Abstract
Background
Poor growth and nutritional status are common in children with chronic diseases. Oral protein calorie supplements are used to improve nutritional status in these children. These expensive products may be associated with some adverse effects, e.g. the development of inappropriate eating behaviour patterns. This is a new update of a Cochrane review last updated in 2009.
Objectives
To examine evidence that in children with chronic disease, oral protein calorie supplements alter daily nutrient intake, nutritional indices, survival and quality of life and are associated with adverse effects, e.g. diarrhoea, vomiting, reduced appetite, glucose intolerance, bloating and eating behaviour problems.
Search methods
Trials of oral protein calorie supplements in children with chronic diseases were identified through comprehensive electronic database searches, handsearching relevant journals and abstract books of conference proceedings. Companies marketing these products were also contacted.
Most recent search of the Group's Trials Register: 24 February 2015.
Selection criteria
Randomised or quasi‐randomised controlled trials comparing oral protein calorie supplements for at least one month to increase calorie intake with existing conventional therapy (including advice on improving nutritional intake from food or no specific intervention) in children with chronic disease.
Data collection and analysis
We independently assessed the outcomes: indices of nutrition and growth; anthropometric measures of body composition; calorie and nutrient intake (total from oral protein calorie supplements and food); eating behaviour; compliance; quality of life; specific adverse effects; disease severity scores; and mortality; we also assessed the risk of bias in the included trials.
Main results
Four studies (187 children) met the inclusion criteria. Three studies were carried out in children with cystic fibrosis and one study included children with paediatric malignant disease. Overall there was a low risk of bias for blinding and incomplete outcome data.Two studies had a high risk of bias for allocation concealment. Few statistical differences were found in the outcomes we assessed between treatment and control groups, except change in total energy intake at six and 12 months, mean difference 304.86 kcal per day (95% confidence interval 5.62 to 604.10) and mean difference 265.70 kcal per day (95% confidence interval 42.94 to 485.46), respectively. However, these were based on the analysis of just 58 children in only one study. Only two chronic diseases were included in these analyses, cystic fibrosis and paediatric malignant disease. No other studies were identified which assessed the effectiveness of oral protein calorie supplements in children with other chronic diseases.
Authors' conclusions
Oral protein calorie supplements are widely used to improve the nutritional status of children with a number of chronic diseases. We identified a small number of studies assessing these products in children with cystic fibrosis and paediatric malignant disease, but were unable to draw any conclusions based on the limited data extracted. We recommend a series of large, randomised controlled trials be undertaken investigating the use of these products in children with different chronic diseases. Until further data are available, we suggest these products are used with caution.
Keywords: Adolescent; Child; Child, Preschool; Humans; Infant; Energy Intake; Nutritional Status; Chronic Disease; Cystic Fibrosis; Cystic Fibrosis/complications; Dietary Proteins; Dietary Proteins/administration & dosage; Dietary Proteins/adverse effects; Dietary Supplements; Dietary Supplements/adverse effects; Neoplasms; Neoplasms/complications; Nutrition Disorders; Nutrition Disorders/etiology; Nutrition Disorders/therapy; Outcome Assessment, Health Care; Quality of Life; Randomized Controlled Trials as Topic; Survival Analysis
Plain language summary
The use of oral protein calorie supplements in children with chronic disease
Background
A lack of growth and poor nutrition are common in children with chronic diseases like cystic fibrosis and paediatric cancer. This may be due to reduced appetite, poor absorption and the need for extra calories due to the disease. Oral protein calorie supplements, either as milk or juices, may improve nutritional status and help children gain weight. Side effects of taking these supplements include the risk that the protein and calories in the supplement end up replacing those from normal food and have a negative effect on eating behaviour and physical side effects (e.g. bloating, vomiting and diarrhoea).
Search date
The evidence is current to: 24 February 2015.
Study characteristics
We looked for trials of oral protein calorie supplements compared to usual treatment or no alternative treatment where the children took the supplements for at least one month. The review included four trials with 187 children; in three of these the children had cystic fibrosis and in one they had cancer. Studies lasted from three months to one year. We recorded the results and judged whether the trials were at risk of being biased based on the design or the way it was run. We looked at outcomes such as weight and height, calorie intake, behaviour and also side effects.
Key results
One study (with 58 children) showed increases in the total calories consumed at both six and 12 months. None of the other outcomes we looked at showed any difference between treatments. This is an updated version of the review, which found no conclusive evidence to support the use of oral protein supplements. We suggest that at least one high quality trial be conducted.Therefore, we suggest that these products are used sparingly and with caution.
Quality of the evidence
Overall the included studies had a low risk of bias, except for two studies in which it was possible that the organisers knew which treatment group in which the children would be placed. These issues are unlikely to change the results as knowing which treatment one receives is unlikely to affect the results of body measurements (e.g. weight, height outcomes). All planned outcomes were reported on, with the exception of one study that did not report on eating behaviour and lipase intake which were measured. The quality of the results for the eating behaviour assessment was questionable and many of the children did not return the food diaries from which the lipase intake could be calculated.
Summary of findings
Summary of findings for the main comparison. Oral protein calorie supplements compared with no intervention, placebo or additional nutritional advice compared to placebo for children with chronic disease.
| Oral protein calorie supplements compared with no intervention, placebo or additional nutritional advice compared to placebo for children with chronic disease | ||||||
| Patient or population: children with chronic disease Settings: Intervention: oral protein calorie supplements Comparison: no intervention, placebo or additional nutritional advice | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control (no intervention, placebo or additional nutritional advice) | Oral protein calorie supplements | |||||
| Change in weight in kg at 6 months | The mean change in weight in kg at 6 months ranged across control groups from 1.33 to 6.55 kg | The mean change in weight in kg at 6 months in the intervention group was 0.42 higher (0.12 lower to 0.96 higher) | ‐ | 125 (3 RCTs) | ⊕⊕⊕ MODERATE | |
| Change in weight Z score at 6 months | The mean change in weight Z score at 6 months ranged across control groups from 0.06 to 0.83 kg | The mean change in weight Z score at 6 months in the intervention group was 0.03 higher (0.1 lower to 0.16 higher) | ‐ | 109 (2 RCTs) | ⊕⊕⊕⊕ HIGH | |
| Change in height in cm at 6 months | The mean change in height in cm at 6 months ranged across control groups from 3.46 to 6.58 cm | The mean change in height in cm at 6 months in the intervention group was 0.4 lower (1.23 lower to 0.42 higher) | ‐ | 109 (2 RCTs) | ⊕⊕⊕⊕ HIGH | |
| Change in BMI at 6 months | The mean change in BMI at 6 months ranged across control groups from 0.14 to 1.12 kg/m2 | The mean change in BMI at 6 months in the intervention group was 0.18 higher (0.12 lower to 0.47 higher) | ‐ | 109 (2 RCTs) | ⊕⊕⊕⊕ HIGH | |
| Change in total energy intake (kcal/day) at 3 months | The mean change in total energy intake (kcal/day) at 3 months ranged across control groups from ‐56.75 to 190.54 kcal | The mean change in total energy intake (kcal/day) at 3 months in the intervention group was 133.43 higher (102.94 lower to 369.79 higher) | ‐ | 53 (2 RCTs) | ⊕⊕⊕⊕ HIGH | |
| Change in total protein intake (g/day) at 3 months | The mean change in total protein intake (g/day) at 3 months ranged across control groups from ‐1.95 to 7.87 g | The mean change in total protein intake (g/day) at 3 months in the intervention group was 3.45 higher (5.87 lower to 12.76 higher) | ‐ | 53 (2 RCTs) | ⊕⊕⊕⊕ HIGH | |
| Disease severity score: change in FEV1 % predicted at 12 months | The mean disease severity score: change in FEV1 % predicted at 12 months in the control group was ‐1.5 % | The mean disease severity score: change in FEV1 % predicted at 12 months in the intervention group was 4.91 higher (1.75 lower to 11.57 higher) | ‐ | 70 (1 RCT) | ⊕⊕⊕⊕ HIGH | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
BMI: body mass index CI: confidence interval FEV1: forced expiratory volume at one second RCT: randomised controlled trial
Background
Description of the condition
Inadequate gains in growth, due to poor nutritional intake, increased nutrient requirements during illness, and reduced appetite are frequently observed features in children with chronic diseases. This has been demonstrated in respiratory disease, chronic renal failure, neuromuscular disease and juvenile chronic arthritis (Hanning 1993; Johansson 1986). Studies also show a prevalence of both acute and chronic malnutrition of children in hospital of between 6% to over 30% in both developed and developing countries globally (Hendricks 1995; Hendrickse 1997; Moy 1990; Pawellek 2008; Joosten 2010; Spagnuolo 2013). Growth failure and poor nutritional status in chronic disease are multi‐factorial in origin. Contributing factors include reduced dietary intake as a result of reduced appetite, malabsorption and increased nutritional requirements associated with some diseases. Poor nutritional status and sub‐optimal growth can have a detrimental effect on both short‐ and long‐term disease outcomes (Corey 1988; CPS 1994; Koscik 2004).
Description of the intervention
Prevention or correction of malnutrition is increasingly recognised as an important component of the management of chronic childhood disease. Several interventions are available to increase nutritional intake thereby improving nutritional status. These include dietetic advice to increase the nutritional content of the child's diet; provision of oral protein calorie supplements; and provision of additional nutrition in the form of tube feeding.
How the intervention might work
Oral protein calorie supplements in the form of either whole protein milk or juice drinks are used to increase the total daily protein and calorie intake in order to improve weight gain and nutritional status. These supplements also contain a range of micronutrients (vitamins and minerals) which may be of benefit to the malnourished child. Supplements which provide only calories with no additional nutrients are also available, however these are infrequently recommended for children with malnutrition as these children require supplementation of protein and other nutrients in addition to calories, rather than calories alone. Provided protein calorie supplements are taken in addition to normal dietary intake from food, then overall nutritional intake should be improved. However, it is possible that these products may replace some of the protein and calories taken as food and their potential effect on overall protein and calorie intake either reduced or eliminated. A further potential adverse consequence of replacing protein and calorie intake from normal food by protein and calories from these supplements may be to have a detrimental effect on eating behaviour which is particularly critical in toddlers and young children who are learning to develop normal eating behaviour. These products may also cause a number of other unpleasant symptoms, for example diarrhoea, vomiting, glucose intolerance and bloating. In addition, these products are expensive especially when compared calorie for calorie to food.
Why it is important to do this review
Given the potential benefits and harms described above it is important to evaluate the effectiveness of oral protein calorie supplements in children with chronic disease. This is an update of a Cochrane review last updated in 2009 (Poustie 2009).
Objectives
To examine the evidence that in children with chronic disease, oral protein calorie supplements:
alter nutritional indices, daily calorie, protein and other nutrient intake, survival and quality of life;
alter protein and calorie intake from normal food and affect overall nutrient intake;
are associated with adverse effects in children with chronic disease which are either important to the child or have long‐term sequelae (these may include diarrhoea, vomiting, reduced appetite, glucose intolerance, bloating and eating behaviour problems).
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) and quasi‐randomised controlled trials, published or unpublished.
Types of participants
Children, aged between one year and 16 years, with any defined chronic disease i.e. a disease which requires medical intervention for a period of six months or more, or for the remainder of the person's lifetime. Children with malnutrition resulting from insufficient dietary intake without any causative disease, e.g. during famine, were excluded.
Types of interventions
Protein calorie supplements administered orally, in any amount and given for a period of at least one month, compared with no intervention, routine nutritional advice or placebo. A period of at least one month has been selected as we are interested in the longer‐term use of these products to improve nutritional status and growth. These products are sometimes provided in the short term to boost nutritional intake during a period when dietary intake is compromised, for example during an acute infection. Studies with an intervention period of less than one month will therefore be excluded. Oral protein calorie supplements provided as either whole protein milk or juice drinks were considered. Those which provide calories alone were not included.
Types of outcome measures
Primary outcomes
-
Change in nutritional indices
weight
height
body mass index
z score
other indices of nutrition (including blood biochemistry if appropriate) or growth, including anthropometric measures of body composition
-
Calorie and nutritional intake from food measured by diet diary or weighed food intake daily, weekly or over some other time interval
total energy intake
total fat intake
total protein intake
-
Calorie and nutritional intake from oral protein calorie supplements measured by diet diary or weighed food intake daily, weekly or over some other time interval
total energy intake
total fat intake
total protein intake
Secondary outcomes
Measures of eating behaviour
Severity scores for individual chronic diseases (e.g. FEV1)
Measures of quality of life
Measures of compliance to dietary treatment
Adverse effects including diarrhoea, vomiting, reduced appetite and abdominal bloating. Other adverse effects, if reported, would also be examined
Number of deaths or age at death in each group
Search methods for identification of studies
Electronic searches
Publications describing RCTs of the use of oral protein calorie supplements in children with chronic diseases were identified from the different trials registers held at the editorial base of the Cochrane Cystic Fibrosis and Genetic Disorders Group.
The search terms used to search this Register were:
CF: nutrition AND supplements AND (protein OR calorie supplements)
HAEM & COAG: (nutritional OR dietary) supplements
OTHERS: (diet* OR calorie* OR protein* OR nutrition*) AND supplement* (ti/abstr)
These registers are compiled from electronic searches of the Cochrane Central Register of Controlled Trials (CENTRAL) (updated each new issue of The Cochrane Library), weekly searches of MEDLINE, a search of Embase to 1995 and the prospective handsearching of two journals ‐ Pediatric Pulmonology and the Journal of Cystic Fibrosis. Unpublished work is identified by searching through the abstract books of three major cystic fibrosis conferences:‐ the International Cystic Fibrosis Conference; the European Cystic Fibrosis Conference and the North American Cystic Fibrosis Conference. For full details of all searching activities for the register, please see the relevant sections of the Cystic Fibrosis and Genetic Disorders Group Module. Date of the most recent search of the Group's trials registers: 24 February 2015.
Detailed computerised searches of MEDLINE from 1966 to October 2013 (using the search strategy described in the Cochrane Handbook for Systematic Reviews of Interventions) and of the Cochrane Central Register of Controlled Trials (Issue 9 of 12 2013) were also undertaken for this review. For the full search strategies employed, please see the additional tables attached to this review (Appendix 1; Appendix 2).
Searching other resources
Unpublished work was identified through the searching of the abstract books of the conferences of the European Society of Parenteral and Enteral Nutrition (ESPEN), 1983 to 1999, the American Society of Parenteral and Enteral Nutrition (ASPEN), 1983 and 1985 to 1999, and the British Association of Parenteral and Enteral Nutrition (BAPEN), 1995, 1997, 1998. Additional RCTs were identified from reference lists. The companies which manufacture oral protein calorie supplements were contacted to ask whether they have data on RCTs of oral protein calorie supplements for children with chronic diseases on file.
Data collection and analysis
Selection of studies
For the original review and up until November 2008, the three authors (VP, RS and RW) independently selected the studies to be included in the review according to criteria set out above and ensured they obtained the full reports of these studies if they were published. As from the 2015 update, two authors (JS and DF) independently decided on which studies to include based on the pre‐stated criteria. In both iterations the authors resolved any disagreements by discussion.
Data extraction and management
For the reviews up to 2009, two authors (VP and RS) independently extracted data and one author (VP) extracted and analysed the individual patient data from one study by calculating mean change from baseline with standard deviations (SDs) for relevant outcomes (Kalnins 1996); the authors used a standard data extraction form adapted to suit this review. From the 2015 update, two authors (JS and DF) carried out data extraction and management. The authors resolved any disagreements regarding extracted data or how it should be handled by discussion.
The authors grouped outcome data into those measured at one, three, six, 12 months and annually thereafter. If study investigators had recorded outcome data at other time periods, then the authors considered examining these as well.
Assessment of risk of bias in included studies
The authors assessed the risk of bias (low, high or unclear) for each study using the 'Risk of bias' assessment tool as documented in chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). They considered the following items:
sequence generation;
allocation concealment;
blinding (or masking) of participants, personnel and outcome assessors;
incomplete outcome data;
selective outcome reporting;
other potential sources of bias.
Again, the authors resolved any differences by discussion.
Measures of treatment effect
For binary outcome measures, the authors calculated a pooled estimate of the treatment effect for each outcome across studies using the Peto odds ratio (OR) (the odds of an outcome among treatment allocated participants to the corresponding odds among controls).
For continuous outcomes, the authors recorded either the mean change from baseline for each group or the mean post‐treatment or intervention values and SD or standard error for each group. They also calculated a pooled estimate of treatment effect by calculating the mean difference (MD). If trial reports had presented standard errors instead of SDs, the authors planned to convert these to SDs in order to enter data into RevMan (RevMan 2014).
For all outcome measures the authors also reported the relevant 95% confidence intervals (CIs).
Unit of analysis issues
If the authors include any cross‐over studies in a future update, ideally they will combine the results in a meta‐analysis using the inverse variance methods that are recommended by Elbourne (Elbourne 2002). If there are restrictions on the data available on the included trials, they will either use first‐arm data only or treat the cross‐over study as if it was a parallel study (assuming a correlation of zero as the most conservative estimate). Elbourne says that this approach produces conservative results as it does not take into account within‐patient correlation (Elbourne 2002). Also each participant appears in both the treatment and control group, so the two groups are not independent. In order to combine these results with the data entered from already included parallel studies, the authors will use the methods recommended by Curtin (Curtin 2002a; Curtin 2002b; Curtin 2002c).
Dealing with missing data
The authors planned to seek data on the number of participants with each outcome event, irrespective of compliance and whether or not the participant was later thought to be ineligible or otherwise excluded from treatment or follow up. Where outcome data or details of the study methodology were missing from the papers, the authors contacted the primary investigators for further details. If the primary investigators had not responded, the authors had planned to contact the co‐investigators.
Assessment of heterogeneity
Heterogeneity was tested using a standard Chi2 test, to assess whether observed differences in results are compatible with chance alone. The authors used the I2 test was used to assess the impact of heterogeneity on the meta‐analysis. It shows the percentage of variability in effect estimates that are due to heterogeneity rather than to chance. Values over 50% indicate a high level of heterogeneity (Higgins 2011).
If a high level of heterogeneity existed, the authors planned to discuss this narratively.
Assessment of reporting biases
In this update, it was the authors intention to investigate publication bias through use of the funnel plot. However, the review did not include the minimum number of studies (n = 10) for any analysis. Furthermore, while funnel plot asymmetry may indicate publication bias, this is not inevitably the case (Egger 1997). This issue will be considered for a future version of this review provided there is a sufficient number of included studies.
Data synthesis
The authors have presented data using MDs and 95% CIs at the pre‐specified time‐points. They have used a fixed‐effect model for the majority of the analyses; however, they analysed data on change in total dietary fat intake using a random‐effects model due to evidence of significant heterogeneity. If the authors are able to add further trials to the meta‐analysis in the future and there is significant heterogeneity between studies, they will use a random‐effects model.
Subgroup analysis and investigation of heterogeneity
To investigate any heterogeneity, if the authors identify sufficient studies (at least four), they plan to perform subgroup analyses stratifying according to type of control group(s) used, age and severity of nutritional status, disease type and type of supplement.
Sensitivity analysis
If, in future, the authors are able to include sufficient studies (at least four) in the review and combine these in a meta‐analysis; they will test the robustness of their results with a sensitivity analysis based on the risk of bias in the studies from randomisation (e.g. randomised controlled trials compared to quasi‐randomised controlled trials).
Results
Description of studies
The included studies are described below.
Results of the search
The original search identified 2105 titles of publications associated with nutrition. From the titles, studies which clearly did not include either children or a nutritional intervention were excluded, leaving 130 publications for which the abstracts were obtained. Two authors then independently selected 39 papers referring to 28 studies, for which the full reports were obtained. These publications were independently checked by both authors to assess eligibility for inclusion in the review. All these references to studies can be obtained from the former lead reviewer (VP).
The same process has been repeated for the additional studies identified in the searches carried out for the annual updates of this review. For the 2015 update of this review, 4891 titles of publication were reviewed (Figure 1) Titles for studies which did not meet the inclusion criteria (children, nutritional intervention) were excluded, as a result 58 abstracts were obtained for further review. Two authors then independently reviewed these abstracts and identified only two studies as needing to obtain full papers.
1.
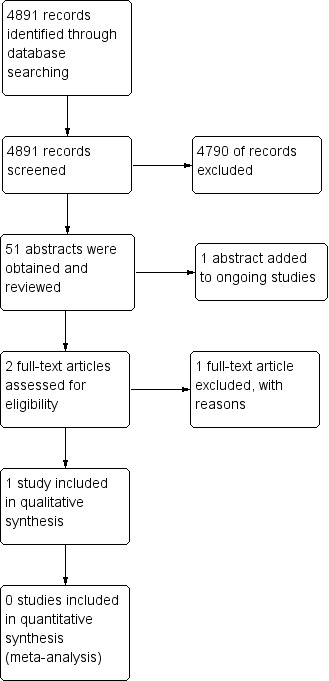
Study flow diagram for 2015 update.
For the 2015 update, three new studies were identified in the searches which are now listed in the review (Bayram 2009; Botrán 2011; Cox 2014). One of these has been included in the updated review (Bayram 2009) and one has been excluded with reasons (Botrán 2011). The third new study is still ongoing and the authors have been contacted to ascertain when the published data will be available (Cox 2014). Also, one reference that was previously listed as 'Awaiting classification' has now been included under the main study ID with confirmation of the paediatric data from the authors (Kalnins 1996). Five further studies that were previously listed as 'Awaiting classification' have now been excluded (Jain 2006; Johnson 2006; Nielson 2007; Powers 2006; Rollins 2007). One study that was previously listed as 'Awaiting classification' has been identified as part of an already excluded study (Rickard 1989).
There are currently a total of 106 studies excluded from the review.
Included studies
Study Design
Four studies of parallel design fulfilled the criteria for inclusion in this review (Bayram 2009; Hanning 1993; Kalnins 1996; Poustie 2006). The study by Hanning, which was an explanatory study, aimed to investigate the relationship between nutritional status and skeletal muscle strength (Hanning 1993). Three studies were single centre (Bayram 2009; Hanning 1993; Kalnins 1996) and one was multicentre (Poustie 2006). Two studies were conducted in Canada (Hanning 1993; Kalnins 1996), one in the UK (the 'CALICO' trial) (Poustie 2006) and one in Turkey (Bayram 2009). One study explicitly stated an open‐label design (Bayram 2009); however, the remaining three studies compared a supplement to dietary advice alone, so the participants could not be blinded (Hanning 1993; Kalnins 1996; Poustie 2006). The duration of the trials ranged from three months (Kalnins 1996) to 12 months (Poustie 2006).
Participants
Three studies were in children with cystic fibrosis (Hanning 1993; Kalnins 1996; Poustie 2006); one study included children with paediatric malignant disease (Bayram 2009). The four studies included a total of 187 participants (Bayram 2009; Hanning 1993; Kalnins 1996; Poustie 2006). The number of participants in each study ranged from 13 (Kalnins 1996) to 126 (Bayram 2009). In the Hanning study, the treatment group appeared to be in better clinical condition at baseline (Hanning 1993). Three of the studies included only children (Hanning 1993; Poustie 2006; Bayram 2009) and one study included both children and adults ‐ for this study, we obtained the individual patient data on those participants who were 16 years or less at the start of the study from the authors (Kalnins 1996).
Interventions
All four studies compared oral protein calorie supplements in addition to a normal diet to a control group. Three of these specified that the supplements were in the form of drinks (Bayram 2009Kalnins 1996; Poustie 2006) and one used drink powders, milk shakes or tinned puddings (Hanning 1993). Two studies used dietary counselling in the control group (Kalnins 1996; Poustie 2006) and two did not have any additional intervention in the control group (Bayram 2009; Hanning 1993). One study included follow up of intervention group by a nurse specialized in nutrition to check whether supplement was taken regularly (Bayram 2009).
Outcomes
The Bayram study included relevant primary and secondary outcomes for inclusion in the review, including loss in body weight, body mass index (BMI) and negative deviation from normal weight percentile; however, the authors only reported on percentage change of each outcome comparing treatment and control groups (Bayram 2009). All efforts made by the review authors to obtain individual patient data or reanalyzed results were unsuccessful.
The Hanning study examined a number of outcome measures relevant to the review (Hanning 1993). Mean change in weight from baseline for treatment and comparison groups was recorded, but results for all other outcomes were expressed as mean post‐treatment values. Since the groups were not similar at baseline, we have not included these results. We have attempted, as yet unsuccessfully, to obtain further information from the authors.
The Kalnins study did not report the results as mean change from baseline. From the individual patient data provided by the authors, we have calculated the mean change in weight, height, BMI, with z scores and percentiles, and mean change in percentage ideal weight for height (% WFH), nutritional intake and forced expiratory volume at one second (FEV1) (% predicted) from baseline (Kalnins 1996).
Poustie reported on nutritional parameters, energy and macronutrient intake, lung function, eating behaviour and activity levels (Poustie 2006).
Excluded studies
In summary, a total of 106 studies were excluded from the review. Of these, the authors identified 69 studies which were not of oral protein calorie supplements, 19 studies were not of RCT design, 10 were not in children with chronic diseases, three of the studies were of short intervention duration and five were not within a specified age range.
Risk of bias in included studies
Allocation
Generation of sequence
Generation of allocation sequence was assessed as having a high risk of bias in one study, which was described as randomised in the published manuscript, but no details of this were provided to appraise the generation of allocation sequence (Kalnins 1996). We have since been informed by the author of the published manuscript that although generation of allocation sequence was adequate for the first participant, alternation was used for subsequent participants. The study was therefore assessed as being at high risk of bias.
The Bayram study was judged to have an unclear risk of bias as it was reported that the participants were consecutively selected but no further details provided as to how this consecutive sequence was generated (Bayram 2009).
Generation of the allocation sequence was assessed as having a low risk of bias in the remaining two studies (Hanning 1993; Poustie 2006). In these studies the allocation sequence was determined by random number tables (Hanning 1993; Poustie 2006). However, in one of the studies, the treated group appeared to be in better clinical condition at baseline (Hanning 1993). To overcome this, we decided not to include any of the data from outcomes which were expressed as mean post‐treatment values in the analysis.
Allocation concealment
Concealment of allocation was assessed as having a high risk of bias in two studies (Bayram 2009; Kalnins 1996). The Bayram study was an open‐label design and therefore did not maintain concealment of allocation. The Kalnins study provided no details of the concealment of the allocation sequence. We have since been informed by the author of the published manuscript that although generation of allocation sequence was adequate for the first participant, alternation was used for subsequent participants. The person undertaking the randomisation was not blinded to the sequence of allocation.
Concealment of allocation was assessed as having a low risk of bias in the other two studies (Hanning 1993; Poustie 2006). In these studies allocation concealment was achieved using sealed envelopes (Hanning 1993; Poustie 2006).
Blinding
In none of the studies were the participants able to be blinded to the treatment: one study compared supplements to counselling (Kalnins 1996); two studies compared dietary supplements to no supplements (Bayram 2009; Hanning 1993); and the final study states that participants were not blinded as there was no satisfactory placebo available (Poustie 2006). With regards to the study investigators, in the Poustie study the research assistant was not masked to allocation group; however, a masked investigator used a computerised growth package when converting weight and height to body mass index centile (Poustie 2006). One study comparing supplements to counselling stated investigators were blinded to treatment allocation (Kalnins 1996).
We classified all four studies as being at low risk of bias despite different levels of blinding as blinding was not deemed to interfere with the outcomes assessed in the studies.
Incomplete outcome data
Intention‐to‐treat analysis was not described in one study where four out of 20 participants were lost to follow up (the time demands for testing or the travelling distance were found to be excessive).and no final data were collected (Hanning 1993). Although intention‐to‐treat analysis was not directly mentioned in the trial publication for two of the studies, the data show that none of the children were lost to follow up and that the number of participants randomised to each group was the same at the start and at the end of the study (Bayram 2009; Kalnins 1996). Statistical analysis of the CALICO Trial was by intention to treat, and no participants were lost to follow up (Poustie 2006). We consider the risk of bias for all four studies to be low.
Selective reporting
All four studies appear to report on all the outcome measures they state they have taken in the 'Methods' section of their individual papers (Bayram 2009; Hanning 1993; Kalnins 1996; Poustie 2006). In three of the studies, we were unable to compare the published study reports with the original study protocols to identify if any planned outcome measures are not reported in the full papers (Bayram 2009; Hanning 1993; Kalnins 1996); however, Dr Kalnins has confirmed that all outcomes measured in the trial were reported. In the CALICO Trial, a number of secondary outcome measures were assessed but not recorded in the key trial publication (Poustie 2006). These were assessment of eating behaviour, activity levels and lipase intake. The authors have confirmed that this was due to the poor quality of the available data on these outcomes which precluded the reporting of these data. Tricep skin‐fold measurement and mid‐upper arm circumference were assessed, but not reported, but were used to calculate mid‐arm muscle circumference which was reported. Where possible, data on these outcomes have been provided by the authors and included in this review. We consider the risk of bias for all four studies to be low (Bayram 2009; Hanning 1993; Kalnins 1996; Poustie 2006).
Effects of interventions
See: Table 1
It should be noted that for studies with such small numbers, it may not be appropriate to use the Review Manager software to analyse the data due to the high variability associated with such small numbers (RevMan 2014). As such, caution should be given to significant results from these findings.
Primary Outcomes
We have listed the outcomes for which we have data to report. Summary statistics for significant results only are provided here.
1. Change nutritional indices
a. Weight
Three studies reported change in weight (kg) (Hanning 1993; Kalnins 1996; Poustie 2006) (Analysis 1.1) and two studies reported change in weight (z score) (Kalnins 1996; Poustie 2006) (Analysis 1.2). Two studies reported change in weight percentile, but as this measure represents the same information as z score but uses different units, and as z score is more commonly used internationally, data on percentile have not been presented (Bayram 2009; Poustie 2006). Additionally, the Bayram study reported the proportion of participants with weight loss, which was significantly higher among the usual dietary intake group compared to the treatment group at six months (P = 0.03) (Bayram 2009). This was the only study to report a significant difference between the treatment and control arms for this outcome, and did not supply data we were able to analyse.
1.1. Analysis.
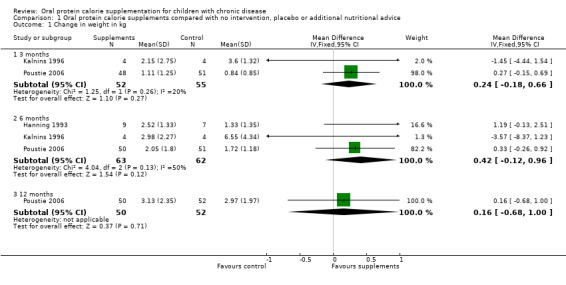
Comparison 1 Oral protein calorie supplements compared with no intervention, placebo or additional nutritional advice, Outcome 1 Change in weight in kg.
1.2. Analysis.
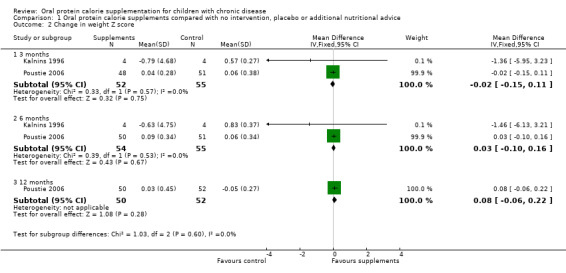
Comparison 1 Oral protein calorie supplements compared with no intervention, placebo or additional nutritional advice, Outcome 2 Change in weight Z score.
b. Height
Two studies reported both change in height (cm) and change in height (z score) (Kalnins 1996; Poustie 2006). One study reported change in percentile, but as this measure represents the same information as z score but uses different units, and as z score is more commonly used internationally, data on percentile have not been presented (Poustie 2006). No significant differences between the treatment and control arms were identified for this outcome (Analysis 1.3; Analysis 1.4).
1.3. Analysis.
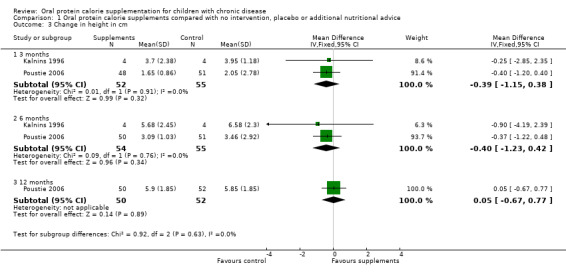
Comparison 1 Oral protein calorie supplements compared with no intervention, placebo or additional nutritional advice, Outcome 3 Change in height in cm.
1.4. Analysis.
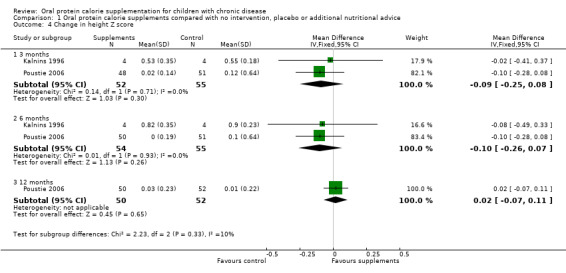
Comparison 1 Oral protein calorie supplements compared with no intervention, placebo or additional nutritional advice, Outcome 4 Change in height Z score.
c. BMI
Two studies reported change in BMI and change in BMI z score or percentile, or both (Bayram 2009; Poustie 2006). Bayram reported a significantly higher loss in BMI among the usual dietary intake group compared to the treatment group at three months (P = 0.002), but again did not provide data we could analyse (Bayram 2009). For the Poutsie study, no significant difference between the treatment and control arms were identified for this outcome (Analysis 1.5; Analysis 1.6).
1.5. Analysis.
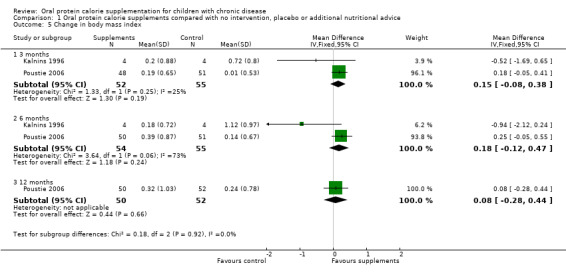
Comparison 1 Oral protein calorie supplements compared with no intervention, placebo or additional nutritional advice, Outcome 5 Change in body mass index.
1.6. Analysis.
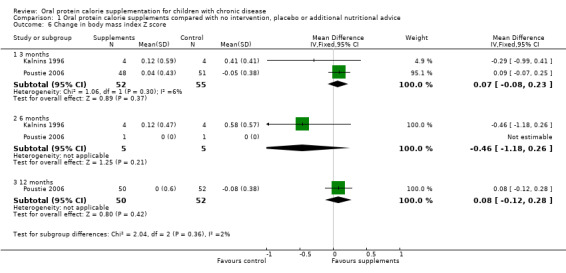
Comparison 1 Oral protein calorie supplements compared with no intervention, placebo or additional nutritional advice, Outcome 6 Change in body mass index Z score.
e. Mid‐arm muscle circumference
One study reported on mid‐arm muscle circumference (calculated from mid‐upper arm circumference and tricep skin‐fold measurements) (Poustie 2006). No significant difference between the treatment and control arms were identified for this outcome (Analysis 1.8).
1.8. Analysis.
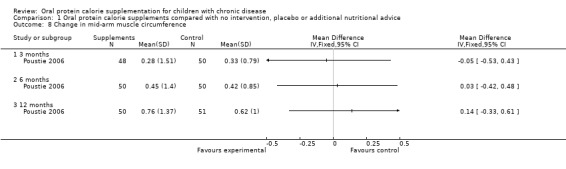
Comparison 1 Oral protein calorie supplements compared with no intervention, placebo or additional nutritional advice, Outcome 8 Change in mid‐arm muscle circumference.
3. Calorie and nutritional intake from food
a. Total energy intake
Two studies reported on total energy intake (Kalnins 1996; Poustie 2006). A significant difference in mean total energy intake at six months, MD 304.86 kcal/day (95% confidence interval (CI) 5.62 to 604.10) and at 12 months, MD 265.70 kcal/day (95% CI 42.94 to 485.46) was found to favour the treatment group (Analysis 1.9). However, the data for these outcomes came from only one study (Poustie 2006).
1.9. Analysis.
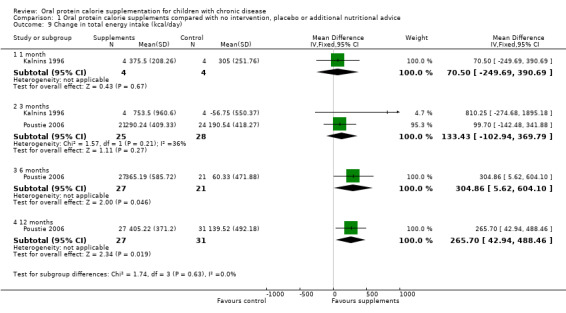
Comparison 1 Oral protein calorie supplements compared with no intervention, placebo or additional nutritional advice, Outcome 9 Change in total energy intake (kcal/day).
b. Total fat intake
Two studies reported on total fat intake (Kalnins 1996; Poustie 2006). No significant difference between the treatment and control arms were identified for this outcome (Analysis 1.10).
1.10. Analysis.
Comparison 1 Oral protein calorie supplements compared with no intervention, placebo or additional nutritional advice, Outcome 10 Change in total fat intake (g/day).
c. Total protein intake
Two studies reported on total protein intake (Kalnins 1996; Poustie 2006). No significant difference between the treatment and control arms were identified for this outcome (Analysis 1.11).
1.11. Analysis.
Comparison 1 Oral protein calorie supplements compared with no intervention, placebo or additional nutritional advice, Outcome 11 Change in total protein intake (g/day).
Secondary Outcomes
Data were reported for just two of these outcomes.
2. Severity scores for individual chronic diseases
We have decided to use lung function data to indicate disease severity in the studies including children with cystic fibrosis. Three studies reported lung function data: Hanning and Kalnins reported absolute forced expiratory volume at one second (FEV1) % predicted (Hanning 1993; Kalnins 1996); and the CALICO study reported change in FEV1 % predicted and change in forced vital capacity (FVC) % predicted (Poustie 2006). No significant difference between the treatment and control arms were identified for this outcome apart from change in FEV1 % predicted at three months in one study with 69 participants, MD ‐7.92 (95% ‐13.89 to ‐1.95) (Poustie 2006) (Analysis 1.12).
1.12. Analysis.
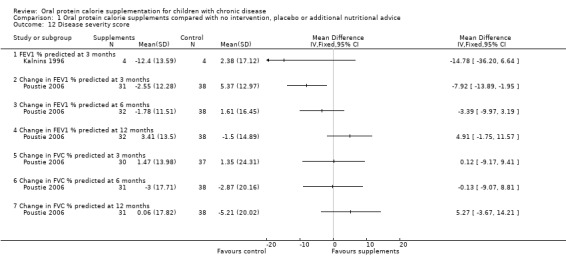
Comparison 1 Oral protein calorie supplements compared with no intervention, placebo or additional nutritional advice, Outcome 12 Disease severity score.
In the study by Bayram, the remission rates were significantly lower in the usual dietary care group compared to the treatment group (P = 0.036) (Bayram 2009). There were no significant differences between the usual dietary care group and the treatment group in the distribution of the febrile neutropenia attacks when assessing both the frequency and the per cent of participants who suffer these attacks (Bayram 2009).
5. Adverse effects
One study reported a gastrointestinal symptom score (Poustie 2006). No significant difference between the treatment and control arms were identified for this outcome (Analysis 1.13). Poustie also reported that there was no significant difference between the treatment and control arms for headache, OR 0.30 (95% CI 0.04 to 2.58) (Analysis 1.14).
1.13. Analysis.
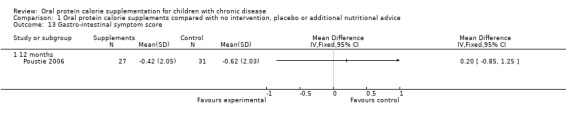
Comparison 1 Oral protein calorie supplements compared with no intervention, placebo or additional nutritional advice, Outcome 13 Gastro‐intestinal symptom score.
1.14. Analysis.
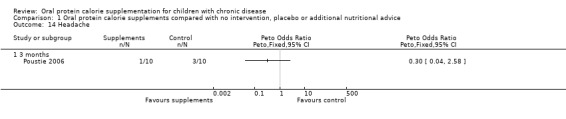
Comparison 1 Oral protein calorie supplements compared with no intervention, placebo or additional nutritional advice, Outcome 14 Headache.
Discussion
Summary of main results
The 2015 updated version of this review screened 4883 studies (abstract and titles) of which just one was included (Bayram 2009); one is still ongoing and may be included in a future update (Cox 2014). Overall four studies with 187 participants in total and ranging in date from 1996 to 2009, are currently included in this review (Bayram 2009; Hanning 1993; Kalnins 1996; Poustie 2006). The main outcomes of interest are summarized below. One study was not included in any of the meta‐analyses as the primary outcomes were reported in dissimilar units to the other studies (Bayram 2009).
Change in weight
Three studies reported on change in weight (kg) (Hanning 1993; Kalnins 1996; Poustie 2006); combined data from all threes studies at six months showed a mean difference in weight of 0.42 kg in the supplemented group compared to the control group, but this was not significant (Analysis 1.1).
Only two studies reported change in weight z score at six months and they found no significant difference in scores between the supplemented group and the control group (Kalnins 1996; Poustie 2006). Although the data from Bayram were not included in the meta‐analyses, the study reported that overall, loss in body weight was 6.1% in the supplemented group compared to 47.4% in the control group. Similarly, negative deviation in weight percentile was significantly lower in the supplemented group compared to the control group (Bayram 2009).
Change in height
Change in height (cm) was reported by two studies at three months (Kalnins 1996; Poustie 2006) and six months (Kalnins 1996; Poustie 2006) and by just one study at 12 months (Poustie 2006). There was no significant difference in change in height between the supplemented group and the control group at any time‐point (Analysis 1.3).
The same two studies reported mean difference in height z score at six months (109 participants); there was no significant difference in the mean change between groups, though the effect estimate favoured the usual diet group (Analysis 1.4).
Change in body mass index (BMI)
Similarly, two studies reported analysable data on the mean difference for the change in BMI at six months (Kalnins 1996; Poustie 2006); the analysis showed no significant difference between the supplemented and usual diet group (Analysis 1.5). The Bayram study reported that the loss in BMI was 12.1% in the supplemented group and 52.6% in the control group (Bayram 2009).
Analysable data for the change in BMI z score was also reported by the same two studies at three months (Kalnins 1996; Poustie 2006). They reported that there was no significant difference in the change in BMI z score between the supplemented and control group (Analysis 1.6).
Overall completeness and applicability of evidence
Oral protein calorie supplements are widely used in an attempt to improve nutritional status in people with a number of chronic diseases, at some considerable cost. It is therefore concerning that their effectiveness has been assessed by adequate clinical trials in very few chronic diseases of childhood .
In this review, just two chronic diseases were addressed in four clinical trials, which highlights the paucity of evidence for rigorous clinical trials addressing appropriate nutritional interventions for children with chronic diseases. The chronic diseases addressed in the trials included cystic fibrosis (Hanning 1993; Kalnins 1996; Poustie 2006) and paediatric malignant disease (Bayram 2009).
There is a link between cystic fibrosis and nutritional status and the role of nutritional management has long been recognized (Corey 1988). Meeting the demand of increased caloric needs in children with cystic fibrosis and cancer may be important in alleviating the increased malnutrition seen in children suffering from these diseases (Florescu 2014). From its comprehensive search of the literature, this review included an explanatory study which aimed to assess the effect of supplementation on skeletal muscle strength, rather than clinical outcomes, although these were also addressed (Hanning 1993). Two other studies were pragmatic in approach to mimic the clinical practice setting (Kalnins 1996; Poustie 2006). In the 2015 update of this review, only one additional study was included in the synthesis which addressed the potential role of oral protein and energy dense supplements in cancer‐related weight loss in children (Bayram 2009). The interventions differed between studies. In the study by Hanning, the participants in the treatment group appear to have been in better clinical condition than the control group, despite the employment of adequate randomisation methods. This is in contrast to the other studies in which both treatment and control groups were similar at baseline. It would appear, that over the last decade there have been few studies addressing the objectives of the review and fitting the inclusion criteria. Furthermore, other potentially eligible studies were run in an older age group and or focused on metabolic outcomes (such as nitrogen balance) as oppose to clinical endpoints (Botrán 2011; Rollins 2007).
No studies were identified in any other conditions (e.g. cholestatic liver disease) for which oral protein calorie supplements are routinely prescribed. The CALICO study resulted from the previous publication of the Cochrane review 'Oral calorie supplements for cystic fibrosis' (Smyth 2014). The search identified many large studies of protein and calorie supplementation for children in the developing world who are malnourished as a result of persistently low nutritional intakes. However, it would not be appropriate to extrapolate data from these studies to children in whom nutritional status is compromised as a consequence of their chronic disease. Although, knowledge of the methods used in these studies may be useful for those planning to investigate supplementation in children with chronic disease.
For reasons of heterogeneity (diversity of outcome measures) only three studies were included in the meta‐analyses (Hanning 1993; Kalnins 1996; Poustie 2006). Analyses showed no significant effect of oral protein supplementation on weight or height change in children with cystic fibrosis. These studies ranged from three months to one year of follow up. Disease severity remained unchanged but there were moderate increases in nutrient intake in those receiving oral protein calorie supplements. In contrast, the study among children with paediatric malignancies found oral protein supplements to be effective in preventing weight loss and negative deviation in weight percentiles (Bayram 2009). The relative inefficacy of oral protein supplements on the reported outcomes in cystic fibrosis may be related to clinical concomitant complications of this chronic disease and methodological issues of the trials. The complex nature of cystic fibrosis affects multiple organs and especially the nutritional status of individuals through the gastrointestinal tract, which usually results in malnutrition as disease progression worsens. The mechanism of the intervention may have been affected by gastrointestinal complications such as diarrhoea, increased metabolic rate, vomiting, fever, and other underlying nutrient deficiencies as a result of malnourished status.
This raises the question of whether prevention of weight loss or correcting nutrient deficiencies related to growth would have been a more sensitive measure of the efficacy of oral protein calorie supplements. Also, none of the studies reported on the appropriateness of the supplements in terms of palatability. None of the three studies of children with cystic fibrosis measured compliance of consumption, but the study in paediatric malignancies reported having a specialised nurse check whether supplements were taken regularly (Bayram 2009).
Quality of the evidence
There were very few published studies that examined the effect of oral protein calorie supplementation on growth in children between one and 16 years of age with chronic diseases. The four included studies only addressed two chronic diseases, malignant disease and cystic fibrosis, highlighting a current gap in the research evidence. The evidence covered in this review marks a slow but important area of work being investigated.
Judgements on the quality of the evidence for the change in weight and height was assessed to be moderate (Table 1). Three randomised controlled trials (RCTs) (125 participants) assessed the change in weight and overall there was a greater weight change in the intervention groups as compared to the control groups. The quality of the evidence for this outcome was moderate, due to the number of studies included and the number of participants who were involved in these studies. For the change in height, two RCTs (109 participants) were assessed and, based on these results, those participants in the intervention group were shorter than those in the control arms. The quality of evidence for this outcome was also moderate due to the number of studies and participants included. For the outcome measure of BMI, only BMI z score was reported in two RCTs (109 participants). Those participants in the intervention group had a higher BMI than those in the control groups; however, the quality of evidence for this outcome was moderate due to the number of studies included and the number of participants assessed.
Potential biases in the review process
In order to reduce bias careful attention was paid to standard systematic review methodology. For example, at least two review authors were involved in every aspect of identifying potential studies, deciding on inclusion and exclusion of studies, extracting data, and conducting analyses. However, a few potential sources of bias may remain.
Publication bias
A comprehensive search of both published and unpublished sources were undertaken to find relevant studies, including making contact with companies which manufacture oral protein calorie supplements for data on RCTs of these products for children with chronic diseases which they might have had on file. It is, however, still possible that some studies were missed. Furthermore, handsearching of relevant journals was also carried out. We attempted to obtain individual patient data from one study, but were unsuccessful.
Due to the fact that this is an update of a previously conducted review, any potential bias (if it exists) included in the initial review such as screening or selection of included studies could not have been corrected.
The original review was only able to access the study protocol for just one study in order to completely assess the risk of bias of included studies (Poustie 2006). However, from the reported available publications risk of bias was assessed as appropriate for all four studies (Figure 2; Figure 3).
2.
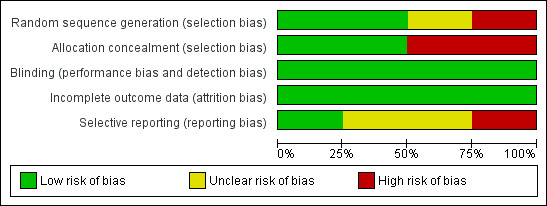
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
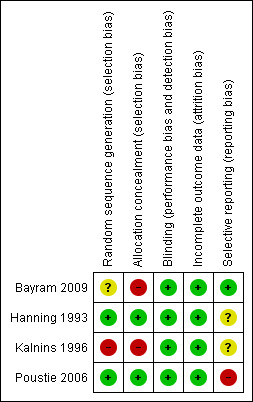
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Agreements and disagreements with other studies or reviews
There are very few studies addressing the efficacy of oral protein calorie supplements in children with chronic diseases and we found no existing review with which to compare our findings.
Authors' conclusions
Implications for practice.
There is still insufficient rigorous evidence on which to base concrete conclusions about the use of oral protein calorie supplements in children with chronic diseases. This does not mean that these products may or may not be efficacious and clinicians must balance potential benefits against possible adverse effects of treatment in making decisions about individual children with chronic disease. A series of large clinical trials to evaluate the effectiveness of this intervention in the different disease areas where these products are commonly used, is urgently needed (see below). Therefore, we would urge caution in their use until this information is available and also since using oral protein calorie supplements in children now may exclude them from participation in a future RCT. The CALICO study of oral protein calorie supplements for children with cystic fibrosis was sufficiently powered to identify a significant effect of this intervention in this population, if one existed. The results of this study have shown that when children with sub‐optimal nutrition receive regular dietary advice, their nutritional status is similar whether or not they have supplements. However, this finding can not be extrapolated to the use of these products in children with other chronic diseases.
Implications for research.
This systematic review has clearly identified the need for a series of well‐designed, adequately‐powered, multicentre RCTs assessing the effectiveness and possible adverse effects of the use of oral protein calorie supplements for children with chronic disease. A number of studies would be required to assess the effectiveness of these products in children with diseases associated with growth and poor nutritional status which lead to the prescription of these products. The CALICO study has contributed important information on the use of these products for children with cystic fibrosis but further research is required in other chronic diseases of childhood. The CALICO trial would be a useful model upon which such studies could be based.
What's new
| Date | Event | Description |
|---|---|---|
| 29 June 2017 | Amended | Contact details updated. |
History
Protocol first published: Issue 1, 2000 Review first published: Issue 3, 2000
| Date | Event | Description |
|---|---|---|
| 20 May 2015 | New citation required but conclusions have not changed | A new review team has taken on this review at the 2015 update. Despite the inclusion of one new trial (in a different disease area than those trials previously included), the conclusions of the review have not changed. |
| 20 May 2015 | New search has been performed | Three new studies were identified in the latest searches; one of these has been included in the updated review (Bayram 2009) and one has been excluded with reasons (Botrán 2011). The third new study is still ongoing and the authors have been contacted to ascertain when the published data will be available (Cox 2014). One reference that was previously listed as 'Awaiting classification' has now been included under the main (already included) study ID with confirmation of the paediatric data from the authors (Kalnins 1996). Five further studies that were previously listed as 'Awaiting classification' have now been excluded (Jain 2006; Johnson 2006; Nielson 2007; Powers 2006; Rollins 2007). One study that was previously listed as 'Awaiting classification' has been identified as part of an already excluded study (Rickard 1989). |
| 12 August 2009 | Amended | Contact details updated. |
| 10 November 2008 | New search has been performed | 17 new trials were identified in the latest searches: 12 new trials have been listed as excluded (Abdulhamid 2008; Bruzzese 2007; Lloyd‐Still 2006; Manguso 2005; Marques 2004; Ndekha 2005; Newby 2007; Oudshoorn 2007; Papas 2007; Soliman 2004; Stark 2005; Teixido‐Planas 2005); five new trials have been listed as 'Awaiting classification' (Jain 2006a; Johnson 2006a; Nielson 2007a; Powers 2006a; Rollins 2007a). One trial which was previously listed as ongoing has now been published in full and has been included in the review (Poustie 2006); one additional reference to an already included trial has also been listed as 'Awaiting classification' until we are able to obtain and analyse data for paediatric participants only (Kalnins 2005). We have obtained the published paper from one trial which was previously listed as 'Awaiting classifcation' and we have now judged this to be not eligible to be listed even as an excluded study. |
| 10 April 2008 | Amended | Converted to new review format. |
| 14 November 2005 | New search has been performed | A search was run in November 2004 and no new references were found which were eligible for inclusion in the review. |
| 27 January 2004 | New search has been performed | A search was run in August 2003 and identified 13 new references. Not all of these were eligible for inclusion in the review, but two of the references (Lepage 2002; Pelekanos 1990) have been added to 'Excluded studies'. |
| 16 October 2002 | New search has been performed | An update of the review was completed in October 2002. No additional studies were found to be eligible for inclusion in the review. |
| 21 May 1999 | New citation required and conclusions have changed | Substantive amendment |
Notes
Please refer to 'Oral calorie supplements for cystic fibrosis' Cochrane review, which assesses the effectiveness of this intervention for children and adults with cystic fibrosis (Smyth 2014).
Acknowledgements
We would like to thank Professor Berthold Koletzko, University of Munich, Germany, for agreeing to provide us with additional data from his study once the report has been accepted for publication, and Daina Kalnins for providing us with the individual patient data from her study.
The new author team (DKF, TS, JS, RW) would like to thank Professor Rosalind Smyth and Dr Vanessa Poustie for their previous input into this review as detailed below.
Appendices
Appendix 1. Search strategy ‐ Medline [Ovid MEDLINE(R) and Ovid OLDMEDLINE(R)]
| Medline ‐ Ovid MEDLINE(R) and Ovid OLDMEDLINE(R) 1947 to Present with Daily Update (Searched 18/10/2013) |
| 1. randomized controlled trial.pt. 2. controlled clinical trial.pt. 3. randomized.ab. 4. placebo.ab. 5. clinical trials as topic.sh. 6. randomly.ab. 7. trial.ti. 8. 1 or 2 or 3 or 4 or 5 or 6 or 7 9. (animals not (humans and animals)).sh. 10. 8 not 9 11. exp Child/ 12. ADOLESCENT/ 13. exp infant/ 14. child hospitalized/ 15. adolescent hospitalized/ 16. (child$ or infant$ or toddler$ or adolescen$ or teenage$).tw. 17. or/11‐16 18. Child Nutrition Sciences/ 19. exp Dietary Proteins/ 20. Dietary Supplements/ 21. Dietetics/ 22. or/18‐21 23. exp Infant, Newborn/ 24. exp Overweight/ 25. exp Eating Disorders/ 26. Athletes/ 27. exp Sports/ 28. exp Pregnancy/ 29. exp Viruses/ 30. (newborn$ or obes$ or eating disorder$ or pregnan$ or childbirth or virus$ or influenza).tw. 31. or/23‐30 32. 10 and 17 and 22 33. 32 not 31 |
Appendix 2. Search strategy ‐ The Cochrane Library 'Clinical Trials'
| The Cochrane Library ‘Clinical Trials’ database, Issue 4 of 4 2011 (searched 18/10/2013) | ||||
| ID | Search | Hits | Edit | Delete |
| #1 | (child* OR infant* OR toddler* OR adolescent* OR teenage*) | 126648 | edit | delete |
| #2 | (supplement*:ti) OR nutrition* | 27096 | edit | delete |
| #3 | (#1 AND #2) | 8463 | edit | delete |
| #4 | (newborn* OR obes* OR eating disorder* OR preg* OR childbirth* OR virus* OR influenza* OR sport* OR athlete*) | 67975 | edit | delete |
| #5 | (#3 AND NOT #4) | 5027 | edit | delete |
| #6 | "accession number" near pubmed | 353977 | edit | delete |
| #7 | (#5 AND NOT #6) | 1424 | edit | delete |
| #8 | SR‐CF | 3532 | edit | delete |
| #9 | (#7 AND NOT #8) | 1364 | edit | delete |
| #10 | (#9) | 984 | edit | delete |
Line #10 restricts search to ‘Clinical Trials’ database only
Data and analyses
Comparison 1. Oral protein calorie supplements compared with no intervention, placebo or additional nutritional advice.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in weight in kg | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 3 months | 2 | 107 | Mean Difference (IV, Fixed, 95% CI) | 0.24 [‐0.18, 0.66] |
| 1.2 6 months | 3 | 125 | Mean Difference (IV, Fixed, 95% CI) | 0.42 [‐0.12, 0.96] |
| 1.3 12 months | 1 | 102 | Mean Difference (IV, Fixed, 95% CI) | 0.16 [‐0.68, 1.00] |
| 2 Change in weight Z score | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 3 months | 2 | 107 | Mean Difference (IV, Fixed, 95% CI) | ‐0.02 [‐0.15, 0.11] |
| 2.2 6 months | 2 | 109 | Mean Difference (IV, Fixed, 95% CI) | 0.03 [‐0.10, 0.16] |
| 2.3 12 months | 1 | 102 | Mean Difference (IV, Fixed, 95% CI) | 0.08 [‐0.06, 0.22] |
| 3 Change in height in cm | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 3 months | 2 | 107 | Mean Difference (IV, Fixed, 95% CI) | ‐0.39 [‐1.15, 0.38] |
| 3.2 6 months | 2 | 109 | Mean Difference (IV, Fixed, 95% CI) | ‐0.40 [‐1.23, 0.42] |
| 3.3 12 months | 1 | 102 | Mean Difference (IV, Fixed, 95% CI) | 0.05 [‐0.67, 0.77] |
| 4 Change in height Z score | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.1 3 months | 2 | 107 | Mean Difference (IV, Fixed, 95% CI) | ‐0.09 [‐0.25, 0.08] |
| 4.2 6 months | 2 | 109 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.26, 0.07] |
| 4.3 12 months | 1 | 102 | Mean Difference (IV, Fixed, 95% CI) | 0.02 [‐0.07, 0.11] |
| 5 Change in body mass index | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5.1 3 months | 2 | 107 | Mean Difference (IV, Fixed, 95% CI) | 0.15 [‐0.08, 0.38] |
| 5.2 6 months | 2 | 109 | Mean Difference (IV, Fixed, 95% CI) | 0.18 [‐0.12, 0.47] |
| 5.3 12 months | 1 | 102 | Mean Difference (IV, Fixed, 95% CI) | 0.08 [‐0.28, 0.44] |
| 6 Change in body mass index Z score | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 6.1 3 months | 2 | 107 | Mean Difference (IV, Fixed, 95% CI) | 0.07 [‐0.08, 0.23] |
| 6.2 6 months | 2 | 10 | Mean Difference (IV, Fixed, 95% CI) | ‐0.46 [‐1.18, 0.26] |
| 6.3 12 months | 1 | 102 | Mean Difference (IV, Fixed, 95% CI) | 0.08 [‐0.12, 0.28] |
| 7 Change in % ideal weight for height | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 7.1 3 months | 1 | 8 | Mean Difference (IV, Fixed, 95% CI) | ‐2.75 [‐9.55, 4.05] |
| 7.2 6 months | 1 | 8 | Mean Difference (IV, Fixed, 95% CI) | ‐5.5 [‐12.73, 1.73] |
| 8 Change in mid‐arm muscle circumference | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 8.1 3 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.2 6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.3 12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9 Change in total energy intake (kcal/day) | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 9.1 1 month | 1 | 8 | Mean Difference (IV, Fixed, 95% CI) | 70.5 [‐249.69, 390.69] |
| 9.2 3 months | 2 | 53 | Mean Difference (IV, Fixed, 95% CI) | 133.43 [‐102.94, 369.79] |
| 9.3 6 months | 1 | 48 | Mean Difference (IV, Fixed, 95% CI) | 304.86 [5.62, 604.10] |
| 9.4 12 months | 1 | 58 | Mean Difference (IV, Fixed, 95% CI) | 265.70 [42.94, 488.46] |
| 10 Change in total fat intake (g/day) | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 10.1 1 month | 1 | 8 | Mean Difference (IV, Random, 95% CI) | 15.48 [‐12.58, 43.54] |
| 10.2 3 months | 2 | 53 | Mean Difference (IV, Random, 95% CI) | 27.31 [‐42.72, 97.33] |
| 10.3 6 months | 1 | 48 | Mean Difference (IV, Random, 95% CI) | 11.71 [‐2.99, 26.41] |
| 10.4 12 months | 1 | 58 | Mean Difference (IV, Random, 95% CI) | 8.85 [‐4.64, 22.34] |
| 11 Change in total protein intake (g/day) | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 11.1 1 month | 1 | 8 | Mean Difference (IV, Fixed, 95% CI) | 4.15 [‐24.62, 32.92] |
| 11.2 3 months | 2 | 53 | Mean Difference (IV, Fixed, 95% CI) | 3.45 [‐5.87, 12.76] |
| 11.3 6 months | 1 | 48 | Mean Difference (IV, Fixed, 95% CI) | 8.77 [‐1.24, 18.78] |
| 11.4 12 months | 1 | 58 | Mean Difference (IV, Fixed, 95% CI) | 6.82 [‐2.36, 16.00] |
| 12 Disease severity score | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 12.1 FEV1 % predicted at 3 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 12.2 Change in FEV1 % predicted at 3 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 12.3 Change in FEV1 % predicted at 6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 12.4 Change in FEV1 % predicted at 12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 12.5 Change in FVC % predicted at 3 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 12.6 Change in FVC % predicted at 6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 12.7 Change in FVC % predicted at 12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 13 Gastro‐intestinal symptom score | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 13.1 12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 14 Headache | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 14.1 3 months | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
1.7. Analysis.
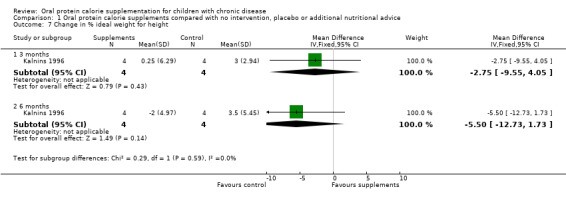
Comparison 1 Oral protein calorie supplements compared with no intervention, placebo or additional nutritional advice, Outcome 7 Change in % ideal weight for height.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bayram 2009.
| Methods | Randomized (2:1 randomization) controlled open‐label study. Duration: 3 months (follow up of a subset of participants for a further 3 months). Parallel design. Single centre. Location: Turkey. No intention‐to‐treat analysis. |
|
| Participants | 52 children (31 boys, 21 girls) with paediatric malignant disease receiving intensive chemotherapy. Mean (SD) age 7.5 (3.0) years. Treatment group n = 33: 18 children were diagnosed with leukaemia (12 acute lymphoblastic leukaemia, 6 acute myeloid leukaemia) and 15 had a solid tumour (4 non‐Hodgkin lymphoma, 3 neuroblastoma, 2 Wilm’s tumour, 2 brain tumour, 2 malignant bone tumour, 1 soft tissue tumour, and 1 hepatoblastoma). Control group n = 19: 11 children were diagnosed with leukaemia (7 acute lymphoblastic leukaemia, 4 acute myeloid leukaemia) and 8 had a solid tumour (3 neuroblastoma, 2 soft tissue tumour, 1 brain tumour, 1 malignant bone tumour, and 1 retinoblastoma). |
|
| Interventions |
Treatment: usual dietary intake plus 2x daily (morning and evening) oral supplement (ProSure™) containing protein and energy dense EPA (banana and vanilla flavoured); each 240 ml container contained 300 kcal, 16 g protein, 1.09 g EPA (derived from deodorized sardine oil). Children supervised by a nurse specialized in nutrition who monitored compliance with supplement. Control: usual dietary care. |
|
| Outcomes | Body weight, BMI, weight percentiles, status of primary disease, attacks of febrile neutropenia and clinical status. Measured monthly for 3 months. Subgroup of 23 children followed for further 3 months (6 months total). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Children were randomized, using a 2:1 randomization scheme. No further details given. |
| Allocation concealment (selection bias) | High risk | Open‐label design and therefore did not maintain concealment of allocation. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Not blinded. Outcomes were not affected by blinding due to the fact that they were objective measures. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There was no loss to follow up. |
| Selective reporting (reporting bias) | Low risk | Outcomes all reported in the Results as outlined in the Methods. |
Hanning 1993.
| Methods | Randomised controlled study. Duration: 6 months (follow up of a subset of participants for a further 3 months). Parallel design. Single centre. Location: Canada. No intention‐to‐treat analysis. |
|
| Participants | 20 children with CF and mild to moderate lung disease aged 7 ‐ 15 years.
Randomised n = 20 (10 treatment group, 10 control group).
Gender split: treatment group 7 males, 3 females; control group 5 male, 5 female. Mean (SD) age: treatment group 10.7 (2.4) years; control group 10.0 (2.8) years. Studied n = 16 (9 treatment group, 7 control group). Gender split: treatment group 6 male, 3 females; control group 4 males, 3 females. Mean (SD) age: treatment group 10.6 (2.5) years; control group 9.5 (2.9) years. |
|
| Interventions | Treatment: dietary supplements, drink powders, milk shakes, tinned puddings, to achieve 25% of normal energy recommendations in addition to normal diet. Control: no intervention. | |
| Outcomes | Skeletal muscle strength and power, pulmonary function and respiratory muscle strength, height, weight and anthropometric measurements, habitual physical activity, body composition, dietary energy and nutrient intake, energy and nutrient intake from supplements. Laboratory measures of nutritional status (e.g. albumin, amino acids). | |
| Notes | Number enrolled was slightly less than the sample size of 24 investigators had estimated they needed. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Paper states that participants were randomly allocated to treatment or control ordered on the basis of a table of random numbers. |
| Allocation concealment (selection bias) | Low risk | Paper states that participants selected a card from within a sealed envelope. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Not explicitly discussed in paper. Groups were dietary supplement or no supplement, so participants at least could not be blinded. Outcomes were not affected by blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Reasons for 4 participants not completing trial given (the time demands for testing or the travelling distance found to be excessive). |
| Selective reporting (reporting bias) | Unclear risk | Paper appears to address all the outcomes measured in the 'Resuts' section. No access to study protocol to double check. |
Kalnins 1996.
| Methods | Quasi‐randomised study. Duration: 3 months. Parallel design. Single centre. Location: Canada. |
|
| Participants | Adolescents and adults (over 10 years of age) with CF. Most recent published report states 13 completed the trial (2 drop‐outs).
Mean (SD) age: treatment group 19.5 (11.3) years; control group 16.4 (6.7) years. Gender split: 3 males, 10 females. Less than 90% ideal WFH or 5% reduction in ideal WFH over 3 months. All participants pancreatic insufficient, except 1 in treatment group. |
|
| Interventions | Treatment: high‐calorie drink to increase energy intake by 20% of predicted energy needs. Control: nutritional counselling to increase energy intake by 20% of predicted energy needs by eating high calorie foods. | |
| Outcomes | Z scores for weight* and height*, WFH* Anthropometric measures* Pulmonary function (FEV1 % predicted)* Energy* and nutrient* intake Faecal balance studies. | |
| Notes | Information from lead author: A modified randomization process was used: 1. males and females were segregated in 2 groups 2. within each gender, participants were separated into groups by age: 10 ‐ 14 years, 15 ‐ 18 years and > 18 years 3. if a participant within his/her age group were to select a sealed envelope card containing supplement, as the arm, then the next patient of the same group would be automatically assigned to diet counselling. If there was a drop out, then the next participant of that gender and age would replace the drop out in that group. The above was done because of the relatively small number of participants in each group. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Paper states "randomized" but gives no further detail on method of randomisation. Personal communication confirmed that participants split into groups according to age and gender and first participant in each group chose a sealed envelope that contained a card allocating to either supplement or dietary advice group. |
| Allocation concealment (selection bias) | High risk | Unclear from paper. However communication with lead author confirmed that allocation was concealed from initial participant as the cards were in sealed envelopes ‐ low risk of bias. But other participants were allocated alternately ‐ high risk of bias as clinician can foresee which treatment next participant will receive. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Participants could not be blinded as treatment options were counselling or dietary supplements. No discussion in paper of whether outcome assessors were blinded to participants' treatment group. However, lead author confirmed that different physicians were used, but she was not sure if patients had discussed their treatment arm during clinic visits therefore we judge that blinding was not present. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Two participants dropped out (one from each group) after baseline measurements. Reasons given (feeling unwell, change of mind). |
| Selective reporting (reporting bias) | Unclear risk | Paper appears to address all the outcomes measured in the 'Resuts' section. No access to study protocol to double check, however the lead author has confirmed that all planned outcome measures were reported. |
Poustie 2006.
| Methods | Randomised controlled study. Duration: 12 months. Parallel design. Multicentre (7 specialist paediatric CF centres and their associated shared care clinics and 7 smaller paediatric CF clinics). Location: UK. |
|
| Participants | 102 children aged 2 ‐ 15 years with CF and at least one of following criteria: BMI < 25th centile but > 0.4th centile; or no increase in weight over the previous 3 months; or 5% decrease in weight from baseline over a period of < 6 months. Treatment group (n = 50). Age (mean (SD)): 8.75 (3.72) years. Gender split: 27 males, 23 females. BMI centile (mean (SD)):34.27 (23.96). Weight centile (mean (SD)): 25.07 (20.37). Height centile (mean (SD)):26.69 (24.83). Energy intake (% EAR) (mean (SD)): 118.43 (28.71). FEV1 (% predicted) (mean (SD)): 81.34 (16.16). Control group (n = 52). Age (mean (SD)): 8.79 (3.67) years. Gender split: 27 males, 25 females. BMI centile (mean (SD)): 31.52 (25.36). Weight centile (mean (SD)): 24.69 (22.79). Height centile (mean (SD)): 28.15 (26.93). Energy intake (% EAR) (mean (SD)): 116.24 (29.59). FEV1 (% predicted) (mean (SD)): 73.67 (18.58). |
|
| Interventions |
Treatment: oral calorie supplements (chosen by the children) in the form of drinks sufficient to increase usual energy intake by 20% plus routine dietetic advice. Control: dietary advice alone. |
|
| Outcomes | Change in BMI*
Change in BMI percentile and z score*
Change in weight*
Change in height*
Change in weight percentile*
Change in height percentile* Mid‐upper arm circumference* Tricep skinfold Mid‐arm muscle circumference Energy* and macro‐nutrient* intake FEV1 & FVC expressed as % predicted for age, sex and height* Gastrointestinal symptoms* Eating behaviour Activity levels Lipase intake |
|
| Notes | Sample size calculation undertaken: with a 5% significance level and 90% power, using a conservatively estimated SD for 1 year change in BMI centile of 15 points, authors needed 47 children in each arm or 94 in total. The children selected the supplements that they liked, and we recommended a daily amount sufficient to increase usual energy intake by 20%. Excluded supplements that provided only energy or protein alone. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Paper states that random number tables were used to generate the randomisation code. |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered, opaque envelopes, administered by the pharmacy of the lead centre, were used for treatment group allocation. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The research assistant was not masked to allocation group, but a masked investigator used a computerised growth package for conversion of weight and height to BMI centile. The children in the trial were not masked, as no satisfactory placebo was available. This however did not influence outcomes due to objectivity of the measures. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Paper states that investigators made every effort to obtain full outcome data on all participants, but replaced any missing data with routinely collected data where appropriate. Full reasons given for any drop outs or missing data (unable to collect interim data on 2 children from the treatment group (owing to parental choice or illness) and 1 child from the standard care group (illness)). |
| Selective reporting (reporting bias) | High risk | Paper appears to address all the outcomes measured in the 'Results' section. The investigators of the study have confirmed that assessment of eating behaviour was planned and undertaken, however due to concerns of the validity and reliability of the tools used to assess this outcome in the three age groups of participants, the data were not reported. Lipase intake was also assessed within the dietary diaries however data were not reported on this outcome in the key publication as the diaries were only returned by approximately half of the study participants. |
BMI: body mass index CF: cystic fibrosis EAR: estimated average requirement for age and sex EPA: eicosapentaenoic acid FEV1: forced expiratory volume in 1 second FVC: forced vital capacity SD: standard deviation WFH : weight for height *: outcomes included in review
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abdulhamid 2008 | Intervention is zinc supplements, not an oral calorie supplement. |
| Agtmaal 1990 | Not children with chronic disease / not OPCS. |
| Akobeng 2000 | Not OPCS. |
| Akobeng 2007 | Not OPCS. |
| Alexander 1980 | Not children with chronic disease / not OPCS. |
| Arora 1998 | Not children with chronic disease / not OPCS. |
| Attard‐Montalto 1998 | Not OPCS. |
| Badaloo 1999 | Not OPCS. |
| Ball 1989 | Not OPCS. |
| Barbosa 1999 | Not OPCS. |
| Bengoa 1985 | Not children with chronic disease. |
| Bennett 1999 | Not OPCS. |
| Bernstein 1999 | Not OPCS. |
| Berry 1975 | Not RCT. |
| Borrelli 2006 | Not OPCS |
| Botrán 2011 | Interevntion duration less than one month |
| Braga 1999 | Adult study. |
| Bruzzese 2007 | Not OPCS. |
| Cassidy 1999 | Not OPCS. |
| Chan 1990 | Not OPCS. |
| Chawla 1983 | Not children with chronic disease / not OPCS / unclear if RCT. |
| Coghlin‐Dickson 2000 | Not OPCS. |
| Dartois 1995 | Not OPCS. |
| Dhanraj 1997 | Not children with chronic disease / not OPCS. |
| Dykman 1998 | Not children with chronic disease / not OPCS. |
| Dziechciarz 2007 | Not RCT |
| Edefonti 1999 | Not RCT / not OPCS. |
| Elhasid 1999 | Not RCT. |
| English 1997 | Not children with chronic disease / not OPCS. |
| Fauveau 1992 | Not children with chronic disease. |
| Fell 2000 | Not RCT. |
| Gianotti 1999 | Not children with chronic disease / not OPCS. |
| Gibson 1998 | Not OPCS. |
| Gottrand 1999 | Not OPCS. |
| Granados 1998 | Not OPCS. |
| Haffejee 1980 | Not OPCS. |
| Hanafy 1967 | Not RCT. |
| Hansen 1996 | Not children with chronic disease / not OPCS. |
| Heikens 1989 | Not children with chronic disease. |
| Hopman 1997 | Not children with chronic disease. |
| Hopman 1998 | Not OPCS. |
| INCAP Study | Not children with chronic disease / not OPCS. |
| Iyengar 1979 | Not children with chronic disease. |
| Jackson 1990 | Not children with chronic disease. |
| Jain 2006 | Not OPCS |
| Jamaican Study 1996 | Not children with chronic disease. |
| Johnson 2006 | Both groups received supplemental amino acids |
| Kabir 1994 | Not children with chronic disease / not OPCS. |
| Karkos 2007 | Not RCT |
| Kashirskaja 1996 | Not RCT / not OPCS. |
| Kaur 1979 | Not children with chronic disease / not OPCS. |
| Keele 1995 | Adult study. |
| Kendell 1982 | Adult study / not OPCS. |
| Kingston Project '93 | Not children with chronic disease. |
| Knops 1999 | Not RCT. |
| Kobayashi 1998 | Not OPCS. |
| Koletzko 1992 | Quasi‐randomised study where treatment & comparison group not comparable. |
| Koretz 2007 | Not RCT |
| Kossmann 2000 | Not children with chronic disease / not OPCS. |
| Ladas 2006 | Not RCT |
| Lagstrom 1999 | Not children with chronic disease / not OPCS. |
| Lambert 1995 | Not OPCS. |
| Lepage 2002 | Not OPCS. |
| Linday 2004 | Not OPCS |
| Lloyd‐Still 2006 | Not OPCS. |
| Lutter 2008 | Not RCT |
| Maes 1997 | Not RCT. |
| Mahlungulu 2007 | Not RCT. |
| Manguso 2005 | Not children with chronic disease/Not OPCS. |
| Marques 2004 | Not RCT / Not OPCS. |
| Martorell 1995 | Not children with chronic disease. |
| Mathisen 1999 | Not RCT. |
| McCargar 1998 | Not children with chronic disease / Not OPCS. |
| McClure 2000 | Not OPCS. |
| McWhirter 1994 | Adult study / OPCS administered for less than 1 month. |
| Mok 2006 | Not OPCS |
| Mora 1981 | Not children with chronic disease. |
| Ndekha 2005 | Not OPCS. |
| Newby 2007 | Not RCT ‐ review article. |
| Nielson 2007 | The median age of the sample was 36 and 35 years in both groups. lowest age range in one group was 15 years but data was not presented disaggregated according to age. |
| Oudshoorn 2007 | Intervention uses micronutrient supplements not oral calorie supplements. |
| Papadopoulou 1998 | Not RCT ‐ review article. |
| Papas 2007 | Pharmacokinetic trial of different formulations of vitamin E supplementation, not oral calorie supplementation. |
| Pelekanos 1990 | Not OPCS. |
| Powers 1999 | Not OPCS. |
| Powers 2006 | Not OPCS. |
| Raynor 1999 | Not OPCS. |
| Reifsnider 1998 | Not children with chronic disease / not OPCS. |
| Rettammel 1995 | Not RCT. |
| Rickard 1983 | Not OPCS. |
| Rickard 1984 | Not OPCS. |
| Rickard 1989 | Not OPCS. |
| Rock 1999 | Not OPCS. |
| Rollins 2007 | Oral protein supplement intervention arm only provided to children under 1 year of age. |
| Saiyed 2000 | Not children with chronic disease / not OPCS. |
| Shaw 1999 | Not OPCS. |
| Shepherd 1988 | Not OPCS. |
| Sirisinha 1973 | Not OPCS. |
| Soliman 2004 | Not RCT/Not OPCS |
| Sondel 1987 | Intervention for less than 1 month. |
| Stark 2005 | Not OPCS. |
| Taylor 1999 | Not children with chronic disease / not OPCS. |
| Teixido‐Planas 2005 | Not children with chronic disease. |
| van Eys 1982 | Not OPCS. |
| Walker 2000 | Not children with chronic disease / not OPCS. |
| Yamamoto 2007 | Not RCT |
OPCS: oral protein calorie supplements RCT : randomised controlled trial
Characteristics of ongoing studies [ordered by study ID]
Cox 2014.
| Trial name or title | Ready‐to‐use supplementary food supplements improve endothelial function, haemoglobin and growth in Tanzanian children with sickle cell anaemia: the vascular function intervention study (V‐FIT), a random order crossover trial. |
| Methods | Randomised cross‐over trial. |
| Participants | Tanzanian children (n = 119) with sickle cell anaemia aged 8 ‐ 11.9 years. |
| Interventions | Daily ready‐to‐use supplementary food providing 500 kcal, 1 RDA of vitamins and minerals and 1 mg folate (Nutriset, France), plus weekly anti‐malarial prophylactic chloroquine syrup (150/225 mg base) (Wallace Manufacturing Chemicals, UK), or a vascular ready‐to‐use supplementary food fortified with arginine and citrulline (average 0.2 g/kg/d and 0.1 g/kg/d) plus daily chloroquine syrup (3 mg base/kg/d). |
| Outcomes | Height, weight and body composition by impedance, endothelium‐dependent and ‐independent vasodilatation. |
| Starting date | November 2012. |
| Contact information | Sharon Cox (sharon.cox@lshtm.ac.uk). |
| Notes | Study author contacted for availability of study findings. |
RDA: recommended daily allowance
Contributions of authors
Original review and updates to 2009
Vanessa Poustie, Ruth Watling and Rosalind Smyth wrote the protocol, independently assessed studies for inclusion in this review and extracted the data. Vanessa Poustie and Rosalind Smyth wrote the remainder of the text.
Vanessa Poustie wrote the updates with comments from Rosalind Smyth and Ruth Watling and she acted as guarantor of the review.
Subsequent updates
Damian Francis and Joanne Smith wrote the updates with comments from Ruth Watling. Joanne Smith acts as guarantor of the review.
Sources of support
Internal sources
No sources of support supplied
External sources
North West NHS Executive R&D Directorate Training Fellowship Scheme, UK.
British Dietetic Association General Education Trust, UK.
-
National Institute for Health Research, UK.
This systematic review was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to the Cochrane Cystic Fibrosis and Genetic Disorders Group.
Declarations of interest
Original review and updates to 2009
Two of the authors are dietitians (VP, RW) who have received travel expenses to attend conferences from the manufacturers of oral protein calorie supplements.
All three of the authors of this review were involved in the CALICO trial (Poustie 2006).
Subsequent updates
Ruth Watling is a dietitian who has received sponsorship to attend meetings and conferences and honorarium to present lectures at conferences and study days from companies who produce oral protein calorie supplements. She declares that this does not influence the use of such products in her clinical practice where the clinical condition, age and nutritional status of the child in conjunction with the scientific evidence is paramount to her decision making. Ruth Watling was also involved in the CALICO trial (Poustie 2006).
The remaining authors declare no conflict of interest.
Edited (no change to conclusions)
References
References to studies included in this review
Bayram 2009 {published data only}
- Bayram I, Erbey F, Celik N, Nelson J L, Tanyeli A. The use of a protein and energy dense eicosapentaenoic acid containing supplement for malignancy‐related weight loss in children. Pediatric Blood & Cancer 2009;52(5):571‐4. [DOI] [PubMed] [Google Scholar]
Hanning 1993 {published data only}
- Hanning RM, Blimkie CJ, Bar‐Or O, Lands LC, Moss LA, Wilson WM. Relationships among nutritional status and skeletal and respiratory muscle function in cystic fibrosis: does early dietary supplementation make a difference?. American Journal of Clinical Nutrition 1993;57(4):580‐7. [DOI] [PubMed] [Google Scholar]
- Lands LC, Heigenhauser GJF, Bar‐Or O, Blimkie C, Hanning R, Wilson WM, et al. The effect of early nutritional supplementation on respiratory function in cystic fibrosis (CF). American Review of Respiratory Disease 1992;145:115. [Google Scholar]
Kalnins 1996 {published data only}
- Kalnins D, Corey M, Ellis L, Pencharz PB, Tullis E, Durie PR. Failure of conventional strategies to improve nutritional status in malnourished adolescents and adults with cystic fibrosis. Journal of Pediatrics 2005;147(3):399‐401. [DOI] [PubMed] [Google Scholar]
- Kalnins D, Durie PR. Oral supplements vs normal food intake in children and adults. Israel Journal of Medical Science 1996;32:S120‐1. [Google Scholar]
- Kalnins D, Durie PR, Corey M, Ellis L, Pencharz P, Tullis E. Are oral dietary supplements effective in the nutritional management of adolescents and adults with cystic fibrosis. Pediatric Pulmonology 1996;Suppl 11:314‐5. [Google Scholar]
Poustie 2006 {published data only}
- Poustie VJ, Russell JE, Watling RM, Ashby D, Smyth RL. Baseline characteristics of children participating in the CALICO trial of oral calorie supplements for cystic fibrosis. Journal of cystic fibrosis 2004;3(Suppl 1):S79. [Google Scholar]
- Poustie VJ, Russell JE, Watling RM, Ashby D, Smyth RL. Oral protein energy supplements for children with cystic fibrosis: CALICO multicentre randomised controlled trial. BMJ 2006;332(7542):632‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poustie VJ, Russell JE, Watling RM, Ashby D, Smyth RL. Recruitment of children to the CALICO trial of oral calorie supplements for cystic fibrosis. Journal of cystic fibrosis 2004;3(Suppl 1):S76. [Google Scholar]
- Poustie VJ, Russell JE, Watling RM, Ashby D, Smyth RS. The CALICO multi‐centre randomised controlled trial of oral calorie supplements for children with cystic fibrosis [abstract]. Pediatric Pulmonology 2004;38(Suppl 27):333. [Google Scholar]
References to studies excluded from this review
Abdulhamid 2008 {published data only}
- Abdulhamid I, Beck FW, Millard S, Chen X, Prasad A. Effect of zinc supplementation on respiratory tract infections in children with cystic fibrosis. Pediatric Pulmonology 2008;43(3):281‐7. [DOI] [PubMed] [Google Scholar]
- Abdulhamid I, Millard S, Beck F, Chen X, Wagnen C, Prasad A. Effect of zinc supplementation on respiratory tract infections in children with cystic fibrosis [abstract]. Pediatric Pulmonology 2005;40(Suppl 28):348. [DOI] [PubMed] [Google Scholar]
Agtmaal 1990 {published data only}
- Agtmaal VE, Egger RJ, Bloem MW. Effects of nutritional supplementation on the protein composition of tear fluid of marginally nourished preschool children. Documenta Ophthalmologica 1990;76:178. [Google Scholar]
Akobeng 2000 {published data only}
- Akobeng AK, Miller V, Stanton J, Elbadri AM, Thomas AG. Double‐blind randomized controlled trial of glutamine‐enriched polymeric diet in the treatment of active Crohn's disease. Journal of Pediatric Gastroenterology and Nutrition 2000;30(1):78‐84. [DOI] [PubMed] [Google Scholar]
Akobeng 2007 {published data only}
- Akobeng AK, Richmond K, Miller V, Thomas AG. Effect of exclusive enteral nutritional treatment on plasma antioxidant concentrations in childhood Crohn's disease. Clinical Nutrition 2007;26(1):51‐6. [DOI] [PubMed] [Google Scholar]
Alexander 1980 {published data only}
- Alexander JW, MacMillan BG, Stinnett JD, Ogle CK, Bozian RC, Fischer JE, et al. Beneficial effects of aggressive protein feeding in severely burned children. Annals of Surgery 1980;192(4):505‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Arora 1998 {published data only}
- Arora NK, Anand NK, Bhan MK, Jailkhani B, Aggarwal A, Meenu R, et al. Nutrient absorption from a fat‐enriched diet in young malnourished children: a randomised controlled trial. Acta Paediatrica 1998;87(2):143‐8. [DOI] [PubMed] [Google Scholar]
Attard‐Montalto 1998 {published data only}
- Attard‐Montalto SP, Hadley J, Kingston JE, Eden OB, Saha V. Ongoing assessment of nutritional status in children with malignant disease. Pediatric Hematology and Oncology 1998;15(5):393‐403. [DOI] [PubMed] [Google Scholar]
Badaloo 1999 {published data only}
- Badaloo A, Boyne M, Reid M, Persaud C, Forrester T, Millward DJ, et al. Dietary protein, growth and urea kinetics in severely malnourished children and during recovery. Journal of Nutrition 1999;129(5):969‐79. [DOI] [PubMed] [Google Scholar]
Ball 1989 {published data only}
- Ball C, Punchard NA, Howell S, Thompson RPH. Essential fatty acid supplementation in children with cystic fibrosis [abstract]. Proceedings of the 16th Annual Meeting of the European Working Group for Cystic Fibrosis. 1989:84. [MEDLINE: ]
Barbosa 1999 {published data only}
- Barbosa E, Moreira EA, Goes JE, Faintuch J. Pilot study with a glutamine‐supplemented enteral formula in critically ill infants. Revista do Hospital das Clinicas 1999;54(1):21‐4. [DOI] [PubMed] [Google Scholar]
Bengoa 1985 {published data only}
- Bengoa JM, Griessen M, Kahn JM, Vadas L, Infante F, LE. Glycemic response and hydrogen production in blenderized and polymeric enteral nutrition formulas [abstract]. Clinical Nutrition 1985;4(Spec Suppl):112. [DOI] [PubMed] [Google Scholar]
Bennett 1999 {published data only}
- Bennett VA, Morales E, Gonzalez J, Peerson JM, Lopez DR, Brown KH. Effects of dietary viscosity and energy density on total daily energy consumption by young Peruvian children. American Journal of Clinical Nutrition 1999;70(2):285‐91. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Bernstein 1999 {published data only}
- Bernstein ML, Baruchel S, Devine S, Markoglou N, Wainer IW, Williams M, et al. Phase I and pharmacokinetic study of CI‐980 in recurrent pediatric solid tumor cases: a Pediatric Oncology Group study. Journal of the American Society of Pediatric Hematology/Oncology 1999;21(6):494‐500. [PubMed] [Google Scholar]
Berry 1975 {published data only}
- Berry HK, Kellogg FW, Hunt MM, Ingberg RL, Gutjahr C. Dietary supplement and nutrition in children with cystic fibrosis. American Journal of Diseases of Childhood 1975;129:165‐71. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Borrelli 2006 {published data only}
- Borrelli O, Cordischi L, Cirulli M, Paganelli M, Labalestra V, Uccini S, Russo PM, Cucchiara S. Polymeric diet alone versus corticosteroids in the treatment of active Crohn's disease: a randomised controlled open‐label trial. Clinical Gastroenterology & Hepatology 2006;4(6):744‐53. [DOI] [PubMed] [Google Scholar]
Botrán 2011 {published data only}
Braga 1999 {published data only}
- Braga M, Gianotti L, Radaelli G, Vignali A, Mari G, Gentilini O, et al. Perioperative immunonutrition in patients undergoing cancer surgery: results of a randomized double‐blind phase 3 trial. Archives of Surgery 1999;134(4):428‐33. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Bruzzese 2007 {published data only}
- Bruzzese E, Raia V, Spagnuolo MI, Volpicelli M, Marco G, Maiuri L, et al. Effect of Lactobacillus GG supplementation on pulmonary exacerbations in patients with cystic fibrosis: a pilot study. Clinical Nutrition 2007;26(3):322‐8. [DOI] [PubMed] [Google Scholar]
Cassidy 1999 {published data only}
- Cassidy JT. Medical management of children with juvenile rheumatoid arthritis. Drugs 1999;58(5):831‐50. [DOI] [PubMed] [Google Scholar]
Chan 1990 {published data only}
- Chan JC, Greifer I, Boineau FG, Mendoza SA, McEnery PT, Strife CF, et al. Rational of the growth failure in children with renal diseases study. Journal of Pediatrics 1990; Vol. 116:S11‐6. [MEDLINE: ] [DOI] [PubMed]
- Massie MD, Strife CF, Foreman JW, Chan JC. Quality control of the nutritional component of the growth failure in children with renal diseases study. Journal of Pediatrics 1990;116(2):S40‐5. [DOI] [PubMed] [Google Scholar]
Chawla 1983 {published data only}
- Chawla P, Puri R. An evaluation of the pre‐school supplementary feeding programme in Chandigarh. Indian Pediatrics 1983;20(5):357‐61. [MEDLINE: ] [PubMed] [Google Scholar]
Coghlin‐Dickson 2000 {published data only}
- Coghlin‐Dickson TM, Wong RM, Offrin RS, Shizuru JA, Johnston LJ, Hu WW, et al. Effect of oral glutamine supplementation during bone marrow transplantation. Journal of Parenteral and Enteral Nutrition 2000;24(2):61‐6. [DOI] [PubMed] [Google Scholar]
Dartois 1995 {published data only}
- Dartois AM, Terzi F, Kleinknecht C, Niaudet P. Comparison of two protein diets in infants with chronic renal failure. Journal of Renal Nutrition 1995;5:52‐61. [Google Scholar]
Dhanraj 1997 {published data only}
- Dhanraj P, Chacko A, Mammen M, Bharathi R. Hospital‐made diet versus commercial supplement in postburn nutritional support. Burns 1997;23(6):512‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Dykman 1998 {published data only}
- Dykman KD, Dykman RA. Effect of nutritional supplements on attentional‐deficit hyperactivity disorder. Integrative Physiological and Behavioral Science : the official journal of the Pavlovian Society 1998;33(1):49‐60. [DOI] [PubMed] [Google Scholar]
Dziechciarz 2007 {published data only}
- Dziechciarz P, Horvath A, Shamir R, Szajewska H. Meta‐analysis: enteral nutrition in active Crohn's disease in children. Alimentary Pharmacology & Theraputics 2007;26(6):795‐806. [DOI] [PubMed] [Google Scholar]
Edefonti 1999 {published data only}
- Edefonti A, Picca M, Damiani B, Loi S, Ghio L, Giani M, et al. Dietary prescription based on estimated nitrogen balance during peritoneal dialysis. Pediatric Nephrology 1999;13(3):253‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Elhasid 1999 {published data only}
- Elhasid R, Laor A, Lischinsky S, Postovsky S, Weyl BA. Nutritional status of children with solid tumors. Cancer 1999;86(1):119‐25. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Massie MD, Strife CF, Foreman JW, Chan JC. Quality control of the nutritional component of the growth failure in children with renal diseases study. Journal of Pediatrics 1990; Vol. 116, issue 2:S40‐5. [MEDLINE: ] [DOI] [PubMed]
English 1997 {published data only}
- English RM, Badcock JC, Giay T, Ngu T, Waters AM, Bennett SA. Effect of nutrition improvement project on morbidity from infectious diseases in preschool children in Vietnam: comparison with control commune. BMJ 1997;315(7116):1122‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fauveau 1992 {published data only}
Fell 2000 {published data only}
- Fell JM, Paintin M, Arnaud BF, Beattie RM, Hollis A, Kitching P, et al. Mucosal healing and a fall in mucosal pro‐inflammatory cytokine mRNA induced by a specific oral polymeric diet in paediatric Crohn's disease. Alimentary Pharmacology & Therapeutics 2000;14(3):281‐9. [DOI] [PubMed] [Google Scholar]
Gianotti 1999 {published data only}
- Gianotti L, Braga M, Fortis C, Soldini L, Vignali A, Colombo S, et al. A prospective, randomized clinical trial on perioperative feeding with an arginine‐, omega‐3 fatty acid‐, and RNA‐enriched enteral diet: effect on host response and nutritional status. Journal of Parenteral and Enteral Nutrition 1999;23(6):314‐20. [DOI] [PubMed] [Google Scholar]
Gibson 1998 {published data only}
- Gibson RA, Makrides M, Neumann MA, SK. Dietary long chain polyunsaturated fatty acids (LCPUFA) do not influence growth of term infants: a randomised clinical trial [abstract]. Proceedings of the Nutrition Society of Australia. 1998; Vol. 22:95.
Gottrand 1999 {published data only}
- Gottrand F, Hankard R, Michaud L, Ategbo S, Dabadie A, Druon D, et al. Effect of glucose to fat ratio on energy substrate disposal in children with cystic fibrosis fed enterally. Clinical Nutrition 1999;18(5):297‐300. [DOI] [PubMed] [Google Scholar]
Granados 1998 {published data only}
- Granados EA. Which treatment should children with recurrent urinary infections, without anatomical anomalies, receive?. Archivos Espanoles de Urologia 1998;51(4):354‐7. [PubMed] [Google Scholar]
Haffejee 1980 {published data only}
- Haffejee A, Angorn IB, Baker LW. Nutritional support in high‐output fistulas of the alimentary tract. South African Medical Journal 1980;57(7):227‐31. [PubMed] [Google Scholar]
Hanafy 1967 {published data only}
- Hanafy MM, Aref MK, Seddik Y, Zein MS, el‐Kashlan KM. Effect of supplementary feeding on the nutritional status of preschool children. Journal of Tropical Medicine and Hygeine 1967;70(10):238‐42. [MEDLINE: ] [PubMed] [Google Scholar]
Hansen 1996 {published data only}
- Hansen GV, Nielson L, Kluger E, Thysen M, Emmertsen H, Stengaard‐Pedersen K, et al. Nutritional status of Danish rheumatoid arthritis patients and effects of a diet adjusted in energy intake, fish‐meal and antioxidants. Scandinavian Journal of Rheumatology 1996;25(5):325‐30. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Heikens 1989 {published data only}
- Heikens GT, Schofield WN, Dawson S, and‐Grantham MS. The Kingston project. I. Growth of malnourished children during rehabilitation in the community, given a high energy supplement. European Journal of Clinical Nutrition 1989;43(3):145‐60. [PubMed] [Google Scholar]
Hopman 1997 {published data only}
- Hopman GD, Pena EG, Broekhof WJ. Nutritional support for children who undergo bone marrow transplantation: enteral or parenteral?. Tijdschrift voor Kindergeneeskunde 1997;65:39. [Google Scholar]
Hopman 1998 {published data only}
- Hopman E, Pena E, Broekhof S, Cessie S, Hullu A, Weel M, et al. Nutritional support in children undergoing bone marrow transplantation: enteral or parenteral? [abstract]. European Journal of Gastroenterology & Hepatology 1998;10:A. [Google Scholar]
INCAP Study {published data only}
- Caulfield LE, Himes JH, Rivera JA. Nutritional supplementation during early childhood and bone mineralization during adolescence. Journal of Nutrition 1995;125(Suppl 4):1104S‐10S. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Haas JD, Martinez EJ, Murdoch S, Conlisk E, Rivera JA, Martorell R. Nutritional supplementation during the preschool years and physical work capacity in adolescent and young adult Guatemalans. Journal of Nutrition 1995;125(Suppl 4):1078S‐89S. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Habicht JP, Martorell R, Rivera JA. Nutritional impact of supplementation in the INCAP longitudinal study: analytic strategies and interences. Journal of Nutrition 1995;125(Suppl 4):1042S‐50S. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Martorell R. Results and implications of the INCAP follow‐up study. Journal of Nutrition 1995;125(Suppl 4):1127S‐38S. [DOI] [PubMed] [Google Scholar]
- Martorell R, Habicht JP, Rivera JA. History and design of the INCAP longitudinal study (1969‐77) and its follow‐up (1988‐89). Journal of Nutrition 1995;125(Suppl 4):1027S‐41S. [DOI] [PubMed] [Google Scholar]
- Pickett KE, Haas JD, Rivera JA, Martorell R. Early nutritional supplementation and skeletal maturation in Guatemalan adolescents. Journal of Nutrition 1995;125(Suppl 4):1097S‐103S. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Rivera JA, Habicht JP. The recovery of Guatemalan children with mild to moderate wasting: factors enhancing the impact of supplementary feeding. American Journal of Public Health 1996;86(10):1430‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera JA, Habicht JP, Robson DS. Effect of supplementary feeding on recovery from mild to moderate wasting in preschool children. American Journal of Clinical Nutrition 1991;54(1):62‐8. [DOI] [PubMed] [Google Scholar]
- Rivera JA, Martorell R, Ruel MT, Habicht JP, Haas JD. Nutritional supplementation during the preschool years influences body size and composition of Guatemalan adolescents. Journal of Nutrition 1995;125(Suppl 4):1068S‐77S. [DOI] [PubMed] [Google Scholar]
- Schroeder DG, Martorell R, Rivera JA, Ruel MT. Age differences in the impact of nutritional supplementation on growth. Journal of Nutrition 1995;125(Suppl 4):1051S‐9S. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Iyengar 1979 {published data only}
- Iyengar AK, Rao BS, Reddy V. Effect of varying protein and energy intakes on nitrogen balance in Indian preschool children. British Journal of Nutrition 1979;42(3):417‐23. [DOI] [PubMed] [Google Scholar]
Jackson 1990 {published data only}
- Jackson AA, Doherty J, Benoist MH, Hibbert J, Persaud C. The effect of the level of dietary protein, carbohydrate and fat on urea kinetics in young children during rapid catch‐up weight gain. British Journal of Nutrition 1990;64(2):371‐85. [DOI] [PubMed] [Google Scholar]
Jain 2006 {published data only}
- Jain MK, Heyland D, Dhaliwal R, Day AG, Drover J, Keefe L, Gelula M. Dissemination of the Canadian clinical practice guidelines for nutrition support: results of a cluster randomized controlled trial. Critical Care Medicine 2006;34(9):2362‐9. [DOI] [PubMed] [Google Scholar]
Jamaican Study 1996 {published data only}
- Grantham‐McGregor SM, Powell CA, Walker SP. Nutritional supplements, stunting and child development (letter). Lancet 1989;2:809‐10. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Grantham‐McGregor SM, Powell CA, Walker SP, Himes JH. Nutritional supplementation, psychosocial stimulation, and mental development of stunted children: the Jamaica study. Lancet 1991;338(8758):1‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Meeks‐Gardner J, Grantham‐McGregor SM, Chang SM, Himes JH, Powell CA. Activity and behavioural development in stunted and non‐stunted children and response to nutritional supplementation. Child Development 1995;66(6):1785‐97. [PubMed] [Google Scholar]
- Walker SP, Grantham‐McGregor SM, Himes JH, Powell CA, Chang SM. Early childhood supplementation does not benefit the long‐term growth of stunted children in Jamaica. Journal of Nutrition 1996;126(12):3017‐24. [DOI] [PubMed] [Google Scholar]
- Walker SP, Powell CA, Grantham‐McGregor SM, Himes JH, Chang SM. Nutritional supplementation, psychosocial stimulation, and growth of stunted children: the Jamaica study. American Journal of Clincial Nutrition 1991;54(4):642‐8. [DOI] [PubMed] [Google Scholar]
Johnson 2006 {published data only}
- Johnson T, MacDonald S, Hill SM, Thomas A, Murphy MS. Treatment of active Crohn's disease in children using partial enteral nutrition with liquid formula: a randomised controlled trial. Gut 2006;55(3):356‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kabir 1994 {published data only}
- Kabir I, Malek MA, Rahman MM, Khaled MA, Mahalanabis D. Changes in body composition of malnourished children after dietary supplementation as measured by bioelectrical impedance. American Journal of Clinical Nutrition 1994;59(1):5‐9. [DOI] [PubMed] [Google Scholar]
Karkos 2007 {published data only}
- Karkos PD, Leong SC, Arya AK, Papouliakos SM, Apostolidou MT, Issing WJ. Complementry ENT: A systematic review of commonly used supplements. Journal of Laryngology & Otology 2007;121(8):779‐82. [DOI] [PubMed] [Google Scholar]
Kashirskaja 1996 {published data only}
- Kashirskaja N, Hill CM, Ilangovan P, Kapranov N, Simonova O, Shabalova L, Rolles CJ. The relative contribution of optimal nutritional support in cystic fibrosis. Journal of the Royal Society of Medicine 1996;89(Suppl 27):48‐50. [MEDLINE: ] [PMC free article] [PubMed] [Google Scholar]
Kaur 1979 {published data only}
- Kaur P, Bhatt CM. Effect of supplementary feeding on the nutritional status of pre‐school children. Indian Pediatrics 1979; Vol. 16, issue 12:1091‐6. [MEDLINE: ] [PubMed]
Keele 1995 {published data only}
- Keele AM, Bray MJ, Emery PW, Silk DBA. Two‐phase randomised controlled clinical trial of oral dietary supplements in surgical patients [abstract]. Proceedings of the 17th European Society of Parenteral & Enteral Nutrition Conference. 1995:8.
Kendell 1982 {published data only}
- Kendell BD, Fonseca RJ, Lee M. Postoperative nutritional supplementation for the orthognathic surgery patient. Journal of Oral and Maxillofacial Surgery 1982;40(4):205‐13. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kingston Project '93 {published data only}
- Heikens GT, Schofield WN, Christie CD, Gernay J, Dawson S. The Kingston Project III. The effects of high energy supplement and metronidazole on malnourished children rehabilitated in the community: morbidity and growth. European Journal of Clinical Nutrition 1993;47(3):174‐91. [MEDLINE: ] [PubMed] [Google Scholar]
- Heikens GT, Schofield WN, Dawson S. The Kingston Project II. The effects of high energy supplement and metronidazole on malnourished children rehabilitated in the community: anthropometry. European Journal of Clinical Nutrition 1993;47(3):160‐73. [MEDLINE: ] [PubMed] [Google Scholar]
- Heikens GT, Schofield WN, Dawson S, Grantham‐McGregor SM. The Kingston Project I. Growth of malnourished children during rehabilitation in the community, given a high energy supplement. European Journal of Clinical Nutrition 1989;43(3):145‐60. [MEDLINE: ] [PubMed] [Google Scholar]
Knops 1999 {published data only}
- Knops N, Wulffraat N, Lodder S, Houwen R, Meer K. Resting energy expenditure and nutritional status in children with juvenile rheumatoid arthritis. Journal of Rheumatology 1999;26(9):2039‐43. [MEDLINE: ] [PubMed] [Google Scholar]
Kobayashi 1998 {published data only}
- Kobayashi K, Katsumata T, Yokoyama K, Takahashi H, Igarashi M, Saigenji K. A randomized controlled study of total parenteral nutrition and enteral nutrition by elemental and polymeric diet as primary therapy in active phase of Crohn's disease. Nippon Shokakibyo Gakkai Zasshi [Japanese Journal of Gastroenterology] 1998;95(11):1212‐21. [PubMed] [Google Scholar]
Koletzko 1992 {published data only}
- Koletzko B, Ruhl‐Bagheri I, Thiel I, Steinkamp G. Effects of a formula supplement rich in linoleic acid on the essential fatty acid status of cystic fibrosis patients [abstract]. Clinical Nutrition 1992;11:39. [DOI] [PubMed] [Google Scholar]
- Steinkamp G, Demmelmair H, Ruhl‐Bagheri I, Hardt H, Koletzko B. Energy supplements rich in linoleic acid improve body weight and essential fatty acid status of cystic fibrosis patients. Journal of Gastroenterology and Nutrition 2000;31(4):418‐23. [DOI] [PubMed] [Google Scholar]
Koretz 2007 {published data only}
- Koretz RL, Avenell A, Lipman TO, Braunschweig CL, Milne AC. Does enteral nutrition affect clinical outcome? A systematic review of the randomised trials. American Journal of Gastroenterology 2007;102(2):412‐429. [DOI] [PubMed] [Google Scholar]
Kossmann 2000 {published data only}
- Kossmann J, Nestel P, Herrera MG, Amin A, Fawzi WW. Undernutrition in relation to childhood infections: a prospective study in the Sudan. European Journal of Clinical Nutrition 2000;54(6):463‐72. [DOI] [PubMed] [Google Scholar]
Ladas 2006 {published data only}
- Ladas EJ, Sacks N, Brophy P, Rogers PC. Standards of nutritional care in pediatric oncology: results from a nationwide survey on the standards of practice in pediatric oncology. A children's oncology groups study. Pediatric blood & cancer 2006;46(3):339‐44. [DOI] [PubMed] [Google Scholar]
Lagstrom 1999 {published data only}
- Lagstrom H, Seppanen R, Jokinen E, Niinikoski H, Ronnemaa T, Viikari J, et al. Influence of dietary fat on the nutrient intake and growth of children from 1 to 5 y of age: the Special Turku Coronary Risk Factor Intervention Project. American Journal of Clinical Nutrition 1999;69(3):516‐23. [DOI] [PubMed] [Google Scholar]
Lambert 1995 {published data only}
- Lambert B, MacDonald A, Booth IW. An open prospective randomised comparison of a newly available high energy enteral feed, with a conventional formula designed for children 1‐6 years [abstract]. Proceedings of the 5th The British Association for Parenteral and Enteral Nutrition Conference. 1995. [MEDLINE: ]
Lepage 2002 {published data only}
- Lepage G, Yesair DW, Ronco N, Champagne J, Bureau N, Chemtob S, et al. Effect of an organized lipid matrix on lipid absorption and clinical outcomes in patients with cystic fibrosis. Journal of Pediatrics 2002;141(2):178‐85. [DOI] [PubMed] [Google Scholar]
Linday 2004 {published data only}
- Linday LA. Nutritional supplements and pediatric upper respiratory tract illnesses [letter]. Journal of Allergy & Clinical Immunology 2006;117(4):953‐4. [DOI] [PubMed] [Google Scholar]
- Linday LA, Shindledecker RD, Tapia‐Mendoza J, Dolitsky JN. Effect of daily cod liver oil and a multivitamin‐mineral supplement with selenium on upper respiratory tract pediatric visits by young, inner‐city, Latino children: randomized pediatric sites.. The Annals of otology, rhinology, and laryngology 2004;113(11):891‐901. [DOI] [PubMed] [Google Scholar]
Lloyd‐Still 2006 {published data only}
- Lloyd‐Still J, Powers CA, Hoffman DR, Boyd‐Trull K, Lester LA, Benisek DC, et al. A randomized, controlled study examining the bioavailability and safety of an algal docosahexaenoic acid (DHA) triacylglycerol in cystic fibrosis (CF) patients [abstract]. Pediatric Pulmonology 2004;38(Suppl 27):331. [Google Scholar]
- Lloyd‐Still JD, Powers CA, Hoffman DR, Arterburn LM, Benisek DC, Lester LA. Bioavailability and safety of an algal docosahexaenoic acid (DHA) triglyceride in cystic fibrosis (CF) [abstract]. Pediatric Research 2001;49(4 Suppl):455a. [Google Scholar]
- Lloyd‐Still JD, Powers CA, Hoffman DR, Boyd‐Trull K, Arterburn LM, Benisek DC, et al. Blood and tissue essential fatty acids after docosahexaenoic acid supplementation in cystic fibrosis [abstract]. Pediatric Pulmonology 2001;32(Suppl 22):263. [Google Scholar]
- Lloyd‐Still JD, Powers CA, Hoffman DR, Boyd‐Trull K, Lester LA, Benisek DC, et al. Bioavailability and safety of a high dose of docosahexaenoic acid triacylglycerol of algal origin in cystic fibrosis patients: a randomized, controlled study. Nutrition 2006;22(1):36‐46. [DOI] [PubMed] [Google Scholar]
- Powers CA, Lloyd‐Still JD, Hoffman DR, Arterburn LM, Benisek DC, Lester LA. Lipid soluble antioxidant status during supplementation with algal docosahexaenoic acid triglyceride in CF [abstract]. Proceedings of 24th European Cystic Fibrosis Conference; 2001 June 6‐9; Vienna, Austria. 2001:P133.
Lutter 2008 {published data only}
- Lutter CK, Rodriguez A, Fuenmayor G, Avila L, Sempertegui F, Escobar J. Growth and micronutrient status in children receiving a fortified complementary food. Journal of nutrition 2008;138(2):379‐88. [DOI] [PubMed] [Google Scholar]
Maes 1997 {published data only}
- Maes M, Sokal E, Otte JB. Growth factors in children with end‐stage liver disease before and after liver transplantation: a review. Pediatric Transplantation 1997;1(2):171‐5. [MEDLINE: ] [PubMed] [Google Scholar]
Mahlungulu 2007 {published data only}
- Mahlungulu S, Grobler LA, Visser ME, Volmink J. Nutritional interventions for reducing morbidity and mortality in people with HIV. Cochrane Database of Systematic Reviews 2007, Issue 3. [DOI: 10.1002/14651858.CD004536.pub2] [DOI] [PubMed] [Google Scholar]
Manguso 2005 {published data only}
- Manguso F, D'Ambra G, Menchise A, Sollazzo R, D'Agostino L. Effects of an appropriate oral diet on the nutritional status of patients with HCV‐related liver cirrhosis: A prospective study. Clinical Nutrition 2005;24(5):751‐9. [PUBMED: 16182039] [DOI] [PubMed] [Google Scholar]
Marques 2004 {published data only}
- Marques IL, Barros Almeida Peres SP, Bettiol H, Barbieri MA, Andrea M, Souza L. Growth of children with isolated Robin sequence treated by nasopharyngeal intubation: Importance of a hypercaloric diet. Cleft Palete‐Craniofacial Journal 2004;41(1):53‐8. [PUBMED: 14697069] [DOI] [PubMed] [Google Scholar]
Martorell 1995 {published data only}
- Martorell R, Habicht JP, Rivera JA. History and design of the INCAP longitudinal study (1969‐77) and its follow‐up (1988‐89). Journal of Nutrition 1995;125:1027S‐41S. [DOI] [PubMed] [Google Scholar]
Mathisen 1999 {published data only}
- Mathisen B, Worrall L, Masel J, Wall C, Shepherd RW. Feeding problems in infants with gastro‐oesophageal reflux disease: a controlled study. Journal of Paediatrics and Child Health 1999;35(2):163‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
McCargar 1998 {published data only}
- McCargar LJ, Innis SM, Bowron E, Leichter J, Dawson K, Toth E, et al. Effect of enteral nutritional products differing in carbohydrate and fat on indices of carbohydrate and lipid metabolism in patients with NIDDM. Molecular & Cellular Biochemistry 1998;188(1‐2):81‐9. [PubMed] [Google Scholar]
McClure 2000 {published data only}
- McClure RJ, Newell SJ. Randomised controlled study of clinical outcome following trophic feeding. Archives of Disease in Childhood. Fetal and Neonatal Edition 2000;82(1):F29‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
McWhirter 1994 {published data only}
Mok 2006 {published data only}
- Mok E, Eleouet‐Da VC, Daubrosse C, Gottrand F, Rigal O, Fontan JE, Cuisset JM, Guilhot J, Hankard R. Oral glutamine and amino acid supplementation inhibit whole‐body protein degradation in children with Duchenne muscular dystrophy. American Journal of Clinical Nutrition 2006;83(4):823‐8. [DOI] [PubMed] [Google Scholar]
Mora 1981 {published data only}
- Mora JO, Herrera MG, Suescun J, Navarro L, Wagner M. The effects of nutritional supplementation on physical growth of children at risk of malnutrition. American Journal of Clinical Nutrition 1981;34(9):1885‐92. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ndekha 2005 {published data only}
- Ndekha MJ, Manary MJ, Ashorn P, Briend A. Home‐based therapy with ready‐to‐use therapeutic food is of benefit to malnourished, HIV‐infected Malawian children. Acta Paediatrica 2005;94(2):222‐5. [PUBMED: 15981758] [DOI] [PubMed] [Google Scholar]
Newby 2007 {published and unpublished data}
- Newby EA, Sawczenko A, Thomas AG, Wilson D. Interventions for growth failure in childhood Crohn's disease. Cochrane Database of Systematic Reviews 2007, Issue 1. [DOI: 10.1002/14651858.CD003873.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Nielson 2007 {published data only}
- Nielsen AA, Nielsen JN, Gronbaek H, Eivindson M, Vind I, Munkholm P, Brandslund I. Impact of enteral supplements enriched with omega‐3 fatty acid and/or omega‐6 fatty acids, arginine and ribonucleic acid compounds on leptin levels and nutritional status in active Crohn's disease treated with prednisolone. Digestion 2007;75(1):10‐6. [DOI] [PubMed] [Google Scholar]
Oudshoorn 2007 {published data only}
- Oudshoorn JH, Klijn PH, Hofman Z, Voorbij HA, Ent CK, Berger R, et al. Dietary supplementation with multiple micronutrients: no beneficial effects in pediatric cystic fibrosis patients. Journal of Cystic Fibrosis 2007;6(1):35‐40. [DOI] [PubMed] [Google Scholar]
Papadopoulou 1998 {published data only}
- Papadopoulou A. Nutritional considerations in children undergoing bone marrow transplantation. European Journal of Clinical Nutrition 1998;52(12):863‐71. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Papas 2007 {published data only}
- Papas K, Kalbfleisch J, Mohon R. Bioavailability of a novel, water‐soluble vitamin E formulation in malabsorbing patients. Digestive Diseases and Sciences 2007;52(2):347‐52. [DOI] [PubMed] [Google Scholar]
Pelekanos 1990 {published data only}
- Pelekanos JT, Holt TL, Ward LC, Cleghorn GJ, Shepherd RW. Protein turnover in malnourished patients with cystic fibrosis: Effects of elemental and non elemental nutritional supplements. Journal of Pediatric Gastroenterology & Nutrition 1990;10(3):339‐43. [DOI] [PubMed] [Google Scholar]
Powers 1999 {published data only}
- Powers SW, Schindler T, Schwarber L, Deeks CM, Byars KC, Arthur S, et al. Behavioral treatment to improve nutrition in toddlers with cystic fibrosis [abstract]. Pediatric Pulmonology 1999;Suppl 19:329. [Google Scholar]
Powers 2006 {published data only}
- Powers SW, Piazza‐Waggoner C, Jones JS, Ferguson KS, Daines C, Acton JD. Examining clinical trial results with single‐subject analysis: an example involving behavioural and nutrition treatment for young children with cystic fibrosis. Journal of Pediatric Psychology 2006;31(6):574‐81. [DOI] [PubMed] [Google Scholar]
Raynor 1999 {published data only}
- Raynor P, Rudolf MC, Cooper K, Marchant P, Cottrell D. A randomised controlled trial of specialist health visitor intervention for failure to thrive. Archives of Disease in Childhood 1999;80(6):500‐6. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Reifsnider 1998 {published data only}
- Reifsnider E. Follow‐up study of children with growth deficiency. Western Journal of Nursing Research 1998;20(1):14‐29. [DOI] [PubMed] [Google Scholar]
- Reifsnider E. Reversing growth deficiency in children: the effect of a community‐based intervention. Journal of Pediatric Health Care 1998;12(6 Pt 1):305‐12. [DOI] [PubMed] [Google Scholar]
Rettammel 1995 {published data only}
- Rettammel AL, Marcus MS, Farrell PM, Sondel SA, Koscil RE, Mischler EH. Oral supplementation with a high‐fat, high‐energy product improves nutritional status and alters serum lipids in patients with cystic fibrosis. Journal of the American Dietetic Association 1996;95:454‐9. [DOI] [PubMed] [Google Scholar]
Rickard 1983 {published data only}
- Rickard KA, Foland BB, Detamore CM, Coates TD, Grosfeld JL, White NM, et al. Effectiveness of central parenteral nutrition versus peripheral parenteral nutrition plus enteral nutrition in reversing protein‐ energy malnutrition in children with advanced neuroblastoma and Wilm's tumor: A prospective randomized study. The American Journal of Clinical Nutrition 1983;38:445‐56. [DOI] [PubMed] [Google Scholar]
Rickard 1984 {published data only}
- Rickard KA, Loghani ES, Grosfeld JL, Detamore CM, White NM, Foland BB, et al. Effectiveness of enteral and parenteral nutrition in preventing and/or reversing PEM in children with advanced neuroblastoma: a prospective randomized study [abstract]. Proceedings of the Annual Meeting of the American Society of Clinical Oncologists. 1984.
Rickard 1989 {published data only}
- Godshall BJ, Rickard KA, Loghmani ES, Coates TD, Grosfeld JL, Weetman RM, et al. Intergration of nutritional support into oncologic treatment protocols for high and low nutritional risk children with Wilm's tumor: a prospective randomized study [abstract]. Proceedings of the Annual Meeting of the American Society of Clinical Oncologists. 1987. [MEDLINE: ]
- Rickard KA, Detamore CM, Coates TD, Grosfeld JL, Weetman RM, White NM, et al. Effect of nutrition staging on treatment delays and outcome in Stage IV neuroblastoma. Cancer 1983;52:587‐98. [DOI] [PubMed] [Google Scholar]
- Rickard KA, Godshall BJ, Loghmani ES, Coates TD, Grosfeld JL, Weetman RM, et al. Integration of nutrition support into oncologic treatment protocols for high and low nutritional risk children with Wilm's tumour. A prospective randomized study. Cancer 1989;64(2):491‐509. [DOI] [PubMed] [Google Scholar]
- Rickard KA, Grosfeld JL, Kirksey A, Ballantine TVN, Baehner RL. Reversal of protein energy malnutrition in children during treatment of advanced neoplastic disease. Annals of Surgery 1979;190(6):771‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rickard KA, Loghmani ES, Grosfeld JL, Detamore CM, White NM, Foland BB, et al. Effectiveness of enteral and parenteral nutrition in preventing and/or reversing PEM in children with advanced neuroblastoma: a prospective randomised study [abstract]. Proceedings of the Annual Meeting of the American Society of Clinical Oncologists. 1984.
- Rickard KA, Loghmani ES, Grosfeld JL, Lingard CD, White NM, Foland BB, et al. Short‐ and long‐term effectiveness of enteral and parenteral nutrition in reversing or preventing protein‐energy malnutrition in advanced neuroblastoma A prospective randomised study. Cancer 1985;56(12):2881‐97. [DOI] [PubMed] [Google Scholar]
Rock 1999 {published data only}
- Rock MJ, Davis L, Marcus M, Lai HC, Douglas J, Kosorok M, et al. Early nutritional intervention in infants with CF [abstract]. Pediatric Pulmonology 1999;Suppl 19:102‐3. [Google Scholar]
Rollins 2007 {published data only}
- Rollins NC, Broeck J, Kindra G, Pent M, Kasambira T, Bennish ML. The effect of nutritional support on weight gain of HIV‐infected children with prolonged diarrhoea. Acta Paediatrica 2007;96(1):62‐8. [DOI] [PubMed] [Google Scholar]
Saiyed 2000 {published data only}
- Saiyed F, Seshadri S. Impact of the integrated package of nutrition and health services. Indian Journal of Pediatrics 2000;67(5):322‐8. [DOI] [PubMed] [Google Scholar]
Shaw 1999 {published data only}
- Shaw WC, Bannister RP, Roberts CT. Assisted feeding is more reliable for infants with clefts‐a randomized trial. The Cleft Palate‐Craniofacial Journal 1999;36(3):262‐8. [DOI] [PubMed] [Google Scholar]
Shepherd 1988 {published data only}
- Shepherd RW, Holt TL, Cleghorn G, Ward LC, Isles A, Francis P. Nutritional supplementation during management of pulmonary exacerbations in CF: a controlled study including effects on protein turnover [abstract]. Proceedings of the 10th International Cystic Fibrosis Congress. 1988. [DOI] [PubMed]
- Shepherd RW, Holt TL, Cleghorn G, Ward LC, Isles A, Francis P. Short‐term nutritional supplementation during management of pulmonary exacerbations in cystic fibrosis: a controlled study, including effects on protein turnover. American Journal of Clinical Nutrition 1988;48(2):235‐9. [DOI] [PubMed] [Google Scholar]
Sirisinha 1973 {published data only}
Soliman 2004 {published data only}
- Soliman AT, El‐Matary W, Abdel Fattah MM, Nasr IS, Alaily RK, Alaa Thabet M. The effect of high‐calorie diet on nutritional parameters of children with beta‐thalassaemia major. Clinical Nutrition 2004;23(5):1153‐8. [PUBMED: 15380908 ] [DOI] [PubMed] [Google Scholar]
Sondel 1987 {published data only}
- Sondel SA, Parrell SW, Becker D, Mischler EH. Oral nutritional supplements in cystic fibrosis. Nutrition Support Services 1987;7(4):20‐2. [Google Scholar]
Stark 2005 {published data only}
- Stark LJ, Hommel KA, Mackner LM, Janicke DM, Davis AM, Pfefferkorn M, et al. Randomized trial comparing two methods of increasing dietary calcium intake in children with inflammatory bowel disease. Journal of Pediatric Gastroenterology & Nutrition 2005;40(4):501‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Taylor 1999 {published data only}
- Taylor SJ, Fettes SB, Jewkes C, Nelson RJ. Prospective, randomized, controlled trial to determine the effect of early enhanced enteral nutrition on clinical outcome in mechanically ventilated patients suffering head injury [see comments]. Critical Care Medicine 1999;27(11):2525‐31. [DOI] [PubMed] [Google Scholar]
Teixido‐Planas 2005 {published data only}
- Teixido‐Planas J, Ortiz A, Coronel F, Montenegro J, Lopez‐Menchero R, Ortiz R, et al. Oral protein‐energy supplements in peritoneal dialysis: A multicentre study. Peritoneal Dialysis International 2005;25(2):163‐72. [PUBMED: 15796145] [PubMed] [Google Scholar]
van Eys 1982 {published data only}
- Eys J, Cangir AC. Effect of nutritional supportive therapy on children with advanced cancer. Cancer Research 1982;42(2 Suppl):713‐4s. [PubMed] [Google Scholar]
Walker 2000 {published data only}
- Walker SP, Grantham‐Mcgregor SM, Powell CA, Chang SM. Effects of growth restriction in early childhood on growth, IQ, and cognition at age 11 to 12 years and the benefits of nutritional supplementation and psychosocial stimulation. Journal of Pediatrics 2000;137(1):36‐41. [DOI] [PubMed] [Google Scholar]
Yamamoto 2007 {published data only}
- Yamamoto T, Nakahigashi M, Umegae S, Kitagawa T, Matsumoto K. Impact of long‐term enteral nutrition on clinical and endoscopic recurrence after resection for Crohn's disease: A prospective, non‐randomised, parallel, controlled study. Alimentary Pharmacology & Theraputics 2007;25(1):67‐72. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
Cox 2014 {published data only}
- Cox SE, Makani J, Walter G, Mtunguja S, Kamala BA, Ellins E, et al. Ready‐to‐use supplementary food supplements improve endothelial function, hemoglobin and growth in Tanzanian children with sickle cell anaemia: the vascular function intervention study (V‐FIT), a random order crossover trial [abstract]. 56th ASH Annual Meeting and Exposition; 2014 Dec 6‐9; San Francisco, California 2014;1:Abstract no: 4087. [CENTRAL: 1017322; CFGD Register: SC262 ; CRS: 5500131000000279] [Google Scholar]
Additional references
Corey 1988
- Corey M, McLaughlin FJ, Williams M, Levison H. A comparison of survival, growth and pulmonary function in patients with cystic fibrosis in Boston and Toronto. Journal of Clinical Epidemiology 1988;41(6):583‐91. [DOI] [PubMed] [Google Scholar]
CPS 1994
- Canadian Paediatric Society Nutrition Committee. Undernutrition in children with a neurodevelopmental disability. Canadian Medical Association Journal 1994;151(6):753‐9. [PMC free article] [PubMed] [Google Scholar]
Curtin 2002a
- Curtin F, Elbourne D, Altman DG. Meta‐analysis combining parallel and cross‐over clinical trials. I: Continuous outcomes. Statistics in medicine 2002;21(15):2131‐44. [DOI] [PubMed] [Google Scholar]
Curtin 2002b
- Curtin F, Elbourne D, Altman DG. Meta‐analysis combining parallel and cross‐over clinical trials. II: Binary outcomes. Statistics in medicine 2002;21(15):2145‐59. [DOI] [PubMed] [Google Scholar]
Curtin 2002c
- Curtin F, Elbourne D, Altman DG. Meta‐analysis combining parallel and cross‐over clinical trials. III: The issue of carry‐over. Statistics in medicine 2002;21(15):2161‐73. [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997;315(7109):629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Elbourne 2002
- Elbourne DR, Altman DG, Higgins JPT, Curtin F, Worthington HV, Vail A. Meta‐analyses involving cross‐over trials: methodological issues. International Journal of Epidemiology 2002;31(1):140‐9. [DOI] [PubMed] [Google Scholar]
Florescu 2014
- Florescu L, Paduraru DT, Mîndru DE, Temneanu OR, Petrariu FD, Matei MC. Epidemiological evaluation regarding the role of cystic fibrosis as a risk factor for child malnutrition.. Revista Medico‐chirurgicala a Societatii de Medici Si Naturalisti Din Iasi 2014;118(2):450‐6. [PubMed] [Google Scholar]
Hendricks 1995
- Hendricks KM, Duggan C, Gallagher L, Carlin AC, Richardson DS, Collier SB, et al. Malnutrition in hospitalised pediatric patients: current perspective. Arhives of Pediatrics and Adolescent Medicine 1995;149(10):1118‐22. [DOI] [PubMed] [Google Scholar]
Hendrickse 1997
- Hendrickse WK, Reilly JJ, Weaver LT. Malnutrition in a children's hospital. Clinical Nutrition 1997;16:13‐8. [Google Scholar]
Higgins 2011
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing the risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org. Wiley Publishers, 2011. [Google Scholar]
Johansson 1986
- Johansson U, Portinsson S, Akesson A, Svantesson H, Ockerman PA, Akesson B. Nutritional status in girls with juvenile chronic arthritis. Human Nutrition: Clinical Nutrition 1986;40(1):57‐67. [PubMed] [Google Scholar]
Joosten 2010
- Joosten K F, Zwart H, Hop W C, Hulst J M. National malnutrition screening days in hospitalised children in The Netherlands. Arch Dis Child 2010;95:141‐5. [DOI] [PubMed] [Google Scholar]
Koscik 2004
- Koscik RL, Farrell PM, Kosorok MR, Zaremba KM, Laxova A, Lai HC, et al. Cognitive function of children with cystic fibrosis: deleterious effect of early malnutrition. Pediatrics 2004;113(6):1549‐58. [DOI] [PubMed] [Google Scholar]
Moy 1990
- Moy RJD, Smallman S, Booth IW. Malnutrition in a UK Children's Hospital. Journal of Human Nutrition and Dietetics 1990;3:93‐100. [Google Scholar]
Pawellek 2008
- Pawellek I, Dokoupil K, Koletzko B. Prevalence of malnutrition in paediatric hospital patients. Clin Nutr 2008;27:72‐6. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Smyth 2014
- Smyth RL, Rayner O. Oral calorie supplements for cystic fibrosis. Cochrane Database of Systematic Reviews 2014, Issue 11. [DOI: 10.1002/14651858.CD000406.pub4] [DOI] [PubMed] [Google Scholar]
Spagnuolo 2013
- Spagnuolo M I, Liguoro I, Chiatto F, Mambretti D, Guarino A. Application of a score system to evaluate the risk of malnutrition in a multiple hospital setting. Ital J Pediatr 2013;39:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Poustie 2009
- Poustie VJ, Smyth RL, Watling RM. Oral protein calorie supplementation for children with chronic disease. Cochrane Database of Systematic Reviews 2009, Issue 3. [DOI: 10.1002/14651858.CD001914] [DOI] [PubMed] [Google Scholar]


