Abstract
Syncytium formation in cells that express herpes simplex virus glycoprotein B (gB), gD, gH, and gL is blocked by gK (E. Avitabile, G. Lombardi, and G. Campadelli-Fiume, J. Virol. 77:6836-6844, 2003). Here, we report the results of two series of experiments. First, UL20 protein (UL20p) expression weakly inhibited cell-cell fusion. Coexpression of UL20p and gK drastically reduced fusion in a cell-line-dependent manner, with the highest inhibition in BHK cells. Singly expressed UL20p and gK localized at the endoplasmic reticulum and nuclear membranes. When they were coexpressed, both proteins relocalized to the Golgi apparatus. Remarkably, in cells that coexpressed UL20p and gK, the antifusion activity correlated with a downmodulation of gD, gB, gH, and gL cell surface expression. Second, gBΔ867 has a partial deletion in the cytoplasmic tail that removed endocytosis motifs. Whereas wild-type (wt) gB was internalized in vesicles lined with the endosomal marker Rab5, gBΔ867 was not internalized, exhibited enhanced cell surface expression, and was more efficient in mediating cell-cell fusion than wt gB. The antifusion activity of UL20p and gK was also exerted when gBΔ867 replaced wt gB in the cell fusion assay. These studies show that the gB C tail carries a functional endocytosis motif(s) and that the removal of the motif correlated with increased gB surface expression and increased fusion activity. We conclude that cell-cell fusion in wt-virus-infected cells is negatively controlled by at least two mechanisms. The novel mechanism described here involves the concerted action of UL20p and gK and correlates with a moderate but consistent reduction in the cell surface expression of the fusion glycoproteins. This mechanism is independent of the one exerted through endocytosis-mediated downmodulation of gB from the plasma membrane.
Membrane fusion events are required at various steps in the herpes simplex virus 1 (HSV-1) replicative cycle. First, fusion takes place at virus entry into the cell and requires glycoprotein D (gD) as the receptor-binding glycoprotein (13, 54) in concert with gB and the gH-gL heterodimer (12, 17, 48). Cumulatively, these glycoproteins are designated the fusion glycoproteins. Second, even though the phenomenon of virus exocytosis is not fully understood, virions likely leave the perinuclear space by fusion of the virion envelope with the outer nuclear membrane and are released from the plasma membrane by fusion of the virion-encasing vesicle with the cytoplasmic side of the plasma membrane. Third, cells infected with HSV syncytial (syn) mutants, but not with wild-type (wt) HSV, fuse with adjacent cells, producing syncytia.
In addition to promoting fusion, HSV regulates membrane fusion negatively, a process less well understood than fusion induction. At least three steps require the negative control of fusion. First, cells infected with wt HSV do not fuse with adjacent cells, despite the fact that they express the fusion glycoproteins at the cell surface. A likely explanation is that mechanisms that prevent the fusion activity of the four glycoproteins exist and that the syn mutations that map to genes other than those for the four glycoproteins target proteins that negatively control fusion. gK and the UL20 protein (UL20p) belong to this group. Second, during virus egress, the virion-encasing vesicles are prevented from fusing with the virion envelope. Third, the luminal faces of the exocytotic pathway membranes are embedded with fusion glycoproteins, yet they do not fuse with each other.
The four glycoproteins required for virus entry are necessary and sufficient to induce cell-cell fusion when expressed from transgenes (10, 42, 56). The cell-cell fusion assay serves as a surrogate for virion-to-cell fusion and infected-cell fusion, even though major differences exist between these systems. Thus, in the cell-cell fusion assay, wt proteins suffice to give rise to syncytia, whereas in infected-cell fusion, syn mutations are required (29, 50, 53). In addition, the cell-cell fusion assay is suitable to investigate the properties of fusion glycoproteins and the entities and mechanisms that regulate HSV-induced fusion. Examples are HSV-1 and HSV-2 gB mutants carrying deletions or substitutions in predicted endocytosis motifs, which exhibited enhanced fusion activity and therefore behaved as gB syn alleles (18, 36).
The HSV genome carries a number of syn mutations located in the gK, gB, UL20, UL24, and gL genes (4, 8, 11, 14, 15, 23, 31, 43-46, 48, 51). Some of them result in cell-cell fusion in a cell-line-independent manner, while others induce fusion in some cells but not in others (3, 29, 50, 53).
gK is a polytopic membrane glycoprotein (24, 32, 47) whose topology has been debated (19, 34, 47). Its high hydrophobicity accounts for poor immunogenicity and the difficulties encountered in studying this protein. It has been reported that in cells that coexpress gK and UL20p and in transfected-infected cells, gK can be detected by immunocytochemical staining at the surfaces of infected cells and cells coexpressing UL20p (19, 21). A prominent feature of gK is that it enables virus exocytosis (20, 25, 27). By applying the cell-cell fusion assay, our laboratory found that gK inhibits fusion; a syn allele blocks fusion to a lesser extent (1).
UL20p shares important properties with gK, including a predicted polytopic structure (33). A UL20 deletion mutant virus is defective in virus exocytosis and in glycoprotein transport to the cell surface (2, 5, 57). This effect was cell line dependent and was apparent in cells in which the Golgi apparatus was fragmented following HSV infection. As was the case with gK, its high hydrophobicity and low immunogenicity hindered a biochemical characterization of UL20p.
The objective of these studies was to investigate whether UL20p is one of the entities that exert antifusion activity and whether this activity is increased when UL20p is coexpressed with gK. We report that ectopic expression of UL20p alone inhibited fusion moderately, while its coexpression with gK inhibited fusion to a high level in a cell-line-dependent fashion and induced the relocalization of both proteins to the Golgi apparatus. Furthermore, an endocytosis-defective gB mutant (gBΔ867) exhibited increased cell surface expression and fusion activity. The fusion mediated by gBΔ867 remained negatively regulated by UL20p and gK.
MATERIALS AND METHODS
Cells and viruses.
BHK, Vero, and COS cells were grown in Dulbecco's modified Eagle minimal essential medium (DMEM) containing 5% fetal bovine serum (FBS). Cells were seeded less than 20 h prior to infection and transfected at about 80% confluence.
Antibodies.
Anti-myc monoclonal antibody (MAb) and anti-V5 MAb were purchased from Invitrogen, anti-myc polyclonal antibody (PAb) was from Sigma-Aldrich, and anti-Rab5a PAb was from Santa Cruz. The following antibodies were previously described: MAb-30 (anti-gD) (9), H233 (anti-gB) (41), 52S (anti-gH) (52), VIII-62-65 (anti-gL) (37), MAb to giantin (30), and PAb to calnexin (55). Anti-UL20 rabbit PAb raised against a UL20-β-galactosidase (β-Gal) fusion protein that contained almost the entire UL20 coding sequence fused to a cro-Escherichia coli LacZ gene was described previously (57). The antibody to rat epidermal growth factor receptor 2Δ (EGFR2Δ) was the anti-p185neu Ab-4 clone 7.16.4 from Oncogene Research Products (San Diego, Calif.).
Plasmids and baculoviruses.
For construction of pUL20-pcDNA, the UL20 gene was amplified from the HSV-1(F) genome by PCR with primers 5′-GTCTCC CCAGCT AGCCCG CACACC CCATGA C (NheI-fw) and 5′-CCCCCC CGCTCG AGCCCC GTTAGA AGCCGA C (XhoI-rev) and cloned into the NheI-XhoI sites of the pcDNA 3.1(−) vector under the immediate-early cytomegalovirus (CMV) promoter. The UL20 gene was also subcloned from pcDNA 3.1 into the pMTS1 vector (pAc-CMV) (59), an empty baculovirus transfer plasmid, as an EcoRI-NotI fragment, generating pUL20-MTS. The gBΔ867-MTS vector carries a truncated form of gB in which the 37 C-terminal amino acids of the C tail are placed downstream of a stop codon. It was generated by insertion of a nonsense mutation into gBwt-MTS (58) by site-directed mutagenesis with the primer 5′-GACCTT GGCGCT GAGGCG GCCGCT CTAGCC CTTCTT CTTGGC-3′, which replaced T867 with a stop codon and introduced a NotI site for screening. The expression plasmids for gB, gH, and gL were described previously (58). Briefly, the genes amplified from the HSV-1(F) genome were cloned in the pMTS1 vector (59) under the control of the CMV early promoter. The wtgK-MTS vector, containing a Myc epitope (10 residues) inserted in frame at residue 283 of HSV-1(F) gK, was previously described (1). The pMTS1 vector is suitable for constitutive expression in mammalian cells and for recombination into baculovirus DNA. Recombinant baculoviruses expressing HSV-1 gK and UL20p, suitable for expression in mammalian cells, were obtained by cotransfection of the corresponding baculovirus transfer vectors (wtgK-MTS or pUL20-MTS) with BaculoGold DNA (Pharmingen) in Sf9 insect cells, according to the manufacturer's instructions. The technologies to grow and titrate baculoviruses were described elsewhere (59). Rab7-green fluorescent protein (GFP) was previously described (39). EGFR2Δ (previously named ECDTM, for extracellular domain and transmembrane) carries the extracellular domain and transmembrane sequences of rat HER-2/neu (nucleotides 25 to 2096) (GenBank accession number NM_017003) and is deleted from the tyrosine kinase domain (49).
Cell-cell fusion assay.
Subconfluent cultures of BHK, COS, and Vero cells grown on glass coverslips in 24-well plates were transfected with DNA mixtures that contained the expression plasmids for gD, gH, gL, and wt gB or gBΔ867, plus the pcDNA 3.1(−) Myc-His/Lac vector (Invitrogen), for constitutive expression of β-Gal. Where appropriate, the DNA from plasmid gK-myc (wtgK-MTS), UL20-pcDNA, or EGFR2Δ was added (160 ng in each transfection mixture unless otherwise indicated). The amounts of DNAs were optimized for each cell line to yield syncytia that could readily be scored by digital microscopy in terms of syncytium size and image contrast. Thus, BHK and COS cells required a smaller amount of the fusion glycoprotein plasmids than did Vero cells. Vero cells also required a larger amount of pcDNA 3.1(−) Myc-His/Lac than the other cells. The amounts of plasmid DNA used for each experiment are specified in the figure legends. The amounts of DNA in the transfection mixtures were made equal by addition of pMTS1, as appropriate. Transfections were performed with Polyfect (QIAGEN) according to the manufacturer's instructions. After incubation at 37°C for 24 or 48 h, cells were fixed with 0.2% glutaraldehyde and 0.2% paraformaldehyde in phosphate-buffered saline (PBS). Syncytia were detected by light microscopy observation of β-Gal-expressing cells with an Axioplan Zeiss microscope equipped with a Kodak DC120 digital camera and Kodak Digital Science 1D LE 3.0 software after the cells were stained with 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal). The entire coverslip was photographed with a low-magnification objective (1.25×). The largest possible square contained in the micrograph (6.35 by 6.35 cm) was defined. The nonblue areas were deselected by means of the “magic tool” and subtracted from the square area with the Photoshop Histogram program. The resulting value represented the blue syncytial areas, expressed in pixels.
Immunofluorescence.
gK and UL20p localization experiments were carried out with subconfluent COS or Vero cells grown on glass coverslips and transfected with 400 ng of each of the two plasmids. Cells were fixed at 30 h after transfection. Confluent BHK cells were infected with baculo-gK and/or baculo-UL20 recombinant baculoviruses at 37°C for 1 h; the culture medium was then replaced with fresh DMEM containing 1% FBS and 3.0 mM sodium butyrate and fixed 16 h later. For gD surface expression, subconfluent COS cells were cotransfected with expression plasmids for gB, gD, gH, and gL (40 ng of each) in the absence or presence of the wtgK-MTS and pUL20-pcDNA vectors (160 ng of each). The amounts of DNA in the transfection mixtures were made equal by the addition of pMTS1, as appropriate. The antibodies and lectins were diluted as follows: giantin, 1:100; calnexin, 1:20; a concanavalin A (ConA)-fluorescein isothiocyanate (FITC) conjugate (Sigma-Aldrich), 1:150; a MAb to myc, 1:200; a PAb to myc, 1:100; a PAb to UL20, 1:1,000; a secondary FITC- or rhodamine-conjugated immunoglobulin (Ig) (Sigma and Jackson Laboratories, respectively), 1:1,000; and MAbs to gB, gD, gH, and gL, 1:400. For the gB endocytosis experiment, subconfluent COS cells were transfected with 250 ng of gBwt-MTS or gBΔ867-MTS per well, starved 24 h later in serum-free DMEM for 2 h, and then incubated with MAb H233 (1:400) (anti-gB) in ice-cold HEPES-buffered DMEM for 1.5 h at 4°C. After being rinsed, the samples were returned to 37°C in DMEM without serum for the indicated times and finally fixed in 4% paraformaldehyde, permeabilized with Triton X-100, and stained with PAb to Rab5a (1:100) and then anti-rabbit FITC-conjugated and anti-mouse tetramethyl rhodamine isothiocyanate-conjugated IgG.
Fluorescence-activated cell sorting (FACS) analysis.
Subconfluent COS cells in T25 flasks were cotransfected with 1 μg of one of the plasmids gBwt-MTS, gBΔ867-MTS, gD-MTS, gH-MTS-gL-MTS, or EGFR2Δ plus gK-myc (2 μg) and pUL20-pcDNA (2 μg) or pMTS1 DNA (4 μg). After 24 h, the transfected cells were harvested with 0.05% EDTA in PBS, pelleted, and washed two times with ice-cold washing buffer (3% FBS in PBS). The cells were divided into two aliquots and incubated for 30 min on ice with MAb to the appropriate glycoprotein (1:150 for MAb to gB, gD, or gH; 1:300 for MAb to gL; 1:20 for MAb to EGFR2Δ) or washing buffer. The cells were then washed two times with washing buffer and incubated for 30 min on ice with anti-mouse fluorescein-conjugated IgG (1:400). The cells were washed two times with ice-cold PBS before being resuspended in 300 μl of PBS. Control samples were reacted with the secondary anti-mouse FITC-IgG for the detection of nonspecific fluorescence. Their fluorescence was subtracted from the specific positive fluorescence. Cells (10,000) were acquired in list mode for each sample with a FacsCalibur flow cytometer (Becton Dickinson, San Jose, Calif.) and analyzed with the Cell Quest program. Analysis was performed in a biparametric way, considering side scattering and green fluorescence (FL-1). The former gives information on cellular granularity, and the latter indicates the presence or absence of the investigated proteins.
RESULTS
Expression and intracellular localization of the UL20 protein.
The UL20 gene amplified from the HSV-1(F) genome by PCR was cloned in the pcDNA 3.1 vector, generating pUL20-pcDNA, and subcloned in the pMTS1 vector under the immediate-early CMV promoter. The resulting plasmid, named pUL20-MTS, was cotransfected with BaculoGold DNA to generate baculo-UL20. UL20 is the only gene expressed in eukaryotic cells infected with baculo-UL20. Some cell types, e.g., BHK cells, were transduced by recombinant baculoviruses at high efficiency and readily expressed the transgene, whereas other cell types, e.g., Vero and COS cells, were fairly resistant to baculovirus transduction.
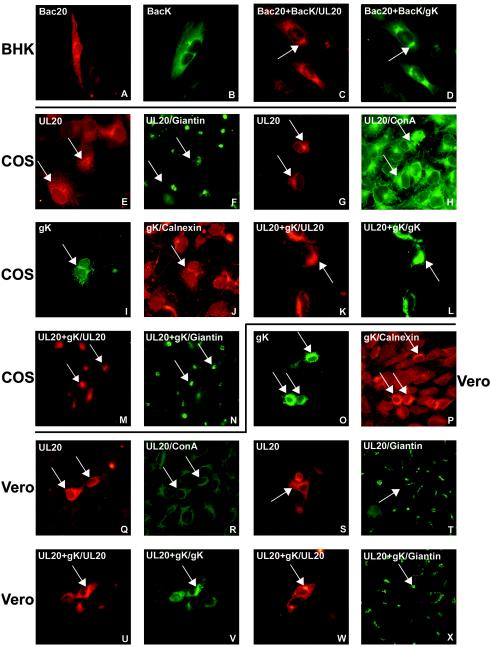
The intracellular localization of UL20p in pUL20-pcDNA-transfected COS and Vero cells and in baculo-UL20-infected BHK cells was investigated by double immunofluorescence with the following markers: antibody to calnexin and ConA, which localize to the endoplasmic reticulum (ER), and antibody to giantin, which localizes to the Golgi apparatus. The results for UL20p localization are shown in Fig. 1. In BHK cells transduced with baculo-UL20, the UL20p localization was reticular and diffuse in the cytoplasm (Fig. 1A). In transfected COS cells, the prominent localization of UL20p was reticular and diffuse toward the cytoplasm; the nuclear membrane was brightly decorated (Fig. 1E). Double immunofluorescence showed colocalization with ConA (Fig. 1G and H) and very modest colocalization with giantin (Fig. 1E and F). Occasionally, in some cells the staining appeared to be located in part in a perinuclear position, typical of the Golgi apparatus marker giantin. In transfected Vero cells, the intracellular distribution of UL20p was very similar to that observed for COS cells, i.e., reticular, or diffusely punctate, toward the cytoplasm (Fig. 1Q). The nuclear envelope was decorated. Altogether, UL20p was predominantly localized at the ER and nuclear membranes.
FIG.1.
Intracellular localization of UL20p and gK detected by immunofluorescence. (A to D) BHK cells transduced with baculovirus-UL20 (Bac20), baculovirus-gK (BacK), or both and stained with PAb to UL20p (A and C) or PAb to myc (B and D). (E to X) Two paired images, one green and one red, are shown for the same photographic field, and the paired panels are E and F, G and H, I and J, K and L, M and N, O and P, Q and R, S and T, U and V, and W and X. (E to H) COS cells transfected with pUL20-pcDNA and stained with PAb to UL20p with (F) and without (E) Ab to giantin and with (H) and without (G) ConA-FITC. (I and J) COS cells transfected with pgK and stained with anti-myc Ab with (J) or without (I) Ab to calnexin. (K to N) COS cells cotransfected with pUL20-pcDNA and pgK and stained with Ab to UL20p (K and M), myc (L), or giantin (N). (O and P) Vero cells transfected with pgK and stained with Abs to myc (O) or calnexin (P). (Q to T) Vero cells transfected with pUL20-pcDNA and stained with Ab to UL20p (Q and S), anti-giantin (T), or ConA-FITC (R). (U to X) Vero cells cotransfected with pUL20-pcDNA and pgK and stained with Ab to UL20p (U and W), myc (V), or giantin (X). Cells were transfected with 250 ng of each plasmid. Cells transfected with a single plasmid received an additional 250 ng of pMTS. The protein indicated after the slash identifies the stained protein in double immunofluorescence.
Coexpression of UL20p and gK induces relocalization of both proteins.
We determined whether the intracellular localization of UL20p was modified by coexpression with gK and, in turn, whether the localization of gK was modified by coexpression with UL20p. Preliminarily, we investigated the intracellular localization of gK in the same cells analyzed as described above for UL20p. The gK construct carries a myc epitope at residue 283 and was employed previously to investigate gK localization in 143tk− and Vero cells (1). It was previously reported that in the transfected cells, gK can be detected in fewer cells than the fusion glycoproteins (1). Here, we observed that gK could be detected in fewer cells than UL20p could be, even though the genes for both were cloned with the same promoter. The intracellular localization of gK did not substantially differ from that of UL20p or from that described in previous reports (1, 26). Specifically, its distribution appeared to be reticular, or punctate, and diffuse toward the cytoplasm in transfected COS and Vero cells as well as in baculo-gK-transduced BHK cells (Fig. 1B, I, and O). The staining of gK colocalized with that of calnexin (Fig. 1I to J and O to P) and colocalized very little with that of the Golgi apparatus marker giantin (data not shown), indicating a predominant localization at the ER. The intracellular distribution of gK-V5, a gK construct that carries the V5 epitope in the N-terminal extracellular domain I (19), did not differ from that of the gK-myc employed throughout these studies (data not shown).
In cells coexpressing UL20p and gK, a substantial relocalization of both proteins was observed. Thus, in BHK cells simultaneously transduced with baculo-UL20 and baculo-gK, both proteins appeared to be less diffuse in the cytoplasm and occupied a perinuclear position. The two proteins colocalized (Fig. 1C and D). A relocalization to a perinuclear position and colocalization were observed in COS and Vero cells (Fig. 1K, L, U, and V). Staining of both proteins overlapped in part with that of giantin (Fig. 1W and X), indicating localization at the Golgi apparatus. Of note is that the localization at the Golgi apparatus and nuclear membranes is typical of UL20p in infected cells (57). We were unable to detect by an immunofluorescence assay (IFA) any cell surface localization of either UL20p or gK, expressed singly or in combination, irrespective of whether the myc- or V5-tagged gK versions were used (data not shown), in contrast with the report from Foster et al. (21). Attempts to detect cell surface expression by FACS were also unsuccessful (data not shown), and the UL20p antiserum was not suitable for FACS analysis. It was previously reported that in a clone of infected Vero cells that overexpresses gK, the Golgi apparatus collapsed into the ER (19). We did not observe any substantial modification in the localization of the Golgi apparatus marker giantin in cells that express gK, UL20p, or both, implying that in our system the Golgi apparatus did not relocalize to the ER (Fig. 1F, N, T, and X). This result suggests that the reported collapse of the Golgi apparatus may have been dependent on infection, the very high level of gK expression, or both.
UL20p moderately reduces HSV-induced cell-cell fusion.
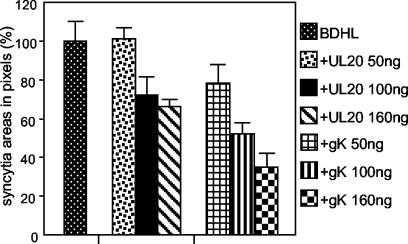
The effect of UL20p on HSV-induced fusion was assessed by cotransfection of pUL20-pcDNA with expression plasmids encoding the four glycoproteins gB, gD, gH, and gL. A plasmid carrying a LacZ gene under the CMV promoter was included in the transfection mixture. The transfected cells, including syncytia, were monitored and quantified following X-Gal staining through digital micrographs (see Materials and Methods). UL20p moderately inhibited cell-cell fusion in BHK cells (Fig. 2 and 3). The effect was dose dependent (Fig. 2). The inhibitory effect was lower than that exerted by transfection of equivalent amounts of the gK plasmid, whose effect was described previously and was included here for comparison (Fig. 2). UL20p also slightly inhibited cell-cell fusion in transfected COS cells: the inhibition was less than that induced by an equivalent amount of gK plasmid (Fig. 4). In Vero cells, the decrease was barely detectable and probably not significant (Fig. 5). We note that Vero cells overall were scarcely prone to fusion even though the amount of DNA coding for the fusion glycoproteins in Vero cells was two times higher than that in BHK or COS cells (Fig. 5, compare syncytial areas expressed in pixels). We also note that the extent of inhibition exerted by gK alone was smaller than that reported previously (1) and reflects an improvement in the detection and quantification of the syncytial areas (see Materials and Methods).
FIG. 2.
Quantification of cell-cell fusion in BHK cells cotransfected with plasmids encoding only gB, gD, gH, and gL (BDHL) (40 ng of each) or gB, gD, gH, and gL plus pUL20-pcDNA or pgK at the indicated amounts and the pcDNA 3.1(−) Myc-His/Lac vector (80 ng). The amounts of DNA in the transfection mixtures were made equal by addition of pMTS1, as appropriate. Syncytia were stained with X-Gal at 48 h. A digital micrograph of the entire coverslip was taken. The blue areas corresponding to syncytia were quantified with the Histogram software of Photoshop. Samples were run three times. The bars indicate standard errors (SE).
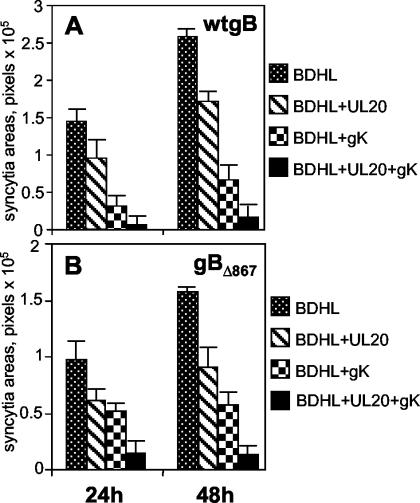
FIG. 3.
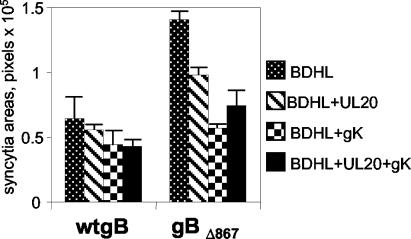
Quantification of cell-cell fusion in BHK cells cotransfected with expression plasmids encoding wt gB, gD, gH, and gL (40 ng of each) (A) or gBΔ867, gD, gH, and gL (20 ng of each) (B) plus pUL20-pcDNA and/or pgK (160 ng of each) and the pcDNA 3.1(−) Myc-His/Lac vector (80 ng). BDHL, gB, gD, gH, and gL. The amounts of DNA in the transfection mixtures were made equal by addition of pMTS1, as appropriate. Syncytium staining and quantification were performed as described in the legend to Fig. 2. The bars indicate SE.
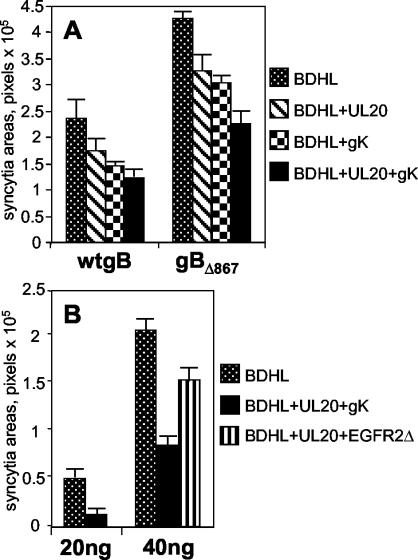
FIG. 4.
(A) Quantification of cell-cell fusion in COS cells cotransfected for 48 h with expression plasmids encoding wt gB, gD, gH, and gL (40 ng of each) (wtgB) or gBΔ867, gD, gH, and gL (20 ng of each) (gBΔ867) plus pUL20-pcDNA and/or pgK (160 ng each). (B) Quantification of cell-cell fusion in COS cells cotransfected for 48 h with expression plasmids encoding wt gB, gD, gH, and gL (20 or 40 ng of each) plus fourfold-higher amounts of pUL20-pcDNA and pgK or EGFR2Δ. BDHL, gB, gD, gH, and gL. The transfection mixtures contained the pcDNA 3.1(−) Myc-His/Lac vector (40 ng), and amounts of DNA in the transfection mixtures were made equal by addition of pMTS1, as appropriate. Syncytium staining and quantification were performed as described in the legend to Fig. 2. The bars indicate SE.
FIG. 5.
Quantification of cell-cell fusion in Vero cells cotransfected for 48 h with expression plasmids encoding wt gB, gD, gH, and gL (80 ng of each) or gBΔ867, gD, gH, and gL (80 ng of each) plus pUL20-pcDNA and/or pgK (160 ng of each) and the pcDNA 3.1(−) Myc-His/Lac vector (240 ng). BDHL, gB, gD, gH, and gL. The amounts of DNA in the transfection mixtures were made equal by addition of pMTS1, as appropriate. Syncytium staining and quantification were performed as described in the legend to Fig. 2. The bars indicate SE.
Coexpression of UL20p and gK reduces fusion to a greater extent than each protein singly in BHK and COS cells but not in Vero cells.
In these experiments, we analyzed the antifusion activity exerted by the coexpression of UL20p and gK. BHK, COS, and Vero cells were cotransfected with pUL20-pcDNA, pgK plasmids, and plasmids encoding the fusion glycoproteins. In BHK cells, the simultaneous presence of UL20p and gK reduced syncytium formation to a greater extent than UL20p alone or gK alone, both at 24 and 48 h after transfection (Fig. 3). At 48 h, the inhibition was almost complete.
In contrast, in Vero cells the simultaneous presence of UL20p and gK did not increase fusion inhibition (Fig. 5). COS cells exhibited an intermediate pattern in that the coexpression of the two proteins increased antifusion activity but the overall inhibition was on the order of 50% (Fig. 4A).
In order to ascertain whether the antifusion activity was an effect specific for UL20p and gK and not a nonspecific effect due to the overexpression of proteins trafficking along the exocytotic pathway and Golgi apparatus, gK was replaced with a heterologous glycoprotein, rat EGFR2Δ (49). This receptor is deleted from the tyrosine phosphorylation sites and lacks signal transduction activity. Figure 4B shows that the replacement of gK with EGFR2Δ induced a moderate inhibition of cell-cell fusion in COS cells. Furthermore, UL20p and gK inhibited fusion even when cells were transfected with half the amount of the plasmids (20 ng) as that used for the fusion glycoproteins (40 ng). Altogether, these experiments show that the overexpression of proteins in the exocytotic pathway and the Golgi apparatus only moderately affects cell-cell fusion and provide evidence that inhibition by UL20p and gK is a specific effect.
A C-tail deletion mutant of gB (gBΔ867) is not endocytosed and exhibits a greater extent of cell surface expression than wt gB.
The cytoplasmic tail of HSV-1 gB carries three potential endocytosis motifs and is the locus of syn mutations (3, 11, 35). gB mutants carrying partial C-tail truncations exhibit enhanced fusion activity in the cell-cell fusion assay and in the context of infected cells (18). Here, the gB C tail was partially deleted by insertion of a stop codon in place of T867. The resulting plasmid was named pMTS-gBΔ867.
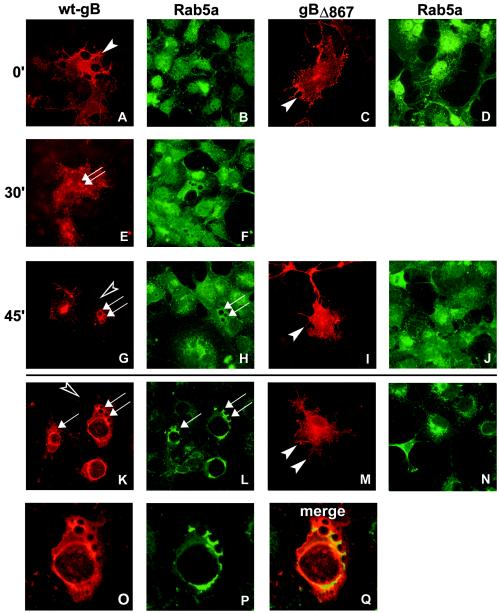
To characterize gBΔ867, we investigated whether its intracellular localization differed from that of wt gB. COS cells were transfected with pMTS-gBΔ867, fixed at 24 h after transfection, and analyzed by IFA. Figure 6 shows that gBΔ867 localized to a large extent to the cell surface (Fig. 6M), in contrast to wt gB, and failed to exhibit the cytoplasmic vesicles that are typical of wt gB in transfected (Fig. 6K) and infected cells. We investigated whether these vesicles originate by endocytosis by reacting the transfected cells with MAb H233 (anti-gB) at 4°C for 90 min and then shifting the cells to 37°C for 0, 30, and 45 min. Cells were simultaneously analyzed for the localization of two Rab proteins that associate with the endocytotic vesicles, Rab5a and Rab7, typical of early and late endosomes, respectively (6, 22). They were detected by staining with anti-Rab5a antibody or by cotransfection with a plasmid encoding Rab7-GFP (39). The results shown in Fig. 6 show that at zero time both wt gB and gBΔ867 were present on the cell surface. At 30 min, wt gB localized at small vesicles (Fig. 6E) which increased in size at 45 min (Fig. 6G). At the steady state, the vesicles and vacuoles were large in size and lined in part with Rab5a (Fig. 6, compare panels K and L with panels O and Q). Simultaneously with vesicle formation, the level of wt gB cell surface expression decreased (Fig. 6G, K, and O). In sharp contrast, in gBΔ867-expressing cells, vesicles were invariably absent, and the level of cell surface expression decreased (Fig. 6C, I, and M). We did not observe any colocalization between gB and Rab7-GFP (data not shown). Altogether, the experiments demonstrate that wt gB is taken into vesicles lined with Rab5a, and its cell surface expression remained high. gBΔ867 was not taken into vesicles, and its cell surface localization did not decrease. The presence of a functional endocytosis motif(s) in the cytoplasmic tail of gB has been reported recently (7).
FIG. 6.
Micrographs of COS cells transfected with pgB-MTS (encoding wt gB) (A, B, E, F, G, H, K, L, O, P, and Q) or pgBΔ867-MTS (encoding gBΔ867) (C, D, I, J, M, and N) and reacted with MAb to gB (A, C, E, G, I, K, M, and O) or MAb to Rab5a (B, D, F, H, J, L, N, and P). (A to J) Endocytosis assays. Cells were reacted with MAb to gB at 4°C and then shifted to 37°C for the indicated times (in minutes), fixed, permeabilized, and reacted with secondary antibodies. Arrows indicate vesicles lined with gB and Rab5a. Filled arrowheads point to gB-labeled cell surfaces. Open arrowheads point to a lack of gB on cell surfaces. (O and P) Higher magnification of panels K and L, respectively. (Q) Merged image of panels O and P shows colocalization of Rab5a and gB.
gBΔ867 exhibits enhanced cell-cell fusion activity relative to wt gB.
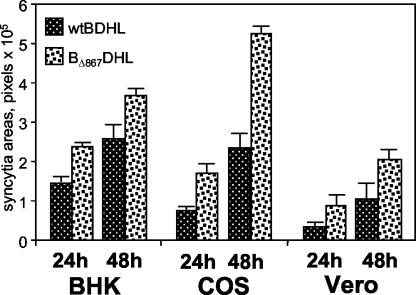
In order to determine whether gBΔ867 displays enhanced syncytium production compared to wt gB, pMTS-gBΔ867 was cotransfected with the expression plasmids carrying the gD, gH, gL, and LacZ genes in BHK, COS, and Vero cells. The results shown in Fig. 7 demonstrate that gBΔ867 mediated the production of larger syncytia than its wt allele in all three cell lines tested. Thus, gBΔ867 behaved functionally as a gB syn allele.
FIG. 7.
Comparison of the levels of cell-cell fusion induced by wt gB and gBΔ867. BHK, COS, and Vero cells cotransfected with expression plasmids encoding wt gB, gD, gH, and gL (wtBDHL) or gBΔ867, gD, gH, and gL (BΔ867DHL) (40 ng of each in BHK and COS cells; 80 ng of each in Vero cells) plus the pcDNA 3.1(−) Myc-His/Lac vector (40 ng in BHK and COS cells; 240 ng in Vero cells). The amounts of DNA in all transfection mixtures were made equal by addition of pMTS1, as appropriate. Syncytium staining and quantification were performed as described in the legend to Fig. 2. The bars indicate SE.
Cell-cell fusion mediated by gBΔ867 is inhibited by UL20p, gK, and the simultaneous presence of both proteins.
Next we asked whether UL20p and gK inhibited fusion when gBΔ867 replaced wt gB. BHK, COS, and Vero cells were cotransfected with the appropriate expression plasmids, and the extent of syncytium formation was quantified. The syncytial areas were so large that in BHK and COS cells the amount of plasmid DNA for each of the fusion glycoproteins was lowered from 40 to 20 ng. The results shown in Fig. 3 to 5 demonstrate that UL20p alone or gK alone inhibited cell-cell fusion mediated by gBΔ867 in all three cell lines. The reduction was largest in BHK cells. The coexpression of UL20p and gK in BHK cells increased the antifusion effect so that fusion was almost eliminated. In COS cells, coexpression of the two proteins augmented the inhibition; however, the total level of reduction was nearly 50%. In Vero cells, we observed not a strong increase in inhibition but rather a slight increase. Altogether, gBΔ867 exhibited enhanced fusion activity relative to wt gB. This activity was subject to the same negative regulation by UL20p and gK as that induced by wt gB, suggesting that the sequences targeted by gK and/or UL20p are present in gBΔ867.
Simultaneous expression of UL20p and gK selectively decreases cell surface expression of HSV glycoproteins.
The objectives of this series of experiments were twofold, i.e., (i) to determine whether the simultaneous expression of UL20p and gK affects the cell surface expression of gB, gD, and gH-gL, and (ii) to determine whether this effect is selective for the viral glycoproteins or is also exerted on a heterologous cell surface protein, EGFR2Δ. For a comparative analysis, cell surface expression was quantified by FACS analysis using transiently expressing cells, even though the assay entailed some limitations. First, COS cells were the only cells that yielded a sufficiently high number of transfected cells. Second, cells expressing the four fusion glycoproteins form syncytia that are excluded by the sorting procedure. Third, the amount of DNA transfected in a given culture cannot be increased beyond certain limits. In preliminary experiments, we observed that a sufficiently high number of transiently expressing cells was obtained when three or, at maximum, four plasmids were cotransfected. Therefore, the effect of UL20p and gK was determined in cells expressing one of the following glycoproteins: gB, gD, or gH-gL. The results are summarized in Table 1. For each glycoprotein, the level of cell surface expression was decreased by the simultaneous expression of UL20p and gK by 20% or more. Analysis by means of a paired t test indicated that the differences were statistically significant (P < 0.05). In contrast, the presence of UL20p alone or gK alone reduced only slightly the cell surface expression of each glycoprotein (data not shown). The cell surface expression of EGFR2Δ was little modified by UL20p and gK, with inhibition below 10%. Altogether, these experiments demonstrate that coexpression of UL20p and gK induces a downmodulation in the cell surface expression of the fusion glycoproteins; the downmodulation is selective for the viral glycoproteins and, although not high, is significant.
TABLE 1.
Effect of UL20p plus gK on the cell surface expression of glycoproteins
| Transfected protein | Cell surface expressiona of:
|
% Inhibition | |
|---|---|---|---|
| Glycoproteinb | Glycoprotein + gK + UL20pc | ||
| wt gB | 13.27 ± 2.01d | 10.51 ± 1.70 | 21 |
| gBΔ867 | 23.23 ± 3.70 | 7.74 ± 1.75 | 24 |
| gD | 26.1 ± 3.51 | 17.04 ± 2.65 | 35 |
| gH | 15.15 ± 3.54 | 9.35 ± 2.13 | 38 |
| gL | 25.05 ± 2.01 | 19.50 ± 2.16 | 22 |
| EGFR2Δ | 24.68 ± 2.05 | 22.71 ± 2.12 | 8 |
Expressed as the percentage of unfixed cells reacting with the appropriate glycoprotein Ab (mean ± SE) as determined by FACS analysis. Values shown are from three or more determinations.
COS cells were transfected with the indicated glycoprotein plasmid.
COS cells were transfected with the indicated glycoprotein plasmid, UL20p, and gK.
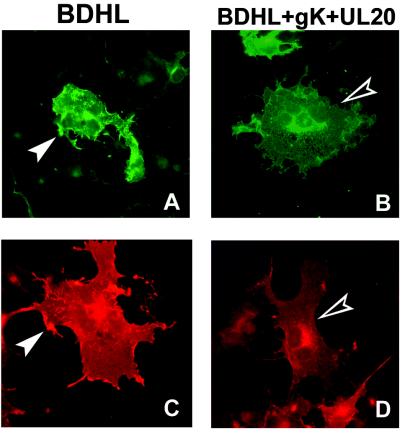
Next we determined whether the cell surface expression of the fusion glycoproteins was downmodulated by UL20p and gK even when all four fusion glycoproteins were simultaneously coexpressed, i.e., under conditions identical to those employed for the fusion assay. To overcome the limitations mentioned above, this determination was carried out with an IFA. The results shown in Fig. 8 demonstrate a marked downmodulation in cell surface expression of gD. The downmodulation in gB, gH, and gL surface expression was not as high as that of gD (data not shown) and did not allow us to draw any firm conclusion.
FIG. 8.
Immunostaining with MAb to gD of COS cells cotransfected with plasmids encoding gB, gD, gH, and gL alone (BDHL) (80 ng of each) (A and C) or gB, gD, gH, and gL plus pUL20-pcDNA and pgK (160 ng of each) (B and D). The amounts of DNA in all transfection mixtures were made equal by addition of pMTS1, as appropriate. Filled arrowheads indicate gD-labeled cell surfaces. Open arrowheads indicate a lack of gD on cell surfaces.
DISCUSSION
The results reported here show the following. (i) UL20p inhibited cell-cell fusion mediated by gB, gD, gH, and gL to a small extent in a cell-line-dependent manner. The inhibition exerted by UL20p was consistently lower than that exerted by gK. (ii) Coexpression of UL20p and gK drastically reduced cell-cell fusion in a cell-line-dependent manner. Thus, fusion was almost eliminated in BHK cells, reduced by nearly 50% in COS cells, and barely inhibited in Vero cells. (iii) The predominant localization of UL20p expressed singly was at the ER and nuclear membranes and closely resembled that of singly expressed gK. This localization was observed in all cell lines tested. (iv) When UL20p was coexpressed with gK, both proteins relocalized, and their predominant site of accumulation became the Golgi apparatus. (v) The coexpression of UL20p and gK in COS cells decreased the surface expression of the fusion glycoproteins gB, gD, gH, and gL by 20 to 40% as determined by FACS. In contrast, singly expressed UL20p and gK reduced the cell surface expression to a small extent despite the fact that gK alone inhibited fusion. (vi) A strong reduction in gD cell surface expression, measured by IFA, was observed in COS cells coexpressing the fusion glycoproteins UL20p and gK. (vii) wt gB was internalized in vesicles and vacuoles lined with the endocytosis marker Rab5a. (viii) gBΔ867, which lacks two predicted endocytosis motifs, was not endocytosed, exhibited an increased level of cell surface expression, and was more efficient in cell fusion. (ix) Syncytium formation by cells expressing gBΔ867 was inhibited by UL20p and gK, indicating that the sequences targeted by UL20p and gK were still present.
Recent studies of varicella-zoster virus gE and gH (38, 40) highlighted that endocytosis is a mechanism that regulates the level of glycoprotein cell surface expression. The results of this study show that wt gB is indeed endocytosed into vesicles lined with the early endosomal marker Rab5a. A truncation in the C tail resulted in a protein that was no longer endocytosed, in agreement with a recent report that functional endocytosis motifs lie downstream of residue 867 (7). At the same time, gBΔ867 was present at higher levels at the cell surface and was associated with increased fusion activity. The latter finding is consistent with earlier observations that the removal of portions of the C tail from gB results in increased fusion activity both in the cell-cell fusion assay and in the context of infected cells (18). Similarly, the deletion of a C-tail portion of pseudorabies virus gB resulted in increased fusion activity (28). In HSV-2, gB truncation of the C tail at residue 862 generated a form of gB with enhanced cell surface expression and fusion activity, even though mutagenesis of the tyrosine residue resulted in a higher level of cell surface expression but no enhancement in fusion activity (16). While the reason for this discrepancy is not clear, collectively these properties indicate that HSV-1 gB carries functional endocytosis motifs in the C tail and that their removal correlates with increased cell surface expression and increased fusion activity.
In this report, we describe a novel mechanism by which HSV blocked cell-cell fusion. Specifically, we show that HSV carries genes that encode at least two proteins, UL20p and gK, that act in concert to block cell-cell fusion mediated by gB, gD, gH, and gL. This mechanism is independent of and differs significantly from the endocytosis of gB from the plasma membrane. Analysis of the mechanism by which UL20p and gK block cell-cell fusion must take into account two fundamental observations. The first of these observations is that viruses with mutations in or deletions of gK or UL20p can cause cell-cell fusion. The existence of these mutants, known for many years, indicates that both proteins play a role in blocking fusion and that in the context of the infected-cell fusion is a recessive phenotype. The studies described in this report reproduce in a more restricted system the predicted role of these proteins in wt-virus-infected cells and identify their ultimate targets.
The second observation is that the fusion activity of gD, gH, gL, and gB is cell type independent, albeit at different efficacies in different cell lines. In this report, we show that the effectiveness of UL20p and gK at blocking cell-cell fusion was cell type dependent. The significance of this observation stems from the notion that the fusogenic activity of syn mutants varies depending on the site of the mutation. A survey of mutants published long ago showed that the abilities of some mutants to fuse cells were strongly cell type dependent, whereas those of a few others were cell type independent (3, 29, 50, 53). Cell type dependence may reflect differences in transcriptional factors that recognize response elements in viral promoters, properties of protein transport machinery, or the abundance of a protein factor involved in cell fusion. The significance of this observation is that both the fusion activity of gB, gD, gH, and gL and the inhibitory activities of UL20p and gK reflect intrinsic phenotypes of cells infected with wild-type or mutant viruses.
On the basis of these observations, at least three hypotheses may explain the mechanisms by which UL20p and gK block cell-cell fusion. The first hypothesis is that gK and UL20p act at the cell surface to block the interaction of fusion glycoproteins. This hypothesis predicts the presence of gK and UL20p at the plasma membrane and a specific interaction with at least one of the viral glycoproteins. At present, there is no direct evidence that UL20p or gK interacts specifically with either gB, gL, gH, or gD; moreover, the bulk of UL20p and gK is found in the Golgi apparatus and not at the plasma membrane. It should be noted, however, that while the syn mutation in gB reflects the abolishment of an antifusion activity of this protein that is independent of gK and UL20p, there exists the possibility that the syn mutations in other loci may reflect a loss of interaction with gK or UL20p. Furthermore, we cannot eliminate the possibility that UL20p and gK shuttle between the Golgi apparatus and the plasma membrane and therefore would have access to the viral glycoproteins at the cell surface.
The second hypothesis is that UL20p and gK modify the steady-state amounts of the fusion glycoproteins at the plasma membranes. Supporting this hypothesis are the observations that in cells transduced with UL20p and gK, the amounts of viral glycoproteins on the surfaces of transfected cells were diminished and that in infected Vero cells overexpressing gK, the cell surface gD was decreased (21). This hypothesis predicts that in infected cells lacking UL20p and gK, there should be an increase in the amount of glycoproteins transported to the surfaces of the cells. The fact that in Vero cells infected with an HSV mutant lacking UL20 the transport of viral glycoproteins was arrested in the trans-Golgi apparatus (2) does not contradict the hypothesis, as a double deletion virus was not constructed, and Vero cells were the least responsive in this study. Third, we cannot exclude the possibility that the dependence of fusion activity of viral proteins on cell type reflects a titration of cellular inhibitors of cell fusion by the viral protein and that UL20p and gK act to facilitate the function of this putative inhibitor. This hypothesis does not predict a specific interaction between gK and UL20p and the fusion glycoproteins.
The most striking phenotype of mutant viruses with deletions of the UL20 or gK genes was a defect in virus egress, suggesting a relationship between virus exocytosis and the blocking of fusion. This raises the question of whether the antifusion activity of UL20p and gK is also exerted at compartments other than the plasma membrane, particularly in the exocytotic pathway. The reason why such a control needs to be postulated is that, in the infected cells, the luminal sides of the membranes of the exocytotic pathway become embedded with fusion glycoproteins, yet these membranes do not fuse. For a number of viruses, the molecular mechanism of control is the cleavage of the fusion glycoprotein that takes place in the trans-Golgi network and prevents the premature activation of fusion activity. HSV glycoproteins do not undergo cleavage; hence, a mechanism other than this must be functional in HSV-infected cells. We propose that the antifusion activity of UL20p or gK may also be exerted on the membranes of the exocytotic pathway in order to preserve the integrity and functionality of this compartment and thus enable virus egress.
In essence, HSV has evolved at least two mechanisms by which it blocks cell-cell fusion and possibly prevents fusion in the exocytotic compartment. The evolution of functional redundancy implies that fusion is inimical to viral replication and is widespread in nature. The mechanisms and relative contributions to the biology of HSV in human cells of these pathways remain to be explored further.
Acknowledgments
We thank K. G. Kousoulas for the gift of the gK-V5 plasmid and M. Zerial for the gift of Rab7-GFP. We thank Elisabetta Romagnoli for invaluable help with cell cultures.
The work was supported by grants from the FIRB autonomous project, the FIRB coordinated project, Cofin-MIUR, CNR-Functional genomics, the University of Bologna, and Fondo Pallotti.
REFERENCES
- 1.Avitabile, E., G. Lombardi, and G. Campadelli-Fiume. 2003. Herpes simplex virus glycoprotein K, but not its syncytial allele, inhibits cell-cell fusion mediated by the four fusogenic glycoproteins, gD, gB, gH, and gL. J. Virol. 77:6836-6844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Avitabile, E., P. L. Ward, C. Di Lazzaro, M. R. Torrisi, B. Roizman, and G. Campadelli-Fiume. 1994. The herpes simplex virus UL20 protein compensates for the differential disruption of exocytosis of virions and viral membrane glycoproteins associated with fragmentation of the Golgi apparatus. J. Virol. 68:7397-7405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baghian, A., L. Huang, S. Newman, S. Jayachandra, and K. G. Kousoulas. 1993. Truncation of the carboxy-terminal 28 amino acids of glycoprotein B specified by herpes simplex virus type 1 mutant amb1511-7 causes extensive cell fusion. J. Virol. 67:2396-2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baines, J. D., and B. Roizman. 1991. The open reading frames UL3, UL4, UL10, and UL16 are dispensable for the replication of herpes simplex virus 1 in cell culture. J. Virol. 65:938-944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baines, J. D., P. L. Ward, G. Campadelli-Fiume, and B. Roizman. 1991. The UL20 gene of herpes simplex virus 1 encodes a function necessary for viral egress. J. Virol. 65:6414-6424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barbieri, M. A., R. L. Roberts, A. Gumusboga, H. Highfield, C. Alvarez-Dominguez, A. Wells, and P. D. Stahl. 2000. Epidermal growth factor and membrane trafficking. EGF receptor activation of endocytosis requires Rab5a. J. Cell Biol. 151:539-550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beitia Ortiz de Zarate, I., K. Kaelin, and F. Rozenberg. 2004. Effects of mutations in the cytoplasmic domain of herpes simplex virus type 1 glycoprotein B on intracellular transport and infectivity. J. Virol. 78:1540-1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bond, V. C., and S. Person. 1984. Fine structure physical map locations of alterations that affect cell fusion in herpes simplex virus type 1. Virology 132:368-376. [DOI] [PubMed] [Google Scholar]
- 9.Brandimarti, R., T. Huang, B. Roizman, and G. Campadelli-Fiume. 1994. Mapping of herpes simplex virus 1 genes with mutations which overcome host restrictions to infection. Proc. Natl. Acad. Sci. USA 91:5406-5410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Browne, H., B. Bruun, and T. Minson. 2001. Plasma membrane requirements for cell fusion induced by herpes simplex virus type 1 glycoproteins gB, gD, gH and gL. J. Gen. Virol. 82:1419-1422. [DOI] [PubMed] [Google Scholar]
- 11.Bzik, D. J., B. A. Fox, N. A. DeLuca, and S. Person. 1984. Nucleotide sequence of a region of the herpes simplex virus type 1 gB glycoprotein gene: mutations affecting rate of virus entry and cell fusion. Virology 137:185-190. [DOI] [PubMed] [Google Scholar]
- 12.Cai, W. H., B. Gu, and S. Person. 1988. Role of glycoprotein B of herpes simplex virus type 1 in viral entry and cell fusion. J. Virol. 62:2596-2604. (Erratum, 62:4438.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Campadelli-Fiume, G., E. Avitabile, S. Fini, D. Stirpe, M. Arsenakis, and B. Roizman. 1988. Herpes simplex virus glycoprotein D is sufficient to induce spontaneous pH-independent fusion in a cell line that constitutively expresses the glycoprotein. Virology 166:598-602. [DOI] [PubMed] [Google Scholar]
- 14.Debroy, C., N. Pederson, and S. Person. 1985. Nucleotide sequence of a herpes simplex virus type 1 gene that causes cell fusion. Virology 145:36-48. [DOI] [PubMed] [Google Scholar]
- 15.Dolter, K. E., R. Ramaswamy, and T. C. Holland. 1994. Syncytial mutations in the herpes simplex virus type 1 gK (UL53) gene occur in two distinct domains. J. Virol. 68:8277-8281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fan, Z., M. L. Grantham, M. S. Smith, E. S. Anderson, J. A. Cardelli, and M. I. Muggeridge. 2002. Truncation of herpes simplex virus type 2 glycoprotein B increases its cell surface expression and activity in cell-cell fusion, but these properties are unrelated. J. Virol. 76:9271-9283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Forrester, A., H. Farrell, G. Wilkinson, J. Kaye, N. Davis-Poynter, and T. Minson. 1992. Construction and properties of a mutant of herpes simplex virus type 1 with glycoprotein H coding sequences deleted. J. Virol. 66:341-348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Foster, T., J. Melancon, and K. Kousoulas. 2001. An alpha-helical domain within the carboxyl terminus of herpes simplex virus type 1 (HSV-1) glycoprotein B (gB) is associated with cell fusion and resistance to heparin inhibition of cell fusion. Virology 287:18-29. [DOI] [PubMed] [Google Scholar]
- 19.Foster, T. P., X. Alvarez, and K. G. Kousoulas. 2003. Plasma membrane topology of syncytial domains of herpes simplex virus type 1 glycoprotein K (gK): the UL20 protein enables cell surface localization of gK but not gK-mediated cell-to-cell fusion. J. Virol. 77:499-510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Foster, T. P., and K. G. Kousoulas. 1999. Genetic analysis of the role of herpes simplex virus type 1 glycoprotein K in infectious virus production and egress. J. Virol. 73:8457-8468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Foster, T. P., G. V. Rybachuk, X. Alvarez, O. Borkhsenious, and K. G. Kousoulas. 2003. Overexpression of gK in gK-transformed cells collapses the Golgi apparatus into the endoplasmic reticulum inhibiting virion egress, glycoprotein transport, and virus-induced cell fusion. Virology 317:237-252. [DOI] [PubMed] [Google Scholar]
- 22.Harrison, R. E., C. Bucci, O. V. Vieira, T. A. Schroer, and S. Grinstein. 2003. Phagosomes fuse with late endosomes and/or lysosomes by extension of membrane protrusions along microtubules: role of Rab7 and RILP. Mol. Cell. Biol. 23:6494-6506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hoggan, M. D., and B. Roizman. 1959. The isolation and properties of a variant of Herpes simplex producing multinucleated giant cells in monolayer cultures in the presence of antibody. Am. J. Hyg. 70:208-219. [DOI] [PubMed] [Google Scholar]
- 24.Hutchinson, L., K. Goldsmith, D. Snoddy, H. Ghosh, F. L. Graham, and D. C. Johnson. 1992. Identification and characterization of a novel herpes simplex virus glycoprotein, gK, involved in cell fusion. J. Virol. 66:5603-5609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hutchinson, L., and D. C. Johnson. 1995. Herpes simplex virus glycoprotein K promotes egress of virus particles. J. Virol. 69:5401-5413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hutchinson, L., C. Roop-Beauchamp, and D. C. Johnson. 1995. Herpes simplex virus glycoprotein K is known to influence fusion of infected cells, yet is not on the cell surface. J. Virol. 69:4556-4563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jayachandra, S., A. Baghian, and K. G. Kousoulas. 1997. Herpes simplex virus type 1 glycoprotein K is not essential for infectious virus production in actively replicating cells but is required for efficient envelopment and translocation of infectious virions from the cytoplasm to the extracellular space. J. Virol. 71:5012-5024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Klupp, B. G., R. Nixdorf, and T. C. Mettenleiter. 2000. Pseudorabies virus glycoprotein M inhibits membrane fusion. J. Virol. 74:6760-6768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee, G. T., and P. G. Spear. 1980. Viral and cellular factors that influence cell fusion induced by herpes simplex virus. Virology 107:402-414. [DOI] [PubMed] [Google Scholar]
- 30.Linstedt, A. D., and H. P. Hauri. 1993. Giantin, a novel conserved Golgi membrane protein containing a cytoplasmic domain of at least 350 kDa. Mol. Biol. Cell 4:679-693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Little, S. P., and P. A. Schaffer. 1981. Expression of the syncytial (syn) phenotype in HSV-1, strain KOS: genetic and phenotypic studies of mutants in two syn loci. Virology 112:686-702. [DOI] [PubMed] [Google Scholar]
- 32.McGeoch, D. J., C. Cunningham, G. McIntyre, and A. Dolan. 1991. Comparative sequence analysis of the long repeat regions and adjoining parts of the long unique regions in the genomes of herpes simplex viruses types 1 and 2. J. Gen. Virol. 72:3057-3075. [DOI] [PubMed] [Google Scholar]
- 33.McGeoch, D. J., M. A. Dalrymple, A. J. Davison, A. Dolan, M. C. Frame, D. McNab, L. J. Perry, J. E. Scott, and P. Taylor. 1988. The complete DNA sequence of the long unique region in the genome of herpes simplex virus type 1. J. Gen. Virol. 69:1531-1574. [DOI] [PubMed] [Google Scholar]
- 34.Mo, C., and T. C. Holland. 1997. Determination of the transmembrane topology of herpes simplex virus type 1 glycoprotein K. J. Biol. Chem. 272:33305-33311. [DOI] [PubMed] [Google Scholar]
- 35.Morse, L. S., L. Pereira, B. Roizman, and P. A. Schaffer. 1978. Anatomy of herpes simplex virus (HSV) DNA. X. Mapping of viral genes by analysis of polypeptides and functions specified by HSV-1 × HSV-2 recombinants. J. Virol. 26:389-410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Muggeridge, M. I. 2000. Characterization of cell-cell fusion mediated by herpes simplex virus 2 glycoproteins gB, gD, gH and gL in transfected cells. J. Gen. Virol. 81:2017-2027. [DOI] [PubMed] [Google Scholar]
- 37.Novotny, M. J., M. L. Parish, and P. G. Spear. 1996. Variability of herpes simplex virus 1 gL and anti-gL antibodies that inhibit cell fusion but not viral infectivity. Virology 221:1-13. [DOI] [PubMed] [Google Scholar]
- 38.Olson, J. K., and C. Grose. 1997. Endocytosis and recycling of varicella-zoster virus Fc receptor glycoprotein gE: internalization mediated by a YXXL motif in the cytoplasmic tail. J. Virol. 71:4042-4054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Papini, E., B. Satin, C. Bucci, M. de Bernard, J. L. Telford, R. Manetti, R. Rappuoli, M. Zerial, and C. Montecucco. 1997. The small GTP binding protein rab7 is essential for cellular vacuolation induced by Helicobacter pylori cytotoxin. EMBO J. 16:15-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pasieka, T. J., L. Maresova, and C. Grose. 2003. A functional YNKI motif in the short cytoplasmic tail of varicella-zoster virus glycoprotein gH mediates clathrin-dependent and antibody-independent endocytosis. J. Virol. 77:4191-4204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pereira, L., D. Dondero, B. Norrild, and B. Roizman. 1981. Differential immunologic reactivity and processing of glycoproteins gA and gB of herpes simplex virus types 1 and 2 made in Vero and HEp-2 cells. Proc. Natl. Acad. Sci. USA 78:5202-5206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pertel, P. E., A. Fridberg, M. L. Parish, and P. G. Spear. 2001. Cell fusion induced by herpes simplex virus glycoproteins gB, gD, and gH-gL requires a gD receptor but not necessarily heparan sulfate. Virology 279:313-324. [DOI] [PubMed] [Google Scholar]
- 43.Pertel, P. E., and P. G. Spear. 1997. Partial resistance to gD-mediated interference conferred by mutations affecting herpes simplex virus type 1 gC and gK. J. Virol. 71:8024-8028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pogue-Geile, K. L., G. T. Lee, S. K. Shapira, and P. G. Spear. 1984. Fine mapping of mutations in the fusion-inducing MP strain of herpes simplex virus type 1. Virology 136:100-109. [DOI] [PubMed] [Google Scholar]
- 45.Pogue-Geile, K. L., and P. G. Spear. 1987. The single base pair substitution responsible for the Syn phenotype of herpes simplex virus type 1, strain MP. Virology 157:67-74. [DOI] [PubMed] [Google Scholar]
- 46.Post, L. E., S. Mackem, and B. Roizman. 1981. Regulation of alpha genes of herpes simplex virus: expression of chimeric genes produced by fusion of thymidine kinase with alpha gene promoters. Cell 24:555-565. [DOI] [PubMed] [Google Scholar]
- 47.Ramaswamy, R., and T. C. Holland. 1992. In vitro characterization of the HSV-1 UL53 gene product. Virology 186:579-587. [DOI] [PubMed] [Google Scholar]
- 48.Roop, C., L. Hutchinson, and D. C. Johnson. 1993. A mutant herpes simplex virus type 1 unable to express glycoprotein L cannot enter cells, and its particles lack glycoprotein H. J. Virol. 67:2285-2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rovero, S., A. Amici, E. D. Carlo, R. Bei, P. Nanni, E. Quaglino, P. Porcedda, K. Boggio, A. Smorlesi, P. L. Lollini, L. Landuzzi, M. P. Colombo, M. Giovarelli, P. Musiani, and G. Forni. 2000. DNA vaccination against rat her-2/Neu p185 more effectively inhibits carcinogenesis than transplantable carcinomas in transgenic BALB/c mice. J. Immunol. 165:5133-5142. [DOI] [PubMed] [Google Scholar]
- 50.Ruyechan, W. T., L. S. Morse, D. M. Knipe, and B. Roizman. 1979. Molecular genetics of herpes simplex virus. II. Mapping of the major viral glycoproteins and of the genetic loci specifying the social behavior of infected cells. J. Virol. 29:677-697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sanders, P. G., N. M. Wilkie, and A. J. Davison. 1982. Thymidine kinase deletion mutants of herpes simplex virus type 1. J. Gen. Virol. 63:277-295. [DOI] [PubMed] [Google Scholar]
- 52.Showalter, S. D., M. Zweig, and B. Hampar. 1981. Monoclonal antibodies to herpes simplex virus type 1 proteins, including the immediate-early protein ICP 4. Infect. Immun. 34:684-692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Spear, P. G. 1992. Membrane fusion induced by herpes simplex virus, p. 201-232. In J. Bentz (ed.), Viral fusion mechanism. CRC Press, Boca Raton, Fla.
- 54.Spear, P. G., R. J. Eisenberg, and G. H. Cohen. 2000. Three classes of cell surface receptors for alphaherpesvirus entry. Virology 275:1-8. [DOI] [PubMed] [Google Scholar]
- 55.Tatu, U., and A. Helenius. 1997. Interactions between newly synthesized glycoproteins, calnexin and a network of resident chaperones in the endoplasmic reticulum. J. Cell Biol. 136:555-565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Turner, A., B. Bruun, T. Minson, and H. Browne. 1998. Glycoproteins gB, gD, and gHgL of herpes simplex virus type 1 are necessary and sufficient to mediate membrane fusion in a Cos cell transfection system. J. Virol. 72:873-875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ward, P. L., G. Campadelli-Fiume, E. Avitabile, and B. Roizman. 1994. Localization and putative function of the UL20 membrane protein in cells infected with herpes simplex virus 1. J. Virol. 68:7406-7417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhou, G., E. Avitabile, G. Campadelli-Fiume, and B. Roizman. 2003. The domains of glycoprotein D required to block apoptosis induced by herpes simplex virus 1 are largely distinct from those involved in cell-cell fusion and binding to nectin1. J. Virol. 77:3759-3767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhou, G., V. Galvan, G. Campadelli-Fiume, and B. Roizman. 2000. Glycoprotein D or J delivered in trans blocks apoptosis in SK-N-SH cells induced by a herpes simplex virus 1 mutant lacking intact genes expressing both glycoproteins. J. Virol. 74:11782-11791. [DOI] [PMC free article] [PubMed] [Google Scholar]