Abstract
Trastuzumab has significantly improved the median survival of patients with HER2-positive breast cancer. In metastatic disease, maintenance trastuzumab is usually given after tumour response has been achieved with the combination of chemotherapy and trastuzumab, with the aim of prolonging time to disease progression. We report a case where a durable complete response (CR) was achieved without maintenance trastuzumab. In the absence of consensus guidelines, it is difficult to recommend which HER2-positive patients with metastatic breast cancer after CR will benefit from withdrawing maintenance trastuzumab therapy and when this could be considered.
Background
Primary metastatic breast cancer (MBC), defined as the presence of distant metastases within 3 months of the initial diagnosis of breast cancer, is rare and comprises 3–10% of all breast cancers.1 The advent of trastuzumab, a humanised monoclonal antibody against the HER2 protein, has revolutionised and greatly improved the natural history and survival of patients with HER2-positive MBC. Prolonged CR from MBC is frequently observed while patients are on extended trastuzumab therapy.2 However, a few reports exist of patients who achieved a sustained CR without subsequent maintenance trastuzumab. What is not known is whether there is a subgroup of patients with HER2-positive MBC who achieve sustained CR, where discontinuation of maintenance trastuzumab could be considered.
Case presentation
A 70-year-old woman presented to The Queen Elizabeth Hospital with a self-detected left (L) breast lump. She had a background history of hypertension, vitamin B12 deficiency and a ventriculoperitoneal shunt for normal pressure hydrocephalus 6 years earlier. She had no family history of breast cancer but had been using hormone replacement therapy for 5 years prior to the diagnosis of breast cancer. Mammography and breast ultrasound confirmed the presence of a suspicious 30–40 mm lesion located in the left upper outer quadrant of the breast associated with (L) axillary lymphadenopathy. Core biopsy of the breast and axillary lymph node (LN) confirmed malignancy. CT staging showed extensive axillary lymphadenopathy in addition to numerous small (1–2 mm) indeterminate bilateral parenchymal lung nodules.
The patient underwent (L) mastectomy and axillary LN dissection. Histology revealed a 70 mm, grade 3, invasive carcinoma of no specific type, which was oestrogen and progesterone receptor negative. A high level of HER2 gene amplification was demonstrated by chromogenic in situ hybridisation (CISH) with >10 HER2 gene copy number per 40 cells detected. All 33 LN analysed were positive for metastatic involvement. The patient was then referred for systemic therapy. In view of the indeterminate nature of the numerous lung nodules evident on the preoperative CT, a repeat CT of the chest/abdomen/pelvis was performed, which showed mediastinal and hilar lymphadenopathy, an increase in number and size of the numerous pulmonary nodules, and multiple hypodensities within the liver, all consistent with metastatic disease (figures 1 and 2). Palliative chemotherapy was started with paclitaxel 80 mg/m2 weekly, and trastuzumab 4 mg/kg loading dose followed by 2 mg/kg weekly. In total, the patient had only five doses of weekly paclitaxel over a 3-month period. Omission of paclitaxel was required on three occasions, due to the toxicities, of grade 2 diarrhoea or fatigue. Weekly trastuzumab was given regularly with no treatment delays. A restaging CT scan prior to week 8 of trastuzumab revealed marked radiological response with near complete resolution of pulmonary and liver metastases. In view of the significant radiological response to treatment and difficulty tolerating paclitaxel, treatment was changed to 3-weekly trastuzumab monotherapy. However, a subsequent gated blood pool scan indicated a decline in left ventricular ejection fraction (LVEF) from a baseline of 70% to 50%. As a result, trastuzumab was not administered for the next 6 weeks. Despite this, CT restaging 2 months later showed complete radiological response with no evidence of metastatic disease. LVEF remained depressed on repeat gated heart pool scan at 50%, and the decision was made to permanently cease trastuzumab after discussion with the patient. The total cumulative dose of trastuzumab administered was 1170 mg. On serial follow-up since ceasing treatment over 6.5 years, the patient has remained progression-free (figures 3 and 4).
Figure 1.
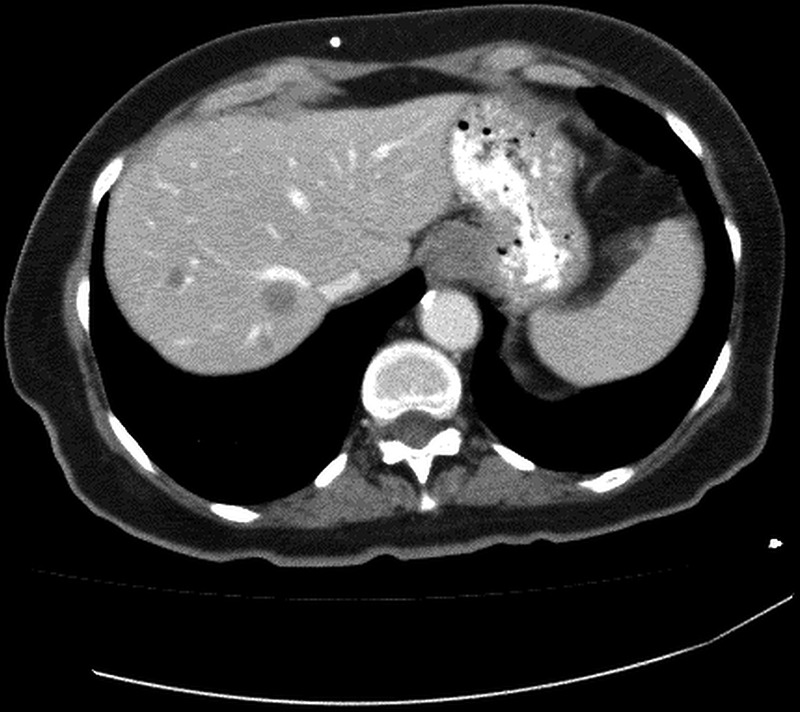
Baseline CT of metastatic liver lesions.
Figure 2.
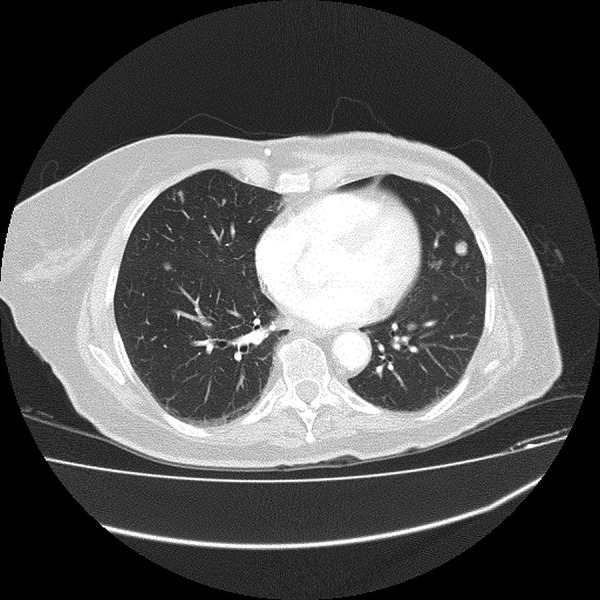
Baseline CT of metastatic lung lesion.
Figure 3.
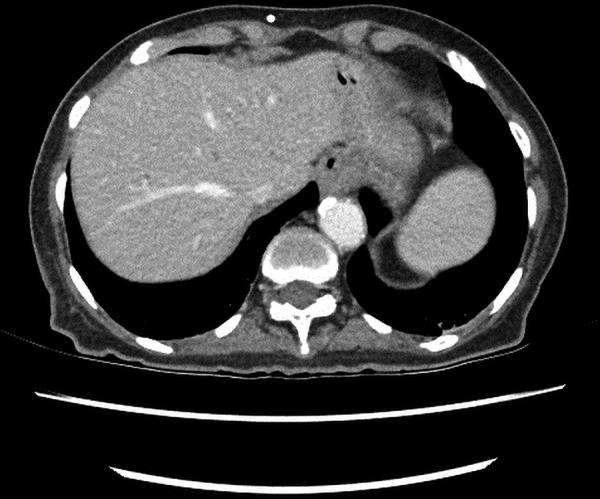
Follow-up CT of liver—no metastases.
Figure 4.
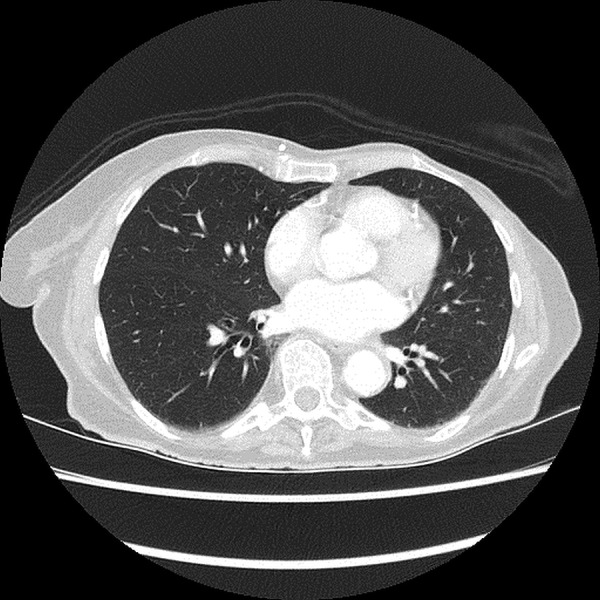
Follow-up CT of lung—no metastases.
Discussion
Despite the advent of many new-targeted therapies, the average survival after diagnosis of MBC is approximately 2 years.3 Patients with HER2-positive breast cancer previously had a poorer prognosis. However, this has significantly improved since the development and routine use of trastuzumab. The combination of trastuzumab and a taxane in the first-line treatment of MBC results in an overall response rate (RR) of 49–73%.4–8 CR rates achieved from the combination of trastuzumab and chemotherapy have been reported to be between 7 and 17% and long-term survivors are increasingly being recognised.9–12 Following combination treatment with trastuzumab and chemotherapy, trastuzumab is traditionally continued until disease progression with many patients remaining on maintenance trastuzumab for many years.12–14
The case presented here is of a patient who achieved a durable CR to trastuzumab-based therapy despite receiving only a short course of treatment and no maintenance therapy. While this is an unusual outcome and not considered standard care, it does illustrate that there is a group of patients who have exquisitely sensitive tumours and respond rapidly to trastuzumab-based therapy. This case raises a number of questions regarding the management of HER2-positive MBC. Can we predict in advance which patients will achieve durable control of their disease with trastuzumab-based therapy? Can trastuzumab maintenance treatment safely be stopped? In which patients and when? And are there any guidelines available to aid clinicians in making these decisions?
The US cohort study, registHER, recently identified 244 long-term survivors from the 1001 patients with HER2-positive MBC entered on the registry. Seventy-one per cent of long-term survivors had achieved a CR to first-line therapy, and the median progression-free survival (PFS) was 37 months. Long-term survivors were more likely to present with de novo MBC, have hormone receptor (HR) positive disease, have bone or local disease (no visceral disease), and have received a taxane and trastuzumab as their first-line therapy.15 A small retrospective study identified 11 patients who achieved a durable CR (lasting ≥36 months) out of a total of 120 (9%) with HER2-positive MBC; these patients were more likely to have HR-negative disease and liver only metastases.11 These results, which fail to define a consistent subgroup based on clinical factors, suggest that those with durable CR likely represent a distinct molecular subgroup, and molecular analysis of these tumour samples will be important.
A number of potential biomarkers and imaging techniques have been assessed in an attempt to predict patients who will achieve the greatest benefit from trastuzumab. The degree of HER 2 gene amplification has been associated with response to trastuzumab with high HER2 gene copy number associated with increased rates of pathological CR (pCR) following neoadjuvant treatment as well as improved CR and progression-free survival in metastatic disease, when compared to those with low HER2 gene copy number.16–18 Interestingly, high levels of HER2 gene amplification are also associated with HR-negative disease and grade 3 tumours, as in this case.19 HER2 enriched tumours, as assessed using the PAM50 intrinsic molecular subtyping, also achieve higher pCR rates to neoadjuvant therapy compared to the other PAM50 molecular subtypes, and in metastatic disease, those with HER2 enriched tumours were more likely to persist on single agent trastuzumab or lapatinib.16 20 In addition, 18F-fluorodeoxyglucose positron emission tomography (PET)/CT to look for ‘metabolic responders’, characterised by reduction in SUVmax <2.1 in the primary tumour as early as 2–6 weeks after trastuzumab administration in the neoadjuvant setting, may identify patients with an increased likelihood of pCR to trastuzumab-based therapy.21–23 Currently, none of these methods are used in clinical decision-making and future confirmatory studies will be required to determine the utility of these biomarkers and the role of PET in selecting patients who may be suitable to cease trastuzumab maintenance.
Historically, chemotherapy for most ‘solid’ cancers was not considered a curative approach. Since the introduction of anti-HER2 therapy, we see a proportion of patients who have prolonged clinical CR. Therefore, in those patients with ongoing durable CR can maintenance trastuzumab safely be stopped and when? Some investigators have attempted to define this. A retrospective study from two institutions included 84 HER2-positive patients with MBC and found 15% achieved a CR to trastuzumab and 9% remained in durable CR. One institution ceased maintenance trastuzumab after 2 years while the other withdrew trastuzumab after at least 5 years of maintenance therapy. A higher proportion of patients with durable CR were observed in the institution that administered maintenance trastuzumab for the longer duration of 5 years as opposed to 2 years (11% vs 6%). This occurred despite the frequency of CR rates achieved in the two institutions being similar at 15–16% post completion of combination therapy with chemotherapy and trastuzumab. Furthermore, the duration of PFS was also longer in the institution administering 5 years of maintenance trastuzumab (7–10.2 years) compared with the institution administering 2 years maintenance trastuzumab (3.1–3.7 years).9 Although the study is not a prospective study and is of relatively small numbers, these findings suggest the duration of maintenance trastuzumab administered may be important in altering the natural course of HER2-positive MBC.
At present, there is a lack of internationally recognised consensus guidelines from any of the recognised cancer organisations available to assist in the decision to cease maintenance trastuzumab.12 The current American Society of Clinical Oncology (ASCO) guidelines conclude that adequate data are not available to provide a statement on when to stop administering trastuzumab.24 Currently, most clinicians do not stop maintenance trastuzumab unless contraindicated.10 13 This case report, however, illustrates that in some circumstances no detrimental effect is seen after stopping trastuzumab and that there is likely to be a subgroup of patients where this is appropriate.
Currently, there are also no biomarkers available to predict those patients likely to achieve a durable CR following trastuzumab-based therapy and, therefore, when to consider ceasing maintenance trastuzumab. Potential predictive profiles may include high HER2 gene amplification, or HER2 enriched tumours on molecular subtyping, but future studies will be required to better define this group. In the meantime, for those patients who have remained in durable CR for a number of years on maintenance trastuzumab, any decision to cease trastuzumab will need to be considered carefully and most would recommend follow-up to involve an ‘active surveillance’ protocol. In the future, increasing numbers of patients will be on dual HER2 blockade, which will further increase the cost of maintenance therapy and the length of maintenance therapy will seriously need to be considered.
Learning points.
Combination therapy with chemotherapy and trastuzumab is beneficial in treating patients with HER2-positive metastatic breast cancer.
Durable complete response can be achieved.
The optimal duration of maintenance trastuzumab prior to considering stopping is yet to be defined.
Predictive biomarkers or robust functional imaging is required, together with guidelines, to assist future decision-making.
Footnotes
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Uyetrub U, Oksuzoglu B, Akman T et al. Assessment of tumour characteristics and factors affecting survival in patients with primary metastatic breast carcinoma: a multicenter study of the Anatolian Society of Medical Oncology. Med Oncol 2014;31:929 10.1007/s12032-014-0929-0 [DOI] [PubMed] [Google Scholar]
- 2.Okada N, Narita Y, Nanno Y et al. Long-term survival of a patient with giant liver metastic breast cancer treated with trastuzumab. Gan To Kagaku Ryoho 2012;39:1399–402. [PubMed] [Google Scholar]
- 3.Vogel CL, Cobleigh MA, Tripathy D et al. First-line herceptin monotherapy in metastatic breast cancer. Oncology 2001;61(Suppl 2):37–42. 10.1159/000055400 [DOI] [PubMed] [Google Scholar]
- 4.Bullock K, Blackwell K. Clinical efficacy of taxane-trastuzumab combination regimens for HER-2-positive metastatic breast cancer. Oncologist 2008;13:515–25. 10.1634/theoncologist.2007-0204 [DOI] [PubMed] [Google Scholar]
- 5.Pronzata P, Fondini M. First line chemotherapy of metastatic breast cancer. Ann Oncol 2006;17(Suppl):165–8. 10.1093/annonc/mdj974 [DOI] [PubMed] [Google Scholar]
- 6.Marty M, Cognetti F, Maraninchi D et al. Randomized phase II trial of efficacy and safety of trastuzumab combined with docetaxel in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer administered as first-line treatment: the M77001 study group. J Clin Oncol 2005;23:4265–74. 10.1200/JCO.2005.04.173 [DOI] [PubMed] [Google Scholar]
- 7.Slamon DJ, Leyland-Jones B, Shak S et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med 2001;344:783–92. 10.1056/NEJM200103153441101 [DOI] [PubMed] [Google Scholar]
- 8.Jackisch C. HER-2-positive metastatic breast cancer: optimizing trastuzumab-based therapy. Oncologist 2006;11(Suppl):34–41. 10.1634/theoncologist.11-90001-34 [DOI] [PubMed] [Google Scholar]
- 9.Gullo G, Zuradelli M, Sclafani F et al. Durable complete response following chemotherapy and trastuzumab for metastatic HER2-positive breast cancer. Ann Oncol 2012;23:2204–8. 10.1093/annonc/mds221 [DOI] [PubMed] [Google Scholar]
- 10.Beda M, Basso U, Ghiotto C et al. When should trastuzumab be stopped after achieving complete response in HER2-positive metastatic breast cancer patients? Tumori 2007;93:491–2. [DOI] [PubMed] [Google Scholar]
- 11.Gullo G, Zuradelli M, Kelleher F et al. 5000 ORAL Long-term outcome of HER2-positive (HER2+) metastatic breast cancer (MBC) patients (pts) achieving durable complete remission (DCR) after trastuzumab (T)- containing chemotherapy (CT). Eur J Cancer 2011;47(Suppl):329–30. 10.1016/S0959-8049(11)71442-3 [DOI] [Google Scholar]
- 12.Pusztai L, Esteva FJ. Continued use of trastuzumab (herceptin) after progression on prior trastuzumab therapy in HER-2-positive metastatic breast cancer. Cancer Invest 2006;24:187–91. 10.1080/07357900500524629 [DOI] [PubMed] [Google Scholar]
- 13.Syrios J, Dokou A, Tsavaris N. Sustained complete remission of human epidermal growth factor receptor 2-positive metastatic breast cancer in the liver during long-term trastuzumab (Herceptin) maintenance therapy in a woman: a case report. J Med Case Rep 2010;4:401 10.1186/1752-1947-4-401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Macia Escalante S, Rodríguez Lescure A, Pons Sanz V et al. A patient with breast cancer with hepatic metastases and a complete response to herceptin as monotherapy. Clin Transl Oncol 2006;8:761–3. 10.1007/s12094-006-0125-6 [DOI] [PubMed] [Google Scholar]
- 15.Yardely DA, Tripathy D, Brusfsky AM et al. Long-term survivor characteristics in HER2-positive metastatic breast cancer from registHER. Br J Cancer 2014;110:2756–64. 10.1038/bjc.2014.174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arnould L, Arveux P, Couturier J et al. Pathologic complete response to trastuzumab-based neoadjuvant therapy is related to the level of HER-2 amplification. Clin Cancer Res 2007;13:6404–9. 10.1158/1078-0432.CCR-06-3022 [DOI] [PubMed] [Google Scholar]
- 17.Fuchs EM, Kostler WJ, Horvat R et al. High-level ERBB2 gene amplification is associated with a particularly short time-to-metastasis, but results in a high rate of complete response once trastuzumab-based therapy is offered in the metastatic setting. Int J Cancer 2014;135:224–31. 10.1002/ijc.28660 [DOI] [PubMed] [Google Scholar]
- 18.Carey LA, Berry DA, Ollila D et al. Clinical and translational results of CALBG 40601: a neoadjuvant phase III trial of weekly paclitaxel and trastuzumab with or without lapatinib for HER2-positive breast cancer. J Clin Oncol 2013. (suppl;abstr 500). [Google Scholar]
- 19.Ludovini V, Gori S, Colozza M et al. Evaluation of serum HER2 extracelluar domain in early breast cancer patients: correlation with clinicopathological parameters and survival. Ann Oncol 2008;19:883–90. 10.1093/annonc/mdm585 [DOI] [PubMed] [Google Scholar]
- 20.Montemurro F, Prat A, Rossi V et al. Potential biomarkers of long-term benefit from single-agent trastuzumab or lapatinib in HER2-positive metastatic breast cancer. Mol Oncol 2014;8:20–6. 10.1016/j.molonc.2013.08.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gebhart G, Gamez C, Holmes E et al. 18F-FDG PET/CT for early prediction of response to neoadjuvant lapatinib, trastuzumab, and their combination in HER2-positive breast cancer: results from Neo-ALTTO. J Nucl Med 2013;54:1862–8. 10.2967/jnumed.112.119271 [DOI] [PubMed] [Google Scholar]
- 22.Groheux D, Giacchetti S, Hatt M et al. HER2-overexpressing breast cancer: FDG uptake after two cycles of chemotherapy predicts the outcome of neoadjuvant treatment. Br J Cancer 2013;109:1157–64. 10.1038/bjc.2013.469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Humbert O, Cochet A, Riendinger JM et al. Her2-positive breast cancer: (18) F-FDG PET for early prediction of response to trastuzumab plus taxane-based neoadjuvant chemotherapy. Eur J Nucl Med Mol Imaging 2014;41: 1525–33. 10.1007/s00259-014-2739-1 [DOI] [PubMed] [Google Scholar]
- 24.Giordano SH, Temin S, Kirshner JJ et al. Systemic therapy for patients with advanced human epidermal growth factor receptor 2-positive breast cancer: American Society of Clinical Practice Guideline. J Clinc Oncol 2014;32:2078–99. 10.1200/JCO.2013.54.0948 [DOI] [PMC free article] [PubMed] [Google Scholar]


