Abstract
The major oncoprotein p53 regulates several cellular antiproliferation pathways that can be triggered in response to a variety of cellular stresses, including viral infection. The stabilization of p53 is a key factor in the ability of cells to initiate an efficient transcriptional response after cellular stress. Here we present data demonstrating that herpes simplex virus type 1 (HSV-1) infection of HFFF-2 cells, a low-passage-number nontransformed human primary cell line, results in the stabilization of p53. This process required viral immediate-early gene expression but occurred independently of the viral regulatory protein ICP0 and viral DNA replication. No specific viral protein could be identified as being solely responsible for the effect, which appears to be a cellular response to developing HSV-1 infections. HSV-1 infection also induced the phosphorylation of p53 at residues Ser15 and Ser20, which have previously been implicated in its stabilization in response to DNA damage. However, an HSV-1 infection of ATM−/− cells, which lack a kinase implicated in these phosphorylation events, did not lead to the phosphorylation of p53 at these residues, but nonetheless p53 was stabilized. We also show that the wild-type p53 expressed by osteosarcoma U2OS cells can be stabilized in response to DNA damage induced by UV irradiation, but not in response to HSV-1 infection. These data suggest that multiple cellular mechanisms are initiated to stabilize p53 during an HSV-1 infection. These mechanisms occur independently of ICP0 and its ability to sequester USP7 and may differ from those initiated in response to DNA damage.
The major oncoprotein p53 is a key regulator of the ability of cells to respond to stress through its control of several important cellular pathways, including growth arrest, apoptosis, and cellular senescence. The activation of p53 is induced by a variety of stresses, including DNA damage, hypoxia, nucleotide deprivation, heat shock, oncogenic activation, and viral infection. The ability of p53 to induce the transcription of several cellular genes is regulated by three principal factors, namely p53's stability, activity, and subcellular localization (for recent reviews, see references 12, 75, 77, and 79). The expression of p53 is normally maintained at low levels through ubiquitination and proteasome-mediated degradation (51). Although it appears that several ubiquitin isopeptide ligases (E3 ubiquitin ligases) are involved in p53 regulation (8, 45), the best characterized of these is mouse double minute 2 (Mdm2). Mdm2 binds to the N terminus of p53, and in conjunction with the E2 ubiquitin-conjugating enzyme UbcH5, mediates the ubiquitination of C-terminal lysine residues of p53 and subsequent p53 degradation by the 26S proteasome (31, 32, 37, 63). Consequently, the stabilization of p53 is critical for its ability to activate transcription. Stabilization of p53 can occur through several mechanisms, including phosphorylation of p53 at N-terminal serine (Ser) and threonine (Thr) residues (6, 41, 68, 69); acetylation of C-terminal lysine residues (38, 47); phosphorylation (15, 35, 42, 53) and sequestration of Mdm2 (40, 49, 61, 73, 83); and competitive binding of p53 by Mdmx, an Mdm2 family member that lacks E3 ubiquitin ligase activity (39, 70). These mechanisms result in the inhibition of Mdm2 interacting with or ubiquitinating p53, thereby promoting p53 stabilization. The targeting of ubiquitinated p53 for degradation also appears to be a tightly regulated process. The ubiquitin-specific protease USP7 (also known as HAUSP) stabilizes ubiquitinated p53 by cleaving the isopeptide-linked ubiquitin chains from the protein (46). De-ubiquitination of p53 therefore provides an additional mechanism by which cells can regulate p53 stability, and ultimately, transcriptional activity.
Herpes simplex virus type 1 (HSV-1) gene expression during a lytic infection occurs in a regulated temporal cascade in which the viral genes can be divided into three broad classes, named immediate early (IE), early (E), and late (L) (for a review, see reference 64). Although the majority of IE proteins have been associated with the regulation of viral gene expression, only ICP0 (also known as Vmw110) is capable of transactivating all three classes of viral genes (for a review, see reference 20). Virus mutants that do not express ICP0 are severely impaired in the ability to replicate in low-passage-number human fibroblast cells at low doses of input virus and are more likely to establish a quiescent infection. Although ICP0 is required to a greater or lesser extent for efficient viral replication in most cell lines, virus mutants that fail to express ICP0 are not impaired in U2OS cells (81). One of the many proteins with which ICP0 has been reported to interact is USP7 (25). This interaction contributes to viral growth in BHK cells and the ability of ICP0 to activate gene expression in Cos7 cells (24, 54). The ability of ICP0 to activate viral gene expression requires its N-terminal zinc binding RING finger motif, a domain that confers E3 ubiquitin ligase activity (11, 19, 27, 34). Directly or indirectly, this activity causes the degradation of several cellular proteins, including the major ND10 (promyelocytic leukemia [PML] nuclear body) constituent proteins PML and SUMO-1 modified isoforms of Sp100 (10, 13, 23, 33, 57, 59), the centromeric proteins CENP-A and CENP-C (22, 50), and the catalytic subunit of DNA-PK (44, 60). The ability of ICP0 to induce the degradation of these and potentially other as yet unidentified cellular proteins through ubiquitination and proteasome-mediated degradation appears to inhibit a cellular response to viral infection and promotes a cellular environment that is conducive to viral replication. Several DNA viruses are known to target p53 either through its sequestration or by ubiquitination and proteasome-mediated degradation, thereby affecting its transcriptional activity. For example, the simian virus 40 large T antigen sequesters p53 (5, 80), whereas the E6/E6-AP complex of human papillomaviruses 16 and 18 ubiquitinates and destabilizes p53 (3, 66, 67; reviewed in reference 4). Several herpesviruses have also been shown to affect the transcriptional activity of p53. For example, the Epstein-Barr virus (EBV) IE protein BZLF1 (52, 82) and the human herpesvirus 8 latency-associated nuclear antigen ORF 73 (30) inhibit p53 transcriptional responses. However, little is known about p53 regulation during alphaherpesvirus infections. Some cellular proteins, including p53 and retinoblastoma protein (Rb), appear to localize to HSV-1 DNA replication compartments, but the significance of this recruitment remains unclear (78). Analyses of HSV-1 mutants restricted in the ability to express IE proteins have shown that HSV-1-induced cell cycle arrest occurs independently of p53 (36) and that the mitotic block caused by ICP0 is a direct result of its ability to induce the degradation of CENP-A and CENP-C (22, 50). We have recently reported that ICP0 can interact with and directly ubiquitinate a proportion of p53 molecules and can protect infected U2OS cells against UV-induced apoptosis (9). Therefore, ICP0 has the potential to modulate p53 expression and/or p53-mediated pathways through its E3 ubiquitin ligase activity and also, potentially, through its interaction with USP7. These observations prompted us to investigate the fate of p53 during productive HSV-1 infections.
In this paper, we show that HSV-1 infection of HFFF-2 cells, a nontransformed human fetal foreskin fibroblast cell line expressing wild-type (wt) p53, results in the phosphorylation and stabilization of p53. The stabilization of p53 required HSV-1 IE gene expression but not viral DNA replication and was therefore not a cellular response to HSV-1 virion binding, uncoating, or the translocation of viral DNA into the nucleus or a cellular DNA damage response activated during viral DNA replication. The stabilization and phosphorylation of p53 occurred in an ICP0-independent manner, suggesting that the sequestration of USP7 by ICP0 is not involved in p53 stabilization during an HSV-1 infection. However, the efficient phosphorylation of p53 required ICP4 expression and its DNA binding activity (and hence its ability to activate viral early gene expression). Although the stabilization of p53 was apparently concordant with its phosphorylation on N-terminal serine residues in HFFF-2 cells, it did not require the phosphorylation of Ser15 or Ser20 by ATM, a cellular kinase known to phosphorylate and promote the stabilization of p53 in response to DNA damage. These effects were found to be cell type dependent since p53 was not stabilized during an infection of U2OS cells, an osteosarcoma cell line that also expresses wt p53. Taken together, these data suggest that a lytic HSV-1 infection induces the stabilization of p53 through several cellular mechanisms but that neither ICP0 nor its ability to interact with USP7 is involved.
MATERIALS AND METHODS
Cells and viruses.
U2OS and HFFF-2 cells (European Collection of Cell Cultures) were grown in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal calf serum. AT−/− (GM02052E) and control AT+/+ (GM03491) (NIGMS Human Genetic Cell Repository) cells were grown in DMEM supplemented with 20% fetal calf serum, 1% nonessential amino acids, and 1% l-glutamine. All cultures were grown at 37°C in an atmosphere containing 5% CO2 unless otherwise stated.
HSV-1 strain 17 syn+ and its derivatives dl1403 (74), FXE (a RING finger deletion mutant lacking residues 106 to 146 of ICP0) (18), substitution mutant M1 (with mutations of ICP0 residues 623R to L and 624K to I) (24), and deletion mutant D12 (lacking residues 594 to 633 of ICP0) (54) were grown in BHK (C13) cells infected at a multiplicity of infection (MOI) of 0.01 PFU per cell. The titers of all viruses, including ICP0 mutant viruses, were determined with U2OS cells. The mutant in1411 virus, which fails to express ICP4 (65), and the ICP27 deletion mutant 17+27-pR19lacZ (48) were grown and titrated in M49 BHK cells, which constitutively express both ICP4 and ICP27. The mutant tsK virus (62) was grown in BHK cells and titrated in U2OS cells at the permissive temperature of 32°C. Virus released into the cell medium was harvested after the cell monolayer showed an extensive cytopathic effect. Supernatants containing the virus were clarified, stored at 4°C, and titrated on appropriate cells in the presence of 2% human serum.
Pulse-chase determination of p53 stability.
Pulse-chase experiments were performed essentially as described previously (28, 71). HFFF-2 cells were seeded in 35-mm-diameter dishes at a cell density of 5 × 105 cells/dish, cultured overnight, and washed twice in 1 ml of phosphate-buffered saline (PBS) before being cultured in methionine-free DMEM (Sigma-Aldrich) for 30 min. The medium was then removed and replaced with methionine-free DMEM supplemented with [35S]methionine (100 μCi/ml) (Amersham) for 1 h (pulse). The medium was subsequently removed and the cells were washed twice with 1 ml of PBS before being mock infected or infected with HSV-1 (strain 17+) at an MOI of 10. After absorption, the cells were returned to the normal culture medium and harvested at the appropriate time points postinfection (p.i.) (chase). The medium was removed and the cells were washed twice with 1 ml of PBS and harvested directly in 1 ml of cell lysis buffer (50 mM Tris [pH 7.4], 500 mM NaCl, 1% NP-40, 0.1% sodium dodecyl sulfate [SDS], 0.1% sodium deoxycholate, 1 mM EDTA, 1 mM EGTA, 1 mM dithiothreitol) containing protease inhibitors (Roche). Samples were subsequently incubated on ice for 30 min, briefly sonicated, and clarified by centrifugation at 20,000 × g for 10 min at 4°C. Soluble fractions were then incubated with 1 μg of a polyclonal p53 antibody (FL-393; Santa Cruz Biotechnology) end-over-end (on a rotary mixer) at 4°C for 2 h. Immune complexes were precipitated by the addition of 40 μl of 50% (wt/vol) protein A-Sepharose for a further 2 h at 4°C end-over-end. The beads were subsequently pelleted by centrifugation and washed three times in 1 ml of ice-cold lysis buffer before being resuspended in 40 μl of SDS-polyacrylamide gel electrophoresis (SDS-PAGE) boiling mix. Radiolabeled proteins were resolved by SDS-8% PAGE, and the gel was subsequently stained with Coomassie brilliant blue and destained before being dried and analyzed by phosphorimaging.
UV irradiation and chemical treatments.
Cells (4 × 105) were seeded in 35-mm-diameter dishes, cultured overnight, washed in PBS, and then subjected to a UV dose of 50 J/m2 (UV cross-linker; Stratagene). Normal medium was then added and the cells were incubated for the indicated time points prior to harvesting. The cells were treated with cycloheximide (CHX) at 10 μg/ml (Sigma-Aldrich), acyclovir (ACG) at 10 μM (Sigma-Aldrich), and wortmannin (Sigma-Aldrich) at various concentrations as described in the text.
Western blots and antibodies.
Cells (4 × 105) were seeded in 35-mm-diameter dishes, incubated overnight, infected with the appropriate viruses and/or treatments, and then incubated for the indicated times. Cell monolayers were then washed twice with 1 ml of PBS before being harvested directly in 200 μl of 1× SDS-PAGE boiling mix buffer containing 3 M urea and 25 mM dithiothreitol. Proteins in 30 μl of cell extract (equivalent to 6 × 104 cells) were resolved in SDS-7.5 and/or 10% PAGE or 4 to 12% Bis-Tris NuPAGE gels (Invitrogen) and then transferred to nitrocellulose membranes by Western blotting. The filters were probed with the following antibodies, as appropriate: anti-ICP0 monoclonal antibody (MAb) 11060 (26), anti-ICP4 MAb 11076 (16), and anti-ICP27 MAb H1113 (1), purchased from the Goodwin Institute for Cancer Research; and an anti-p53 MAb (oncogene Ab-6), anti-actin MAb AC-40 (Sigma-Aldrich), and phospho-specific (p-) anti-p53 rabbit antibodies p-p53(Ser15) (Oncogene) and p-p53(hser20) (Santa Cruz Biotechnology).
RESULTS
HSV-1 infection results in the stabilization of p53.
We recently reported that ICP0 can interact with and ubiquitinate p53 both in vitro and in vivo, implying that ICP0 has the potential to regulate p53 expression and its transcriptional activity through ubiquitination and proteasome-mediated degradation (9). However, while ICP0 could ubiquitinate p53 in vivo, its activity was significantly lower than that of Mdm2, a negative regulator of p53 expression. We concluded that ICP0 may target only a subset of posttranslationally modified p53 molecules or p53 molecules that are associated with other known cellular targets of ICP0 as part of a complex of proteins targeted for degradation. Since little is known about the fate of p53 during HSV-1 infection, we decided to analyze the metabolism of p53 in HSV-1-infected cells.
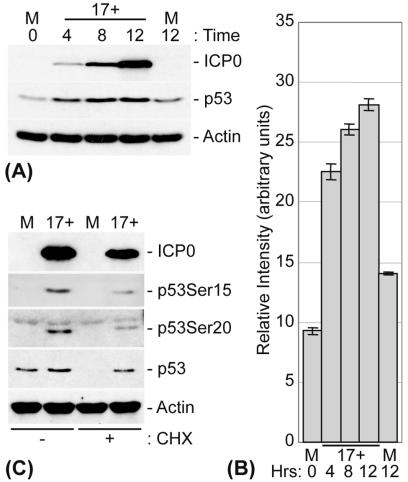
HFFF-2 cells were infected at an MOI of 1, and p53 levels were analyzed at various times p.i. (Fig. 1). Infected cells exhibited increased levels of p53 as early as 4 h p.i., correlating with increased levels of IE gene expression (Fig. 1A). Densitometry analysis demonstrated that p53 levels had increased more than twofold at 4 h p.i. and continued to increase over the time course of the infection (Fig. 1A and B). The increased levels of p53 appeared to be a result of its stabilization, since infected cells maintained their p53 levels upon a subsequent treatment with CHX, whereas parallel mock-infected cells showed a significant decrease in p53, presumably due to turnover by ubiquitination and proteasome-mediated degradation (Fig. 1C). The increase in p53 stability during infection also correlated with its phosphorylation at N-terminal serine residues Ser15 and Ser20 (Fig. 1C). Phosphorylation of these residues reduces the ability of Mdm2 to bind to p53 and thereby contributes to its stabilization (6, 41, 68, 69). Since HSV-1 inhibits cellular protein synthesis through the activities of the tegument-associated protein vhs and the IE protein ICP27 (reviewed in reference 64) and since p53 levels increased during the initial stages of viral infection, it appears that the increase in p53 expression during infection is a result of p53 stabilization rather than an increase in its transcription. Indeed, previous microarray analysis of HSV-1-infected HeLa cells showed that the level of p53 mRNA transcripts does not increase during infection (72).
FIG. 1.
HSV-1 infection results in stabilization of p53. (A) HFFF-2 cells were either mock infected or infected with HSV-1 (strain 17+) at an MOI of 1 PFU/cell for the indicated times. (B) Histogram representing the relative increases in p53 levels during HSV-1 infection of HFFF-2 cells. Densitometry analysis was performed on p53 Western blots from a series of experiments, and the results are expressed as relative changes in intensity (arbitrary units). Error bars represent the standard errors for three independent experiments. (C) HFFF-2 cells were either mock infected or infected with HSV-1 for 8 h at an MOI of 1 PFU/cell. The cells were then either harvested directly in SDS-PAGE loading buffer or treated with CHX (final concentration, 10 μg/ml) for an additional 10 h before being harvested. The cell extracts were analyzed by Western blotting with monoclonal antibodies that recognize ICP0, p53, and actin and with phospho-specific p53-Ser15 and p53-Ser20 rabbit polyclonal antibodies, as indicated. Note that in this and subsequent figures, the relative intensities of bands of the different proteins in the vertical columns vary according to trivial factors and cannot therefore be used for quantitative purposes (for example, for comparing the apparent absolute levels of p53 to actin).
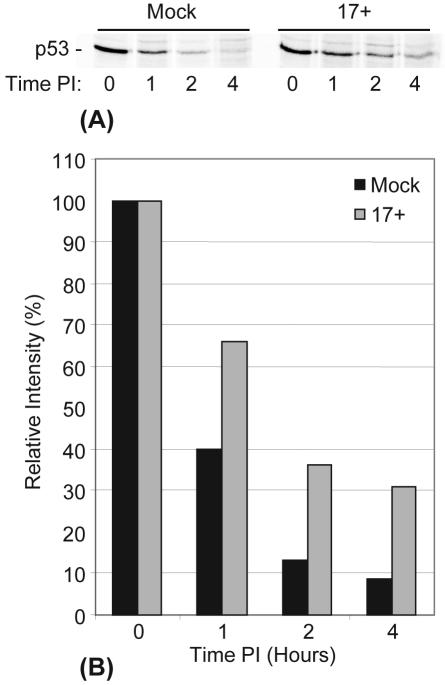
To confirm that HSV-1 infection stabilizes p53, we conducted a pulse-chase experiment. Cells were incubated with a medium containing [35S]methionine before being mock infected or infected with HSV-1 (strain 17+, as described in Materials and Methods). Replicate plates were harvested at the indicated times points p.i., and soluble cell extracts were prepared for the immunoprecipitation of p53. The samples were analyzed by SDS-PAGE and phosphorimaging (Fig. 2A). Mock-infected cells showed a dramatic decrease (approximately 60%) in the amount of metabolically labeled p53 recovered after 1 h of chase (consistent with the published half-life of approximately 25 min), with a similar decrease after 2 h (Fig. 2B). In HSV-1-infected cells, however, p53 was clearly more stable, with a decrease of only 35% of the metabolically labeled p53 after 1 h, with a similar relative decrease after 2 h, indicating that the half-life of p53 had been increased to at least an hour. Due to technical considerations, this experiment had to be performed at early times of infection, probably before the maximum stabilization effect of HSV-1 on metabolically labeled p53 could be established, and therefore this increase in half-life is probably a minimum estimate. In normal cells, p53 lost by degradation is replaced by newly synthesized proteins, leading to stable total levels of the protein. Therefore, an increase in the p53 half-life during HSV-1 infection leads to an increased total amount of p53 protein (as seen in Fig. 1A), provided that its rate of synthesis is maintained. Taken together with the results of the CHX experiment, the results of the pulse-chase experiment confirm that HSV-1 causes a relative stabilization of p53, resulting in increased total p53 levels in HFFF-2 cells, and most significantly, in the retention of p53 in the absence of de novo synthesis.
FIG. 2.
HSV-1 infection decreases rate of p53 degradation. (A) HFFF-2 cells were pulse labeled (as described in Materials and Methods) prior to being mock infected or infected with HSV-1 (strain 17+) at an MOI of 10 PFU/cell. The cellular levels of metabolically labeled p53 were determined by immunoprecipitation and SDS-PAGE analysis (as described in Materials and Methods). (B) Histogram representing quantification of metabolically labeled p53 from mock-infected or HSV-1-infected HFFF-2 cells (as described for panel A) by phosphorimaging analysis. Relative intensity values for both mock-infected and HSV-1 17+-infected cells are given as percentages of the total amounts of metabolically labeled p53 at time zero.
HSV-1-induced stabilization of p53 requires IE gene expression but not DNA replication.
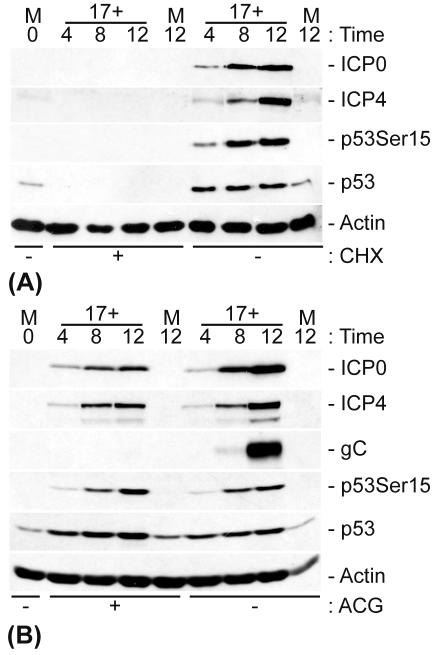
In order to investigate the basis of HSV-1-induced p53 stabilization, we carried out infections in the presence or absence of either CHX to inhibit viral protein synthesis or ACG to inhibit the onset of viral DNA replication. The cells were harvested and analyzed by Western blotting for the presence of both viral and cellular proteins (Fig. 3).
FIG. 3.
p53 stabilization requires IE gene expression but not viral DNA replication. HFFF-2 cells were either mock infected or infected with HSV-1 (strain 17+) at an MOI of 1 PFU/cell in the presence or absence (+ and −, respectively) of CHX (final concentration, 10 μg/ml) (A) or ACG (final concentration, 10 μg/ml) (B).
CHX efficiently inhibited de novo viral protein expression and resulted in a decrease in the levels of p53 expression below the threshold of detection by Western blot analysis (Fig. 3A). Since CHX does not inhibit HSV-1 entry, virus uncoating, the translocation of viral DNA to the nucleus, or IE gene transcription, these data clearly indicate that viral (and potentially also cellular) protein expression is required to induce both the phosphorylation and stabilization of p53 during infection. The stabilization of p53 is therefore independent of any pre-existing stress-activated pathways that may be triggered due to virion-receptor binding, virus entry and uncoating, the presence of any virion-associated proteins, e.g., tegument-associated kinase that may potentially phosphorylate p53, or a response to viral DNA entry into the nucleus.
Treatment with the viral DNA replication inhibitor ACG had no effect on the expression of ICP0 or ICP4 or on the phosphorylation and stabilization of p53 during HSV-1 infection (Fig. 3B). The lack of the late protein glycoprotein C (gC) in the ACG-treated samples clearly demonstrate that the drug treatment had successfully inhibited viral DNA replication. The phosphorylation and stabilization of p53 must therefore occur prior to the onset of viral DNA replication and are therefore not responses to replicative intermediates that may be recognized as damaged DNA.
The stabilization of p53 occurs in an ICP0-independent manner.
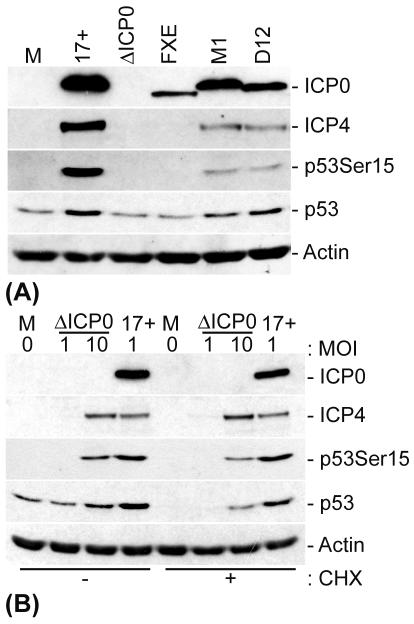
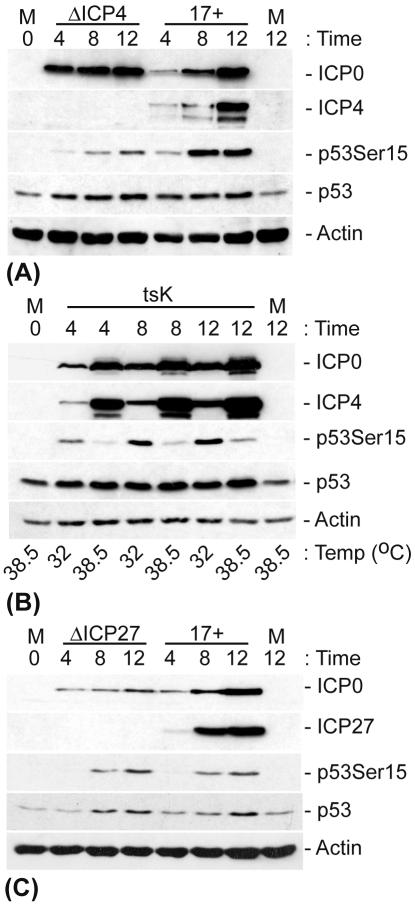
Virus mutants that fail to express functional ICP0 are severely impaired in the ability to replicate in low-passage-number human fibroblasts at low MOIs (21; for a review, see reference 20). ICP0 also binds strongly and specifically to USP7 (25, 54, 55), a ubiquitin-specific protease that can stabilize p53 by cleaving isopeptide-linked ubiquitin chains from the C terminus of the protein (46). In order to determine whether ICP0 could be involved in the stabilization of p53 through its interaction with USP7 or whether it was simply required for the efficient onset of viral gene expression, which in turn results in the stabilization of p53, we infected cells with several HSV-1 ICP0 mutants at a low or high MOI and analyzed the stability of p53 by Western blotting (Fig. 4). The HSV-1 ICP0 null mutant dl1403 and the RING finger deletion mutant FXE, which are less efficient than the wt virus at inducing the onset of IE gene expression at an MOI of 1 in HFFF-2 cells (18, 21, 74), showed similar profiles in low-MOI infections, with no detectable ICP4 expression, no phosphorylation of p53 at Ser15, and no increase in the stabilization of p53 compared to mock-infected cells (Fig. 4A).
FIG. 4.
ICP0 is not required for phosphorylation and stabilization of p53 during HSV-1 infection. HFFF-2 cells were either mock infected or infected with HSV-1 (strain 17+) and the indicated mutants. (A) The mutants used were HSV-1 IE-1 mutants that expressed no ICP0 (dl1403/ΔICP0) or had a deletion in the RING finger domain of ICP0 (FXE) and two ICP0 USP7-negative binding mutants, M1 (a substitution mutant carrying the mutations 623R to L and 624K to I in ICP0) and D12 (a deletion mutant lacking amino acids 594 to 633 of ICP0). These mutants were used at an MOI of 1 PFU/cell. (B) The mutant used was dl1403 (ΔICP0), which was added at various MOIs (as indicated). At 8 h p.i., the cells were either harvested or treated with CHX, as designated (final concentration, 10 μg/ml), for an additional 10 h before being harvested. The cell extracts were then analyzed by Western blotting with anti-ICP0, -ICP4, -p53, and -actin monoclonal antibodies and a phospho-specific p53-Ser15 rabbit polyclonal antibody.
HSV-1 mutants M1 and D12, which express forms of ICP0 that are unable to bind to USP7 (17, 54), showed a reduced ability to induce the phosphorylation of p53 compared to that of wt HSV-1 in low-MOI infections. Nonetheless, total p53 levels were increased in the mutant virus infections, which is indicative of stabilization (Fig. 4A). The reduction in p53 phosphorylation in these infections correlated with a reduction in ICP4 expression (Fig. 4A). Therefore, in order to determine whether the ability of ICP0 to bind to USP7 was specifically required for the stabilization of p53, we infected cells with dl1403 at a high MOI, thereby overcoming the requirement for ICP0-mediated viral gene transactivation (Fig. 4B). Infection with dl1403 at an MOI of 10 resulted in levels of IE gene expression that were similar to those for a wt virus infection at an MOI of 1, and this correlated with equivalent levels of phosphorylation of p53 at Ser15. The treatment of cells with CHX 8 h after infection with dl1403 at a high but not a low MOI resulted in a significant stabilization of p53. Therefore, the stabilization and phosphorylation of p53 during HSV-1 infection require the ability of ICP0 to induce efficient viral gene expression, but the stabilization of p53 occurs independently of ICP0 and therefore of any potential mutual interactions between ICP0, USP7, and p53.
ICP4, but not ICP27, is required for efficient p53 phosphorylation at Ser15.
ICP4 is essential for HSV-1 replication due to its ability to bind to viral DNA and activate the transcription of early and late genes (7, 14, 56, 62). Since the HSV-1-induced N-terminal phosphorylation of p53 appears to correlate with the induction of IE gene expression and since p53 has been reported to be recruited to viral DNA replication compartments associated with ICP4 (78), we determined whether ICP4 expression was required for the phosphorylation and stabilization of p53 during infection. Cells were infected at an MOI of 1 with in1411, an HSV-1 mutant that fails to express ICP4, and the stability and phosphorylation status of p53 was analyzed by Western blotting (Fig. 5A). In the absence of ICP4, there was a significant reduction in the extent of p53 phosphorylation at Ser15 compared to that in wt HSV-1-infected cells. However, since there were similar increases in the level of p53 stabilization in in1411-infected cells compared to that in wt HSV-1-infected cells, the phosphorylation of p53 at Ser15 does not appear to correlate with its induced stabilization during infection. Note that infections in the absence of functional ICP4 lead to an enhanced expression of the other IE genes compared to similar low-MOI infections carried out in the absence of ICP0. Therefore, in1411 infections are considerably more biologically productive, at least with regard to IE protein synthesis, than low-multiplicity dl1403 infections.
FIG. 5.
Efficient phosphorylation of p53 at Ser15 requires ICP4 expression and its DNA binding activity, but not ICP27 expression. HFFF-2 cells were either mock infected or infected with HSV-1 (strain 17+) and an HSV-1 mutant that fails to express ICP4 (in1411/ΔICP4) (A), a temperature-sensitive ICP4 mutant (tsK) (B), or an HSV-1 mutant that fails to express ICP27 (17+27-pR19lacZ/ΔICP27) (C) at an MOI of 1 PFU/cell. The cells were incubated at 37°C, unless stated otherwise, and were harvested at the indicated time points. The cell extracts were then analyzed by Western blotting with the appropriate anti-ICP0, -ICP4, -ICP27, -p53, and -actin monoclonal antibodies and a phospho-specific p53-Ser15 rabbit polyclonal antibody.
To determine if p53 metabolism was affected by the DNA binding activity of ICP4, and consequently by its ability to transactivate viral gene expression, we monitored the phosphorylation and stabilization of p53 after an infection with a temperature-sensitive tsK mutant virus that expresses a form of ICP4 that fails to bind to DNA at the nonpermissive temperature of 38.5°C (62) (Fig. 5B). Infection with tsK at the permissive temperature of 32°C resulted in the phosphorylation of p53 at Ser15 to an extent that was comparable to that in previous wt HSV-1 infections (for a comparison, see Fig. 5A). In contrast, cells infected with tsK at the nonpermissive temperature showed levels of p53 phosphorylation at Ser15 that were reduced and similar to those in in1411 infections. Therefore, the efficiency of phosphorylation of p53 at Ser15 correlates more with the ability of ICP4 to induce viral early gene expression than with any direct effect of ICP4 on p53 itself. This result is consistent with the reduced levels of p53 phosphorylation during the infection of HFFF-2 cells with HSV-1 ICP0 mutants M1 and D12 (Fig. 4A).
We next tested any possible effect of ICP27 on p53 metabolism by infecting cells with the HSV-1 mutant 17+27-pR19lacZ (ΔICP27) (Fig. 5C). The lack of ICP27 expression did not affect the rate of phosphorylation or the stabilization of p53 compared to those in wt HSV-1-infected cells.
Taken together, these data indicate that rather than being direct effects of any of the individual IE proteins tested, the stabilization and phosphorylation of p53 appear to be independent mechanisms that correlate with the extent and activity of viral IE and early gene expression, respectively. Although it remains possible that another specific IE or early gene product has a direct effect upon p53, the in1411 data imply that no early gene product is essential for p53 stabilization. Thus, the stabilization of p53 is induced during the onset of IE gene expression, but changes in the p53 phosphorylation status may be a consequence of a developing viral infection and the intracellular changes that ensue.
Phosphorylation of p53 at Ser15 and Ser20 requires ATM but is not essential for its stabilization.
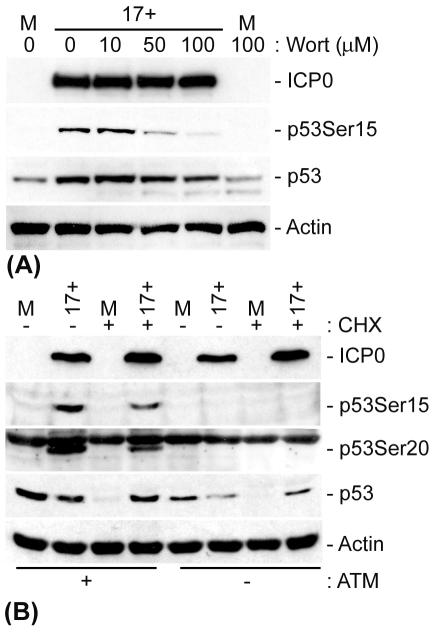
Several phosphatidylinositol 3-kinases (PI-3 kinases), including DNA-PK, ATM, and ATR, have been implicated in the phosphorylation of p53, thereby promoting its stabilization in response to DNA damage (for a review, see reference 43). In order to determine if a PI-3 kinase was responsible for the phosphorylation of p53 in response to HSV-1 infection and if this phosphorylation was required for p53 stabilization, we infected cells in the presence of increasing concentrations of wortmannin, a nonspecific PI-3 kinase inhibitor (Fig. 6A). Wortmannin inhibited the phosphorylation of p53 at Ser15 in a dose-dependent manner while having no effect on HSV-1 IE (ICP0) gene expression levels. These data therefore implicate a PI-3 kinase in the HSV-1-induced phosphorylation of p53 at Ser15. However, consistent with the in1411 infection data, the decrease in p53 Ser15 phosphorylation did not eliminate the increase in p53 stabilization during infection. Since ICP0 has been shown to degrade the catalytic subunit of the PI-3 kinase DNA-PK (44, 60), we decided to analyze the role of the PI-3 kinase ATM in p53 phosphorylation during an HSV-1 infection. AT−/− (GM02052E) and AT+/+ (GM03491) cells were infected with HSV-1, and the stability of p53 was analyzed by Western blotting (Fig. 6B). In the absence of ATM, no phosphorylation of p53 at Ser15 or Ser20 could be detected during HSV-1 infection. In contrast, ATM+/+ control cells showed a phosphorylation profile similar to that of infected HFFF-2 cells, with p53 becoming phosphorylated at both Ser15 and Ser20. These data indicate that ATM is required, either directly or indirectly, for the phosphorylation of p53 at Ser15 and Ser20 during infection. Despite the lack of Ser15 and Ser20 phosphorylation, CHX treatment of infected AT−/− cells demonstrated that HSV-1 still induced the stabilization of p53 compared to uninfected cells. In contrast to wt HSV-1 infections of HFFF-2 cells, for which we routinely observed increases in total p53 levels, such infections of AT+/+ and AT−/− cells did not appear to increase the amount of p53 (Fig. 6B). Total p53 levels are a consequence of the relative rates of synthesis and degradation of p53, both of which may be affected by HSV-1 infection in a cell-type-dependent manner. The effect of HSV-1 on the balance of these pathways may be insufficient to cause an increase in total p53 in the AT cells, but nonetheless p53 must have become stabilized since it was retained despite the inhibition of new synthesis by CHX.
FIG. 6.
ATM is required for HSV-1-induced phosphorylation of p53 at Ser15 and Ser20 but is not essential for p53 stabilization. (A) HFFF-2 cells were pretreated with various concentrations (as indicated) of wortmannin (Wort) 1 h prior to infection. The cells were subsequently mock infected or infected with HSV-1 (strain 17+) at an MOI of 1 PFU/cell for 8 h before being harvested. (B) AT−/− or AT+/+ cells were either mock infected or infected with HSV-1 (strain 17+) for 8 h before being harvested or treated with CHX (final concentration, 10 μg/ml), as indicated, for an additional 10 h prior to harvesting. The cell extracts were then analyzed by Western blotting with anti-ICP0, -p53, and -actin monoclonal antibodies and phospho-specific p53-Ser15 and p53-Ser20 rabbit polyclonal antibodies.
Therefore, the phosphorylation of p53 at Ser15 or Ser20 induced by ATM in response to HSV-1 infection is not solely responsible for p53 stabilization. These results are consistent with the data from in1411 infections (Fig. 5A) and tsK infections carried out at the nonpermissive temperature (Fig. 5B) and with the effect of wortmannin (Fig. 6A), all of which showed a reduced phosphorylation of p53 at Ser15 without compromising the increased p53 stability induced by HSV-1 infection. Therefore, additional mechanisms are responsible for, or at least contribute to, the stabilization of p53 during HSV-1 infection.
HSV-1 infection of U2OS cells fails to induce stabilization of p53.
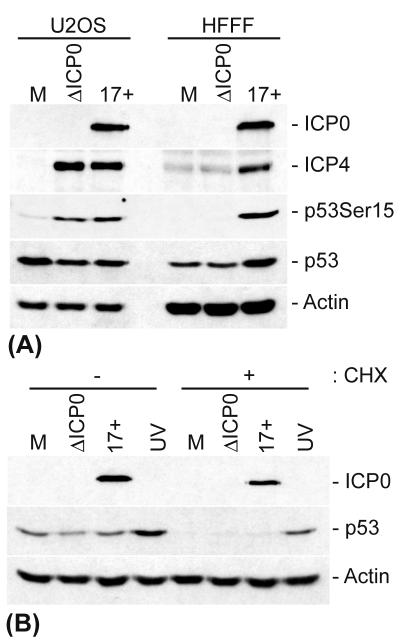
During the course of these experiments, we noted that HSV-1 strain 17+ and ICP0 null mutant dl1403 caused similar increases in p53 Ser15 phosphorylation (and ICP4 expression) during low-MOI infections of U2OS cells, which also express wt p53, confirming that ICP0 is not required for the promotion of phosphorylation of p53 at Ser15. However, the total levels of p53 were reduced during infection of this cell type in the presence or absence of ICP0 (Fig. 7A), contrasting with the increased p53 levels seen in wt HSV-1-infected HFFF-2 cells. Since wt HSV-1 and dl1403 gave similar results in U2OS cells, these data indicate that the reduction in p53 levels in these infected cells cannot be attributed to the ability of ICP0 to ubiquitinate p53 (9) during this infection situation. Instead, the decreased levels of p53 in HSV-1-infected U2OS cells were concordant with the failure to induce the stabilization of p53 that was observed in infected HFFF-2 cells. Thus, while a CHX treatment revealed an increased stability of p53 in infected HFFF-2 cells (Fig. 1C and 4B), infected and CHX-treated U2OS cells showed no stabilization of p53 during an HSV-1 or dl1403 infection (Fig. 7B). As a control, U2OS cells showed a significant increase in p53 stability after UV irradiation, indicating that these cells can induce the stabilization of p53 after DNA damage. These data imply that the mechanism by which p53 is stabilized during HSV-1 infections of HFFF-2 cells is absent from or fails to be initiated during infections of U2OS cells and differs from that induced by UV irradiation in the latter cell type. Since p53 was phosphorylated but not stabilized during the infection of U2OS cells, these results provide further evidence to suggest that N-terminal phosphorylation, and more specifically Ser15 phosphorylation, is neither responsible nor required for p53 stabilization during HSV-1 infection. Furthermore, as a productive HSV-1 infection of U2OS cells failed to induce p53 stabilization, it is clear that no single viral protein alone and of itself is responsible for this effect.
FIG. 7.
HSV-1 infection of U2OS cells fails to induce stabilization of p53. (A) U2OS and HFFF-2 cells were mock infected or infected with either HSV-1 (strain 17+) or an HSV-1 IE-1 mutant that fails to express ICP0 (dl1403/ΔICP0) at an MOI of 1 PFU/cell for 8 h before being harvested. (B) U2OS cells were infected as described above or were UV irradiated and harvested at 8 h posttreatment or treated with CHX (final concentration, 10 μg/ml), as indicated, for an additional 10 h prior to being harvested. The cell extracts were then analyzed by Western blotting with anti-ICP0, -ICP4, -p53, and -actin monoclonal antibodies and a phospho-specific p53-Ser15 rabbit polyclonal antibody.
DISCUSSION
The major oncoprotein p53 is a key regulator in the stress response and controls many important pathways involved in growth arrest, apoptosis, and senescence (for a review, see reference 76). Levels of p53 in unstressed cells are maintained at a low level through ubiquitination and proteasome-mediated degradation (31, 32, 37, 51, 63). Consequently, the stabilization of p53 is a critical factor in its ability to initiate a rapid response to stress or DNA damage through the transcriptional activation of p53-responsive genes. Since a viral infection often induces both cellular stress and DNA damage, these activities of p53 are significant during the replication of many viruses. For example, if a virus is unable to inhibit p53 responses, the infected cell may be subjected to processes that are detrimental to viral replication, e.g., the induction of apoptosis.
Although it is becoming increasingly clear that many viruses, including several herpesviruses, directly affect the transcriptional activity of p53, there is limited information regarding the fate of p53 during alphaherpesvirus infections. We recently reported that the HSV-1 IE protein ICP0 can directly interact with and ubiquitinate a proportion of p53 molecules in a USP7-independent manner (9). Moreover, ICP0 expression in the context of an HSV-1 infection was sufficient to protect U2OS cells from UV-induced apoptosis. These data suggest that ICP0 can directly affect p53 stability and/or p53-mediated pathways during infection. However, during the course of this study, we noted that HSV-1 infections of HFFF-2 cells, a nontransformed cell line expressing wt p53, resulted in an increase in p53 levels. Therefore, any potential destabilization of p53 by ICP0 during the infection of these cells must be secondary to virus-induced mechanisms that result in the stabilization of p53. Accordingly, we analyzed the potential mechanisms by which p53 becomes stabilized during HSV-1 infection. In particular, we investigated whether the ability of ICP0 to recruit USP7, a ubiquitin-specific protease known to stabilize p53 (46), had any direct effect upon the stabilization of p53 during infection.
The increase in p53 levels during lytic HSV-1 infections correlated with an increase in p53 stability. While microarray studies have indicated that p53 transcription is not increased during a normal lytic infection (72), we note that an HSV-1 virus with defects in several IE genes has been shown to increase the transcription of both p53 and p53-responsive genes after the prolonged expression of ICP0 in the absence of other IE proteins (36). However, the infections in these previous studies were carried out at a high MOI for extended periods of nonproductive infection (typically 12 to 48 h). Here we addressed the level of p53 expression during typical low-MOI productive infections. Under these conditions, we found that the increase in p53 expression was due to p53 stabilization. We found that de novo HSV-1 IE protein expression was necessary to induce p53 stabilization and phosphorylation and that virion-receptor binding, virion uncoating, the release of associated virion proteins, viral DNA nuclear import, and the transcription of IE genes were not responsible for the stabilization of p53. Indeed, a similar stabilization and phosphorylation of p53 have been observed for a human cytomegalovirus infection (28, 29; Lee Fortunato, personal communication). Although the infection of HFFF-2 cells with an HSV-1 ICP0 null mutant virus at a low MOI did not lead to the stabilization of p53, this was not a direct effect of ICP0, but a consequence of the requirement for ICP0 to stimulate viral gene expression in low-MOI infections (20, 21). The infection of HFFF-2 cells at a high MOI with dl1403 resulted in p53 stabilization, demonstrating that neither ICP0 nor its ability to recruit USP7 is directly responsible or required for p53 stabilization or phosphorylation. Moreover, infections carried out in U2OS cells, which express wt p53, demonstrated that p53 was not stabilized in either the presence or absence of ICP0 or any additional viral gene product (Fig. 7B). Taken together with the results of the studies using the ICP4 and ICP27 mutant viruses, these data clearly indicate that neither individual viral IE proteins nor the recruitment of USP7 by ICP0 is directly involved in p53 stabilization. Thus, the stabilization of p53 during a productive HSV-1 infection appears to be a result of viral IE protein expression in general, which triggers a cellular response that inhibits the ubiquitination and proteasome-mediated turnover of p53, as opposed to any specific viral protein mediating its stabilization through sequestration or de-ubiquitination. This cellular response is augmented as the infection progresses to early protein expression.
The stabilization of p53 after DNA damage can occur through a number of mechanisms involving the posttranslational modification of p53. Several cellular kinases, including ATM, ATR, DNA-PK, Chk1, and Chk2, have been shown to phosphorylate N-terminal p53 serine residues that have been implicated in the stabilization of p53 by the blocking of its interaction with its negative regulator Mdm2. Although the HSV-1-induced stabilization of p53 appeared in our initial experiments to correlate with its phosphorylation at N-terminal serine residues, a more detailed examination suggested that these events were not necessarily linked. The phosphorylation of p53 occurred prior to the onset of viral DNA replication, indicating that it did not occur due to the replicating viral DNA triggering a DNA damage response. Although the phosphorylation of both Ser15 and Ser20 has been implicated in the stabilization of p53 in response to DNA damage (6, 41, 68, 69), infections carried out in AT−/− cells showed that these phosphorylation events, although they required ATM, were not essential for the stabilization of p53 in response to an HSV-1 infection (Fig. 6B). Indeed, similar findings have been observed concerning the stabilization of p53 during human cytomegalovirus infection (Lee Fortunato, personal communication). Since ATM has also been implicated in the phosphorylation of Mdm2 (15, 42), an event that in turn also contributes to the stabilization of p53, these data indicate that the stabilization of p53 during HSV-1 infection is not directly linked to these phosphorylation events and that other mechanisms must also be involved. Indeed, since HSV-1 infection can induce p53 phosphorylation without concomitant stabilization (in U2OS cells) and stabilization without N-terminal serine phosphorylation (in AT−/− cells), it appears that these two processes may be triggered by independent mechanisms during HSV-1 infection.
The sequestration of Mdm2 and the Mdmx-mediated sequestration of p53, as well other posttranslational modifications of p53, e.g., the acetylation of C-terminal lysine residues, have been implicated in the stabilization of p53. Although these mechanisms have not been directly addressed in this paper, it is likely that one or more of these factors are required for the stabilization of p53 during an HSV-1 infection. Interestingly, the EBV protein BZLF1, a DNA binding IE protein that is required for the efficient onset of EBV lytic replication and the reactivation of latent EBV genomes, has been shown to have a direct effect on the posttranslational modification status of p53, inducing extensive phosphorylation of N-terminal residues as well as the acetylation of C-terminal lysine residues (52). It is therefore likely that p53 undergoes similar multiple modifications during the course of an HSV-1 infection. If so, it is unlikely that one particular modification is directly responsible for p53 stabilization, but rather that each modification (and potentially the initiation of other pathways [for example, the sequestration of Mdm2 or p53 by Mdmx]) contributes to the overall increase in the stability of p53 during infection. During the course of this work, we also investigated the phosphorylation of p53 at serine residues 46 and 392. Of these, only serine 46 had a significant modification during HSV-1 infection (data not shown); the phosphorylation of this residue has been implicated in p53's ability to induce apoptosis after DNA damage (58). We attempted to investigate p53 acetylation at lysine residues 320 and 373, but these experiments failed for technical reasons. Unfortunately, given the myriad of modifications, proteins, and pathways that affect p53, it is impractical to investigate all of the possible mechanisms for p53 stabilization during HSV-1 infection.
The results presented in this paper demonstrate that several aspects of p53 metabolism are affected during HSV-1 infections of cultured cells. The extent and consequences of these effects on p53 vary between different cell types, implying that viral gene expression modulates several pathways that impinge upon p53. While the results were clear and highly reproducible, the overall biological significance for the cell and for viral infections was less apparent. The stabilization of p53 does not appear to increase p53-responsive gene expression in HFFF-2 cells infected with wt HSV-1 under the conditions of our experiments, since neither p21 nor GADD45 showed increased levels of expression, in contrast to the substantial increase in both proteins that was seen after the UV irradiation of HFFF-2 cells (data not shown). Some viruses appear to target p53 to inhibit apoptotic responses. While this may be true of HSV-1 in certain situations, HSV-1 also expresses many other defenses against a variety of steps in the apoptotic pathways (for a review, see reference 2). A DNA damage response via stabilized p53 and the reported p53 recruitment into replication compartments could in principle have effects on the efficiency of viral DNA replication. However, no obvious differences in HSV-1 replication in p53-positive or -negative cells have been reported to date. Given that a productive HSV-1 infection causes such widespread and drastic changes to the cell status, it is perhaps unsurprising that a cellular protein that plays important roles in the cellular stress response is affected by viral replication. It is likely that the effects that we have observed are significant for as yet undefined aspects of natural HSV-1 infections.
Acknowledgments
We are very grateful for our informative and constructive discussions with Lee Fortunato (Department of Microbiology, Molecular Biology and Biochemistry, University of Idaho) and for the constructive comments made by Duncan McGeoch during the preparation of the manuscript.
This work was supported by the Medical Research Council and the Human Frontiers Science Programme (a fellowship awarded to C.B. to work in the laboratory of R.D.E.).
REFERENCES
- 1.Ackermann, M., D. K. Braun, L. Pereira, and B. Roizman. 1984. Characterization of herpes simplex virus 1 alpha proteins 0, 4, and 27 with monoclonal antibodies. J. Virol. 52:108-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aubert, M., and J. A. Blaho. 2001. Modulation of apoptosis during herpes simplex virus infection in human cells. Microbes Infect. 3:859-866. [DOI] [PubMed] [Google Scholar]
- 3.Band, V., S. Dalal, L. Delmolino, and E. J. Androphy. 1993. Enhanced degradation of p53 protein in HPV-6 and BPV-1 E6-immortalized human mammary epithelial cells. EMBO J. 12:1847-1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Banks, L., D. Pim, and M. Thomas. 2003. Viruses and the 26S proteasome: hacking into destruction. Trends Biochem. Sci. 28:452-459. [DOI] [PubMed] [Google Scholar]
- 5.Bargonetti, J., P. N. Friedman, S. E. Kern, B. Vogelstein, and C. Prives. 1991. Wild-type but not mutant p53 immunopurified proteins bind to sequences adjacent to the SV40 origin of replication. Cell 65:1083-1091. [DOI] [PubMed] [Google Scholar]
- 6.Bean, L. J., and G. R. Stark. 2002. Regulation of the accumulation and function of p53 by phosphorylation of two residues within the domain that binds to Mdm2. J. Biol. Chem. 277:1864-1871. [DOI] [PubMed] [Google Scholar]
- 7.Beard, P., S. Faber, K. W. Wilcox, and L. I. Pizer. 1986. Herpes simplex virus immediate early infected-cell polypeptide 4 binds to DNA and promotes transcription. Proc. Natl. Acad. Sci. USA 83:4016-4020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bech-Otschir, D., R. Kraft, X. Huang, P. Henklein, B. Kapelari, C. Pollmann, and W. Dubiel. 2001. COP9 signalosome-specific phosphorylation targets p53 to degradation by the ubiquitin system. EMBO J. 20:1630-1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boutell, C., and R. D. Everett. 2003. The herpes simplex virus type 1 (HSV-1) regulatory protein ICP0 interacts with and ubiquitinates p53. J. Biol. Chem. 278:36596-36602. [DOI] [PubMed] [Google Scholar]
- 10.Boutell, C., A. Orr, and R. D. Everett. 2003. PML residue lysine 160 is required for the degradation of PML induced by herpes simplex virus type 1 regulatory protein ICP0. J. Virol. 77:8686-8694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boutell, C., S. Sadis, and R. D. Everett. 2002. Herpes simplex virus type 1 immediate-early protein ICP0 and its isolated RING finger domain act as ubiquitin E3 ligases in vitro. J. Virol. 76:841-850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brooks, C. L., and W. Gu. 2003. Ubiquitination, phosphorylation and acetylation: the molecular basis for p53 regulation. Curr. Opin. Cell Biol. 15:164-171. [DOI] [PubMed] [Google Scholar]
- 13.Chelbi-Alix, M. K., and H. de The. 1999. Herpes virus induced proteasome-dependent degradation of the nuclear bodies-associated PML and Sp100 proteins. Oncogene 18:935-941. [DOI] [PubMed] [Google Scholar]
- 14.DeLuca, N. A., A. M. McCarthy, and P. A. Schaffer. 1985. Isolation and characterization of deletion mutants of herpes simplex virus type 1 in the gene encoding immediate-early regulatory protein ICP4. J. Virol. 56:558-570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Toledo, S. M., E. I. Azzam, W. K. Dahlberg, T. B. Gooding, and J. B. Little. 2000. ATM complexes with HDM2 and promotes its rapid phosphorylation in a p53-independent manner in normal and tumor human cells exposed to ionizing radiation. Oncogene 19:6185-6193. [DOI] [PubMed] [Google Scholar]
- 16.Everett, R., A. Cross, J. Tyler, and A. Orr. 1993. An epitope within the DNA-binding domain of the herpes simplex virus immediate early protein Vmw175 is conserved in the varicella-zoster virus gene 62 protein. J. Gen. Virol. 74:1955-1958. [DOI] [PubMed] [Google Scholar]
- 17.Everett, R. D. 1988. Analysis of the functional domains of herpes simplex virus type 1 immediate-early polypeptide Vmw110. J. Mol. Biol. 202:87-96. [DOI] [PubMed] [Google Scholar]
- 18.Everett, R. D. 1989. Construction and characterization of herpes simplex virus type 1 mutants with defined lesions in immediate early gene 1. J. Gen. Virol. 70:1185-1202. [DOI] [PubMed] [Google Scholar]
- 19.Everett, R. D. 2000. ICP0 induces the accumulation of colocalizing conjugated ubiquitin. J. Virol. 74:9994-10005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Everett, R. D. 2000. ICP0, a regulator of herpes simplex virus during lytic and latent infection. Bioessays 22:761-770. [DOI] [PubMed] [Google Scholar]
- 21.Everett, R. D., C. Boutell, and A. Orr. 2004. Phenotype of a herpes simplex virus type 1 mutant that fails to express immediate-early regulatory protein ICP0. J. Virol. 78:1763-1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Everett, R. D., W. C. Earnshaw, J. Findlay, and P. Lomonte. 1999. Specific destruction of kinetochore protein CENP-C and disruption of cell division by herpes simplex virus immediate-early protein Vmw110. EMBO J. 18:1526-1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Everett, R. D., P. Freemont, H. Saitoh, M. Dasso, A. Orr, M. Kathoria, and J. Parkinson. 1998. The disruption of ND10 during herpes simplex virus infection correlates with the Vmw110- and proteasome-dependent loss of several PML isoforms. J. Virol. 72:6581-6591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Everett, R. D., M. Meredith, and A. Orr. 1999. The ability of herpes simplex virus type 1 immediate-early protein Vmw110 to bind to a ubiquitin-specific protease contributes to its roles in the activation of gene expression and stimulation of virus replication. J. Virol. 73:417-426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Everett, R. D., M. Meredith, A. Orr, A. Cross, M. Kathoria, and J. Parkinson. 1997. A novel ubiquitin-specific protease is dynamically associated with the PML nuclear domain and binds to a herpesvirus regulatory protein. EMBO J. 16:1519-1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Everett, R. D., A. Orr, and M. Elliott. 1991. High level expression and purification of herpes simplex virus type 1 immediate early polypeptide Vmw110. Nucleic Acids Res. 19:6155-6161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Everett, R. D., A. Orr, and C. M. Preston. 1998. A viral activator of gene expression functions via the ubiquitin- proteasome pathway. EMBO J. 17:7161-7169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fortunato, E. A., and D. H. Spector. 1998. p53 and RPA are sequestered in viral replication centers in the nuclei of cells infected with human cytomegalovirus. J. Virol. 72:2033-2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fortunato, E. A., and D. H. Spector. 2003. Viral induction of site-specific chromosome damage. Rev. Med. Virol. 13:21-37. [DOI] [PubMed] [Google Scholar]
- 30.Friborg, J., Jr., W. Kong, M. O. Hottiger, and G. J. Nabel. 1999. p53 inhibition by the LANA protein of KSHV protects against cell death. Nature 402:889-894. [DOI] [PubMed] [Google Scholar]
- 31.Fuchs, S. Y., V. Adler, T. Buschmann, X. Wu, and Z. Ronai. 1998. Mdm2 association with p53 targets its ubiquitination. Oncogene 17:2543-2547. [DOI] [PubMed] [Google Scholar]
- 32.Grossman, S. R., M. E. Deato, C. Brignone, H. M. Chan, A. L. Kung, H. Tagami, Y. Nakatani, and D. M. Livingston. 2003. Polyubiquitination of p53 by a ubiquitin ligase activity of p300. Science 300:342-344. [DOI] [PubMed] [Google Scholar]
- 33.Gu, H., and B. Roizman. 2003. The degradation of promyelocytic leukemia and Sp100 proteins by herpes simplex virus 1 is mediated by the ubiquitin-conjugating enzyme UbcH5a. Proc. Natl. Acad. Sci. USA 100:8963-8968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hagglund, R., C. Van Sant, P. Lopez, and B. Roizman. 2002. Herpes simplex virus 1-infected cell protein 0 contains two E3 ubiquitin ligase sites specific for different E2 ubiquitin-conjugating enzymes. Proc. Natl. Acad. Sci. USA 99:631-636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hay, T. J., and D. W. Meek. 2000. Multiple sites of in vivo phosphorylation in the MDM2 oncoprotein cluster within two important functional domains. FEBS Lett. 478:183-186. [DOI] [PubMed] [Google Scholar]
- 36.Hobbs, W. E., II, and N. A. DeLuca. 1999. Perturbation of cell cycle progression and cellular gene expression as a function of herpes simplex virus ICP0. J. Virol. 73:8245-8255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Honda, R., H. Tanaka, and H. Yasuda. 1997. Oncoprotein MDM2 is a ubiquitin ligase E3 for tumor suppressor p53. FEBS Lett. 420:25-27. [DOI] [PubMed] [Google Scholar]
- 38.Ito, A., Y. Kawaguchi, C. H. Lai, J. J. Kovacs, Y. Higashimoto, E. Appella, and T. P. Yao. 2002. MDM2-HDAC1-mediated deacetylation of p53 is required for its degradation. EMBO J. 21:6236-6245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jackson, M. W., and S. J. Berberich. 2000. MdmX protects p53 from Mdm2-mediated degradation. Mol. Cell. Biol. 20:1001-1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kamijo, T., J. D. Weber, G. Zambetti, F. Zindy, M. F. Roussel, and C. J. Sherr. 1998. Functional and physical interactions of the ARF tumor suppressor with p53 and Mdm2. Proc. Natl. Acad. Sci. USA 95:8292-8297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kapoor, M., R. Hamm, W. Yan, Y. Taya, and G. Lozano. 2000. Cooperative phosphorylation at multiple sites is required to activate p53 in response to UV radiation. Oncogene 19:358-364. [DOI] [PubMed] [Google Scholar]
- 42.Khosravi, R., R. Maya, T. Gottlieb, M. Oren, Y. Shiloh, and D. Shkedy. 1999. Rapid ATM-dependent phosphorylation of MDM2 precedes p53 accumulation in response to DNA damage. Proc. Natl. Acad. Sci. USA 96:14973-14977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lakin, N. D., and S. P. Jackson. 1999. Regulation of p53 in response to DNA damage. Oncogene 18:7644-7655. [DOI] [PubMed] [Google Scholar]
- 44.Lees-Miller, S. P., M. C. Long, M. A. Kilvert, V. Lam, S. A. Rice, and C. A. Spencer. 1996. Attenuation of DNA-dependent protein kinase activity and its catalytic subunit by the herpes simplex virus type 1 transactivator ICP0. J. Virol. 70:7471-7477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Leng, R. P., Y. Lin, W. Ma, H. Wu, B. Lemmers, S. Chung, J. M. Parant, G. Lozano, R. Hakem, and S. Benchimol. 2003. Pirh2, a p53-induced ubiquitin-protein ligase, promotes p53 degradation. Cell 112:779-791. [DOI] [PubMed] [Google Scholar]
- 46.Li, M., D. Chen, A. Shiloh, J. Luo, A. Y. Nikolaev, J. Qin, and W. Gu. 2002. Deubiquitination of p53 by HAUSP is an important pathway for p53 stabilization. Nature 416:648-653. [DOI] [PubMed] [Google Scholar]
- 47.Li, M., J. Luo, C. L. Brooks, and W. Gu. 2002. Acetylation of p53 inhibits its ubiquitination by Mdm2. J. Biol. Chem. 277:50607-50611. [DOI] [PubMed] [Google Scholar]
- 48.Lilley, C. E., F. Groutsi, Z. Han, J. A. Palmer, P. N. Anderson, D. S. Latchman, and R. S. Coffin. 2001. Multiple immediate-early gene-deficient herpes simplex virus vectors allowing efficient gene delivery to neurons in culture and widespread gene delivery to the central nervous system in vivo. J. Virol. 75:4343-4356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lohrum, M. A., R. L. Ludwig, M. H. Kubbutat, M. Hanlon, and K. H. Vousden. 2003. Regulation of HDM2 activity by the ribosomal protein L11. Cancer Cell 3:577-587. [DOI] [PubMed] [Google Scholar]
- 50.Lomonte, P., K. F. Sullivan, and R. D. Everett. 2001. Degradation of nucleosome-associated centromeric histone H3-like protein CENP-A induced by herpes simplex virus type 1 protein ICP0. J. Biol. Chem. 276:5829-5835. [DOI] [PubMed] [Google Scholar]
- 51.Maki, C. G., J. M. Huibregtse, and P. M. Howley. 1996. In vivo ubiquitination and proteasome-mediated degradation of p53(1). Cancer Res. 56:2649-2654. [PubMed] [Google Scholar]
- 52.Mauser, A., S. Saito, E. Appella, C. W. Anderson, W. T. Seaman, and S. Kenney. 2002. The Epstein-Barr virus immediate-early protein BZLF1 regulates p53 function through multiple mechanisms. J. Virol. 76:12503-12512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mayo, L. D., J. J. Turchi, and S. J. Berberich. 1997. Mdm-2 phosphorylation by DNA-dependent protein kinase prevents interaction with p53. Cancer Res. 57:5013-5016. [PubMed] [Google Scholar]
- 54.Meredith, M., A. Orr, M. Elliott, and R. Everett. 1995. Separation of sequence requirements for HSV-1 Vmw110 multimerisation and interaction with a 135-kDa cellular protein. Virology 209:174-187. [DOI] [PubMed] [Google Scholar]
- 55.Meredith, M., A. Orr, and R. Everett. 1994. Herpes simplex virus type 1 immediate-early protein Vmw110 binds strongly and specifically to a 135-kDa cellular protein. Virology 200:457-469. [DOI] [PubMed] [Google Scholar]
- 56.Muller, M. T. 1987. Binding of the herpes simplex virus immediate-early gene product ICP4 to its own transcription start site. J. Virol. 61:858-865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Muller, S., and A. Dejean. 1999. Viral immediate-early proteins abrogate the modification by SUMO-1 of PML and Sp100 proteins, correlating with nuclear body disruption. J. Virol. 73:5137-5143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Oda, K., H. Arakawa, T. Tanaka, K. Matsuda, C. Tanikawa, T. Mori, H. Nishimori, K. Tamai, T. Tokino, Y. Nakamura, and Y. Taya. 2000. p53AIP1, a potential mediator of p53-dependent apoptosis, and its regulation by Ser-46-phosphorylated p53. Cell 102:849-862. [DOI] [PubMed] [Google Scholar]
- 59.Parkinson, J., and R. D. Everett. 2000. Alphaherpesvirus proteins related to herpes simplex virus type 1 ICP0 affect cellular structures and proteins. J. Virol. 74:10006-10017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Parkinson, J., S. P. Lees-Miller, and R. D. Everett. 1999. Herpes simplex virus type 1 immediate-early protein Vmw110 induces the proteasome-dependent degradation of the catalytic subunit of DNA-dependent protein kinase. J. Virol. 73:650-657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pomerantz, J., N. Schreiber-Agus, N. J. Liegeois, A. Silverman, L. Alland, L. Chin, J. Potes, K. Chen, I. Orlow, H. W. Lee, C. Cordon-Cardo, and R. A. DePinho. 1998. The Ink4a tumor suppressor gene product, p19Arf, interacts with MDM2 and neutralizes MDM2's inhibition of p53. Cell 92:713-723. [DOI] [PubMed] [Google Scholar]
- 62.Preston, C. M. 1979. Control of herpes simplex virus type 1 mRNA synthesis in cells infected with wild-type virus or the temperature-sensitive mutant tsK. J. Virol. 29:275-284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rodriguez, M. S., J. M. Desterro, S. Lain, D. P. Lane, and R. T. Hay. 2000. Multiple C-terminal lysine residues target p53 for ubiquitin-proteasome-mediated degradation. Mol. Cell. Biol. 20:8458-8467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Roizman, B., and D. M. Knipe. 2001. Herpes simplex viruses and their replication, 4th ed. Lippincott Williams and Wilkins, Philadelphia, Pa.
- 65.Russell, J., E. C. Stow, N. D. Stow, and C. M. Preston. 1987. Abnormal forms of the herpes simplex virus immediate early polypeptide Vmw175 induce the cellular stress response. J. Gen. Virol. 68:2397-2406. [DOI] [PubMed] [Google Scholar]
- 66.Scheffner, M., J. M. Huibregtse, R. D. Vierstra, and P. M. Howley. 1993. The HPV-16 E6 and E6-AP complex functions as a ubiquitin-protein ligase in the ubiquitination of p53. Cell 75:495-505. [DOI] [PubMed] [Google Scholar]
- 67.Scheffner, M., B. A. Werness, J. M. Huibregtse, A. J. Levine, and P. M. Howley. 1990. The E6 oncoprotein encoded by human papillomavirus types 16 and 18 promotes the degradation of p53. Cell 63:1129-1136. [DOI] [PubMed] [Google Scholar]
- 68.Schon, O., A. Friedler, M. Bycroft, S. M. Freund, and A. R. Fersht. 2002. Molecular mechanism of the interaction between MDM2 and p53. J. Mol. Biol. 323:491-501. [DOI] [PubMed] [Google Scholar]
- 69.Shieh, S. Y., M. Ikeda, Y. Taya, and C. Prives. 1997. DNA damage-induced phosphorylation of p53 alleviates inhibition by MDM2. Cell 91:325-334. [DOI] [PubMed] [Google Scholar]
- 70.Shvarts, A., W. T. Steegenga, N. Riteco, T. van Laar, P. Dekker, M. Bazuine, R. C. van Ham, W. van der Houven van Oordt, G. Hateboer, A. J. van der Eb, and A. G. Jochemsen. 1996. MDMX: a novel p53-binding protein with some functional properties of MDM2. EMBO J. 15:5349-5357. [PMC free article] [PubMed] [Google Scholar]
- 71.Song, J. Y., J. W. Lim, H. Kim, T. Morio, and K. H. Kim. 2003. Oxidative stress induces nuclear loss of DNA repair proteins Ku70 and Ku80 and apoptosis in pancreatic acinar AR42J cells. J. Biol. Chem. 278:36676-36687. [DOI] [PubMed] [Google Scholar]
- 72.Stingley, S. W., J. J. Ramirez, S. A. Aguilar, K. Simmen, R. M. Sandri-Goldin, P. Ghazal, and E. K. Wagner. 2000. Global analysis of herpes simplex virus type 1 transcription using an oligonucleotide-based DNA microarray. J. Virol. 74:9916-9927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Stott, F. J., S. Bates, M. C. James, B. B. McConnell, M. Starborg, S. Brookes, I. Palmero, K. Ryan, E. Hara, K. H. Vousden, and G. Peters. 1998. The alternative product from the human CDKN2A locus, p14(ARF), participates in a regulatory feedback loop with p53 and MDM2. EMBO J. 17:5001-5014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Stow, N. D., and E. C. Stow. 1986. Isolation and characterization of a herpes simplex virus type 1 mutant containing a deletion within the gene encoding the immediate early polypeptide Vmw110. J. Gen. Virol. 67:2571-2585. [DOI] [PubMed] [Google Scholar]
- 75.Vousden, K. H. 2002. Activation of the p53 tumor suppressor protein. Biochim. Biophys. Acta 1602:47-59. [DOI] [PubMed] [Google Scholar]
- 76.Vousden, K. H. 2000. p53: death star. Cell 103:691-694. [DOI] [PubMed] [Google Scholar]
- 77.Vousden, K. H., and X. Lu. 2002. Live or let die: the cell's response to p53. Nat. Rev. Cancer 2:594-604. [DOI] [PubMed] [Google Scholar]
- 78.Wilcock, D., and D. P. Lane. 1991. Localization of p53, retinoblastoma and host replication proteins at sites of viral replication in herpes-infected cells. Nature 349:429-431. [DOI] [PubMed] [Google Scholar]
- 79.Woods, D. B., and K. H. Vousden. 2001. Regulation of p53 function. Exp. Cell Res. 264:56-66. [DOI] [PubMed] [Google Scholar]
- 80.Xing, J., H. M. Sheppard, S. I. Corneillie, and X. Liu. 2001. p53 stimulates TFIID-TFIIA-promoter complex assembly, and p53-T-antigen complex inhibits TATA binding protein-TATA interaction. Mol. Cell. Biol. 21:3652-3661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yao, F., and P. A. Schaffer. 1995. An activity specified by the osteosarcoma line U2OS can substitute functionally for ICP0, a major regulatory protein of herpes simplex virus type 1. J. Virol. 69:6249-6258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhang, Q., D. Gutsch, and S. Kenney. 1994. Functional and physical interaction between p53 and BZLF1: implications for Epstein-Barr virus latency. Mol. Cell. Biol. 14:1929-1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhang, Y., Y. Xiong, and W. G. Yarbrough. 1998. ARF promotes MDM2 degradation and stabilizes p53: ARF-INK4a locus deletion impairs both the Rb and p53 tumor suppression pathways. Cell 92:725-734. [DOI] [PubMed] [Google Scholar]