Abstract
Over the past two decades, extent of resection has emerged as a significant prognostic factor in patients with low-grade gliomas. Greater extent of resection has been shown to improve overall survival, progression-free survival, and time to malignant transformation. The operative goal in the majority of low-grade glioma cases is to maximize extent of resection while avoiding post-operative neurologic deficits. Several advanced surgical techniques have been developed in an attempt to better achieve maximal safe resection. Intraoperative magnetic resonance imaging, fluorescence-guided surgery, intraoperative functional pathway mapping, and neuro-navigation are some of the most commonly utilized techniques with multiple studies to support their efficacy in glioma surgery. By utilizing these techniques either alone or in combination, patients harboring low-grade gliomas have a better prognosis with less surgical morbidity following tumor resection.
Introduction
Low-grade gliomas (LGGs) constitute approximately 15% of the nearly 19,000 primary brain tumors diagnosed in adults each year.1 The majority of LGGs are detected in healthy patients with good neurologic status following a seizure 2. LGG tend to occur in locations adjacent to eloquent areas of the cortex.3 A common location for these tumors in adults is in the supratentorial region, frequently involving the supplementary motor cortex and insula. This presents a formidable operative challenge for neurosurgeons, as the location of tumors near eloquent cortex limits extent of resection and increases the likelihood of post-operative neurologic deficits.4-7
While recent advances have been made in chemotherapy and radiation therapy for LGG, surgical resection remains essential to its management. A growing body of literature supports the claim that greater extent of resection leads to a significant survival benefit.8-19 Extent of tumor resection has become a strong predictor of patient outcomes, alongside patient age, performance status, tumor histology, and molecular genetics (isocitrate dehydrogenase-1 and 1p/19q co-deletion status).4,6,20 Over the past two decades, surgeons have emphasized the importance of maximizing extent of resection and its impact on overall survival, progression-free survival, and time to malignant transformation.
Maximizing the extent of resection while preserving neurologic function is the central tenet of LGG surgery. In light of the fact that LGGs occur in younger patients with good neurologic function near eloquent areas of brain, advanced surgical techniques have been developed to aid in improving the extent of resection of LGG due to the difficulties in distinguishing tumor tissue from normal brain intra-operatively. In the following review, we examine the current literature describing the role of extent of resection in the management of LGG, highlighting the most significant studies that demonstrate its importance for overall prognosis. In addition, we provide an overview as well as supporting evidence for the use of advanced surgical techniques in the operative treatment of LGG.
Evidence for Extent of Resection
A review of the neurosurgical literature since 1990 produced 17 studies analyzing the efficacy of extent of resection on progression-free and overall survival in LGG (Table 1).9-19,21-26 The evidence prior to 1990 is ambivalent; although, many studies demonstrated a trend toward greater overall survival in patients who received gross total resection, these trends did not reach statistical significance.27,28 It should be noted that the majority of pre-1990 studies relied on the neurosurgeon’s intraoperative judgment to gauge extent of resection. Several studies have since suggested that a surgeon’s intraoperative assessment of tumor removal is unreliable when compared to residual tumor identified on post-operative imaging.29,30 With the advent of magnetic resonance imaging (MRI) and the routine practice of obtaining post-operative imaging, it is now feasible to obtain a more reliable assessment of post-operative residual tumor volume and extent of resection.
Table 1.
Overview of Literature on Extent of Resection in Low-Grade Glioma
| Overall Survival |
Non-Volumetric Studies | No. Patients |
Volumetric Studies | No. Patients |
|---|---|---|---|---|
| Benefit | ||||
| North et al., 199070 | 77 | van Veelen et al., 199818 | 75 | |
| Philippon et al., 199313 | 179 | Glaus et al., 20058 | 156 | |
| Rajan et al., 199414 | 82 | Smith et al., 200817 | 216 | |
| Leighton et al., 199711 | 167 | Sanai et al., 201015 | 104 | |
| Nakamura et al., 200012 | 88 | |||
| Yeh et al., 200519 | 93 | |||
| McGirt et al., 200825 | 170 | |||
| Ahmadi et al., 200971 | 130 | |||
| Chaichana et al., 201022 | 191 | |||
| Jakolaet al., 201210 | 153 | |||
| No Benefit | ||||
| Whitton & Bloom, 199026 | 88 | None to date | ||
| Bauman et al., 199921 | 401 | |||
| Johannesen et al., 200324 | 993 |
Studies evaluating extent of resection can be divided into volumetric (ie, quantitative volumetric analysis used to determine percentage of extent of resection) and non-volumetric (qualitative assessment of residual tumor, commonly divided into gross-total, subtotal, and biopsy) categories. Of the 13 non-volumetric studies, 10 demonstrated that extent of resection is associated with improved 5- and 10-year rates of overall survival in a statistically significant fashion.8-19,22,25 Statistical significance was reached on both univariate and multivariate analysis for all studies showing a positive benefit for extent of resection. The 3 studies that were not statistically significant did reveal a consistent trend toward improved overall survival with greater extent of resection.21,24,26
A recent study by Jakola and colleagues examined different LGG treatment approaches by comparing 2 population-based parallel cohorts treated at either hospital A (favoring biopsy and observation) or hospital B (favoring early resection following diagnosis).10 A total of 153 patients were included in the study (66 from hospital A, 87 from hospital B). Median follow-up was approximately 7 years in both groups. Overall survival was significantly better in patients receiving early resection,, (5.9-year median survival in the cohort favoring biopsy vs. no median survival reached in the cohort favoring early resection, as over 50% of patients were still alive). The estimated 5-year survival was 60% and 74%, respectively. The survival benefit remained after multivariate analysis using known prognostic factors, including age greater than 40, maximum tumor diameter 6 cm or greater, tumor crossing midline, neurologic deficit, and astrocytoma histology.6 Moreover, malignant transformation was significantly decreased in the group favoring early resection, indicating that early surgical intervention may improve overall survival by altering the natural history of LGG.
The four volumetric based studies reviewed determined that extent of resection is a prognostic factor for overall survival.8,15,17,18 The largest study examined 216 patients with hemispheric LGGs.17 Patients with greater than or equal to 90% tumor resection had a 5- and 8-year overall survival rate of 97% and 91%, respectively; patients with less complete resection had a survival rate of 76% and 60%, respectively. After correcting for multiple confounding variables (patient age, Karnofsky performance status, tumor location, and histologic subtype), the extent of resection was significantly correlated with overall survival, along with pre-operative and post-operative tumor volume. This was the first study to demonstrate an improved outcome in LGG patients as predicted by greater extent of resection. Collectively, the body of literature examining extent of resection supports operative intervention as a mainstay treatment for LGG. Maximizing extent of resection while preserving functional brain regions should be the operative goal in the majority of LGG patients.
Advanced Surgical Techniques
Understanding the role of advanced surgical techniques
The objective of LGG surgery is twofold: 1) maximize tumor removal, and 2) minimize surgical morbidity and post-operative neurologic deficits. Modern surgical techniques have emerged in an attempt to better navigate these two operative obstacles. Several randomized controlled trials have now been reported, supporting their efficacy.31-34 The major techniques are summarized in Table 2. Most techniques have been developed to preferentially serve one objective over the other. For example, intraoperative MRI (iMRI) is a technique that was developed in the 1990s in order to identify residual tumor radiographically during tumor resection. This technique serves the purpose of maximizing tumor removal by providing updated data on extent of resection. However, currently available iMRI does not provide real-time information on functional pathways.32 When tumors occur near eloquent areas, functional mapping serves to delineate a safe operative margin, and as a result largely determines and improves extent of resection.5
Table 2.
Advanced Surgical Techniques in Glioma Surgery
| Identification of Tumor | |
| Fluorescence-guided surgery | |
| Intraoperative MRI | |
| Intraoperative ultrasound | |
| Intraoperative microscopy | |
| Mapping Functional Pathways | |
| Direct Stimulation | |
| • Cortical stimulation | |
| • Subcortical stimulation | |
| Awake craniotomy with stimulation | |
| Functional imaging | |
| • Functional MRI | |
| • Magnetoencephalography | |
| • Positron emission tomography | |
| Neuronavigation with functional imaging |
A review of the most promising surgical techniques and technologies that aid in the identification of tumor tissue will be described In addition, several techniques dedicated to identifying functional pathways and eloquent areas will be reviewed. In conclusion, the utility of a multimodal approach combining several of these techniques will be discussed.
Intraoperative MRI
For over two decades, iMRI has been used to detect tumor intra operatively..35,36 The advantage of iMRI over traditional neuro-navigation (which uses pre-operative imaging) is its ability to provide real-time accuracy, addressing brain shifts during surgery secondary to cerebrospinal fluid (CSF) loss, tumor removal, and brain edema. Multiple studies have shown increased extent of resection in LGG surgery using iMRI.8,32,37-40 Claus et al. performed a retrospective study evaluating progression-free and overall survival rates for 156 patients who underwent resection of LGG using iMRI compared to age- and histology-adjusted controls obtained from the Surveillance, Epidemiology, and End Results (SEER) registry.8,23 The 1-year, 2-year, and 5-year death rates were 1.9%, 3.6%, and 17.6%, respectively. These results show a significant decrease in death rates when compared to SEER matched controls (10%, 16%, and 29%, respectively).
Senft et al. performed a randomized, controlled trial evaluating the effect of iMRI in glioma surgery. Patients were randomized to either iMRI-guided surgery (study group) or conventional microsurgery with neuro-navigation (control group).32 The primary endpoint was extent of resection, with secondary endpoints of post-operative tumor volume and progression-free survival at 60 months. A total of 49 patients were included in the data analysis, with 24 patients in the iMRI group and 25 patients in the control group. Significantly more patients who underwent iMRI-guided surgery had complete tumor resection (96%) compared to the control group (68%). While there was no statistically significant difference in progression-free survival between the two groups, the data did show complete resection was a strong predictor of 6-month progression-free survival. There was no difference in post-operative neurologic deficits between the two groups. Importantly, no patient in whom residual tumor was identified intra-operatively and subsequently underwent further resection after scanning developed post-operative neurologic deficits. An early concern that iMRI may lead to greater risk of postoperative neurologic deficits due to more aggressive resections has not been realized. A review of surgical results did not show iMRI-guided surgery to result in additional neurologic deficits, including cases where intraoperative imaging resulted in further, more aggressive resection.37 Results to date support the use of iMRI as an important tool for maximizing extent of resection without producing additional surgical morbidity.
Fluorescence-guided glioma surgery
Low-grade primary brain neoplasms frequently resemble surrounding tissue, making it difficult to delineate normal tissue from abnormal tissue. Fluorescence-guided surgery was developed in an attempt to label tumor tissue with a fluorescent biomarker to aid in the intra-operative identification of tumor tissue. Two agents have received the greatest amount of attention: fluorescein sodium and 5-aminolevulinic acid (5-ALA). While fluorescein sodium has been studied in glioma surgery,41 it suffers from significant non-specific staining. At the present time it has minimal value in LGG surgery and will not be discussed in detail here.
5-ALA is a naturally occurring amino acid precursor in the heme biosynthesis pathway. Exogenous administration of 5-ALA acts as a pro-drug to the production of fluorescent porphyrins (especially protoporphyrin IX) within glioma cells as well as other malignant tumors. 5-ALA fluorescence is particularly high in malignant glioma for 2 major reasons. As a water-soluble amino acid, 5-ALA does not readily pass the blood-brain barrier, thereby preventing accumulation in healthy brain. The breakdown of the blood-brain barrier that occurs in glioma results in intracellular accumulation of 5-ALA in glioma tissue. Additionally, the heme biosynthesis pathway is up-regulated in malignant glioma, producing a favorable kinetic environment for the production of fluorescent porphyrins from exogenous 5-ALA.
5-ALA is given either intravenously or orally 3 hours prior to the induction of general anesthesia. This allows for tumor resection to occur at the peak fluorescent time of 6 hours following administration. Protoporphyrin IX emits a red-violet light (wavelength = 635-704 nm) when excited by blue light (400-410 nm).42-44 The operative microscope can be equipped with white light for standard microsurgery as well as a blue light both for fluorescent excitation and optimal visualization of red-violet light emitted from tumor tissue (Figure 1).
Figure 1.
Fluorescence-guided surgery using 5-ALA. Left: Standard white-light intraoperative photograph using the operative microscope. Corticectomy has been performed and the tumor is internally debulked. Resection cavity is visualized without clear tumor-brain interface. Right: Red-violet 5-ALA fluorescence is clearly visualized under blue filter within residual tumor. Note this photograph was taken of a malignant glioma. 5-ALA fluorescence in LGG requires confocal microscopy.
In 2006, Stummer et al. completed a randomized, controlled trial comparing resection of malignant glioma using 5-ALA vs. resection under standard white light.33 Primary endpoints were number of patients without contrast-enhancing tumor on early post-operative MRI and 6-month progression-free survival. A total of 270 patients were included in the final analysis, 139 in the 5-ALA group and 131 in the white-light group. The trial was terminated at interim analysis as defined by the study. Complete resection of contrast-enhancing tumor was achieved in 90% of patients receiving 5-ALA compared to 36% of patients in the white-light group. The 5-ALA group had a statistically significant greater 6-month progression-free survival (41%) compared to the white-light group (21%). No difference was noted in post-operative neurologic deficits or adverse events between the two groups. The study was not powered to assess overall survival.
The literature on 5-ALA and fluorescence-guided glioma surgery has been largely limited to high-grade glioma. In the majority of LGGs, no visible fluorescence can be detected under standard microsurgical conditions. Intraoperative confocal microscopy has been used in an attempt to visualize 5-ALA tumor fluorescence in LGGs during microsurgical resection.45 An initial study involving 10 patients confirmed that no macroscopic fluorescence was detected at any point during the procedure. All patients in this study had intraoperative fluorescence detected using the confocal microscope, and greater than 90% extent of resection was achieved in 9/10 patients. While these results are promising, a limitation of this technique is that in 4 patients, no fluorescence was detected at the tumor/cavity margin. A possible explanation for this limitation lies in the inherent problem with any technique that relies on dye delivery. In addition to heterogeneous delivery and nonspecific staining, labeling specificity for tumor tissue will progressively diminish as resection proceeds toward the tumor-normal brain margin in infiltrative tumors such as glioma. For this reason, various approaches to label-free techniques for tumor detection are under development.46 Despite these limitations, fluorescence-guided surgery has emerged as an important component in the brain tumor surgeon’s armamentarium for the treatment of glioma.
Intraoperative functional mapping
The variability of functional pathways in the human brain has been well described.47-53 Reliance upon anatomic landmarks for localization of eloquent cortex, such as the primary motor cortex, has proven to be inadequate in clinical practice and lacks the precision necessary to minimize postoperative deficits.54,55 Mass effect from adjacent tumor can distort tissues and the plasticity of functional pathways allows for significant reorganization. Moreover, functional motor fibers have been found to travel directly through tumor tissue in LGG, making intratumoral resection vulnerable to post-operative deficits.56,57 This variability has necessitated the use of techniques that can reliably localize functional pathways when tumor tissue is located near eloquent areas.
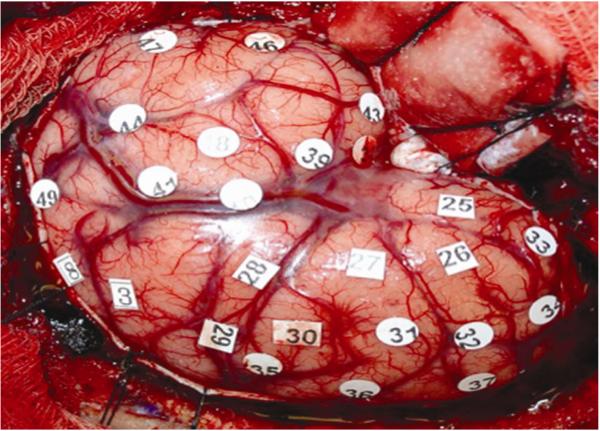
The oldest and best-described technique for identifying functional pathways is by direct cortical stimulation. The technique was developed in the 1930s by Wilder Penfield and others, and was largely utilized in epilepsy surgery.58 This technique involves the application of a depolarization current using a bipolar stimulation device. Direct stimulation depolarizes a focal area of cortex, exciting local neurons and inducing either focal excitation or inhibition of function. Numerically marked stimulation sites separated by 1 cm are placed on the surgical field (Figure 2). To improve accuracy and limit subclinical seizure activity, continuous electrocorticography is used to determine the threshold intensity at which potential epileptiform activity occurs. All language testing is repeated at least 2 times and a positive site is defined as the inability to reliably count, name objects, or read words during stimulation.51 Language testing identifies sites responsible for speech arrest, anomia, and alexia with stimulation testing. Speech arrest is defined as discontinuation in number counting without simultaneous motor response. Dysarthria can be distinguished from speech arrest by an absence of involuntary muscle contractions affecting speech. To identify reading sites, the same stimulation is applied during a slide presentation of words.
Figure 2.
Intraoperative mapping of functional pathways by direct cortical stimulation. A left pterional craniotomy was performed in order to expose inferior frontal and posterior temporal regions for language mapping. Stimulation sites are numerically marked and recorded as either positive or negative mapping sites. Resection proceeds after a safe location for the corticectomy has been identified.
Awake craniotomy for functional pathway mapping requires an experienced neuroanesthesiologist to administer the anesthesia. Patients are typically pre-medicated with midazolam and fentanyl prior to positioning for patient comfort and anxiolysis. Sedation is achieved with propofol and remifentanil. A scalp block using lidocaine and bupivacaine with sodium bicarbonate and epinephrine is applied by the neurosurgeon, to reduce discomfort. When positioning, prepping, and draping the patient, it is important to allow the patient direct visual access to the anesthesiologist and intraoperative tester. After skin incision and removal of the bone flap, all sedatives are discontinued. Topical ice-cold Ringers lactate solution is made available on the surgical field, as it has been shown to prevent or stop stimulation-induced seizure activity.59
The use of awake craniotomies in brain tumor surgery has been shown to decrease post-operative neurologic deficits and surgical morbidity while safely delineating an operative margin for resection.5 Early mapping techniques used in epilepsy surgery involved large craniotomies to widely expose the cortex in order to identify positive controls that generated speech arrest, motor stimulation, or inhibition. This technique has been largely abandoned in brain tumor surgery in favor of “negative” mapping, in which cortical stimulation proceeds by identifying areas that fail to produce neurologic symptoms.51 Intraoperative stimulation and identification of eloquent cortex increases the risk of post-operative neurologic deficits, likely due to tumor proximity to these areas. Negative mapping is a safe alternative, allowing for tailored craniotomies, shorter operative times, and fewer post-operative deficits.5,51 Utilizing this technique, long-term language function after intraoperative stimulation was evaluated in a series of 250 consecutive glioma patients with World Health Organization (WHO) grade II-IV dominant hemisphere gliomas.51 Fifty-eight percent of patients had at least 1 functional language site identified, and long-term language disability was 1.6%.
A recent meta-analysis of 90 studies and 8,091 patients was completed to determine the impact of intraoperative stimulation (cortical and subcortical) brain mapping on patient outcomes after glioma surgery.60 Severe neurologic deficits were observed in 3.4% of patients after resections with intraoperative stimulation mapping, and 8.2% of patients after resections without mapping. In addition to this significant decrease in surgical morbidity, gross total resection was achieved in operations with intraoperative stimulation in 75% of cases, whereas only 58% of operations performed without intraoperative mapping resulted in gross total resection. This finding provides evidence that mapping functional pathways in tumors adjacent to eloquent cortex serves not only to achieve its primary objective of reducing post-operative neurologic deficits, but also to increase extent of resection and reduce post-operative residual tumor.
Frameless stereotactic neuronavigation
Neuro-navigation has become a ubiquitous tool in brain tumor surgery.61 Improvements in cross-sectional imaging modalities, such as computed tomography (CT) and MRI, have made possible 3-dimensional reconstructions of anatomic structures. Neuro-navigation is the process by which anatomic landmarks in and around the operative field are spatially registered to a corresponding reconstructed 3-dimensional model generated from preoperative or intraoperative cross-sectional imaging. Using a handheld optical or electromagnetic detector probe, anatomic landmarks are localized using a tracking system. The most common tracking system uses dual infrared cameras to track the position of the handheld surgical probe relative to a reference frame fixed either to the patient or a rigid head-holder.
Standard neuro-navigation in brain tumor surgery is used mainly for optimizing the surgical approach to the tumor prior to opening the dura. Scalp incision and craniotomies can be accurately tailored to allow for minimal tissue injury and bony removal. A randomized, controlled trial was completed to evaluate the importance of neuro-navigation for cytoreduction of solitary intra-cerebral contrast-enhancing tumors.62 A total of 45 patients were enrolled in the study. There was no statistically significant difference in extent of resection between patients who underwent surgery with or without standard neuro-navigation. The results of this study highlight some of the limitations of neuro-navigation in brain tumor surgery. With opening of the dura, CSF egress allows for significant shifts in intracranial contents. Additionally, as tumor resection proceeds, a tumor cavity develops, leading to distortion of surrounding structures, including eloquent cortex and functional pathways. In the absence of an updated navigational dataset, traditional rigid registration methods do not account for these intra-operative anatomic changes.
Because of these inherent limitations, neuro-navigation is best used as an adjunct to other surgical or imaging techniques. One particular strategy involves the integration of standard neuro-navigation with functional non-invasive neuroimaging such as functional MRI, diffusion tensor imaging (DTI), or magnetoencephalography (MEG). Wu and colleagues performed a randomized, controlled trial evaluating the use of DTI-based functional neuronavigation in patients with gliomas involving the pyramidal tracts.34 The study demonstrated a significant decrease in post-operative motor deterioration in patients using DTI-based functional neuronavigation (9.8%) compared to standard neuronavigation (18.6%) in patients with LGG. The study group also had 13% of patients show improvement in their post-operative motor function compared to only 1% in the control group. There was no difference in extent of resection in patients with LGG.
Resting state coherence measured with MEG is capable of mapping functional connectivity of the brain. Tarapore et al. measured resting whole brain MEG recordings from 79 patients with unilateral gliomas near or within sensory, motor, or language areas during the preoperative and postoperative period.63 Patients with baseline decreased functional connectivity had a 29% rate of new neurological deficits 1 week after surgery, and 0% at 6-month follow-up. However, patients with increased functional connectivity had a 60% rate of new deficits at 1 week and 25% at 6 months. Tumors with decreased resting state connectivity have a relatively low risk of post-operative neurological deficits, while those with increased resting state connectivity are associated with higher risk of post-operative neurological deficits.
Neuro-navigation is an essential component of brain tumor surgery. The technique will continue to improve, as methods are developed to integrate functional imaging into navigational datasets that are able to account for intra-operative brain shift and anatomic changes during tumor resection.
Conclusion
Each of the advanced techniques described above has a role in safely maximizing extent of resection in LGG surgery. Deciding which technique is optimal for a given surgery remains nuanced and complex. Lesions located near eloquent areas should prompt neurosurgeons to pursue non-invasive functional imaging and/or intraoperative mapping. Candidacy for awake craniotomy depends on tumor location and the patient’s ability to cooperate during surgery. Awake mapping should be strongly considered in dominant hemisphere LGG with involvement of language pathways. Intraoperative MRI and fluorescence-guided surgery are ideal for large LGGs occurring in non-eloquent areas. This strategy facilitates maximal resection in patients at lower risk for post-operative neurologic deficits. Several studies have examined use of a multimodal approach in glioma surgery.64-69 Good operative results have been reported with iMRI or 5-ALA-guided surgery integrated with functional mapping techniques. This allows both for delineation of tumor tissue and identification of functional pathways in a single operation. The decision to utilize advanced surgical techniques for any given patient harboring a LGG can only be made by an experienced neurosurgeon following an extensive pre-operative evaluation in order to provide optimal surgical care.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURE STATEMENT:
The authors have no conflicts of interest to report pertaining to the materials or methods used in this study or the findings specified in this paper.
References
- 1.Barnholtz-Sloan JS, Sloan AE, Schwartz AG. Chapter 25: Cancer of the brain and other central nervous system. In: Gloeckler-Ries LA, Young JL, Eisner MP, Lin YD, Horner MJD, editors. SEER Survival Monograph: Cancer Survival Among Adults: U.S. SEER Program, 1988-2001, Patient and Tumor Characteristics. National Cancer Institute; Bethesda, MD: 2007. pp. 204–215. SEER Program, NIH Pub. No. 07-6215. http://seer.cancer.gov/archive/publications/survival/ [Google Scholar]
- 2.Bondy ML, Scheurer ME, Malmer B, et al. Brain tumor epidemiology: consensus from the Brain Tumor Epidemiology Consortium. Cancer. 2008;113:1953–1968. doi: 10.1002/cncr.23741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Duffau H, Capelle L. Preferential brain locations of low-grade gliomas. Cancer. 2004;100:2622–2626. doi: 10.1002/cncr.20297. [DOI] [PubMed] [Google Scholar]
- 4.Chang EF, Clark A, Jensen RL, et al. Multiinstitutional validation of the University of California at San Francisco Low-Grade Glioma Prognostic Scoring System. Clinical article. J Neurosurg. 2009;111:203–210. doi: 10.3171/2009.2.JNS081101. [DOI] [PubMed] [Google Scholar]
- 5.Kim SS, McCutcheon IE, Suki D, et al. Awake craniotomy for brain tumors near eloquent cortex: correlation of intraoperative cortical mapping with neurological outcomes in 309 consecutive patients. Neurosurgery. 2009;64:836–846. doi: 10.1227/01.NEU.0000342405.80881.81. [DOI] [PubMed] [Google Scholar]
- 6.Pignatti F, van den Bent M, Curran D, et al. Prognostic factors for survival in adult patients with cerebral low-grade glioma. J Clin Oncol. 2002;20:2076–2084. doi: 10.1200/JCO.2002.08.121. [DOI] [PubMed] [Google Scholar]
- 7.Sawaya R, Hammoud M, Schoppa D, et al. Neurosurgical outcomes in a modern series of 400 craniotomies for treatment of parenchymal tumors. Neurosurgery. 1998;42:1044–1056. doi: 10.1097/00006123-199805000-00054. [DOI] [PubMed] [Google Scholar]
- 8.Claus EB, Horlacher A, Hsu L, et al. Survival rates in patients with low-grade glioma after intraoperative magnetic resonance image guidance. Cancer. 2005;103:1227–1233. doi: 10.1002/cncr.20867. [DOI] [PubMed] [Google Scholar]
- 9.Ius T, Isola M, Budai R, et al. Low-grade glioma surgery in eloquent areas: volumetric analysis of extent of resection and its impact on overall survival. A single-institution experience in 190 patients: clinical article. J Neurosurg. 2012;117:1039–1052. doi: 10.3171/2012.8.JNS12393. [DOI] [PubMed] [Google Scholar]
- 10.Jakola AS, Myrmel KS, Kloster R, et al. Comparison of a strategy favoring early surgical resection vs a strategy favoring watchful waiting in low-grade gliomas. JAMA. 2012;308:1881–1888. doi: 10.1001/jama.2012.12807. [DOI] [PubMed] [Google Scholar]
- 11.Leighton C, Fisher B, Bauman G, et al. Supratentorial low-grade glioma in adults: an analysis of prognostic factors and timing of radiation. J Clin Oncol. 1997;15:1294–1301. doi: 10.1200/JCO.1997.15.4.1294. [DOI] [PubMed] [Google Scholar]
- 12.Nakamura M, Konishi N, Tsunoda S, et al. Analysis of prognostic and survival factors related to treatment of low-grade astrocytomas in adults. Oncology. 2000;58:108–116. doi: 10.1159/000012087. [DOI] [PubMed] [Google Scholar]
- 13.Philippon JH, Clemenceau SH, Fauchon FH, et al. Supratentorial low-grade astrocytomas in adults. Neurosurgery. 1993;32:554–559. doi: 10.1227/00006123-199304000-00010. [DOI] [PubMed] [Google Scholar]
- 14.Rajan B, Pickuth D, Ashley S, et al. The management of histologically unverified presumed cerebral gliomas with radiotherapy. Int J Radiat Oncol Biol Phys. 1994;28:405–413. doi: 10.1016/0360-3016(94)90064-7. [DOI] [PubMed] [Google Scholar]
- 15.Sanai N, Polley MY, Berger MS. Insular glioma resection: assessment of patient morbidity, survival, and tumor progression. J Neurosurg. 2010;112:1–9. doi: 10.3171/2009.6.JNS0952. [DOI] [PubMed] [Google Scholar]
- 16.Shaw E, Arusell R, Scheithauer B, et al. Prospective randomized trial of low-versus high-dose radiation therapy in adults with supratentorial low-grade glioma: initial report of a North Central Cancer Treatment Group/Radiation Therapy Oncology Group/Eastern Cooperative Oncology Group study. J Clin Oncol. 2002;20:2267–2276. doi: 10.1200/JCO.2002.09.126. [DOI] [PubMed] [Google Scholar]
- 17.Smith JS, Chang EF, Lamborn KR, et al. Role of extent of resection in the long-term outcome of low-grade hemispheric gliomas. J Clin Oncol. 2008;26:1338–1345. doi: 10.1200/JCO.2007.13.9337. [DOI] [PubMed] [Google Scholar]
- 18.van Veelen ML, Avezaat CJ, Kros JM, et al. Supratentorial low grade astrocytoma: prognostic factors, dedifferentiation, and the issue of early versus late surgery. J Neurol Neurosurg Psychiatry. 1998;64:581–587. doi: 10.1136/jnnp.64.5.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yeh SA, Ho JT, Lui CC, et al. Treatment outcomes and prognostic factors in patients with supratentorial low-grade gliomas. Br J Radiol. 2005;78:230–235. doi: 10.1259/bjr/28534346. [DOI] [PubMed] [Google Scholar]
- 20.Weiler M, Wick W. Molecular predictors of outcome in low-grade glioma. Curr Opin Neurol. 2012;25:767–773. doi: 10.1097/WCO.0b013e32835a0217. [DOI] [PubMed] [Google Scholar]
- 21.Bauman G, Lote K, Larson D, et al. Pretreatment factors predict overall survival for patients with low-grade glioma: a recursive partitioning analysis. Int J Radiat Oncol Biol Phys. 1999;45:923–929. doi: 10.1016/s0360-3016(99)00284-9. [DOI] [PubMed] [Google Scholar]
- 22.Chaichana KL, McGirt MJ, Laterra J, et al. Recurrence and malignant degeneration after resection of adult hemispheric low-grade gliomas. J Neurosurg. 2010;112:10–17. doi: 10.3171/2008.10.JNS08608. [DOI] [PubMed] [Google Scholar]
- 23.Claus EB, Black PM. Survival rates and patterns of care for patients diagnosed with supratentorial low-grade gliomas: data from the SEER program, 1973-2001. Cancer. 2006;106:1358–1363. doi: 10.1002/cncr.21733. [DOI] [PubMed] [Google Scholar]
- 24.Johannesen TB, Langmark F, Lote K. Progress in long-term survival in adult patients with supratentorial low-grade gliomas: a population-based study of 993 patients in whom tumors were diagnosed between 1970 and 1993. J Neurosurg. 2003;99:854–862. doi: 10.3171/jns.2003.99.5.0854. [DOI] [PubMed] [Google Scholar]
- 25.McGirt MJ, Chaichana KL, Attenello FJ, et al. Extent of surgical resection is independently associated with survival in patients with hemispheric infiltrating low-grade gliomas. Neurosurgery. 2008;63:700–708. doi: 10.1227/01.NEU.0000325729.41085.73. [DOI] [PubMed] [Google Scholar]
- 26.Whitton AC, Bloom HJ. Low grade glioma of the cerebral hemispheres in adults: a retrospective analysis of 88 cases. Int J Radiat Oncol Biol Phys. 1990;18:783–786. doi: 10.1016/0360-3016(90)90397-3. [DOI] [PubMed] [Google Scholar]
- 27.Laws ER, Taylor WF, Clifton MB, et al. Neurosurgical management of low-grade astrocytoma of the cerebral hemispheres. J Neurosurg. 1984;61:665–673. doi: 10.3171/jns.1984.61.4.0665. [DOI] [PubMed] [Google Scholar]
- 28.Piepmeier JM. Observations on the current treatment of low-grade astrocytic tumors of the cerebral hemispheres. J Neurosurg. 1987;67:177–181. doi: 10.3171/jns.1987.67.2.0177. [DOI] [PubMed] [Google Scholar]
- 29.Albert FK, Forsting M, Sartor K, et al. Early postoperative magnetic resonance imaging after resection of malignant glioma: objective evaluation of residual tumor and its influence on regrowth and prognosis. Neurosurgery. 1994;34:45–61. doi: 10.1097/00006123-199401000-00008. [DOI] [PubMed] [Google Scholar]
- 30.Orringer D, Lau D, Khatri S, et al. Extent of resection in patients with glioblastoma: limiting factors, perception of resectability, and effect on survival. J Neurosurg. 2012;117:851–859. doi: 10.3171/2012.8.JNS12234. [DOI] [PubMed] [Google Scholar]
- 31.Barone DG, Lawrie TA, Hart MG. Image guided surgery for the resection of brain tumours. Cochrane Database Syst Rev. 2014;1:CD009685. doi: 10.1002/14651858.CD009685.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Senft C, Bink A, Franz K, et al. Intraoperative MRI guidance and extent of resection in glioma surgery: a randomised, controlled trial. Lancet Oncol. 2011;12:997–1003. doi: 10.1016/S1470-2045(11)70196-6. [DOI] [PubMed] [Google Scholar]
- 33.Stummer W, Pichlmeier U, Meinel T, et al. Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: a randomised controlled multicentre phase III trial. Lancet Oncol. 2006;7:392–401. doi: 10.1016/S1470-2045(06)70665-9. [DOI] [PubMed] [Google Scholar]
- 34.Wu JS, Zhou LF, Tang WJ, et al. Clinical evaluation and follow-up outcome of diffusion tensor imaging-based functional neuronavigation: a prospective, controlled study in patients with gliomas involving pyramidal tracts. Neurosurgery. 2007;61:935–949. doi: 10.1227/01.neu.0000303189.80049.ab. [DOI] [PubMed] [Google Scholar]
- 35.Black PM, Alexander E, Martin C, et al. Craniotomy for tumor treatment in an intraoperative magnetic resonance imaging unit. Neurosurgery. 1999;45:423–433. doi: 10.1097/00006123-199909000-00001. [DOI] [PubMed] [Google Scholar]
- 36.Knauth M, Wirtz CR, Tronnier VM, et al. Intraoperative MR imaging increases the extent of tumor resection in patients with high-grade gliomas. AJNR Am J Neuroradiol. 1999;20:1642–1646. [PMC free article] [PubMed] [Google Scholar]
- 37.Nimsky C, Fujita A, Ganslandt O, et al. Volumetric assessment of glioma removal by intraoperative high-field magnetic resonance imaging. Neurosurgery. 2004;55:358–371. doi: 10.1227/01.neu.0000129694.64671.91. [DOI] [PubMed] [Google Scholar]
- 38.Nimsky C, Ganslandt O, Tomandl B, et al. Low-field magnetic resonance imaging for intraoperative use in neurosurgery: a 5-year experience. Eur Radiol. 2002;12:2690–2703. doi: 10.1007/s00330-002-1363-9. [DOI] [PubMed] [Google Scholar]
- 39.Schneider JP, Schulz T, Schmidt F, et al. Gross-total surgery of supratentorial low-grade gliomas under intraoperative MR guidance. AJNR Am J Neuroradiol. 2001;22:89–98. [PMC free article] [PubMed] [Google Scholar]
- 40.Schneider JP, Trantakis C, Rubach M, et al. Intraoperative MRI to guide the resection of primary supratentorial glioblastoma multiforme--a quantitative radiological analysis. Neuroradiology. 2005;47:489–500. doi: 10.1007/s00234-005-1397-1. [DOI] [PubMed] [Google Scholar]
- 41.Li Y, Rey-Dios R, Roberts DW, et al. Intraoperative fluorescence-guided resection of high-grade gliomas: a comparison of the present techniques and evolution of future strategies. World Neurosurg. 2013 Jul 9; doi: 10.1016/j.wneu.2013.06.014. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 42.Ishihara R, Katayama Y, Watanabe T, et al. Quantitative spectroscopic analysis of 5-aminolevulinic acid-induced protoporphyrin IX fluorescence intensity in diffusely infiltrating astrocytomas. Neurol Med Chir (Tokyo) 2007;47:53–57. doi: 10.2176/nmc.47.53. [DOI] [PubMed] [Google Scholar]
- 43.Stummer W, Reulen HJ, Novotny A, et al. Fluorescence-guided resections of malignant gliomas--an overview. Acta Neurochir Suppl. 2003;88:9–12. doi: 10.1007/978-3-7091-6090-9_3. [DOI] [PubMed] [Google Scholar]
- 44.Stummer W, Stocker S, Wagner S, et al. Intraoperative detection of malignant gliomas by 5-aminolevulinic acid-induced porphyrin fluorescence. Neurosurgery. 1998;42:518–526. doi: 10.1097/00006123-199803000-00017. [DOI] [PubMed] [Google Scholar]
- 45.Sanai N, Snyder LA, Honea NJ, et al. Intraoperative confocal microscopy in the visualization of 5-aminolevulinic acid fluorescence in low-grade gliomas. J Neurosurg. 2011;115:740–748. doi: 10.3171/2011.6.JNS11252. [DOI] [PubMed] [Google Scholar]
- 46.Ji M, Orringer DA, Freudiger CW, et al. Rapid, label-free detection of brain tumors with stimulated Raman scattering microscopy. Sci Transl Med. 2013;5:3005954. doi: 10.1126/scitranslmed.3005954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Herholz K, Thiel A, Wienhard K, et al. Individual functional anatomy of verb generation. Neuroimage. 1996;3:185–194. doi: 10.1006/nimg.1996.0020. [DOI] [PubMed] [Google Scholar]
- 48.Ojemann G, Ojemann J, Lettich E, et al. Cortical language localization in left, dominant hemisphere. An electrical stimulation mapping investigation in 117 patients. J Neurosurg. 1989;71:316–326. doi: 10.3171/jns.1989.71.3.0316. [DOI] [PubMed] [Google Scholar]
- 49.Ojemann GA. Individual variability in cortical localization of language. J Neurosurg. 1979;50:164–169. doi: 10.3171/jns.1979.50.2.0164. [DOI] [PubMed] [Google Scholar]
- 50.Ojemann GA, Whitaker HA. Language localization and variability. Brain Lang. 1978;6:239–260. doi: 10.1016/0093-934x(78)90061-5. [DOI] [PubMed] [Google Scholar]
- 51.Sanai N, Mirzadeh Z, Berger MS. Functional outcome after language mapping for glioma resection. N Engl J Med. 2008;358:18–27. doi: 10.1056/NEJMoa067819. [DOI] [PubMed] [Google Scholar]
- 52.Seitz RJ, Huang Y, Knorr U, et al. Large-scale plasticity of the human motor cortex. Neuroreport. 1995;6:742–744. doi: 10.1097/00001756-199503270-00009. [DOI] [PubMed] [Google Scholar]
- 53.Wunderlich G, Knorr U, Herzog H, et al. Precentral glioma location determines the displacement of cortical hand representation. Neurosurgery. 1998;42:18–27. doi: 10.1097/00006123-199801000-00005. [DOI] [PubMed] [Google Scholar]
- 54.FitzGerald DB, Cosgrove GR, Ronner S, et al. Location of language in the cortex: a comparison between functional MR imaging and electrocortical stimulation. AJNR Am J Neuroradiol. 1997;18:1529–1539. [PMC free article] [PubMed] [Google Scholar]
- 55.Quiñones-Hinojosa A, Ojemann SG, Sanai N, et al. Preoperative correlation of intraoperative cortical mapping with magnetic resonance imaging landmarks to predict localization of the Broca area. J Neurosurg. 2003;99:311–318. doi: 10.3171/jns.2003.99.2.0311. [DOI] [PubMed] [Google Scholar]
- 56.Ojemann JG, Miller JW, Silbergeld DL. Preserved function in brain invaded by tumor. Neurosurgery. 1996;39:253–259. doi: 10.1097/00006123-199608000-00003. [DOI] [PubMed] [Google Scholar]
- 57.Skirboll SS, Ojemann GA, Berger MS, et al. Functional cortex and subcortical white matter located within gliomas. Neurosurgery. 1996;38:678–685. [PubMed] [Google Scholar]
- 58.Penfield W, Boldrey E. Somatic motor and sensory representation in the cerebral cortex of man as studied by electrical stimulation. Brain. 1937;60:389–443. [Google Scholar]
- 59.Sartorius CJ, Berger MS. Rapid termination of intraoperative stimulation-evoked seizures with application of cold Ringer's lactate to the cortex. Technical note. J Neurosurg. 1998;88:349–351. doi: 10.3171/jns.1998.88.2.0349. [DOI] [PubMed] [Google Scholar]
- 60.De Witt Hamer PC, Robles SG, Zwinderman AH, et al. Impact of intraoperative stimulation brain mapping on glioma surgery outcome: a meta-analysis. J Clin Oncol. 2012;30:2559–2565. doi: 10.1200/JCO.2011.38.4818. [DOI] [PubMed] [Google Scholar]
- 61.Orringer DA, Golby A, Jolesz F. Neuronavigation in the surgical management of brain tumors: current and future trends. Expert Rev Med Devices. 2012;9:491–500. doi: 10.1586/erd.12.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Willems PW, Taphoorn MJ, Burger H, et al. Effectiveness of neuronavigation in resecting solitary intracerebral contrast-enhancing tumors: a randomized controlled trial. J Neurosurg. 2006;104:360–368. doi: 10.3171/jns.2006.104.3.360. [DOI] [PubMed] [Google Scholar]
- 63.Tarapore PE, Tate MC, Findlay AM, et al. Preoperative multimodal motor mapping: a comparison of magnetoencephalography imaging, navigated transcranial magnetic stimulation, and direct cortical stimulation. J Neurosurg. 2012;117:354–362. doi: 10.3171/2012.5.JNS112124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Della Puppa A, De Pellegrin S, d'Avella E, et al. 5-aminolevulinic acid (5-ALA) fluorescence guided surgery of high-grade gliomas in eloquent areas assisted by functional mapping. Our experience and review of the literature. Acta Neurochir (Wien) 2013;155:965–972. doi: 10.1007/s00701-013-1660-x. [DOI] [PubMed] [Google Scholar]
- 65.Feigl GC, Ritz R, Moraes M, et al. Resection of malignant brain tumors in eloquent cortical areas: a new multimodal approach combining 5-aminolevulinic acid and intraoperative monitoring. J Neurosurg. 2010;113:352–357. doi: 10.3171/2009.10.JNS09447. [DOI] [PubMed] [Google Scholar]
- 66.González-Darder JM, González-López P, Talamantes F, et al. Multimodal navigation in the functional microsurgical resection of intrinsic brain tumors located in eloquent motor areas: role of tractography. Neurosurg Focus. 2010;28:E5. doi: 10.3171/2009.11.FOCUS09234. [DOI] [PubMed] [Google Scholar]
- 67.Nossek E, Korn A, Shahar T, et al. Intraoperative mapping and monitoring of the corticospinal tracts with neurophysiological assessment and 3-dimensional ultrasonography-based navigation. Clinical article. J Neurosurg. 2011;114:738–746. doi: 10.3171/2010.8.JNS10639. [DOI] [PubMed] [Google Scholar]
- 68.Prabhu SS, Gasco J, Tummala S, et al. Intraoperative magnetic resonance imaging-guided tractography with integrated monopolar subcortical functional mapping for resection of brain tumors. Clinical article. J Neurosurg. 2011;114:719–726. doi: 10.3171/2010.9.JNS10481. [DOI] [PubMed] [Google Scholar]
- 69.Schucht P, Beck J, Abu-Isa J, et al. Gross total resection rates in contemporary glioblastoma surgery: results of an institutional protocol combining 5-aminolevulinic acid intraoperative fluorescence imaging and brain mapping. Neurosurgery. 2012;71:927–936. doi: 10.1227/NEU.0b013e31826d1e6b. [DOI] [PubMed] [Google Scholar]
- 70.North CA, North RB, Epstein JA, et al. Low-grade cerebral astrocytomas. Survival and quality of life after radiation therapy. Cancer. 1990;66:6–14. doi: 10.1002/1097-0142(19900701)66:1<6::aid-cncr2820660103>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 71.Ahmadi R, Dictus C, Hartmann C, et al. Long-term outcome and survival of surgically treated supratentorial low-grade glioma in adult patients. Acta Neurochir. 2009;151:1359–1365. doi: 10.1007/s00701-009-0435-x. [DOI] [PubMed] [Google Scholar]