Abstract
Background
Mesenchymal stem cells (MSCs) can differentiate into chondroblasts, adipocytes, or osteoblasts under appropriate stimulation. Ghrelin, an endogenous ligand for the growth hormone secretagogue receptor (GHSR), stimulates growth hormone (GH) secretion, and has both orexigenic and adipogenic effects. This study sought to understand the potential involvement of members of MAPK serine/threonine kinases in the ghrelin-induced growth of rabbit MSCs ( rBMSC).
Methods
We applied various concentrations of ghrelin to cultured rBMSC and observed the growth rate of the cells by MTT, changes in the phosphorylation state of ERK1/2, JNK and p38, and the expression levels of ALP, Runx2, and Osterix by wetern blot.
Results
We found that the growth and osteogenic differentiation of ghrelin-treated rBMSC are promoted primarily by phosphorylated ERK1/2, and that this phosphorylation, as well p38 phosphorylation, is mediated by GHSR.
Conclusions
Our study suggests that ghrelin promotes the growth and osteogenic differentiation of rBMSC primarily through the ERK1/2 pathway.
Keywords: rBMSC, MAPK, Ghrelin, Osteogenic differentiation
Background
Mesenchymal stem cells (MSCs) are regarded as one of the promising candidates for cell therapy by either autologous or allogeneic transplantation [1]. MSCs can differentiate into a variety of cell types, including osteocytes, chondrocytes, adipocytes, cardiomyocytes, neurons, and endothelial cells [2–4]. Transplanted MSCs can mobilize and become integrated within the host to replace pathological or damaged tissue [5]. In addition, MSCs can modulate immune responses and inflammation, providing growth factors or cytokines that can prevent cells from undergoing apoptosis in certain environments, notably in the central nervous system [6]. During the last two decades, an increasing number of studies have proved the therapeutic potential of MSCs in the treatment of neurodegenerative diseases, spinal cord and brain injuries, cardiovascular diseases, diabetes mellitus, and diseases of the skeleton [7–9]. Basic research on MSCs in certain fields has led to the initiation of clinical trials worldwide. To provide therapeutic benefits and further understand the mechanisms responsible for them, large numbers of cells are needed [10, 11]. Thus, it is important to find efficient, economical and scalable methods to generate large numbers of MSCs without altering their multipotency [12].
Ghrelin, an endogenous ligand for the growth hormone secretagogue receptor (GHSR), is a 28-amino acid peptide produced from a 117-amino acid preprohormone. The mature form of ghrelin may undergo several post-translational modifications, including the addition of a fatty acid chain (n-octanoic acid) to the serine residue at position 3 [13]. Ghrelin has been shown to stimulate growth hormone (GH) secretion, to have both orexigenic and adipogenic effects [14], and to influence the metabolism of both glucose and lipids. Most ghrelin is produced in the stomach, by a distinct group of endocrine cells located within the gastric oxyntic mucosa [15, 16], with smaller amounts produced by other organs. Small amounts of ghrelin have also been observed elsewhere in the gastrointestinal tract and in the pancreas.
The MAPKs are members of a family of serine/threonine kinases that play an essential role in transmitting the activation of cell-surface receptors to effect downstream changes in transcriptional programs. They are expressed ubiquitously and are involved in the regulation of a wide variety of critical cellular functions, including proliferation, differentiation, migration and apoptosis [17]. In humans, there are at least 11 members of the MAPK superfamily, which can be divided into six distinct subgroups based on sequence similarity: ERK1 and ERK2; JNK1, JNK2 and JNK3; and the p38 MAPKs. Each group of MAPKs is activated by a distinct kinase cascade in which a MAP3K (or MEKK) phosphorylates and activates a downstream dual-specificity MAP2K (or MEK), which in turn stimulates MAPK activity through dual phosphorylation on threonine and tyrosine residues within a conserved tripeptide motif (Thr-X-Tyr). Phosphorylation of these threonine and tyrosine residues results in a conformational change that increases the accessibility of the active site and enhances catalysis [18–20]. ERKs are activated in response to various cytokines and growth factors and mediate primarily mitogenic and anti-apoptotic signals [21].
While several studies have focused on ghrelin’s effect on the growth of neural stem cells and embryonic stem cells, few have examined its influence on MSCs. Here, we used ghrelin to investigate the molecular mechanisms underlying rabbit MSC differentiation to osteoblasts and to enhance the osteogenic potential of rabbit MSCs. Our data revealed that ghrelin triggers osteogenic differentiation of rabbit bone marrow-derived mesenchymal stromal cells through ERK1/2 signaling pathways.
Results
Identification of rBMSC
At day ten, cells reached 80 % confluence. At day 13, the cells displayed a uniform spindle shape and reached 100 % confluence. To further identify the rBMSC, the expression of CD34 and CD44 was examined by immunofluorescence. The cells were CD44 positive but CD34 negative (Fig. 1a).
Fig. 1.
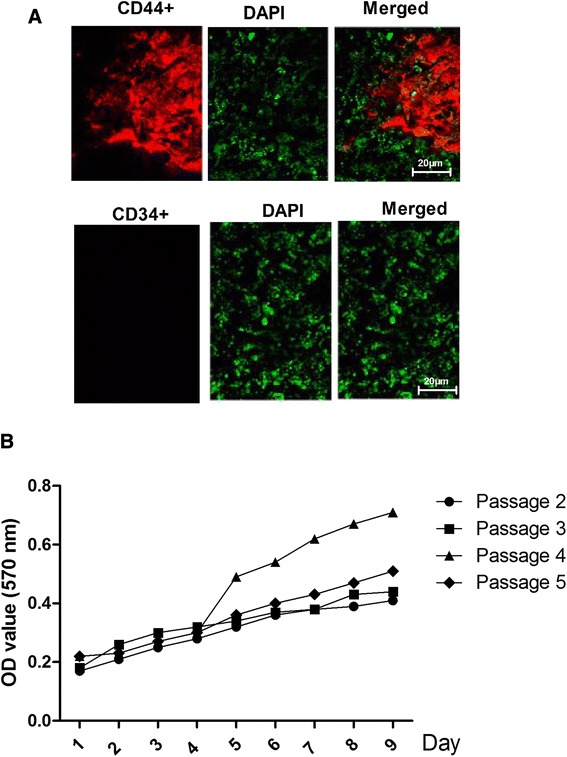
Identification of rBMSC. a Immunofluorescence staining showing the expression of CD34 and CD44 in cultured rBMSC. b Cellular proliferation during the second through fifth cell passages (as determined at an absorbance of 570 nm)
The proliferation of the cells in the second through the fifth cell passages was analyzed using an automated microplate reader at an absorbance of 570 nm on days 1 to 10 (Fig. 1b). The cells from the fourth and fifth passages were 100 % confluent at seven days and then entered a lag phase. However, the cells from the second and third passages entered the lag phase two days later. Thus, the cells from the fourth passage displayed the greatest ability to proliferate (P < 0.05).
GHSR expression in rBMSC
RT-PCR was used To detect the expression level of GHSR mRNA in the rBMSC,. GHSR was expressed at a high level in the rBMSC (Fig. 2).
Fig. 2.
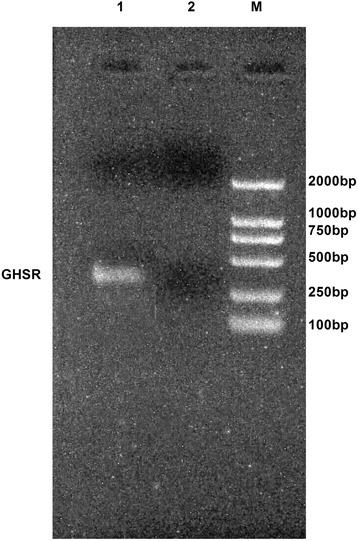
Expression of GHSR mRNA in rBMSCs by RT-PCR
Optimal Concentrations and Timing of Ghrelin and D-Lys3-GHRP-6Treatment of rBMSC
The MTT assay was used to assess cell growth and viability following treatment of rBMSC with varying concentrations of ghrelin, and to determine the appropriate duration of treatment to achieve the desired level of growth.
Ghrelin was added to the growth medium to final concentrations of 400, 500, 600, 700 and 800 ng/ml. The numbers of viable cells were assessed at 1 to 6 day (Fig. 3a). The results showed that the optimal concentration and duration of ghrelin treatment for rBMSC was 3days of treatment at 600ng/ml ghrelin.
Fig. 3.
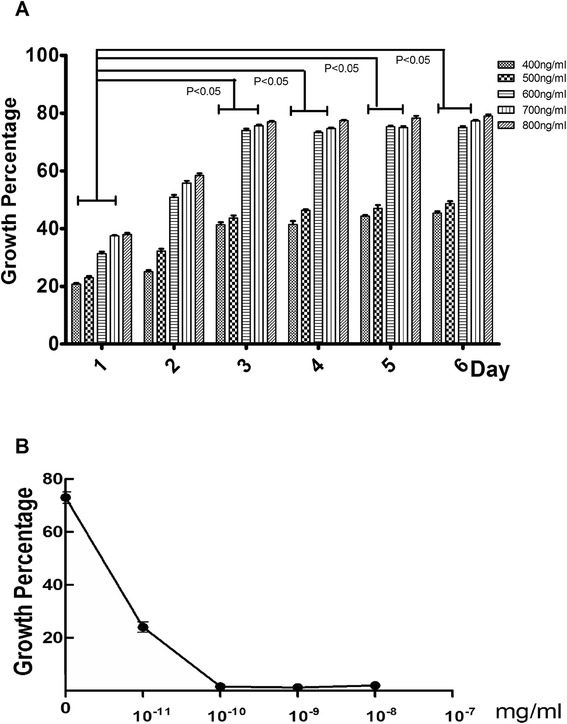
Growth of rBMSCs following treatment with ghrelin and with ghrelin plus D-Lys3-GHRP-6. a: Growth percentage at 1 to 6 days following treatment with increasing concentrations (400, 500, 600, 700, and 800 ng/ml) of ghrelin. Presented as mean ± SD (n = 5). b: The effect of D-Lys3-GHRP-6 (10−11, 10−10, 10−9, and 10−8 mg/ml) treatment on cell growth following ghrelin treatment
Next, we determined the concentration of D-Lys3-GHRP-6 necessary to promote the growth caused by 600ng/ml ghrelin. rBMSC that had been treated with 600 ng/ml ghrelin were treated with D-Lys3-GHRP-6 at concentrations of 10−8, 10−9, 10−10, and 10−11mg/ml, and cell numbers were evaluated at day three (Fig. 3b). The result showed that 10−10mg/ml D-Lys3-GHRP-6 could inhibit the growth caused by 600ng/ml ghrelin.
Ghrelin accelerates the Growth of rBMSC via the ERK1/2 Pathway
Phosphorylation states of the MAPKs ERK1/2, JNK, and p38 were detected by their phosphorylation antibody which mainly combinate to the phosphorylation part in these protein. To determine which pathway mediates ghrelin’s acceleration of rBMSC growth, the phosphorylation states of the MAPKs ERK1/2, JNK, and p38 were detected at 0, 20, 40, and 60 min (Fig. 4a) following treatment with 600ng/ml ghrelin. The resulting increase in ERK1/2 phosphorylation was greater than that of JNK or p38 after 40min (Fig. 4b). To explore the function of ghrelin on rBMSC-derived osteoblasts, the rBMSC were allowed to differentiate into osteoblasts, and then treated with ghrelin for 0, 20, 40, and 60 min. The expression levels of ALP, RUNX2, and Osterix were then examined. The expression of these proteins at 40 min was higher than at the other time points (Fig. 5).
Fig. 4.
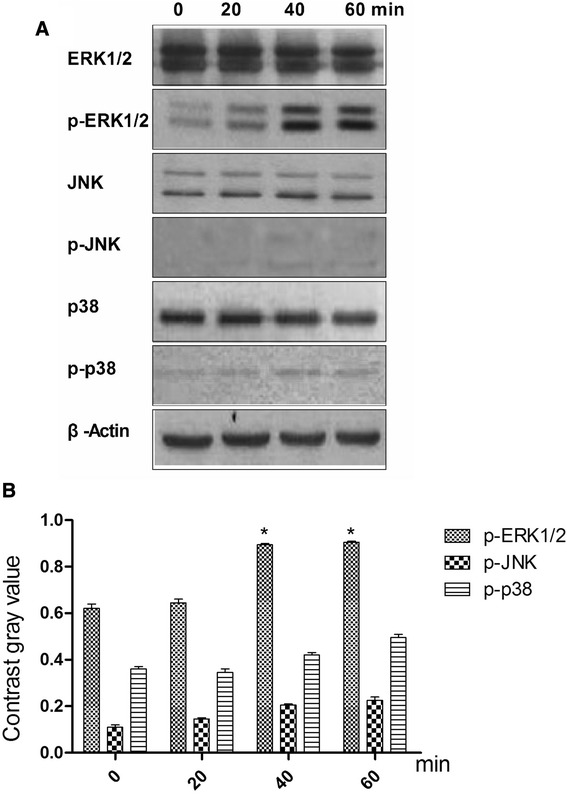
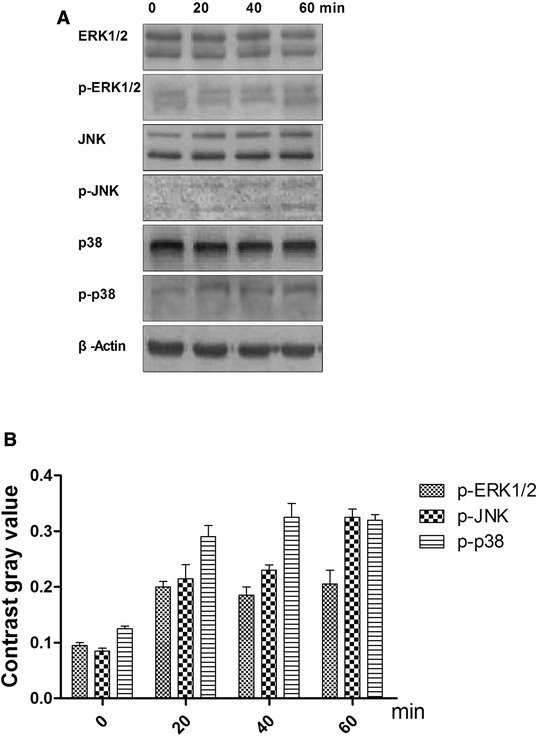
Effects of 600 ng/ml ghrelin on mitogen-activated protein kinase (MAPK) activation in rBMSCs cells. a: The expression of total and phosphorylated ERK1/2, JNKs, and p38 proteins. b: Contrast gray value of phosphorylation of ERK1/2, JNKs, and p38 based on the western blot. Presented as mean ± SD (n = 5). *Significantly different from the 0 min group (P < 0.05)
Fig. 5.
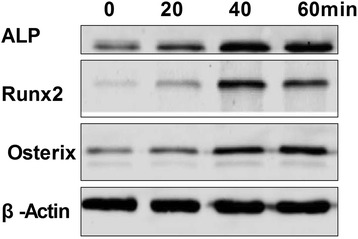
Expression levels of ALP, Runx2, and Osterix proteins in osteoblasts
When this treatment was repeated with the addition of 10−9mg/ml D-Lys3-GHRP-6, compared with treatment with ghrelin only, the phosphorylation of ERK1/2 was reduced (Fig. 6a). The phosphorylation status of the JNKs and p38 were not significantly changed (Fig. 6b), nor were the levels of ALP, RUNX2, and Osterix (Fig. 7).
Fig. 6.
Effects of ghrelin receptor inhibitor (D-Lys3-GHRP-6) on ghrelin-mediated MAPK activation in rBMSC cells. a: The expression of total and phosphorylated ERK1/2, JNKs, and p38 proteins. b: Contrast gray value of phosphorylation of ERK1/2, JNKs and p38 based on the western blot. Presented as mean ± SD (n = 5). * Significantly different from the 0 min group (P < 0.05)
Fig. 7.
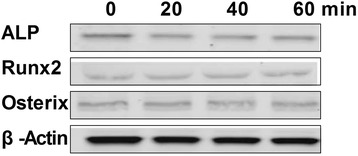
Expression of ALP, Runx2, and Osterix proteins by osteoblasts after treatment with the ghrelin receptor inhibitor (D-Lys3-GHRP-6)
These results suggested that ERK1/2 plays a key role in ghrelin’s ability to accelerate the growth of rBMSC. To test this, U0126 (a specific inhibitor of ERK1/2 phosphorylation) was used to silence ERK1/2 expression. In the presence of U0126, ghrelin (600ng/ml) did not accelerate rBMSC growth (Fig. 8a), and the growth rate was significantly lower than in the presence of ghrelin alone. The phosphorylation of p90rsk (p-p90rsk) was reduced (Fig. 8b), as were the levels of ALP, RUNX2, and Osterix (Fig. 8c).
Fig. 8.
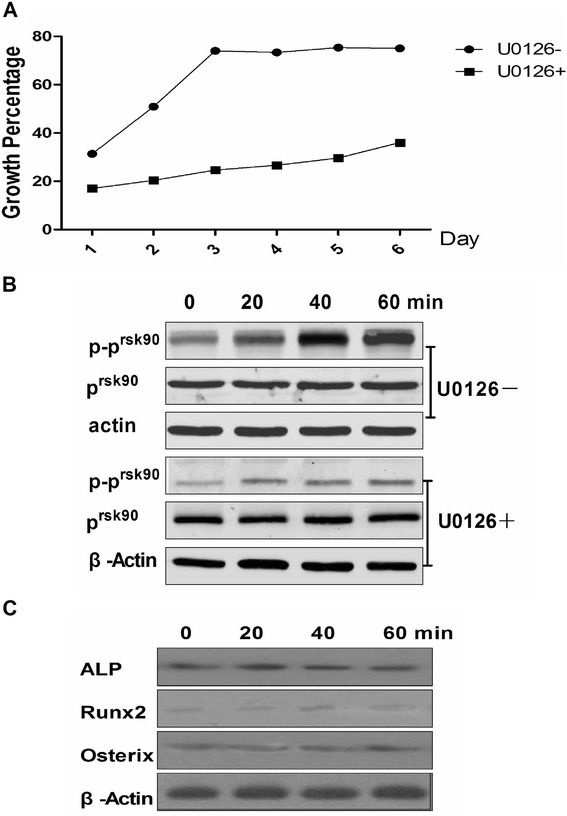
Effect on rBMSCs following treatment with U0126 (a specific inhibitor of ERK1/2 phosphorylation). a The growth rate of rBMSCs following treatment with U0126. U0126-: medium plus ghrelin; U0126+: medium plus ghrelin and U0126. b: Effects on p90rsk and phosphorylation of p90rsk after the phosphorylation ERK1/2 was inhibited at (0, 20, 40, and 60 min). U0126-: medium plus ghrelin; U0126+: medium plus ghrelin and U0126. c: Expression of ALP, Runx2, and Osterix proteins by osteoblasts after the phosphorylation ERK1/2 was inhibited, at 0, 20, 40 and 60 min
Discussion
Ghrelin is produced mainly by the stomach, although lesser amounts are also produced by the bowel, pancreas, pituitary, kidney, and placenta. The ghrelin receptor, GHSR, is a typical G-protein-coupled seven-pass transmembrane receptor [22]. Several studies have identified the protective effects of ghrelin on the reproductive system [23–26]. Other studies have shown that ghrelin affects the function of embryonic stem cells [27, 28] via the ERK1/2 pathway, but have not focused on rBMSC.
In this study, we verified the identity of rBMSC by their morphology and the finding that they expressed CD44, a marker of rBMSC, but not CD34, a closely related molecule not expressed by rBMSC. we found that ghrelin accelerates the growth of rBMSC through activation of the ERK1/2 branch of the MAPK pathway. Cellular proliferation following the second through fifth passages was analyzed on days 1 to 10 following the passages. The fourth generation rBMSC showed the greatest capacity for proliferation, whereas the second generation showed the least. The optimal ghrelin treatment to obtain maximum growth was 600ng/ml of ghrelin for 3days. We also demonstrated that this effect of ghrelin is mediated through its receptor, GHSR [29], which we showed to be expressed at high levels in rBMSC. Experiments in which the GHSR inhibitor D-Lys3-GHRP-6 blocked the ghrelin-mediated growth provided further support for the role of GHSR.
To gain further insight into the mechanism by which ghrelin accelerate rBMSC growth, we evaluated the activity of signaling pathways downstream of GHSR. The MAPKs are a super-family of serine/threonine kinases that includes ERK, JNK, and p38. These kinases are involved primarily in the activation of nuclear transcription factors that control cell proliferation, differentiation, and apoptosis [30]. Our results suggest that ghrelin accelerates rBMSC growth via the ERK signaling pathway, and not through the activation of JNK or p38. We found that 20 to 60 min of ghrelin treatment was required to stimulate phosphorylation of ERK, and therefore that the stimulus is time-dependent. Furthermore, both blockade of GHSR and ERK by chemical inhibition suppressed the ghrelin-mediated acceleration of rBMSC growth and promoted rBMSC differentiation to osteoblasts.
Conclusions
Our results provide evidence that the ghrelin/GHSR signaling pathway accelerate rBMSC growth and promotes rBMSC differentiation to osteoblasts mainly through an ERK-dependent pathway. This study only obtained the ghrelin function on rBMSC in vitro, in future will be in vivo. Thus, our findings suggest that ghrelin might be useful in growing large numbers of rBMSC. Further study is necessary before any clinical application is considered.
Methods
Unless otherwise specified, all chemicals and reagents were purchased from Sigma-Aldrich (St. Louis, MO, USA). Antibodies to IgG, GAPDH, ALP, Runx2, Osterix, CD44, CD34, U0126 (an inhibitor of phospho-ERK1/2) ERK1/2, JNK, p90rsk,phospho-ERK1/2, phospho-JNK and phospho-p90rsk1(Ser380) were purchased from the Abcam Corporation, USA.
Isolation and culture of rBMSC
To obtain the rBMSC, the rabbit was used. The femur from a neonatal New Zealand white rabbit was isolated, and the ends of the femur were opened. The bone marrow was flushed from the femur with low glucose Dulbecco’s modified Eagle’s medium (DMEM) using a 1mL syringe. Cells were harvested into a culture dish, suspended using a Pasteur pipette, seeded into a flask containing DMEM and 15 % fetal bovine serum, and cultured in an incubator with 5 % CO2 at 37 °C. The medium was replaced every 2days. When cells grew to a confluence of approximately 85 %, they were passaged with 0.25 % trypsin and 0.1 % EDTA (1:2). Cell growth was monitored using an inverted phase contrast microscope (Nikon Co.). The animal experimental protocols were approved by the Chongqing medical university experimental animal management committee.
RNA Extraction and RT-PCR
To detected the GHSR expression status in the rBMSC, the RT-PCR was used. Total RNA was isolated from o cells using the RNeasy kit (Qiagen, Hilden, Germany). All RNA samples were treated with RNase-free DNase I to remove genomic DNA contamination. The RNA content of samples was too low to be accurately quantified by spectrometry, and thus, 6.5-μL RNA aliquots were converted to cDNA by reverse transcription, then amplified (TaKaRa, Inc., Dalian, China). The ghrelin receptor PCR primers were: sense, 5’-TCTTCCTTCCTGTCTTCTGTC-3’;antisense, 5’-AGTCTGAACACTGCCACC-3’and the PCR condition was 95 °C 5 min, 95 °C 30 s 57 °C 30 s 72 °C 30 s 30 cycles, 72 °C 10 min.
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltertrazolium bromide (MTT) assay
To determine the cell growth percentage, the MTT assay was operated. Cells were grown in 96-well plates (1 × 103 cells/well) supplemented with MGF. Control cells were switched from RPMI-1640 to DMEM containing 0.1 % dimethyl sulfoxide (DMSO). At 1 to 6 days following ghrelin treatment (400, 500, 600, 700 and 800ng/ml ghrelin), 20μL of MTT was added to each well to a final concentration of 0.5 %. After a 4h incubation at 37 °C in the dark, 150μL DMSO was added to each well for 10 min to dissolve the formazan crystals. The absorbance was measured using a microplate reader (EXL800, Cole-Parmer, Vernon Hills, IL, USA) at 490nm. All experiments were repeated three times. The viability of the MGF treated cells was expressed as percentage of population growth plus the standard error of the mean (SEM) relative to that of untransfected control cells. Cell growth was calculated as follows:
Immunofluorescence
To detected the CD44 and CD34 expressin status, the immunofluorescence was used. The rBMSC were fixed in 3.7 % paraformaldehyde for 30 min at room temperature, permeabilized with 0.5 % Triton X-100 in PBS for 15 min, and blocked with 1 % BSA in phosphate-buffered saline (PBS) with 10 % goat serum overnight at 4 °C. The samples were then stained with primary antibodies diluted in PBS. The primary antibody binding was detected with an Alexa Fluor 488 goat anti-rabbit IgG (H + L) secondary antibody. Images were captured with a Nikon A1 confocal microscope. Experiments were performed in triplicate.
Western blotting
The detected the protein in MAPK pathway and osteogenic, the western blot was used. The protein homogenates from rBMSC were separated using electrophoresis on 8–12 % sodium dodecyl sulphate/polyacrylamide gels and transferred to nitrocellulose membranes. Membranes were blocked for 30 min at room temperature in PBS buffer containing 5 % fat-free milk and 0.1 % Tween 20. Membranes were then incubated with primary antibody for at least 1 h at room temperature or overnight at 4 °C. The membranes were subsequently washed three times with PBS containing 0.1 % Tween 20, incubated with peroxidase-conjugated secondary antibodies, and developed using ECL reagents (Pierce, Rockford, IL, USA).
Osteogenic differentiation
To detect the rBMSC differentiation to osteogenic, this experiments was operated. The rBMSC were plated at a density of 5000 cells/cm2 and exposed to standard differentiation-inducing media for 21 days. The medium was changed twice per week. Osteogenic differentiation was achieved following standard in vitro protocols. Endothelial differentiation was stimulated by culturing the cells in endothelial growth medium-2 (EGM-2) [31].
Statistical analysis
Statistically significant differences between gene expression levels were determined using one-way analysis of variance (ANOVA) followed by a Newman–Keuls test with GraphPad Prism version five software (GraphPad Software, La Jolla, CA, USA, www.graphpad.com/company/). Replicates were included in the statistical model. Differences were considered statistically significant at the 95 % confidence level (P < 0.05). Data are presented as mean ± S.D.
Acknowledgements
This work was acknowledge to the Dianming Jiang.
Abbreviations
- MSCs
Mesenchymal stem cells
- rBMSC
Rabbit MSCs
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
The authors declare that they have no competing interests. Authors’ contributions NY carried out experimental design, statistical analysis, manuscript and figures preparation. DJ carried out experimental design, Western blot experiments, figure preparations, NY carried out cell culture, DJ carried out experimental design. All authors read and approved the final manuscript.
Contributor Information
Nan Ye, Email: ynlovearn2004@163.com.
Dianming Jiang, Email: jiangdianming1122@126.com.
Reference
- 1.Zhou S, Chiang C, Liu K, Li Q. Intravenous Transplantation of Bone Marrow Mesenchymal Stem Cell Could Effectively Promote Vascularization and Skin Regeneration in Mechanical Stretched Skin. Br J Dermatol 2015;172(5):1278-85. [DOI] [PubMed]
- 2.Wollmer E, Neubauer A. [Complications after allogeneic bone marrow and stem cell transplantation] Internist (Berl) 2014;55:547–560. doi: 10.1007/s00108-013-3432-3. [DOI] [PubMed] [Google Scholar]
- 3.Wilson A, Trumpp A. Bone-marrow haematopoietic-stem-cell niches. Nat Rev Immunol. 2006;6:93–106. doi: 10.1038/nri1779. [DOI] [PubMed] [Google Scholar]
- 4.Suzuki S, Narita Y, Yamawaki A, Murase Y, Satake M, Mutsuga M, Okamoto H, Kagami H, Ueda M, Ueda Y. Effects of extracellular matrix on differentiation of human bone marrow-derived mesenchymal stem cells into smooth muscle cell lineage: utility for cardiovascular tissue engineering. Cells Tissues Organs. 2010;191:269–280. doi: 10.1159/000260061. [DOI] [PubMed] [Google Scholar]
- 5.Sreejit P, Dilip KB, Verma RS. Generation of mesenchymal stem cell lines from murine bone marrow. Cell Tissue Res. 2012;350:55–68. doi: 10.1007/s00441-012-1458-9. [DOI] [PubMed] [Google Scholar]
- 6.Sharma S, Kumar L, Mohanty S, Kumar R, Datta Gupta S, Gupta DK. Bone marrow mononuclear stem cell infusion improves biochemical parameters and scintigraphy in infants with biliary atresia. Pediatr Surg Int. 2011;27:81–89. doi: 10.1007/s00383-010-2712-4. [DOI] [PubMed] [Google Scholar]
- 7.Sadat K, Ather S, Aljaroudi W, Heo J, Iskandrian AE, Hage FG. The effect of bone marrow mononuclear stem cell therapy on left ventricular function and myocardial perfusion. J Nucl Cardiol. 2014;21:351–367. doi: 10.1007/s12350-013-9846-4. [DOI] [PubMed] [Google Scholar]
- 8.Ringden O, Labopin M, Beelen DW, Volin L, Ehninger G, Finke J, Greinix HT, Kyrcz-Krzemien S, Bunjes D, Brinch L, et al. Bone marrow or peripheral blood stem cell transplantation from unrelated donors in adult patients with acute myeloid leukaemia, an Acute Leukaemia working party analysis in 2262 patients. J Intern Med. 2012;272:472–483. doi: 10.1111/j.1365-2796.2012.02547.x. [DOI] [PubMed] [Google Scholar]
- 9.Rimmele P, Lofek-Czubek S, Ghaffari S. Resveratrol increases the bone marrow hematopoietic stem and progenitor cell capacity. Am J Hematol. 2014;89(12):E235–8. doi: 10.1002/ajh.23837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ricart E. Current status of mesenchymal stem cell therapy and bone marrow transplantation in IBD. Dig Dis. 2012;30:387–391. doi: 10.1159/000338134. [DOI] [PubMed] [Google Scholar]
- 11.Ratajczak MZ, Lee H, Wysoczynski M, Wan W, Marlicz W, Laughlin MJ, Kucia M, Janowska-Wieczorek A, Ratajczak J. Novel insight into stem cell mobilization-plasma sphingosine-1-phosphate is a major chemoattractant that directs the egress of hematopoietic stem progenitor cells from the bone marrow and its level in peripheral blood increases during mobilization due to activation of complement cascade/membrane attack complex. Leukemia. 2010;24:976–985. doi: 10.1038/leu.2010.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qian H, Tryggvason K, Jacobsen SE, Ekblom M. Contribution of alpha6 integrins to hematopoietic stem and progenitor cell homing to bone marrow and collaboration with alpha4 integrins. Blood. 2006;107:3503–3510. doi: 10.1182/blood-2005-10-3932. [DOI] [PubMed] [Google Scholar]
- 13.Kojima M, Kangawa K. Ghrelin: from gene to physiological function. Results Probl Cell Differ. 2010;50:185–205. doi: 10.1007/400_2009_28. [DOI] [PubMed] [Google Scholar]
- 14.Kojima M, Kangawa K. Ghrelin: more than endogenous growth hormone secretagogue. Ann N Y Acad Sci. 2010;1200:140–148. doi: 10.1111/j.1749-6632.2010.05516.x. [DOI] [PubMed] [Google Scholar]
- 15.Kojima M, Kangawa K. Structure and function of ghrelin. Results Probl Cell Differ. 2008;46:89–115. doi: 10.1007/400_2007_049. [DOI] [PubMed] [Google Scholar]
- 16.Kojima M. The discovery of ghrelin–a personal memory. Regul Pept. 2008;145:2–6. doi: 10.1016/j.regpep.2007.09.023. [DOI] [PubMed] [Google Scholar]
- 17.Zhang C, Shi J, Mao SY, Xu YS, Zhang D, Feng LY, Zhang B, Yan YY, Wang SC, Pan JP, et al. Role of p38 MAPK in enhanced human cancer cells killing by the combination of aspirin and ABT-737. J Cell Mol Med. 2015;19:408–417. doi: 10.1111/jcmm.12461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu L, Zhang Y, Wang H, Zhang G, Ding Y, Zhao L. Tumor suppressor miR-1 restrains epithelial-mesenchymal transition and metastasis of colorectal carcinoma via the MAPK and PI3K/AKT pathway. J Transl Med. 2014;12:244. doi: 10.1186/s12967-014-0244-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wojtera E, Konior A, Fedoryszak-Kuska N, Beresewicz A. Obligatory role of intraluminal O2- in acute endothelin-1 and angiotensin II signaling to mediate endothelial dysfunction and MAPK activation in guinea-pig hearts. Int J Mol Sci. 2014;15:19417–19443. doi: 10.3390/ijms151119417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang Z, Li P, Zhang Q, Lv H, Liu J, Si J. Interleukin-1beta regulates the expression of glucocorticoid receptor isoforms in nasal polyps in vitro via p38 MAPK and JNK signal transduction pathways. J Inflamm (Lond) 2015;12:3. doi: 10.1186/s12950-014-0046-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu YY, Ma TL, Ge ZJ, Lin J, Ding WL, Feng JK, Zhou SJ, Chen GC, Tan YF, Cui GX. Gene regulates PANC-1 pancreatic cancer cell behaviors through MEK-ERK1/2 of the MAPK signaling pathway. Oncol Lett. 2014;8:1859–1863. doi: 10.3892/ol.2014.2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fujitsuka N, Asakawa A, Amitani H, Hattori T, Inui A. Efficacy of ghrelin in cancer cachexia: clinical trials and a novel treatment by rikkunshito. Crit Rev Oncog. 2012;17:277–284. doi: 10.1615/CritRevOncog.v17.i3.50. [DOI] [PubMed] [Google Scholar]
- 23.Bai R, Zhao P, Cao G, Wen S, Li Q, Meng Q. Ghrelin promotion of oocyte maturation via ERK1/2 pathway in ovis aries. Cell Mol Biol (Noisy-le-grand) 2012;58(Suppl):OL1797–1802. [PubMed] [Google Scholar]
- 24.Hosoda H, Kangawa K. [Role of ghrelin in cancer] Nihon Rinsho. 2004;62(Suppl 9):421–423. [PubMed] [Google Scholar]
- 25.Milewski L, Wojtowicz K, Roszkowski PI, Barcz E, Ziarkiewicz-Wroblewska B, Kaminski P, Malejczyk J. Expression of ghrelin and its receptors in ovarian endometrioma. Gynecol Endocrinol. 2012;28:310–313. doi: 10.3109/09513590.2011.631628. [DOI] [PubMed] [Google Scholar]
- 26.Rak A, Szczepankiewicz D, Gregoraszczuk EL. Expression of ghrelin receptor, GHSR-1a, and its functional role in the porcine ovarian follicles. Growth Horm IGF Res. 2009;19:68–76. doi: 10.1016/j.ghir.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 27.Yang J, Liu GQ, Wei R, Hou WF, Gao MJ, Zhu MX, Wang HN, Chen GA, Hong TP. Ghrelin promotes differentiation of human embryonic stem cells into cardiomyocytes. Acta Pharmacol Sin. 2011;32:1239–1245. doi: 10.1038/aps.2011.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gao M, Yang J, Wei R, Liu G, Zhang L, Wang H, Wang G, Gao H, Chen G, Hong T. Ghrelin induces cardiac lineage differentiation of human embryonic stem cells through ERK1/2 pathway. Int J Cardiol. 2013;167:2724–2733. doi: 10.1016/j.ijcard.2012.06.106. [DOI] [PubMed] [Google Scholar]
- 29.Kamegai J, Wakabayashi I, Miyamoto K, Unterman TG, Kineman RD, Frohman LA. Growth hormone-dependent regulation of pituitary GH secretagogue receptor (GHS-R) mRNA levels in the spontaneous dwarf Rat. Neuroendocrinology. 1998;68:312–318. doi: 10.1159/000054379. [DOI] [PubMed] [Google Scholar]
- 30.Delhanty PJ, van der Eerden BC, van der Velde M, Gauna C, Pols HA, Jahr H, Chiba H, van der Lely AJ, van Leeuwen JP. Ghrelin and unacylated ghrelin stimulate human osteoblast growth via mitogen-activated protein kinase (MAPK)/phosphoinositide 3-kinase (PI3K) pathways in the absence of GHS-R1a. J Endocrinol. 2006;188:37–47. doi: 10.1677/joe.1.06404. [DOI] [PubMed] [Google Scholar]
- 31.Zhang Z, Luo X, Xu H, Wang L, Jin X, Chen R, Ren X, Lu Y, Fu M, Huang Y, et al. Bone marrow stromal cell-derived extracellular matrix promotes osteogenesis of adipose-derived stem cells. Cell Biol Int 2015;39(3):291-9. [DOI] [PubMed]


