SUMMARY
Background
Diffuse large-B-cell lymphoma (DLBCL) is curable but when treatment fails, outcome is poor. Imaging scans help identify patients at risk of treatment failure but are often imprecise, and the radiation exposure is a potential health risk. Specific, sensitive and readily available biomarkers of treatment failure are needed.
Methods
We retrospectively analyzed cell-free circulating tumor DNA (ctDNA) in patients treated on one of 3 treatment protocols using quantitative next-generation DNA sequencing. Eligible patients had DLBCL, no evidence of indolent lymphoma and were previously untreated. Serial serum samples and concurrent computed tomography scans were obtained at specified times during most treatment cycles and 5-years of follow-up. VDJ gene segments of the rearranged immunoglobulin receptor genes were amplified and sequenced from pre-treatment specimens and serum ctDNA encoding the VDJ rearrangements was quantitated.
Findings
Tumor clonotype(s) were identified in pretreatment specimens from 126 patients who were followed for a median (interquartile range) of 11 (6.8 to 14.2) years. Interim ctDNA monitoring at the end of 2 treatment cycles in 108 patients showed a time to progression (TTP) of 41.7% (95% Confidence Interval (CI): 22.2% to 60.1%) and 80.2% (95% CI: 69.6% to 87.3%), at 5-years (p<0.0001) in patients with and without detectable ctDNA, respectively, and a positive and negative predicative value (PPV and NPV) of 63% and 80%, respectively. Surveillance ctDNA monitoring was performed in 107 patients who achieved complete remission. A Cox proportional hazards model showed patients who developed detectable ctDNA during surveillance had a hazard ratio 228 times that of patients with undetectable ctDNA for clinical disease progression (95% CI: 51 to 1022) (p<0.0001). Surveillance ctDNA had a PPV and NPV of 88% and 98%, respectively, and identified recurrence a median (range) of 3.5 months (0 to 200) before evidence of clinical disease.
Interpretation
Surveillance ctDNA identifies patients at risk of recurrence before clinical evidence of disease in most patients and results in lower disease burden at relapse. Interim ctDNA is a promising biomarker to identify patients at high risk of treatment failure.
INTRODUCTION
Diffuse large B-cell lymphoma (DLBCL) is the most common lymphoma.1 Most patients achieve remission after frontline therapy and undergo surveillance imaging for disease recurrence. However, disease recurs in up to 40% of patients and most are incurable, particularly those who progress early and/or have significant tumor burdens.2 A reliable biomarker that detects subclinical disease offers the potential to improve long-term survival.
Relapse detection entails computerized tomography (CT) and/or positron emission/CT (PET/CT) scans to detect disease at an asymptomatic stage.3, 4 More recently, interim PET (iPET) scans during treatment have been investigated to predict treatment failure.5–7 The clinical utility of surveillance and interim imaging, however, is limited by significant imprecision.8–10 Further, imaging-associated ionizing radiation carries potential health risk, limiting their use, and adds significant health care costs.4, 11
DLBCL relapses most likely originate from the persistence of minimal residual disease below the detection of imaging. Apoptosis and necrosis of the malignant cells leads to the release of tumor DNA into the circulation.12 Next-generation sequencing (NGS) can detect and quantify circulating tumor DNA (ctDNA) and can non-invasively assess tumor dynamics.13–15 The VDJ immunoglobulin genes contain unique sequences that are markers of clonality.16 We hypothesized the malignant cell VDJ gene sequences could be detected in the serum of DLBCL patients and used to predict clinical disease recurrence in frontline treatment.17
We employed a quantitative high-throughput method that combines amplification of immunoglobulin gene segments with NGS to detect ctDNA in serum.18 Circulating tumor-specific DNA was quantitated in serial samples obtained during treatment and follow-up of patients with newly diagnosed DLBCL. Herein, we show ctDNA identifies patients at risk of recurrence prior to imaging.
METHODS
Study Framework
We performed a retrospective analysis of ctDNA in patients with DLBCL enrolled on one of 3 frontline protocols of EPOCH (etoposide, prednisone, vincristine, cyclophosphamide and doxorubicin) with or without rituximab (based on protocol era), between May 1993 and December 2013 (ClinicalTrials.gov NCT00001563, NCT00001337, and NCT00006436).19–23 Eligibility included a diagnosis of DLBCL without evidence of an indolent histology, no prior treatment, negative pregnancy test, and normal laboratory values unless due to respective organ involvement by lymphoma. Eligibility required at least stage II disease except patients with bulky stage I mediastinal B-cell lymphoma or all stages in patients with human immunodeficiency virus infection (HIV). Patients with other systemic malignancies, serious infections, recent myocardial infection or inadequate cardiac function (ejection fraction < 40%) were ineligible. Eligibility evaluation included standard laboratory tests for organ function, whole body CT scans and history and physical examination.
All 3 protocols included the prospective collection and banking of research serum samples pre-treatment, before each chemotherapy cycle, at the end of treatment and at every staging evaluation for analysis of outcome biomarkers. At each predetermined time point, 10 cc of blood was drawn into a red top serum separator tube, centrifuged, aliquoted into 1-milliliter eppendorf tubes and stored at least minus 20 degrees centigrade. Due to evolving technology, the biomarker assays were not stipulated in the protocols. The protocols also included scheduled evaluations with whole body CT scans pre-treatment, after cycle 2 or 4 (depending on study), after the last cycle of treatment, and post-treatment surveillance evaluation every 3 months for the first year, every 4 months for the second year, every 6 months for the third year and yearly for the next 2 years. Beyond 5 years, surveillance was yearly and only included a clinical evaluation and a research serum sample. Research serum samples were always drawn at the same time as the evaluation, which included CT scans as outlined above. No research samples were drawn at other time points and all times points with samples were analyzed for ctDNA, which was blinded to clinical outcome.
Patients provided written informed consent in accordance with the Declaration of Helsinki, and the institutional review board (IRB) approved the protocols. Exemption from IRB review was obtained for the coded analysis of ctDNA performed at Sequenta Inc, South San Francisco. Treatment response followed the International Working Group criteria.24
Isolation and quantification of circulating tumor DNA
Pre-treatment formalin-fixed paraffin embedded biopsy specimens were analyzed for tumor-specific clonotypes based on the LymphoSIGHT™ method (Figure 1).15 Inpatients without biopsies, tumor clonotypes were determined using pre-treatment serum samples. ctDNA unit of quantitation was the number of lymphoma molecules (one tumor cell equivalent) per 106 diploid genomes (106 cells equivalent) with a limit of detection of 1 lymphoma molecule per sample. The tumor clonotype analysis was blinded. For surveillance analysis, we measured ctDNA in 980 serum samples, which included 889 in non-progressors and 91 in progressors, drawn at pre-determined time points. In addition, for interim analysis, we measured ctDNA in 578 serum samples, which included 410 in non-progressors and 168 in progressors.
Figure 1. Tumor Clonotype Analysis Work flow.
Step 1a. Tumor DNA was amplified using locus-specific primer sets for the immunoglobulin heavy-chain locus (IGH) complete (IGH-VDJ), IGH incomplete (IGH-DJ), and immunoglobulin kappa locus (IGK). The amplified product was sequenced, and the frequencies of the different clonotypes determined. Step 1b. Tumor clone(s) that comprised at least 5% of the B-cell repertoire were identified for analysis of minimal residual disease (ctDNA). Step 2a. ctDNA in serum samples was calculated based on the number of lymphoma molecules (cell equivalent) per 106 diploid genomes (cell equivalent). The lower limit of detection was 1 lymphoma molecule per 106 diploid genomes. In cases with 2 or more tumor clones, the highest frequency clone was reported. Step 2b. ctDNA was quantitatively analyzed in serial serum samples over multiple time points.
Circulating tumor DNA and Clinical monitoring
ctDNA monitoring was performed on serial serum samples. Interim monitoring was based on 108 patient-samples obtained after 2 treatment cycles (i.e. day 1 of cycle 3), and surveillance monitoring was based on 107 patients with serial samples from end-of-treatment until disease progression and only included patients in complete remission at the end of treatment (Figure 2). Patients underwent whole body CT scans between cycles 2 and 4, end-of-treatment, and every 3–4 months for the first 2 years and every 4–6 months for the next 3 years. After 5-years, annual evaluation occurred without routine imaging.
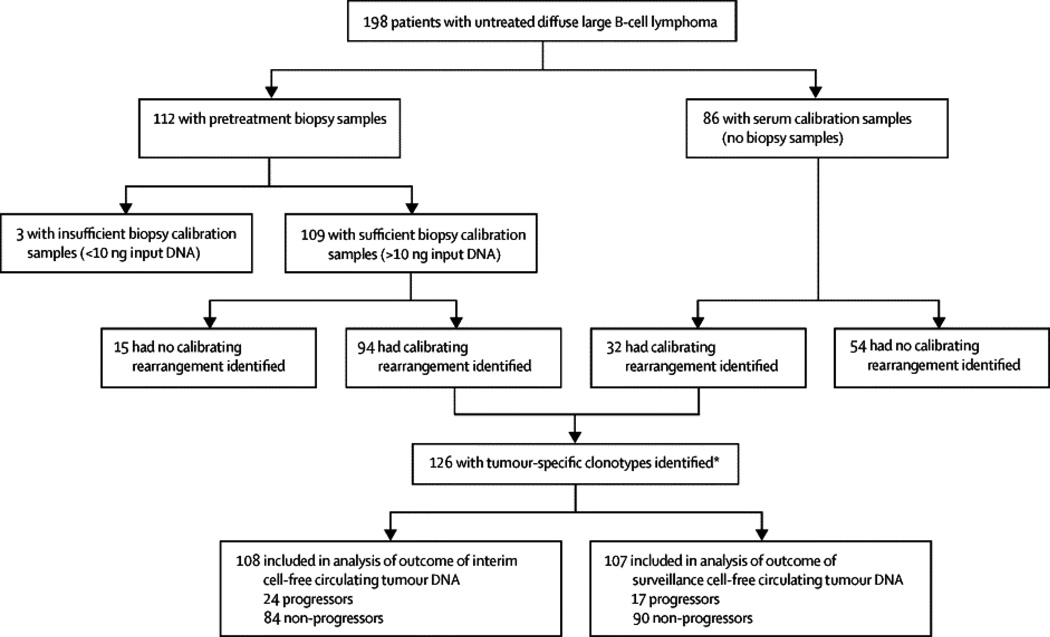
Figure 2. Patient Sample Flow Diagram.
Tree diagram showing outcome of tumor clonotype analysis of pretreatment biopsy and serum specimens. One hundred and ninety-eight pretreatment samples were analyzed for tumor clonotypes and 126 patients had identifiable clones. The time to progression and overall survival of 108 patients with and without detectable interim ctDNA at the end of cycle 2 (i.e. day 1 of cycle 3) were determined by a landmark analysis. Among 107 patients who achieved complete remission at the end of treatment, the hazard risk of developing clinical progression based on detection of ctDNA was calculated using a Cox proportional hazard model.
Statistical analysis
For the interim analysis, time to progression (TTP) and survival were calculated from day 1 of cycle 3 as a land-mark analysis until disease progression/radiotherapy for active disease or death, respectively, or last follow-up by the Kaplan-Meier method.25 The log-rank test was used to determine the significance of the difference between Kaplan-Meier curves. The median follow-up was calculated from the on-study date to death or last follow-up.26 For the surveillance analysis, a Cox proportional hazards model of ctDNA as a time-varying covariate was used to assess the association of this factor on TTP. For determining differences in progression time according to pattern of ctDNA for those who progressed early, a Kruskal-Wallis test was used. Sensitivity, specificity, and positive and negative predictive (PPV and NPV) of ctDNA detection of clinical disease were calculated based on the final determinations of ctDNA and clinical progression or not as dichotomous outcomes.27 All p-values are two-tailed. Statistical analyses were performed using SAS version 9.3 and Prism version 6.0.
Role of the funding source
The clinical studies were funded by the National Cancer Institute and the sample analysis was funded by Sequenta, Inc. Investigators from the National Cancer Institute participated in study design, patient management, sample collection, data analysis and interpretation and writing the manuscript. Investigators from Sequenta, Inc. participated in study design, sample analysis, data analysis and interpretation and writing the manuscript. MR, KK, MF and WHW had access to the raw data. The corresponding author, WHW, had full access to all of the data and final responsibility to submit for publication.
RESULTS
Patient characteristics
Among 198 patients with untreated DLBCL, a tumor-specific clonotype was identified in 126 (64%) study cases (Figure 1). The characteristics of the study cases and the excluded cases, due to inadequate DNA samples, were similar except for the distribution of DLBCL variants (Figure 1, Table 1). Characteristics of the 126 study patients included a median age of 44 years, high-risk disease in 31 (25%), and male sex in 78 (62%) patients. All patients received EPOCH-based chemotherapy, which included rituximab in 99 (79%) patients. Treatment failure occurred in 36 (29%) patients with 25 (69%) early (≤ 6 months) and 11 (31%) (> 6 months) late progressions. Overall 126 study patients, there were 34 (27%) deaths, which included 11 (32%) deaths without progression due to second malignancy (N=2), Acquired Immunodeficiency Syndrome (N=2), infection (N=2), liver failure (N=1), drug overdose (N=1), unknown (N=3). With a median (interquartile range) follow-up of 11 (6.8 to 14.2) years, the TTP and survival at 5-years were 72.1% (95% CI: 63.5% to 79.3%) and 77.4% (95% CI: 69.2% to 83.9%), respectively.
Table 1.
Patient Characteristics and Monitoring
| Patients Screened |
Study Patients | Clinical Progressors |
Clinical Non-Progressors | |
|---|---|---|---|---|
| N | 198 | 126 | 36 (29%) | 90 (71%) |
| Median age, y | 43 | 44 | 50 | 42 |
| Age range | (12–93) | (14–85) | (17–70) | (14–85) |
| Gender, male, % | 113 (57%) | 78 (62%) | 24 (67%) | 54 (60%) |
| DLBCL variants (%)* | ||||
| Not otherwise specified | 97 (49%) | 58 (46%) | 17 (29%) | 41 (71%) |
| HIV-associated | 25 (13%) | 24 (19%) | 6 (25%) | 18 (75%) |
| Mediastinal B-cell lymphomas | 76 (38%) | 44 (35%) | 13 (30%) | 31 (70%) |
| International Prognostic Index score | ||||
| (%) | 98 (49%) | 56 (44%) | 13 (23%) | 43 (77%) |
| Low risk (0–1) | 57 (29%) | 39 (31%) | 11 (28%) | 28 (72%) |
| Low-intermediate risk (2) | 29 (15%) | 20 (16%) | 8 (40%) | 12 (60%) |
| High-intermediate risk (3) | 14 (7%) | 11 (9%) | 4 (26%) | 7 (64%) |
| High risk (4–5) | ||||
| Ann Arbor Stage (%) | ||||
| I–II | 88 (20%) | 50 (40%) | 13 (26%) | 37 (74%) |
| III–IV | 110 (56%) | 76 (60%) | 23 (30%) | 53 (70%) |
| Treatment regimen (%) | ||||
| EPOCH | 40 (20%) | 27 (21%) | 11 (41%) | 16 (59%) |
| EPOCH with rituximab | 158 (80%) | 99 (79%) | 25 (25%) | 74 (75%) |
| Interim monitoring | 108 | 32 | 76 | |
| Surveillance monitoring | 112 | 22 | 90 | |
| Clinical Progressors | 36 | |||
| Early (≤ 6 months from treatment) | 25 (69%) | |||
| Late (> 6 months from treatment) | 11 31% |
Abbreviations: DLBCL: Diffuse large B-cell lymphoma; HIV: Human immunodeficiency virus; EPOCH: adriamycin, vincristine, etoposide, cyclophosphamide, and prednisone.
Patients with a calibrating rearrangement (126 study patients) and without a calibrating rearrangement (72 ineligible patients) had statistically similar characteristics except for distribution of DLBCL variants, designated as:
(p<0.05).
Identification of tumor clonotypes
Among 112 patients with pretreatment biopsy samples, adequate DNA for calibration was available in 109 (97%) patients. Within this group, 94 (86%) patients had a calibrating rearrangement, of which 30 (32%) were obtained from core biopsies. In 86 patients without a biopsy sample, a calibrating rearrangement was detected in pretreatment serum specimens from 32 (37%) cases. Including all 126 patients, 71 (56%) calibrated for one receptor (18 IGH-VDJ; 20 IGH-DJ; 33 IGK), 46 (37%) calibrated for 2 receptors (9 IGH-DJ and IGK; 20 IGH-VDJ and IGK; 17 IGH-VDJ and IGH-DJ) and 9 (7%) calibrated for all 3 receptors. Tumor clonotype was also assessed by sample type. Among the 94 biopsies, 47 (50%) calibrated for one receptor (15 IGH-VDJ; 15 IGH-DJ; 17 IGK), 39 (41%) calibrated for 2 receptors (5 IGH-DJ and IGK; 18 IGH-VDJ and IGK; 16 IGH-VDJ and IGH-DJ) and 8 (9%) calibrated for all 3 receptors. In the 32 serum specimens, 24 (75%) calibrated for one receptor (3 IGH-VDJ; 5 IGH-DJ; 16 IGK), 7 (22%) calibrated for 2 receptors (4 IGH-DJ and IGK; 2 IGH-VDJ and IGK; 1 IGH-VDJ and IGH-DJ) and 1 (3%) calibrated for all 3 receptors.
Of 83 patient-biopsy samples with clonotypes that could potentially undergo somatic hypermutation (IGH-VDJ and IGK), 24 (29%) had mutated clones in the pretreatment biopsies. In these 24 cases, 13 (54%) also had detectable mutated clones in the follow-up serum, and in 7 (54%) of these cases, the serum sample had a mutated clone not found in the biopsy sample. A further 5 cases had no mutated clones in the biopsy sample but had mutated clones in the serum samples. Among 28 patients with only pretreatment serum samples with clonotypes that potentially undergo somatic hypermutation, 3 (11%) had mutated clones.
A feature of the technology is the ability to detect clonotypes that are similar but not identical to the highest frequency (index) clone(s), allowing the detection of ctDNA despite clonal evolution. In one case (# 210), the index clone contained a 17 bp deletion, which was not present in the clone detected at relapse, indicating these two clones likely evolved from a common ancestor.
Circulating tumor DNA and tumor burden
We hypothesized that the pretreatment concentration of ctDNA would correlate with tumor burden. There was a significant association between the international prognostic index (IPI) and level of circulating tumor DNA with median (range) concentrations of 416 (0 to 2.4 × 104), 5095 (0–1 × 106) and 7226 (3 to 1.6 × 105) lymphoma molecules per 106 diploid genomes for patients with low (0/1), intermediate (2/3) and high (4/5) risk IPI scores, respectively (p<0.0001). Several of the IPI characteristics reflect tumor burden including disease stage and lactate dehydrogenase (LDH). Specifically, patients with early stage (1 or 2) compared to advanced stage (3 or 4) disease had significantly lower ctDNA, median 1038 versus 3254 lymphoma molecules per 106 diploid genomes, respectively (p=0.014), and there was a moderately strong correlation between LDH and ctDNA levels (Spearman correlation 0.629; p<0.0001).
Circulating tumor DNA monitoring
In the 36 patients with clinical progression, 25 (69%) progressed early (Figure 3A). Three patterns of ctDNA kinetics were observed. The most common pattern was no ctDNA clearance before clinical progression, observed in 10 (40%) patients. The second pattern was transient ctDNA clearance followed by ctDNA recurrence before clinical progression, observed in 9 (36%) patients, and the least frequent pattern was ctDNA clearance followed by clinical progression, which occurred in 6 (24%) patients. Patients who never cleared ctDNA had the shortest time to progression of 3.84 months (range: 0.76 to 7.16), compared to 5.81 (range: 4 to 9.9) and 5.3 (range: 4.2 to 9.7) months, respectively, for patients with transient or persistent ctDNA clearance (p=0.0043 by Kruskal-Wallis test). Although not statistically significant, patients who never cleared ctDNA also had the shortest survival following recurrence with a median survival of 2.3 months compared to 9.3 and 15 months for patients with transient or persistent ctDNA clearance, respectively. In 11 (31%) patients, disease progressed late after treatment (Figure 3B). While all 11 cases had undetectable ctDNA after treatment, 10 (91%) subsequently developed detectable ctDNA with 8 (80%) occurring prior to clinical evidence of disease. Among 90 patients without clinical progression, 88 (98%) never developed detectable ctDNA, whereas 2 cases developed low-level and non-reproducible ctDNA on a single measurement as discussed below.
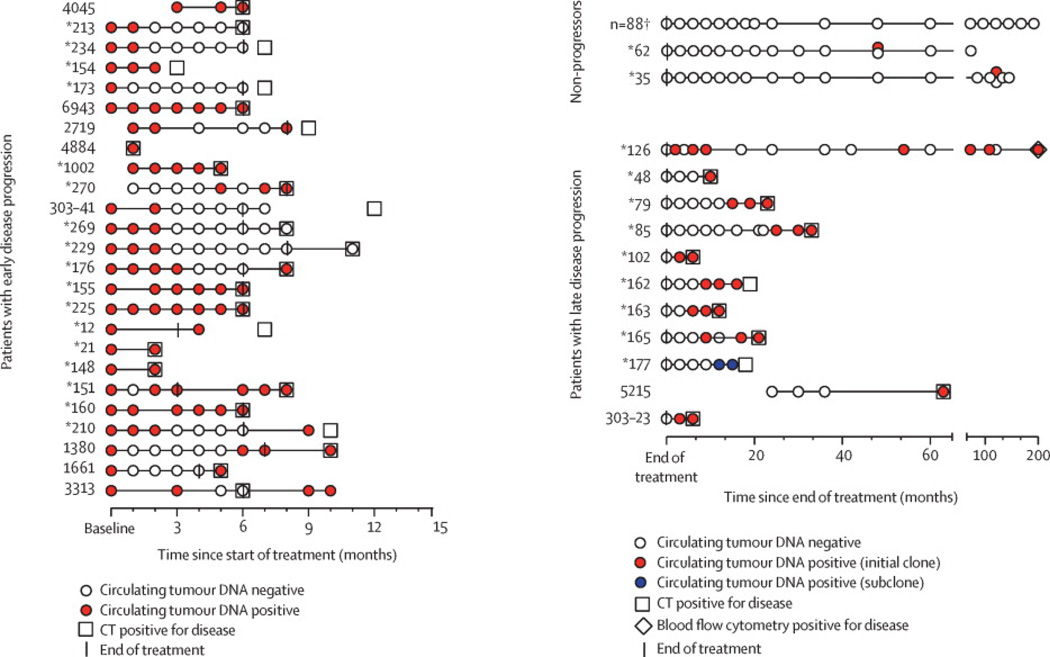
Figure 3. Circulating Tumor DNA Time Points and Kinetics.
A. Tram stop showing ctDNA outcome of patients with early progression within 6 months of treatment completion. Time starts from pretreatment. Coded identification number shown for each patient. (*) Identifies patients who received rituximab. B. Tram stop showing outcome of all patients who completed treatment and either did not progress or had progression at least 6 months after treatment. Time starts from end of treatment (EoT). Coded identification number shown for each patient. (*) Identifies patients who received rituximab. One patient recurred with a leukemic clone detected by flow cytometry (FCM). In case #177, two clonal sequences (IGH-DJ and IGK) were initially detected, but the IGK clonotype sequence (subclone) was not considered sufficiently unique to ensure a low false positive rate (< 0.001). The biopsy sample at relapse and the two previous samples only contained the IGK clonotype, which was detected after the analysis was unblinded. The case was classified as ctDNA negative for the overall analysis as the clone was intentionally not prospectively followed. This case illustrates clonal heterogeneity and the utility of following more than one clonotype. This is an area for further optimization of the technology. Two patients (#35, 62) developed single low-level positive ctDNA during surveillance and did not have clinical progression. Separate samples from the same time points were reanalyzed in both cases and were negative for ctDNA as indicated on the tram stop, suggesting these were false positive. C. Quantitative ctDNA dynamics during treatment for all patients with detectable ctDNA. D. Quantitative ctDNA dynamics for patients who recurred post-treatment (N=17). Color and symbols represent individual patient ctDNA kinetics. X-axis shows time (months) to relapse normalized to time of clinical recurrence.
Considering all 126 patients on the study, the blinded ctDNA results were discordant with the clinical outcome in 10 (8%) patients. Seven of these 10 patients (70%) patients did not have detectable ctDNA at clinical progression: 4 patients (# 213, 234, 269, 229) with mediastinal lymphoma each had an area of residual disease less than 1 cm identified by PET scan at the end of therapy and received radiotherapy; one patient (#173) had an isolated central nervous system recurrence one month after treatment; one patient (#303-41) progressed 5 months after treatment and had no serum sample and; one patient (#177) had a detectable clonotype, which was not detected in the blinded analysis (Figure 3B). Three (30%) patients with discordant outcomes developed positive ctDNA late in their course without progression on CT imaging. One patient (#126) had 4 positive ctDNA samples over a 5-year period, but on restaging a tumor clone was detected in the blood. Two cases (# 35, 62) had single samples with detectable ctDNA followed by at least 2 negative samples over 2 years. In both cases, the ctDNA was at the detection limit (1 and 2 lymphoma molecules per sample, respectively) and no clinical disease was detected on restaging. Analysis of new samples from the same time points in both patients had no detectable ctDNA, suggesting the initial results were incorrect.
Circulating tumor DNA dynamics
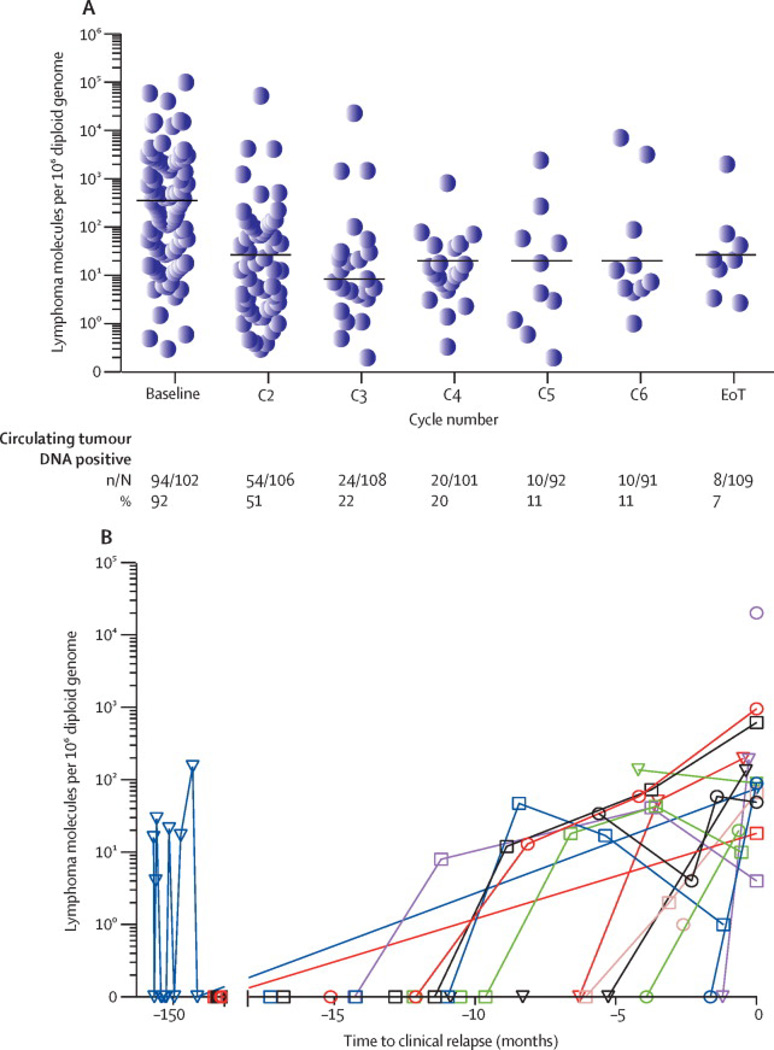
ctDNA was quantitatively assessed during treatment (Figure 3C). In 102 patients with a pretreatment sample, the tumor clone was detected in 94 (92%) cases with a median (range) of 2710 (3 to 1×106) lymphoma molecules per 106 diploid genomes. After two treatment cycles, 24 of 108 (22%) patients with samples had detectable ctDNA and by the end-of-treatment, 8 of 109 (7.3%) patients with samples had detectable ctDNA. The median number of molecules significantly declined after the first treatment cycle and remained relatively unchanged over subsequent cycles (Figure 3C). We looked at ctDNA dynamics in 17 patients who completed treatment and clinically recurred (Figure 3D). In 9 of 15 (60%) patients that had more than one time point with detectable ctDNA, the serum concentration of ctDNA increased over time, consistent with expanding tumor burden.
Interim circulating tumor DNA monitoring during therapy
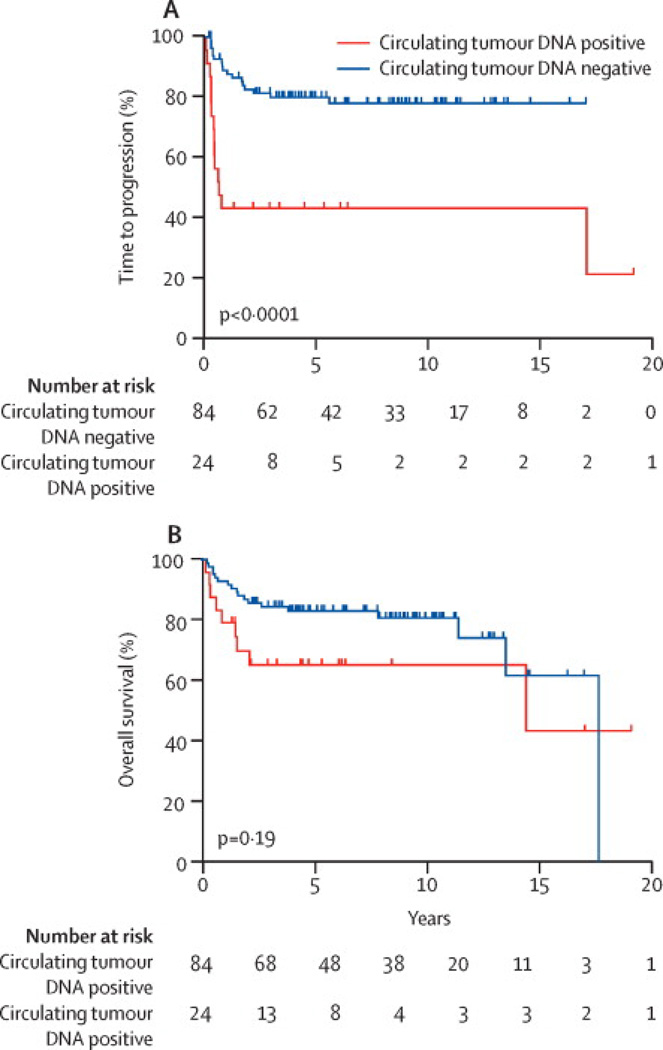
We examined the association of interim ctDNA and disease progression. Based on studies showing a prognostic role of interim PET scans in DLBCL at the end of 2 treatment cycles, we analyzed ctDNA after 2 cycles (i.e. day 1 of cycle 3) in 108 patients with available serum.6, 7 Among 24 patients with detectable interim ctDNA, 15 (63%) clinically progressed with 14 occurring early. By contrast, only 17 (20%) patients with undetectable interim ctDNA clinically progressed, 7 of which were early. At 5-years, the TTP was 41.7% (95% CI: 22.2% to 60.1%) and 80.2% (95% CI: 69.6% to 87.3%) in patients with and without detectable interim ctDNA (p<0.0001), respectively, and the overall survival was 65.4% (95% CI: 42.4% to 81.1%) and 83% (95% CI: 73.1% to 89.6%) (p=0.19)(Figure 4A, 4B). Detectable interim ctDNA had a PPV of 63% and NPV of 80%, and a sensitivity and specificity of 47% and 88%, respectively.
Figure 4. Kaplan-Meier Outcome Estimates and Lead Time.
A. Time to progression (TTP) landmark analysis based on interim ctDNA result drawn on day 1 of cycle 3 (N= 108). The TTP at 5-years was 41.7% (95% CI: 22.2% to 60.1%) and 80.2% (95% CI: 69.6% to 87.3%), respectively, for patients who were ctDNA positive and negative. B. Survival landmark analysis based on interim ctDNA result drawn on day 1 of cycle 3 (N=108). The survival at 5-years was 65% (95% CI: 42.4% to 81.1%) and 83% (95% CI: 73.1% to 89.6%), respectively, for patients with and without detectable ctDNA. C. The median (range) lead-time between detection of disease by ctDNA and CT or flow cytometry in all 15 patients who relapsed after treatment (surveillance group) was 3.5 (0 to 200) months.
Surveillance monitoring of circulating tumor DNA after therapy
The association of ctDNA and disease progression was assessed in 107 patients who achieved a complete remission at the end of treatment (surveillance group). Overall, 17 (16%) patients had clinical progression following treatment. Of these, 17 patients developed detectable ctDNA and 15 clinically progressed. The 2 patients (#35, 62) (Figure 3B) who did not relapse had the non-reproducible ctDNA tests, as discussed above.
A Cox proportional hazards model showed that patients who developed detectable ctDNA had a hazard ratio that was 228 times that of patients who never had detectable ctDNA for developing clinical progression (95% CI: 51 to 1022) (p<0.0001). Furthermore, surveillance ctDNA had a PPV of 88% and NPV of 98%, and a sensitivity and specificity of 88% and 98%, respectively. Overall, patients developed detectable ctDNA a median (range) of 3.5 (0 to 200) months before clinical evidence of disease (Figure 4C). The median (range) lead-time was 1.6 (0.3 to 4.2) months in the 5 patients with early relapse and 7.4 (0 to 200) months in 10 patients with late relapse.
DISCUSSION
Herein, we show the capacity of ctDNA to effectively monitor disease status at the molecular level in DLBCL. We characterized the tumor-specific clonotype in 94 of 109 (86%) patients who had adequate archival biopsies, demonstrating the technical ability to identify the malignant clonotype in most patients. Given a detection limit of one lymphoma molecule per 106 diploid genomes, we hypothesized ctDNA would be a sensitive and specific measure of disease and compared serial ctDNA samples to the standard of CT imaging. When serially employed to monitor disease status following treatment, surveillance ctDNA showed a PPV of 88% and NPV of 98%. Patients who developed detectable ctDNA during surveillance had a hazard risk 228 times greater than patients with undetectable ctDNA for developing clinical progression. The higher sensitivity of ctDNA surveillance compared to CT imaging resulted in a median (range) detection lead-time of 3.5 (0 to 200) months for all patients who relapsed, and 7.4 (0 to 200) months for patients with late recurrences. Due to the interest in risk-adaptive strategies based on early assessment of tumor response, we also assessed the performance of interim ctDNA after two treatment cycles. Interim ctDNA had a PPV of 63% and NPV of 80% with a 5-year TTP of 41.7% (95% CI: 22.2% to 60.1%) and 80.2% (95% CI: 69.6% to 87.3%), respectively, for patients with and without detectable ctDNA on day 1 of cycle 3.
The standard of CT imaging for disease monitoring is limited by low sensitivity, whereas PET imaging, which improves sensitivity, is limited by low specificity and an unacceptable false positive rate. Both imaging modalities are further restricted by radiation exposure, invasiveness, and limits on frequency of use. In the present study, monitoring ctDNA overcame many of these limitations. The unique molecular sequences associated with the tumor immunoglobulin genes should theoretically lead to 100% specificity. It was not clear prior to this study whether low level tumor immunoglobulin sequences shed from pre-malignant or malignant cells would be present in the serum of patients who do not relapse. This study clearly demonstrates that DLBCL patients with long-term remission are essentially free of ctDNA. Out of almost 1000 samples tested in patients without recurrence, there were only 2 discordantly positive samples. In both cases, the positive ctDNA samples were at the detection limit and could not be confirmed on reanalysis, highlighting the need to confirm low-level positives.
We were uncertain at the study’s inception whether ctDNA assays would be sufficiently sensitive to detect tumor recurrence. Indeed, it was unknown if ctDNA encoding the tumor immunoglobulin gene could be detected in serum, particularly given that patients with DLBCL rarely have detectable circulating tumor cells. However, we demonstrated that ctDNA is detectable in the serum before clinical progression in most patients. Though 7 patients had persistent or progressive disease after treatment without ctDNA detection, 5 cases had focal disease at the end of treatment, one patient lacked a proximal serum sample before a recurrence and one patient recurred with a subclone that had not been followed. These results suggest that ctDNA may not be sufficiently sensitive to detect small foci of persistent disease in patients with mediastinal B-cell lymphoma.
Our results suggest that molecular monitoring of ctDNA improves upon conventional surveillance imaging. Unlike indolent lymphomas, where polymerase chain reaction-based ctDNA assays are prognostic, detection of ctDNA in DLBCL is a potentially actionable finding. Indolent lymphomas are rarely curable and patients are often observed at relapse, whereas patients with DLBCL are typically treated at first recurrence in an effort to improve outcome. Thus, based on current guidelines and standard of care, DLBCL patients undergo surveillance CT imaging for 2 years after treatment in an effort to identify recurrences with minimal disease burden. While the benefit of surveillance imaging has not been prospectively validated, low disease burden at relapse is associated with a significantly improved outcome to salvage therapy.28,29 Even with frequent CT imaging, administered a median (range) of 11 (1–16) times per patient in our study, early disease detection is suboptimal. Indeed, a recent retrospective study suggested that surveillance CT scans might be no better than a careful history and physical, supporting the need for more effective monitoring technologies.30
In addition to clinical uses, ctDNA monitoring has research implications. Interim tumor response monitoring using iPET for risk adaptive treatment is an active area of research. While this approach has not proven useful so far, our study showed that interim ctDNA might provide a favorable alternative or complement to iPET.6 Although it would be of interest to compare interim ctDNA and iPET, our patients did not undergo iPET scans. Nonetheless, early disease detection based on ctDNA could be employed as a biomarker to test novel targeted agents with monitoring of molecular response. ctDNA also has multiple clinical research applications including tumor response assessment, risk adapted treatment, monitoring maintenance therapy and institution of salvage therapy such as autologous transplant. Analysis of the immunoglobulin genes may provide insight into the antigen receptor repertoire of DLBCL and help identify tumors that are dependent on B-cell receptor signaling, and analysis of clonogenic evolution may allow characterization of tumor molecular heterogeneity.31
In conclusion, monitoring ctDNA in DLBCL accurately identifies patients at clinical risk of disease progression. Unlike imaging, ctDNA is a non-invasive and dynamic test that can be employed as often as indicated to detect subclinical disease. Early disease detection using ctDNA has important clinical and research applications and provides a potential alternative and/or adjunct to imaging for the management of DLBCL.
Supplementary Material
RESEARCH IN CONTEXT.
Evidence before this study
Detection of tumor cells in blood and bone marrow has been reported using polymerase chain reaction (PCR) technology for tumor specific gene sequences. These studies have shown that detection of disease by PCR in indolent lymphomas and mantle cell lymphoma are predictive of recurrence. To our knowledge, no molecular biomarker studies for detection of tumor cells have been performed in diffuse large B-cell lymphoma (DLBCL). Unlike indolent lymphomas, DLBCL is associated with a relatively low incidence of circulating tumor cells. Hence, we applied next generation sequencing to assess if cell free tumor DNA (ctDNA) encoding the clonal immunoglobulin gene sequence could be detected in the serum of patients with DLBCL and performed a study to assess its positive and negative predictive value for detecting disease recurrence.
Added value of this study
To our knowledge, this study provides the first evidence that ctDNA can be measured and detects disease progression months before conventional imaging in DLBCL. Given the limitations of conventional imaging due to sensitivity, specificity and radiation exposure, a sensitive and specific molecular biomarker like tumor ctDNA will likely provide an important test for surveillance and may significantly reduce the dependence on conventional imaging and identify recurrences at a lower tumor burden. Furthermore, ctDNA may be a useful research biomarker for assessment of drug activity, early treatment intervention with targeted agents and monitoring of disease during maintenance treatment.
Implications of all the available evidence
At the present time, serum tumor ctDNA may be used as an adjunct to conventional imaging. Although further studies are needed to assess the scope of tumor ctDNA applications, this test may reduce the need for conventional imaging and improve the monitoring of disease recurrence.
Acknowledgments
FP, JZ, MM, KK, TW and MF are employees and stockholders of Sequenta, Inc. FP, JZ, MM, TW and MF are inventors on Sequenta patent applications.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
AUTHOR CONTRIBUTIONS
Mark Roschewski participated in study design, data collection, data analysis, data interpretation and writing. Kieron Dunleavy participated in patient management, data interpretation and reviewing the manuscript. Stefania Pittaluga participated in study design, data analysis, data interpretation and reviewing the manuscript. Martin Moorhead participated in data analysis, data interpretation and reviewing the manuscript. Francois Pepin participated in data analysis, data interpretation and reviewing the manuscript. Katherine Kong participated in data analysis, data interpretation, data collection, figures and reviewing the manuscript. Margaret Shovlin participated in data collection, sample handling, data analysis, and reviewing the manuscript. Elaine S. Jaffe participated in data analysis, data interpretation and reviewing the manuscript. Louis M. Staudt participated in data interpretation and reviewing the manuscript. Catherine Lai participated in patient management, data interpretation and reviewing the manuscript. Seth M. Steinberg participated in data analysis, data interpretation and reviewing the manuscript. Clara C. Chen participated in data analysis, data interpretation and reviewing the manuscript. Jianbiao Zheng participated in data analysis, data interpretation and reviewing the manuscript. Thomas D. Willis participated in study design, data interpretation and reviewing the manuscript. Malek Faham participated in study design, data analysis, data interpretation and writing. Wyndham H Wilson participated in study design, protocol implementation, sample handling, data analysis, data interpretation, figures and writing.
DECLARATION OF INTEREST
MR, KD, SP, MS, ESJ, LMS, CL, SMS, CCC and WHW have no conflicts to declare.
REFERENCES
- 1.Menon MP, Pittaluga S, Jaffe ES. The histological and biological spectrum of diffuse large B-cell lymphoma in the World Health Organization classification. Cancer J. 2012;18(5):411–420. doi: 10.1097/PPO.0b013e31826aee97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gisselbrecht C, Glass B, Mounier N, et al. Salvage regimens with autologous transplantation for relapsed large B-cell lymphoma in the rituximab era. J Clin Oncol. 2010;28(27):4184–4190. doi: 10.1200/JCO.2010.28.1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liedtke M, Hamlin PA, Moskowitz CH, Zelenetz AD. Surveillance imaging during remission identifies a group of patients with more favorable aggressive NHL at time of relapse: a retrospective analysis of a uniformly-treated patient population. Ann Oncol. 2006;17(6):909–913. doi: 10.1093/annonc/mdl049. [DOI] [PubMed] [Google Scholar]
- 4.Armitage JO. Who benefits from surveillance imaging? J Clin Oncol. 2012;30(21):2579–2580. doi: 10.1200/JCO.2012.42.6189. [DOI] [PubMed] [Google Scholar]
- 5.Moskowitz CH, Schoder H, Teruya-Feldstein J, et al. Risk-Adapted Dose-Dense Immunochemotherapy Determined by Interim FDG-PET in Advanced-Stage Diffuse Large B-Cell Lymphoma. Journal of Clinical Oncology. 2010;28(11):1896–1903. doi: 10.1200/JCO.2009.26.5942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cashen AF, Dehdashti F, Luo J, Homb A, Siegel BA, Bartlett NL. 18F-FDG PET/CT for early response assessment in diffuse large B-cell lymphoma: poor predictive value of international harmonization project interpretation. J Nucl Med. 2011;52(3):386–392. doi: 10.2967/jnumed.110.082586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Safar V, Dupuis J, Itti E, et al. Interim [18F] fluorodeoxyglucose positron emission tomography scan in diffuse large B-cell lymphoma treated with anthracycline-based chemotherapy plus rituximab. J Clin Oncol. 2012;30(2):184–190. doi: 10.1200/JCO.2011.38.2648. [DOI] [PubMed] [Google Scholar]
- 8.Horning SJ, Juweid ME, Schoder H, et al. Interim positron emission tomography scans in diffuse large B-cell lymphoma: an independent expert nuclear medicine evaluation of the Eastern Cooperative Oncology Group E3404 study. Blood. 2010;115(4):775–777. doi: 10.1182/blood-2009-08-234351. quiz 918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheah CY, Hofman MS, Dickinson M, et al. Limited role for surveillance PET-CT scanning in patients with diffuse large B-cell lymphoma in complete metabolic remission following primary therapy. Br J Cancer. 2013;109(2):312–317. doi: 10.1038/bjc.2013.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thompson CA, Ghesquieres H, Maurer MJ, et al. Utility of routine post-therapy surveillance imaging in diffuse large B-cell lymphoma. J Clin Oncol. 2014;32(31):3506–3512. doi: 10.1200/JCO.2014.55.7561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brenner DJ, Hall EJ. Computed tomography--an increasing source of radiation exposure. N Engl J Med. 2007;357(22):2277–2284. doi: 10.1056/NEJMra072149. [DOI] [PubMed] [Google Scholar]
- 12.Jahr S, Hentze H, Englisch S, et al. DNA fragments in the blood plasma of cancer patients: quantitations and evidence for their origin from apoptotic and necrotic cells. Cancer Res. 2001;61(4):1659–1665. [PubMed] [Google Scholar]
- 13.Bettegowda C, Sausen M, Leary RJ, et al. Detection of circulating tumor DNA in early and late-stage human malignancies. Sci Transl Med. 2014;6(224):224ra24. doi: 10.1126/scitranslmed.3007094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Diehl F, Schmidt K, Choti MA, et al. Circulating mutant DNA to assess tumor dynamics. Nat Med. 2008;14(9):985–990. doi: 10.1038/nm.1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Faham M, Zheng J, Moorhead M, et al. Deep-sequencing approach for minimal residual disease detection in acute lymphoblastic leukemia. Blood. 2012;120(26):5173–5180. doi: 10.1182/blood-2012-07-444042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arnold A, Cossman J, Bakhshi A, Jaffe ES, Waldmann TA, Korsmeyer SJ. Immunoglobulin-gene rearrangements as unique clonal markers in human lymphoid neoplasms. N Engl J Med. 1983;309(26):1593–1599. doi: 10.1056/NEJM198312293092601. [DOI] [PubMed] [Google Scholar]
- 17.Roschewski M, Pittaluga S, Dunleavy K, et al. DNA sequencing-based monitoring of serum predicts clinical relapse before CT imaging in diffuse large B-cell lymphoma. 2013 [Google Scholar]
- 18.Armand P, Oki Y, Neuberg DS, et al. Detection of circulating tumour DNA in patients with aggressive B-cell non-Hodgkin lymphoma. Br J Haematol. 2013;163(1):123–126. doi: 10.1111/bjh.12439. [DOI] [PubMed] [Google Scholar]
- 19.Wilson WH, Grossbard ML, Pittaluga S, et al. Dose-adjusted EPOCH chemotherapy for untreated large B-cell lymphomas: a pharmacodynamic approach with high efficacy. Blood. 2002;99(8):2685–2693. doi: 10.1182/blood.v99.8.2685. [DOI] [PubMed] [Google Scholar]
- 20.Wilson WH, Dunleavy K, Pittaluga S, et al. Phase II study of dose-adjusted EPOCH and rituximab in untreated diffuse large B-cell lymphoma with analysis of germinal center and post-germinal center biomarkers. J Clin Oncol. 2008;26(16):2717–2724. doi: 10.1200/JCO.2007.13.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dunleavy K, Little RF, Pittaluga S, et al. The role of tumor histogenesis, FDG-PET, and short-course EPOCH with dose-dense rituximab (SC-EPOCH-RR) in HIV-associated diffuse large B-cell lymphoma. Blood. 2010;115(15):3017–3024. doi: 10.1182/blood-2009-11-253039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dunleavy K, Pittaluga S, Maeda LS, et al. Dose-adjusted EPOCH-rituximab therapy in primary mediastinal B-cell lymphoma. N Engl J Med. 2013;368(15):1408–1416. doi: 10.1056/NEJMoa1214561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wilson WH, Pittaluga S, Nicolae A, et al. A prospective study of mediastinal gray-zone lymphoma. Blood. 2014;124(10):1563–1569. doi: 10.1182/blood-2014-03-564906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cheson BD, Horning SJ, Coiffier B, et al. Report of an international workshop to standardize response criteria for non-Hodgkin's lymphomas. NCI Sponsored International Working Group. J Clin Oncol. 1999;17(4):1244. doi: 10.1200/JCO.1999.17.4.1244. [DOI] [PubMed] [Google Scholar]
- 25.Kaplan EL, Meier P. Nonparametric-Estimation from Incomplete Observations. J Am Stat Assoc. 1958;53(282):457–481. [Google Scholar]
- 26.Schemper M, Smith TL. A note on quantifying follow-up in studies of failure time. Control Clin Trials. 1996;17(4):343–346. doi: 10.1016/0197-2456(96)00075-x. [DOI] [PubMed] [Google Scholar]
- 27.Altman DG, Bland JM. Diagnostic tests. 1: Sensitivity and specificity. BMJ. 1994;308(6943):1552. doi: 10.1136/bmj.308.6943.1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rapoport AP, Rowe JM, Kouides PA, et al. One hundred autotransplants for relapsed or refractory Hodgkin's disease and lymphoma: value of pretransplant disease status for predicting outcome. J Clin Oncol. 1993;11(12):2351–2361. doi: 10.1200/JCO.1993.11.12.2351. [DOI] [PubMed] [Google Scholar]
- 29.Hamlin PA, Zelenetz AD, Kewalramani T, et al. Age-adjusted International Prognostic Index predicts autologous stem cell transplantation outcome for patients with relapsed or primary refractory diffuse large B-cell lymphoma. Blood. 2003;102(6):1989–1996. doi: 10.1182/blood-2002-12-3837. [DOI] [PubMed] [Google Scholar]
- 30.Thompson CA, Ghesquieres H, Maurer MJ, et al. Utility of Routine Post-Therapy Surveillance Imaging in Diffuse Large B-Cell Lymphoma. J Clin Oncol. 2014 doi: 10.1200/JCO.2014.55.7561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Agathangelidis A, Darzentas N, Hadzidimitriou A, et al. Stereotyped B-cell receptors in one-third of chronic lymphocytic leukemia: a molecular classification with implications for targeted therapies. Blood. 2012;119(19):4467–4475. doi: 10.1182/blood-2011-11-393694. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.