Abstract
The purpose of this study was to examine biological and behavioral explanations for gender differences in leukocyte telomere length (LTL), a biomarker of cell aging that has been hypothesized to contribute to women’s greater longevity. Data are from a subsample (n = 851) of the Multi-Ethnic Study of Atherosclerosis, a population-based study of women and men aged 45 to 84. Mediation models were used to examine study hypotheses. We found that women had longer LTL than men, but the gender difference was smaller at older ages. Gender differences in smoking and processed meat consumption partially mediated gender differences in telomere length, whereas gender differences in estradiol, total testosterone, oxidative stress, and body mass index did not. Neither behavioral nor biological factors explained why the gender difference in LTL was smaller at older ages. Longitudinal studies are needed to assess gender differences in the rate of change in LTL over time; to identify the biological, behavioral, and psychosocial factors that contribute to these differences throughout the life course; and to determine whether gender differences in LTL explain the gender gap in longevity.
On average, women in the United States outlive men by 4.9 years (Minnino 2011). Studies suggest that gender differences in leukocyte telomere length (LTL), a marker of cell aging, may contribute to women’s greater longevity (Aviv et al. 2005; Stindl 2004; Barrett and Richardson 2011). While a growing body of evidence has demonstrated that women have longer telomeres than men (see Sanders and Newman 2013 for a review), prior research has not examined the mechanisms linking gender to telomere length. Using data from the Multi-Ethnic Study of Atherosclerosis (MESA), a population-based study of women and men aged 45 to 84, the current study examined biological and behavioral explanations for gender differences in LTL.
The Telomere Hypothesis of Aging
Telomeres naturally shorten with mitosis; every time a cell divides, a portion of the telomeric DNA fails to replicate as a result of the “end replication problem” (Blackburn 2005). Oxidative stress also contributes to telomere loss (von Zglinicki 2002). Although telomerase can counteract shortening by elongating and protecting telomeres (Blackburn 1997), this enzyme is expressed at very low levels in normal somatic human cells. When telomeres become critically shortened, cellular senescence is triggered, and cells lose their ability to divide (Blackburn 2000; Blasco 2005).
In addition to being a key mechanism of cellular aging, telomere shortening has been hypothesized to contribute to organismal aging as well (Aubert and Lansdorp 2008). Human mutations causing short telomeres have been linked to a group of conditions collectively called “telomere syndromes” that resemble the premature onset of common age-related diseases, including cancer and liver disease (Armanios and Blackburn 2012). Moreover, epidemiologic studies have found that shorter telomere length is associated with numerous diseases of aging, including cardiovascular disease (Samani et al. 2001; Brouilette et al. 2007; Fitzpatrick et al. 2007; Willeit, Willeit, Brandstatter, et al. 2010), type 2 diabetes (Zee et al. 2010; Salpea et al. 2010), dementia (von Zglinicki et al. 2000; Panossian et al. 2003; Yaffe et al. 2011), and cancer (Willeit, Willeit, Mayr, et al. 2010; Wentzensen et al. 2011), independent of chronological age. While some studies have failed to find an association between telomere length and survival (Harris et al. 2006; Njajou et al. 2009; Strandberg et al. 2011; Bischoff et al. 2006), the majority of epidemiologic studies have found that shorter telomere length is associated with increased odds of mortality (Astrup et al. 2010; Cawthon et al. 2003; Epel et al. 2009; Fitzpatrick et al. 2011; Honig et al. 2012; Kimura et al. 2008; Lee et al. 2012; Martin-Ruiz et al. 2006; Weischer et al. 2012; Willeit, Willeit, Mayr, et al. 2010; Bakaysa et al. 2007; Farzaneh-Far et al. 2008).
Consistent with the telomere hypothesis of aging, older people tend to have shorter telomeres than younger people, but there is substantial inter-individual variation in telomere length (Muezzinler, Zaineddin, and Brenner 2013). Evidence from twin studies suggests that telomere length is heritable, with estimates of heritability ranging from .36 (Andrew et al. 2006) to .44 (Njajou et al. 2007). Of note to social and behavioral scientists, there is also evidence that telomere length is modified by environmental risk factors, such as exposure to lead (Wu et al. 2012) and air pollution (Hou et al. 2012); behavioral risk factors, such as smoking (McGrath et al. 2007), physical activity (Ludlow and Roth 2011), and processed meat consumption (Nettleton et al. 2008); and psychosocial risk factors, such as low socioeconomic status (SES) (Needham et al. 2013), depression (Simon et al. 2006), and perceived stress (Epel et al. 2004).
Gender Differences in Telomere Length
A number of studies have found that women have longer telomeres than men during adulthood (Benetos et al. 2001; Cawthon et al. 2003; Jeanclos et al. 2000; Nawrot et al. 2004; Slagboom, Droog, and Boomsma 1994; Mayer et al. 2006; Moller et al. 2009; Diez Roux et al. 2009), and a recent review of the literature on telomere epidemiology concluded that the association between gender and LTL has been observed consistently across studies (Sanders and Newman 2013). Explanations for gender differences in LTL have focused primarily on biological factors. There is some evidence that estrogen may reduce oxidative stress, which has been shown to accelerate telomere shortening (von Zglinicki 2002), and that estrogen stimulates the production of telomerase (Bayne et al. 2007), which protects and lengthens telomeres. Conversely, testosterone appears to have fewer antioxidant properties and may even increase oxidative stress, which could contribute to shorter telomere lengths in men (see Aviv et al. 2005; and Barrett and Richardson 2011 for detailed reviews on sex hormones and other biological factors that may contribute to gender differences in telomere length).
At least one prior study found that women’s midlife advantage in LTL diminished for older adults (Diez Roux et al. 2009). Because of the cross-sectional nature of the study, it is unclear whether the observed decline was due to aging, cohort differences, or survivor bias. If the diminishment was, in fact, due to aging, then the narrowing of the gender gap in telomere length during the post-reproductive years may be attributable to the loss of the protective effect of estrogen after menopause. Notably, as women age and transition through menopause, estradiol (the primary estrogen secreted from the ovary) declines dramatically. Thus, age-related declines in estradiol may lead to accelerated telomere shortening among postmenopausal women. Despite the plausibility of biological explanations for gender and gender-by-age differences in LTL, no prior studies have directly tested hypotheses related to sex hormones or oxidative stress.
While biological mechanisms have received the most attention in the literature on LTL, health behavior may also contribute to gender and gender-by-age differences in telomere length. Courtenay (2000b) has argued that men engage in risky behaviors in order to demonstrate hegemonic masculinity, which is characterized by strength, power, and invulnerability. Men tend to have worse health behaviors than women, including greater rates of smoking, greater processed meat consumption, and higher levels of overweight and obesity (which results from behaviors related to diet and activity) (Courtenay 2000a), and these behaviors are associated with reduced telomere length (McGrath et al. 2007; Nettleton et al. 2008; Lee et al. 2011). While demonstrating masculinity is important to men of all ages, a Swedish study found that risk-taking was not a component of the dominant masculinities constructed by men aged 85 and older (Alex et al. 2008). This finding is consistent with previous research that found that gender differences in risky health behavior are smaller at older ages because younger men are more likely to engage in risky behaviors than older men (Liang et al. 1999). Thus, narrowing of the gender gap in telomere length during the post-reproductive years may be due to greater age-related declines in health risk behavior among men compared to women.
Hypotheses
Previous research using data from the MESA study reported that women had significantly longer telomeres than men, and that the gender difference was smaller at older ages (Diez Roux et al. 2009). The purpose of the current study was to examine biological and behavioral explanations for these observed differences. First, we hypothesized that the effect of gender on LTL would be partially mediated by estradiol, testosterone, oxidative stress, smoking, processed meat consumption, and body mass index (BMI)—all factors previously shown or hypothesized to be related to telomere length. We expected that there would be an indirect relationship in which gender would be related to the proposed biological and behavioral mediators, which would in turn be related to LTL. Given that we hypothesized partial mediation, we also expected to observe a direct effect of gender on LTL in addition to its indirect effect through sex hormones, oxidative stress, and key health behaviors. We expected that the direct and indirect effects would be observed for respondents at all ages. The total effect model is shown in Panel A of Figure 1, and the mediation model, which includes direct and indirect effects, is shown in Panel B of Figure 1.
Figure 1.
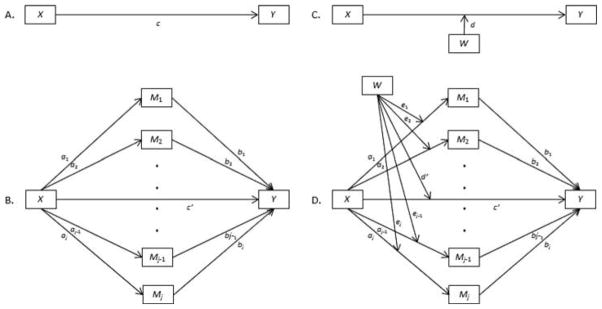
Conceptual models. Panel A shows the total effect model; Panel B shows the mediation model; Panel C shows the total conditional effect model; Panel D shows the mediated moderation model; X is gender; Y is LTL; M1–Mj are estradiol, total testosterone, oxidative stress (GGT), pack-years smoking, processed meat consumption, and BMI; W is age; c = total effect of X on Y; c′ = direct effect of X on Y; a1b1 = indirect effect of X on Y through M1; a2b2 = indirect effect of X on Y through M2; aj–1bj–1 = indirect effect on X on Y through Mj–1; ajbj = indirect effect of X on Y through Mj; a1b1+ a2b2+ … + aj–1bj–1 + ajbj = total indirect effect of X on Y through M1, M2, … Mj–1, Mj; d = total effect of X on Y conditional on W; d′ = direct effect of X on Y conditional on W; (a1+e1W)b1 = indirect effect of X on Y through M1 conditional on W; (a2 + e2W)b2 = indirect effect of X on Y through M2 conditional on W; (aj–1 + ej1W)bj–1 = indirect effect of X on Y through Mj–1 conditional on W; (aj + ejW)bj = indirect effect of X on Y through Mj conditional on W; e1b1 = indirect effect of XW on Y through M1; e2b2 = indirect effect of XW on Y through M2; ej–1bj–1 = indirect effect of XW on Y through Mj–1; ejbj = indirect effect of XW on Y through Mj.
Next, we hypothesized that the narrowing of the gender gap in LTL with increasing age would be partially mediated by estradiol, testosterone, oxidative stress, smoking, processed meat consumption, and BMI. We expected that gender differences in LTL would be greater at younger ages because gender differences in the proposed biological and behavioral mediators would also be greater at younger ages. The total conditional effect model (i.e., the model in which the effect of gender on LTL varies by age) is shown in Panel C of Figure 1, and the mediated moderation model, which includes conditional indirect and conditional direct effects, is shown in Panel D of Figure 1.
Data and Methods
Data
MESA is a population-based longitudinal study designed to identify risk factors for the progression of subclinical cardiovascular disease (CVD) (Bild et al. 2002). Between July 2000 and August 2002, 6,814 white, African American, Latino, and Chinese American women and men aged 45 to 84 without clinically apparent CVD were recruited from six regions in the United States, including Forsyth County, North Carolina; northern Manhattan and the Bronx, New York; Baltimore City and Baltimore County, Maryland; St. Paul, Minnesota; Chicago, Illinois; and Los Angeles County, California. Each field site recruited from locally available sources, which included lists of residents, lists of dwellings, and telephone exchanges. Telomeres were assessed on a random subsample of 978 white, black, and Latino MESA participants aged 45 to 84 years from the New York and Los Angeles sites who agreed to participate in an ancillary study examining the effects of stress on cardiovascular outcomes (the MESA Stress Study). We excluded 81 premenopausal women, since sex hormones were only measured in men and postmenopausal women, and an additional 46 respondents with missing data on one or more study variables (final n = 851).
Measures
Dependent Variable
LTL was measured by quantitative polymerase chain reaction (Q-PCR) in serum obtained at the baseline examination (Cawthon 2002). Each sample was amplified for telomeric DNA and for 36B4, a single-copy control gene that provided an internal control to normalize the starting amount of DNA. A four-point standard curve (twofold serial dilutions from 10 to 1.25 ng DNA) was used to transform cycle threshold into nanograms of DNA. Baseline background subtraction was performed by aligning amplification plots to a baseline height of 2 percent in the first five cycles. The cycle threshold was set at 20 percent of maximum product. All samples were run in triplicate, and the median was used for calculations. The amount of telomeric DNA (T) was divided by the amount of single-copy control gene DNA (S), producing a relative measurement of the telomere length (T/S ratio). Two control samples were run in each experiment to allow for normalization between experiments, and periodical reproducibility experiments were performed to guarantee correct measurements. The intra- and inter-assay coefficients of variation for Q-PCR were 6 percent and 7 percent, respectively.
Independent Variable
Gender was coded 1 for female and 0 for male.
Biological Mediators
Sex hormones were measured in serum obtained at the baseline visit in men and postmenopausal women. Serum estradiol (nmol/L) was measured using an ultrasensitive radioimmunoassay kit (Diagnostic System Laboratories, Webster, Texas). Total testosterone (nmol/L) was measured directly with a radioimmunoassay kit (Diagnostic Products Corporation, Los Angeles, California). The intra-assay coefficients of variation for estradiol and total testosterone were 10.5 percent and 12.3 percent, respectively. Sex hormones were measured in the Steroid Hormone Laboratory at the University of Massachusetts Medical Center (Worcester, Massachusetts). Gamma-glutamyl transferase (GGT) (U/L), a biomarker of oxidative stress, was measured at the Fletcher Allen Health Care laboratory at the University of Vermont (Burlington, Vermont) using an assay based on methods by Silber, Gandolfi, and Brendel (1986). The intra-assay coefficient of variation for GGT was less than 5 percent.
Behavioral Mediators
Pack-years of smoking was calculated as the average number of cigarettes smoked per day times the number of years smoked, divided by 20. Processed meat consumption was the average number of servings per day during the past year of processed meat, including ham, hot dogs, lunch meat, sausage, organ meats, and ham hocks. BMI was the ratio of weight to height squared in kg/m2.
Moderator
Age was measured in years.
Covariates
All models included controls for the following potential confounders of hypothesized mediator-outcome relationships: age, race/ethnicity (dummy variables for African American and Latino, with white as the reference category), education (dummy variables for less than high school, high school, and some college, with college as the reference category), and per capita household income (total income/number of people supported). Adjusting for these factors also allowed us to estimate gender differences in LTL net of differences in sociodemographic characteristics between women and men. Descriptive statistics for all study variables are shown in Table 1.
Table 1.
Descriptive statistics for all study variables
| Full sample
|
Aged 45–64
|
Aged 65–84
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Female (n = 416) | Male (n = 435) | p | Female (n = 215) | Male (n = 247) | p | Female (n = 201) | Male (n = 188) | p | |
| LTL (T/S ratio) | .85 (.22) | .80 (.23) | .0007 | .91 (.24) | .83 (.22) | <.0001 | .81 (.23) | .79 (.23) | .16 |
| Estradiol (nmol/L) | .07 (.08) | .11 (.06) | <.0001 | .08 (.13) | .12 (.06) | <.0001 | .06 (.05) | .11 (.06) | <.0001 |
| Total testosterone (nmol/L) | .94 (.75) | 13.91 (6.32) | <.0001 | .97 (.80) | 14.64 (6.73) | <.0001 | .90 (.69) | 13.55 (6.40) | <.0001 |
| Oxidative stress (GGT [U/L]) | 7.89 (7.34) | 11.20 (11.45) | <.0001 | 8.07 (7.56) | 11.93 (12.48) | <.0001 | 7.47 (6.87) | 10.18 (8.75) | <.0001 |
| Pack-years smoking | .00 (3.90) | 2.55 (17.00) | <.0001 | .00 (3.50) | 1.15 (12.60) | <.0001 | .00 (6.40) | 5.20 (20.50) | <.0001 |
| Processed meat consumption (servings per day) | .03 (.14) | .09 (.17) | <.0001 | .05 (.14) | .11 (.18) | <.0001 | .02 (.12) | .08 (.16) | <.0001 |
| Body mass index (BMI) | 28.59 (7.41) | 27.78 (5.29) | .0009 | 29.44 (7.58) | 28.01 (5.87) | .003 | 28.05 (7.31) | 27.66 (4.70) | .08 |
| Age (in years) | 64.00 (14.00) | 62.00 (15.00) | .04 | 56.00 (8.00) | 55.00 (10.00) | .002 | 70.00 (7.00) | 70.00 (8.00) | .24 |
| White | .17 | .19 | .45 | .15 | .17 | .48 | .20 | .21 | .61 |
| African American | .30 | .27 | .27 | .33 | .30 | .46 | .27 | .22 | .34 |
| Latino | .53 | .54 | .67 | .53 | .54 | .86 | .53 | .57 | .68 |
| Less than high school | .29 | .27 | .52 | .24 | .25 | .91 | .33 | .31 | .52 |
| High school | .23 | .18 | .06 | .22 | .15 | .20 | .24 | .18 | .16 |
| Some college | .28 | .30 | .56 | .33 | .34 | .97 | .24 | .28 | .46 |
| College | .20 | .25 | .06 | .21 | .26 | .17 | .19 | .24 | .18 |
| Per capita household income (in 10,000s) | 1.50 (1.89) | 1.50 (2.38) | .98 | 1.50 (2.11) | 1.40 (2.17) | .81 | 1.40 (1.83) | 1.63 (2.41) | .62 |
Notes: Proportions are shown for categorical variables; medians with interquartile ranges in parentheses are shown for continuous variables; p-value for comparison across gender groups is based on chi-square for proportions and Wilcoxon-Mann-Whitney test for medians.
Analytical Design
The first hypothesis was that the effect of gender on LTL would be partially mediated by estradiol, testosterone, oxidative stress, smoking, processed meat consumption, and BMI. First, LTL was regressed on gender and the covariates to generate an estimate of the total effect of gender on telomere length. Next, each mediator was regressed on gender, along with the covariates. Finally, LTL was regressed on gender, the mediators, and the covariates. The indirect effect of each mediator was calculated by multiplying the path coefficient from gender to the mediator by the path coefficient from the mediator to LTL. The direct effect was calculated as the difference between the total effect and the total indirect effect (i.e., the sum of the indirect effects for each mediator) (Hayes 2009). This approach assumed no residual confounding of the mediator-outcome relation and no interaction between the mediator and gender (MacKinnon, Fairchild, and Fritz 2007; VanderWeele 2009). We used a directed acyclic graph (DAG), which uses formal rules to depict assumptions regarding causal relationships between variables, to select the appropriate covariates for the models (Glymour 2006). In addition, we regressed LTL on each mediator, gender, an interaction term between gender and each mediator, and the covariates. None of the interactions were significant, which suggests that the associations between the proposed mediators and LTL do not differ for women and men (results available from the authors upon request).
The second hypothesis was that the interactive effect of gender and age on LTL would be partially mediated by estradiol, testosterone, oxidative stress, smoking, processed meat consumption, and BMI. First, LTL was regressed on gender, gender*age, and the covariates; this model provided an estimate of the total effect of gender on LTL conditional on age. Next, each mediator was regressed on gender, gender*age, and the covariates. Finally, LTL was regressed on gender, gender*age, the mediators, and the covariates. The indirect effect of the interaction of gender and age on LTL through each mediator was calculated by multiplying the path coefficient from gender*age to the mediator by the path coefficient from the mediator to LTL (Hayes 2012).
We used PROCESS, a SAS macro developed by Hayes (2012), to test the study hypotheses. PROCESS can accommodate mediation models, including mediated moderation models, with up to 10 mediators operating in parallel (Hayes 2012). We used bias-corrected bootstrap confidence intervals to test the significance of the indirect effects in the mediation and mediated moderation models (Hayes 2012; Bollen and Stine 1990). If the 95 percent confidence interval does not contain zero, then the indirect effect is significant at the p < .05 level.
Results
First, we examined whether biological and behavioral factors mediate gender differences in LTL. The results of the mediation model are shown in Table 2. There was a significant total effect of gender on LTL, controlling for age, race/ethnicity, education, and per capita household income. In fully adjusted models, estradiol did not differ by gender, but women had significantly lower total testosterone, oxidative stress (GGT), smoking, and processed meat consumption than men, as well as significantly higher BMI than men. Of the six proposed mediators, only smoking and processed meat consumption were significantly associated with shorter LTL. The direct effect of gender on LTL was not significant after accounting for the hypothesized mediators, but the bias-corrected bootstrap confidence interval for the total indirect effect of gender on LTL through all the proposed mediators included zero and was therefore not statistically significant. Although the total indirect effect was not significant, there were significant specific indirect effects of gender on LTL through smoking and processed meat consumption. In contrast, there were no significant specific indirect effects of gender on LTL through estradiol, total testosterone, oxidative stress (GGT), or BMI.
Table 2.
Regression results for the mediation of the effect of female gender on LTL (T/S ratio) by estradiol, total testosterone, oxidative stress (GGT), pack-years smoking, processed meat consumption, and body mass index (BMI), n = 851
| Model | Estimate | SE | p | CI (lower) | CI (upper) |
|---|---|---|---|---|---|
| Total effect of gender on LTL | |||||
| Female → LTL (c) | .049 | .011 | <.001 | .026 | .071 |
| Effect of gender on mediators | |||||
| Female → Estradiol (a1) | .004 | .007 | .527 | −.009 | .017 |
| Female → Total testosterone (a2) | −13.649 | .284 | .000 | −14.205 | −13.092 |
| Female → GGT (a3) | −3.427 | .930 | .000 | −5.253 | −1.602 |
| Female → Smoking (a4) | −7.821 | 1.068 | .000 | −9.917 | −5.725 |
| Female → Processed meat (a5) | −.088 | .016 | .000 | −.121 | −.056 |
| Female → BMI (a6) | 1.421 | .355 | .000 | .725 | 2.117 |
| Effect of mediators on LTL | |||||
| Estradiol → LTL (b1) | .038 | .061 | .534 | −.082 | .158 |
| Total testosterone → LTL (b2) | −.000 | .001 | .823 | −.003 | .003 |
| GGT → LTL (b3) | −.000 | .000 | .785 | −.001 | .001 |
| Smoking → LTL (b4) | −.001 | .000 | .040 | −.002 | −.000 |
| Processed meat → LTL (b5) | −.057 | .024 | .018 | −.104 | −.010 |
| BMI → LTL (b6) | .001 | .001 | .395 | −.001 | .003 |
| Direct effect of gender on LTL | |||||
| Female → LTL (c′) | .031 | .023 | .171 | −.014 | .076 |
| Indirect effect of gender on LTL | |||||
| Total indirect effect through all mediators ((a1b1)+(a2b2)+(a3b3)+(a4b4)+(a5b5)+(a6b6)) | .017 | .021 | −.028 | .053 | |
| Indirect effect through estradiol (a1b1) | .000 | .001 | −.000 | .002 | |
| Indirect effect through total testosterone (a2b2) | .004 | .020 | −.040 | .039 | |
| Indirect effect through GGT (a3b3) | .000 | .002 | −.003 | .004 | |
| Indirect effect through smoking (a4b4) | .006 | .003 | .001 | .012 | |
| Indirect effect through processed meat (a5b5) | .005 | .003 | .001 | .010 | |
| Indirect effect through BMI (a6b6) | .001 | .002 | −.001 | .005 | |
| R2Y,X | .091 | ||||
| R2M1,X | .039 | ||||
| R2M2,X | .739 | ||||
| R2M3,X | .054 | ||||
| R2M4,X | .083 | ||||
| R2M5,X | .065 | ||||
| R2M6,X | .073 | ||||
| R2Y,M1M2M3M4M5M6X | .111 | ||||
Notes: Regression weights a1-aj, b1-bj, c, and c′ are shown in Panels A and B of Figure 1. R2Y,X is the proportion of variance in LTL (Y) explained by female gender (X); R2M1,X is the proportion of the variance in estradiol (M1) explained by female gender (X); R2 M2,X is the proportion of the variance in total testosterone (M2) explained by female gender (X); R2 M3,X is the proportion of the variance in GGT (M3) explained by female gender (X); R2 M4,X is the proportion of the variance in smoking (M4) explained by female gender (X); R2 M5,X is the proportion of the variance in processed meat consumption (M5) explained by female gender (X); R2 M6,X is the proportion of the variance in BMI (M6) explained by female gender (X); and R2 Y,M1M2M3M4M5M6X is the proportion of the variance in LTL (Y) explained by female gender (X), estradiol (M1), total testosterone (M2), GGT (M3), smoking (M4), processed meat consumption (M5), and BMI (M6). The 95 percent CIs for the indirect effects are obtained by the bias-corrected bootstrap with 5,000 resamples. CI (lower) = lower bound of a 95 percent confidence interval; CI (upper) = upper bound; → = affects. Models include controls for age, race/ethnicity, education, and per capita household income.
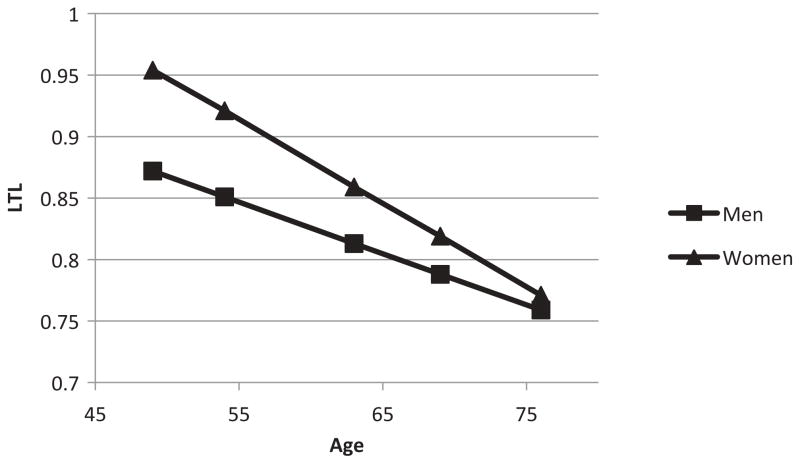
Next, we examined whether biological and behavioral factors mediate gender-by-age differences in LTL. The results of the conditional mediation model are shown in Table 3. Controlling for age, race/ethnicity, education, and per capita household income, both the main effect of gender on LTL and the interaction of gender with age were significant. As shown in Figure 2, probing the interaction revealed that the effect of gender on LTL declined with age and was no longer significant after age 70 (estimates were based on setting covariates to their sample means). In fully adjusted models, the main effect of gender on estradiol and the interaction of gender with age were significant; the main effect of gender on total testosterone and the interaction of gender with age were significant; neither the main effect of gender on oxidative stress (GGT) nor the interaction of gender with age was significant; the main effect of gender on smoking was not significant, but there was a significant interaction of gender with age; neither the main effect of gender on processed meat consumption nor the interaction of gender with age was significant; and neither the main effect of gender on BMI nor the interaction of gender with age was significant. Of the six proposed mediators, only smoking and processed meat consumption were significantly associated with LTL.
Table 3.
Regression results for the conditional direct and indirect effects of female gender on LTL (T/S ratio) by estradiol, total testosterone, oxidative stress (GGT), pack-years smoking, processed meat consumption, and body mass index (BMI), n = 851
| Model | Estimate | SE | p | CI (lower) | CI (upper) |
|---|---|---|---|---|---|
| Total conditional effect of gender on LTL | |||||
| Female → LTL (c) | .210 | .074 | .005 | .064 | .356 |
| Female × Age → LTL (d) | −.003 | .001 | .027 | −.005 | −.000 |
| Effect of gender and the interaction of gender and age on mediators | |||||
| Female → Estradiol (a1) | .154 | .043 | .000 | .070 | .238 |
| Female × Age → Estradiol (e1) | −.002 | .001 | .000 | −.004 | −.001 |
| Female → Total testosterone (a2) | −17.675 | 1.886 | .000 | −21.377 | −13.973 |
| Female × Age → Total testosterone (e2) | .064 | .030 | .031 | .006 | .123 |
| Female → GGT (a3) | −9.498 | 6.200 | .126 | −21.667 | 2.670 |
| Female × Age → GGT (e3) | .097 | .098 | .322 | −.095 | .289 |
| Female → Smoking (a4) | 11.293 | 7.091 | .112 | −2.626 | 25.211 |
| Female × Age → Smoking (e4) | −.305 | .112 | .007 | −.525 | −.086 |
| Female → Processed meat (a5) | −.040 | .109 | .718 | −.254 | .175 |
| Female × Age → Processed meat (e5) | −.001 | .002 | .651 | −.004 | .003 |
| Female → BMI (a6) | 4.086 | 2.636 | .084 | −.553 | 8.724 |
| Female × Age → BMI (e6) | −.043 | .034 | .255 | −.116 | .031 |
| Effect of mediators on LTL | |||||
| Estradiol → LTL (b1) | .020 | .061 | .748 | −.101 | .140 |
| Total testosterone → LTL (b2) | −.000 | .001 | .958 | −.003 | .003 |
| GGT → LTL (b3) | −.000 | .000 | .852 | −.001 | .001 |
| Smoking → LTL (b4) | −.001 | .000 | .024 | −.0020 | −.000 |
| Processed meat → LTL (b5) | −.057 | .024 | .018 | −.105 | −.010 |
| BMI → LTL (b6) | .001 | .001 | .412 | −.001 | .003 |
| Conditional direct effect of gender on LTL | |||||
| Female → LTL (c′) | .221 | .081 | .006 | .063 | .379 |
| Female × Age → LTL (d′) | −.003 | .001 | .015 | −.005 | −.001 |
| Indirect effect of the interaction of gender and age on LTL | |||||
| Indirect effect through estradiol (e1b1) | −.000 | .000 | −.000 | .000 | |
| Indirect effect through total testosterone (e2b2) | −.000 | .000 | −.000 | .000 | |
| Indirect effect through GGT (e3b3) | −.000 | .000 | −.000 | .000 | |
| Indirect effect through smoking (e4b4) | .000 | .000 | .000 | .001 | |
| Indirect effect through processed meat (e5b5) | .000 | .000 | −.000 | .000 | |
| Indirect effect through BMI (e6b6) | −.000 | .000 | −.000 | .000 | |
| R2Y,XW*X | .107 | ||||
| R2M1,XW*X | .053 | ||||
| R2M2,XW*X | .740 | ||||
| R2M3,XW*X | .055 | ||||
| R2M4,XW*X | .092 | ||||
| R2M5,XW*X | .065 | ||||
| R2M6,XW*X | .074 | ||||
| R2Y,M1M2M3M4M5M6XW*X | .118 | ||||
Notes: Regression weights a1–aj, b1–bj, c, and c′, d, d′, and e1–ej are shown in Panels A, C, and D of Figure 1. R2Y,XW*X is the proportion of variance in LTL (Y) explained by female gender (X) and the interaction of female gender (X) with age (W); R2M1,XW*X is the proportion of the variance in estradiol (M1) explained by female gender (X) and the interaction of female gender (X) with age (W); R2M2,XW*X is the proportion of the variance in total testosterone (M2) explained by female gender (X) and the interaction of female gender (X) and age (W); R2M3,XW*X is the proportion of the variance in GGT (M3) explained by female gender (X) and the interaction of female gender (X) and age (W); R2M4,XW*X is the proportion of the variance in smoking (M4) explained by female gender (X) and the interaction of female gender (X) and age (W); R2M5,XW*X is the proportion of the variance in processed meat consumption (M5) explained by female gender (X) and the interaction of female gender (X) and age (W); R2M6,XW*X is the proportion of the variance in BMI (M6) explained by female gender (X) and the interaction of female gender (X) and age (W); and R2Y,M1M2M3M4M5M6XW*X is the proportion of the variance in LTL (Y) explained by female gender (X), the interaction of female gender (X) and age (W), estradiol (M1), total testosterone (M2), GGT (M3), smoking (M4), processed meat consumption (M5), and BMI (M6). The 95 percent CIs for the indirect effects are obtained by the bias-corrected bootstrap with 5,000 resamples. CI (lower) = lower bound of a 95 percent confidence interval; CI (upper) = upper bound; → = affects. Models include controls for age, race/ethnicity, education, and per capita household income.
Figure 2.
LTL (T/S ratio) by gender and age (n = 851).
After accounting for the hypothesized mediators, the direct effect of gender on LTL and the direct effect of the interaction of gender with age remained significant. There were no significant indirect effects of the interaction between gender and age on LTL through estradiol, total testosterone, oxidative stress (GGT), processed meat consumption, or BMI. Although the indirect effect of the interaction between gender and age on LTL through smoking was significant, gender differences in smoking were larger rather than smaller at older ages. Consequently, controlling for smoking led to a marginal increase in the gender-by-age effect on LTL rather than a decrease (from −.0026 to −.0030). This pattern suggests that moderation of the effect of gender on LTL by age was not mediated by smoking, since mediated moderation requires that the direct effect be smaller in absolute value than the total effect (see Muller, Judd, and Yzerbyt 2005).
Discussion
The purpose of this study was to determine whether biological factors, including sex hormones and oxidative stress, and/or behavioral factors, including smoking, processed meat consumption, and BMI, explained previously reported gender and gender-by-age differences in LTL in a population-based study of adults aged 45 to 84 (Diez Roux et al. 2009). Explanations for gender differences in telomere length have largely focused on biological factors (see Aviv et al. 2005; Barrett and Richardson 2011), although no prior studies have directly tested hypothesized mechanisms underlying male/female differences in LTL.
First, we hypothesized that biological and behavioral factors would partially mediate the association between gender and LTL. We expected that there would be an indirect relationship in which gender would be related to the proposed mediators, which would in turn be related to LTL. Consistent with this hypothesis, we found that women had significantly lower levels of total testosterone, oxidative stress, smoking, and processed meat consumption. Contrary to expectations, however, we found no gender differences in estradiol, and we found that women had significantly higher BMI than men. None of the biological mediators were related to LTL, and there were no significant indirect effects of gender on LTL through sex hormones or oxidative stress. In contrast, two health behaviors were significantly associated with LTL: specifically, pack-years smoking and processed meat consumption (but not BMI) were related to shorter LTL, and both of these behavioral factors appeared to be significant mediators of the longer LTL observed among women. Sensitivity analyses stratified by age revealed significant indirect effects of gender on LTL through smoking and processed meat consumption among those aged 65 to 84 but not among those aged 45 to 64.
Next, we hypothesized that the interactive effect of gender and age on LTL would be partially mediated by estradiol, testosterone, oxidative stress, smoking, processed meat consumption, and BMI. We expected that gender differences in LTL would be greater at younger ages because gender differences in the proposed biological and behavioral mediators would also be greater at younger ages. Consistent with this hypothesis, we found that women had significantly higher levels of estradiol up to age 58 and significantly lower levels of estradiol after age 71 (results available from the authors upon request). In addition, we found that men had significantly higher levels of testosterone at all ages, but this gender difference was smaller at older ages. Contrary to expectations, we found that gender differences in smoking were larger at older ages and that gender differences in oxidative stress, processed meat consumption, and BMI did not vary by age. The interactive effect of gender and age on LTL was not mediated by estradiol, total testosterone, oxidative stress, processed meat consumption, or BMI. Although the indirect effect of the interaction between gender and age on LTL through smoking was significant, this should not be interpreted as evidence of mediated moderation because controlling for smoking led to an increase in the gender by age effect on LTL rather than a decrease (Muller, Judd, and Yzerbyt 2005).
Limitations, Strengths, and Directions for Future Research
This study had several limitations. First, we used cross-sectional data to examine causal models. In order for the conclusions from a mediation analysis to be valid, causal order must be correctly specified (MacKinnon, Fairchild, and Fritz 2007). While we are obviously confident that gender is not caused by LTL or the mediators, we cannot rule out the possibility that shorter LTL led to changes in the biological and/or behavioral mediators. Furthermore, we were unable to establish time order in the relationships among the mediators. The models assumed that the mediators were operating in parallel, but this may not be a valid assumption. For example, sex hormones, smoking, processed meat consumption, and/or BMI may be upstream determinants of oxidative stress. To the extent that the hypothesized mediators are linked together in a serial rather than parallel causal sequence, we may have underestimated the indirect effects of upstream determinants. Although sensitivity analyses excluding oxidative stress produced substantively equivalent results, longitudinal data are required to validate the assumptions of the parallel multiple mediator model. Longitudinal data are also needed to determine whether the observed patterns reflect either cohort effects (e.g., greater gender differences in smoking in older cohorts) or selection effects (e.g., greater mortality among female than male smokers).
Next, measurement error in the proposed hormonal mediators may have led to bias in our estimates of the indirect effects of estradiol and testosterone. The laboratory assays may not have provided optimal sensitivity to detect the low levels of estradiol common among postmenopausal women and men and testosterone among women (Rosner et al. 2013). The use of more sensitive sex hormone assays could help further refine the understanding of any meditational role of these sex hormones in gender differences in LTL.
Finally, we were unable to test several hypothesized biological mechanisms underlying gender differences in telomere length, including variation in telomere maintenance alleles on the X chromosome, skewed X-inactivation, sex-specific parental imprinting of telomere maintenance genes, and sexual size dimorphism (see Barrett and Richardson 2011). In addition to exploring these additional biological hypotheses, future studies should examine other potential behavioral mechanisms, such as heavy drinking and sedentary behavior, as well as potential social/psychosocial mechanisms, such as social support and social integration.
Balancing these study limitations were several key strengths. First and foremost, most prior research on gender differences in LTL has been conducted in small, homogenous samples. While larger, more representative studies have begun to add measures of telomere length, MESA is the only population-based study that we are aware of that includes data on LTL, sex hormones, oxidative stress, and health behavior for women and men—data that are necessary to test hypotheses regarding the mechanisms underlying gender differences in LTL. Another strength of this study was the application of new methods to study mediation and mediated moderation hypotheses (Hayes 2012). These methods overcame the limitations of earlier methods, such as the causal steps approach, by quantifying and testing the significance of indirect effects and by combining mediation and moderation analytically (Hayes 2009; Edwards and Lambert 2007).
Future work can refine the present analyses with more extensive data than are currently available. Most notably, longitudinal data could help distinguish aging effects from possible cohort effects, particularly with regard to the significant interaction of gender with age. While our results suggest that women may experience a faster rate of decline in telomere length during the transition from mid to late life, longitudinal data are needed to determine whether there are gender differences in the intra-individual rate of change in LTL over time or whether the cross-sectional results were confounded with cohort effects or affected by survivor bias (since men have a higher mortality rate than women at all ages, unobserved factors, which could be related to telomere length, may have conferred a survival advantage to the men who were older at the baseline examination).
Future studies should also examine the study hypotheses in a younger sample of women and men, as our findings are not generalizable beyond the age range of the population studied. Moreover, because we did not have sex hormone measures for premenopausal women, we were not able to determine whether biological (or behavioral) factors contribute to gender differences in LTL during the reproductive years, when estradiol levels are high. Future studies should include women across reproductive stages. Finally, given prior evidence that hormone replacement therapy protects against telomere shortening in female APOE-ε4 carriers (Jacobs et al. 2013), future research should consider the extent to which genetic factors moderate associations between sex hormones and telomere length.
Conclusions
In summary, our findings suggest that gender differences in smoking and processed meat consumption contribute to gender differences in telomere length during mid to late life, whereas gender differences in sex hormones and oxidative stress do not. Neither behavioral nor biological factors explain why the gender difference in telomere length seen in this study was smaller at older ages. Longitudinal studies that begin in early adulthood (or even in childhood or adolescence) are needed to assess gender differences in the rate of change in LTL over time; to identify the biological, behavioral, and psychosocial factors that contribute to these differences throughout the life course; and to determine whether gender differences in LTL explain the gender gap in longevity.
Acknowledgments
Funding
MESA was supported by contracts N01-HC-95159 through N01-HC-95169 from the National Heart, Lung, and Blood Institute (NHLBI) and by grants UL1-RR-024156 and UL1-RR-025005 from the National Center for Research Resources (NCRR). This work was also supported by grants R01-HL-076831 and R01-HL-101161 from NHLBI (PI: Ana Diez Roux), grant R01-HL-074406 from NHLBI (PI: Pamela Ouyang), grant R01-HL-074338 from NHLBI (PI: Susan Gapstur), and grant UL1-RR-02501401 from NCRR (PI: Mary Disis). The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
References
- Alex L, Hammarstrom A, Norberg A, Lundman B. Construction of masculinities among men aged 85 and older in the north of Sweden. J Clin Nurs. 2008;17(4):451–459. doi: 10.1111/j.1365-2702.2007.01961.x. [DOI] [PubMed] [Google Scholar]
- Andrew T, Aviv A, Falchi M, Surdulescu GL, Gardner JP, Lu X, Kimura M, Kato BS, Valdes AM, Spector TD. Mapping genetic loci that determine leukocyte telomere length in a large sample of unselected female sibling pairs. Am J Hum Genet. 2006;78(3):480–486. doi: 10.1086/500052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armanios M, Blackburn EH. The telomere syndromes. Nat Rev Genet. 2012;13(10):693–704. doi: 10.1038/nrg3246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astrup AS, Tarnow L, Jorsal A, Lajer M, Nzietchueng R, Benetos A, Rossing P, Parving HH. Telomere length predicts all-cause mortality in patients with type 1 diabetes. Diabetologia. 2010;53(1):45–48. doi: 10.1007/s00125-009-1542-1. [DOI] [PubMed] [Google Scholar]
- Aubert G, Lansdorp PM. Telomeres and aging. Physiol Rev. 2008;88(2):557–579. doi: 10.1152/physrev.00026.2007. [DOI] [PubMed] [Google Scholar]
- Aviv A, Shay J, Christensen K, Wright W. The longevity gender gap: are telomeres the explanation? Sci Aging Knowledge Environ. 2005;23:16. doi: 10.1126/sageke.2005.23.pe16. [DOI] [PubMed] [Google Scholar]
- Bakaysa SL, Mucci LA, Slagbloom PE, Boomsma DI, McClearn GE, Johansson B, Pedersen NL. Telomere length predicts survival independent of genetic influences. Aging Cell. 2007;6:769–774. doi: 10.1111/j.1474-9726.2007.00340.x. [DOI] [PubMed] [Google Scholar]
- Barrett ELB, Richardson DS. Sex differences in telomeres and lifespan. Aging Cell. 2011;10:913–921. doi: 10.1111/j.1474-9726.2011.00741.x. [DOI] [PubMed] [Google Scholar]
- Bayne S, Jones ME, Li H, Liu JP. Potential roles for estrogen regulation of telomerase activity in aging. Ann N Y Acad Sci. 2007;1114:48–55. doi: 10.1196/annals.1396.023. [DOI] [PubMed] [Google Scholar]
- Benetos A, Okuda K, Lajemi M, Kimura M, Thomas F, Skurnick J, Labat C, Bean K, Aviv A. Telomere length as an indicator of biological aging: the gender effect and relation with pulse pressure and pulse wave velocity. Hypertension. 2001;37:381–385. doi: 10.1161/01.hyp.37.2.381. [DOI] [PubMed] [Google Scholar]
- Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, Greenland P, et al. Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol. 2002;156(9):871–881. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- Bischoff C, Petersen HC, Graakjaer J, Andersen-Ranberg K, Vaupel JW, Bohr VA, Kolvraa S, Christensen K. No association between telomere length and survival among the elderly and oldest old. Epidemiology. 2006;17(2):190–194. doi: 10.1097/01.ede.0000199436.55248.10. [DOI] [PubMed] [Google Scholar]
- Blackburn EH. The telomere and telomerase: nucleic acid-protein complexes acting in a telomere homeostasis system. A review. Biochemistry (Mosc) 1997;62(11):1196–1201. [PubMed] [Google Scholar]
- Blackburn EH. Telomere states and cell fates. Nature. 2000;408(6808):53–56. doi: 10.1038/35040500. [DOI] [PubMed] [Google Scholar]
- Blackburn EH. Telomeres and telomerase: their mechanisms of action and the effects of altering their functions. FEBS Lett. 2005;579(4):859–862. doi: 10.1016/j.febslet.2004.11.036. [DOI] [PubMed] [Google Scholar]
- Blasco MA. Telomeres and human disease: ageing, cancer and beyond. Nat Rev Genet. 2005;6(8):611–622. doi: 10.1038/nrg1656. [DOI] [PubMed] [Google Scholar]
- Bollen KA, Stine R. Direct and indirect effects: classical and bootstrap estimates of variability. Sociol Methodol. 1990;20:115–140. [Google Scholar]
- Brouilette S, Moore J, McMahon AD, Thompson JR, Ford I, Shepherd J, Packard CJ, Samani NJ. Telomere length, risk of coronary heart disease, and statin treatment in the West of Scotland Primary Prevention Study: a nested case-control study. Lancet. 2007;369:107–114. doi: 10.1016/S0140-6736(07)60071-3. [DOI] [PubMed] [Google Scholar]
- Cawthon RM. Telomere measurement by quantitative PCR. Nucleic Acids Res. 2002;30(10):e47. doi: 10.1093/nar/30.10.e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cawthon RM, Smith K, O’Brien E, Sivatchenko A, Kerber R. Association between telomere length in blood and mortality in people aged 60 years or older. Lancet. 2003;361:393–395. doi: 10.1016/S0140-6736(03)12384-7. [DOI] [PubMed] [Google Scholar]
- Courtenay WH. Behavioral factors associated with disease, injury, and death among men: evidence and implications for prevention. J Men Stud. 2000a;9(1):81–142. [Google Scholar]
- Courtenay WH. Constructions of masculinity and their influence on men’s well-being: a theory of gender and health. Soc Sci Med. 2000b;50:1385–1401. doi: 10.1016/s0277-9536(99)00390-1. [DOI] [PubMed] [Google Scholar]
- Diez Roux AV, Ranjit N, Jenny NS, Shea S, Cushman M, Fitzpatrick A, Seeman T. Race/ethnicity and telomere length in the multi-ethnic study of atherosclerosis. Aging Cell. 2009;8(3):251–257. doi: 10.1111/j.1474-9726.2009.00470.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards JR, Lambert LS. Methods for integrating moderation and mediation: a general analytical framework using moderated path analysis. Psychol Methods. 2007;12(1):1–22. doi: 10.1037/1082-989X.12.1.1. [DOI] [PubMed] [Google Scholar]
- Epel ES, Blackburn EH, Lin J, Dhabhar FS, Adler NE, Morrow JD, Cawthon RM. Accelerated telomere shortening in response to life stress. Proc Natl Acad Sci U S A. 2004;101(49):17,312–17,315. doi: 10.1073/pnas.0407162101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epel E, Merkin SS, Cawthon R, Blackburn EH, Adler NE, Pletcher MJ, Seeman TE. The rate of telomere shortening predicts mortality from cardiovascular disease in elderly men. Aging. 2009;1(1):81–88. doi: 10.18632/aging.100007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farzaneh-Far R, Cawthon RM, Na B, Browner WS, Schiller NB, Whooley MA. Prognostic value of leukocyte telomere length in patients with stable coronary artery disease: data from the Heart and Soul Study. Arterioscler Thromb Vasc Biol. 2008;28(7):1379–1384. doi: 10.1161/ATVBAHA.108.167049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick AL, Kronmal RA, Gardner JP, Psaty BM, Jenny NS, Tracy RP, Walston J, Kimura M, Aviv A. Leukocyte telomere length and cardiovascular disease in the cardiovascular health study. Am J Epidemiol. 2007;165(1):14–21. doi: 10.1093/aje/kwj346. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick AL, Kronmal RA, Kimura M, Gardner JP, Psaty BM, Jenny NS, Tracy RP, Hardikar S, Aviv A. Leukocyte telomere length and mortality in the Cardiovascular Health Study. J Gerontol A Biol Sci Med Sci. 2011;66(4):421–429. doi: 10.1093/gerona/glq224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glymour MM. Using causal diagrams to understand common problems in social epidemiology. In: Oakes JM, Kaufman JS, editors. Methods in social epidemiology. San Francisco: Jossey-Bass; 2006. pp. 393–428. [Google Scholar]
- Harris SE, I, Deary J, MacIntyre A, Lamb KJ, Radhakrishnan K, Starr JM, Whalley LJ, Shiels PG. The association between telomere length, physical health, cognitive ageing, and mortality in non-demented older people. Neurosci Lett. 2006;406(3):260–264. doi: 10.1016/j.neulet.2006.07.055. [DOI] [PubMed] [Google Scholar]
- Hayes AF. Beyond Baron and Kenny: statistical mediation analysis in the new millennium. Commun Monogr. 2009;76(4):408–420. [Google Scholar]
- Hayes AF. PROCESS: a versatile computational tool for observed variable mediation, moderation, and conditional process modeling [white paper] 2012 Accessed at http://www.afhayes.com/public/process2012.pdf.
- Honig LS, Kang MS, Schupf N, Lee JH, Mayeux R. Association of shorter leukocyte telomere repeat length with dementia and mortality. Arch Neurol. 2012;23:1–8. doi: 10.1001/archneurol.2012.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou L, Wang S, Dou C, Zhang X, Yu Y, Zheng Y, Avula U, et al. Air pollution exposure and telomere length in highly exposed subjects in Beijing, China: a repeated-measure study. Environ Int. 2012;48:71–77. doi: 10.1016/j.envint.2012.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs EG, Kroenke C, Lin J, Epel ES, Kenna HA, Blackburn EH, Rasgon NL. Accelerated cell aging in female APOE-epsilon4 carriers: implications for hormone therapy use. PLoS One. 2013;8(2):e54713. doi: 10.1371/journal.pone.0054713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeanclos E, Schork NJ, Kyvik KO, Kimura M, Skurnick JH, Aviv A. Telomere length inversely correlates with pulse pressure and is highly familial. Hypertension. 2000;36(2):195–200. doi: 10.1161/01.hyp.36.2.195. [DOI] [PubMed] [Google Scholar]
- Kimura M, Hjelmborg J, Gardner JP, Bathum L, Brimacombe M, Lu X, Christiansen L, Vaupel LW, Aviv A, Christensen K. Short leukocyte telomeres forecast mortality: a study in elderly Danish twins. Am J Epidemiol. 2008;167(7):799–806. doi: 10.1093/aje/kwm380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Sandford AJ, Connett JE, Yan J, Mui T, Li Y, Daley D, et al. The relationship between telomere length and mortality in chronic obstructive pulmonary disease (COPD) PLoS One. 2012;7(4):e35567. doi: 10.1371/journal.pone.0035567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M, Martin H, Firpo MA, Demerath EW. Inverse association between adiposity and telomere length: the Fels Longitudinal Study. Am J Hum Biol. 2011;23(1):100–6. doi: 10.1002/ajhb.21109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang W, Shediac-Rizkallah MC, Celentano DD, Rohde C. A population-based study of age and gender differences in patterns of health-related behaviors. Am J Prev Med. 1999;17(1):8–17. doi: 10.1016/s0749-3797(99)00040-9. [DOI] [PubMed] [Google Scholar]
- Ludlow AT, Roth SM. Physical activity and telomere biology: exploring the link with aging-related disease prevention. J Aging Res. 2011:790378. doi: 10.4061/2011/790378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKinnon DP, Fairchild AJ, Fritz MS. Mediation analysis. Annu Rev Psychol. 2007;58:593–614. doi: 10.1146/annurev.psych.58.110405.085542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Ruiz C, Dickinson HO, Keys B, Rowan E, Kenny RA, Von Zglinicki T. Telomere length predicts poststroke mortality, dementia, and cognitive decline. Ann Neurol. 2006;60(2):174–180. doi: 10.1002/ana.20869. [DOI] [PubMed] [Google Scholar]
- Mayer S, Bruderlein S, Perner S, Waibel I, Holdenried A, Ciloglu N, Hasel C, Mattfeldt T, Nielsen KV, Moller P. Sex-specific telomere length profiles and age-dependent erosion dynamics of individual chromosome arms in humans. Cytogenet Genome Res. 2006;112(3–4):194–201. doi: 10.1159/000089870. [DOI] [PubMed] [Google Scholar]
- McGrath M, Wong JY, Michaud D, Hunter DJ, De Vivo I. Telomere length, cigarette smoking, and bladder cancer risk in men and women. Cancer Epidemiol Biomarkers Prev. 2007;16(4):815–819. doi: 10.1158/1055-9965.EPI-06-0961. [DOI] [PubMed] [Google Scholar]
- Minnino AM. NCHS data brief, no. 65. Hyattsville, MD: National Center for Health Statistics; 2011. Death in the United States, 2009. [Google Scholar]
- Moller P, Mayer S, Mattfeldt T, Muller K, Wiegand P, Bruderlein S. Sex-related differences in length and erosion dynamics of human telomeres favor females. Aging. 2009;1(8):733–739. doi: 10.18632/aging.100068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muezzinler A, Zaineddin AK, Brenner H. A systematic review of leukocyte telomere length and age in adults. Ageing Res Rev. 2013;12(2):509–519. doi: 10.1016/j.arr.2013.01.003. [DOI] [PubMed] [Google Scholar]
- Muller D, Judd CM, Yzerbyt VY. When moderation is mediated and mediation is moderated. J Pers Soc Psychol. 2005;89(6):852–863. doi: 10.1037/0022-3514.89.6.852. [DOI] [PubMed] [Google Scholar]
- Nawrot TS, Staessen JA, Gardner JP, Aviv A. Telomere length and possible link to X chromosome. Lancet. 2004;363(9408):507–510. doi: 10.1016/S0140-6736(04)15535-9. [DOI] [PubMed] [Google Scholar]
- Needham BL, Adler N, Gregorich S, Rehkopf D, Lin J, Blackburn EH, Epel ES. Socioeconomic status, health behavior, and leukocyte telomere length in the National Health and Nutrition Examination Survey, 1999–2002. Soc Sci Med. 2013;85:1–8. doi: 10.1016/j.socscimed.2013.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nettleton JA, Diez-Roux A, Jenny NS, Fitzpatrick AL, Jacobs DR., Jr Dietary patterns, food groups, and telomere length in the Multi-Ethnic Study of Atherosclerosis (MESA) Am J Clin Nutr. 2008;88(5):1405–1412. doi: 10.3945/ajcn.2008.26429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Njajou OT, Cawthon RM, Damcott CM, Wu SH, Ott S, Garant MJ, Blackburn EH, Mitchell BD, Shuldiner AR, Hsueh WC. Telomere length is paternally inherited and is associated with parental lifespan. Proc Natl Acad Sci U S A. 2007;104(29):12,135–12,139. doi: 10.1073/pnas.0702703104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Njajou OT, Hsueh WC, Blackburn EH, Newman AB, Wu SH, Li R, Simonsick EM, et al. Association between telomere length, specific causes of death, and years of healthy life in health, aging, and body composition, a population-based cohort study. J Gerontol A Biol Sci Med Sci. 2009;64(8):860–864. doi: 10.1093/gerona/glp061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panossian LA, V, Porter R, Valenzuela HF, Zhu X, Reback E, Masterman D, Cummings JL, Effros RB. Telomere shortening in T cells correlates with Alzheimer’s disease status. Neurobiol Aging. 2003;24(1):77–84. doi: 10.1016/s0197-4580(02)00043-x. [DOI] [PubMed] [Google Scholar]
- Rosner W, Hankinson SE, Sluss PM, Vesper HW, Wierman ME. Challenges to the measurement of estradiol: an endocrine society position statement. J Clin Endocrinol Metab. 2013;98(4):1376–1387. doi: 10.1210/jc.2012-3780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salpea KD, Talmud PJ, Cooper JA, Maubaret CG, Stephens JW, Abelak K, Humphries SE. Association of telomere length with type 2 diabetes, oxidative stress and UCP2 gene variation. Atherosclerosis. 2010;209(1):42–50. doi: 10.1016/j.atherosclerosis.2009.09.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samani NJ, Boultby R, Butler R, Thompson JR, Goodall AH. Telomere shortening in atherosclerosis. Lancet. 2001;358:472–473. doi: 10.1016/S0140-6736(01)05633-1. [DOI] [PubMed] [Google Scholar]
- Sanders JL, Newman AB. Telomere length in epidemiology: a biomarker of aging, age-related disease, both, or neither? Epidemiol Rev. 2013;35:112–131. doi: 10.1093/epirev/mxs008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silber PM, Gandolfi AJ, Brendel K. Adaptation of a gamma-glutamyl transpeptidase assay to microtiter plates. Anal Biochem. 1986;158(1):68–71. doi: 10.1016/0003-2697(86)90590-7. [DOI] [PubMed] [Google Scholar]
- Simon NM, Smoller JW, McNamara KL, Maser RS, Zalta AK, Pollack MH, Nierenberg AA, Fava M, Wong KK. Telomere shortening and mood disorders: preliminary support for a chronic stress model of accelerated aging. Biol Psychiatry. 2006;60:432–435. doi: 10.1016/j.biopsych.2006.02.004. [DOI] [PubMed] [Google Scholar]
- Slagboom PE, Droog S, Boomsma DI. Genetic determination of telomere size in humans: a twin study of three age groups. Am J Hum Genet. 1994;55(5):876–882. [PMC free article] [PubMed] [Google Scholar]
- Stindl R. Tying it all together: telomeres, sexual size dimorphism and the gender gap in life expectancy. Med Hypotheses. 2004;62(1):151–154. doi: 10.1016/s0306-9877(03)00316-5. [DOI] [PubMed] [Google Scholar]
- Strandberg TE, Saijonmaa O, Tilvis RS, Pitkala KH, Strandberg AY, Miettinen TA, Fyhrquist F. Association of telomere length in older men with mortality and midlife body mass index and smoking. J Gerontol A Biol Sci Med Sci. 2011;66(7):815–820. doi: 10.1093/gerona/glr064. [DOI] [PubMed] [Google Scholar]
- VanderWeele TJ. Mediation and mechanism. Eur J Epidemiol. 2009;24(5):217–224. doi: 10.1007/s10654-009-9331-1. [DOI] [PubMed] [Google Scholar]
- von Zglinicki T. Oxidative stress shortens telomeres. Trends Biochem Sci. 2002;27(7):339–344. doi: 10.1016/s0968-0004(02)02110-2. [DOI] [PubMed] [Google Scholar]
- von Zglinicki T, Serra V, Lorenz M, Saretzki G, Lenzen-Grossimlighaus R, Gessner R, Risch A, Steinhagen-Thiessen E. Short telomeres in patients with vascular dementia: an indicator of low antioxidative capacity and a possible risk factor? Lab Invest. 2000;80(11):1739–1747. doi: 10.1038/labinvest.3780184. [DOI] [PubMed] [Google Scholar]
- Weischer M, Bojesen SE, Cawthon RM, Freiberg JJ, Tybjaerg-Hansen A, Nordestgaard BG. Short telomere length, myocardial infarction, ischemic heart disease, and early death. Arterioscler Thromb Vasc Biol. 2012;32(3):822–829. doi: 10.1161/ATVBAHA.111.237271. [DOI] [PubMed] [Google Scholar]
- Wentzensen IM, Mirabello L, Pfeiffer RM, Savage SA. The association of telomere length and cancer: a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2011;20(6):1238–1250. doi: 10.1158/1055-9965.EPI-11-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willeit P, Willeit J, Brandstatter A, Ehrlenbach S, Mayr A, Gasperi A, Weger S, et al. Cellular aging reflected by leukocyte telomere length predicts advanced atherosclerosis and cardiovascular disease risk. Arterioscler Thromb Vasc Biol. 2010;30(8):1649–1656. doi: 10.1161/ATVBAHA.110.205492. [DOI] [PubMed] [Google Scholar]
- Willeit P, Willeit J, Mayr A, Weger S, Oberhollenzer F, Brandstatter A, Kronenberg F, Kiechl S. Telomere length and risk of incident cancer and cancer mortality. JAMA. 2010;304(1):69–75. doi: 10.1001/jama.2010.897. [DOI] [PubMed] [Google Scholar]
- Wu Y, Liu Y, Ni N, Bao B, Zhang C, Lu L. High lead exposure is associated with telomere length shortening in Chinese battery manufacturing plant workers. Occup Environ Med. 2012;69(8):557–563. doi: 10.1136/oemed-2011-100478. [DOI] [PubMed] [Google Scholar]
- Yaffe K, Lindquist K, Kluse M, Cawthon R, Harris T, Hsueh WC, Simonsick EM, et al. Telomere length and cognitive function in community-dwelling elders: findings from the Health ABC Study. Neurobiol Aging. 2011;32(11):2055–2060. doi: 10.1016/j.neurobiolaging.2009.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zee RY, Castonguay AJ, Barton NS, Germer S, Martin M. Mean leukocyte telomere length shortening and type 2 diabetes mellitus: a case-control study. Transl Res. 2010;155(4):166–169. doi: 10.1016/j.trsl.2009.09.012. [DOI] [PubMed] [Google Scholar]