Abstract
This meta-analysis sets out to systematically assess the efficacy of short course radiation (SRT) for rectal cancer patients based on randomized, controlled trials. Eight randomized controlled trials involving 6894 patients were ultimately included in this meta-analysis. Three trials (n = 2574) compared SRT with surgery alone. Local recurrence was improved (HR = 0.48, 95% CI 0.40 to 0.58). Overall survival was marginally improved with an HR of 0.90 (95% CI 0.81 to 1.00), but the magnitude of benefit was heterogeneous across trials. An additional three trials (n = 3682) compared SRT with selective postoperative radiation ± chemotherapy. A significant reduction of local recurrence (HR = 0.44, 95% CI 0.35 to 0.56) was also found after SRT. However, no benefit in overall survival was observed. Moreover, two trials (n = 638) compared SRT with long course chemoradiation. There was no statistically significant local recurrence or overall survival difference observed between the two strategies. Patients receiving SRT had lower grade 3 or 4 acute treatment related toxicity (RR 0.11, 95% CI 0.05 to 0.22) whereas no difference in late toxicity was observed. Overall, SRT is a reasonable alternative for resectable rectal cancer patients and should be part of an informed discussion of treatment options for this group of patients.
Colorectal cancer has been ranked as the third most common cancer in the world1. Radical surgery is the mainstay of therapy for resectable colorectal cancer. Unlike colon cancer, however, the risk of local recurrence is higher in patients with rectal cancer2. The addition of radiation to surgery has been successfully used to improve local control3,4. There are two radiation regimens typically used in clinical practice: short course radiation (5 fractions of 5 Gy delivered over 1 week) and long course radiation (25–30 fractions of 1.8 or 2 Gy over 5–6 weeks).
Most randomized trials of short course radiation have been performed in the United Kingdom, Scandinavia and the Netherlands5,6,7,8. As the smaller number of fractions makes short course radiation (SRT) less expensive and more convenient than long course radiation, this schedule is preferred in these countries. However, it is not favoured in certain other European countries and North America. Unlike long course radiation combined with chemotherapy (CRT), SRT cannot be safely combined concurrently with adequate doses of systemic chemotherapy. The value of introducing a short course of preoperative radiotherapy before surgery for rectal cancer is still subject for debate. Among the unanswered questions are the effect of short course radiation on local recurrence or overall survival and, in particular, the relative merits compared with long course radiation combined with chemotherapy or other adjuvant approaches.
Meta-analysis provides a useful tool by which to summarize and analyse a body of research, particularly when apparent benefits are marginal or research results are conflicting9. No comprehensive meta-analysis on randomized trials related to short course radiation in rectal cancer has been performed to this point. Therefore, the current meta-analysis will provide an up-to-date exhaustive summary on the role of short course radiation in localized rectal cancer, including comparisons with surgery alone and other adjuvant approaches.
Methods
Aims
The specific aims were to systematically review the published literature of randomized controlled trials, comparing the following therapies: short course radiation versus surgery alone; short course radiation versus selective postoperative radiation ± chemotherapy; and short course radiation versus long course radiation combined with chemotherapy.
Search Strategy
Trials were identified by searching PubMed, EMBASE, Chinese Biomedical Literature Database and the Cochrane library. Moreover, the important trial registries and conference proceedings (ASCO, ASCO GI, ECCO, ESTRO and AACR) were also searched. The following medical subject heading (MeSH) terms and key words were variably combined: rectal cancer, rectal carcinoma, neoadjuvant, chemoradiotherapy, radiation, short course radiation, randomized and trial. We used the “related articles” function to broaden the search. The deadline for trial inclusion was publication by June 30, 2014. The reference lists from the selected articles were also scanned for additional relevant trials. Each study was subjected to a quality assessment using a 14-point checklist by two investigators10.
Selection Criteria
Two independent reviewers assessed the eligibility of abstracts identified by the search. If the eligibility was unclear from the abstract, the full article was retrieved for clarification. Any disagreements were resolved by discussion. The inclusion criteria were randomized controlled trials including patients with localized rectal cancer treated with short course radiation. Studies that were nonrandomized, were single-arm phase II trials, or had inadequate statistical analysis or information missing were excluded.
Statistical Analysis
The data extracted from the trials were entered into RevMan version 5.2 software (Cochrane Collaboration, Oxford, United Kingdom) for meta-analysis to calculate the pooled estimates. Meta-analyses were conducted on local recurrence (LR), overall survival (OS) and toxicity. The results regarding LR and OS were analysed by calculating the pooled hazard ratio (HR) estimated from published trial data or from curve analysis, as they were both censoring and time to an event data11 In particular, we determined the value of observed minus estimated number of event (O–E) and its variance for each trial. The results are expressed as HRs with 95% CIs. An HR > 1.0 indicates that patients receiving SRT had a higher probability of experiencing an event. The results for treatment related toxicity were expressed as risk ratio (RR) with 95% CIs using the Mantel–Haenszel method12.
Heterogeneity was evaluated using Q statistics and quantified by the I2 statistic13,14. If the P value was less than 0.10 or I2 was greater than 50%, there was statistically significant between-study heterogeneity. Forest plots were used to display hazard ratios and risk ratios within individual trials. Visual examination of the funnel plots was performed to evaluate the likelihood of publication bias15. A two-sided P-value of <0.05 was considered significant for all analyses except the heterogeneity tests.
Results
Eligible studies
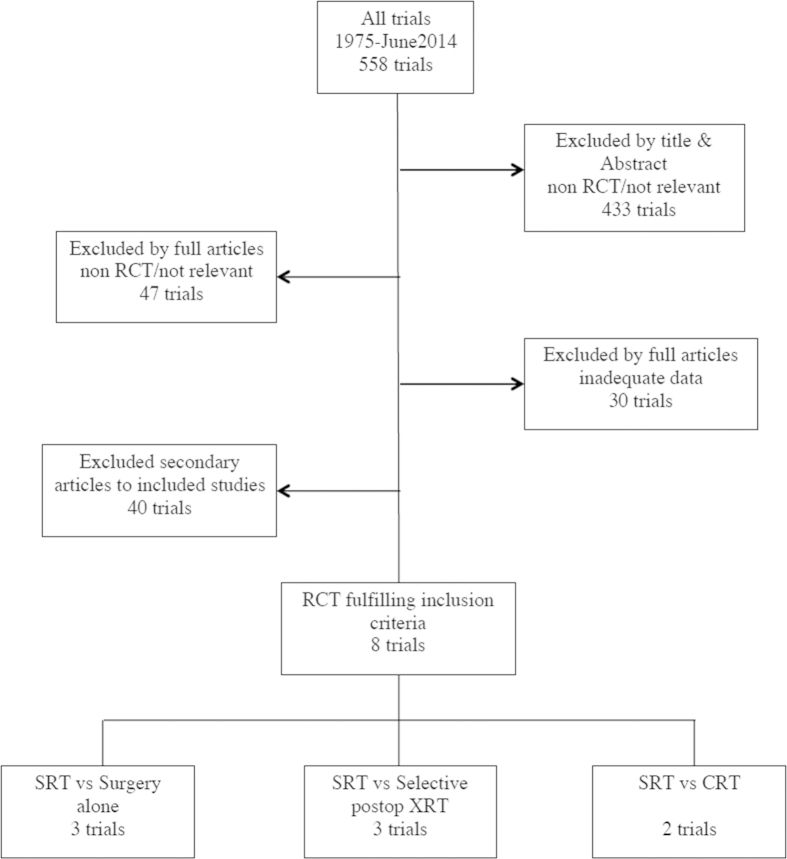
After excluding 550 studies (selection process is shown in Fig. 1), a total of 8 trials5,6,7,8,16,17,18,19 were eligible. The regimens of included trials are listed in Table 1. Short course radiation was delivered followed by immediate surgery within 1 week in all eight trials. Three published randomized trials involving 2574 patients compared neoadjuvant short course radiation with surgery alone (Stockholm 1, Stockholm 2, Swedish trial). The Swedish RCT trial contained some patients (316) who were previously in Stockholm 2. As both studies were included in our analysis, these 316 patients have been accounted for twice. Another three published randomized trials involving 3682 patients compared neoadjuvant short course radiation with selective postoperative radiation ± chemotherapy (Uppsala, Dutch TME trial, MRC CR07). In addition, two randomized trials comparing short course radiation with long course chemoradiation group involving 638 total patients were eligible (Polish trial, TROG 01.04). The baseline characteristics of these studies and the results of assessing the quality of all included studies are listed in Table 2. Visual examination of the funnel plots with respect to all end points did not reveal any evidence of publication bias.
Figure 1. Flow chart of the literature search and study selection process.
Table 1. Trials studying short course radiation in localized rectal cancer.
| Study | Regimen | No. of Patients |
|---|---|---|
| Stockholm 1 | Arm 1: 5×5 Gy with immediate surgery | 424 |
| Arm 2: surgery alone | 425 | |
| Stockholm 2 | Arm 1: 5 ×5 Gy with immediate surgery | 272 |
| Arm 2: surgery alone | 285 | |
| Swedish trial | Arm 1: 5×5 Gy with immediate surgery | 585 |
| Arm 2: surgery alone | 583 | |
| Uppsala | Arm 1: 5 ×5.1 Gy with immediate surgery | 235 |
| Arm 2: selective postoperative radiotherapy for patients with stage B/C, 60 Gy in 30 fr | 236 | |
| Dutch TME trial | Arm 1: 5 ×5 Gy with immediate surgery | 924 |
| Arm 2: selective postoperative radiotherapy for patients with positive margins (11%), 50.4 Gy in 28 fr | 937 | |
| MRC CR07 | Arm 1: 5 ×5 Gy with immediate surgery | 674 |
| Arm 2: selective postoperative CRT for patients with positive circumferential margin(9%), 45 Gy in 25 fr +5-FU | 676 | |
| Polish trial | Arm 1: 5 ×5 Gy with immediate surgery | 155 |
| Arm 2: 50.4 Gy in 28 fr with concomitant CT weeks 1 & 5 | 157 | |
| TROG 01.04 | Arm 1: 5 ×5 Gy with immediate surgery | 163 |
| Arm 2: 50.4 Gy and 5-FU 225 mg/m2/day followed by surgery at 4-6 weeks | 163 |
Table 2. Baseline characteristics of patients included in the meta-analysis.
| Study | Year | Stage | Criteria used to define rectal cancer | Median follow-up(year) | Surgery | Quality score |
|---|---|---|---|---|---|---|
| Stockholm 1 | 1980–1987 | Any stage resectable | below sacral promontory | 4.5 | AP/anterior resection | 0.74 |
| Stockholm 2 | 1987–1993 | Any stage resectable | below sacral promontory | 9 | AP/anterior resection | 0.88 |
| Swedish trial | 1987–1990 | T1-3 | below sacral promontory | 13 | AP/anterior resection | 0.82 |
| Uppsala | 1980–1985 | Dukes A/B/C | NA | Minimum 5 | AP/anterior resection | 054 |
| Dutch TME trial | 1996–1999 | Any stage resectable | 15 cm from anal verge, below S1-2 | 12 | AP/anterior resection with TME technique | 0.88 |
| MRC CR07 | 1998–2005 | I-III | NA | 4 | APR/non-APR with TME technique | 0.83 |
| Polish trial | 1999–2002 | T3 or resectable T4 | inferior edge palpable of digital exam | 4 | AP/anterior resection or Hartman with TME | 1 |
| TROG 01.04 | 2001–2006 | cT3N any | within 12 cm of the anal verge | 5.9 | APR/non-APR with TME technique | 0.81 |
SRT versus Surgery alone
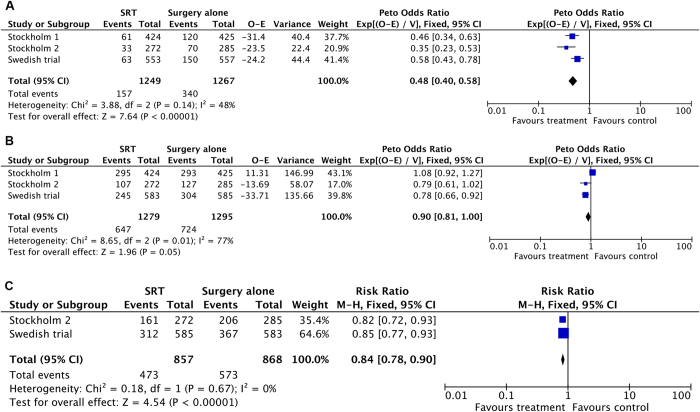
Local recurrence (Fig. 2A) was significantly improved in patients who received short course radiation compared with patients who received surgery alone. The risk of local recurrence was reduced by 52% in patients who received short course radiation (three trials, 2516 patients; HR = 0.48; 95% CI, 0.40 to 0.58; P < 0.00001). No statistically significant heterogeneity was present among these studies (I2 = 48%, P = 0.14).
Figure 2. SRT versus surgery alone:
(A) Forest plot of hazard ratio for local recurrence (B) Forest plot of hazard ratio for overall survival (C) Forest plot of risk ratio for no toxicity.
The pooled data reached borderline significance in support for overall survival advantage in favour of SRT with an HR of 0.90 [95% CI, 0.81 to 1.00; P = 0.05] (three trials, 2574 patients; Fig. 2B). However, there was evidence of heterogeneity in the magnitude of the effect across the included studies (I2 = 77%; P = 0.01). Unfortunately, due to the small number of studies, a sensitivity analysis cannot be performed.
Acute toxicities after surgery were listed by type but were not graded in all three studies. The toxicities include anastomotic leak, wound dehiscence, bowel obstruction, septicaemia, wound infection, postoperative ileus and others. The proportion of patients with no toxicities post operatively favoured the surgery alone group with an RR of 0.84 [95%CI, 0.78 to 0.90; P < 0.00001] (Stockholm 2 and Swedish trial, 1725 patients; Fig. 2C). Significant heterogeneity was not evident (I2 = 0%; P = 0.67). Long term toxicity data were available from all 3 studies (Stockholm 1, Stockholm 2 and Swedish trial), though they each reported on different aspects of long term outcomes. Therefore, it was not possible to do a quantitative analysis across studies. A follow-up of 23 preoperatively irradiated patients and 43 non-irradiated patients from the Stockholm 1 and 2 trials revealed more frequent faecal incontinence (57%) and soiling (38%) in irradiated patients than in non-irradiated patients (26% faecal incontinence and 16% soiling)20. The long-term follow-up of the Stockholm trials also revealed a higher risk for small bowel obstruction (RR 1.6; 95% CI, 1.1 to 2.2), urinary incontinence, venous thromboembolism (RR 2; 95% CI, 1.2 to 3.4), and femoral neck and pelvic fractures (RR 2; 95%CI, 1.1 to 3.7) in irradiated patients when the Stockholm 1 and 2 trials were analysed together21,22. In the Swedish trial, there were more patients with increased stool frequency (20% versus 8%) and continence problems (50% versus 24%) in the SRT group23.
SRT versus selective postoperative radiation ± chemotherapy
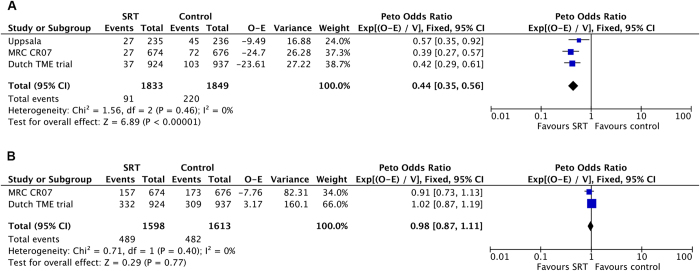
Local control was significantly improved, with a risk reduction of 56% for recurrence (Fig. 3A) in favour of SRT (3 trials, 3682 patients; HR = 0.44; 95% CI, 0.35 to 0.56; P < 0.00001). There was no evidence for heterogeneity between the trials (I2 = 0%; P = 0.46).
Figure 3. SRT versus selective postoperative radiation ± chemotherapy:
(A) Forest plot of hazard ratio for local recurrence (B) Forest plot of hazard ratio for overall survival.
Data regarding overall survival were reported in two of the three trials (MRC CR07 and Dutch TME trial). Overall survival (Fig. 3B) was not significantly changed in patients who received short course radiation compared with selective postoperative radiation ± chemotherapy (two trials; HR = 0.98; 95% CI, 0.87 to 1.11; P = 0.77). There was no evidence for heterogeneity between the studies (I2 = 0%; P = 0.40).
Acute toxicities after surgery were only reported in the Dutch TME trial for Dutch subgroup (1530/1861 patients). The proportion of patients with no toxicities post operatively was 51.6% in the SRT group and 59.6% in the control group. Long term toxicity data were available; however, it was not possible to perform quantitative analyses across the studies. In Uppsala, there were no significant differences in bowel dysfunction, gastrointestinal disorders or urinary dysfunction between the two treatment groups8. During long term follow-up of the Dutch TME trial, no significant differences were observed in the rates of small bowel obstruction, sexual dysfunction, fractures and venous, arterial or cardiovascular diseases between the two patient groups24,25. However, a higher risk of bowel dysfunction was observed in the SRT group compared with the control group (62% vs 38%)25. Longer term follow-up (in patients with an abdomino-perineal excision) at 12 and 24 months showed that rates of small bowel obstruction, bowel dysfunction and lumbar or sacral neuropathy did not differ between the two treatment groups in the MRC CR07 trial. However, a significant increase in faecal incontinence was observed in patients treated with SRT17,26.
SRT versus CRT
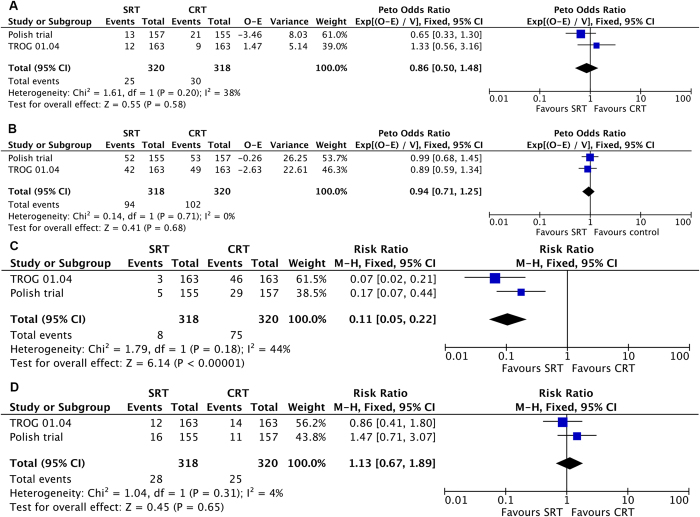
No statistically significant difference in local recurrence was observed in the short course irradiation group when compared with the chemoradiation group (HR 0.86; 95%CI, 0.50 to 1.48; P = 0.58; Fig. 4A), and there was no heterogeneity between studies (I2 = 38%, P = 0.20). In the analysis of overall survival, there was also no difference between these two groups (HR 0.94; 95% CI, 0.71 to 1.25; P = 0.68; Fig. 4B) and no heterogeneity was observed (I2 = 0%, P = 0.71).
Figure 4. SRT versus long course radiation combined with chemotherapy:
(A) Forest plot of hazard ratio for local recurrence (B) Forest plot of hazard ratio for overall survival (C) Forest plot of risk ratio for grade 3 or 4 acute treatment related toxicity (D) Forest plot of risk ratio for grade 3 or 4 late treatment related toxicity.
In the SRT group, 8 of 318 patients (2.5%) developed a grade 3 or 4 acute treatment related toxicity, while this grade of toxicity was observed in 75 out of 320 patients (23.4%) in the CRT group. This difference was statistically significant (RR 0.11; 95% CI, 0.05 to 0.22; P < 0.00001; Fig. 4C). There was no statistically significant heterogeneity among the studies (I2 = 44%, P = 0.18). Grade 3 or 4 late treatment related toxicity developed in 28 of 318 patients (8.8%) treated with SRT, while this occurred in 25 of 320 patients (7.8%) treated with CRT. There was no statistically significant difference in this late toxicity rate (RR 1.13; 95% CI, 0.67 to 1.89; P = 0.65; Fig. 4D) nor was there significant heterogeneity between the studies (I2 = 4%, P = 0.31).
Discussion
To our knowledge, the Colorectal Cancer Collaborative Group4 and Wong et al.3 conducted literature-based meta-analyses that focused on preoperative radiation, and they found beneficial effects on local control compared to surgery alone. Then, meta-analyses by De Caluwé et al.27 and Ceelen et al.28 demonstrated that preoperative CRT improves local control in resectable stage II and III rectal cancer compared to preoperative RT alone. None of the previous meta-analyses analysed short course radiation separately. Although short course radiation for rectal cancer has been investigated over the past two decades, many clinicians in the United States and China have not adopted a short course radiation approach. Nevertheless, we comprehensively searched for literature examining the outcomes after short course radiation.
In this systematic review of data from 6894 patients in 8 trials of short course radiation for rectal cancer, short course radiotherapy substantially reduced the risk of local recurrence compared with surgery alone or selective postoperative radiation ± chemotherapy. In the past, some clinicians questioned the results in Stockholm trials and Swedish trial as the surgery used in these slightly older studies was not consistent with optimal current surgical plans. However, the effect on reducing local recurrence remains consistent after implementation of the total mesorectal excision (TME) technique in the Dutch TME trial and MRC CR07 trial. The effect is also comparable when compared with long course chemoradiation, which is in contrast with the meta-analysis findings by De Caluwé et al.27and Ceelen et al.28 that demonstrated a significant benefit in local control by adding chemotherapy to radiotherapy. The aforementioned meta-analyses also included studies that compared conventionally fractionated preoperative radiation with long course chemoradiation in patients with resectable rectal cancer. As uncontrolled local recurrence can have a destructive effect on a patient’s quality of life, the improved local control with SRT might be a sufficient benefit to justify its use.
Although SRT has been shown to marginally improve survival over surgery alone in our meta-analysis, it must be emphasized that the Swedish trial was the only included trial that reported a significant improvement in survival, and there was evidence of heterogeneity across the included studies. Therefore, this result should be interpreted with caution. Contrary to the belief that reduction in local recurrence after radiation increases the likelihood of overall survival, no significant effect in terms of OS was found from SRT approach compared with selective postoperative radiation ± chemotherapy. The result was similar with prior meta-analyses3,4,27,28. This finding seems to be reasonable, considering the following facts. First, the reduction in mortality from rectal cancer may be largely counterbalanced by the increased risk of death from other causes. Second, the number of patients may not be large enough to reliably detect small but significant differences in long-term survival. Furthermore, we did not find a statistically significant difference in overall survival between the SRT group and the long course chemoradiation group.
It was reported in several studies that SRT is associated with an increased risk of certain toxicities at long-term follow-up compared with patients treated with surgery alone or selective postoperative radiation. Due to significant improvements in radiation treatment plans and because of different radiation techniques and irradiated volumes, it is difficult to interpret the results of toxicity across different studies. However, treatment related toxicity was generally acceptable as evidenced by the relatively high compliance rates in the mentioned studies. When compared with CRT, grade 3 and 4 acute treatment related toxicity was more pronounced in the CRT group. With respect to late toxicity, no significant differences were observed between patients who received short course radiotherapy and those who received chemoradiotherapy. However, the duration of follow-up in both trials was too short to draw definitive conclusions regarding late adverse effects. Longer follow-up is required to fully assess late effects.
Finally, what are the implications of these results for current practice? On one hand, SRT has certain advantages compared to long course radiation, including a small number of fractions that makes short course radiation less expensive, more convenient and improves compliance. On the other hand, because cancer cells sterilized by radiation require time to undergo necrosis29, short course radiation with immediate surgery has limited effect on tumour shrinkage and downstaging. However, the problem may be solved by short course radiation with delayed surgery. An interim analysis of the Stockholm III trial comparing 3 preoperative radiotherapy schemes showed a pathologic complete response (pCR) in 12.5% of patients in the short course radiation with delayed-surgery group whereas the rate of pCR after short course radiotherapy with immediate surgery was only 0.8%. As shown in other nonrandomized studies30,31, the rate of pCR after short course radiation and delayed surgery is not largely different from that which is observed after conventionally fractionated chemoradiation. Therefore, short course radiation with a delayed interval to surgery can be a reasonable option for elderly patients with comorbidities, who are often unfit for chemotherapy and can be used when tumour shrinkage is necessary prior to resection.
In addition to a limited effect on tumour shrinkage and downstaging, short course radiation was called into question given its inability to safely be combined with adequate doses of systemic chemotherapy. As has been shown previously, the local efficacy of 5 × 5Gy and conventionally fractionated chemoradiation is similar. However, the dose of chemotherapy must be reduced in order to keep the level of toxicity acceptable. This may negatively affect the systemic efficacy of chemotherapy. Therefore, short course radiation with sequential consolidation chemotherapy during the interval to surgery may enable intensification of both systemic treatment and radiation. It is feasible and promising particularly in patients with a rectal cancer and potentially resectable synchronous metastatic disease. In a study reported by van Dijk et al.32, 48 patients with rectal cancer and synchronous distant metastases received such management. The result was promising with a complete pathologic response in 22.5% of patients and curative surgery in 85% of patients. Prospective studies to validate this treatment strategy are needed in the future.
This analysis had specific limitations. First, our meta-analysis is not based on individual patient data and therefore differences in individual baseline characteristics cannot be integrated. The second limitation is the relatively small number of enrolled patients. Finally, but importantly, the duration of follow-up for some studies was too short to draw definitive conclusions. Regardless of the limitations, this meta-analysis demonstrates that SRT provided significant benefit for resectable rectal cancer patients in local control, the effect of which is equivalent with long course chemoradiation. However, SRT does not benefit overall survival when compared with either selective postoperative radiation ± chemotherapy or long course chemoradiation. SRT related toxicity was generally acceptable and SRT decreased short-term toxicity compared to preoperative chemoradiation in resectable rectal cancer. In conclusion, SRT is a reasonable alternative for resectable rectal cancer patients and should be part of an informed discussion of treatment options for this group.
Additional Information
How to cite this article: Chen, C. et al. Short Course Radiation in the Treatment of Localized Rectal cancer: A Systematic Review and Meta-Analysis. Sci. Rep. 5, 10953; doi: 10.1038/srep10953 (2015).
Footnotes
Author Contributions All authors contributed significantly to this work. C.C., S.Y., J.R. and P.S. performed the research study and collected the data; C.C., Q.D. and H.W. analysed the data; S.Y. and P.S. designed the research study; C.C., P.S., Q.D. and H.W. wrote the paper, and J.R. and S.Y. prepared Fig. 1, 2, 3, 4 and Tables 1, 2. All authors reviewed the manuscript. In addition, all authors approved the final draft.
References
- Ferlay J. et al. GLOBOCAN 2012, Cancer Incidence and Mortality Worldwide: IARC CancerBase [Internet]. http://globocan.iarc.fr. (accessed June 2014).
- Kapiteijn E. et al. Local recurrence in patients with rectal cancer diagnosed between 1988 and 1992: a population-based study in the west Netherlands. Eur J Surg Oncol 24, 528–35 (1998). [DOI] [PubMed] [Google Scholar]
- Wong R. K. et al. Pre-operative radiotherapy and curative surgery for the management of localized rectal carcinoma. Cochrane Database of Systematic Reviews 18, CD002102 (2007). [DOI] [PubMed] [Google Scholar]
- Colorectal Cancer Collaborative Group. Adjuvant radiotherapy for rectal cancer:a systematic overview of 8507 patients from 22 randomised trials. Lancet 358, 1291–304 (2001). [DOI] [PubMed] [Google Scholar]
- Cedermark B. et al. The Stockholm I Trial of preoperative short term radiotherapy in operable rectal carcinoma. Cancer 75, 2269–75 (1995). [DOI] [PubMed] [Google Scholar]
- Martling A. et al. The Stockholm II trial on preoperative radiotherapy in rectal carcinoma - long term follow up of a population based study. Cancer 92, 896–902 (2001). [DOI] [PubMed] [Google Scholar]
- Swedish rectal trial. Local recurrence rate in a randomised multicentre trial of preoperative radiotherapy compared with operation alone in resectable rectal carcinoma. Eur J Surg 162, 397–402 (1996). [PubMed] [Google Scholar]
- Frykholm G. J., Glimelius B. & Pahlman L. Preoperative or postoperative irradiation in adenocarcinoma of the rectum: final treatment results of a randomized trial and an evaluation of late secondary effects. Dis Colon Rectum 36, 564–72 (1993). [DOI] [PubMed] [Google Scholar]
- Sacks H. et al. Meta-analysis of randomised controlled trials. N Engl J Med 316, 450–5 (1987). [DOI] [PubMed] [Google Scholar]
- Detsky A. S. et al. Incorporating variations in the quality of individual randomized trials into meta-analysis. J Clin Epidem 45, 255–65 (1992). [DOI] [PubMed] [Google Scholar]
- Parmar M. K., Torri V. & Stewart L. Extracting summary statistics to perform meta- analyses of the published literature for survival endpoints. Stat Med 17, 2815–34 (1998). [DOI] [PubMed] [Google Scholar]
- Mantel N. & Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst 22, 719–48 (1959). [PubMed] [Google Scholar]
- Higgins J. P. & Thompson S. G. Quantifying heterogeneity in a meta-analysis. Stat Med 21, 1539–58 (2002). [DOI] [PubMed] [Google Scholar]
- Higgins J. P. et al. Measuring inconsistency in meta-analyses. BMJ 327, 557–60 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau J., Ioannidis J. P. A & Schmid C. H. Quantitative synthesis in systematic reviews. Ann Int Med YR 107, 820–6 (1997). [DOI] [PubMed] [Google Scholar]
- Kapiteijn E. et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. J England Journal of Medicine 345, 690–2 (2001). [DOI] [PubMed] [Google Scholar]
- Sebag-Montefiore D. et al. Preoperative radiotherapy versus selective postoperative chemoradiotherapy in patients with rectal cancer (MRC CR07 and NCIC-CTG C016): a multicentre, randomised trial. Lancet 373, 811–20 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bujko K. et al. Long-term results of a randomized trial comparing preoperative short-course radiotherapy with preoperative conventionally fractionated chemoradiation for rectal cancer. British Journal of Surgery 93, 1215–23 (2006). [DOI] [PubMed] [Google Scholar]
- Ngan S. Y. et al. Randomized Trial of Short-Course Radiotherapy Versus Long-Course Chemoradiation Comparing Rates of Local Recurrence in Patients With T3 Rectal Cancer: Trans-Tasman Radiation Oncology Group Trial 01.04. J Clin Oncol 30, 3827–33 (2012). [DOI] [PubMed] [Google Scholar]
- Pollack J. et al. Long-term effect of preoperative radiation therapy on anorectal function. Dis Colon Rectum 49, 345–52 (2006). [DOI] [PubMed] [Google Scholar]
- Pollack J. et al. Late adverse effects of short-course preoperative radiotherapy in rectal cancer. Br J Surg 93, 519–25 (2006). [DOI] [PubMed] [Google Scholar]
- Holm T. et al. Adjuvant preoperative radiotherapy in patients with rectal carcinoma. Adverse effects during long term follow-up of two randomized trials. Cancer 78, 968–76 (1996). [DOI] [PubMed] [Google Scholar]
- Birgisson H. et al. Adverse Effects of Preoperative Radiation Therapy for Rectal Cancer: Long Term Follow-up of the Swedish Rectal Cancer Trial. Journal of Clinical Oncology 23, 8697–9705 (2005). [DOI] [PubMed] [Google Scholar]
- Marijnen C. A. et al. Impact of short-term preoperative radiotherapy on health-related quality of life and sexual functioning in primary rectal cancer: Report of a multicenter randomized trial. J Clin Oncol 23, 1847–58 (2005). [DOI] [PubMed] [Google Scholar]
- Peeters K. C. et al. Late side effects of short-course preoperative radiotherapy combined with total mesorectal excision for rectal cancer: Increased bowel dysfunction in irradiated patients–a Dutch colorectal cancer group study. J Clin Oncol 23, 6199–206 (2005). [DOI] [PubMed] [Google Scholar]
- Stephens R. J. et al. Impact of short-course preoperative radiotherapy for rectal cancer on patients’ quality of life: data from the Medical Research Council CR07/National Cancer Institute of Canada Clinical Trials Group C016 randomized clinical trial. J Clin Oncol 28, 4233–39 (2010). [DOI] [PubMed] [Google Scholar]
- De Caluwé L., Van Nieuwenhove Y. & Ceelen W. P. Preoperative chemoradiation versus radiation alone for stage II and III resectable rectal cancer. Cochrane Database Syst Rev 2, CD006041 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceelen W. et al. Preoperative chemoradiation versus radiation alone for stage II and III resectable rectal cancer. Int J Cancer 124, 2966–72 (2009). [DOI] [PubMed] [Google Scholar]
- Suit H. D. & Gallager H. S. Intact tumor cells in irradiated tissue. Arch Pathol 78, 648–51 (1964). [PubMed] [Google Scholar]
- Radu C. et al. Short course preoperative radiotherapy with delayed surgery in rectal cancer—A retrospective study. Radiother Oncol 87, 343–9 (2008). [DOI] [PubMed] [Google Scholar]
- Hatfield P. et al. Short-course radiotherapy, with elective delay prior to surgery, in patients with unresectable rectal cancer who have poor performance status or significant comorbidity. Radiother Oncol 92, 210–4 (2009). [DOI] [PubMed] [Google Scholar]
- Van Dijk T. H., Hospers G. A. P. & Beukema J. C. Short-course radiation therapy, neoadjuvant bevacizumab, capecitabine and oxaliplatin, and radical resection of primary tumour and metastases in primary stage IV rectal cancer: A phase II multicenter study of the Dutch colorectal cancer Group [abstract]. Ann Oncol 21(suppl 1), i51 (2010). [Google Scholar]