Abstract
Nicotine exposure in adolescent rats has been shown to cause learning impairments that persist into adulthood long after nicotine exposure has ended. This study was designed to assess the extent to which the effects of adolescent nicotine exposure on learning in adulthood can be accounted for by adolescent injection stress experienced concurrently with adolescent nicotine exposure. Female rats received either 0.033 mg/hr nicotine (expressed as the weight of the free base) or bacteriostatic water vehicle by osmotic pump infusion on postnatal days 25-53 (P25-53). Half of the nicotine-exposed rats and half of the vehicle rats also received twice-daily injection stress consisting of intraperitoneal saline injections on P26-53. Together these procedures produced 4 groups: No Nicotine / No Stress, Nicotine / No Stress, No Nicotine / Stress, and Nicotine / Stress. On P65-99, rats were trained to perform a structurally complex 24-element serial pattern of responses in the serial multiple choice (SMC) task. Four general results were obtained in the current study. First, learning for within-chunk elements was not affected by either adolescent nicotine exposure, consistent with past work (Pickens, Rowan, Bevins, & Fountain, 2013), or adolescent injection stress. Thus, there were no effects of adolescent nicotine exposure or injection stress on adult within-chunk learning typically attributed to rule learning in the SMC task. Second, adolescent injection stress alone (i.e., without concurrent nicotine exposure) caused transient but significant facilitation of adult learning restricted to a single element of the 24-element pattern, namely, the “violation element,” that was the only element of the pattern that was inconsistent with pattern structure. Thus, adolescent injection stress alone facilitated violation element acquisition in adulthood. Third, also consistent with past work (Pickens et al., 2013), adolescent nicotine exposure, in this case both with and without adolescent injection stress, caused a learning impairment in adulthood for the violation element in female rats. Thus, adolescent nicotine impaired adult violation element learning typically attributed to multiple-item learning in the SMC task. Fourth, a paradoxical interaction of injection stress and nicotine exposure in acquisition was observed. In the same female rats in which violation-element learning was impaired by adolescent nicotine exposure, adolescent nicotine experienced without adolescent injection stress produced better learning for chunk-boundary elements in adulthood compared to all other conditions. Thus, adolescent nicotine without concurrent injection stress facilitated adult chunk-boundary element learning typically attributed to concurrent stimulus-response discrimination learning and serial-position learning in the SMC task. To the best of our knowledge, the current study is the first to demonstrate facilitation of adult learning caused by adolescent nicotine exposure.
1. Introduction
Survey research has shown that cigarette smoking has been on a decreasing trend among adolescents since the late 1990's though 40% of 12th graders still admit to have smoked at least once in their life (Johnston, 2011). However, from 2011 to 2012, electronic cigarette use doubled for middle and high school students and every day, more than 3,200 U.S. adolescents smoke their first cigarette with an estimated 2,100 becoming daily smokers (Center for Disease Control and Prevention, 2014). Though smoking in adolescence may not be as prevalent as it was in the early 1990's, adolescents continue to be exposed to nicotine, perhaps in growing numbers.
Research in rats has shown that nicotine exposure during adolescence can cause long-lasting physiological and morphological changes to the brain that cause persistent changes in adult neural and behavioral function (Abreu-Villaça, Seidler, Qiao, Tate, Counsins, Thillai, & Slotkin, 2003a; Abreu-Villaça, Seidler, Tate, & Slotkin, 2003b). Nicotine exposure in adolescence has also been shown to cause cognitive deficits in adults such as decreased attentional performance, impairments of stimulus-response (S-R) learning, and impairments of memory in several behavioral paradigms (Counotte, Spijker, Burgwal, Hogenboom, Schoffelmeer, Vries, Smit, & Pattij, 2009; Fountain, Rowan, Kelley, Willey, & Nolley, 2008; Jacobsen, Krystal, Einar, Westerveld, Frost, & Pugh, 2005; Schochet, Kelley, & Landry, 2004; Slawecki, Thorsell, & Ehlers, 2004; Spaeth, Barnet, Hunt, & Burk, 2010).
For experimental purposes in animal models, controlled nicotine exposure in adolescence is typically achieved by subcutaneous or intraperitoneal injections, by transdermal patch, or by implantable osmotic pump. Administration can thus be intermittent via single or multiple bolus injections distributed through time, or continuous via chronic absorption or infusion. Very few indications are given by experimenters as to why one procedure is chosen over another. However, all of the foregoing methods of adolescent nicotine exposure in rats have been shown to cause neural and behavioral changes that last into adulthood (Abreu-Villaça et al., 2003a; Abreu-Villaça et al., 2003b; Adriani, Spijker, Deroche-Gamonet, Laviola, Le Moal, Smit, & Piazza, 2003; Barron, White, Swartzwelder, Bell, Rodd, Slawecki, Ehlers, Levin, Rezvani, & Spear, 2005; Belluzzi, Lee, Oliff, & Leslie, 2004; Brielmaier, McDonald, & Smith, 2007; Counotte et al., 2009; Dwyer, McQuown, & Leslie, 2009; Fountain et al., 2008; McDonald, Dailey, Bergstrom, Wheeler, Eppolito, Smith, & Smith, 2005; Natividad, Torres, Friedman, & O'Dell, 2013; Philpot, Engberg, & Wecker, 2014; Pickens et al., 2013; Polesskaya, Fryxell, Merchant, Locklear, Ker, McDonald, Eppolito, Smith, Wheeler, & Smith, 2007; Quick, Olausson, Addy, & Taylor, 2014; Schochet et al., 2004; Slawecki et al., 2004; Slotkin, 2002; Slotkin, Bodwell, Ryde, & Seidler, 2008; Slotkin, MacKillop, Rudder, Ryde, Tate, & Seidler, 2007; Spaeth et al., 2010; Trauth, McCook, Seidler, & Slotkin, 2000; Trauth, Seidler, McCook, & Slotkin, 1999; Trauth, Seidler, & Slotkin, 2000a, 2000b; Wheeler, Smith, Bachus, McDonald, Fryxell, & Smith, 2013). Furthermore, recent research has found that various forms of stress in adolescence can have long-term effects on cognition (Green & McCormick, 2013; Isgor, Kabbaj, Akil, & Watson, 2004; Morrissey, Mathews, & McCormick, 2011; Torregrossa, Xie, & Taylor, 2012). Since daily injections and other stress-inducing activities related to injections such as handling and weighing have been shown to be a source of stress in rats (Dilsaver & Majchrzak, 1990; Sharp, Zammit, Azar, & Lawson, 2003), it is possible that the behavioral effects that have been observed in adult rats after adolescent nicotine exposure are a result of chronic injection stress experienced during adolescence. Injection stress alone, or through an interaction with nicotine, may result in cognitive impairments. The current study assessed the effects of injection stress and adolescent nicotine exposure on adult rat learning.
Prior research has shown that serial pattern learning in a serial multiple choice (SMC) task in rats is sensitive to learning impairments in adulthood caused by earlier adolescent nicotine exposure (Fountain et al., 2008; Pickens et al., 2013). The SMC task is modeled after a nonverbal task that has been used to study human associative versus rule learning (Fountain & Rowan, 1995; Kundey, De Los Reyes, Rowan, Lee, Delise, Molina, & Cogdill, 2013; Restle & Brown, 1970) and we have used it to study complex cognitive processes in rats (Fountain, Rowan, Muller, Kundey, Pickens, & Doyle, 2012). When rats learn sufficiently complex serial patterns in this task, they have been shown to employ multiple cognitive systems concurrently, including simple S-R discrimination learning, multiple-item memory, and abstract rule learning processes (for a review, see Fountain et al., 2012). In addition, sex differences in acquisition rates have been observed in this paradigm for adult vehicle rats and for adult rats previously exposed to nicotine during adolescence (Pickens et al., 2013). Taken together, these characteristics make serial pattern learning in the SMC task attractive as a cognitive assessment tool.
Fountain et al. (2008) found that once-daily intraperitoneal injections of 1.0 mg/kg of nicotine during adolescence resulted in persistent retardation of S-R discrimination learning and a very brief retardation of rule learning in the SMC task. Pickens et al. (2013) extended the Fountain et al. (2008) study by increasing group size, adding to the complexity of the pattern rats were required to learn, extending training to examine acquisition to asymptotic levels, and including both male and female rats to examine possible sex differences in vehicle versus adolescent nicotine exposed rats’ acquisition during adulthood. Sex differences were observed in different aspects of S-R learning in this paradigm, including sex differences in adolescent nicotine effects on adult serial pattern learning. However, since these studies used daily injections, it is possible that drug-injection stress interacted with nicotine effects to produce the detrimental effects that were observed.
To determine whether daily injection stress during adolescence played a role in the observed nicotine effects on serial pattern learning in adulthood we observed in past studies (Fountain et al., 2008; Pickens et al., 2013), the current study utilized subcutaneously implanted osmotic pumps as the nicotine delivery system. All of the rats in the reported study were implanted with an osmotic pump during adolescence that delivered either nicotine or vehicle. In order to increase the amount of injection stress rats experienced relative to our earlier studies, half of the nicotine-exposed and half of the vehicle rats were given twice-daily injections of saline. Remaining rats received no injections. These procedures allowed for an evaluation of the effects of daily adolescent nicotine exposure on adult learning independent of adolescent injection stress. Female rats were used in this experiment because prior work has shown that they display greater impairments in some aspects of serial pattern learning caused by adolescent nicotine exposure compared to males (Pickens et al., 2013). If the previously reported cognitive impairments after adolescent nicotine exposure were due to injection stress instead of nicotine exposure, nicotine-exposed rats that were not subject to chronic injection stress should make no more errors than vehicle rats.
2. Materials and methods
2.1. Animal care and nicotine treatment
Sixty female Long Evans rats (Rattus norvegicus) that were bred in-house served as subjects for this experiment. They were randomly assigned to one of four conditions. Rats were received on postnatal day 21 (P21) and housed in groups of 3 in plastic shoe-box cages (40 cm wide × 85 cm long × 40 cm high) and given free access to water and food (LabDiet 5P00 - ProLab® RMH 3000). Rats were housed so that each individual in a cage was from a different litter but part of the same experimental condition. Rats caged together were differentiated from one another by tail markings.
Delivery of nicotine (0.033 mg/hr expressed as the weight of the free base) or vehicle occurred from P25 to P53 via osmotic pumps (Alzet®, model 2004). This dose was chosen based on previous work reported by Trauth et al. (1999), though their dose was based on the weight of the rat at the onset of exposure. Because adolescence is a period of rapid growth in rats, the concentration of nicotine we used was determined by estimating the average weight of female rats during the entire period of exposure (P25-53) based on a Long Evans rat growth chart (“Long Evans (Blue Spruce),” 2006) with a target average exposure of 6.0 mg/kg/day over the entire period of exposure. Because osmotic pumps deliver contents at a constant rate, rats received 0.033 mg/hr of nicotine or vehicle throughout the period of exposure. Weight data for nicotine-exposed rats during the period of exposure closely approximated the expected weights from the growth chart (“Long Evans (Blue Spruce),” 2006) for the days in the growth chart closest to the beginning and end of the exposure period. The group mean weight of nicotine-exposed rats near the beginning of exposure on P28 was 61.1 g (ranging from 34-75 g), which was comparable to a mean weight of 62.8 g from the growth chart for the same day. The group mean weight of nicotine-exposed rats near the end of exposure on P49 was 160.9 g (ranging from 141-181 g), which was comparable to a mean weight of 156.7 g from the growth chart for the same day. The overall mean nicotine dose for these rats based on constant nicotine exposure of 0.033 mg/hr throughout the period of exposure and the overall mean body weight for the period of exposure was 6.79 mg/kg/day of nicotine expressed as the weight of the free base.
On P24 pumps were prepared with either nicotine bitartrate dissolved in bacteriostatic water (Sigma Chemical, Saint Louis, MO, expressed as the weight of the free base) or bacteriostatic water alone and left to prime in a saline solution for 24 hours. On P25, rats were anesthetized under isoflurane and a pump was implanted subcutaneously in the mid-scapular region of the animal's back. Subjects were then allowed to recover from surgery in their home cages. Beginning the following day, P26, rats in the injection stress condition were weighed daily and received twice-daily intraperitoneal injections of 1.0 ml/kg of saline until P53 twelve hours apart. Rats in the No Stress condition were left undisturbed until P53 except for regular cage maintenance. On P53 the surgical procedure was repeated for all rats to remove their osmotic pumps. It should be noted that the osmotic pump, marketed as a four-week infusion device, actually takes approximately 33.3 days to be exhausted completely (information supplied by the manufacturer). After the osmotic pumps were removed, rats were housed individually (and remained so for the rest of the experiment) and allowed to recover in their home cages. This resulted in 4 groups of rats in this experiment: No Nicotine / No Stress, Nicotine / No Stress, No Nicotine / Stress, and Nicotine / Stress. Two rats were removed from the study prior to water restriction due illness. Therefore, the current study used only 58 subjects: 15 subjects in the No Nicotine / No Stress group, 14 subjects in the Nicotine / No Stress group, 14 subjects in the No Nicotine / Stress group, and 15 in the Nicotine / Stress group.
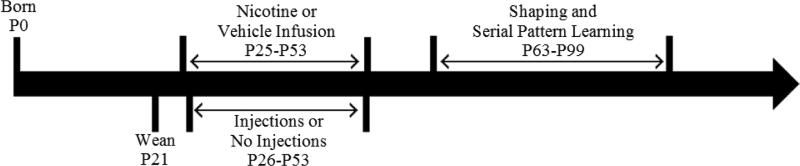
At P61, access to water was restricted to prepare for shaping and experimental testing. Rats continued to receive free access to food in their home cage and after training received 5 minutes of access to water daily throughout the experiment. Rats were monitored for signs of dehydration, such as yellowing of the belly, loss of skin elasticity, and lethargy. If any rats displayed signs of dehydration their participation in the experiment was paused and they were given supplemental water. No rats in the current experiment displayed signs of dehydration, therefore supplemental water was not necessary. All rats were kept on a 15:9-h light-dark cycle, which is the standardized light-dark cycle for our facility, with testing occurring during the light portion of the cycle. A timeline of experimental procedures is depicted in Fig. 1.
Fig. 1.
Timeline of experimental methods and procedures. Rats were weaned on postnatal day 21 (P21). Osmotic pumps containing either nicotine or vehicle were implanted on P26 and rats in the Stress groups began receiving twice-daily injections of saline on P26. Osmotic pumps were removed and stress injections were discontinued on P53. Starting on P63, all rats completed 2 days of shaping followed by 35 days of serial pattern learning training (ending in P99).
2.2. Apparatus
Three clear Plexiglas® shaping chambers (15 cm wide × 30 cm long × 30 cm high) with stainless steel wire mesh flooring and a single nose poke receptacle (2.5-cm diameter PVC pipe end caps painted flat black) centered on one end wall 5.0 cm above the floor were used in this study. This receptacle contained an infra-red emitter and detector which were located on the left and right side as well as a white LED cue light positioned on the back of the receptacle.
Six clear ¼-inch Plexiglas® octagonal test chambers (15 cm wide × 30 cm tall walls with 40 cm between opposite walls) with wire mesh floors were used as the experimental apparatus. Each of the eight walls was equipped with a nose-poke receptacle described above centered 5.0 cm above the floor.
An opening located at the bottom of each receptacle, connected to a solenoid and syringe by plastic tubing, served to deliver water to the chamber. All chambers were enclosed within sound attenuating chambers with 10-ml syringes attached to an internal wall of the enclosure that served as water reservoirs. Syringes were connected by Tygon tubing (VWR Scientific, Performance Plastics 1/32-inch, #R-3603) to solenoids (General Valve Corp. Vac. 20 psig. 24 volts) and then to the receptacles. The solenoid controlled the delivery of water droplets to the nose-poke receptacles.
Background white noise masked extraneous noise. All chambers were controlled by a computer running the MedPC interface (Med Associates interface; Grayson Stadler power supply Model E 783 DA) which was located in a separate room of the laboratory. Rats were monitored from the computer room via closed circuit cameras mounted inside the enclosures.
2.3. Procedure
2.3.1. Shaping procedure
On P63 and P64, all rats received two consecutive days of nose-poke shaping in shaping chambers. On each trial, the receptacle light was illuminated. After each nose-poke response, a reinforcer consisting of a 0.025-ml droplet of water was delivered through the bottom of the receptacle and the receptacle light was extinguished for the intertrial interval. Intertrial intervals were 1 s on P63 and 2 s on P64. Criterion for being included in the study was set at 240 responses within one hour on each of these two consecutive days. All rats met criterion on both shaping days.
2.3.2. Testing procedure
Testing in the SMC task began on P65, the day after rats completed nose poke shaping. All testing was conducted in our experimental 8-walled chamber. At the beginning of each trial, all 8 nose poke receptacles were illuminated and the rat was allowed to make a response at one of the 8 receptacles. If the rat's response was correct, all lights were extinguished and reinforcement consisting of a 0.025-ml droplet of water was delivered to the correct receptacle. If an incorrect choice was made at any time during experimental sessions, the correction procedure was initiated where all lights were extinguished except for the light of the correct receptacle and the rat was reinforced only after a nose-poke response in the correct receptacle. After the correction procedure resulted in the rat's response to the indicated correct receptacle, the sequence continued as if a correct response had been produced on the trial. The computer recorded number of correct and incorrect responses as well as the location of the rats’ response on each trial. Rats had to perform the following pattern:
where digits represent the clockwise position of the correct receptacles in the octagonal chamber on successive trials. Dashes indicate the location of 3-s pauses that served as phrasing cues separating structural chunks of the pattern. All other intertrial intervals were 1 s. The location of the receptacle designated as “1” was constant throughout the experiment for each rat but counterbalanced between rats. The first digit of each chunk is called a chunk-boundary element, the two digits following the chunk boundary are called within-chunk elements, and the last digit “8” in the pattern (underlined) is called the violation element because it violates pattern structure. Rats performed the pattern 20 times a day without interruption, 7 days a week, for 35 days. Rats were allowed unlimited time to complete 20 pattern repetitions daily, resulting in sessions that lasted as long as approximately 2 hours early in training, but average session length quickly decreased to approximately 30 minutes.
2.4 Statistical analysis
Analysis of variance (ANOVA) was used to examine the effects of adolescent exposure to nicotine and injection stress on rats’ acquisition for each element type (within-chunk, chunk-boundary, and violation elements) across the 35 days of the experiment. Main effects and interactions were considered significant if p < .05. To assess differences in acquisition of pattern elements, a 2 (drug condition) × 2 (stress condition) × 35 (days) repeated measures ANOVA was conducted on rats’ daily total correct responses for each element type. When significant effects were observed, planned comparisons based on the appropriate error term of the ANOVA (Fisher's Least Significant Difference tests) were conducted to determine the direction of the effect as well as specific days within acquisition when groups differed. Multiple regression analysis was used to assess differences in asymptotic levels of performance over the last 10 days of acquisition. Lastly, “intrusion analysis” examined the types of errors committed by element type to assess possible changes in learning strategies caused by adolescent exposure to nicotine or injection stress.
3. Results
Generally, the results show that adolescent injection stress and adolescent nicotine exposure interacted in producing somewhat paradoxical effects in adulthood on some, but not all, aspects of serial pattern learning in adult female rats. Correct choice data showed that adolescent exposure to 0.033 mg/hr of nicotine while not receiving stress from the injection procedure resulted in faster learning of the chunk-boundary element. However this exposure also resulted in impaired learning of the violation element on the last 10 days of acquisition.
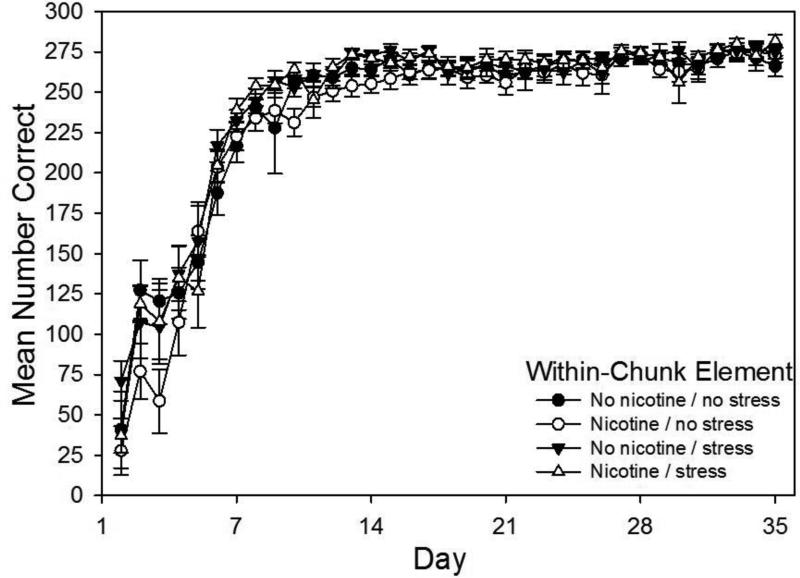
Acquisition curves for within-chunk elements, which are the second and third elements of each chunk, are shown in Fig. 2. A drug × stress × day repeated measures ANOVA conducted on rats’ daily mean correct response rates on within-chunk elements revealed a significant main effect of days, F(34,1839)=184.04, p < 0.001, but all other main effects and interactions were not significant (p > 0.05), indicating that there were no significant differences in learning rate for within-chunk elements between groups.
Fig. 2.
Acquisition curves for within-chunk elements of the pattern showing daily mean total correct responses over 35 days of training beginning on P65 for four groups (N = 14-15 per group). Rats received prior adolescent exposure to either 0.033 mg/hr nicotine or an equivalent volume of saline via implanted osmotic pumps from P25-53. From P26-53 rats in the Stress conditions also received twice-daily injection stress resulting from intraperitoneal injections of 1.0 ml/kg saline. Error bars: ±SEM.
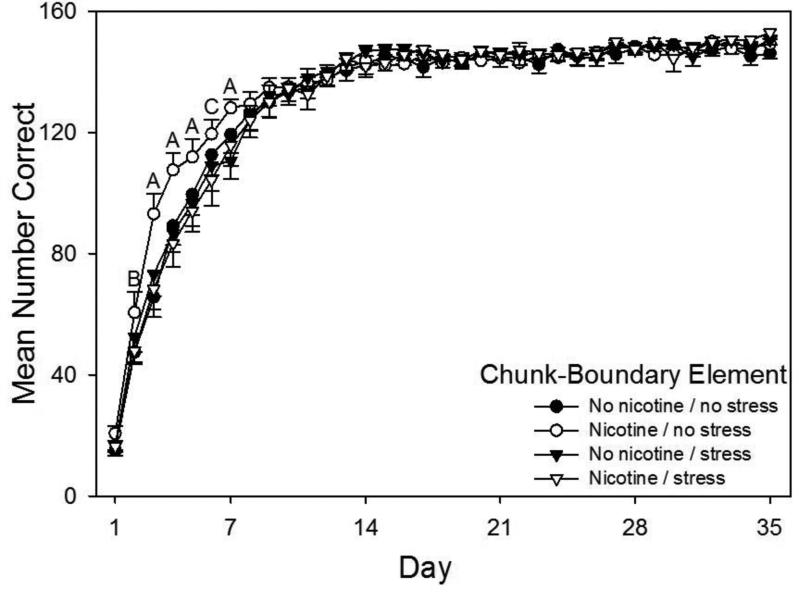
Acquisition curves for chunk-boundary elements, that is, the first element of chunks that always immediately followed phrasing cues, are shown in Fig. 3. A drug × stress × day repeated measures ANOVA conducted on rats’ daily mean correct response rates on chunk-boundary elements revealed a significant main effect of day of the experiment, F(34,1836)=415.11, p < 0.001. The ANOVA also revealed significant interactions for stress × day, F(34,1839)=2.21, p < 0.001, and a drug × stress × day, F(34,1836)=1.75, p = 0.005. The drug and stress main effects, however, were not significant (p > 0.05). Planned comparisons based on the appropriate error term from the ANOVA showed that female rats in the Nicotine / No Stress group made significantly more correct responses than all other groups on days 3, 4, 5, and 7, more correct responses than the No Nicotine / No Stress and the Nicotine / Stress groups on day 2, and more correct responses than rats in both Stress conditions on day 6. This result indicates that adolescent nicotine without concurrent injection stress facilitated learning of the chunk-boundary element.
Fig. 3.
Acquisition curves for chunk-boundary elements of the pattern showing daily mean total correct responses over 35 days of training beginning on P65 for all four groups (N = 14-15 per group). Rats received prior adolescent exposure to either 0.033 mg/hr nicotine or an equivalent volume of saline via implanted osmotic pumps from P25-53. From P26-53 rats in the Stress conditions also received twice daily injections of 1 ml/kg saline. Letters above the Nicotine / No Stress acquisition curve indicate significant differences relative to other groups. “A” indicates days when Nicotine / No Stress rats made significantly more correct responses than rats in all other groups, “B” indicates 1 day when Nicotine / No Stress rats made more correct responses than the No Nicotine / No Stress and the Nicotine / Stress groups, and “C” indicates 1 day when Nicotine / No Stress rats made more correct responses than No Nicotine / Stress and the Nicotine / Stress rats (ps < 0.05). To summarize, female rats in the Nicotine / No Stress group made significantly more correct responses than all other groups on days 3, 4, 5, and 7, more correct responses than the No Nicotine / No Stress and the Nicotine / Stress groups on day 2, and more correct responses than rats in both Stress conditions on day 6. Error bars: ±SEM.
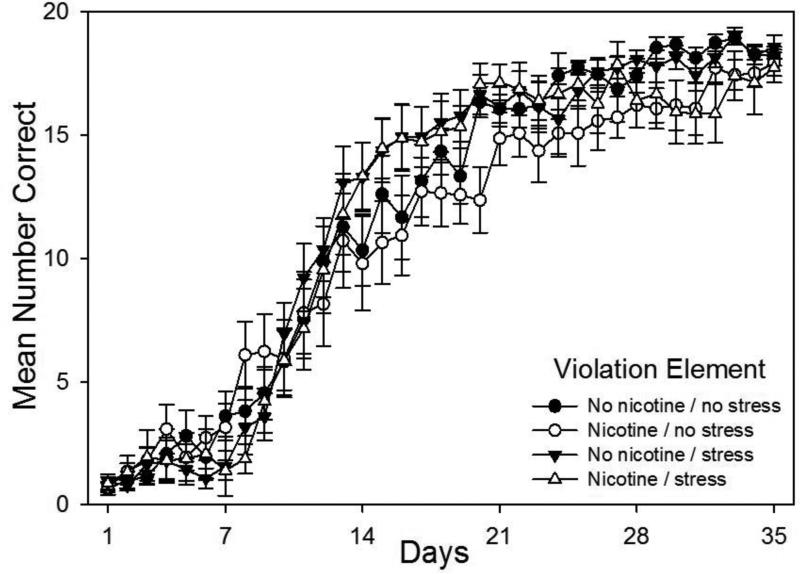
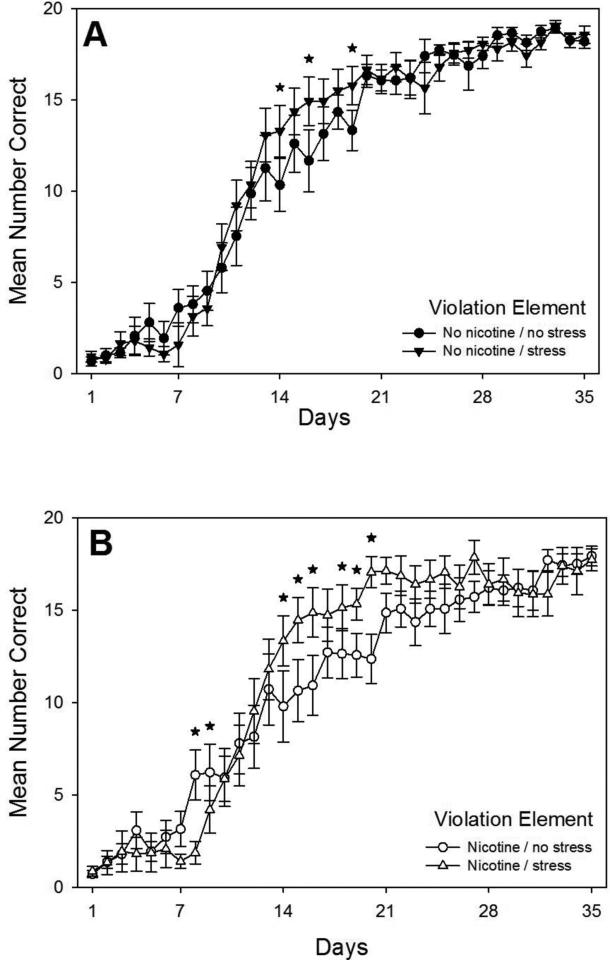
Finally, acquisition curves for the violation element, that is, the last element of the pattern which violated the rules set forth in the rest of the pattern, are shown in Fig. 4. A drug × stress × day repeated measures ANOVA conducted on rats’ daily mean correct response rates on violation elements revealed a significant main effect of days, F(34,1839)=209.70, p < 0.001, as well as a significant interaction of stress × day, F(34,1839)=2.68, p < 0.001. The drug and stress main effects were not significant (p > 0.05), and the drug × stress interaction and drug × stress × day interaction were not significant (p >0.05). Planned comparisons based on the appropriate error term from the ANOVA were performed to analyze the effects of nicotine and injection stress independently on violation element learning, as shown in Figs. 5 and 6, respectively.
Fig. 4.
Acquisition curves for the violation element of the pattern showing daily mean total correct responses over 35 days of training beginning on P65 for all four groups (N = 14-15 per group). Rats received prior adolescent exposure to either 0.033 mg/hr nicotine or an equivalent volume of saline via implanted osmotic pumps from P25-53. From P26-53 rats in the Stress conditions also received twice daily injections of 1.0 ml/kg saline. Error bars: ±SEM.
Fig. 5.
Acquisition curves for the violation element of the pattern showing daily mean total correct responses over 35 days of training beginning on P65 replotted from Fig. 4 to assess the effects of injection stress for rats in the No Nicotine condition that were exposed to saline (Panel A) or for rats exposed to nicotine during adolescence (Panel B) (N = 14-15 per group). Error bars: ±SEM. * p < 0.05.
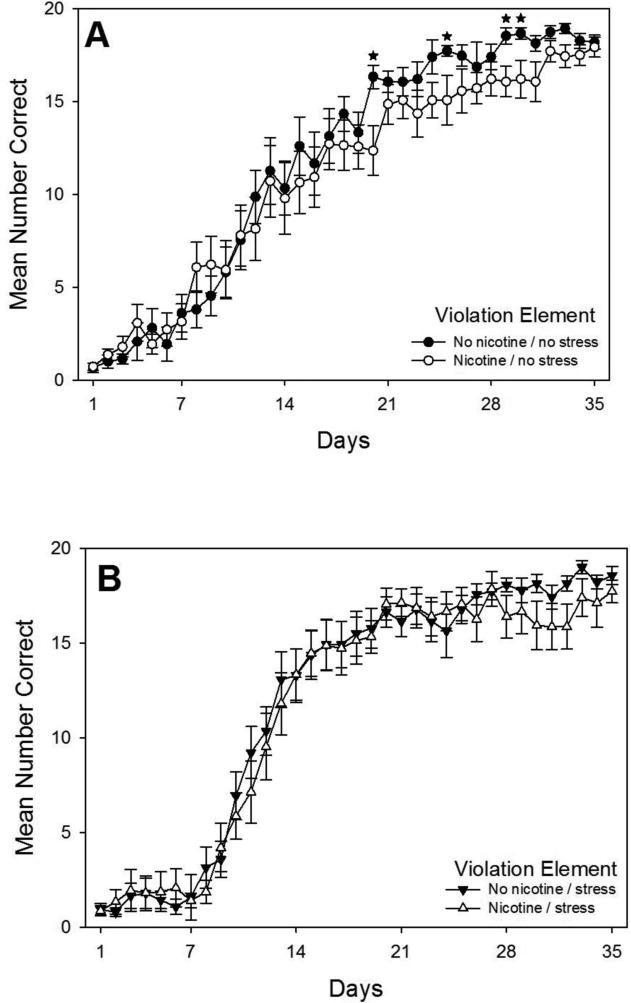
Fig. 6.
Acquisition curves for the violation element of the pattern showing daily mean total correct responses over 35 days of training beginning on P65 replotted from Fig. 4 to assess the effects of adolescent nicotine for rats in the No Stress condition that received no injection stress (Panel A) or for rats that received stress via twice daily injections during adolescence (Panel B) (N = 14-15 per group). Error bars: ±SEM. * p < 0.05.
First, planned comparisons were performed to examine the effects of adolescent injection stress on violation element learning in adulthood independent of nicotine exposure. Planned comparisons revealed that adolescent injection stress alone caused a transient but significant facilitation of learning to anticipate the violation element in adulthood in both No Nicotine and Nicotine groups. As shown in Fig. 5A, adolescent injection stress facilitated learning of the violation element for rats in No nicotine groups that did not receive adolescent nicotine exposure. Rats in the No Nicotine / Stress group made significantly more correct responses than rats in the No Nicotine / No Stress group on days 14, 16, and 19. Similarly, as shown in Fig. 5B, adolescent injection stress also facilitated learning of the violation element for rats in the Nicotine groups. Rats in the Nicotine / Stress group made significantly more correct responses than rats in the Nicotine / No Stress group on days 14-16 and 18-20. It should be noted, however, that the opposite was true on days 8 and 9, that is, rats in the Nicotine / No Stress group made significantly more correct responses than rats in the Nicotine / Stress group. Overall, these results indicate that adolescent injection stress caused a transient but significant facilitation of violation element learning in adulthood, the opposite of what was found for chunk-boundary element acquisition.
Second, planned comparisons were performed to examine the effects of adolescent nicotine exposure on violation element learning in adulthood independent of adolescent injection stress. Planned comparisons showed that adolescent nicotine caused a transient but significant impairment of violation element acquisition in adulthood in rats that did not receive adolescent injection stress, but not in rats that did receive adolescent injection stress. As shown in Fig. 6A comparing data for No Stress groups, rats in the Nicotine / No Stress group made significantly fewer correct responses than rats in the No Nicotine / No Stress group on days 20, 25, 29, and 30. However, as shown in Fig. 6B comparing data for Stress groups, no differences were observed in violation element acquisition in adulthood between the Nicotine and No Nicotine groups in rats that received adolescent injection stress. Overall, these results indicate that adolescent nicotine exposure caused a transient but significant impairment of violation element learning in adulthood, but only in rats that did not experience adolescent injection stress.
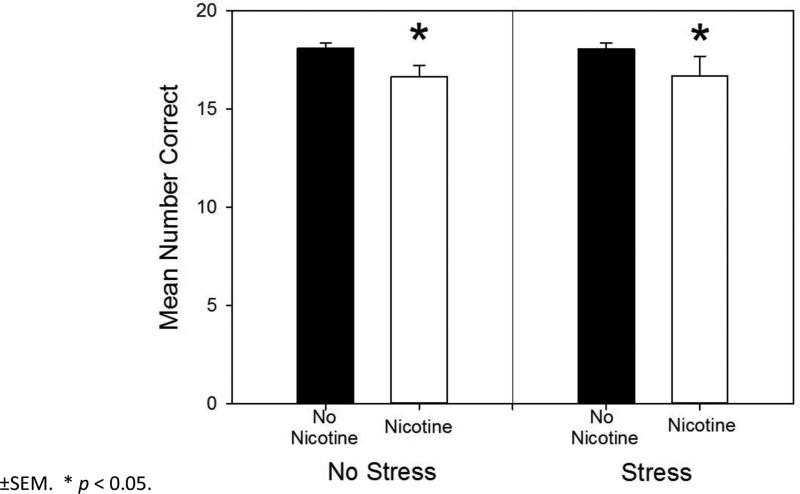
In the earlier Pickens et al. (2013) study using the same serial pattern, in female rats the most profound learning impairment caused by adolescent nicotine exposure was observed for the violation element late in training. In order to determine if adolescent exposure to nicotine had an effect on violation element performance late in training, a multiple regression was performed for the last 10 days of training. This set of days was chosen because planned comparisons detected no significant differences in daily mean performance by rats in the No Nicotine / No Stress control group during this period. The multiple regression found that only the original violation error model (which did not include the interaction term) was significant. It accounted for 32% of the variance in violation errors. According to the regression model, only nicotine was a significant predictor of violation errors (β=.32, t(57) = 2.53, p =.014). This variable also accounted for a significant amount of the variance in the outcome (R2 = .105, F(2, 55) = 3.23, p =.047). As shown in Fig. 7, further analysis of the data, determined that rats not exposed to nicotine made fewer errors on the violation element (Mean (M) = 1.09, Standard Deviation (SD) = 1.01) than rats that were exposed to nicotine (M = 3.32, SD = 3.09). No other significant main effects or interactions were found for the models (p >.05).
Fig. 7.
Mean number of correct violation responses made collapsed across the last 10 days of training. Rats received prior adolescent exposure to either 0.033 mg/hr nicotine or an equivalent volume of saline via implanted osmotic pumps from P25-56. From P26-56 rats in the Stress conditions also received twice daily injections of 1 ml/kg saline (N = 14-15 per group). Error bars: ±SEM. * p < 0.05.
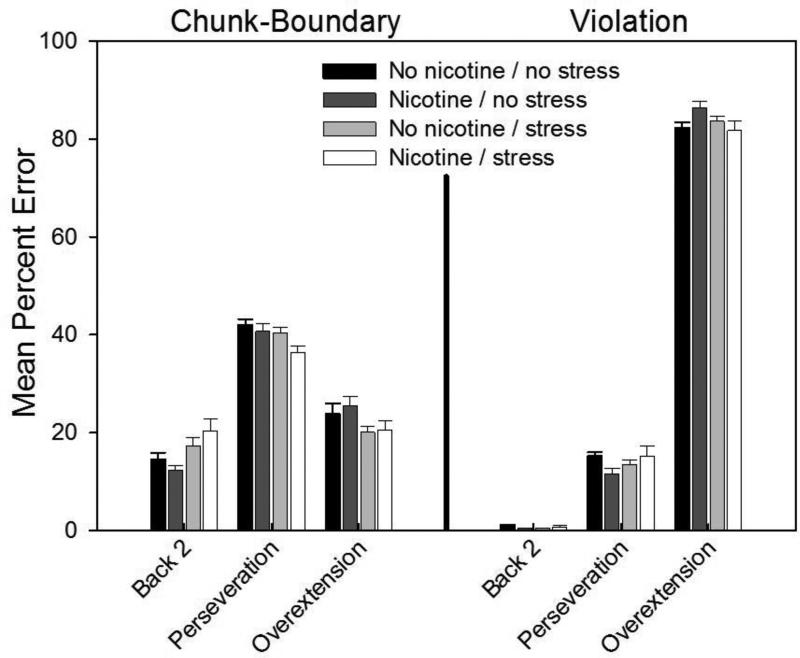
As shown in Fig. 8, error data were further analyzed with an intrusion analysis to determine types of errors committed for the different element types. For chunk-boundary elements, the most frequent type of error for all conditions was a perseveration response, that is, a repetition of the last correct response. An example of such an error after a 2-3-4 chunk would be a “4” response on the chunk boundary trial following the phrasing cue when a “3” response was correct. Across all groups, this type of error accounted for 36-42% of all errors made on chunk-boundary trials. For the violation element, rats in all conditions responded similarly, with all groups producing more than 80% of their errors as overextensions. This error occurs when the rats extrapolates the within-chunk “+1” rule; that is, on the third element of the violation chunk, 8-1-8, all rats tended to respond with a rule-consistent but incorrect “2”--rather than “8--to produce an 8-1-2 chunk that was structurally consistent with the rest of the chunks of the pattern. These results suggest that adolescent injection stress and nicotine exposure do not create changes in behavioral strategy. Number of errors varied between groups but the cognitive strategies employed remained the same.
Fig. 8.
Rates of different types of intrusion errors committed on trials for chunk-boundary elements (left panel) and violation elements (right panel) (N = 14-15 per group). Error bars: ±SEM.
4. Discussion
This study was designed to examine the effects of chronic injection stress and concurrent chronic nicotine exposure experienced during adolescence on adult learning in female rats. Four general results were obtained in the current study. First, learning for within-chunk elements was not affected by either adolescent nicotine exposure, consistent with past work (Pickens et al., 2013), or adolescent injection stress. Thus, there were no effects of adolescent nicotine exposure or injection stress on adult within-chunk learning typically attributed to rule learning in the SMC task, as shown in Fig. 2. Second, adolescent injection stress alone (i.e., without concurrent nicotine exposure) caused transient but significant facilitation of adult learning restricted to a single element of the 24-element pattern, namely, the “violation element,” that was the only element of the pattern that was inconsistent with pattern structure. Thus, adolescent injection stress alone facilitated violation element acquisition in adulthood, as shown in Fig. 6A. Third, also consistent with past work (Pickens et al., 2013), adolescent nicotine exposure, in this case both with and without adolescent injection stress, caused a learning impairment in adulthood for the violation element in female rats , as show in Fig. 7. Thus, adolescent nicotine impaired adult violation element learning typically attributed to multiple-item learning in the SMC task. Fourth, a paradoxical interaction of injection stress and nicotine exposure in acquisition was observed. In the same female rats in which violation-element learning was impaired (Fig. 7), adolescent nicotine experienced without adolescent injection stress produced better learning for chunk-boundary elements in adulthood compared to all other conditions (Fig. 3).
Previous research has shown that serial pattern learning in the SMC task recruits multiple cognitive systems concurrently, including associative S-R learning, serial position learning involving timing or counting processes, and rule abstraction processes (Fountain & Benson Jr, 2006; Fountain et al., 2008; Fountain et al., 2012; Kundey & Fountain, 2010; Muller & Fountain, 2010). Learning to anticipate chunk-boundary elements has been shown to depend on both associative stimulus response (S-R) learning and serial-position learning concurrently (Muller & Fountain, 2010; Stempowski, Carman, & Fountain, 1999). Learning to anticipate the violation element has been shown to depend on associative multiple-item learning involving cues from several preceding trials and “intra-box” apparatus cues that signal the upcoming violation trial (Kundey & Fountain, 2010; Muller & Fountain, 2010). Learning to anticipate within-chunk elements, on the other hand, has been shown to depend on learning a motor program or abstract rules that are independent of external stimuli (Muller & Fountain, 2010). Since the different pattern element types are learned using distinct cognitive mechanisms, it is not surprising to find that the same drug or toxic agent can result in differential facilitation of learning, impairment of learning, and no effect on learning for different element types in individual rats in the SMC task. Dissociations in learning and performance consistent with the foregoing behavioral and cognitive distinctions have been observed in rats following acute systemic treatment with MK-801, an N-methyl-D-aspartate receptor (NMDAr) antagonist, and with atropine, a muscarinic cholinergic antagonist (Fountain & Rowan, 2000; Fountain, Rowan, & Wollan, 2013).
Similar dissociations have also been observed in adolescent nicotine effects on adult learning. Pickens et al. (2013) demonstrated sex-specific impairments of discrimination learning for chunk-boundary elements in male rats, but not female rats, and impairments of multiple-item discrimination learning for violation elements in female rats, but not male rats. Neither adult male nor female rats were impaired in rule-based learning for within-chunk elements after adolescent nicotine exposure. The current study replicated the dissociation of effects observed by Pickens et al. (2013) where female rats exposed to nicotine in adolescence had impaired learning of multiple-item learning for violation elements whereas S-R and serial-position learning for chunk-boundary elements was not impaired. Thus, based on the results of Pickens et al. (2013), a dissociation of nicotine effects on multiple-item learning for violation elements versus S-R and serial-position learning of chunk-boundary elements in female rats in the current study was expected. However, facilitated learning for chunk-boundary elements in adulthood after adolescent nicotine exposure was unanticipated and, given the growing literature on learning impairments caused by adolescent nicotine exposure in rodent models, it was indeed surprising.
The current study found that when nicotine was experienced without concurrently experiencing injection stress, nicotine in adolescence facilitated learning in adulthood long after chronic adolescent nicotine exposure ceased. The critical behavioral effect supporting this claim is the fact that the group receiving adolescent nicotine without stress (i.e., the Nicotine / No Stress group) learned chunk-boundary elements faster during adulthood than all other groups including its control group (the No Nicotine / No Stress group), as shown in Fig. 3. Taken together, the evidence indicates that adolescent nicotine exposure without accompanying adolescent injection stress caused facilitation of S-R and/or serial position learning involved in learning to anticipate chunk-boundary elements in the SMC task. It should be noted that, although facilitated learning or performance is commonly observed after acute nicotine exposure in adulthood (Attaway, Compton, & Turner, 1999; Barron et al., 2005; Levin & Torry, 1996; Semenova, Stolerman, & Markou, 2007) evidence of facilitated learning in adulthood after adolescent nicotine exposure appears to be unprecedented in the literature on adolescent nicotine exposure effects on adult learning.
It is possible that the unusual pattern of behavioral effects observed in this study may have been related to the method used for adolescent nicotine exposure. Whereas earlier studies employing the SMC task used “bolus intraperitoneal” (c.f. Silverstone, Williams, McMahon, Fleming, & Fogarty, 2008) injections of nicotine (Fountain et al., 2008; Pickens et al., 2013), the current study used continuous subcutaneous infusion of nicotine during adolescence. Intraperitoneal injections of nicotine cause a rapid surge in the concentration of nicotine followed by a gradual return to baseline levels and since injections were adjusted as a function of rats’ daily weight in prior studies, the concentration of nicotine at the time of injections was a constant dose throughout the experiment. In contrast, continuous subcutaneous infusion of nicotine over many days via osmotic pump, as used in the present study, delivered a steady infusion of a nicotine solution of consistent concentration throughout the exposure period of many days so that as a rat's body weight increased during adolescence, the daily nicotine dose decreased as a proportion of body weight over the period of infusion. It is also important to note that the rats in the present study received a nicotine dose of 0.033 mg/hr, which is an average over the course of the entire experiment of approximately 6.79 mg/kg nicotine daily via infusion. This dose was on average six times higher than the 1.0 mg/kg daily dose delivered via bolus intraperitoneal injection in our previous research (Fountain et al., 2008; Pickens et al., 2013). However, because continuous infusion by osmotic pump would deliver nicotine at only 0.033 mg/hr, the highest tissue concentration of nicotine actually experienced at any time in the present study would have been much lower than the peak concentration experienced in earlier studies employing 1.0 mg/kg intraperitoneal injections. Perhaps a higher daily dose of nicotine or the fact that it was delivered via continuous subcutaneous infusion rather than bolus intraperitoneal injections contributed to the interaction of adolescent nicotine and adolescent injection stress that produced facilitated learning for chunk-boundary elements.
It should also be noted that the current experiment found that in the absence of adolescent nicotine exposure, there was no effect of adolescent injection stress on adult learning for within-chunk and chunk-boundary elements. However, injection stress during adolescence did cause transient but significant facilitation of violation element learning for both Nicotine and No Nicotine groups (Fig. 5A and 5B). No Nicotine / Stress rats made significantly more correct violation element responses for 3 days of acquisition compared to No Nicotine / No Stress rats. Furthermore, Nicotine / No Stress rats made significantly more correct violation responses on 6 days of acquisition compared to Nicotine / Stress rats. One question raised by the current experiment is how best to study the effects of adolescent nicotine while controlling stress as a factor. Methods for exposing rats to nicotine while minimizing stress due to handling, surgery, or injection—such as by adding nicotine to drinking water or food—also have methodological drawbacks.
The results of the current study demonstrated that injection stress did interact with adolescent nicotine exposure to produce paradoxical effects on adult learning. Adolescent nicotine exposure in the absence of concurrent adolescent injection stress resulted in facilitated S-R discrimination learning for chunk-boundary elements while concurrently impairing multiple-item memory of cues important for anticipating the violation element in adulthood in female rats. Previous behavioral and neurobiological work has shown that learning to anticipate chunk-boundary elements depends on different cognitive/behavioral mechanisms than learning to anticipate violation elements, and that these different behavioral mechanisms depend on dissociable neural mechanisms (for a summary, see Fountain et al., 2012). For that reason it is not surprising that the same manipulation—in this case, adolescent nicotine exposure—might affect multiple brain/behavioral mechanisms differentially in the same animal, perhaps even in opposite directions as observed in the current study in chunk-boundary element versus violation element learning. One reviewer of this paper suggested that perhaps adolescent injection stress may have one or more general effects, for example, that adolescent injection stress reverses or interferes with adolescent nicotine effects. Two pieces of evidence from the current study are consistent with this idea in the context of our multiple-processes view. First, adolescent injection stress appeared to reduce nicotine-induced facilitation of learning for chunk-boundary elements in Fig. 3. Second, adolescent injection stress reduced the nicotine-induced impairment of learning the violation element in Fig. 6. One hypothesis is that adolescent nicotine exposure can produce differential effects—facilitation or impairment—depending on the system, but that adolescent stress always interferes with the expression of adolescent nicotine effects. Although this view of adolescent injection stress has its appeal, data such as those of Fig. 7, where adolescent nicotine effects appear to be the same across different levels of adolescent injection stress, are not consistent with this hypothesis. Interestingly, adolescent injection stress when considered alone appears to facilitate learning of violation elements, as shown in both Fig. 5A and 5B, but has no such effect on learning chunk-boundary elements, as shown in Fig. 3. One conclusion is that like the effects of adolescent nicotine exposure, the effects of adolescent injection stress may vary across different neural, behavioral, and cognitive systems.
How to interpret the results of the current study from a public health perspective is an open question. However, adolescent smokers may encounter a wide variety of acute and chronic stressors as they go about their lives. Thus, the effects of adolescent nicotine exposure under different conditions of concurrent adolescent stress deserve further scrutiny, especially with regard to possible effects of both of these factors on cognitive capacity in adulthood. Furthermore, other studies have found that exposure to nicotine can cause cognitive enhancement when nicotine is administered in adulthood and acutely just prior to behavioral assessment (Attaway et al., 1999; Barron et al., 2005; Levin & Torry, 1996; Semenova et al., 2007). Numerous studies with rodent models, including our own demonstrating sex differences in adolescent nicotine effects on adult learning in rats, have already shown that adolescent exposure to nicotine causes cognitive impairments in adulthood. To the best of our knowledge, the current study is the first to demonstrate facilitation of learning in adulthood caused by adolescent exposure to nicotine. Following on this discovery, to be able to properly predict the sequelae of adolescent nicotine exposure it will be of great importance to identify the conditions under which adolescent nicotine causes cognitive facilitation or enhancement in adulthood versus cognitive impairment. This, in turn, will provide a necessary foundation for identifying the relevant neurobiological mechanisms responsible for both these effects.
Supplementary Material
Highlights.
We examined effects of adolescent nicotine and injection stress on adult rat learning.
Adolescent nicotine and stress caused paradoxical effects on adult rat learning.
Adolescent nicotine impaired adult multiple-item learning in rats.
Adolescent nicotine without stress facilitated adult S-R and serial position learning.
This is the first report of adolescent nicotine facilitation of adult learning.
Acknowledgments
The project described was supported in part by Award Number R15DA023349 from the National Institute on Drug Abuse to S. B. Fountain. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Drug Abuse or the National Institutes of Health. We thank Carissa Martin for assistance with injections and data collection.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abreu-Villaça Y, Seidler FJ, Qiao D, Tate CA, Counsins MM, Thillai I, Slotkin TA. Short-term adolescent nicotine exposure has immediate and persistent effects on cholinergicsystems: Critical periods, patterns of exposure, dose thresholds. Neuropsychopharmacology. 2003a;28(11):1935–1949. doi: 10.1038/sj.npp.1300221. doi: 10.1038/sj.npp.1300221. [DOI] [PubMed] [Google Scholar]
- Abreu-Villaça Y, Seidler FJ, Tate CA, Slotkin TA. Nicotine is a neurotoxin in the adolescent brain: Critical periods, patterns of exposure, regional selectivity, and dose thresholds for macromolecular alterations. Brain Research. 2003b;979(1–2):114–128. doi: 10.1016/s0006-8993(03)02885-3. doi: 10.1016/s0006-8993(03)02885-3. [DOI] [PubMed] [Google Scholar]
- Adriani W, Spijker S, Deroche-Gamonet V, Laviola G, Le Moal M, Smit AB, Piazza PV. Evidence for enhanced neurobehavioral vulnerability to nicotine during periadolescence in rats. The Journal of Neuroscience. 2003;23(11):4712–4716. doi: 10.1523/JNEUROSCI.23-11-04712.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attaway CM, Compton DM, Turner MD. The Effects of Nicotine on Learning and Memory: A neuropsychological assessment in young and senescent Fischer 344 rats. Physiology & Behavior. 1999;67(3):421–431. doi: 10.1016/s0031-9384(99)00081-5. [DOI] [PubMed] [Google Scholar]
- Barron S, White A, Swartzwelder HS, Bell RL, Rodd ZA, Slawecki CJ, Ehlers CL, Levin ED, Rezvani AH, Spear LP. Adolescent vulnerabilities to chronic alcohol or nicotine exposure: Findings from rodent models. Alcoholism: Clinical and Experimental Research. 2005;29(9):1720–1725. doi: 10.1097/01.alc.0000179220.79356.e5. doi: 10.1097/01.alc.0000179220.79356.e5. [DOI] [PubMed] [Google Scholar]
- Belluzzi JD, Lee AG, Oliff HS, Leslie FM. Age-dependent effects of nicotine on locomotor activity and conditioned place preference in rats. Psychopharmacology. 2004;174(3):389–395. doi: 10.1007/s00213-003-1758-6. doi: 10.1007/s00213-003-1758-6. [DOI] [PubMed] [Google Scholar]
- Brielmaier JM, McDonald CG, Smith RF. Immediate and long-term behavioral effects of a single nicotine injection in adolescent and adult rats. Neurotoxicology and Teratology. 2007;29(1):74–80. doi: 10.1016/j.ntt.2006.09.023. doi: 10.1016/j.ntt.2006.09.023. [DOI] [PubMed] [Google Scholar]
- Center for Disease Control and Prevention Estimates of current tobacco use among youth. Smoking and Tobacco Use. 2014 from http://www.cdc.gov/tobacco/data_statistics/fact_sheets/youth_data/tobacco_use/
- Counotte DS, Spijker S, Burgwal L. H. V. d., Hogenboom F, Schoffelmeer ANM, Vries TJD, Smit AB, Pattij T. Long-lasting cognitive deficits resulting from adolescent nicotine exposure in rats. Neuropsychopharmacology. 2009;34(2):299–306. doi: 10.1038/npp.2008.96. doi: 10.1038/npp.2008.96. [DOI] [PubMed] [Google Scholar]
- Dilsaver SC, Majchrzak MJ. Effects of placebo (saline) injections on core temperature in the rat. Progress Neuropsychopharmacology Biological Psychiatry. 1990;14(3):417–422. doi: 10.1016/0278-5846(90)90029-g. [DOI] [PubMed] [Google Scholar]
- Dwyer JB, McQuown SC, Leslie FM. The dynamic effects of nicotine on the developing brain. Pharmacology & therapeutics. 2009;122(2):125–139. doi: 10.1016/j.pharmthera.2009.02.003. doi: 10.1016/j.pharmthera.2009.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fountain SB, Benson DM., Jr Chunking, rule learning, and multiple item memory in rat interleaved serial pattern learning. Learning and Motivation. 2006;37(2):95–112. [Google Scholar]
- Fountain SB, Rowan JD. Coding of hierarchical versus linear pattern structure in rats and humans. Journal of Experimental Psychology: Animal Behavior Processes. 1995;21(3):187–202. doi: 10.1037//0097-7403.21.3.187. doi: 10.1037//0097-7403.21.3.187. [DOI] [PubMed] [Google Scholar]
- Fountain SB, Rowan JD. Differential impairments of rat serial pattern learning and retention induced by MK-801, and NMDA receptor antagonist. Psychobiology. 2000;28(1):32–44. [Google Scholar]
- Fountain SB, Rowan JD, Kelley BM, Willey AR, Nolley EP. Adolescent exposure to nicotine impairs adult serial pattern learning in rats. Experimental Brain Research. 2008;187:651–656. doi: 10.1007/s00221-008-1346-4. doi: 10.1007/s00221-008-1346-4. [DOI] [PubMed] [Google Scholar]
- Fountain SB, Rowan JD, Muller MD, Kundey SMA, Pickens LRG, Doyle KE. The organization of sequential behavior: Conditioning, memory, and abstraction. In: Zentall TR, Wasserman EA, editors. Handbook of Comparative Cognition. Oxford University Press; Oxford: 2012. pp. 594–614. [Google Scholar]
- Fountain SB, Rowan JD, Wollan MO. Central cholinergic involvement in sequential behavior: Impairments of performance by atropine in a serial multiple choice task for rats. Neurobiology of learning and memory. 2013;106:118–126. doi: 10.1016/j.nlm.2013.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green MR, McCormick CM. Effects of social instability stress in adolescence on long-term, not short-term, spatial memory performance. Behavioural Brain Research. 2013;256:165–171. doi: 10.1016/j.bbr.2013.08.011. [DOI] [PubMed] [Google Scholar]
- Isgor C, Kabbaj M, Akil H, Watson SJ. Delayed effects of chronic variable stress during peripubertal-juvenile period on hippocampal morphology and on cognitive and stress axis functions in rats. Hippocampus. 2004;14(5):636–648. doi: 10.1002/hipo.10207. [DOI] [PubMed] [Google Scholar]
- Jacobsen LK, Krystal JH, Einar W, Westerveld M, Frost SJ, Pugh KR. Effects of Smoking and Smoking Abstinence on Cognition in Adolescent Tobacco Smokers. Biological Psychiatry. 2005;57(1):56–66. doi: 10.1016/j.biopsych.2004.10.022. doi: 10.1016/j.biopsych.2004.10.022. [DOI] [PubMed] [Google Scholar]
- Johnston LD, O'Malley PM, Bachman JG, Schulenberg JE. Monitoring the Future national results on adolescent drug use: Overview of key findings. Institute for Social Research: University of Michigan; Ann Arbor: 2011. pp. 1–84. 2010. [Google Scholar]
- Kundey S, De Los Reyes A, Rowan JD, Lee B, Delise J, Molina S, Cogdill L. Involvement of working memory in college students’ sequential pattern learning and performance. Learning and Motivation. 2013;44(2):114–126. [Google Scholar]
- Kundey S, Fountain SB. Blocking in rat serial pattern learning. Journal of Experimental Psychology: Animal Behavior Processes. 2010;36(2):307. doi: 10.1037/a0016523. [DOI] [PubMed] [Google Scholar]
- Levin ED, Torry D. Acute and chronic nicotine effects on working memory in aged rats. Psychopharmacology. 1996;123(1):88–97. doi: 10.1007/BF02246285. doi: 10.1007/bf02246285. [DOI] [PubMed] [Google Scholar]
- Long Evans (Blue Spruce) Growth Curve. 2006 from http://www.harlan.com/products_and_services/research_models_and_services/research_models/long_evans_blue_spruce_outbred_rat.hl.
- McDonald CG, Dailey VK, Bergstrom HC, Wheeler TL, Eppolito AK, Smith LN, Smith RF. Periadolescent nicotine administration produces enduring changes in dendritic morphology of medium spiny neurons from nucleus accumbens. Neuroscience Letters. 2005;385(2):163–167. doi: 10.1016/j.neulet.2005.05.041. doi: 10.1016/j.neulet.2005.05.041. [DOI] [PubMed] [Google Scholar]
- Morrissey MD, Mathews IZ, McCormick CM. Enduring deficits in contextual and auditory fear conditioning after adolescent, not adult, social instability stress in male rats. Neurobiology of learning and memory. 2011;95(1):46–56. doi: 10.1016/j.nlm.2010.10.007. [DOI] [PubMed] [Google Scholar]
- Muller MD, Fountain SB. Concurrent cognitive processes in rat serial pattern learning: Item memory, serial position, and pattern structure. Learning and Motivation. 2010;41(4):252–272. doi: 10.1016/j.lmot.2010.08.003. doi: 10.1016/j.lmot.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natividad LA, Torres OV, Friedman TC, O’Dell LE. Adolescence is a period of development characterized by short- and long-term vulnerability to the rewarding effects of nicotine and reduced sensitivity to the anorectic effects of this drug. Behav Brain Res. 2013;257(0):275–285. doi: 10.1016/j.bbr.2013.10.003. doi: http://dx.doi.org/10.1016/j.bbr.2013.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philpot RM, Engberg ME, Wecker L. Ethanol conditioned place preference and alterations inΔFosB following adolescent nicotine administration differ in rats exhibiting high or low behavioral reactivity to a novel environment. Behav Brain Res. 2014;262(0):101–108. doi: 10.1016/j.bbr.2013.12.014. doi: http://dx.doi.org/10.1016/j.bbr.2013.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickens LRG, Rowan JD, Bevins RA, Fountain SB. Sex differences in adult cognitive deficits after adolescent nicotine exposure in rats. Neurotoxicology and Teratology. 2013;38:72–78. doi: 10.1016/j.ntt.2013.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polesskaya OO, Fryxell KJ, Merchant AD, Locklear LL, Ker K-F, McDonald CG, Eppolito AK, Smith LN, Wheeler TL, Smith RF. Nicotine causes age-dependent changes in gene expression in the adolescent female rat brain. Neurotoxicology and Teratology. 2007;29(1):126–140. doi: 10.1016/j.ntt.2006.11.005. doi: 10.1016/j.ntt.2006.11.005. [DOI] [PubMed] [Google Scholar]
- Quick SL, Olausson P, Addy NA, Taylor JR. Repeated nicotine exposure during adolescence alters reward-related learning in male and female rats. Behav Brain Res. 2014;261(0):171–176. doi: 10.1016/j.bbr.2013.12.001. doi: http://dx.doi.org/10.1016/j.bbr.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Restle F, Brown ER. Serial pattern learning. Journal of Experimental Psychology. 1970;83:120–125. [Google Scholar]
- Schochet TL, Kelley AE, Landry CF. Differential behavioral effects of nicotine exposure in adolescent and adult rats. Psychopharmacology. 2004;175(3):265–273. doi: 10.1007/s00213-004-1831-9. doi: 10.1007/s00213-004-1831-9. [DOI] [PubMed] [Google Scholar]
- Semenova S, Stolerman IP, Markou A. Chronic nicotine administration improves attention while nicotine withdrawal induces performance deficits in the 5-choice serial reaction time task in rats. Pharmacology Biochemistry and Behavior. 2007;87(3):360–368. doi: 10.1016/j.pbb.2007.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp J, Zammit T, Azar T, Lawson D. Stress-like responses to common procedures in individually and group-housed female rats. Contemporary Topics in Laboratory Animal Science. 2003;42(1):9–18. [PubMed] [Google Scholar]
- Silverstone PH, Williams R, McMahon L, Fleming R, Fogarty S. Effect of increasing intraperitoneal infusion rates on bupropion hydrochloride-induced seizures in mice. Annals of general psychiatry. 2008;7(1):27. doi: 10.1186/1744-859X-7-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slawecki CJ, Thorsell A, Ehlers CL. Long-term neurobehavioral effects of alcohol or nicotine exposure in adolescent animal models. Annals of the New York Academy of Sciences. 2004;1021:448–452. doi: 10.1196/annals.1308.062. doi: 10.1196/annals.1308.062. [DOI] [PubMed] [Google Scholar]
- Slotkin TA. Nicotine and the adolescent brain: Insights from an animal model. Neurotoxicology and Teratology. 2002;24(3):369–384. doi: 10.1016/s0892-0362(02)00199-x. doi: 10.1016/s0892-0362(02)00199-x. [DOI] [PubMed] [Google Scholar]
- Slotkin TA, Bodwell BE, Ryde IT, Seidler FJ. Adolescent nicotine treatment changes the response of acetylcholine systems to subsequent nicotine administration in adulthood. Brain Research Bulletin. 2008;76(1–2):152–165. doi: 10.1016/j.brainresbull.2007.12.009. doi: 10.1016/j.brainresbull.2007.12.009. [DOI] [PubMed] [Google Scholar]
- Slotkin TA, MacKillop EA, Rudder CL, Ryde IT, Tate CA, Seidler FJ. Permanent, sex-selective effects of prenatal or adolescent nicotine exposure, separately or sequentially, in rat brain regions: indices of cholinergic and serotonergic synaptic function, cell signaling, and neural cell number and size at 6 months of age. Neuropsychopharmacology. 2007;32(5):1082–1097. doi: 10.1038/sj.npp.1301231. doi: 10.1038/sj.npp.1301231. [DOI] [PubMed] [Google Scholar]
- Spaeth AM, Barnet RC, Hunt PS, Burk JA. Adolescent nicotine exposure disrupts context conditioning in adulthood in rats. Pharmacology Biochemistry and Behavior. 2010;96(4):501–506. doi: 10.1016/j.pbb.2010.07.011. doi: 10.1016/j.pbb.2010.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stempowski NK, Carman HM, Fountain SB. Temporal phrasing and overshadowing in rat serial-pattern learning. Learning and Motivation. 1999;30:74–100. [Google Scholar]
- Torregrossa MM, Xie M, Taylor JR. Chronic corticosterone exposure during adolescence reduces impulsive action but increases impulsive choice and sensitivity to yohimbine in male Sprague-Dawley rats. Neuropsychopharmacology. 2012;37(7):1656–1670. doi: 10.1038/npp.2012.11. doi: 10.1038/npp.2012.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trauth JA, McCook EC, Seidler FJ, Slotkin TA. Modeling adolescent nicotine exposure: Effects on cholinergic systems in rat brain regions. Brain Research. 2000;873(1):18–25. doi: 10.1016/s0006-8993(00)02465-3. [DOI] [PubMed] [Google Scholar]
- Trauth JA, Seidler FJ, McCook EC, Slotkin TA. Adolescent nicotine exposure causes persistent upregulation of nicotinic cholinergic receptors in rat brain regions. Brain Research. 1999;851(1-2):9–19. doi: 10.1016/s0006-8993(99)01994-0. [DOI] [PubMed] [Google Scholar]
- Trauth JA, Seidler FJ, Slotkin TA. An animal model of adolescent nicotine exposure: Effects on gene expression and macromolecular constituents in rat brain regions. Brain Research. 2000a;867(1-2):29–39. doi: 10.1016/s0006-8993(00)02208-3. [DOI] [PubMed] [Google Scholar]
- Trauth JA, Seidler FJ, Slotkin TA. Persistent and delayed behavioral changes after nicotine treatment in adolescent rats. Brain Research. 2000b;880(1-2):167–172. doi: 10.1016/s0006-8993(00)02823-7. [DOI] [PubMed] [Google Scholar]
- Wheeler TL, Smith LN, Bachus SE, McDonald CG, Fryxell KJ, Smith RF. Low-dose adolescent nicotine and methylphenidate have additive effects on adult behavior and neurochemistry. Pharmacology Biochemistry and Behavior. 2013;103(4):723–734. doi: 10.1016/j.pbb.2012.12.005. doi: http://dx.doi.org/10.1016/j.pbb.2012.12.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.