Abstract
Goggles that degrade the retinal image produce axial enlargement of the ocular globe and large myopic refractive errors. Many authors have assumed that visual image degradation itself leads to myopia. Hodos and co-authors have shown, however, that goggled eyes in chicks are considerably warmer than normal. Such temperature changes may either underlie or be a consequence of alterations in choroidal blood flow (CBF). Since alterations in CBF could affect eye growth, we explored the effect of monocular goggling on CBF in chicks. Plastic goggles were glued over one eye in four-day old chicks and the goggles were left in place for 12 or 14 days. Fourteen days after the goggling, CBF was measured using laser Doppler velocimetry. Three groups of chicks were studied: 1) chicks with goggles for 14 days; 2) chicks with goggles for 12 days followed by no goggles for the two days; 3) age matched non-goggled chicks. A-scan ultrasonography confirmed that the visual deprivation produced vitreous chamber elongation in the goggled eye and that the degree of elongation for the goggled eye was the same for the two goggled groups. The results were : 1) blood flow in non-goggled chicks was similar in both eyes; 2) blood flow was significantly reduced in the goggled eye in chicks wearing goggles for 14 days- 37% of control; and 3) blood flow was still significantly reduced in the goggled eye in chicks whose goggles were removed two days before measurement- 51% of control. These results show that CBF is reduced by goggles that result in myopic eye growth. These findings have implications for the mechanisms underlying myopic eye growth and for the retinopathy that often attends high myopia.
INTRODUCTION
Myopia is a highly prevalent condition, and in extreme cases it is associated with retinal pathology leading to blindness; yet the mechanisms underlying development of myopia remain largely unknown. The relationship of changes in choroidal blood flow (CBF) to the occurrence of myopia is of interest both because of the role that changes in CBF could play in the genesis of myopia and because of the possible role that alterations in CBF could have in the retinopathy that often occurs in high myopes. In terms of the former point, the mechanisms underlying the occurrence of myopia have been extensively studied during the last several years in various animal models in which myopia is induced by experimental manipulations that produce eye growth that is excessive for the lens and corneal curvature (1-8). For example, in chicks (which have been used extensively in myopia research), high myopia and eye enlargement stemming from vitreous chamber elongation can be induced by lid suturing or by goggles or occluders that impair form vision (2, 4, 5, 7, 8). Depending on the precise shape of the goggles or the part of the visual field occluded, either equatorial or both axial and equatorial ocular enlargement is produced. The mechanism by which goggles that degrade the visual image result in axial elongation of the eye is unknown. There is evidence that the degraded retinal image somehow causes the retina to send a signal to the sclera that promotes eye growth (9). Hodos et at. (10), however, have found that ocular enlargement in young chicks produced by dome-like plastic goggles glued onto the skin surrounding the orbit is accompanied by temperature elevations in the occluded eye. Elevated ocular temperature has been implicated previously in the etiology of myopia by others (11-13). For example, Tokoro (11) showed that elevation of body temperature in rabbits yields myopia and Finger et al. (13), showed that elevation of scleral temperature in rabbits by 2°C for several hours per day results in increased proliferation of fibroblasts. Since CBF is thought to be involved in ocular temperature regulation (14), we therefore thought it possible that alterations in CBF might occur in ocular manipulations that yield myopia and might facilitate eye growth.
Altered CBF could also play a role in the retinopathy that occurs in high myopia. Myopic chorioretinopathy is accompanied by atrophy of the retina and choroid, and it is one of the major clinical features occurring with high myopia (15). The retina of the posterior pole in myopic retinopathy thins and degenerates, affecting mainly the outer retinal layers, which receive their vascular supply mainly from the choroidal vessels. Photoreceptors are particularly dependent on the choroid since disruptions of CBF lead to pathological changes in photoreceptors, as well as in other outer retinal cell types (16-20). Because of the possible role altered CBF might play in the genesis of myopia and/or the occurrence of outer retinal degeneration in high myopia, we sought to explore the effect of goggles on CBF and myopia in chicks.
METHODS
White Leghorn chicks (Gallus domesticus) were hatched in our laboratory. To induce myopia by means of form deprivation in these animals, dome-shaped, acrylic plastic goggles were glued to the circumorbital feathers and skin of the right eye of 4 day old chicks, using collodion and cyanoacrylate adhesive, following the method of Hodos and Kuenzel (7). Three groups were studied: 1) control birds that wore no goggles and were age matched with the two groups of goggled birds (n=11); 2) birds with a goggle that remained on for 14 days (n=13); and 3) birds with a goggle that remained on for 12 days and was then removed, followed by measurement of CBF two days later (n=11). In the second group of birds, the goggles remained on throughout the course of the blood flow measurements. All birds were maintained on 12 hour light - 12 hour dark cycle.
After the two weeks, the birds were anesthetized with Ketamine (0.66 ml / kg) and Xylazine (0.33 ml / kg) and positioned in a Kopf small animal stereotaxic device. Body temperature was maintained at 38°C with a Harvard heating blanket and a thermoprobe placed under the wing. The scalp and skin were incised and reflected to each side to expose the upper part of both eyes, and the fascia of the eyeballs was cut to expose the sclera. Laser Doppler velocimetry (LDV) using a Laserflo® blood perfusion monitor (Model BPM 403 A TSI Inc.) was employed to measure the CBF in both eyes. Although a variety of approaches have been used to measure CBF, including the radio-labelled microsphere method (21), methods relying on clearance of a detectable substance (22) and LDV (23, 24), LDV is advantageous because it is suited for continuously and quantitatively monitoring blood flow in a shallow vascular network such as the choroid. The assumptions underlying the operation of the Laserflo® and underlying LDV itself have been described in detail previously by others (25, 26). In brief, a noninvasive probe connected to the Laserflo® instrument illuminates the approximately 1mm hemisphere of tissue under its lip with an infrared laser beam. Within the tissue, the photons of the beam are scattered and frequency shifted by moving red blood cells. Some of this frequency shifted light is reflected back into a photodetector in the probe head. The electrical output of this photodetector, which contains information on the number of shifted photons and the degree of their shift, is then used by a microprocessor in the Laserflo® to calculate flow, volume, and velocity for the blood in the measured bed. The algorithms used for these calculations are specifically designed for microvascular beds (such as in the skin) that randomly scatter and reflect the incident infrared light. These requirements are met by the choroid since the diverse orientations of the choroidal vessels and the scleral interface promote random scattering of photons. Nonrandom scattering would affect the number of photons returning to the detector and yield a consistent over- or underestimate ot blood volume (and thus flow also) across animals or eyes. Thus, nonrandom photon scattering and reflection affects the absolute but not relative accuracy of the measurements obtained. In this context it is important to note that we use the flow, volume and velocity data from our Laserflo® as quantitative information for purposes of between eye and between group comparisons1. Thus, even if the values obtained are not absolutely accurate, their relative accuracy suffices for our purposes. Note however that several studies suggest that the TSI Laserflo® provides comparable flow values to those obtained with other methodologies in various microvascular beds, including the choroid (23-26). Also note that we call our approach LDV (as many authors do) even though we measure flow, volume and velocity.
The Laserflo® gives blood flow measurements in ml/min/100g tissue, which is displayed both digitally and on a chart recorder. Volume data and velocity data from the Laserflo® are expressed as Doppler shifts per photon and the mean frequency of the Doppler shifted light, respectively. Thus, although the Laserflo® provides quantitative blood volume & velocity information, this information is not expressed in blood flow units. The Laserflo® laser Doppler probe was held in a stereotaxic carrier and the tip of the Laserflo® probe was positioned close (approximately 1-3mm) to the scleral surface, with a small amount of ultrasound gel used in the interface between sclera and probe tip to increase signal transmission.
The data were recorded from two sites for each eye in each bird: 1) an area of the superior-anterior part of the eye, located anterior to the superior rectus muscle; and 2) a large vortex vein medial to site #1, into which the other veins of the superior choroid drain. Five pairs of measurement were made of blood flow, five pairs were made of blood velocity and five pairs were made of blood volume for each eye for sile #1. Each pair of these recordings consisted of the initial and final blood flow values during a thirty second session. Following a pair of flow measurements, initial and final blood volume values during the next thirty second session were recorded for the same eye, and then initial and final blood velocity values during the next thirty second session were recorded for the same eye. At this point, the probe was then positioned over the other eye and the same three pairs of flow, volume and velocity measurements taken for that eye, after which the probe was then repositioned over the initial eye, and so on, until all measurements had been completed for both eyes. To ensure repeatability of probe placement for each eye of an individual animal, stereotaxic coordinates were used for the probe positioning. The mean value of the five pairs of recordings of CBF, volume and velocity were thereby calculated for each eye in each bird. Following completion of the recording from site #1, one measurement of flow was taken of the vortex vein for each eye. Blood flow was measured from the vortex vein site as a confirmation of the values obtained from site #1, which could in principle appear to be reduced (even if there was no overall diminution in CBF) if there was reduction in the depth of the choroid attending putative stretching and thinning of the wall of the eye during ocular enlargement. Note that there was some degree of variability in flow, volume or velocity data for the untreated eyes among groups. Such differences presumably stem from slight differences in age, eye size, body size, or probe placement between animals. There was also evidence that the manipulation on the treated eye affected the untreated eye to a variable extent. Despite such variability (which appears inherent to physiological measurements in a wide variety ot systems), ANOVA revealed a number of clearly significant effects, as discussed below.
After the CBF measurements, all birds were transcardially perfused with 6% dextran followed by a fixative consisting of 4% paraformaldehyde in 0.1M lysine-0.01M sodium periodate-0.1M phosphate buffer (pH 7.4). The heads were then stored in 0.1M phosphate buffer with 0.02% sodium azide at 4°C until postmortem ultrasound measurements were made. Measurements of anterior chamber depth, lens thickness, vitreous chamber depth and axial length were made in the fixed eyes using A-scan ultrasonography as described previously (27). The identity of the control and deprived eyes and the group to which each animal belonged was concealed during the measurements. The eyelids were trimmed away and the A-scan measures made with the eyes in place in the head. The average value from 6 individual measures were calculated for each eye. Time values were converted to distance using the following conduction velocities: anterior segment 1,534 m/s; lens 1,6078 m/s; vitreous chamber 1,534 m/s (28). These values are for living eyes at body temperature and thus may be slightly in error for perfused eyes at room temperature. However, any errors should be similar for both treated and nontreated eyes and have a minimal effect on the estimates of differences between treated and nontreated eyes. Also of concern was the frequent dimpling of the cornea as a result of the fixation. Because of this dimpling, we believed the anterior chamber measurements, and thus also the total axial length measurements, were unreliable and they are not presented here.
The ultrasonography confirmed that vitreous chamber elongation occurred in the right eye in both groups of goggled birds. Since studies by others have shown that the types of goggles we used result in concurrent ocular elongation and myopia (7), we assume that the ocular elongation in our birds resulted in myopia even though we did not confirm this with refraction. Data were analyzed using a two-way ANOVA for repeated measures with a priori planned comparisons to test the differences between right and left eye within groups and between right eye of different groups or left eye of different groups (29). In this study, all procedures were carried out in accordance with the The Guiding Principles in the Care and Use of Animals (DHEW Publication, NIH 86-23) guidelines.
RESULTS
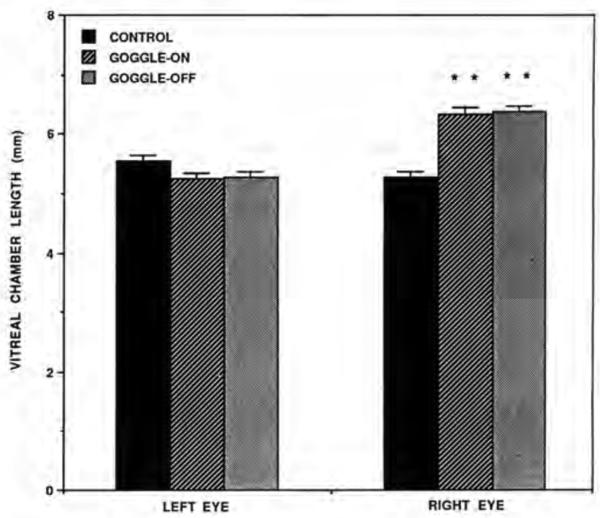
The results of the ultrasonography show that ocular elongation was induced in the deprived eye in both the goggle-on group (birds that had the goggles on for 14 days prior to CBF measurements) and the goggle-off group (birds that had the goggles on for 12 days then had them removed 2 days prior to blood flow measurements)(Table 1, Fig. 1). Further, the vitreous chamber length of the deprived eyes in the goggle-on and goggle-off groups did not differ significantly (p = 0.7772). In both groups, the ocular elongation of the deprived eye was attributable to increased length of the vitreous chamber. The mean right minus left difference in vitreous chamber length in the control group was −0.275 ± 0.104 mm, while for the goggle-on and goggle-off groups it was 1.090 ± 0.095 mm and 1.088 ± 0.104 mm, respectively. These differences between the goggled groups and the control group were statistically significant (p< 0.0001). The vitreous chamber lengths of the left eyes (the nongoggled eyes) in the goggled chicks were similar to and statistically indistinguishable (p>0.82l) from those of the right eyes of the control (nongoggled) chicks, suggesting that the experimental treatment on the right eye had no evident effect on vitreous chamber length in the opposite eye in the goggled groups. Significant effects of goggle wear were observed in lens thickness when the right eye was compared with the left eye of each goggled group (p< 0.05), with the lens being thicker in the right eye. As noted previously, because of corneal dimpling, the data on anterior chamber length and total axial length were considered unreliable and are not presented.
Table 1.
Ocular dimensions of chicks in different groups. The right eye was the deprived eye in the goggled groups. Each value represents mean (±SE)
| Ocular Parameter | Control (n=11) | Goggle-on (n=13) | Goggle-off (n = 11) | |||
|---|---|---|---|---|---|---|
| Right | Left | Right | Left | Right | Left | |
| Lens Thickness (mm) | 2.902±0.040 | 2.916±0.042 | 3.104±0.066 | 3.047±0.057 | 3.097±0.058 | 3.047±0.058 |
| Vitreal Chamber Length (mm) | 5.266±0.106 | 5.541±0.103 | 6.334±0.112 | 5.244±0.092 | 6.363±0.098 | 5.275+0.089 |
Figure 1.
Comparisons of the vitreal chamber length of the left eye vs. the right eye in the control, goggle-on and goggle-off groups. Note that vitreous chamber length of the goggled eye (right eye) is significantly increased (asterisks) in both goggled groups when compared wilh the control right eye. Error bars represent standard error of the mean.
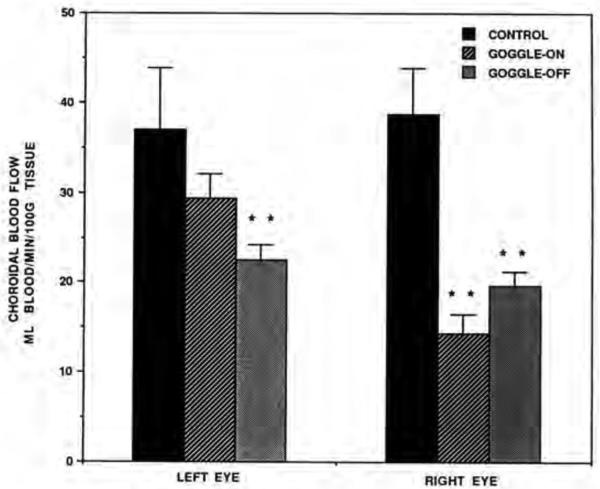
Choroidal blood flow in control chicks was similar in both eyes. In contrast, the CBF was decreased in goggled eyes compared to the opposite non-goggled eyes within both the goggle-on and goggle-off groups (Table 2, Fig. 2). Reductions in CBF were even more evident when comparing only the right eye across groups. In this comparison, the mean CBF in the right eye of the control birds for site #1 was 38.62±5.19 ml/min/100gm, but significantly less in the goggled eye of goggle-on birds (14.31±2.16) (P= 0.0001) and the deprived eyes of the goggle-off birds (19.58±1.67) (P=0.0004). Comparing the left eyes across groups for site # 1 (Table 2, Fig. 2), the results suggest that CBF showed a trend toward reduction in the nongoggled eyes of the goggled birds, though this effect was only statistically significant in the goggle-off group (p=0.0056).
Table 2.
Choroidal blood flow, volume and velocity of chicks in different groups. The right eye was the deprived eye in the goggled groups. Each value represents mean (±SE)
| Blood Flow Parameter | Control (n=11) | Goggle-on (n=13) | Goggle-off (n = 11) | |||
|---|---|---|---|---|---|---|
| Right | Left | Right | Left | Right | Led | |
| Flow (Site #1) | 38.62±5.19 | 36.91±6.98 | 14.31±2.16 | 29.39±2.77 | 19.58±1.67 | 22.45±1.78 |
| Volume (Site #1) | 0.56±0.07 | 0.57±0.09 | 0.31±0.03 | 0.55±0.08 | 0.28±0.02 | 0.46±0.06 |
| Velocity (Site #1) | 1.41±0.18 | 1.15±0.12 | 0.99±0.06 | 1.20±0.12 | 1.22±0.14 | 0.98±0.11 |
| Flow (Vortex Vein) | 163.78±17.41 | 175.23±24.03 | 108.23±14.18 | 163.54±21.58 | 118.55±15.61 | 111.73±18.98 |
Figure 2.
Comparisons of the choroidal blood flow in the left eye vs. right eye of the control, goggle-on and goggle-off groups of birds. Note that choroidal blood flow is significantly reduced compared to the control in the right eye of both goggle groups (asterisks) and in the left eye of the goggle-off group (asterisks). The right eye was the goggled eye. Error bars represent standard error of the mean.
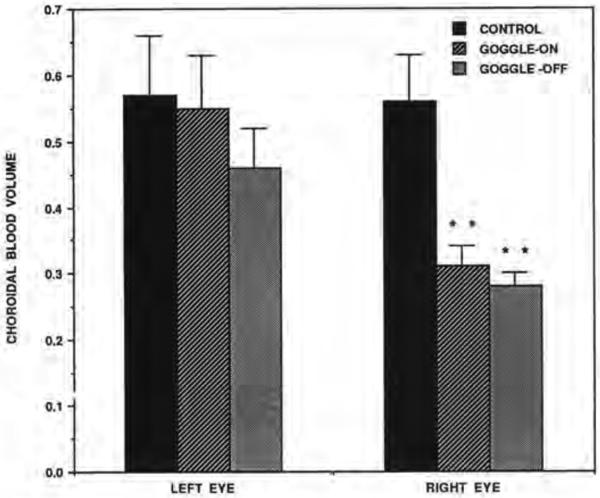
The effects of goggling on the volume of the CBF were also clearcut. Significantly decreased choroidal blood volume was found in the deprived eyes of goggle-on birds compared to the right eyes of the control birds (p=0.0052) and in the deprived eyes of goggle-off birds compared to the right eyes of the control birds (p=0.0031) (Fig. 3). The effects of goggling on the velocity of the CBF were less clearcut. Significantly decreased choroidal blood velocity was found in the deprived eyes of goggle-on birds compared to the right eyes of the control birds (p=0.0174) but not in the deprived eyes of goggle-off birds compared to the right eyes of the control birds (p=0.3l44) (Fig. 3).
Figure 3.
Comparisons of choroidal blood volume in the left eye vs. right eye of the control, goggle-on and goggle-off birds. Note that choroidal blood volume is significantly reduced compared to control in the right eye (goggled eye) of both goggled groups. Error bars represent standard error of the mean.
For the vortex vein site, the CBF results were fundamentally similar to those at site #1. The CBF was statistically indistinguishable between the two eyes in the control birds (p=0.5931), but the CBF in the goggled eye was significantly less (approximately 70%) in both goggled groups than that in either eye in the control birds (p<0.0468). In the nongoggled eye in the goggle-off group, the CBF at the vortex vein site was also significantly reduced compared to either eye in the control birds (p<0.0229). This was not true, however, for the nongoggled eye in the goggle-on birds (p>0.5852).
DISCUSSION
The present results clearly show that CBF is greatly reduced (to approximately 40-60% of normal) in eyes in which myopia has been induced by visual deprivation (by means of wearing goggles). We base our statement that the eyes were myopic on the large increase in vitreous chamber depth that occurred in the deprived eyes. Even though no direct measurement was made of refractive state and even though possible changes in corneal curvature were not measured, this large increase in vitreous chamber depth, which is comparable to that seen in other studies of chick in which myopia was found (30, 31), would surely have produced a large myopic refractive state. Removal of the goggles two days prior to measurements of CBF appears to result in no evident return of vitreous chamber length toward normal, but does result in some recovery of CBF toward normal. Interestingly, goggling of the right eye resulted in some apparent decreases in CBF in the nongoggled eye (60-70% of normal) in both groups of goggled birds, but no effects of such altered CBF were observed on eye lengths in the nongoggled eyes of the goggled birds. Although our findings do not completely clarify whether the reduced CBF is an outcome of the myopia or whether it plays a role in the development of the myopia, these various results are more consistent with the notion that the reduced CBF is a consequence of the myopia, as discussed in more detail below. The implications of our findings for the genesis of myopia itself and for the genesis of myopic retinopathy are also discussed below.
Basis of reduced choroidal blood flow with goggle-wearing
There appear to be two general possibilities as to why CBF is reduced in chicks that have worn goggles for 12-14 days: 1) the goggles themselves somehow lead to an alteration in CBF, which then either may or may not secondarily affect eye growth; or 2) the vitreous chamber elongation induced by the goggles may somehow affect CBF. The results of this study suggest that although the goggles themselves may directly affect CBF, the majority of the reduction in CBF appears attributable to the myopia induced by the goggles. This conclusion is supported by the observation that removal of the goggles two days prior to CBF measurements does not result in a return of CBF to normal - rather it remains substantially reduced (51% of normal). Nonetheless, the goggles themselves have some impact on CBF independent of any induced myopia because there appears to be some recovery of CBF by two days after goggle removal (from 37% of normal in goggle-on birds to 51% in goggle-off birds). The mechanism by which the goggles themselves or myopia might affect CBF are uncertain, but disturbances in ocular temperature regulation attending goggle-wearing or choroidal and retinal thinning attending myopia may be the factors in operation. Hodos et al. (10) showed that wearing hemispheric dome goggles (such as those used here) could elevate ocular temperature by up to 5 °C. This phenomenon presumably occurs because the goggle over the eye traps radiated heat from the eye and thereby interferes with normal ocular heat dissipation mechanisms (10). Since the choroid is a source of heat to the eye (14), CBF may decrease as part of a response to mitigate the increased intraocular temperature caused by the goggle. This is the most likely explanation of the slight increase in CBF that occurs in the chicks that had their goggles removed two days prior to CBF measurements, i.e. heat dissipation mechanisms return to normal and CBF then increases toward normal. A similar reflexive downregulation in CBF may occur with lid suture in chicks and explain the decreases in temperature at the rear pole of the eye observed with this manipulation (31). The neural mechanisms by which the choroid senses ocular temperature and that lead to an appropriate adjustment in CBF are uncertain, but it is possible that temperature sensing nerves are present directly in the choroid since substance P-containing sensory fibers are present in the choroid (32-35) and substance P-containing sensory fibers have been proposed to be involved in cutaneous temperature sensation (36).
The main part of the decrease in CBF observed in this study, however, appears attributable to the induced myopic ocular enlargement, since CBF does not recover fully by 48 hrs after goggle removal (by which time ocular temperature regulation should have recovered) (31). The results of another recent study of ours in chicks also strongly favor the view that ocular enlargement leads to reduced CBF. In this other study, we found that corneal manipulations that induce ocular enlargement without disturbing ocular temperature regulation (as goggles or eyelid suture do) also result in dramatically reduced CBF (37). There appear to be two possible mechanisms by ocular enlargement could lead to decreased CBF: 1) choroidal stretching and thinning accompanying overall ocular stretching in myopia: or 2) neurally mediated reductions in CBF, possibly due to decreased need for high CBF for a now thinner retina. Both types of changes would lead to reduced CBF by reduction of the volume of the vascular bed, as observed to occur in the present study. A general thinning of all retinal layers is observed in myopic chicks and some (but not all) other animals in whom myopia is induced (1, 38), and choroidal and scleral thinning are features of human degenerative myopia. (15). Thus, to the extent myopic eye growth involves passive stretching of the eye, choroidal thinning in myopia may be the cause of reduced CBF in myopic chicks. No thinning of the choroid and sclera, however, has been found in chicks made myopic by visual deprivation (38) and myopic eye growth in chicks is reported to involve active mechanisms such as increased protein synthesis and cellular proliferation in the sclera (39). Thus, the reduced CBF in myopia may be a neurally mediated adaptive response. The normal high CBF rate is known to be essential as the driving force for oxygen and nutrients to diffuse through the depth of the outer retina (and to the inner retina in the case of the avian retina and the primate macula) and it is possible that with a thinner retina, as occurs in myopia, the CBF may no longer need to be as high to provide such a driving force (20).
On a final note, it should be added that we cannot be certain that CBF would not have recovered to normal if we had examined chicks more than 48 hours after goggle removal. Wildsoet and Wallman (40) have found that choroidal thickening during recovery from induced myopia develops after several days. Such a structural change might be associated with increased CBF. It would be useful to study the relative rates in chicks at which myopic eyes recover from myopia and at which CBF returns to normal to better determine the relationship between reduced CBF and myopia.
Implications for genesis of myopia
The mechanisms by which form deprivation (caused by eyelid suture or goggle wearing) lead to axial and equatorial elongation are not clear. An initial impetus of ours for exploring the relationship of CBF to myopia was the possibility that CBF changes might be involved as part of the mechanism. As noted, Hodos el al., (10) showed that wearing hemispheric dome-type goggles could elevate ocular temperature, raising the possibility that such elevated temperature might encourage scleral growth, resulting in an elongated globe and myopic refractive error. We initially believed it possible that such increased ocular temperatures might stem from putative increases in CBF, with both temperature and CBF increases then favoring the eye growth toward myopia. As discussed above, however, our current data suggests that CBF is decreased with ocular enlargement of the type that commonly occurs in myopia and the altered CBF obtained with goggle-wearing is largely an effect of and not a cause of the myopia. Further, Hodos (31) has shown that the sutured eyes of lid-sutured chicks, which also exhibit ocular elongation and myopia, are not warmer than normal when closed and sutured. Thus, neither ocular temperature elevation nor increased CBF is necessary for myopia. In support of this, we have found that corneal manipulations that have no effect on ocular temperature yield ocular elongation and reduced CBF in chicks (37). Finally, although it is possible that reduced CBF (irrespective of its effect on temperature regulation) could favor ocular enlargement (perhaps by altering the rigidity of the wall of the eye), the available evidence does not favor this notion. In the present study, the nongoggled eyes in both experimental groups showed apparent reductions in CBF (60-70% of normal), but no evidence was seen of axial elongation in the nongoggled eyes of the goggled birds. Further, in a separate study we have found that reducing CBF by severing the choroidal nerves (which provide vasodilatory tone to the choroidal vessels (23)) in chicks does not yield ocular enlargement (41).
Implications for myopic retinopathy
Choroidal blood flow might also be of importance in myopia because of a role that altered CBF could play in myopic retinopathy, which is one of the major clinical problems occurring with high myopia (15). The principal function of the choroid is to nourish the outer retina and normal blood flow rates through the choroid are high, with the CBF alone accounting for 85% of all ocular blood flow (42). In myopic retinopathy, the retina of the posterior pole thins and degenerates, affecting mainly the outer layers, which receive their principal vascular supply from the choroidal vessels. A reduction ot CBF has been noted in high myopic patients (43). The basis of such reductions is uncertain, but it is possible that some of the same mechanisms may be in operation as in the myopic chicks described in the present study. Passive stretching and attendant thinning of the choroid, neurally mediated down regulation of CBF and/or rarefaction of choroidal vessels may all be involved in the decreases in CBF in human myopia. Such reduced CBF could be damaging for the retina, since disruptions of CBF have been shown to lead to pathological changes in photoreceptors as well as in other outer retinal cell types (16-20).
ACKNOWLEDGEMENTS
We gratefully thank: Betty Cook, Sherry Cuthbertson, Hadley Hamilton, Anne Kimrey, Charity Stewart Brown, Jennifer While, Gaynell Cole and Stevanna Mason for their technical assistance and Kris Arheart for his expertise in statistics. We would also like to express appreciation to John Borgos and Dr. Robert Bonner for their discussions on the theory and technical nuances of laser Doppler methodology. A brief report of this work has been presented previously (Invest. Ophthalmol. Vis. Sci. 32(Suppl.), 2614, 1991). Supported by: A fellowship from the National Taiwan University Hospital (YFS), NIH-AG-10538 (MECF), NIH-EY-05298 (AR), NIH-EY-05922 (TTN), NIH-EY-07558 (PDRG), NIH-EY-09380 (PDRG), and NIH-EY-04742 (WH).
Footnotes
Data presented in this paper are in values as displayed on Laserflo®. No other computations were conducted.
REFERENCES
- 1.Wiesel TN, Raviola E. Neural control of eye growth and experimental myopia. In: Bock GR, Widdows K, editors. Myopia and the Control of Eye Growth. Wiley; Chichester, England: 1990. pp. 22–44. [DOI] [PubMed] [Google Scholar]
- 2.Wallman J, Turkel J, Tractman J. Extreme myopia produced by modest change in early visual experience. Science. 1978;201:1249–1251. doi: 10.1126/science.694514. [DOI] [PubMed] [Google Scholar]
- 3.Sherman SM, Norton TT, Casagrande VA. Myopia in the lid-sutured tree shrew (Tupaia glis). Brain Res. 1977;124:154–157. doi: 10.1016/0006-8993(77)90872-1. [DOI] [PubMed] [Google Scholar]
- 4.Yinon U, Rose L, Shapiro A. Myopia in the eye of developing chicks following monocular and binocular lid closure. Vis. Res. 1980;20:137–141. doi: 10.1016/0042-6989(80)90155-8. [DOI] [PubMed] [Google Scholar]
- 5.Lauber JK, Boyd JE, Boyd TAS. Intraocular pressure and aqueous outflow facility in light induced avian buphthalmos. Exp. Eye Res. 1970;9:181–187. doi: 10.1016/s0014-4835(70)80074-4. [DOI] [PubMed] [Google Scholar]
- 6.Smith EL, III, Maguire GW, Watson JT. Axial lengths and refractive errors in kittens with an optically induced anisometropia. Invest. Ophthalmol. Vis. Sci. 1980;19:1250–1255. [PubMed] [Google Scholar]
- 7.Hodos W, Kuenzel WJ. Retinal-image degradation produces ocular enlargement in chicks. Invest. Ophthalmol. Vis. Sci. 1984;25:652–659. [PubMed] [Google Scholar]
- 8.Lauber JK. Review: light-induced avian glaucoma as an animal model for human primary glaucoma. J. Ocular Pharmacol. 1987;3:77–100. doi: 10.1089/jop.1987.3.77. [DOI] [PubMed] [Google Scholar]
- 9.Wallman J. Retinal influences on sclera underlie visual deprivation myopia. In: Bock GR, Widdows K, editors. Myopia and the Control of Eye Growth. Wiley; Chichester, England: 1990. pp. 126–141. [DOI] [PubMed] [Google Scholar]
- 10.Hodos W, Revzin AM, Kuenzel WJ. Thermal gradients in the chick eye: A contributing factor in experimental myopia. Invest. Ophthalmol. Vis. Sci. 1987;28:1859–1866. [PubMed] [Google Scholar]
- 11.Tokoro T. Experimental myopia in rabbits. Invest. Ophthalmol. 1970;12:926–934. [PubMed] [Google Scholar]
- 12.Maham M, Rav VA, Dada VK. Experimental myopia in the rabbit. Exp. Eye Res. 1977;25:33–38. doi: 10.1016/0014-4835(77)90243-3. [DOI] [PubMed] [Google Scholar]
- 13.Finger PT, Curtin BJ, Packer S, Svrita PP, Iwamoto T, Whitmore W, Jacobiec FA. Scleral hyperplasia induced by heat. Invest. Ophthalmol. Vis. Sci. 1986;27(Suppl.):338. doi: 10.1016/0002-9394(86)90204-7. [DOI] [PubMed] [Google Scholar]
- 14.Parver L, Auker C, Carpenter D. Choroidal blood flow as a heat dissipating mechanism in the macula. Am. J. Ophthalmol. 1980;89:641–646. doi: 10.1016/0002-9394(80)90280-9. [DOI] [PubMed] [Google Scholar]
- 15.Curtin BJ. The Myopias: Basic and Clinical Management. Harper & Row; Philadelphia: 1985. [Google Scholar]
- 16.Fitzgerald MEC, Reiner A. Lesions of the nucleus of Edinger-Westphal deleteriously affect photoreceptors in avian retina. Invest. Ophthalmol. Vis. Sci. 1989;30(Suppl.):464. [Google Scholar]
- 17.Fitzgerald MEC, Vana BA, Reiner A. Evidence for retinal pathology following interruption of neural regulation of choroidal blood flow: Müller cells express GFAP following lesions of the nucleus of Edinger-Westphal in pigeons. Curr. Eye Res. 1990;9:583–598. doi: 10.3109/02713689008999598. [DOI] [PubMed] [Google Scholar]
- 18.Gay AJ, Golder H, Smith M. Chorioretinal vascular occlusions wilh latex spheres. Invest. Ophthalmol. 1964;3:647–656. [PubMed] [Google Scholar]
- 19.Golder H, Gay AJ. Chorioretinal vascular occlusions with latex microspheres (a long lerm study). Part II. Invest. Ophthalmol. 1967;6:51–58. [PubMed] [Google Scholar]
- 20.Yancey CM, Linsenmeier RA. The electroretinogram and choroidal P02 in the cat during elevated intraocular pressure. Invest. Ophthalmol. Vis. Sci. 1988;29:700–707. [PubMed] [Google Scholar]
- 21.Stjernschantz J, Bill A. Effect of intracranial stimulation of the oculomotor nerve on ocular blood flow in the monkey, cat and rabbit. Invest. Ophthalmol. Vis. Sci. 1979;18:99–103. [PubMed] [Google Scholar]
- 22.Ernest J, Goldstick T. Choroidal blood flow measurement in the monkey by clearance of indocyanine green dye. Exp. Eye Res. 1979;29:7–14. doi: 10.1016/0014-4835(79)90162-3. [DOI] [PubMed] [Google Scholar]
- 23.Fitzgerald MEC, Vana BA, Reiner A. Control of choroidal blood flow by the nucleus of Edinger-Westphal: A laser-Doppler study. Invest. Ophthalmol. Vis. Sci. 1990;31:2483–2492. [PubMed] [Google Scholar]
- 24.Gherezghiher T, Okubo H, Koss MC. Choroidal and ciliary body blood flow analysis: Application of laser Doppler flowmetry in experimental animals. Exp. Eye Res. 1991;53:151–156. doi: 10.1016/0014-4835(91)90068-p. [DOI] [PubMed] [Google Scholar]
- 25.Borgos JA. TSI's LDV blood flowmeter. In: Shepherd AP, Öberg PÅ, editors. Laser-Doppler Blood Flowmetry. Kluwer Academic Publishers; Norwell, Massachusetts: 1990. pp. 73–92. [Google Scholar]
- 26.Bonner RF, Nossal R. Principals of Laser-Doppler flowmetry. In: Shepherd AP, Öberg PÅ, editors. Laser-Doppler Blood Flowmetry. Kluwer Academic Publishers; Norwell, Massachusetts: 1990. pp. 17–45. [Google Scholar]
- 27.Norton TT, McBrien NA. Normal development of refractive state and ocular component dimensions in the tree shrew (Tupaia belangeri). Vis. Res. 1992;32:833–842. doi: 10.1016/0042-6989(92)90026-f. [DOI] [PubMed] [Google Scholar]
- 28.Wallman J, Adams JI. Developmental aspects of experimental myopia in chicks: susceptibility, recovery and relations to emmetropization. Vis. Res. 1987;27:1139–1163. doi: 10.1016/0042-6989(87)90027-7. [DOI] [PubMed] [Google Scholar]
- 29.Kirk RE. Experimental Design: Procedures for the Behavioral Sciences. Brooks/Cole Publishing; Monterey, California: 1982. Multiple comparison tests. pp. 106–110. [Google Scholar]
- 30.Hodos W, Fitzke F, Hayes BP, Holden AL. Experimental myopia in chicks: ocular refraction by electroretinography. Invest. Ophthalmol. Vis. Sci. 1985;26:1423–1430. [PubMed] [Google Scholar]
- 31.Hodos W. Avian models of experimental myopia: environmental factors in the regulation of eye growth. In: Bock GR, Widdows K, editors. Myopia and the Control of Eye Growth. John Wiley and Sons; Chichester, England: 1990. pp. 149–159. [DOI] [PubMed] [Google Scholar]
- 32.Terenghi G, Polak JM, Probert L, McGregor GP, Ferri GL, Blank MA, Butler JM, Unger WG, Zhang S, Cole DF, Bloom SR. Mapping, quantitative distribution and origin of Substance P and VIP-containing nerves in the uvea of guinea pig eye. Histochemistry. 1982;75:399–417. doi: 10.1007/BF00496742. [DOI] [PubMed] [Google Scholar]
- 33.Stone RA, Kuwayama Y. Substance P-like immunoreactive nerves in the human eye. Arch. Ophthalmol. 1985;103:1207–1211. doi: 10.1001/archopht.1985.01050080119031. [DOI] [PubMed] [Google Scholar]
- 34.Corvetti G, Pignocchino P, Daneo LS. Distribution and development of Substance P immunoreactive axons in the chick cornea and uvea. Bas. Appl. Histochem. 1988;32:187–192. [PubMed] [Google Scholar]
- 35.Denis P, Fardin V, Nordmann JP, Elena PP, Laroche L, Saraux H, Rostene W. Localization and characterization of Substance P binding sites in rat and rabbit eyes. Invest. Ophthalmol. Vis. Sci. 1991;32:1894–1902. [PubMed] [Google Scholar]
- 36.Phillips JW. Substance P in the central nervous system. In: Barker JL, Smith TG, editors. The Role of Peptides in Neuronal Function. Marcel Dekker Inc.; New York: 1980. pp. 616–652. [Google Scholar]
- 37.Shih YF, Fitzgerald MEC, Reiner A. Choroidal blood flow is reduced in chicks with ocular enlargement induced by corneal incisions. Curr. Eye Res. 1993;12:229–237. doi: 10.3109/02713689308999468. [DOI] [PubMed] [Google Scholar]
- 38.Hayes BP, Fitzke FW, Hodos W, Holden AL. A morphological analysis of experimental myopia in young chickens. Invest. Ophthalmol. Vis. Sci. 1986;27:981–991. [PubMed] [Google Scholar]
- 39.Christensen AM, Wallman J. Evidence that increased scleral growth underlies visual deprivation myopia in chicks. Invest. Ophthalmol. Vis. Sci. 1991;32:2143–2150. [PubMed] [Google Scholar]
- 40.Wildsoet C, Wallman J. Optic nerve section affects ocular compensation for spectacle lens. Invest. Ophthalmol. Vis. Sci. 1992;33(Suppl.):1053. [Google Scholar]
- 41.Fitzgerald MEC, Shih YF, Reiner AR. Effects of choroidal and ciliary nerve cuts on choroidal blood flow and ocular growth. Invest. Ophthalmol. Vis. Sci. 1992;33(Suppl.):1053. [Google Scholar]
- 42.Bill A. The circulation in the eye. In: Renkin EM, Michel CC, editors. The Microcirculation. Part 2, Handbook of Physiology, Section 2. The American Physiological Society; Baltimore, MD: 1984. pp. 1001–1035. [Google Scholar]
- 43.Shih YF, Horng IH, Yang CH, Lin LLK, Peng Y, Hung PT. Ocular pulse amplitude in myopia. J. Ocular Pharmacol. 1991;7:83–87. doi: 10.1089/jop.1991.7.83. [DOI] [PubMed] [Google Scholar]