Pharmacol. 1994 Feb 9;47(3):429–441. doi: 10.1016/0006-2952(94)90172-4. [DOI] [PubMed] [Google Scholar]
- Kennedy E. D., Challiss R. A., Nahorski S. R. Lithium reduces the accumulation of inositol polyphosphate second messengers following cholinergic stimulation of cerebral cortex slices. J Neurochem. 1989 Nov;53(5):1652–1655. doi: 10.1111/j.1471-4159.1989.tb08566.x. [DOI] [PubMed] [Google Scholar]
- Lee C. H., Dixon J. F., Reichman M., Moummi C., Los G., Hokin L. E. Li+ increases accumulation of inositol 1,4,5-trisphosphate and inositol 1,3,4,5-tetrakisphosphate in cholinergically stimulated brain cortex slices in guinea pig, mouse and rat. The increases require inositol supplementation in mouse and rat but not in guinea pig. Biochem J. 1992 Mar 1;282(Pt 2):377–385. doi: 10.1042/bj2820377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer M. L., Miller R. J. Excitatory amino acid receptors, second messengers and regulation of intracellular Ca2+ in mammalian neurons. Trends Pharmacol Sci. 1990 Jun;11(6):254–260. doi: 10.1016/0165-6147(90)90254-6. [DOI] [PubMed] [Google Scholar]
- Nowak G., Trullas R., Layer R. T., Skolnick P., Paul I. A. Adaptive changes in the N-methyl-D-aspartate receptor complex after chronic treatment with imipramine and 1-aminocyclopropanecarboxylic acid. J Pharmacol Exp Ther. 1993 Jun;265(3):1380–1386. [PubMed] [Google Scholar]
- Schoepp D., Bockaert J., Sladeczek F. Pharmacological and functional characteristics of metabotropic excitatory amino acid receptors. Trends Pharmacol Sci. 1990 Dec;11(12):508–515. doi: 10.1016/0165-6147(90)90052-a. [DOI] [PubMed] [Google Scholar]
- Sherman W. R., Gish B. G., Honchar M. P., Munsell L. Y. Effects of lithium on phosphoinositide metabolism in vivo. Fed Proc. 1986 Oct;45(11):2639–2646. [PubMed] [Google Scholar]
- Whitworth P., Kendall D. A. Lithium selectively inhibits muscarinic receptor-stimulated inositol tetrakisphosphate accumulation in mouse cerebral cortex slices. J Neurochem. 1988 Jul;51(1):258–265. doi: 10.1111/j.1471-4159.1988.tb04865.x. [DOI] [PubMed] [Google Scholar]