Abstract
Background
Oral involvement is often associated with inflammatory bowel disease (IBD). Recent evidence suggests a high incidence of periodontal disease in patients with Crohn disease (CD). To the best of the authors’ knowledge, no animal model of IBD that displays associated periodontal disease was reported previously. The aim of this study is to investigate the occurrence and progression of periodontal disease in SAMP1/YitFc (SAMP) mice that spontaneously develop a CD-like ileitis. In addition, the temporal correlation between the onset and progression of periodontal disease and the onset of ileitis in SAMP mice was studied.
Methods
At different time points, SAMP and parental AKR/J (AKR) control mice were sacrificed, and mandibles were prepared for stereomicroscopy and histology. Terminal ilea were collected for histologic assessment of inflammation score. Periodontal status, i.e., alveolar bone loss (ABL) and alveolar bone crest, was examined by stereomicroscopy and histomorphometry, respectively.
Results
ABL increased in both strains with age. SAMP mice showed greater ABL compared with AKR mice by 12 weeks of age, with maximal differences observed at 27 weeks of age. AKR control mice did not show the same severity of periodontal disease. Interestingly, a strong positive correlation was found between ileitis severity and ABL in SAMP mice, independent of age.
Conclusions
The present results demonstrate the occurrence of periodontal disease in a mouse model of progressive CD-like ileitis. In addition, the severity of periodontitis strongly correlated with the severity of ileitis, independent of age, suggesting that common pathogenic mechanisms, such as abnormal immune response and dysbiosis, may be shared between these two phenotypes.
Keywords: Crohn disease, inflammatory bowel diseases, models, animal, periodontal diseases, periodontitis
Inflammatory bowel disease (IBD) is a chronic, relapsing and remitting disorder of the gastrointestinal tract, manifesting in two main clinical forms: 1) Crohn disease (CD) and 2) ulcerative colitis (UC).1 UC commonly involves the rectum but may also affect either part of or the entire colon in a continuous pattern, whereas CD primarily affects the ileum and colon but can also discontinuously involve any region of the gastrointestinal tract.1–3 It is widely accepted that IBD results from a dysregulated mucosal immune response to environmental factors in genetically susceptible hosts.3 However, the precise cause of the disease was not fully elucidated.1,2,4
Although IBD primarily affects the gastrointestinal tract, both CD and UC share a number of extraintestinal manifestations generally related to inflammatory disease activity. These include musculoskeletal, dermatologic, hepatopancreatobiliary, ocular, renal, and pulmonary inflammation.1–4 Oral involvement is also common, usually in the form of aphthous stomatitis. Less commonly, patients may experience pyostomatitis vegetans and the more specific aspects of tag-like lesions, mucogingivitis, or “cobblestone” lesions.5,6 A higher prevalence of dental caries was also described in individuals with CD.7,8
Previous studies reported an association between the occurrence of periodontal disease in patients with CD, suggesting that the two inflammatory conditions may share common pathogenic pathways.9–12 Similar to IBD, periodontal disease is considered a multifactorial condition, wherein a chronic inflammatory response is triggered and maintained by a combination of genetic and environmental factors, including a prominent role for an abnormal microflora and impaired host responses against the soft and hard tissues surrounding the teeth.10 Cigarette use and metabolic impairment (as observed in diabetes mellitus) are other common determinants of periodontal disease.10,13,14 Mechanistic studies to investigate the relationship between CD and periodontitis relied heavily on the use of chemically induced mouse models of colitis, which are generated through administration of either dextran sodium sulfate (DSS) or trinitrobenzenesulfonic acid (TNBS) enemas to otherwise healthy mice.11,15 Although these chemically induced models can be used to understand specific mechanistic aspects of intestinal inflammation and periodontal disease pathogenesis, they do not fully represent the multifactorial and complex nature of these two immune-mediated diseases.
The goal of the present study is to investigate the presence and progression of periodontal disease in a well-characterized spontaneous mouse model of chronic intestinal inflammation. SAMP1/YitFc (SAMP) mice develop severe, chronic ileitis by 20 weeks of age without chemical, genetic, or immunologic manipulation. Furthermore, the resulting ileitis in these mice bears remarkable phenotypic similarities to human CD with regard to disease location, histologic features, response to conventional CD therapies, and, notably, the occurrence of extraintestinal manifestations.16
In the present study, evidence is provided that SAMP mice develop a spontaneous periodontitis that temporally correlates with the onset, progression, and severity of ileitis. The present results provide new insight into the pathogenic association between CD and periodontitis and describe, to the best of the authors’ knowledge, for the first time a unique animal model of spontaneous periodontal disease.
MATERIALS AND METHODS
Animals
SAMP mice were bred at Case Western Reserve University, and parental AKR/J (AKR) control mice were purchased.§ All experimental mice were maintained under specific-pathogen-free conditions, fed standard laboratory chow,‖ and kept on 12-hour light/dark cycles. All procedures were approved by the Case Western Reserve University Institutional Animal Care and Use Committee (animal protocol 2011-0196) and conducted in accordance with the Association for Assessment and Accreditation of Laboratory Animal Care guidelines.
Alveolar Bone Loss (ABL) and Ileal Histologic Analyses
SAMP and AKR mice (n = 20 per group) were euthanized by inhalation of CO2 at 4, 12, and 27 weeks of age for evaluation of ABL and ileal inflammation. Additionally, older SAMP mice of 30 to 60 weeks of age (n = 11) were sacrificed for ABL evaluation only, using the same method.
Briefly, mandibles were collected, and soft tissues and gingiva were removed after 10 minutes of boiling. Samples were then defleshed, treated overnight with 3% H2O2, cleaned, and stained with 1%methylene blue. ABL was evaluated using the trough morphometric approach because other methods, such as histomorphometry and microcomputer tomography, revealed similar results.17 Digital photographs were taken using stereomicroscopy on a custom-made stage holder with an unglued jaw to enhance the visualization of the cemento-enamel junction (CEJ) and bone levels, as described previously.18 ABL was defined by computer software¶ as the area bordered by the CEJ, the alveolar bone crest, and the mesial and distal lines on the lingual side of the first (M1) and second (M2) mandibular molars.19 All measurements were performed in triplicate in a masked manner. In addition, to minimize interanimal and age-related variability, normalization by the mesio-distal axis of the teeth was performed. Data are expressed in square millimeters for ABL and millimeters for tooth axis.
For histologic evaluation of intestinal inflammation, ilea from experimental mice were removed, flushed of fecal contents, opened longitudinally, and placed in Bouin fixative for 24 hours, followed by 70% ethanol. Tissues were embedded in paraffin and stained with hematoxylin and eosin (H&E). Inflammation was evaluated by a trained pathologist (Prof. Wei Xin, Case Medical Center, Cleveland, OH) in a masked manner using an established and validated scoring system.20 The total inflammatory index for ileal specimens represents the sum of three individual indices: 1) active inflammation; 2) chronic inflammation; and 3) villous distortion.
Histologic Evaluation of the Periodontal Tissues
Four- and 30-week-old SAMP and AKR mice were euthanized by inhalation of CO2 for histologic evaluation of periodontal tissues. Mandibles were collected, fixed in 10% formalin for 24 hours, placed in 70% ethanol for 24 hours, decalcified by hydrochloric acid solution,# washed, dehydrated, and embedded in paraffin. Serial sections (five semi-serial sections of each hemimandible) with 5- µm thickness were cut and stained with H&E.21
The same morphometric approach was used to evaluate the area of the alveolar bone crest and the hemiproximal and hemidistal coronal-to-apical cementum layer of the first and second mandibular molars, respectively. The cementum layer was measured on the hemidistal root of the first molar and the hemiproximal root of the second molar, and a total cementum area was calculated as a sum of the two. A trained pathologist (DP) evaluated each section in a masked manner, and all measurements were performed in triplicate.
Statistical Analyses
All experiments were performed at least twice, and statistical analysis was conducted using the collective data from replicated experiments. Data that revealed a normal distribution by the Shapiro-Wilk test were compared using the Student t test, one-way or two-way analysis of variance (ANOVA), linear regression, or receiver operating characteristic (ROC) analysis. Alternative nonparametric tests were used for data that were not normally distributed. The level of significance was set at P <0.05, and standard error of the means are shown in the figures. Statistical analysis and graph plots were performed using a statistical program.**
RESULTS
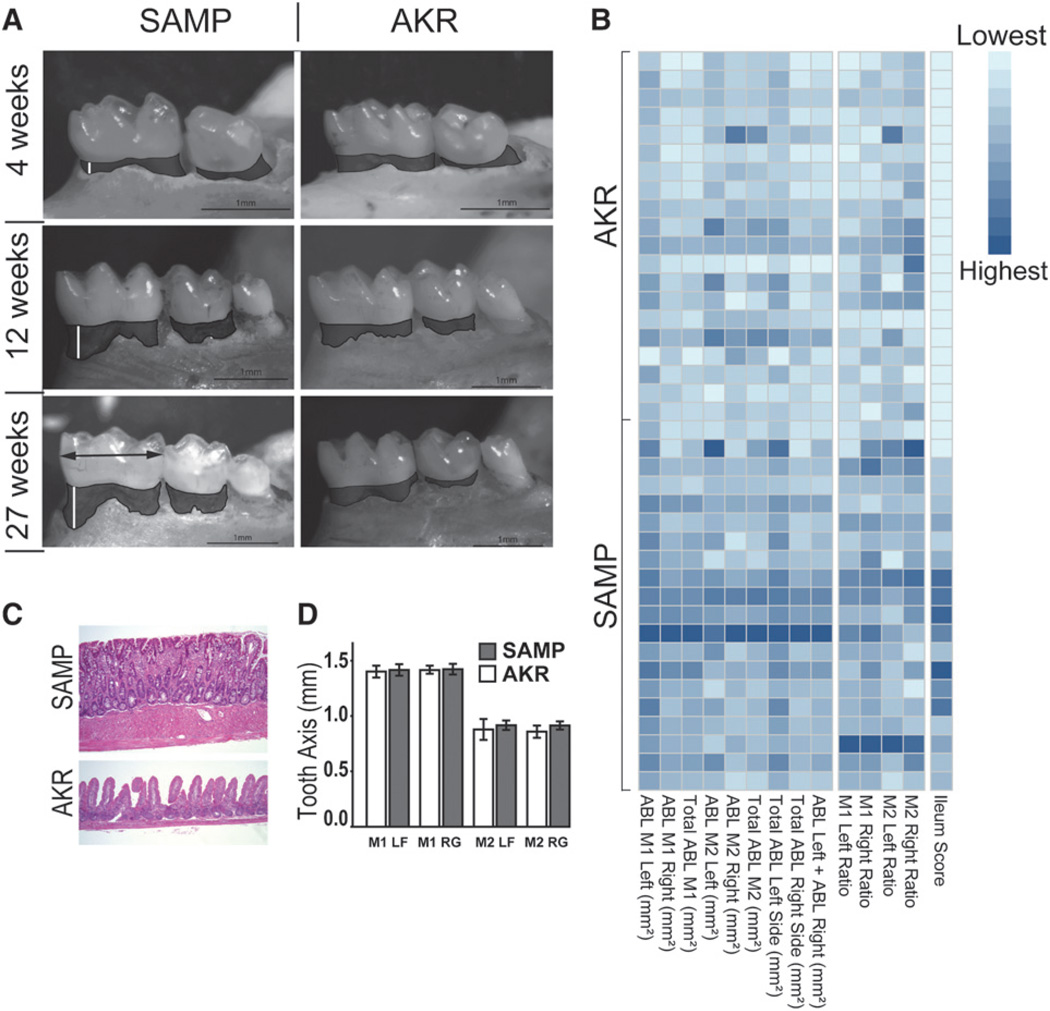
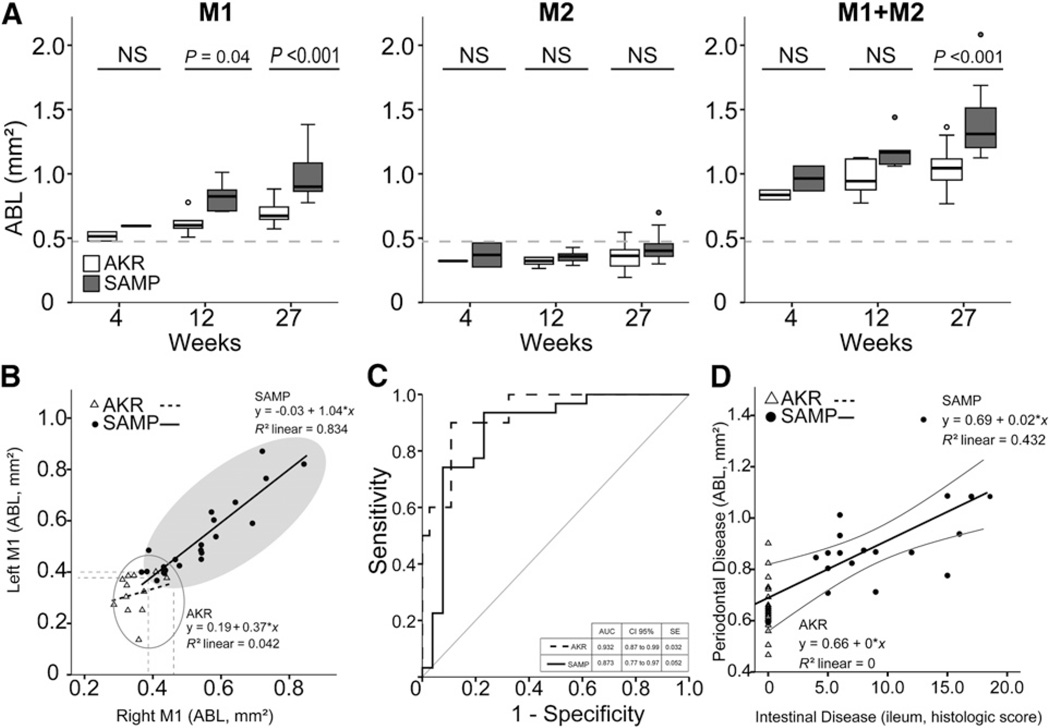
Stereomicroscopic results are shown in Figures 1 and 2. Stereomicroscopy analysis revealed more severe ABL in SAMP mice compared with AKR mice starting at 12 weeks of age (Fig. 1A), and the severity at each site is shown in the heat map (Fig. 1B). Total ABL of M1 and M2 of both sides (M1 ABL and M2 ABL, respectively) were analyzed individually and together according to age in both SAMP and AKR control mice, as shown in Figure 2A. M1 ABL increased in both strains according to age. Elevated levels were observed in SAMP mice compared with AKR mice beginning as early as 12 weeks of age (P = 0.04) and further increased at 27 weeks (P <0.001). No differences in ABL of M2 were found between the two strains or within strain at any age (Fig. 2A). Total ABL for the entire resorption area of M1 and M2 (M1+M2 ABL) was increased in SAMP mice versus AKR mice at 27 weeks (P <0.001) but not at 12 weeks of age. The same comparisons performed with normalized data (ratio of ABL [square millimeters] to teeth major axis [millimeters]) provided comparable results, and, as expected, teeth axis lengths were statistically similar between strains (Fig. 1D). Based on these results, M1 ABL was determined to have the highest level of discrimination between the two strains (versus M2 ABL or M1+M2 ABL) and was used for all subsequent analyses.
Figure 1.
A) Stereomicroscopy images showing ABL in SAMP and AKR mice at 4, 12, and 27 weeks of age. B) A heat map is a graphical way of displaying a table of numbers by using colors to represent numerical values. Specifically, low severity scores tend toward lighter colors, whereas high severity scores are represented by darker colors. The heat map herein shows single-tooth and total ABL, ABL ratios, and ileum score for each sample in both strains, thus summarizing the relations of periodontal disease to ileal inflammation severity. Each row represents samples from a single animal, with AKR mice clustered in the top half of the picture and SAMP mice in the bottom. Darker colors occur more frequently in the bottom half (SAMP mice), thus representing higher severity scores of both periodontal disease and ileitis in SAMP mice compared with AKR mice. C) Ileal sections from 27-week-old SAMP mice and AKR control mice (H&E; magnification ×10). SAMP mice develop spontaneous, transmural inflammation of the terminal ileum characterized by discontinuous inflammatory infiltrates, villous architecture alterations, and bowel wall thickening, with hypertrophy of the muscular layers, villous blunting and distortion, and infiltration of acute and chronic immune cells. A normal structure in uninflamed control AKR mice is also shown. D) Comparison of tooth axis of left/right M1 and M2 showed no statistical difference between strains (ANOVA), thus indicating similar tooth dimensions in the two strains. LF = left; RG = right.
Figure 2.
A) ANOVA of ABL on M1, M2, and M1+M2 according to age. M1 alone was able to discriminate the strains by 12 weeks of age, M1+M2 ABL only at 27 weeks, and M2 alone was not able to identify differences between strains. NS = not significant. B) Regression analysis of ABL in SAMP and AKR mice. SAMP mice showed a comparable ABL value in both sides of the mouth, suggesting a very reproducible model (R2 = 0.834); AKR did not show the same features. C) ROC analysis ofM1 ABL and M1 ABL ratio showed high sensitivity to differentiate between the groups, with SAMP AUC = 0.873 and AKR AUC = 0.932. SE = standard error. D) A positive significant correlation between ileal disease and periodontal disease was found in SAMP mice (P = 0.002; R2 = 0.432). The severity of periodontal disease was directly related to the severity of ileal damage.
Combined cluster analyses on right and left M1 ABL in SAMP and AKR mice clearly showed the segregation of samples into two groups according to strain (Fig. 2B), with SAMP mice showing more bone resorption compared with AKR mice. Regression analysis yielded a very reproducible correlation between right and left M1 ABL in SAMP mice (y = −0.03 + 1.04 × x; R2 = 0.834; P <0.001), indicating that the same process occurs in both sides of the mouth, and is suggestive of either a systemic disease or a phenomenon driven by a systemic factor. In contrast, AKR mice tended to show a small correlation between left and right M1 ABL (M1 left, 0.39 mm2; M1 right, 0.47 mm2) and did not display a clear trend toward increased variability (Fig. 2B). To determine the discriminatory ability of M1 ABL and M1 ratio (M1 ABL [square millimeters] to M1 major axis [millimeters]) to differentiate between the two strains, sensitivity/specificity ROC curve analysis was conducted, indicating that both methods have a high sensitivity to differentiate the groups (AKR: area under the curve [AUC] = 0.932, 95% confidence interval (CI) = 0.87 to 0.99; SAMP: AUC = 0.873, 95% CI = 0.77 to 0.97) (Fig. 2C).
To test the hypothesis that the occurrence of periodontal disease correlates with the severity of intestinal inflammation in SAMP mice (Fig. 1C), a regression analysis was performed between the ileal histologic scores and M1 ABL. The results showed a significant positive correlation between the two parameters (y = 0.69 + 0.02 × x; R2 = 0.432; P = 0.002), with periodontal disease severity increasing as the severity of ileal scores in SAMP mice also increased (Fig. 2D).
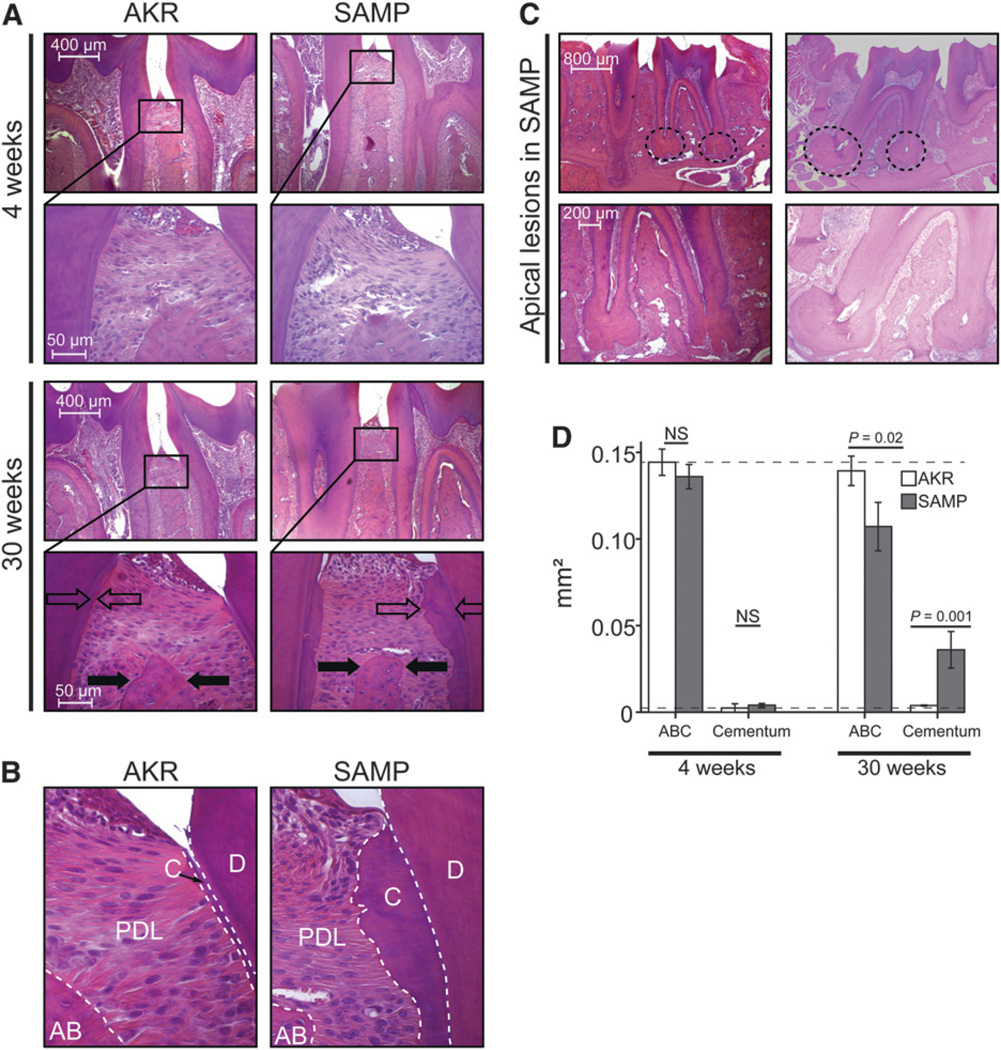
Histologic results are shown in Figure 3. Histologic evaluation of mandibles from SAMP mice showed the presence of periodontal disease (Fig. 3A). Evidence of alveolar bone resorption was observed in SAMP mice as early as 4 weeks, with gradual worsening over time. A significant increase in polymorphonuclear leukocyte (PMN) and mononuclear cell (MN) infiltration was noted in the alveolar bone crest over time in SAMP mice compared with AKR control mice (Fig. 3A). Alveolar bone morphology was not preserved, and mosaic-like remodeling was present in SAMP mice but not in AKR mice (Figs. 3A and 3C). Cementum layer and granuloma-like lesions with substantial infiltration of inflammatory cells was observed in SAMP mice at all ages but remarkably increased only at 30 weeks compared with 4 weeks (Figs. 3A through 3C). AKR mice did not show the same features in the aforementioned described structures. Computer-assisted morphometric analysis showed no difference for SAMP versus AKR mice in alveolar bone crest and cementum area at 4 weeks of age. In contrast, SAMP mice displayed lower alveolar bone crest area (P = 0.02) and increased cementum area compared with AKR control mice at 30 weeks of age (P = 0.001) (Fig. 3D).
Figure 3.
A) Sagittal mandibular sections of SAMP and AKR mice at 4 and 30 weeks of age. Selected areas of interest (black squares) are presented as magnified images below the corresponding section. No notable alveolar bone resorption or signs of inflammation were observed at 4 weeks in either strain. Thirty-week-old SAMP mice showed reduction in the thickness of the alveolar bone crest compared with AKR mice (filled arrows). Surprisingly, severe hypercementosis was found in SAMP mice but not in AKR mice (open arrows) (H&E; original magnification ×10). B) Higher magnification (×40) of the CEJ area shown in Fig. 3A reveals the major thickness of cementum layer (C). PMN and MN cells were present in the periodontal ligament (PDL) layer of SAMP mice. The alveolar bone crest (AB) was markedly reduced in SAMP mice but not in AKR mice at 30 weeks of age. Root dentin (D) appears normal. (H&E stain.) C) Hypercementosis in SAMP mice was accompanied by severe apical lesions (dotted circles) in the absence of apparent endodontic causes. Magnification of apical lesions is shown under the corresponding original image. Numerous inflammatory cells surround the apical region, and alveolar bone remodeling was visible between the roots (H&E; original magnification ×10). D) Ranked Kruskal-Wallis one-way ANOVA showed statistical differences in terms of alveolar bone crest area (ABC) (P = 0.02) and cementum area (P = 0.001) only at 30 weeks of age. NS = not significant.
DISCUSSION
This study demonstrates the spontaneous occurrence of periodontal disease in a mouse model of CD-like terminal ileitis, in the absence of any exogenous proinflammatory stimuli. The present results are consistent with data from clinical studies showing a high prevalence of periodontitis in patients with IBD.9–12 In addition, many case-control studies and case reports observed this relationship.6,22,23 Interestingly, periodontal disease and hypercementosis in SAMP mice occurs spontaneously, with similar alveolar bone resorption on both sides of the mouth, suggesting a systemic disease or phenomenon.
Moreover, the present study provides strong evidence of a correlation between the severity of both inflammatory disorders. Notably, although the data confirm the progression of periodontal disease with age in both SAMP and AKR mice as described previously,15 the association of periodontal disease with ileitis severity was found to be independent of age, a finding that is-consistent with human disease wherein severe periodontitis was reported in young patients with IBD.24
To the best of the authors’ knowledge, this is the first study to evaluate the occurrence and severity of periodontal disease in a mouse model of spontaneous CD-like ileitis. A recent study described a mouse model of chemically induced periodontitis that uses prolonged oral administration of TNBS and DSS to induce a status of non-specific chronic inflammation and ABL. DSS is commonly used to chemically induce colitis in mice, and the same study describes how the administration of low doses of DSS is able to induce a chronic inflammatory state in both the intestine and periodontal tissue, suggesting an imbalance in the antioxidant functional reserve as a common link.11 However, the limitations of chemically induced models lie in the dose and time dependence of the clinical manifestations; consequently, these models do not represent the best system for evaluating shared pathogenic mechanisms between periodontal disease and intestinal lesions in IBD.
One possible common immunopathogenic mechanism may be a shared abnormal cytokine pattern and intercellular crosstalk that leads to T-helper 1 polarization and progressive tissue damage, which is exacerbated by coexistent predisposing genetic mutations, poor health conditions, malnutrition, and commensal bacterial load, and pathogenicity.10 In the 1980s, Van Dyke et al.12 first described the presence of a peculiar oral microflora in patients with IBD with concomitant periodontal disease that was mainly composed of motile Gram-negative rods, referred to as Wolinella genus. This study also observed an important defect in neutrophil chemotaxis in all patients with IBD with periodontal disease that was not associated with impaired phagocytic function in the same cells. It was proposed that this oral pathogen may play a role in IBD pathogenesis, possibly as a trigger for an infectious agent or as an immune response modifier.12
The present results in the SAMP mouse model are consistent with this hypothesis. Previous in vivo and in vitro studies with the SAMP mice show that these mice have a reduced capacity for bacterial clearance, although their phagocytic ability is preserved.25 Although they do not harbor genetic mutations in the nucleotide-binding oligomerization domain containing 2 (NOD2) (which were associated with CD in a subset of patients), they do display abnormal immune responses and bacterial clearance in response to muramyl dipeptide stimulation, suggesting an underlying functional defect in the NOD2 molecular pathway. This immunologic dysfunction may contribute to the pathogenesis of both the observed intestinal and oral lesions. Moreover, SAMP mice also show a defect in intestinal permeability, demonstrated through ex vivo studies on portions of terminal ileum. Interestingly, the use of probiotics ameliorates the mucosal permeability defect in SAMP mice, further suggesting a role for the microbiota in the clinical course of chronic ileitis.26
CONCLUSIONS
The SAMP mouse model of spontaneous CD-like ileitis represents a powerful tool for the study not only of the natural history of IBD but also of the associated periodontal disease. These two conditions likely share similar etiopathogenic features, and multiple pathogenic mechanisms that were identified as contributing to SAMP ileitis conceptually may also be involved in the development of SAMP periodontal disease. These findings suggest an intriguing pathogenic hypothesis linking the two conditions and provide a new tool for the development of novel therapeutic interventions for both diseases. Future research will aim at defining the role of cytokines and the function of the oral microbiota in SAMP mice and in humans with CD.
ACKNOWLEDGMENTS
This research was supported by National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases Grants P01DK091222, R01DK055812, and R01DK04219 (to FC). The authors thank Mitchell Guanzon, Dennis Guszka, Joshua Weber, and Lindsey Kaydo for technical assistance (Department of Medicine and Pathology, and Digestive Health Research Center, Case Western Reserve University, Cleveland, Ohio) and Kristen Arseneau (Department of Medicine and Pathology, and Digestive Health Research Center, Case Western Reserve University) and Theresa Pizarro (Department of Pathology, Case Western Reserve University School of Medicine) for critical revision of this manuscript.
Footnotes
The Jackson Laboratory, Bar Harbor, ME.
Teklad, Harlan, Indianapolis, IN.
Image J v1.46r, National Institutes of Health, Bethesda, MD.
Rapid Decalcifier, VWR International, Radnor, PA.
The R packages “stat” and “ggplot” v.3.0.3, R Foundation for Statistical Computing, Vienna, Austria.
The authors report no conflicts of interest related to this study.
REFERENCES
- 1.Larsen S, Bendtzen K, Nielsen OH. Extraintestinal manifestations of inflammatory bowel disease: Epidemiology, diagnosis, and management. Ann Med. 2010;42:97–114. doi: 10.3109/07853890903559724. [DOI] [PubMed] [Google Scholar]
- 2.Su CG, Judge TA, Lichtenstein GR. Extraintestinal manifestations of inflammatory bowel disease. Gastroenterol Clin North Am. 2002;31:307–327. doi: 10.1016/s0889-8553(01)00019-x. [DOI] [PubMed] [Google Scholar]
- 3.Danese S, Semeraro S, Papa A, et al. Extraintestinal manifestations in inflammatory bowel disease. World J Gastroenterol. 2005;11:7227–7236. doi: 10.3748/wjg.v11.i46.7227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Urlep D, Mamula P, Baldassano R. Extraintestinal manifestations of inflammatory bowel disease. Minerva Gastroenterol Dietol. 2005;51:147–163. [PubMed] [Google Scholar]
- 5.Lankarani KB, Sivandzadeh GR, Hassanpour S. Oral manifestation in inflammatory bowel disease: A review. World J Gastroenterol. 2013;19:8571–8579. doi: 10.3748/wjg.v19.i46.8571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lourencxo SV, Hussein TP, Bologna SB, Sipahi AM, Nico MM. Oral manifestations of inflammatory bowel disease: A review based on the observation of six cases. J Eur Acad Dermatol Venereol. 2010;24:204–207. doi: 10.1111/j.1468-3083.2009.03304.x. [DOI] [PubMed] [Google Scholar]
- 7.Grössner-Schreiber B, Fetter T, Hedderich J, Kocher T, Schreiber S, Jepsen S. Prevalence of dental caries and periodontal disease in patients with inflammatory bowel disease: A case-control study. J Clin Periodontol. 2006;33:478–484. doi: 10.1111/j.1600-051X.2006.00942.x. [DOI] [PubMed] [Google Scholar]
- 8.Sundh B, Emilson CG. Salivary and microbial conditions and dental health in patients with Crohn’s disease: A 3-year study. Oral Surg Oral Med Oral Pathol. 1989;67:286–290. doi: 10.1016/0030-4220(89)90356-3. [DOI] [PubMed] [Google Scholar]
- 9.Vavricka SR, Manser CN, Hediger S, et al. Periodontitis and gingivitis in inflammatory bowel disease: A casecontrol study. Inflamm Bowel Dis. 2013;19:2768–2777. doi: 10.1097/01.MIB.0000438356.84263.3b. [DOI] [PubMed] [Google Scholar]
- 10.Brandtzaeg P. Inflammatory bowel disease: Clinics and pathology. Do inflammatory bowel disease and periodontal disease have similar immunopathogeneses? Acta Odontol Scand. 2001;59:235–243. doi: 10.1080/00016350152509265. [DOI] [PubMed] [Google Scholar]
- 11.Oz HS, Chen T, Ebersole JL. A model for chronic mucosal inflammation in IBD and periodontitis. Dig Dis Sci. 2010;55:2194–2202. doi: 10.1007/s10620-009-1031-x. [DOI] [PubMed] [Google Scholar]
- 12.Van Dyke TE, Dowell VR, Jr, Offenbacher S, Snyder W, Hersh T. Potential role of microorganisms isolated from periodontal lesions in the pathogenesis of inflammatory bowel disease. Infect Immun. 1986;53:671–677. doi: 10.1128/iai.53.3.671-677.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pietropaoli D, Monaco A, Del Pinto R, Cifone MG, Marzo G, Giannoni M. Advanced glycation end products: Possible link between metabolic syndrome and periodontal diseases. Int J Immunopathol Pharmacol. 2012;25:9–17. doi: 10.1177/039463201202500102. [DOI] [PubMed] [Google Scholar]
- 14.Maddi A, Scannapieco FA. Oral biofilms, oral and periodontal infections, and systemic disease. Am J Dent. 2013;26:249–254. [PubMed] [Google Scholar]
- 15.Oz HS, Ebersole JL. A novel murine model for chronic inflammatory alveolar bone loss. J Periodontal Res. 2010;45:94–99. doi: 10.1111/j.1600-0765.2009.01207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pizarro TT, Pastorelli L, Bamias G, et al. SAMP1/YitFc mouse strain: A spontaneous model of Crohn’s disease-like ileitis. Inflamm Bowel Dis. 2011;17:2566–2584. doi: 10.1002/ibd.21638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li CH, Amar S. Morphometric, histomorphometric, and microcomputed tomographic analysis of periodontal inflammatory lesions in a murine model. J Periodontol. 2007;78:1120–1128. doi: 10.1902/jop.2007.060320. [DOI] [PubMed] [Google Scholar]
- 18.Rivaldo EG, Padilha DM, Hugo FN, Hilgert JB, Rybu BR. Reproducibility of a hemi mandible positioning device and a method for measuring alveolar bone loss area in mice. J Oral Sci. 2007;49:13–17. doi: 10.2334/josnusd.49.13. [DOI] [PubMed] [Google Scholar]
- 19.Wang Q, Li H, Xie H, et al. 25-Hydroxyvitamin D3 attenuates experimental periodontitis through down-regulation of TLR4 and JAK1/STAT3 signaling in diabetic mice. J Steroid Biochem Mol Biol. 2013;135:43–50. doi: 10.1016/j.jsbmb.2013.01.008. [DOI] [PubMed] [Google Scholar]
- 20.Bamias G, Martin C, Mishina M, et al. Proinflammatory effects of TH2 cytokines in a murine model of chronic small intestinal inflammation. Gastroenterology. 2005;128:654–666. doi: 10.1053/j.gastro.2004.11.053. [DOI] [PubMed] [Google Scholar]
- 21.Shibata Y, Fujita S, Takahashi H, Yamaguchi A, Koji T. Assessment of decalcifying protocols for detection of specific RNA by non-radioactive in situ hybridization in calcified tissues. Histochem Cell Biol. 2000;113:153–159. doi: 10.1007/s004180050434. [DOI] [PubMed] [Google Scholar]
- 22.Habashneh RA, Khader YS, Alhumouz MK, Jadallah K, Ajlouni Y. The association between inflammatory bowel disease and periodontitis among Jordanians: A case-control study. J Periodontal Res. 2012;47:293–298. doi: 10.1111/j.1600-0765.2011.01431.x. [DOI] [PubMed] [Google Scholar]
- 23.Flemmig TF, Shanahan F, Miyasaki KT. Prevalence and severity of periodontal disease in patients with inflammatory bowel disease. J Clin Periodontol. 1991;18:690–697. doi: 10.1111/j.1600-051x.1991.tb00111.x. [DOI] [PubMed] [Google Scholar]
- 24.Sigusch BW. Periodontitis as manifestation of Crohn’s disease in primary dentition: A case report. J Dent Child (Chic) 2004;71:193–196. [PubMed] [Google Scholar]
- 25.Corridoni D, Kodani T, Rodriguez-Palacios A, et al. Dysregulated NOD2 predisposes SAMP1/YitFc mice to chronic intestinal inflammation. Proc Natl Acad Sci USA. 2013;110:16999–17004. doi: 10.1073/pnas.1311657110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Corridoni D, Pastorelli L, Mattioli B, et al. Probiotic bacteria regulate intestinal epithelial permeability in experimental ileitis by a TNF-dependent mechanism. PLoS One. 2012;7:e42067. doi: 10.1371/journal.pone.0042067. [DOI] [PMC free article] [PubMed] [Google Scholar]