Abstract
Background
Transrectal ultrasound-guided prostate biopsies are prone to detection errors. Multi-parametric MRI (MP-MRI) may improve the diagnostic pathway.
Methods
PROMIS is a prospective validating paired-cohort study that meets criteria for level 1 evidence in diagnostic test evaluation. PROMIS will investigate whether multi-parametric (MP)-MRI can discriminate between men with and without clinically-significant prostate cancer who are at risk prior to first biopsy. Up to 714 men will have MP-MRI (index), 10–12 core TRUS-biopsy (standard) and 5 mm transperineal template mapping (TPM) biopsies (reference). The conduct and reporting of each test will be blinded to the others.
Results
PROMIS will measure and compare sensitivity, specificity, and positive and negative predictive values of both MP-MRI and TRUS-biopsy against TPM biopsies. The MP-MRI results will be used to determine the proportion of men who could safely avoid biopsy without compromising detection of clinically-significant cancers. For the primary outcome, significant cancer on TPM is defined as Gleason grade >/= 4 + 3 and/or maximum cancer core length of ≥ 6 mm. PROMIS will also assess inter-observer variability among radiologists among other secondary outcomes. Cost-effectiveness of MP-MRI prior to biopsy will also be evaluated.
Conclusions
PROMIS will determine whether MP-MRI of the prostate prior to first biopsy improves the detection accuracy of clinically-significant cancer.
Keywords: Prostate cancer, Transrectal ultrasound guided biopsy, Template transperineal mapping biopsy, Magnetic resonance imaging, Multi-parametric MRI, Triage diagnostic test
1. Background & introduction
Prostate cancer is the most common male cancer, with a doubling in incidence over the last 15 years in the UK. Currently over 40,000 new cases are diagnosed every year in the UK [1,2] and 223,307 new cases in the USA [3]. Many prostate cancers currently detected are clinically insignificant and do not have any clinical impact on the individual during his remaining life if left untreated [4,5]. This assertion has received considerable support from a number of large randomised controlled trials of prostate cancer screening and treatment. Whilst the PLCO (Prostate, Lung, Colon and Ovaries) screening trial in the USA showed no evidence of a survival benefit it was criticised for significant contamination (i.e., PSA testing) in the control arm [6]. The European Randomized Study of Screening for Prostate Cancer (ERSPC) showed a modest reduction in risk of death from prostate cancer in those screened [7]. The number needed to invite for screening was 936 and the number of prostate cancers needed to detect and/or treat was 33 to extend the life of one man over a 12 year period [8–10]. Further, the benefit of PSA screening was diminished by loss of quality-adjusted life-years owing to post-diagnosis long-term effects [10].
The role of treatment, once diagnosed, has also been questioned. The Scandinavian Prostate Cancer Group-4 (SPCG-4) and the Prostate Cancer Intervention Versus Observation Trial (PIVOT) randomised controlled trials of radical prostatectomy compared to watchful waiting have shown that the benefit of treatment in terms of improved overall survival was restricted to those men with higher risk – clinically significant – prostate cancer [11–14].
In order to minimise over-diagnosis and over-treatment, the optimal diagnostic pathway should be able to discriminate reliably between men with and without clinically significant cancer.
2. The current diagnostic pathway
Men deemed at risk of prostate cancer are those with an elevated PSA level, an abnormal digital rectal examination, a positive family history of prostate cancer or a specific ethnic profile [15]. Once a risk factor is identified, patients are advised to have a trans-rectal ultrasound (TRUS) guided biopsy. Annually, between 59,000 and 80,000 men have a TRUS-biopsy in the UK and about one million in the USA [16]. TRUS-biopsy can be problematic in that the ultrasound is used to identify the prostate itself and is unable to identify suspicious lesions with any degree of accuracy; this results in biopsies being taken blindly throughout the prostate. This is in contrast to the approach taken in most other solid organ cancers where the lesion is identified, usually on imaging, in order to direct biopsies to the area of suspicion. The random and systematic errors in diagnosis which are inherent in TRUS-biopsies lead to a number of problems.
2.1. TRUS biopsies over-diagnose clinically insignificant prostate cancer
A man who undergoes TRUS-biopsy has a 1 in 4 chance of being diagnosed with prostate cancer [17,18]. This compares with a 6–8% lifetime risk of having prostate cancer that will impact life expectancy. The over-detection of these small low-grade lesions is due in part to the random deployment of the needles [17–19] (Fig. 1).
Fig. 1.
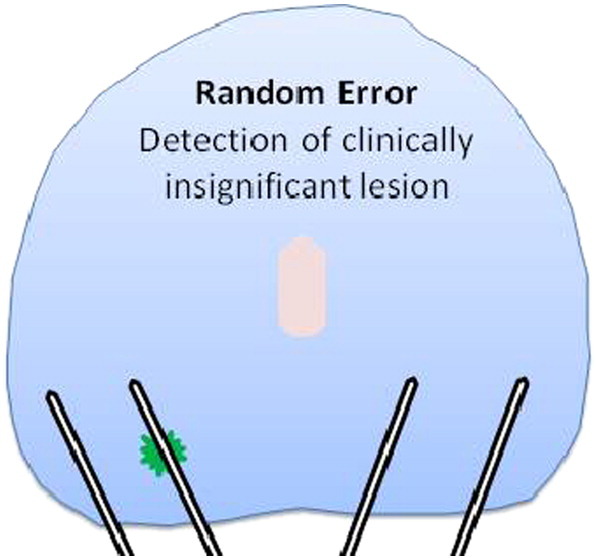
Over-detection of insignificant prostate cancer resulting from TRUS-guided biopsies.
2.2. TRUS-guided biopsies miss clinically significant cancers
They have an estimated false negative rate of 30%–45% [19,20]. The clinician takes 10–12 biopsies in a manner that attempts to obtain representative tissue from the peripheral zone (Fig. 2a). However, this systematic error leads to significant cancer being missed in the peripheral zone. Further, several parts of the prostate are systematically under-sampled. First, the anterior gland is missed as a result of its greater distance from the rectum (Fig. 2b). Second, areas in the midline are under-sampled due to efforts to avoid the urethra. Third, the prostate apex is often difficult to access by the transrectal route [21–24].
Fig. 2.
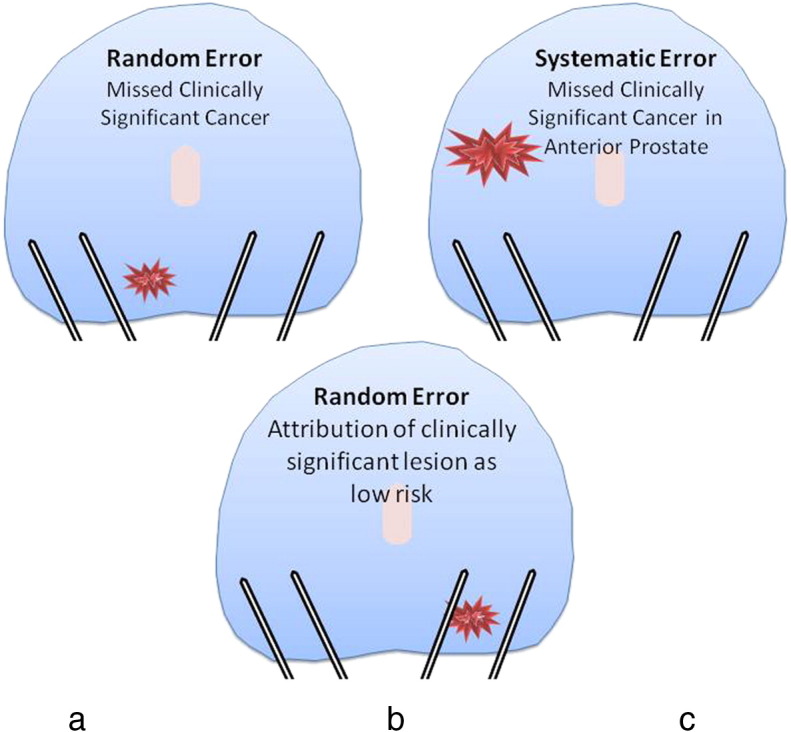
Under-detection of clinically significant prostate cancer resulting from TRUS-guided biopsies.
2.3. TRUS-biopsies can misrepresent the true burden of cancer
The random sampling error (Fig. 2c) can mean that a biopsy does not hit the cancer lesion through its greatest diameter leading to either or both the size or grade of cancer being underestimated [25] (Fig. 2c). Up to half of men deemed low risk on TRUS-biopsies can have higher burden or grade, or both, when a more accurate biopsy test is applied [22–24,26–28]. As a result of the poor risk attribution, many men with true low risk disease cannot be sure of their risk attribution so they and their physicians often choose radical therapies from which they derive little to no survival benefit [11].
2.4. TRUS-biopsy has harms
It is associated with a number of complications, the most important being urinary tract infection (1–8%) that can result in life-threatening sepsis (1–4%). Haematuria (50%), haematospermia (30%), pain/discomfort (most), dysuria (most) and urinary retention (1%) can also be expected [29–32].
3. The proposed new diagnostic pathway
A novel pathway (Fig. 3) in which imaging is used as a triage test [33] for men at risk of prostate cancer might enable physicians to decide if the patient should or should not have a prostate biopsy, whilst those patients with a visible lesion could have a targeted biopsy.
Fig. 3.
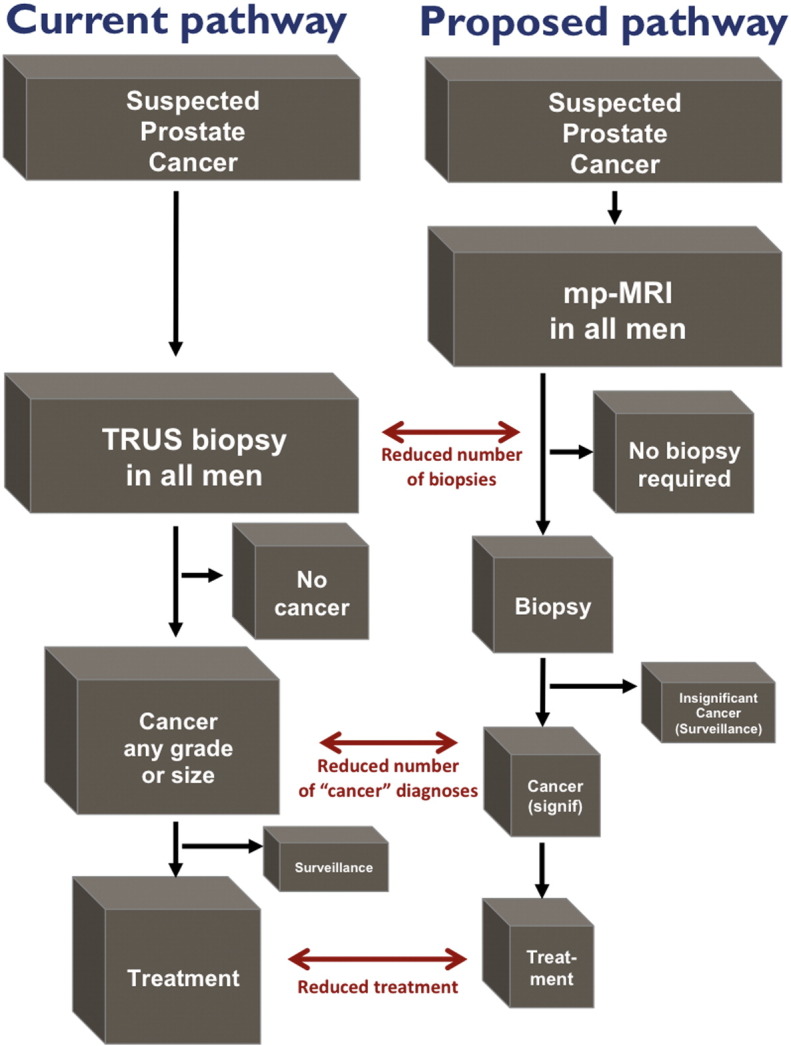
Current and proposed diagnostic pathways should MP-MRI prove favourable.
This proposed new pathway might offer several advantages. Should the results of the trial be in favour of MP-MRI, we hypothesise three corrections in the current pathway that might occur. First, over-diagnosis might be reduced because fewer clinically insignificant prostate cancers will be detected by chance as only patients with positive MRIs will undergo a biopsy. Second, as a result there might be less over-treatment. Third, the detection of clinically significant prostate cancers might be improved. Fourth, there might be better treatment allocation to active surveillance or radical therapies due to more representative biopsy sampling and better risk stratification. Fifth, complications might be reduced if fewer men are biopsied [34,35].
At present multi-parametric MRI (MP-MRI) appears to have the desired attributes of a test that could be used in the prostate cancer diagnostic pathway [36]. The evidence base available at the time of conceiving PROMIS suggested that MP-MRI can achieve both a sensitivity and specificity between 70–90% for the detection of clinically significant prostate cancer [37]. However, a systematic review of the literature [38] found the quality of the initial studies evaluating MP-MRI to be disappointing [36]. Early reports repeatedly showed low sensitivity and specificity as well as high inter-observer variability, even when using high-resolution endorectal MRI [39–45]. Since these early reports, much has changed including an appreciation of the impact of post-biopsy changes on MR-image quality, technological improvements such as increasing magnetic field strength (from 0.5 T to 1.5 T and 3.0 T), shorter pulse sequences enabling faster image acquisition, and the introduction of functional imaging in the form of diffusion weighting (DW) and dynamic contrast-enhancement (DCE).
Multi-parametric approaches in which sequences are combined together have found an advantage for using two or three MRI sequences rather than just one. When PROMIS started, none had evaluated the clinical validity of MP-MRI in the population of interest – men at risk – against an accurate and appropriate reference standard within a prospective multi-centre study (Table 1) [46–56].
Table 1.
Sensitivity and specificity of MRI parameters as reported in the literature.
| Parameter | Number (mean) | Sensitivity | Specificity |
|---|---|---|---|
| T2 | 12–320 (97) | 37–96% | 21–67% |
| DW | 11–95 (42) | 57–90% | 79–88% |
| DCE | 23–54 (41) | 71–87% | 61–89% |
There were a number of limitations to the early studies investigating the diagnostic accuracy of MRI for prostate cancer that probably account for the limited acceptance of MRI into the prostate cancer diagnostic pathway [57]. These include:
-
a)
biopsy artefact: studies mostly evaluate MRI after biopsy. This results in biopsy-related haemorrhage and inflammation, which can lead to false positives or negative results or scans which are not interpretable.
-
b)
Limited application: many studies evaluated only the peripheral zone of the prostate, ignoring up to one-third of prostate cancers that lie anteriorly.
-
c)
Segmentation: most studies segmented the prostate into a number of sectors for analysis in order to both achieve sufficient matched datasets. These studies classify each sector of analysis as ‘independent’. Segmentation is usually employed because the reference used is radical whole-mount prostatectomy.
-
d)
Inaccurate or inappropriate reference standard: most studies used radical prostatectomy (RP), leading to selection bias as men have to test positive on a biopsy first and then choose to have surgery. Those undergoing surgery tend to have burdens of cancer that are distinct from patients choosing other treatments [58]. Further, as the prostate is usually segmented, co-registration of an image to an RP specimen is challenging because of shrinkage (10–20%), distortion, tissue loss as a result of ‘trimming’ (10%), orientation, absent perfusion and sector boundaries [59,60].
4. Design and methods: the PROMIS protocol
4.1. Design
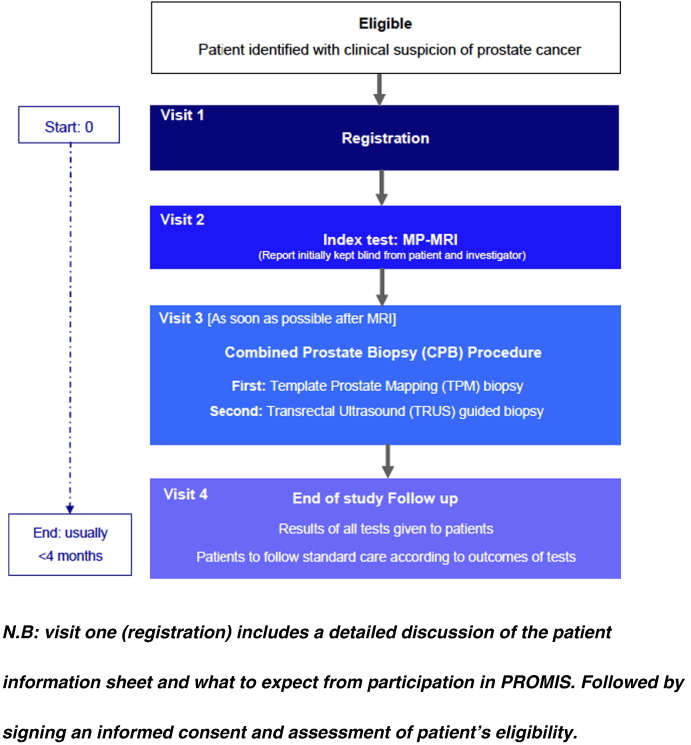
PROMIS is a validating paired cohort study representing level 1 evidence for diagnostic studies [61]. The primary objectives of PROMIS are to assess the ability of MP-MRI to identify men who can safely avoid unnecessary biopsy and to assess the ability of the MP-MRI based pathway to improve the rate of detection of clinically significant cancer by evaluating the diagnostic accuracy of MP-MRI (the index test) against an accurate reference standard, template prostate mapping (TPM) biopsies, which can be applied to all men under evaluation. The current TRUS-biopsy (standard test) will also be evaluated against TPM. All men consenting to the study will have a MP-MRI, followed by a combined prostate biopsy procedure in which TPM biopsies will be followed by TRUS-guided biopsies. Each test will be conducted and reported independent of the other tests (Fig. 4).
Fig. 4.
PROMIS Trial Schema. N.B.: visit one (registration) includes a detailed discussion of the patient information sheet and what to expect from participation in PROMIS. Followed by signing an informed consent and assessment of patient's eligibility.
PROMIS is designed to overcome shortcomings highlighted in the current literature. First, since all patients will be biopsy-naïve and undergo MP-MRI prior to any biopsy, there will be no biopsy artefact. Second, MP-MRI will be evaluated for all anatomical zones of the prostate including peripheral and transition zones. Third, the study is powered so that the primary outcome will be derived using the whole prostate as the sector of analysis rather than segmented sectors of the prostate. Fourth, PROMIS employs an accurate reference test that can be applied to all men at risk.
Furthermore, the study has been designed to ensure avoidance or minimisation of a number of biases that are inherent in the current literature. First, spectrum and selection biases will be avoided by recruiting men at risk of prostate cancer and applying all tests to all men. Second, work-up bias will be eliminated by ensuring that patients and clinicians remain blinded to all imaging test results until the biopsies have been carried out and reported. Third, reviewer/reporter bias will be avoided by ensuring that the radiologist is blinded to the reference test and the pathologist is blinded to the imaging. The radiology report in particular will be submitted prior to the biopsies. Last, incorporation bias is minimised by ensuring that TPM biopsies and TRUS-guided biopsies follow a standard accepted protocol.
4.2. Patient population
Men who have never had a prostate biopsy before are eligible for the study if there is a clinical suspicion that they may be harbouring prostate cancer. This essentially includes men with an elevated PSA and/or a suspicious digital rectal examination, family history of prostate cancer or an ethnic risk group. Men with a PSA above 15 ng/ml are excluded from PROMIS as physicians are unlikely to use MP-MRI as a triage test to avoid a biopsy due to the higher incidence of prostate cancer in this sub-group. The inclusion and exclusion criteria are described in Table 2. Patients who do not want to participate in PROMIS or who are ineligible are returned to the normal clinical pathway employed in the participating centre.
Table 2.
Patient inclusion and exclusion criteria.
| Patient inclusion criteria |
|---|
| Men at least 18 years or over at risk of prostate cancer who have been advised to have a prostate biopsy |
| Serum PSA ≤ 15 ng/ml within the previous 3 months |
| Suspected stage ≤ T2 on rectal examination (organ confined) |
| Fit for general/spinal anaesthesia |
| Fit to undergo all protocol procedures including a transrectal ultrasound |
| Signed informed consent |
| Patient exclusion criteria |
| Treated using 5-alpha-reductase inhibitors at time of registration or during the prior 6 months |
| Previous history of prostate biopsy, prostate surgery or treatment for prostate cancer (interventions for benign prostatic hyperplasia/bladder outflow obstruction is acceptable) |
| Evidence of a urinary tract infection or history of acute prostatitis within the last 3 months |
| Contraindication to MRI (e.g., claustrophobia, pacemaker, estimated GFR ≤ 50) |
| Any other medical condition precluding procedures described in the protocol |
| Previous history of hip replacement surgery, metallic hip replacement or extensive pelvic orthopaedic metal work. |
4.3. Study interventions
4.3.1. The index test — multi-parametric magnetic resonance imaging
Although experts in the field generally regard the performance characteristics of MP-MRI of the prostate as promising [62], there exists professional disagreement on its accuracy and usefulness in clinical practice [35], limiting wider adoption. These concerns relate in part to the variable quality and methodology of studies that have resulted in marked variation in indication, conduct, interpretation, and reporting [46,54,60,63–66].
As a result, MP-MRI in PROMIS will be standardised to the minimal requirements advised by a European consensus meeting [67], the European Society of Uro-Radiology [68] and the British Society of Uro-Radiology guidelines [69]; this will entail acquiring T1-weighted, T2-weighted, diffusion-weighted (apparent diffusion coefficient maps and long-b scan) and dynamic gadolinium contrast-enhanced imaging using a 1.5 Tesla scanner and a pelvic phased array (Table 3).
Table 3.
Standard operating procedure for MRI parameters for all centres to follow.
| TR | TE | Flip angle/degrees | Plane | Slice thickness (gap) | Matrix size | Field of view/mm | Time for scan | |
|---|---|---|---|---|---|---|---|---|
| T2 TSE | 5170 | 92 | 180 | Axial, coronal, sagittal | 3 mm (10% gap) | 256 × 256 | 180 × 180 | 3 min 54 s (ax) |
| VIBE at multiple flip angles for T1 calculation (optional) | Will be included in the Phoenix file | |||||||
| VIBE fat sat | 5.61 | 2.52 | 15 | Axial | 3 mm | 192 × 192 | 260 × 260 | Continue for at least 5 min 30 s after contrast |
| Diffusion (b values: 0, 150, 500, 1000) | 2200 | Min (< 98) | Axial | 5 mm | 172 × 172 | 260 × 260 | 5 min 44 s (16 averages) | |
| Diffusion (b = 1400) | 2200 | Min (< 98) | Axial | 5 mm | 172 × 172 | 320 × 320 | 3 min 39 s (32 averages) |
Use of endorectal coils will be avoided, as there is no consensus on its role in minimal scanning requirements [67]. It was decided to not include magnetic resonance spectroscopy as evidence from a large prospective multicentre study at the time showed no benefit of spectroscopy for prostate cancer localization compared with T2-weighted imaging alone [70].
In order to maintain the quality of scans and ensure uniformity across all centres, optimization of the conduct of scans will be applied to all centres through a robust quality control process. This will be undertaken by a separate independent commercial sub-contractor (Ixico Ltd, UK) selected through an open competition compliant with the European Union guidelines on the tender process. Scans deemed of insufficient quality by the commercial partner or the reporting radiologist will be repeated.
4.3.1.1. Standardization of MRI reporting
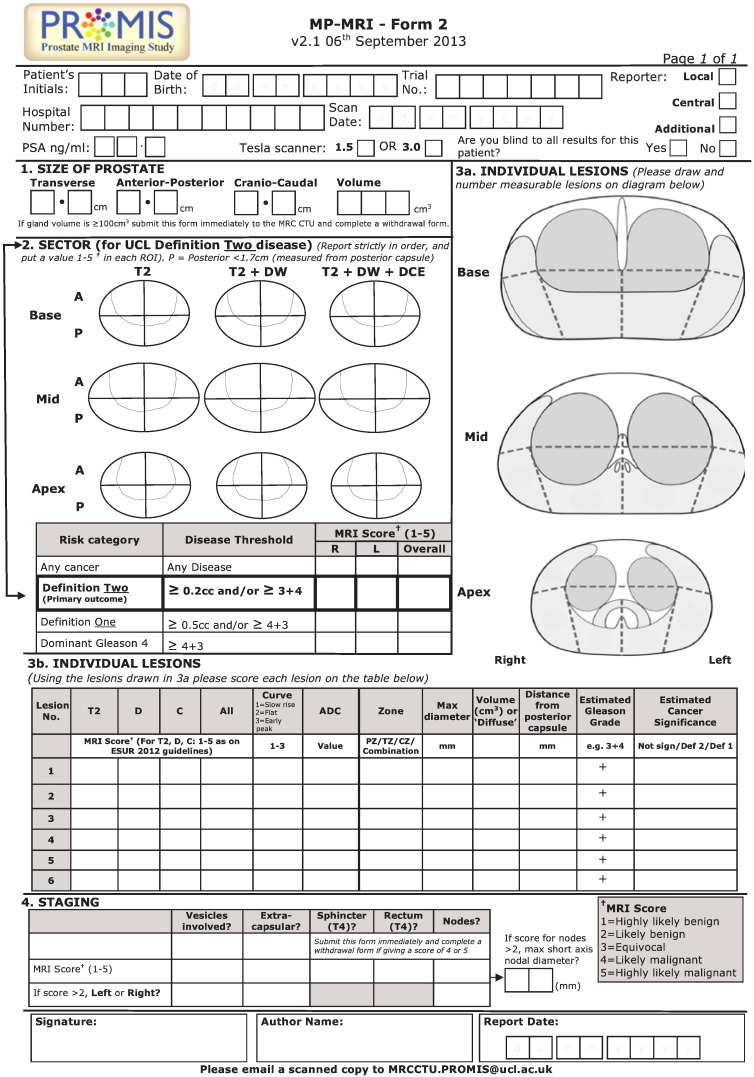
In order to avoid variation in method of interpretation, a standardised operating procedure for MP-MRI reporting has been adopted in line with the recommendations of the European consensus meeting and the European Society of Uro-Radiology prostate MRI guidelines [68]. All radiologists will undergo training and standardisation of reporting by the lead radiologist centrally prior to reporting within the trial. The actual reporting will require all radiologists being provided with the same clinical details including PSA, DRE findings and any other risk factors. Images will be reported in sequence so that T2-weighted images will be reported first, T2-weighted and diffusion-weighted together and then a third report issued for T2-weighted with diffusion and dynamic contrast enhanced scans together. A separate report will be produced for each combination of sequences in order to secondarily investigate whether both diffusion-weighted and DCE are both required. As DCE requires contrast agent (with its need for intravenous access, medical supervision and contrast-related risks) and an additional 10–15 min of scan time there is considerable merit in determining whether this additional resource and cost is necessary (Fig. 5).
Fig. 5.
MP-MRI reporting form.
A 1 to 5 Likert scoring system [67,68,71] will be used to indicate probability of cancer (1 — highly likely to be benign, 2 — likely to be benign, 3 — equivocal, 4 — likely to be malignant and 5 — highly likely to be malignant) with the prostate divided into 12 regions of interest (ROI) and each region scored from 1 to 5. Further, each lesion will be identified and scored on the 1 to 5 scale separately and the longest axial diameter, lesion volume, ADC value and contrast enhancement curve type will be recorded [72–75]. An overall 1 to 5 score of the whole prostate will be recorded for each level of cancer burden that the radiologist thinks might be present. This will be ‘all cancer’, ‘definition 1 clinically significant cancer’ and ‘definition 2 clinically significant cancer’ (see below).
With respect to the primary outcome, an overall score of 3 or more will be used to indicate the possible presence of clinically significant cancer (i.e., a positive MP-MRI score). This reflects the level at which further tests (e.g., biopsy) would be considered if MP-MRI were to be introduced into the diagnostic pathway in the future.
4.3.1.2. Assessing for inter-observer variability and quality control and assessment
In order to establish if a diagnostic test can improve or change the diagnostic pathway in prostate cancer, it must be assessed for intra- and inter-observer variability. Thus, a subset of scans will be reported by another experienced central radiologist. A subset of scans will also be reported by the same reporter again at a different time-point to assess intra-observer variability.
In order to make sure that the result of the MP-MRI does not influence the conduct of the biopsy, the results of the MP-MRI will not be revealed to either the men having the biopsies or to the clinicians undertaking the biopsies until after the results of the TRUS-biopsy and TPM biopsies are available (with the exceptions for un-blinding given below). This blinding is necessary to prevent the results of the MP-MRI influencing whether men are biopsied and if they are, how the biopsies are conducted.
For safety purposes, the results of the MP-MRI can be un-blinded by the radiologist if the MP-MRI reveals an enlarged prostate > 100 ml in volume or there is evidence of T4 prostate cancer or involved lymph nodes or colorectal/bladder invasion. The presence of other cancers such as bladder or colorectal cancers will also be a criterion for withdrawal. This information will be provided to the treating clinician for appropriate clinical decision making.
4.3.2. The standard test — transrectal ultrasound-guided biopsy
TRUS-biopsy of the prostate is to be performed after TPM biopsies, under the same general/spinal anaesthetic. This helps ensure that results are obtained for the reference test in an optimal fashion in a biopsy naïve gland that has not undergone swelling and distortion. It also theoretically minimises the risk of infection as the potential for faecal contamination is restricted to the end of the procedure. The surgeon performing the biopsy procedure will be blind to the MRI results so no targeting of suspicious areas will occur. TRUS-guided biopsies incorporate 10–12 core biopsies taken as per international guidelines [76]. Each core will be identified and potted separately. The TPM biopsies and TRUS-guided biopsy sets from a particular patient will be sent to different pathologists to minimise review and work-up biases.
4.3.3. Comparator
4.3.3.1. Transperineal Template Prostate Mapping biopsies
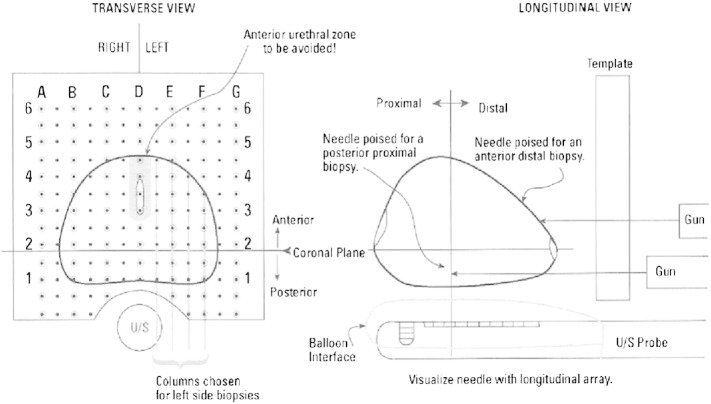
Transperineal Template Prostate Mapping (TPM) biopsies (Fig. 6) has been selected as the reference test; when using 5 mm-sampling it meets the required specification as a reference test for our defined population [24,26,77–80].
Fig. 6.
Illustration of how Transperineal Template Prostate Mapping biopsies are conducted.
TPM biopsies produce a histological map of the entire prostate in 3-dimensions with an estimated sensitivity and negative predictive value (NPV) in the order of 95% for clinically significant cancers when assessed against radical prostatectomy [21,24]. TPM biopsies have a similar side-effect profile to that of TRUS-biopsy with three important differences. First, they carry a significantly lower risk of urosepsis (< 0.5%) – the most serious complication of TRUS-biopsy – as the needles do not traverse the rectal mucosa. Second, TPM biopsies confer a higher risk of self-limiting failure to void urine (5–10%) as a result of greater gland swelling [78,81,82] compared to 1–2% risk associated with TRUS-biopsy. Third, TPM biopsies require a general/spinal anaesthetic. The accuracy of TPM biopsies is high and has been recently validated against radical whole-mount specimens [83]. We chose to combine TPM biopsies with TRUS-biopsies under the same general/spinal anaesthetic in order to reduce patient burden (from two visits and two procedures to one visit) and minimise drop-out of patients between tests. Training will be provided to all centres to conduct TPM biopsies according to the PROMIS protocol although all centres have been selected for their prior experience in carrying out TPM biopsies.
4.3.3.2. Side effect profile of a combined TPM and TRUS-biopsy procedure
The expected side-effects of combining both procedures is detailed in the patient information sheet which is discussed with all patients prior to registration (Table 4). Our rate of serious adverse events is monitored by an independent trial steering committee on a weekly basis.
Table 4.
Combined prostate biopsy procedure side effect profile as stated in the patient information sheet and consent documentation.
| Side effect | Procedure |
|
|---|---|---|
| TRUS alone (standard care) | Combined biopsy: TPM + TRUS (in the PROMIS study) | |
| Pain/discomfort | Almost all men experience temporary discomfort in the rectum | Almost all men experience temporary discomfort in the rectum |
| Burning when passing urine | Almost all men | Almost all men |
| Bloody urine | 1 in 2 men (self-resolving, 2–3 days) | Almost all men (self-resolving, 2–3 days) |
| Bloody sperm | 3 in 10 men (2–3 months to resolve) | Almost all men (lasting up to 3 months) |
| Poor erections | 3 in 10 men (self-resolving after 6–8 weeks). Rarely, tablets may be needed to help the erections improve. | Almost all men (self-resolving after 6–8 weeks). Rarely, tablets may be needed to help the erections improve. |
| Infection of skin or urine | 1–8 in 100 men | 1–8 in 100 men |
| Infection of skin or urine requiring admission and intravenous antibiotics | Between 1–4 in 100 men | Between 1–4 in 100 men |
| Difficulty passing urine | 1 in 100 men | 1–3 in 20 men |
| Bruising of skin | None | Almost all men |
| Bruising spread to scrotum | None | Between 1 in 20 to 1 in 10 men |
4.4. The target condition for detection — defining clinically significant prostate cancer
Despite the high accuracy of TPM biopsies in detecting clinically significant disease there is no widespread agreement on the criteria to define clinically significant prostate cancer on TRUS-biopsies or TPM biopsies. Whilst some definitions exist, such as the Epstein criteria [84], they have been developed for TRUS-biopsy; indeed, if these were applied to TPM biopsies the prevalence of intermediate and high risk disease would be artificially inflated given the different sampling densities used [28,85]. As a consequence, the best evidence to select the risk classification for analysing TPM biopsy results has been derived by a simulation study using TPM-biopsies with a 5 mm sampling density. By combining maximum cancer core length and Gleason score, this simulation study stratified patients into three groups: low risk, intermediate risk and high risk (Fig. 7). The target definition for clinically significant disease on TPM biopsies for the primary outcome will be set at a maximum cancer core involvement >/= 6 mm and/or Gleason >/= 4 + 3 (UCL definition 1). The cancer core length in particular relates to an area of cancer on TPM biopsies that approximates to a lesion volume of >/= 0.5 ml [17]. We chose this target condition as the primary outcome on the basis that few physicians would disagree that any man having this burden of cancer would require treatment. A further threshold for clinically significant disease will also be used (cancer core length involvement >/= 4 mm and/or Gleason >/= 3 + 4) (UCL definition 2).
Fig. 7.
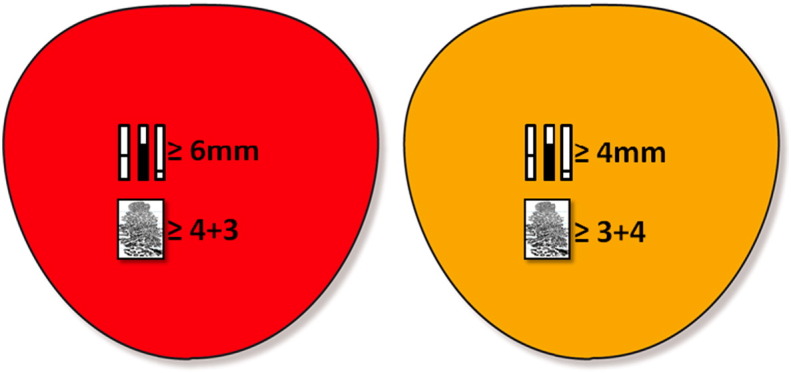
Definitions of clinical significance on TTPM-biopsy. Red signifies UCL definition 1 against which the primary outcome will be validated. Yellow signifies UCL definition 2 and is a secondary outcome.
From Ahmed HU et al., J Urol, 2011; 186(2):458-64.
4.5. Outcomes
4.5.1. Primary outcomes
The primary outcomes in this trial are of fundamental importance to decisions regarding the future use of MP-MRI in the diagnostic pathway for the prostate cancer. First, they include the proportion of men who could safely avoid biopsy as determined by specificity and negative predictive values (NPVs) for clinically significant cancer. Second, the proportion of men correctly identified by MP-MRI to have clinically significant prostate cancer as determined by sensitivity and positive predictive values (PPV). For the primary outcomes, UCL definition one criteria will be used to set the target definition of clinically significant prostate cancer on TPM biopsy and a score of 3 or more on MP-MRI will be used to define a positive index test. Further, the accuracy of TRUS-biopsy will also be reported in terms of sensitivity, specificity, NPV, and PPV as listed in the section below. In addition, a head-to-head comparison of the accuracy of MP-MRI versus TRUS-guided biopsy (current standard) will be performed. All primary and secondary outcomes are presented in Table 5.
Table 5.
Primary and secondary outcomes for the PROMIS trial.
| Primary outcomes: |
|---|
| Proportion of men who could safely avoid a biopsy as determined by specificity and negative predictive values (NPV), based on definition one of clinical significance as assessed by TPM. |
| Proportion of men correctly identified by MP-MRI to have clinically significant prostate cancer as determined by sensitivity and positive predictive value, based on definition one of clinical significance as assessed by TPM. |
| Secondary outcomes: |
| The proportion of men who could safely avoid biopsy, given that they do not have definition two prostate cancer as assessed by TPM. |
| The proportion of men testing positive on MP-MRI out of those with DEFINITION TWO prostate cancer assessed by TPM. |
| Performance characteristics of TRUS versus TPM (sensitivity, specificity, NPV, PPV) according to definitions one and two |
| Evaluation of the optimal combination of MP-MRI functional parameters (T2, DW, DCE) to detect or rule-out clinically significant prostate cancer. |
| Intra-observer variability in the reporting of MP-MRI. |
| Inter-observer variability in the reporting of MP-MRI. |
| Evaluation of socio-demographic, clinical, imaging and radiological variables in relation to the detection of clinically significant prostate cancer. |
| Patients' health-related quality of life using the EQ-5D instrument. |
| Resource use and costs for further economic evaluation (see section on Cost-effectiveness analyses). |
4.6. Translational objectives
PROMIS is ideal for assessing the utility of biomarkers (from urine and blood) to identify men with clinically significant prostate cancer. This is the first time that a broad spectrum of men at risk will be evaluated using an optimal biopsy technique that accurately characterises the presence, size and grade of prostate cancer. We will collect, process and store a comprehensive bank of tissue samples (serum, plasma, germ-line DNA, urine) from men prior to biopsy, to analyse urinary and serum biomarkers with respect to the detection of clinically significant prostate cancer on TPM biopsy.
4.7. Trial conduct
The study has been set up to run in two stages: the pilot phase, followed by the main phase. The pilot study has already completed and recruited 50 patients over one year to allow testing of safety and recruitment. At the end of the pilot in May 2013, few safety concerns emerged and the Trial Steering Committee recommended continuation of the study into the main phase [86].
If the combination of TRUS-biopsy and TPM biopsies leads to more than a 4% risk of sepsis at any time, there could be cause for concern and a requirement for modification of the study design. If deemed appropriate, recruitment to the study will be suspended until any safety concerns have been resolved.
4.8. Long-term follow-up through linkage
The long-term outcomes of the PROMIS cohort will be of interest and contribute to our understanding of the epidemiology of prostate cancer. Men who specifically consent to long-term data collection will be flagged and followed up using the Office for National Statistics and NHS databases. For example, linkage to Hospital Episode Statistics (HES) may give valuable information on further diagnoses, treatments and outcomes beyond the timeframe of the study for future analyses. Consenting men may additionally be contacted in the future to assess their willingness to respond to questionnaires. This allows the potential for research that would complement the planned long-term follow-up in terms of health status, for example picking up future biopsies not included in HES, and allows assessment of quality of life.
4.9. Statistical considerations
4.9.1. Sample size
Power calculations were performed in relation to: (1) precision around the estimates for the accuracy of MP-MRI in terms of the joint primary outcomes of specificity and sensitivity, and (2) a head-to-head comparison of the MP-MRI versus TRUS. The largest sample size from (1) and (2) was 714 (as detailed below) and this was taken as the maximum number of men required to have all 3 tests (MP-MRI, TRUS biopsy and TTPM-biopsies).
4.9.2. Prevalence of clinically significant cancer
For all calculations we have assumed [19,20,28,87] that 15% of the study population will have clinically significant prostate cancer as detected by the reference standard according to UCL definition one and 25% will have clinically significant prostate cancer as detected by the reference standard according to UCL definition two. These estimates act as inflation factors for the total number of men required for the study. All calculations are based on 90% power and 5% significance (2-sided). The specified estimates of sensitivity and specificity are considered realistic based on current unpublished and published literature [88,89] (Fig. 8).
Fig. 8.
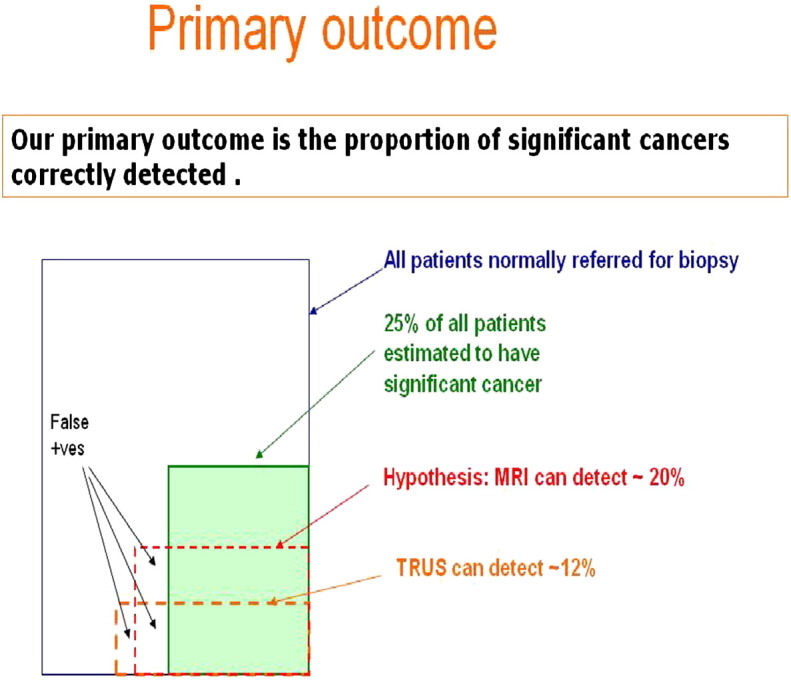
Illustration summarizing some of the assumptions made in determining sample size calculations for the primary outcome.
4.9.3. Specificity of MP-MRI
Assuming a specificity of 77%, in order to demonstrate that the lower 95% confidence interval of specificity is at least 70% or greater, we would require 407 cases of negative or clinically insignificant prostate cancer. This is equivalent to a total of 479 men for UCL definition one and 543 men for UCL definition two.
4.9.4. Sensitivity of MP-MRI
Assuming a sensitivity of 75%, in order to demonstrate that the lower 95% confidence interval of sensitivity is at least 60% or greater, we would require 97 cases of clinically significant prostate cancer. This is equivalent to a total of 647 men for UCL definition one and 388 men for UCL definition two. Since the number of men without clinically significant prostate cancer will be much higher than the number with, the precision for estimating specificity and NPV is much greater.
4.9.5. MP-MRI versus TRUS-biopsy
We have assumed that TRUS-biopsy detects 48% of clinically significant prostate cancer [28,90] and MP-MRI will detect at least 70% (conservative estimates). Using McNemar's test for paired binary observations [91], in order to show an absolute increase in the proportion of clinically significant cancers detected of at least 22% (from 48% to 70%) with a power of 90% and a 2-sided alpha of 5%, a total of 107 cases are required. This is equivalent to a total study population of 714 men for UCL definition one and 428 men for UCL definition two.
4.9.6. Cost effectiveness analyses
A model will be populated from the study as well as a review of secondary sources of epidemiological, clinical and economic evidence together with appropriately elicited expert opinion [92]. The use of probabilistic sensitivity analysis, value of information methods and scenario analysis [93] will quantify the uncertainty associated with identifying the most cost-effective diagnostic strategy, the costs of that uncertainty (in health and resource terms) and the key uncertainties to resolve with further research. This will inform the inputs into the main economic model. This cost-effectiveness model will seek to quantify the long-term implication of changes to the diagnostic classification of prostate cancer that result from adoption of alternative diagnostic pathways within the NHS. The implications will relate to the health effects (in terms of quality adjusted life expectancy) and NHS costs of a given diagnostic pathway placing patients into each of the four groups: 1. MRI test positive, clinically significant disease; 2. MRI test negative, clinically significant disease; 3. MRI test positive, clinically insignificant disease; and 4. MRI test negative, clinically insignificant disease. By altering the likelihood of a man falling into any one of these groups, the value of MP-MRI will be assessed by the changes in average outcomes experienced by men and the costs that result. The model will also include the implications of a positive result in the index test concurrent with a negative result in the current standard as well as accounting for the side effect profile of different diagnostic pathways. Structurally, the model will consist of a diagnostic element that will model the probabilities of a given patient falling into each of the diagnostic groups above, and a prognostic element that will estimate the long term implications for health and costs. The specific details of model structure will be informed by a review of existing prostate cancer models including those relating to screening, diagnosis and treatment. In general terms the modelling will adhere to the methods advocated to inform guidance by the UK National Institute for Health and Clinical Excellence [94].
We will also collect data on the costs of tests and the management of adverse events, and the health-related quality of life (HRQL) implications of any adverse events experienced with tests. The latter will be assessed using the EQ-5D instrument as part of the main clinical study. This is a widely used generic measure of HRQL which can be used to derive quality adjusted life years (QALYs) [95]. Ultimately, this work will provide an assessment of the implications of any change that the use of MP-MRI has on under-detection and over-detection. These implications will be in terms of expected quality adjusted survival duration and long-term health service costs. This will allow the value for money of MP-MRI in this context to be assessed using the same metrics employed to evaluate therapeutic technologies by organisations such as NICE.
4.10. Ethical considerations
The study abides by the principles of the Declaration of Helsinki and the UK Research Governance Framework version 2 and received UK Research Ethics Committee approval on 16th March 2011 by the NRES Committee London—Hampstead. PROMIS is published on clinical trials.gov [96]
5. Discussion and limitations
The PROMIS protocol has some potential limitations. First, the thresholds we have used for clinically significant disease are open to debate as no universally accepted definition exists. It is widely accepted that some prostate cancer lesions are clinically significant and others are not [97,99]. Volume thresholds of 0.5 ml and 1.3 ml for low grade Gleason 6 lesions have been supported by recent data from the European Prostate Cancer Screening trial [98]. There are even some calls for such lesions to be re-designated as something other than malignant, such is their indolent behaviour [97,99,100]. However, we recognize that there is legitimate professional disagreement on what constitutes clinically significant prostate cancer, so we decided to reflect this by using other disease burden thresholds to define the target condition on the reference test for the purpose of validating mpMRI.
Second, TPM biopsies may not be as accurate as whole-mount prostatectomy, but a number of studies point to its accuracy being sufficiently high to use as a reference test for the specific population we will recruit. Indeed, for men with no cancer diagnosis or for those not choosing surgery, it is the best available reference standard.
Third, the sequence of TPM biopsy first followed by TRUS-guided biopsy might compromise the standard test. However, the decision for this sequence was one primarily based around safety — inoculation of bacteria by TRUS-guided biopsy into the gland followed by numerous TPM biopsies may theoretically increase sepsis risk. We also wanted to ensure that the reference test was not compromised by swelling caused by the TRUS-guided biopsy if the latter was performed first. Further, because TRUS-guided biopsies were being performed under general/spinal anaesthetic with rectal cleansing performed using 2% chlorhexidine solution might actually be better than standard care in terms of sepsis and test accuracy.
6. Conclusion
PROMIS will determine whether introduction of MP-MRI prior to biopsy can safely allow men to avoid a biopsy and its associated harms. It will also determine whether MP-MRI can better identify men with clinically significant disease that requires a biopsy to confirm diagnosis using accurate targeting to the lesion. The evidence produced by PROMIS will aid current research interest investigating the possibility of directing biopsies only to the suspicious areas on MRI without deploying systemic biopsies.
Much research has focused on developing and validating novel imaging and tissue biomarkers for early detection of clinically significant prostate cancer. These programmes of research have used TRUS-biopsy as the reference test with any volume, grade and risk of cancer taken as a ‘positive’. PROMIS aims to overcome the problems of TRUS-biopsy as a reference test by using TPM biopsies that have a very high degree of accuracy and can be applied to all eligible men. It therefore represents an opportunity to develop and validate numerous imaging and tissue biomarkers in their performance characteristics to discriminate between men at risk who have absence of clinically significant cancer and those men who have clinically significant cancer.
Trial status
Currently PROMIS is open for recruitment across 11 centres in the United Kingdom with 3 more undergoing site setup (Appendix 1). We are on target to conclude our recruitment. Recruitment commenced in May 2012 and is expected to come to an end in October 2015.
Funding
-
National Institute of Health Research — Health Technology Assessment: Project Number 09/22/67.
Department of Health Disclaimer:
The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the health technology assessment programme, NIHR, NHS or the Department of Health.
Prostate Cancer UK: funding blood and urine collection and processing for the translational aspect of PROMIS (PROMIS-T).
Author contributions
PROMIS has been fortunate to receive input and advice from a wide range of experts in their respective fields detailed in Appendix 1.
Study concept and initial design: Parker, Emberton, Ahmed.
Study design and statistical analysis: Kaplan, Brown, Gaibe.
Acquisition of data and Data analysis and interpretation: Hindley, El-Shater Bosaily.
Acknowledgement
We acknowledge funding from the NIHR HTA and Prostate Cancer UK (PG10-17).
Prof. Mark Emberton is partially supported by core funding from the NIHR UCH/UCL Biomedical Research Centre.
Hashim Ahmed receives funding from the Medical Research Council (UK).
Footnotes
This project is also supported and partially funded by UCLH/UCL Biomedical Research Centre and The Royal Marsden and Institute for Cancer Research Biomedical Research Centre and is coordinated by the Medical Research Council Clinical Trials Unit (MRC CTU) at UCL. It is sponsored by University College London (UCL).
Appendix 1. The PROMIS Study Group
| Trial sponsor: | University College London (UCL) |
| Trial coordination: | Medical Research Council Clinical Trial Unit (MRC CTU) at UCL |
| Funders: | National Institute for Health Research (NIHR) Health Technology Assessment (HTA) & Prostate Cancer UK |
| Trial Management Group: | |
| Professor Mark Emberton (Chief Investigator) | UCLH (Urologist) |
| Mr Hashim Ahmed (Co-chief Investigator) | UCLH (Urologist) |
| Dr Ahmed El-Shater Bosaily | UCLH (Clinical Research Fellow) |
| Dr Alex Kirkham | UCLH (Radiologist) |
| Dr Alex Freeman | UCLH (Pathologist) |
| Dr Charles Jameson | UCLH (Pathologist) |
| Mr Richard Hindley | Basingstoke (Urologist) |
| Dr Christopher Parker | Royal Marsden (Translational Research) |
| Professor Colin Cooper | Royal Marsden (Translational Research) |
| Robert Oldroyd | Patient representative |
| Professor Richard Kaplan | MRC CTU |
| (Programme Lead/Oncologist) | |
| Dr Louise Brown | MRC CTU |
| (Project Lead/statistician) | |
| Dr Rhian Gabe | University of York (Statistician) |
| Dr Yolanda Collaco-Moraes | MRC CTU (Clinical Operations Manager) |
| Cybil Adusei, Katie Ward | MRC CTU (Trial Manager) |
| Sophie Stewart, Katie Thompson | |
| Claire Mulrenan, Hannah Gardner | MRC CTU (Data Managers) |
| Carlos Diaz-Montana | MRC CTU (Data programmer) |
| Dr Chris Coyle | MRC CTU (Clinical Fellow) |
| Professor Mark Sculpher | University of York (Health Economics) |
| Dr Rita Faria | University of York (Health Economics) |
| Trial Steering Committee (also act as Data Monitoring Committee): | |
| Dr David Guthrie (Chair) | Derbyshire Royal Infirmary (Oncologist) |
| Professor John Chester | University of Cardiff (Oncologist) |
| Professor Richard Cowan | Christie Hospital Manchester (Oncologist) |
| Professor Michael Jewitt | University of Toronto (Urologist) |
| Participating centres: | |
| University College London Hospital: H. Ahmed (PI), J. Coe, A. El-Shater Bosaily, M. Emberton, A. Freeman, M. Hung, C. Jameson, A. Kirkham, S. Punwani, R. Scott. | |
| Basingstoke General Hospital: Richard Hindley (PI), A. Edwards, H. El-Mahallawi, D. Peppercorn, J. Smith, A. Thrower. | |
| Imperial College London: M. Winkler (PI), K. Ansu, T. Barwick, S. Edwards, L. Honeyfield, N. Qazi, B. Statton, V. Stewart, E. Temple. | |
| Musgrove Park Hospital: N. Burns-Cox (PI), P. Burn, K. Gordon, H. Routley, A. Maccormick, D. Paterson. | |
| Maidstone and Tunbridge Wells Hospital: A. Henderson (PI), E. Bernsten, R. Casey, D. Day, S. Ghosh, J. James, P.J. McMillan, G. Russell. | |
| Southmead Hospital, Bristol: R. Persad (PI), J. Ash-Miles, M. Elmahdy, S. Pandian and C. Shiridzinomwa, M. Sohail, A. Treasure. | |
| Whittington Hospital, London: M. Ghei (PI), V. Conteh, L. Harbin, R. Katz, J. Kumaradevan, A. Trinidade, A. Verjee. | |
| Southampton General Hospital: T. Dudderidge (PI), J. Smart | |
| Sheffield University Hospitals: D. Rosario (PI), J. Catto, F. Selem | |
| Wrexham Maelor Hospital, Betsi Cadwaladr University Health Board, Wrexham, Wales: I. Shergill (PI), S. Agarwal | |
| Frimley Park Hospital: S. Bott (PI), H. Evans | |
References
- 1.Wilt T.J., Ahmed H.U. Prostate cancer screening and the management of clinically localized disease. BMJ. 2013;346:f325. doi: 10.1136/bmj.f325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cancer prevalence. http://www.cancerresearchuk.org/cancer-info/cancerstats/incidence/prevalence/
- 3.CDC data & statistics|feature: new cases of prostate cancer by race and ethnicity. http://www.cdc.gov/Features/dsProstateCancer/
- 4.Ahmed H.U., Hindley R.G., Dickinson L., Freeman A., Kirkham A.P., Sahu M. Focal therapy for localised unifocal and multifocal prostate cancer: a prospective development study. Lancet Oncol. 2012;13(6):622–632. doi: 10.1016/S1470-2045(12)70121-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Djulbegovic M., Beyth R.J., Neuberger M.M., Stoffs T.L., Vieweg J., Djulbegovic B. Screening for prostate cancer: systematic review and meta-analysis of randomised controlled trials. BMJ. 2010;341:c4543. doi: 10.1136/bmj.c4543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Andriole G.L., Crawford E.D., Grubb R.L., 3rd, Buys S.S., Chia D., Church T.R. Mortality results from a randomized prostate-cancer screening trial. N Engl J Med. 2009;360:1310–1319. doi: 10.1056/NEJMoa0810696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hugosson J., Carlsson S., Aus G., Bergdahl S., Khatami A., Lodding P. Mortality results from the Goteborg randomised population-based prostate-cancer screening trial. Lancet Oncol. 2010;11:725–732. doi: 10.1016/S1470-2045(10)70146-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schroder F.H., Hugosson J., Roobol M.J., Tammela T.L., Ciatto S., Nelen V. Prostate-cancer mortality at 11 years of follow-up. N Engl J Med. 2012;366:981–990. doi: 10.1056/NEJMoa1113135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schroder F.H., Hugosson J., Roobol M.J., Tammela T.L., Ciatto S., Nelen V. Screening and prostate-cancer mortality in a randomized European study. N Engl J Med. 2009;360:1320–1328. doi: 10.1056/NEJMoa0810084. [DOI] [PubMed] [Google Scholar]
- 10.Heijnsdijk E.A., Wever E.M., Auvinen A., Hugosson J., Ciatto S., Nelen V. Quality-of-life effects of prostate-specific antigen screening. N Engl J Med. 2012;367(7):595–605. doi: 10.1056/NEJMoa1201637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wilt T.J. The Prostate Cancer Intervention Versus Observation Trial: VA/NCI/AHRQ Cooperative Studies Program #407 (PIVOT): design and baseline results of a randomized controlled trial comparing radical prostatectomy with watchful waiting for men with clinically localized prostate cancer. JNCI Monogr. 2012;2012:184–190. doi: 10.1093/jncimonographs/lgs041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barry M.J. Screening for prostate cancer — the controversy that refuses to die. N Engl J Med. 2009;360:1351–1354. doi: 10.1056/NEJMe0901166. [DOI] [PubMed] [Google Scholar]
- 13.Parker C., Emberton M. Screening for prostate cancer appears to work, but at what cost? BJU Int. 2009;104:290–292. doi: 10.1111/j.1464-410X.2009.08689.x. [DOI] [PubMed] [Google Scholar]
- 14.Bill-Axelson A., Holmberg L., Garmo H., Rider J.R., Taari K., Busch C. Radical prostatectomy or watchful waiting in early prostate cancer. N Engl J Med. 2014;370:932–942. doi: 10.1056/NEJMoa1311593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wilt T.J., Ahmed H.U. Prostate cancer screening and the management of clinically localized disease. BMJ. 2013;346 doi: 10.1136/bmj.f325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.A report to the National Collaborating Centre for Cancer. 2008. Cross T MPS: Clinical Guideline Prostate Cancer: diagnosis and treatment. An assessment of need. [PubMed] [Google Scholar]
- 17.Bangma C.H., Roemeling S., Schroder F.H. Overdiagnosis and overtreatment of early detected prostate cancer. World J Urol. 2007;25:3–9. doi: 10.1007/s00345-007-0145-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thompson I.M., Goodman P.J., Tangen C.M., Lucia M.S., Miller G.J., Ford L.G. The influence of finasteride on the development of prostate cancer. N Engl J Med. 2003;349:215–224. doi: 10.1056/NEJMoa030660. [DOI] [PubMed] [Google Scholar]
- 19.Djavan B., Ravery V., Zlotta A., Dobronski P., Dobrovits M., Fakhari M. Prospective evaluation of prostate cancer detected on biopsies 1, 2, 3 and 4: when should we stop? J Urol. 2001;166:1679–1683. [PubMed] [Google Scholar]
- 20.Scattoni V., Zlotta A., Montironi R., Schulman C., Rigatti P., Montorsi F. Extended and saturation prostatic biopsy in the diagnosis and characterisation of prostate cancer: a critical analysis of the literature. Eur Urol. 2007;52:1309–1322. doi: 10.1016/j.eururo.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 21.Lecornet E., Ahmed H.U., Hu Y., Moore C.M., Nevoux P., Barratt D. The accuracy of different biopsy strategies for the detection of clinically important prostate cancer: a computer simulation. J Urol. 2012;188:974–980. doi: 10.1016/j.juro.2012.04.104. [DOI] [PubMed] [Google Scholar]
- 22.Barzell W.E., Melamed M.R., Cathcart P., Moore C.M., Ahmed H.U., Emberton M. Identifying candidates for active surveillance: an evaluation of the repeat biopsy strategy for men with favorable risk prostate cancer. J Urol. 2012;188:762–768. doi: 10.1016/j.juro.2012.04.107. [DOI] [PubMed] [Google Scholar]
- 23.Onik G., Miessau M., Bostwick D.G. Three-dimensional prostate mapping biopsy has a potentially significant impact on prostate cancer management. J Clin Oncol. 2009;27:4321–4326. doi: 10.1200/JCO.2008.20.3497. [DOI] [PubMed] [Google Scholar]
- 24.Crawford E.D., Wilson S.S., Torkko K.C., Hirano D., Stewart J.S., Brammell C. Clinical staging of prostate cancer: a computer-simulated study of transperineal prostate biopsy. BJU Int. 2005;96:999–1004. doi: 10.1111/j.1464-410X.2005.05801.x. [DOI] [PubMed] [Google Scholar]
- 25.Kulkarni G.S., Al-Azab R., Lockwood G., Toi A., Evans A., Trachtenberg J. Evidence for a biopsy derived grade artifact among larger prostate glands. J Urol. 2006;175:505–509. doi: 10.1016/S0022-5347(05)00236-3. [DOI] [PubMed] [Google Scholar]
- 26.Barzell W.E., Melamed M.R. Appropriate patient selection in the focal treatment of prostate cancer: the role of transperineal 3-dimensional pathologic mapping of the prostate—a 4-year experience. Urology. 2007;70:S27–S35. doi: 10.1016/j.urology.2007.06.1126. [DOI] [PubMed] [Google Scholar]
- 27.Onik G., Barzell W. Transperineal 3D mapping biopsy of the prostate: an essential tool in selecting patients for focal prostate cancer therapy. Urol Oncol. 2008;26:506–510. doi: 10.1016/j.urolonc.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 28.Taira A.V., Merrick G.S., Galbreath R.W., Andreini H., Taubenslag W., Curtis R. Performance of transperineal template-guided mapping biopsy in detecting prostate cancer in the initial and repeat biopsy setting. Prostate Cancer Prostatic Dis. 2010;13:71–77. doi: 10.1038/pcan.2009.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Loeb S., Vellekoop A., Ahmed H.U., Catto J., Emberton M., Nam R. Systematic review of complications of prostate biopsy. Eur Urol. 2013;64:876–892. doi: 10.1016/j.eururo.2013.05.049. [DOI] [PubMed] [Google Scholar]
- 30.Batura D., Gopal Rao G. The national burden of infections after prostate biopsy in England and Wales: a wake-up call for better prevention. J Antimicrob Chemother. 2013;68:247–249. doi: 10.1093/jac/dks401. [DOI] [PubMed] [Google Scholar]
- 31.Abdelkhalek M.A., Abdelshafy M., Elhelaly H.A., El Nasr M.K. Hemospermia after transrectal ultrasound (TRUS)-guided prostatic biopsy: a prospective study. J Egypt Soc Parasitol. 2012;42:63–70. doi: 10.12816/0006295. [DOI] [PubMed] [Google Scholar]
- 32.Pepe P., Aragona F. Morbidity after transperineal prostate biopsy in 3000 patients undergoing 12 vs 18 vs more than 24 needle cores. Urology. 2013;81:1142–1146. doi: 10.1016/j.urology.2013.02.019. [DOI] [PubMed] [Google Scholar]
- 33.Bossuyt P.M., Irwig L., Craig J., Glasziou P. Comparative accuracy: assessing new tests against existing diagnostic pathways. BMJ. 2006;332:1089–1092. doi: 10.1136/bmj.332.7549.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.NCC-C . Feb 2008. NIfHaCENNCCfC: prostate cancer: diagnosis and treatment. [Google Scholar]
- 35.Kelloff G.J., Choyke P., Coffey D.S. Challenges in clinical prostate cancer: role of imaging. AJR Am J Roentgenol. 2009;192:1455–1470. doi: 10.2214/AJR.09.2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ahmed H.U., Kirkham A., Arya M., Illing R., Freeman A., Allen C. Is it time to consider a role for MRI before prostate biopsy? Nat Rev Clin Oncol. 2009;6:197–206. doi: 10.1038/nrclinonc.2009.18. [DOI] [PubMed] [Google Scholar]
- 37.Kurhanewicz J., Vigneron D., Carroll P., Coakley F. Multiparametric magnetic resonance imaging in prostate cancer: present and future. Curr Opin Urol. 2008;18:71–77. doi: 10.1097/MOU.0b013e3282f19d01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kirkham A.P., Emberton M., Allen C. How good is MRI at detecting and characterising cancer within the prostate? Eur Urol. 2006;50:1163–1174. doi: 10.1016/j.eururo.2006.06.025. [discussion 1175] [DOI] [PubMed] [Google Scholar]
- 39.Bernstein M.R., Cangiano T., D'Amico A., Chittams J., Hardy C., Whittington R.D. Endorectal coil magnetic resonance imaging and clinicopathologic findings in T1c adenocarcinoma of the prostate. Urol Oncol. 2000;5:104–107. doi: 10.1016/s1078-1439(99)00049-6. [DOI] [PubMed] [Google Scholar]
- 40.Cornud F., Flam T., Chauveinc L., Hamida K., Chretien Y., Vieillefond A. Extraprostatic spread of clinically localized prostate cancer: factors predictive of pT3 tumor and of positive endorectal MR imaging examination results. Radiology. 2002;224:203–210. doi: 10.1148/radiol.2241011001. [DOI] [PubMed] [Google Scholar]
- 41.May F., Treumann T., Dettmar P., Hartung R., Breul J. Limited value of endorectal magnetic resonance imaging and transrectal ultrasonography in the staging of clinically localized prostate cancer. BJU Int. 2001;87:66–69. doi: 10.1046/j.1464-410x.2001.00018.x. [DOI] [PubMed] [Google Scholar]
- 42.Outwater E.K., Petersen R.O., Siegelman E.S., Gomella L.G., Chernesky C.E., Mitchell D.G. Prostate carcinoma: assessment of diagnostic criteria for capsular penetration on endorectal coil MR images. Radiology. 1994;193:333–339. doi: 10.1148/radiology.193.2.7972739. [DOI] [PubMed] [Google Scholar]
- 43.Rifkin M.D., Zerhouni E.A., Gatsonis C.A., Quint L.E., Paushter D.M., Epstein J.I. Comparison of magnetic resonance imaging and ultrasonography in staging early prostate cancer. Results of a multi-institutional cooperative trial. N Engl J Med. 1990;323:621–626. doi: 10.1056/NEJM199009063231001. [DOI] [PubMed] [Google Scholar]
- 44.Schiebler M.L., Yankaskas B.C., Tempany C., Spritzer C.E., Rifkin M.D., Pollack H.M. MR imaging in adenocarcinoma of the prostate: interobserver variation and efficacy for determining stage C disease. AJR Am J Roentgenol. 1992;158:559–562. doi: 10.2214/ajr.158.3.1738994. [discussion 563–554] [DOI] [PubMed] [Google Scholar]
- 45.Schnall M.D., Pollack H.M. Magnetic resonance imaging of the prostate gland. Urol Radiol. 1990;12:109–114. doi: 10.1007/BF02923982. [DOI] [PubMed] [Google Scholar]
- 46.Chen M., Dang H.D., Wang J.Y., Zhou C., Li S.Y., Wang W.C. Prostate cancer detection: comparison of T2-weighted imaging, diffusion-weighted imaging, proton magnetic resonance spectroscopic imaging, and the three techniques combined. Acta Radiol. 2008;49:602–610. doi: 10.1080/02841850802004983. [DOI] [PubMed] [Google Scholar]
- 47.Futterer J.J., Heijmink S.W., Scheenen T.W., Veltman J., Huisman H.J., Vos P. Prostate cancer localization with dynamic contrast-enhanced MR imaging and proton MR spectroscopic imaging. Radiology. 2006;241:449–458. doi: 10.1148/radiol.2412051866. [DOI] [PubMed] [Google Scholar]
- 48.Kozlowski P., Chang S.D., Jones E.C., Berean K.W., Chen H., Goldenberg S.L. Combined diffusion-weighted and dynamic contrast-enhanced MRI for prostate cancer diagnosis—correlation with biopsy and histopathology. J Magn Reson Imaging. 2006;24:108–113. doi: 10.1002/jmri.20626. [DOI] [PubMed] [Google Scholar]
- 49.Kumar V., Jagannathan N.R., Kumar R., Das S.C., Jindal L., Thulkar S. Correlation between metabolite ratios and ADC values of prostate in men with increased PSA level. Magn Reson Imaging. 2006;24:541–548. doi: 10.1016/j.mri.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 50.Macura K.J. Multiparametric magnetic resonance imaging of the prostate: current status in prostate cancer detection, localization, and staging. Semin Roentgenol. 2008;43:303–313. doi: 10.1053/j.ro.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 51.Mazaheri Y., Shukla-Dave A., Hricak H., Fine S.W., Zhang J., Inurrigarro G. Prostate cancer: identification with combined diffusion-weighted MR imaging and 3D 1H MR spectroscopic imaging—correlation with pathologic findings. Radiology. 2008;246:480–488. doi: 10.1148/radiol.2462070368. [DOI] [PubMed] [Google Scholar]
- 52.Noworolski S.M., Henry R.G., Vigneron D.B., Kurhanewicz J. Dynamic contrast-enhanced MRI in normal and abnormal prostate tissues as defined by biopsy, MRI, and 3D MRSI. Magn Reson Med. 2005;53:249–255. doi: 10.1002/mrm.20374. [DOI] [PubMed] [Google Scholar]
- 53.Reinsberg S.A., Payne G.S., Riches S.F., Ashley S., Brewster J.M., Morgan V.A. Combined use of diffusion-weighted MRI and 1H MR spectroscopy to increase accuracy in prostate cancer detection. AJR Am J Roentgenol. 2007;188:91–98. doi: 10.2214/AJR.05.2198. [DOI] [PubMed] [Google Scholar]
- 54.Tanimoto A., Nakashima J., Kohno H., Shinmoto H., Kuribayashi S. Prostate cancer screening: the clinical value of diffusion-weighted imaging and dynamic MR imaging in combination with T2-weighted imaging. J Magn Reson Imaging. 2007;25:146–152. doi: 10.1002/jmri.20793. [DOI] [PubMed] [Google Scholar]
- 55.van Dorsten F.A., van der Graaf M., Engelbrecht M.R., van Leenders G.J., Verhofstad A., Rijpkema M. Combined quantitative dynamic contrast-enhanced MR imaging and (1)H MR spectroscopic imaging of human prostate cancer. J Magn Reson Imaging. 2004;20:279–287. doi: 10.1002/jmri.20113. [DOI] [PubMed] [Google Scholar]
- 56.Yoshimitsu K., Kiyoshima K., Irie H., Tajima T., Asayama Y., Hirakawa M. Usefulness of apparent diffusion coefficient map in diagnosing prostate carcinoma: correlation with stepwise histopathology. J Magn Reson Imaging. 2008;27:132–139. doi: 10.1002/jmri.21181. [DOI] [PubMed] [Google Scholar]
- 57.Ahmed H.U., Calleary J., Arya M., Emberton M., Illing R.O., Allen C. Re: Dynamic contrast enhanced, pelvic phased array magnetic resonance imaging of localized prostate cancer for predicting tumor volume: correlation with radical prostatectomy findings. A. Villers, P. Puech, D. Mouton, X. Leroy, C. Ballereau and L. Lemaitre. J Urol. 2006;176:2432–2437. doi: 10.1016/j.juro.2006.08.007. [J Urol 2007, 177:2395; author reply 2395–2396] [DOI] [PubMed] [Google Scholar]
- 58.Harlan S.R., Cooperberg M.R., Elkin E., Lubeck D.P., Meng M., Mehta S.S. Time trends and characteristics of men choosing watchful waiting for initial treatment of localized prostate cancer: results from CaPSURE. J Urol. 2003;170:1804–1807. doi: 10.1097/01.ju.0000091641.34674.11. [DOI] [PubMed] [Google Scholar]
- 59.Turkbey B., Pinto P.A., Mani H., Bernardo M., Pang Y., McKinney Y.L. Prostate cancer: value of multiparametric MR imaging at 3 T for detection—histopathologic correlation 1. Radiology. 2010;255:89–99. doi: 10.1148/radiol.09090475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Villers A., Puech P., Mouton D., Leroy X., Ballereau C., Lemaitre L. Dynamic contrast enhanced, pelvic phased array magnetic resonance imaging of localized prostate cancer for predicting tumor volume: correlation with radical prostatectomy findings. J Urol. 2006;176:2432–2437. doi: 10.1016/j.juro.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 61.2013. Oxford CfebMUo: levels of evidence 1. [Google Scholar]
- 62.Puech P., Huglo D., Petyt G., Lemaitre L., Villers A. Imaging of organ-confined prostate cancer: functional ultrasound, MRI and PET/computed tomography. Curr Opin Urol. 2009;19:168–176. doi: 10.1097/MOU.0b013e328323f5ed. [DOI] [PubMed] [Google Scholar]
- 63.Puech P., Potiron E., Lemaitre L., Leroy X., Haber G.-P., Crouzet S. Dynamic contrast-enhanced-magnetic resonance imaging evaluation of intraprostatic prostate cancer: correlation with radical prostatectomy specimens. Urology. 2009;74:1094–1099. doi: 10.1016/j.urology.2009.04.102. [DOI] [PubMed] [Google Scholar]
- 64.Lemaitre L., Puech P., Poncelet E., Bouye S., Leroy X., Biserte J. Dynamic contrast-enhanced MRI of anterior prostate cancer: morphometric assessment and correlation with radical prostatectomy findings. Eur Radiol. 2009;19:470–480. doi: 10.1007/s00330-008-1153-0. [DOI] [PubMed] [Google Scholar]
- 65.Mazaheri Y., Hricak H., Fine S.W., Akin O., Shukla-Dave A., Ishill N.M. Prostate tumor volume measurement with combined T2-weighted imaging and diffusion-weighted MR: correlation with pathologic tumor volume. Radiology. 2009;252:449–457. doi: 10.1148/radiol.2523081423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Heijmink S.W., Futterer J.J., Hambrock T., Takahashi S., Scheenen T.W., Huisman H.J. Prostate cancer: body-array versus endorectal coil MR imaging at 3 T—comparison of image quality, localization, and staging performance. Radiology. 2007;244:184–195. doi: 10.1148/radiol.2441060425. [DOI] [PubMed] [Google Scholar]
- 67.Louise D., Hashim U.A., Clare A., Jelle O.B., Brendan C., Jurgen J.F. Magnetic resonance imaging for the detection, localisation, and characterisation of prostate cancer: recommendations from a European consensus meeting. Eur Urol. 2010;59:477–494. doi: 10.1016/j.eururo.2010.12.009. [DOI] [PubMed] [Google Scholar]
- 68.Barentsz J.O., Richenberg J., Clements R., Choyke P., Verma S., Villeirs G. ESUR prostate MR guidelines 2012. Eur Radiol. 2012;22:746–757. doi: 10.1007/s00330-011-2377-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kirkham A., Haslam P., Keanie J., McCafferty I., Padhani A., Punwani S. Prostate MRI: who, when, and how? Report from a UK consensus meeting. Clin Radiol. 2013;68:1016–1023. doi: 10.1016/j.crad.2013.03.030. [DOI] [PubMed] [Google Scholar]
- 70.Weinreb J.C., Blume J.D., Coakley F.V., Wheeler T.M., Cormack J.B., Sotto C.K. Prostate cancer: sextant localization at MR imaging and MR spectroscopic imaging before prostatectomy—results of ACRIN Prospective Multi-institutional Clinicopathologic Study 1. Radiology. 2009;251:122–133. doi: 10.1148/radiol.2511080409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dickinson L., Ahmed H.U., Allen C., Barentsz J.O., Carey B., Futterer J.J. Scoring systems used for the interpretation and reporting of multiparametric MRI for prostate cancer detection, localization, and characterization: could standardization lead to improved utilization of imaging within the diagnostic pathway? J Magn Reson Imaging. 2013;37:48–58. doi: 10.1002/jmri.23689. [DOI] [PubMed] [Google Scholar]
- 72.Li B., Du Y., Yang H., Huang Y., Meng J., Xiao D. Magnetic resonance imaging for prostate cancer clinical application. Chin J Cancer Res. 2013;25:240–249. doi: 10.3978/j.issn.1000-9604.2013.03.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Franiel T., Hamm B., Hricak H. Dynamic contrast-enhanced magnetic resonance imaging and pharmacokinetic models in prostate cancer. Eur Radiol. 2011;21:616–626. doi: 10.1007/s00330-010-2037-7. [DOI] [PubMed] [Google Scholar]
- 74.Ocak I., Bernardo M., Metzger G., Barrett T., Pinto P., Albert P.S. Dynamic contrast-enhanced MRI of prostate cancer at 3 T: a study of pharmacokinetic parameters. AJR Am J Roentgenol. 2007;189:849. doi: 10.2214/AJR.06.1329. [DOI] [PubMed] [Google Scholar]
- 75.Ito H., Kamoi K., Yokoyama K., Yamada K., Nishimura T. Visualization of prostate cancer using dynamic contrast-enhanced MRI: comparison with transrectal power Doppler ultrasound. Br J Radiol. 2003;76:617–624. doi: 10.1259/bjr/52526261. [DOI] [PubMed] [Google Scholar]
- 76.Heidenreich A., Bellmunt J., Bolla M., Joniau S., Mason M., Matveev V. EAU guidelines on prostate cancer. Part 1: screening, diagnosis, and treatment of clinically localised disease. Eur Urol. 2011;59:61–71. doi: 10.1016/j.eururo.2010.10.039. [DOI] [PubMed] [Google Scholar]
- 77.Li H., Yan W., Zhou Y., Ji Z., Chen J. Transperineal ultrasound-guided saturation biopsies using 11-region template of prostate: report of 303 cases. Urology. 2007;70:1157–1161. doi: 10.1016/j.urology.2007.07.072. [DOI] [PubMed] [Google Scholar]
- 78.Merrick G.S., Taubenslag W., Andreini H., Brammer S., Butler W.M., Adamovich E. The morbidity of transperineal template-guided prostate mapping biopsy. BJU Int. 2008;101:1524–1529. doi: 10.1111/j.1464-410X.2008.07542.x. [DOI] [PubMed] [Google Scholar]
- 79.Bott S.R., Henderson A., Halls J.E., Montgomery B.S., Laing R., Langley S.E. Extensive transperineal template biopsies of prostate: modified technique and results. Urology. 2006;68:1037–1041. doi: 10.1016/j.urology.2006.05.033. [DOI] [PubMed] [Google Scholar]
- 80.Furuno T., Demura T., Kaneta T., Gotoda H., Muraoka S., Sato T. Difference of cancer core distribution between first and repeat biopsy: in patients diagnosed by extensive transperineal ultrasound guided template prostate biopsy. Prostate. 2004;58:76–81. doi: 10.1002/pros.10298. [DOI] [PubMed] [Google Scholar]
- 81.Buskirk S.J., Pinkstaff D.M., Petrou S.P., Wehle M.J., Broderick G.A., Young P.R. Acute urinary retention after transperineal template-guided prostate biopsy. Int J Radiat Oncol Biol Phys. 2004;59:1360–1366. doi: 10.1016/j.ijrobp.2004.01.045. [DOI] [PubMed] [Google Scholar]
- 82.Miller J., Perumalla C., Heap G. Complications of transrectal versus transperineal prostate biopsy. ANZ J Surg. 2005;75:48–50. doi: 10.1111/j.1445-2197.2005.03284.x. [DOI] [PubMed] [Google Scholar]
- 83.Crawford E.D., Rove K.O., Barqawi A.B., Maroni P.D., Werahera P.N., Baer C.A. Clinical-pathologic correlation between transperineal mapping biopsies of the prostate and three-dimensional reconstruction of prostatectomy specimens. Prostate. 2013;73:778–787. doi: 10.1002/pros.22622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Epstein J.I., Walsh P.C., Carmichael M., Brendler C.B. Pathologic and clinical findings to predict tumor extent of nonpalpable (stage t1 c) prostate cancer. JAMA. 1994;271:368–374. [PubMed] [Google Scholar]
- 85.Taira A.V., Merrick G.S., Bennett A., Andreini H., Taubenslag W., Galbreath R.W. Transperineal template-guided mapping biopsy as a staging procedure to select patients best suited for active surveillance. Am J Clin Oncol. 2013;36:116–120. doi: 10.1097/COC.0b013e31823fe639. [DOI] [PubMed] [Google Scholar]
- 86.Barzell W. 2010. Personal communication. [Google Scholar]
- 87.Dall'Era M.A., Cooperberg M.R., Chan J.M., Davies B.J., Albertsen P.C., Klotz L.H. Active surveillance for early‐stage prostate cancer. Cancer. 2008;112:1650–1659. doi: 10.1002/cncr.23373. [DOI] [PubMed] [Google Scholar]
- 88.Lim H.K., Kim J.K., Kim K.A., Cho K.S. Prostate cancer: apparent diffusion coefficient map with T2-weighted images for detection—a multireader study. Radiology. 2009;250:145–151. doi: 10.1148/radiol.2501080207. [DOI] [PubMed] [Google Scholar]
- 89.Turkbey B., Thomasson D., Pang Y., Bernardo M., Choyke P.L. The role of dynamic contrast-enhanced MRI in cancer diagnosis and treatment. Diagn Interv Radiol. 2010;16:186–192. doi: 10.4261/1305-3825.DIR.2537-08.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Eichler K., Hempel S., Wilby J., Myers L., Bachmann L.M., Kleijnen J. Diagnostic value of systematic biopsy methods in the investigation of prostate cancer: a systematic review. J Urol. 2006;175:1605–1612. doi: 10.1016/S0022-5347(05)00957-2. [DOI] [PubMed] [Google Scholar]
- 91.Machin D., Campbell M.J., Tan S.B., Tan S.H. Sample size tables for clinical studies. Wiley-Blackwell; 2009. Basic design considerations; pp. 1–13. [Google Scholar]
- 92.O'Hagan A.B.C., Daneshkhah A., Eiser J.R., Garthwaite P.H., Jenkinson D.J., Oakley J.E. John Wiley and Sons; 2006. Uncertain judgments: eliciting expert probabilities. [Google Scholar]
- 93.Andrew Briggs M.S. 1st ed. Oxford University Press; August 17 2006. Karl Claxton decision modelling for health economic evaluation. [Google Scholar]
- 94.Guide to methods of technology appraisal. http://www.nice.org.uk/media/b52/a7/tamethodsguideupdatedjune2008.pdf
- 95.Kind P SB: The EuroQoL instrument: an index of health-related quality of life. Quality of life and pharmacoeconomics in clinical trials. Philadelphia: Lippincott-Raven.
- 96.MRI in diagnosing prostate cancer – full text View – ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT01292291
- 97.Carter H.B., Partin A.W., Walsh P.C., Trock B.J., Veltri R.W., Nelson W.G. Gleason score 6 adenocarcinoma: should it be labeled as cancer? J Clin Oncol. 2012;30:4294–4296. doi: 10.1200/JCO.2012.44.0586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wolters T., Roobol M.J., van Leeuwen P.J., van den Bergh R.C.N., Hoedemaeker R.F., van Leenders G.J.L.H. A critical analysis of the tumor volume threshold for clinically insignificant prostate cancer using a data set of a randomized screening trial. J Urol. 2011;185:121–125. doi: 10.1016/j.juro.2010.08.082. [DOI] [PubMed] [Google Scholar]
- 99.Ahmed H.U., Arya M., Freeman A., Emberton M. Do low-grade and low-volume prostate cancers bear the hallmarks of malignancy? Lancet Oncol. 2012;13:e509–e517. doi: 10.1016/S1470-2045(12)70388-1. [DOI] [PubMed] [Google Scholar]
- 100.Esserman L.J., Thompson I.M., Reid B. Overdiagnosis and overtreatment in cancer: an opportunity for improvement. JAMA. 2013;310:797–798. doi: 10.1001/jama.2013.108415. [DOI] [PubMed] [Google Scholar]