Abstract
A hydrogen polysulfide mediated aziridine ring-opening reaction was discovered. Based on this reaction, a novel H2Sn-specific chemosensor (AP) was developed. AP showed high sensitivity and selectivity for H2Sn. Notably, the fluorescent turn-on product (1) exhibited excellent two-photon photophysical properties, a large Stokes shift, and high solid state luminescent efficiency.
Reactive sulfur species (RSS), including biothiols, hydrogen sulfide (H2S), sulfane sulfurs, and S-modified cysteine adducts (i.e., S-nitrosothiols, sulfenic acids, etc.), play important roles in redox biology.1−4 Among these species, hydrogen polysulfides (H2Sn, n > 1) have recently received particular attention as they are believed to be involved in H2S-mediated signaling transduction.5−10 Although H2S has been extensively studied as a new signaling molecule in the past decade, the fundamental chemistry and physiological function of H2Sn are still poorly understood. H2Sn belong to the sulfane sulfur family and have very unique chemistry. H2Sn can be derived from endogenous H2S by the action of reactive oxygen species,4,7,11−13 as redox partners of H2S. In this regard, H2Sn very likely coexist with H2S in vivo and they work together to regulate sulfur redox balance. Recent studies suggested that H2Sn might act as the real regulators in cellular signaling transduction.5−10 Some biological activities that were originally ascribed to H2S may actually be mediated by H2Sn. One such case is S-sulfhydration (i.e., conversion of protein cysteine residues (−SH) to persulfides (−S–SH)).4,14−20 This reaction was originally ascribed to H2S. However, H2Sn are recently found to be more effective than H2S in S-sulfhydration.6,7,12,13,21
The field of H2Sn is now rapidly growing, and more exciting biological activity exhibited by H2Sn are to be discovered. In order to better understand the roles of H2Sn, it is critical to develop efficient methods that can distinguish H2Sn from other reactive sulfur species, especially H2S and biothiols. This is still an underdeveloped field. So far the most commonly used method for H2Sn detection is to measure UV absorption at 290–300 and 370 nm.7 This low-sensitivity method is unsuitable for biological samples. Fluorescence-based methods could be ideal due to their rapid, sensitive fluorescent responses and spatiotemporal resolution capability.22,23 Our laboratory recently discovered a H2Sn-mediated aromatic substitution–cyclization and reported the first H2Sn-specific fluorescent sensors based on this reaction.24 The sensors utilize 2-fluoro-5-nitro-benzoic ester to trap H2Sn and release the fluorophores. Although the sensors proved to be highly selective for H2Sn, the competing reaction of the 2-fluoro-5-nitro-benzoic ester template with biothiols could cause the consumption of the sensors and higher sensor loading may be required. To solve this problem, further improvements of the sensor template or the discovery of novel H2Sn specific reaction templates would be needed. Herein we report a unique reaction between aziridines and H2Sn. Based on this reaction, a novel H2Sn-specific sensor (AP) was prepared and evaluated. The physical properties of the actual fluorescent species generated from the reaction of H2Sn were also studied.
In order to develop reaction-based fluorescent sensors for H2Sn detection, the key is to identify specific reactions that only react with H2Sn, but not react with biothiols such as glutathione (GSH) and cysteine (Cys) (which are ubiquitous in biological systems and concentrations can reach mM levels). The presence of H2S (at μM levels) should also be taken into consideration. Due to α-effects, H2Sn are expected to be weak acids. The estimated pKa values of H2Sn are in the range of 3 to 5.25,26 For comparison, the pKa values of H2S and biothiols are in the range of 7 to 9.2 (H2S, 7.0; Cys, 8.30; GSH, 9.2). Under physiological pH, H2Sn should be stronger and more reactive nucleophiles than biothiols and H2S. As such, our focus was on possible electrophiles which are reactive enough for H2Sn, but not reactive toward H2S and thiols.
Aziridines are popular electrophilic synthons for making amine-containing molecules. Ring-opening reactions of aziridines by many nucleophiles, including thiol-derivatives, have been reported.27−30 In most cases the reactions were for synthetic purposes. High concentrations of reactants, organic solvents/bases, long reaction times, and elevated temperatures are often employed. It should be noted that although those studies revealed good reactivity of aziridines toward certain nucleophiles such as thiols, whether or not such reactions could be used for the detection of thiols or related biological molecules are still unclear. The low concentrations of analytes in biological systems and the mild, neutral, and aqueous reaction environments may make the reactions slow and nonproductive so the reaction signals cannot be visualized. So far the use of aziridine-based sensors for the detection and distinction of reactive sulfur species has not been well studied. We wondered if the strong nucleophilicity of H2Sn under physiological conditions could be recognized by activated aziridine-based chemosensors. A N-sulfonylaziridine chemosensor was selected for this study.
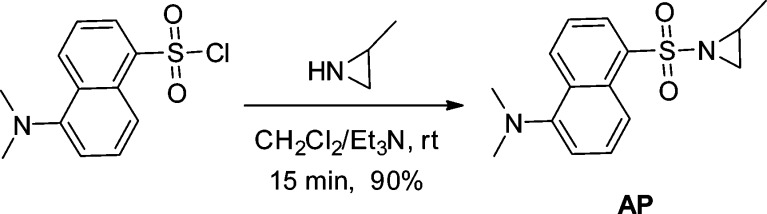
In the design of the proposed chemosensor, we expected the aziridine moiety and strongly fluorescent dansyl group to be the recognition unit and signaling unit, respectively. The fluorescence of dansyl group should be effectively quenched or weakened via the twisted intramolecular charge transfer (TICT) effect. If the aziridine ring of the sensor could be opened upon reacting with H2Sn, the resultant dansylamide derivative should possess strong fluorescence. With this idea in mind, an off–on fluorescent chemosensor, AP, was prepared from a simple coupling between dansyl chloride and 2-methylaziridine (Scheme 1).
Scheme 1. Preparation of AP.
With the sensor in hand, we first tested the time-dependent fluorescence changes of AP (10 μM) in the absence and presence of H2Sn (25 μM). Freshly prepared Na2S2 was used in buffer solutions as the equivalent of H2Sn. As shown in Figure 1A, AP showed very weak fluorescence with a low quantum yield (Φ = 0.01) in PBS buffer (pH 7.4), which was due to the TICT effect. The sensor appeared to be quite stable, as no fluorescence change was noted in 45 min. Upon the treatment of Na2S2, the fluorescence intensity at 530 nm increased dramatically, presumably due to a ring-opening reaction and the formation of the dansylamide derivative. This was also accompanied by a distinct fluorescence color change from weak yellow to bright green (see the inset of Figure 1A), which might be useful for the simple detection of H2Sn by the naked eye. The maximum intensity could reach a plateau in 15 min, suggesting this was a fast detection. To ensure reproducibility, a reaction time of 30 min was used in all the experiments in this study. It should be noted that 50 μM CTAB was used in our experiments. Without CTAB, the reaction between AP and H2Sn was found to be slow and nonproductive. This might be due to the poor stability of H2Sn in water. The use of CTAB significantly increased reaction rates. The effects of CTAB could be attributed to the fact that (1) CTAB can increase the solubility of the sensor in aqueous buffers and (2) CTAB is a cationic surfactant, which may absorb a polysulfide anion (HSn–) and facilitate the reaction. We next studied the fluorescence responses of AP to Na2S2 at varied concentrations. Upon gradual introduction of Na2S2 (1 to 40 μM), increases in fluorescence emission at 530 nm were observed. This spectroscopic property displayed a large Stokes shift (180 nm), which should prevent serious self-quenching and fluorescence detection error due to excitation backscattering effects. We also noted that the fluorescence intensity increased linearly with the concentrations of Na2S2 in the range of 0–20 μM. The detection limit31,32 was calculated to be 0.3 μM, suggesting the sensor was highly sensitive to H2Sn. In addition, we studied the pH effects on this reaction and found that AP was a stable sensor in aqueous buffers and worked effectively in a pH range from 6 to 10 (Figure S1 in the Supporting Information).
Figure 1.
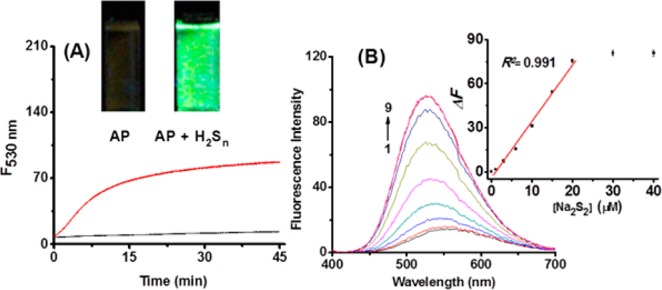
(A) Time-dependent fluorescence intensity changes of AP (10 μM) in the absence (black line) or presence (red line) of Na2S2 (25 μM). The inset depicts the fluorescence color change of the detection system after AP reacts with Na2S2. (B) Fluorescence emission spectra of AP (10 μM) with varied concentrations of Na2S2 (0, 1, 3, 6, 10, 15, 20, 30, 40 μM for curves 1–9, respectively). The inset depicts the plot of fluorescence increase value (ΔF) of the reaction system at λex/em = 350/530 nm against the corresponding reagent blank (without Na2S2).
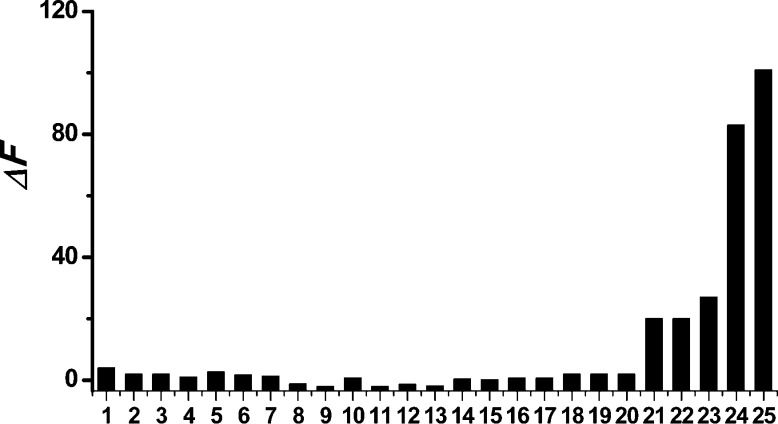
Having proven the sensitivity of AP toward H2Sn, we then turned our attention to examine the specificity of AP. In this study AP (10 μM) was treated with a series of biologically relevant sulfur species including GSH, Cys, Hcy, GSSG, H2S, SO32–, S2O32–, CH3SSSCH3, and S8. The concentrations of these species were selected based on their biological relevance. As shown in Figure 2, none of these molecules gave a significant fluorescence response (columns 1–9). We also tested the responses of AP to common reactive oxygen species and some representative amino acids, including hydrogen peroxide (H2O2), hypochlorite (ClO–), superoxide (O2–), the hydroxyl radical (·OH), singlet oxygen (1O2), alanine, proline, serine, lysine, tryptophan, and histidine. Again these species did not exhibit any significant fluorescence response (columns 10–20). H2Sn are highly reactive species and may react with thiols to form persulfides. We then wondered if AP could effectively identify H2Sn in the presence of thiols. To this end, AP was applied in the mixtures of Na2S2 and thiols (columns 21–23). We still observed satisfactory fluorescence increases, albeit the values (ΔF) were lower as compared to that of Na2S2 only. This could be attributed to the loss of H2Sn in the reactions with thiols.6,7,13,33 Moreover, we tested the detection of in situ H2Sn formation by AP. Nagy et al. reported that hypochlorite (ClO–) could rapidly react with H2S to form H2Sn.11 When Na2S (100 μM) and ClO– (25 μM) coexisted, AP gave a very strong fluorescence enhancement (column 25) and the value was even higher than that of Na2S2. Taken together, these results demonstrated the specificity and sensitivity of AP for H2Sn.
Figure 2.
Fluorescence intensity increases (ΔF) of AP (10 μM) in the presence of various RSS, ROS, amino acids. (1) 10 mM GSH; (2) 500 μM Cys; (3) 100 μM Hcy; (4) 100 μM GSSG; (5) 100 μM Na2S; (6) 100 μM Na2S2O3; (7) 100 μM Na2SO3; (8) 100 μM CH3SSSCH3; (9) 100 μM S8; (10) 250 μM H2O2; (11) 25 μM ClO–; (12) 25 μM O2–; (13) 25 μM ·OH; (14) 25 μM 1O2; (15) 100 μM alanine; (16) 100 μM proline; (17) 100 μM serine; (18) 100 μM lysine; (19) 100 μM tryptophan; (20) 100 μM histidine; (21) 100 μM GSH + 50 μM Na2S2; (22) 100 μM Cys + 50 μM Na2S2; (23) 100 μM Hcy + 50 μM Na2S2; (24) 25 μM Na2S2; (25) 25 μM ClO– + 100 μM Na2S.
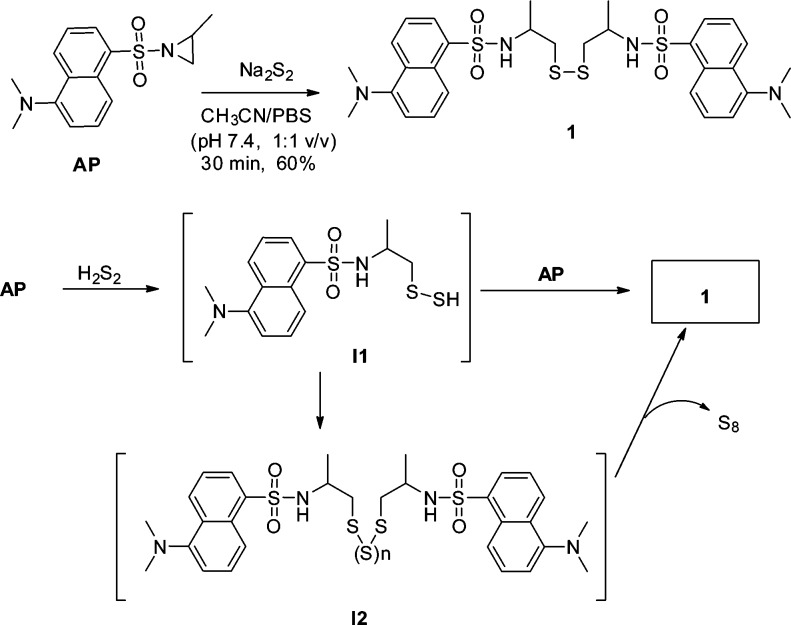
To understand the fluorescence turn-on mechanism, the reaction between AP and Na2S2 was studied at the semisynthetic scale (60 mM AP; 68 mM Na2S2) (Scheme 2). The reaction was found to be fast which completed within 30 min at room temperature. The major isolated product was disulfide 1 (60% yield). This result indicated that H2S2 could effectively open the aziridine ring of AP to form an intermediate I1. It was possible that I1 reacted with another molecule of AP to form the final product 1. Another possibility was that I1 decomposed to form polysulfide I2, which was eventually converted to a stable disulfide product.7 Given the good yield obtained in this reaction, we expected it could be used for the synthesis of disulfide derivatives.
Scheme 2. Reaction between AP and H2Sn.
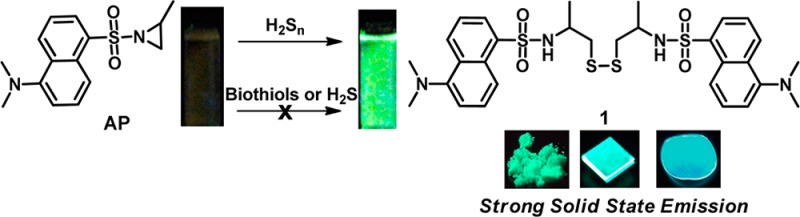
The isolation of the disulfide product 1 allowed us to carefully study its fluorescence properties as a new fluorophore. As shown in Figure 3, this molecule displayed a strong green fluorescence with a moderate quantum yield (Φ = 0.25) in PBS buffer (pH 7.4). In addition, compound 1 exhibited interesting two-photon photophysical properties. The shape of the two-photon emission spectrum (λex = 740 nm) closely resembles that obtained by single-photon excitation (λex = 350 nm) (Figure 3A and Figure S2 in the Supporting Information). This characteristic suggested that AP might be a useful two-photon fluorescent sensor. Of particular interest is that compound 1 is a highly emissive fluorophore in solid states, such as in powder form and spin-coated thin films, and even in poly(methyl methacrylate) (PMMA) films. Both powder form and a spin-coated thin film of 1 were found to have bright green fluorescence (λem = 502 nm) under UV light (λex = 365 nm). A PMMA film dispersing 0.8% compound 1 also emitted a strong blue-green fluorescence (λem = 466 nm), bearing the excellent efficiency of the solid-state emission (Φ = 1.00, calculated by using an integrating sphere34,35). The development of organic molecules bearing high solid state luminescent efficiency with a high absolute quantum yield remains a difficult task in the field of optoelectronic devices.36 Currently available molecules with such properties are still quite scarce. Compared to most known organic solid-state luminescence molecules, the unique properties of fluorophore 1, such as good solubility, easy and low-cost synthesis, and high absolute quantum yield, may endow it as a potential candidate for organic emitters and for application to solid-state lighting devices.
Figure 3.
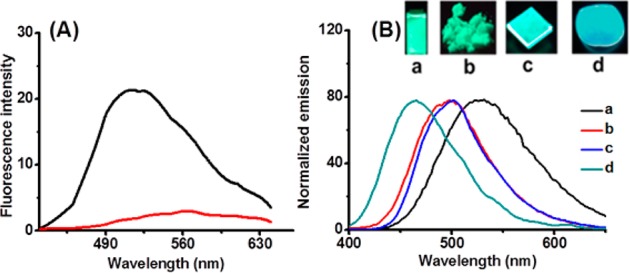
(A) Two-photon fluorescence emission spectra of AP (red line) and 1 (black line) (λex = 740 nm). (B) Fluorescence emission spectra of 1 in different forms and corresponding fluorescence photos under UV light (λex = 365 nm): (a) PBS buffer (pH 7.4); (b) solid powder; (c) spin-coated thin films; (d) PMMA solid films (film thickness is 50 μm) doped with 0.8% compound 1.
To further evaluate the application of AP in biological samples, the detection of H2Sn in diluted deproteinized bovine plasma37 was performed (Figure S3 in the Supporting Information). Upon gradual introduction of Na2S2, steady fluorescence enhancements appearing at 530 nm were observed. The fluorescence response signals were lower than those obtained in PBS buffers. This may be due to the consumption of H2S2 by biothiols. Nevertheless a good linear correlation between the H2S2 concentration and the fluorescence intensity at 530 nm was obtained.
In summary, we have reported a unique ring-opening reaction of N-sulfonylaziridine by Na2S2 under mild conditions. This reaction was used to develop a specific fluorescent sensor AP for the detection of H2Sn. The sensor was found to be selective and sensitive for H2Sn while other reactive sulfur/oxygen species and amino acids could not turn on the fluorescence. Moreover, the fluorophore, i.e. compound 1, exhibited excellent two-photon photophysical properties and a large Stokes shift. Given its high solid state luminescent efficiency, this molecule may be a potential candidate for organic emitters and for application to solid-state lighting devices.
Acknowledgments
This work is supported by an American Chemical Society Teva USA Scholar Grant and the NIH (R01GM088226 and R01HL116571). A portion of the research was performed at William R. Wiley Environmental Molecular Science Laboratory, a national scientific user facility sponsored by the Department of Energy’s Office of Biological and Environmental Research and located at PNNL.
Supporting Information Available
Detailed synthetic procedures, characteristic data, and experimental procedures. The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.orglett.5b01194.
The authors declare no competing financial interest.
Supplementary Material
References
- Giles G. I.; Tasker K. M.; Jacob C. Free Radical Biol. Med. 2001, 31, 1279. [DOI] [PubMed] [Google Scholar]
- Tangerman A. J. Chromatogr., B: Anal. Technol. Biomed. Life Sci. 2009, 877, 3366. [DOI] [PubMed] [Google Scholar]
- Gruhlke M. C. H.; Slusarenko A. J. Plant Physiol. Biochem. 2012, 59, 98. [DOI] [PubMed] [Google Scholar]
- Paulsen C. E.; Carroll K. S. Chem. Rev. 2013, 113, 4633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toohey J. I. Anal. Biochem. 2011, 413, 1. [DOI] [PubMed] [Google Scholar]
- Koike S.; Ogasawara Y.; Shibuya N.; Kimura H.; Ishii K. FEBS Lett. 2013, 587, 3548. [DOI] [PubMed] [Google Scholar]
- Greiner R.; Pálinkás Z.; Bäsell K.; Becher D.; Antelmann H.; Nagy P.; Dick T. P. Antioxid. Redox Signaling 2013, 19, 1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toohey J. I.; Cooper A. J. L. Molecules 2014, 19, 12789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono K.; Akaike T.; Sawa T.; Kumagai Y.; Wink D.; Tantillo D. J.; Hobbs A. J.; Nagy P.; Xian M.; Lin J.; Fukuto J. M. Free Radical Biol. Med. 2014, 77, 82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura H. Antioxid. Redox Signaling 2015, 22, 362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy P.; Winterbourn C. C. Chem. Res. Toxicol. 2010, 23, 1541. [DOI] [PubMed] [Google Scholar]
- Nagy P.; Pálinkás Z.; Nagy A.; Budai B.; Tóth I.; Vasas A. Biochim. Biophys. Acta 2014, 1840, 876. [DOI] [PubMed] [Google Scholar]
- Kimura Y.; Mikami Y.; Osumi K.; Tsugane M.; Oka J.; Kimura H. FASEB J. 2013, 27, 2451. [DOI] [PubMed] [Google Scholar]
- Mustafa A. K.; Gadalla M. M.; Sen N.; Kim S.; Mu W.; Gazi S. K.; Barrow R. K.; Yang G.; Wang R.; Snyder S. H. Sci. Signal 2009, 2, ra72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan N.; Fu C.; Pappin D. J.; Tonks N. K. Sci. Signal 2011, 4, ra86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen N.; Paul B. D.; Gadalla M. M.; Mustafa A. K.; Sen T.; Xu R.; Kim S.; Snyder S. H. Mol. Cell 2012, 45, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang G.; Zhao K.; Ju Y.; Mani S.; Cao Q.; Puukila S.; Khaper N.; Wu L.; Wang R. Antioxid. Redox Signaling 2013, 15, 1906. [DOI] [PubMed] [Google Scholar]
- Pan J.; Carroll K. S. ACS Chem. Biol. 2013, 8, 1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ida T.; Sawa T.; Ihara H.; Tsuchiya Y.; Watanabe Y.; Kumagai Y.; Suematsu M.; Motohashi H.; Fujii S.; Matsunaga T.; Yamamoto M.; Ono K.; Devarie-Baez N. O.; Xian M.; Fukuto J. M.; Akaike T. Proc. Natl. Acad. Sci. U.S.A. 2014, 111, 7606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D.; Macinkovic I.; Devarie-Baez N. O.; Pan J.; Park C.-M.; Carroll K. S.; Filipovic M. R.; Xian M. Angew. Chem., Int. Ed. 2014, 53, 575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabil O.; Motl N.; Banerjee R. Biochim. Biophys. Acta 2014, 1844, 1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X.; Gao X.; Shi W.; Ma H. Chem. Rev. 2014, 114, 590. [DOI] [PubMed] [Google Scholar]
- Lin V. S.; Chen W.; Xian M.; Chang C. J. Chem. Soc. Rev. 2015, 10.1039/C4CS00298A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C.; Chen W.; Shi W.; Peng B.; Zhao Y.; Ma H.; Xian M. J. Am. Chem. Soc. 2014, 136, 7257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann M. R. Environ. Sci. Technol. 1977, 11, 61. [Google Scholar]
- Schwarzenbach G.; Fischer A. Helv. Chim. Acta 1960, 43, 1365. [Google Scholar]
- Hu X. E. Tetrahedron 2004, 60, 2701. [Google Scholar]
- Lu P. Tetrahedron 2010, 66, 2549. [Google Scholar]
- Maligres P. E.; See M. M.; Askin D.; Reider P. J. Tetrahedron Lett. 1997, 38, 5253. [Google Scholar]
- Bera M.; Pratihar S.; Roy S. J. Org. Chem. 2011, 76, 1475. [DOI] [PubMed] [Google Scholar]
- Li Z.; Li X.; Gao X.; Zhang Y.; Shi W.; Ma H. Anal. Chem. 2013, 85, 3926. [DOI] [PubMed] [Google Scholar]
- Chen W.; Liu C.; Peng B.; Zhao Y.; Pacheco A.; Xian M. Chem. Sci. 2013, 4, 2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toohey J. I. Biochem. J. 1989, 264, 625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pålsson L.-O.; Monkman A. P. Adv. Mater. 2002, 14, 757. [Google Scholar]
- Li Y.; Li Z.; Wang Y.; Compaan A.; Ren T.; Dong W. Energy Environ. Sci. 2013, 6, 2907. [Google Scholar]
- Shimizu M.; Takeda Y.; Higashi M.; Hiyama T. Angew. Chem., Int. Ed. 2009, 48, 3653. [DOI] [PubMed] [Google Scholar]
- Yang X.; Guo Y.; Strongin R. Angew. Chem., Int. Ed. 2011, 50, 10690. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.