Abstract
Between 1975 and 2014, housing conditions for laboratory-housed marmosets changed dramatically after the introduction of new guidelines designed to improve their care and wellbeing. According to these guidelines, our facility provided marmosets with outside enclosures, switched to deep litter as bedding material, and discontinued the use of disinfectant agents in animal enclosures. However, both deep litter and access to outside enclosures hypothetically increase the risk of potential exposure to pathogenic microorganisms. We evaluated whether these housing and husbandry modifications constituted an increased veterinary risk for laboratory-housed common marmosets (Callithrix jacchus). After the animals had been exposed to these new housing conditions for 2.5 y, we examined their intestinal bacterial flora and feces, the deep litter, and insects present in the housing. In addition, we assessed the marmosets’ general health and the effect of outdoor housing on, for example, vitamin D levels. Although numerous bacterial strains—from nonpathogenic to potentially pathogenic—were cultured, we noted no increase in illness, mortality, or breeding problems related to this environmental microflora. Housing laboratory marmosets in large enriched cages, with both indoor and outdoor enclosures, providing them with deep litter, and eliminating the use of disinfectants present an increased veterinary risk. However, after evaluating all of the collected data, we estimate that the veterinary risk of the new housing conditions is minimal to none in terms of clinical disease, disease outbreaks, abnormal behavior, and negative effects on reproduction.
The Biomedical Primate Research Centre (Rijswijk, The Netherlands) houses a self-sustaining breeding colony of common marmosets (Callithrix jacchus) for the purpose of conducting biomedical research on life-threatening human diseases. The marmoset colony was formed in 1975 and has been used mainly for research on autoimmune diseases, neurodegenerative disorders, and comparative genetics.3,5,6,14,21,29
After the introduction of new European and Dutch guidelines regarding animal care and welfare, animal housing conditions changed markedly between 1975 and 2014. Our facility responded promptly to these new guidelines, by providing larger and more complex cages comprising outdoor enclosures, each with an attached heated indoor enclosure, where the animals are housed in family groups to improve animal wellbeing.22,35 The concept of environmental enrichment continued to be developed and optimized over the years.
Potential benefits of outdoor enclosures are exposure to seasonal fluctuations in light and climate and increased sensory stimulation. These enclosures provide opportunities for exploration and manipulation that are considered to contribute positively to the animals’ wellbeing. Furthermore, marmosets housed indoors with no access to UV light are susceptible to metabolic bone diseases. Marmosets cannot synthesize vitamin D3 (cholecalciferol) from the plant form of the vitamin (ergocalciferol, vitamin D2). Without access to UVB radiation, they cannot form vitamin D3 from 7-dehydroxycholesterol in the skin.31 In addition to dietary supplementation with vitamin D3, we surmised that access to unfiltered sunlight in outside enclosures would limit or prevent vitamin D deficiency.
Another change initiated in response to BPRC's new housing guidelines was the cleaning of the housing facilities. Scent marking is an important aspect of the natural behavior of marmosets. In laboratory settings, marmosets scent-mark their cages constantly.11 To minimize the removal of scents, disinfectants are no longer used to clean the enclosures. In addition to effects on scent, limiting disinfectant use could have other beneficial effects. For example, the chemical disinfection of their environment was suggested to be one cause of chromosomal disorders in marmosets.13
A third important housing-related change was the provision of deep litter in the outdoor and indoor enclosures. Deep litter is a floor covering, preferably of organic origin, that promotes activities including locomotion, foraging, and playing. In general, the changes associated with providing deep litter typically involved a shift in the animals’ behavioral profiles toward those that might be observed in their wild counterparts; therefore, the provision of deep litter is seen as environmental enrichment.7,9,10,24,30,32
Although some of these changes have been implemented in zoos, primate centers that breed marmosets for research purposes have been more reticent because of potential health issues. To evaluate whether the new housing conditions enhance the animals’ wellbeing, we studied their benefits and potential threats to the animals, the practical consequences for personnel and management, and the influence on experimental results. In particular, the health risks for the marmosets due to increased microbiologic exposure because of the new housing conditions were examined. The aim of the study was to determine whether these changes in their housing constituted not only an improvement in their wellbeing but also a possible increased veterinary risk for laboratory-housed common marmosets.
Materials and Methods
Animals.
Formed in 1975, the marmoset colony at the Biomedical Primate Research Centre (Rijswijk, The Netherlands) consisted initially of animals obtained from various accredited suppliers (only captive-bred animals were included). Later, new breeding lines were introduced on several occasions to maintain the outbred character of the colony. Imported animals were released into the colony after a 12-wk quarantine period, which included monthly bacteriologic examinations of rectal swabs, parasitologic examinations of feces, tuberculin skin tests, physical examinations, and hematology and serum biochemistry analyses.
The colony continuously includes around 30 breeding groups comprising a total of approximately 150 animals, ranging from infants to adults older than 12 y. Marmosets were maintained as monogamous breeding pairs, sharing their accommodation with successive sets of offspring. The offspring remained with their family group for as long as possible, that is, until either the dam or sire or both parents rejected them or until they were selected for experimental use (at least 1.5 y old).
Before the guideline-associated changes, the marmosets were housed in wire-mesh cages with a solid bottom and contact bedding (Lignocel 3-4S, J Rettenmaier and Sohne, Rosenberg, Germany). Cages were cleaned with hot water once each week; the surrounding corridors were disinfected once weekly with Halamid-d (Chloramine-T, Veip, Wijk bij Duurstede, the Netherlands). Beginning in 2005, animals were housed in outdoor enclosures with heated indoor enclosures; the marmosets were able to move freely between the 2 environments. Both enclosures measured 300 × 200 × 300 cm (Figure 1 A and B). The marmosets’ environmental enrichment was optimized by using a complex system of fixed and swinging branches, ropes, nets, and wooden runways. The bedding in the enclosures was deep litter consisting of French pine (Pinus pinastre; Van Dijk Groothandel Biofiltratie, Veenendaal, the Netherlands Figure 2). Neither enclosure was cleaned; however, in the inside enclosure, the surface of the deep litter was raked and sprinkled with water twice each month.
Figure 1.
(A) Outdoor and (B) indoor marmoset enclosures at the BPRC. Environmental enrichment consists of a system of fixed and swinging branches, ropes, nets, and wooden runways. The animals are able to move freely between the 2 environments at all times.
Figure 2.
Deep litter as bedding in the indoor enclosure. The insert shows mealworms (Tenebrio molitor) that the marmosets can pick out of the bedding. Other insects that can be found in the bedding include woodlice, small spiders, and ants.
The temperature in the indoor enclosure was maintained between 26 °C and 28 °C with a relative humidity between 50% and 60% and a 12:12-h The light:dark cycle (lights on, 0700 to 1900). Lighting in the indoor enclosures was provided by full-spectrum fluorescent bulbs placed close to the cages. The room ventilation rate was around 8 air changes hourly.
The daily diet consisted of commercial primate pellets for New World Monkeys (Sniff, Soest, Germany) offered free choice and supplemented daily with limited amounts of fresh fruit, gum Arabic, and a homemade porridge. Vitamin D3 levels provided in the pellets were 3000 IU per kg. Additional vitamin D3 (Davitamon Vitamine D Aquosum, Omega Pharma Nederland, Rotterdam, the Netherlands) was provided in the porridge (12 IU per marmoset). In 2003, a new feeding regimen including live insects (for example, mealworms, crickets, and grasshoppers) was introduced, with insects being supplied by a regular pet shop. Tap water was provided free choice by way of automatic watering nipples.
All procedures performed in this study were in accordance with the regulations for animal handling as described in European Union Directive 63/2010 and the Weatherall report (2006).33
Veterinary risk evaluation.
Approximately 2.5 y after the introduction of the new housing conditions (July 2008), microbiologic profiles of the deep litter, insects, rectal swabs, and fresh stool samples were evaluated. Data from the rectal swabs and fresh stool samples were compared with those of samples collected in 2008 and 2009. In addition, health records and reproduction parameters during 2004 (old housing conditions) and 2008 (new housing conditions) were compared. Health records collected before 2004 were not analyzed, because initiation of pseudotuberculosis (Pseudovac) vaccination at the end of 20034 positively influenced mortality and health records tremendously.
Veterinary care and health monitoring.
Every year, each marmoset underwent complete physical, hematologic, and biochemical examination and tuberculin skin test for tuberculosis. In addition, the colony was monitored annually for presence of various bacteria and parasites (examination of rectal swabs and fresh stool samples). To discern any outward signs of disease, animal caretakers examined marmosets at least twice daily for injuries and for changes in behavior and fecal consistency. Abnormalities were reported to the veterinarians, and daily health records were kept for each animal.
Acute outbreaks of Yersinia spp. infection occurred in 2001, 2002, and 2003.4 Therefore, from 2003 onward, all marmosets were vaccinated every 6 mo with Pseudovac (obtained from the Department of Veterinary Pathology, Zoo and Exotic Animals Section, Utrecht University, Utrecht, The Netherlands), which effectively prevented additional outbreaks of Yersinia.
Pathology.
All animals in the breeding colony that died or were euthanized were examined by our veterinary pathologist.
Microbiologic tests.
During the annual veterinary check-up in July 2008 (n = 167 animals), 2.5 y after introduction of the new housing conditions, we collected samples of deep litter, insects, rectal swabs, and fresh stool for microbiologic examination. Beginning in 2009, only rectal swabs and fresh stool samples were collected.
Rectal swabs were obtained from sedated marmosets by inserting a swab 2 cm and spinning the swab for several full rotations. The swab was placed immediately in a tube filled with charcoal transport medium (Copan Italia, Brescia, Italy). In addition, 5 fresh fecal samples were picked randomly from every indoor enclosure (34 cages) and fixed in sodium acetate–acetic acid–formalin.
We collected a total of 10 samples of deep litter (5 superficial, 5 deep) from every indoor enclosure (34 cages). The samples were added to 0.9% saline solution (sterile) and fixed in sodium acetate–acetic acid–formalin.
Insects were identified according to their morphologic features by an expert in this field. Crickets (n = 5 per cage), mealworms (n = 5 per cage), and woodlice (n = 5 per cage) were collected at random from every cage. In addition, 3 grasshoppers were collected directly from the delivery box when it arrived at our facility. The insects were squeezed directly and fixed in sodium acetate–acetic acid–formalin.
All fixed samples of litter were centrifuged at 2.8 × g for 10 min. The pellets were inoculated on Columbia agar with 5% sheep blood (Becton Dickinson, Heidelberg, Germany) for growth of facultative anaerobic bacteria. The rectal swabs were inoculated on xylose–lysine–deoxycholate agar (Becton Dickinson) for growth of both Salmonella spp. and Shigella spp., cefsulodin–irgasan–novobiocin agar (Becton Dickinson) for Yersinia spp., CHROM O157 (Becton Dickinson) for growth of Escherichia coli O157, cefoperazone–vancomycin–amphotericin agar (Becton Dickinson) for Campylobacter spp., and CDC Anaerobe Blood Agar with 5% sheep blood (Becton Dickinson) for obligate anaerobe bacteria. All agar plates were examined after 24 and 48 h incubation at 35 °C. API bacterial identification products (BioMérieux, Boxtel, The Netherlands) were used to identify gram-positive and gram-negative bacteria.
In addition, fixed stool specimens were concentrated according to the modified Ridley–Allen technique.1 After iodine staining, the concentrate was examined microscopically for metazoans and metazoan eggs. Trichrome staining was done for the microscopic examination of protozoan cysts and trophozoites, and Ziehl–Neelsen staining was used to identify Microsporidia spp., Cryptosporidium spp., Cyclospora spp., and Isospora spp.
Reproduction.
Reproductive data including birth interval, number of offspring per parturition, and number of abortions and stillbirths were obtained for 7 female marmosets (age at first parturition, 1.7 to 5.4 y) of breeding pairs that had been housed under both the old and new conditions.
Vitamin D level.
For a separate study (DEC-BPRC no. 676; January 2013 through February 2014), a group of 21 marmosets was sedated for monthly radiographs, and monthly blood sampling was performed to determine vitamin D level. All blood samples (2 mL each) were drawn during alphaxalone sedation (12 mg/kg IM). Samples were collected from the vena femoralis into serum tubes (Greiner Bio-one, Linz, Austria), left for 30 min, and centrifuged at 1.9 × g for 10 min. Serum was transferred to polypropylene tubes, frozen within 1 h of collection, and stored upright below –20 °C for 0 to 2 y until assayed. All measurements were performed by using an automated clinical chemistry analyzer in a medical laboratory (Reinier de Graaf Groep Diagnostisch Centrum SSDZ, Delft, the Netherlands).
Use of outside enclosures by the animals.
In November and December 2008, a behavioral study was conducted to determine how often the marmosets used their indoor and outdoor enclosures. A total of 48 animals living in 12 different groups of 2, 4 and 6 animals were monitored for 7 wk by 2 students; one observed the indoor enclosure, while the other simultaneously observed the outside enclosure. Every animal was observed 8 times for 15 min. During those 15 min the time spent in the indoor or outdoor enclosure was registered using focal animal sampling.
Results
Microorganisms in the litter, insects, and feces and components of the intestinal bacterial flora.
Numerous microorganisms were detected in the July 2008 samples of litter from the marmosets’ enclosures: Acinetobacter baumannii, Aerococcus viridans, Aeromonas hydrophilia, Aeromonas sobria, Alcaligenes faecalis, Aspergillus flavus, Aspergillus nidulans, Bacillus cereus, Burkholderia cepacia, Chryseobacterium indologenes, Citrobacter freundii, Clostridium perfringens, Comamonas testosterone, Enterobacter aerogenes, Enterobacter amnigenus, Enterococcus avium, Enterococcus durans, Enterobacter cloacae, Enterococcus faecalis, Escherichia coli, Ewingella americana, Klebsiella pneumonia, Lactococcus lactis, Listeria spp., Micrococcus spp., Microsporidia spores, Moraxella spp., Ochromobactrum anthropic, Pantoea spp., Pasteurella pneumotropica, Proteus mirabilis, Proteus vulgaris, Pseudomonas fluorescens, Pseudomonas luteola, Pseudomonas orzyhabitans, Pseudomonas putida, Ralstonia picketti, Raoultella terrigena, Rhizobium radiobacter, Serratia plymuthica, Serratia rubidaea, Shewanella putrefaciens, Sphingomonas paucimobilis, Staphylococcus cohnii, Staphylococcus saprophyticus, Staphylococcus sciuri, Staphylococcus xylosus, Stenotrophomonas maltophilia, Vibrio parahaemolyticus, and Weeksella virosa. Most microorganisms that were cultured belong to the normal microflora expected in marmosets living in new, open, natural-setting, housing conditions.16 Percentages of potential pathogens, including facultative pathogenic bacteria and opportunistic pathogenic bacteria, cultured from the tested substrates are shown in Table 1. More than 50% of the rectal swabs were positive for Escherichia coli, but E. coli O157:H7 was never cultured. Although the presence of various microorganisms shifted somewhat when the tests were repeated 1 y later, most of the results were similar (Table 2).
Table 1.
Microbiologic results (% of samples positive for pathogens, including facultative and opportunistic pathogenic bacteria) of the substrates tested in 2008
| Deep litter | Cricket | Grasshopper | Woodlice | Feces | Rectal swab | |
| Aeromonas spp. | 24.0 | 0 | 66.7 | 18.2 | 15.4 | 6.5 |
| Aspergillus flavus | 16.0 | 16.7 | 0 | 9.1 | 2.2 | 0 |
| Burkholderia cepacia | 20.0 | 0 | 0 | 18.2 | 11.0 | 0 |
| Citrobacter freundii | 0 | 0 | 0 | 0 | 0 | 21.0 |
| Clostridium perfringens | 12.0 | 0 | 0 | 0 | 19.2 | 11.0 |
| Enterobacter aerogenes | 0 | 0 | 0 | 0 | 0 | 11.0 |
| Enterobacter cloacae | 0 | 0 | 0 | 0 | 0 | 0.9 |
| Enterococcus faecium | 0 | 0 | 0 | 0 | 0 | 4.0 |
| Escherichia coli | 0 | 0 | 0 | 0 | 0 | 54.9 |
| Klebsiella oxytoca | 0 | 0 | 0 | 0 | 0 | 1.8 |
| Klebsiella pneumoniae | 0 | 0 | 0 | 0 | 0 | 6.4 |
| Proteus spp. | 32.0 | 16.7 | 0 | 18.2 | 45.6 | 22.9 |
| Pseudomonas spp. | 4.0 | 0 | 0 | 0 | 9.6 | 5.5 |
| Vibrio parahaemolyticus | 20.0 | 16.7 | 66.7 | 27.3 | 41.9 | 0 |
Table 2.
Overview of microbiologic results from fecal samples and rectal swabs tested in 2008 and 2009
| Feces |
Rectal swab |
|||
| 2008 | 2009 | 2008 | 2009 | |
| Aeromonas hydrophilia | + | + | - | + |
| Enterobacter aerogenes | + | - | + | + |
| Klebsiella pneumoniae | + | - | + | + |
| Pseudomonas luteola | + | - | + | - |
| Vibrio parahaemolyticus | + | - | - | - |
+, ≥ 5% of samples tested positive for bacteria; –, < 5% of samples were positive for bacterial growth.
Clinical illness.
Animal caretakers and veterinarians did not note any changes in behavior or fecal consistency, monitored as overt signs of disease, when comparing previous with new housing conditions. In addition, no abnormalities were found during the annual veterinary check-up.
Pathology.
The percentage of marmosets that presented each year for necropsy after implemention of the new housing requirements (2007, 9.8%; 2008, 7.5%; 2009, 5.2%; 2010, 7.2%; 2011, 5.9%; 2012, 3.0%; and 2013, 4.0%) was similar to that under the previous conditions (2004, 7.3%; 2005, 6.4%).
Histopathologic examination of tissue samples from euthanized (sick) marmosets with deteriorating health or those that died suddenly revealed lesions consistent with mild to moderate chronic enterocolitis, chronic fibrosing glomerulonephritis, and lymphocytic interstitial nephritis. These lesions are common incidental findings in marmosets (so-called ‘wasting marmoset syndrome’).8,12,19,23 We noted no negative differences when we compared samples from marmosets housed under the new compared with previous conditions.
Vitamin D levels.
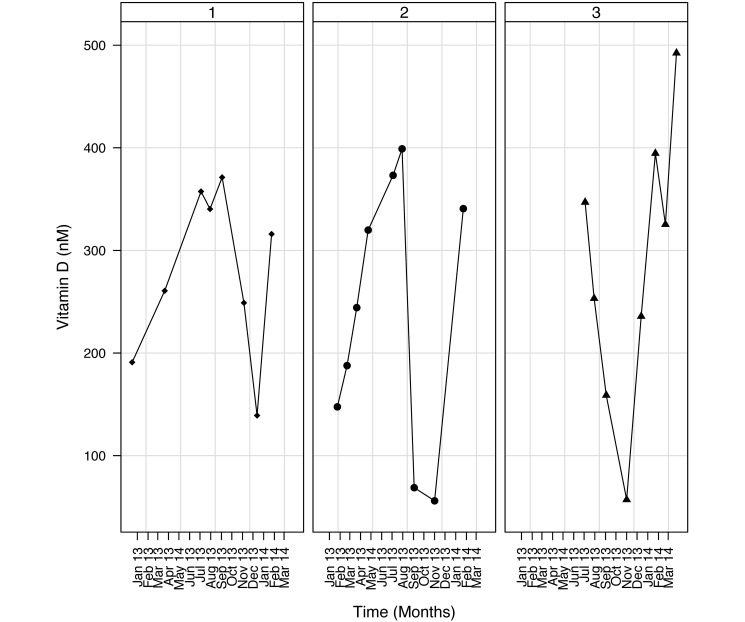
Determination of the vitamin D levels for 14 mo in the marmosets of the breeding colony showed that the peak concentrations of vitamin D were during the summer months, whereas the lowest values were observed during the winter months. This pattern occurred consistently in all animals studied (Figure 3).
Figure 3.
Data of 3 randomly selected animals. All sampled marmosets were housed in the breeding colony and had unlimited access to an outdoor enclosure. Vitamin D levels were monitored monthly between January 2013 and March 2014.
Reproduction.
Under the former housing conditions, the average interbirth interval was 168 d, with 2.3 offspring per parturition (n = 29 parturitions). The first parturition in the new housing conditions occurred a mean of 211 d after the last one in the previous housing, with 2.9 off spring per parturition (n = 7 parturitions). Subsequent parturitions showed an interbirth interval of 168 d, with 2.6 offspring per parturition (n = 32 parturitions). Under both conditions, the births were not seasonally restricted, nor were there any abortions or stillbirths.
Macroscopic observation of the deep litter of the indoor enclosures.
No large areas with feces and dropped food were present. Woodlice, crickets, and ants (Hypoponera schauinslandi emery) were seen daily on the surface of the deep litter (data not shown). Grasshoppers were present only after they had been offered to the marmosets as food (probably escaped insects). Fungi were macroscopically visible as well.
Use of outside enclosures by the animals.
We did not expect the marmosets to enter the outside enclosures during winter. However, all 48 animals spent an average of 3.2% outside while they were being observed, albeit this duration is much shorter than that during spring and summer, when the permanent animal caretakers report that the marmosets spend substantial time in the outdoor enclosures.
Discussion
Over the past couple of decades, major changes in the husbandry and housing conditions for common marmosets at our facility have included the provision of indoor–outdoor enclosures with deep litter and the limited use of disinfectants. After evaluating all of the collected data, we estimate that the veterinary risk of the new housing conditions is minimal to none in terms of clinical disease, disease outbreaks, abnormal behavior, and negative effects on reproduction.
Deep litter has been used for some time on pig farms,18,25,26,34 but is fairly new to both zoos15 and laboratory facilities. Bedding is normally used to bind excretions to keep the animals’ living environment dry and comfortable. In addition to feces and urine, the soiled bedding contains animal hair and dander and spoiled food, thus providing a breeding environment for bacteria and fungi. The microorganisms in the bedding may be spread by way of dust particles to the breathing zone of both personnel and animals, constituting a health risk during various care or experimental procedures.20 Adequate ventilation in animal rooms minimizes this exposure. Although fungi were present inside the enclosures, no disease-associated side effects were noted in either the marmosets or their caretakers.
Most of the bacteria we obtained were harmless environmental bacteria; several were facultatively or opportunistically pathogenic.16 Having colony animals that act as ‘carriers’ of pathogenic to facultatively pathogenic bacteria is a potential veterinary risk; for example, Klebsiella-related mortality has been described.17 However, we noted no increase in clinical disease, disease outbreaks, abnormal behavior, or negative effects on reproduction during the study period; therefore no treatment was deemed necessary. Currently, the marmosets have been housed in the enriched environment for more than 8 y, during which time no negative veterinary and behavioral effects due to the described housing conditions have occurred.
The disadvantages of using deep litter do not outweigh its advantages. The potential negative of providing deep litter as bedding on animal wellbeing include possible excessive vermin reproduction (which can promote disease), increased ammonia and moisture levels, and the presence of pathogenic microorganisms. In contrast, providing deep litter creates enrichment for the animals, a welfare benefit. Furthermore, the decrease in the frequency with which cages need to be cleaned means less frequent handling and disturbance of the animals, thus allowing the marmosets to remain within their scent-marked environment. Potentially the litter may never need to be changed; however, in the absence of supporting data, we completely replaced the litter after 5 y. When deep litter is used, the system must be kept as natural as possible to allow the feces, urine, and food deposited on top of the litter to decompose biologically, which occurs at the bottom of the litter layer. Therefore, several factors need to be considered, especially those that affect the decomposition rate, such as type and number of microorganisms, temperature, humidity, the percentage of oxygen, and pH. To optimize these factors, the surface of the deep litter must be raked and sprinkled with water twice each month. Macroscopically, the deep litter appeared to have absorbed the feces and dropped food, because no large areas of feces and dropped food were apparent.
Deep litter in the indoor enclosures seems to be a good environment for some insect species to live and even to reproduce in. This environment includes not only physical factors, such as climate and available food, but also the presence or absence of predators and other members of a population. The origin of crickets, grasshoppers, and mealworms was probably escaped insects, because these species were offered weekly as food. Other species, like woodlice, were not offered as food but migrated into the deep litter. We experienced several limited outbreaks of small spiders (species not determined) and ants (Hypoponera schauinslandi emery), which might be bothersome for the animal caretakers. However, we noted no effect on the marmosets themselves.
The microbial ecosystem in the deep litter is acquired naturally: the litter itself is not sterilized, and the marmosets have their own microbiota. In addition, their fecal waste contains high amounts of intestinal bacteria, which may or may not grow in the bedding material. Cross-infection between insects, animals, and deep litter should not be excluded, given that we cultured Aeromonas spp. and Vibrio parahaemolyticus from most of the tested samples. In addition, these genera of microorganisms were cultured from fecal samples but not from rectal swabs. Furthermore, Shigella spp. was cultured once from crickets that were obtained from a pet shop and that were provided weekly as part of the marmosets’ diet. Therefore, in 2009, we decided to discontinue providing crickets, even though Shigella was not cultured from any of the substrates tested in 2008 and 2009.
Opinions vary concerning the benefits and disadvantages of outdoor enclosures. In addition to food supplementation with vitamin D3, providing the marmosets access to unfiltered sunlight in outside enclosures might limit or prevent the potential problem of vitamin D deficiency. The results of the monthly blood sampling demonstrate season-dependent changes in vitamin D levels, strongly indicating the influence of sunlight. This seasonal influence doesn't occur in marmosets with access to indoor facilities only.2 This finding strongly supports the observation that outdoor facilities are beneficial for marmosets in regard to vitamin D levels. Another benefit of outdoor enclosures is exposure to seasonal fluctuations in light and climate, which produce physiologic and behavioral changes and may contribute positively to the animals’ wellbeing. These conditions contrast with the very stable temperature, humidity, and light conditions inside laboratory holding rooms. In addition, outdoor enclosures provide the animals with more sensory stimulation and more complex environments, which provide greater opportunities for exploration and manipulation, for example forage for insects and watching birds.28 An additional advantage of free access to outdoor enclosures is that partition of the available space into 2 separate enclosures may reduce stress, because of the increased opportunities to avoid aggressive encounters.27 However, outdoor enclosures pose some disadvantages. First, outdoor enclosures provide the potential risk of disease transmission from outside vectors. The animals should be vaccinated to protect them against infections that might be present in bird and rodent droppings, such as Yersinia spp.4 Another veterinary risk involves potential access to toxic living plants. Although all of these points are valid, these risks likely occur at very low frequencies, and the disadvantages are outweighed by positive behavioral changes.
One study that included chromosomal analyses of marmosets from 2 colonies showed that the levels of chromosomal disorders differed between the colonies.13 One of the biggest differences between the 2 colonies is that chlorine-based disinfectants were used at the center with more chromosomal disorders, whereas no chemical disinfection was applied at the other center.13 The authors suggested that an increased rate of chromosomal disorders in marmosets might be related to the chemical disinfection of their environment.13
The size of the living space provided for each marmoset has increased considerably over past decades, and we now house our colony animals in large enriched cages that have both indoor and outdoor enclosures. The modified housing conditions improve animal wellbeing in conjunction with an acceptable level of increased veterinary risk. The minimal increased veterinary risk associated with these new housing conditions is far outweighed by the improvement in the marmosets’ wellbeing.
Acknowledgments
We thank Donna Devine and Thea de Koning for editing the manuscript and Fred Reeder for determination of the ant species. This study was funded in part by EUPRIM-NET 2, European Community grant agreement number 262443.
References
- 1.Allen AV, Ridley DS. 1970. Further observations on the formol–ether concentration technique for faecal parasites. J Clin Pathol 23:545–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Angeliewa A. 2004. Optimization of the diet of the marmoset (Callithrix jacchus) under special consideration of the osseous metabolism and its monitoring by means of biochemical and densitometric methods, p 177. Berlin (Germany): Free University of Berlin, Institute for Animal Welfare and Behavior; [Article in German]. [Google Scholar]
- 3.Antunes SG, de Groot NG, Brok H, Doxiadis G, Menezes AA, Otting N, Bontrop RE. 1998. The common marmoset: a New World primate species with limited MHC class II variability. Proc Natl Acad Sci U S A 95:11745–11750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bakker J, Kondova I, de Groot C, Remarque EJ, Heidt PJ. 2007. A report on Yersinia-related mortality in a colony of New World Monkeys. Laboratory primate newsletter 46:11–16. [Google Scholar]
- 5.Brok HP, Uccelli A, Kerlero De Rosbo N, Bontrop RE, Roccatagliata L, de Groot NG, Capello E, Laman JD, Nicolay K, Mancardi GL, Ben-Nun A, Hart BA. 2000. Myelin/oligodendrocyte glycoprotein-induced autoimmune encephalomyelitis in common marmosets: the encephalitogenic T cell epitope pMOG24-36 is presented by a monomorphic MHC class II molecule. J Immunol 165:1093–1101. [DOI] [PubMed] [Google Scholar]
- 6.Brok HP, van Meurs M, Blezer E, Schantz A, Peritt D, Treacy G, Laman JD, Bauer J, ’t Hart BA. 2002. Prevention of experimental autoimmune encephalomyelitis in common marmosets using an antiIL12p40 monoclonal antibody. J Immunol 169:6554–6563. [DOI] [PubMed] [Google Scholar]
- 7.Byrne GD, Suomi SJ. 1991. Effects of woodchips and buried food on behavior patterns and psychological wellbeing of captive rhesus monkeys. A J Primatol 23:141–151. [DOI] [PubMed] [Google Scholar]
- 8.Chalifoux LV, Bronson RT, Escajadillo A, McKenna S. 1982. An analysis of the association of gastroenteric lesions with chronic wasting syndrome of marmosets. Vet Pathol Suppl 19:141–162. [PubMed] [Google Scholar]
- 9.Chamove AS, Anderson JR. 1989. Examining environmental enrichment, p 183–202. In: Segal EF. Housing, care, and psychological wellbeing of captive and laboratory primates. Norwich (NY): William Andrew. [Google Scholar]
- 10.Chamove AS, Anderson JR, Morgan-Jones SC, Jones SP. 1982. Deep woodchip litter: Hygiene, feeding, and behavioral enhancement in 8 primates species. Int J Study Anim Probl 3:308–318. [Google Scholar]
- 11.Cordeiro de Sousa MBC, Moura SLN, Menezes AAL. 2006. Circadian variation with a diurnal bimodal profile on scent-marking behavior in captive common marmosets (Callitrhix jacchus). Int J Primatol 27:263–272. [Google Scholar]
- 12.David JM, Dick EJ, Jr, Hubbard GB. 2009. Spontaneous pathology of the common marmoset (Callithrix jacchus) and tamarins (Saguinus oedipus, Saguinus mystax). J Med Primatol 38:347–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Delimitreva S, Wedi E, Bakker J, Tkachenko OY, Nikolova V, Nayudu PL. 2013. Numerical chromosome disorders in the common marmoset (Callithrix jacchus)—comparison between 2 captive colonies. J Med Primatol 42:177–185. [DOI] [PubMed] [Google Scholar]
- 14.Doxiadis GG, van der Wiel MK, Brok HP, de Groot NG, Otting N, ’t Hart BA, van Rood JJ, Bontrop RE. 2006. Reactivation by exon shuffling of a conserved HLA-DR3-like pseudogene segment in a New World primate species. Proc Natl Acad Sci U S A 103:5864–5868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fuller G, Sadowski L, Cassella C, Lukas KE. 2010. Examining deep litter as environmental enrichment for a family group of Wolf guenons, Cercopithecus wolfi. Zoo Biol 29:626–632. [DOI] [PubMed] [Google Scholar]
- 16.Gibson SV. 1998. Bacterial and mycotic disease, p 59–110. In: Benett BT, Abee CR, Henrickson R. Nonhuman primates in biomedical research: diseases. San Diego (CA): Academic Press. [Google Scholar]
- 17.Gozalo A, Montoya E. 1992. Klebsiella pneumoniae infection in a New World nonhuman primate center. Lab Primate Newsletter 30:13–20. [Google Scholar]
- 18.Groenestein CM, Van Faassen HG. 1996. Volatilization of ammonia, nitrous oxide and nitric oxide in deep-litter systems for fattening pigs. J Agric Eng Res 65:269–274. [Google Scholar]
- 19.Isobe K, Adachi K, Hayashi S, Ito T, Miyoshi A, Kato A, Suzuki M. 2012. Spontaneous glomerular and tubulointerstitial lesions in common marmosets (Callithrix jacchus). Vet Pathol 49:839–845. [DOI] [PubMed] [Google Scholar]
- 20.Kaliste E, Linnainmaa M, Meklin T, Torvinen E, Nevalainen A. 2004. The bedding of laboratory animals as a source of airborne contaminants. Lab Anim 38:25–37. [DOI] [PubMed] [Google Scholar]
- 21.Kap YS, Smith P, Jagessar SA, Remarque E, Blezer E, Strijkers GJ, Laman JD, Hintzen RQ, Bauer J, Brok HP, ’t Hart BA. 2008. Fast progression of recombinant human myelin/oligodendrocyte glycoprotein (MOG)-induced experimental autoimmune encephalomyelitis in marmosets is associated with the activation of MOG34-56-specific cytotoxic T cells. J Immunol 180:1326–1337. [DOI] [PubMed] [Google Scholar]
- 22.Kitchen AM, Martin AA. 1996. The effects of cage size and complexity on the behaviour of captive common marmosets, Callithrix jacchus jacchus. Lab Anim 30:317–326. [DOI] [PubMed] [Google Scholar]
- 23.Logan AC, Khan KN. 1996. Clinical pathologic changes in 2 marmosets with wasting syndrome. Toxicol Pathol 24:707–709. [DOI] [PubMed] [Google Scholar]
- 24.Ludes-Fraulob E, Anderson JR. 1999. Behaviour and preferences among deep litters in captive capuchin monkeys (Cebus capucinus). Anim Welf 8:127–134. [Google Scholar]
- 25.Morrison RS, Hemsworth PH, Cronin GM, Campbell RG. 2003. The social and feeding behaviour of growing pigs in deep-litter, large group-housing systems. Appl Anim Behav Sci 82:173–188. [Google Scholar]
- 26.Morrison RS, Johnston LJ, Hilbrands AM. 2007. The behaviour, welfare, growth performance, and meat quality of pigs housed in a deep-litter, large group housing system compared to a conventional confinement system. Appl Anim Behav Sci 103:12–24. [Google Scholar]
- 27.Novak MA, Suomi SJ. 1988. Psychological wellbeing of primates in captivity. Am Psychol 43:765–773. [DOI] [PubMed] [Google Scholar]
- 28.O'Neill PL, Novak MA, Suomi SJ. 1991. Normalizing laboratory-reared rhesus macaques (Macaca mulatta) behavior with exposure to complex outdoor enclosures. Zoo Biol 10:237–245. [Google Scholar]
- 29.Philippens IH, Wubben JA, Finsen B, ’ t Hart BA. 2013. Oral treatment with the NADPH oxidase antagonist apocynin mitigates clinical and pathological features of parkinsonism in the MPTP marmoset model. J Neuroimmune Pharmacol 8:715–726. [DOI] [PubMed] [Google Scholar]
- 30.Poole TB. 1987. Behaviour, housing, and welfare of nonhuman primates, p 231–237. In: Beynen AC, Solleveld HA. New developments in biosciences: their implications for laboratory animal science. Dordrecht (The Netherlands): Martinus Nijhoff. [Google Scholar]
- 31.Power ML, Oftedal OT, Tardif SD, Allen ME. 1995. Vitamin D and primates: recurring problems on a familiar theme, p 188–194. First annual conference of the Nutrition Advisory Group (NAG) of the American Zoo and Aquarium Association (AZA). Toronto (Canada): American Zoo and Aquarium Association. [Google Scholar]
- 32.Reinhardt V, Roberts A. 1997. Effective feeding enrichment for nonhuman primates: a brief review. Anim Welf 6:265–272. [Google Scholar]
- 33.The Royal Society. [Internet] 2006. The Weatherall report on the use of nonhuman primates in research. [Cited day/mo/year]. Available at: https://royalsociety.org/policy/publications/2006/weatherall-report/
- 34.Turner SP, Ewen M, Rooke JA, Edwards SA. 2000. The effect of space allowance on performance, aggression, and immune competence of growing pigs housed on straw deep litter at different group sizes. Livest Prod Sci 66:47–55. [Google Scholar]
- 35.Ventura R, Buchanan-Smith HM. 2003. Physical environmental effects on infant care and development in captive Callithrix jacchus. Int J Primatol 24:399–413. [Google Scholar]