Abstract
Environmental enrichment provides laboratory-housed species the opportunity to express natural behavior and exert control over their home environment, thereby minimizing stress. We sought to determine whether providing an artificial plant in the holding tank as enrichment influenced anxiety-like behaviors and place-preference choice in adult zebrafish. Fish were housed singly or in social groups of 5 for 3 wk in 1 of 4 experimental housing environments: single-housed enriched (n = 30), single-housed barren (n = 30), group-housed enriched (n = 30), and group-housed barren (n = 30). On week 4, individual fish were selected randomly from each of the experimental housing environments and tested by using novel-tank, light–dark, and place-preference tests. Housing fish singly in a barren environment increased anxiety-like behaviors in the novel-tank and light–dark behavioral tests. Single-housed zebrafish in barren tanks as well as zebrafish group-housed with conspecifics, both with and without plant enrichment, spent more time associating with conspecifics than with the artificial plant enrichment device during the place-preference test. Single-housed fish maintained in enriched tanks displayed no preference between a compartment with conspecifics or an artificial plant. Our results suggest the addition of an artificial plant as enrichment may benefit single-housed zebrafish when social housing is not possible.
Environmental enrichment promotes the psychologic and physiologic health and wellbeing of laboratory animals and is provided routinely to laboratory-reared species.5,9,21 Increasing housing complexity allows laboratory-housed species to exert control over their immediate surroundings and encourages species-specific behaviors.5,9,18,28 Group housing in barren tanks is the current housing standard for laboratory zebrafish.5,11,14 However, maintaining fish in barren environments may promote stereotypies and increased aggression,6,9,14 and the UK Royal Society for the Prevention of Cruelty to Animals recommends providing zebrafish with environmental enrichment.21 Because the natural habitats of zebrafish include aquatic vegetation, providing an artificial plant has been suggested as enrichment, although its use has not been evaluated objectively.21
In a laboratory environment, zebrafish may be housed singly for genotyping or breeding needs.11 Because zebrafish are a shoaling species, isolation from conspecifics may increase stress. There is minimal literature on the effects of single compared with group housing on adult zebrafish.19 Given that zebrafish are typically reared in a group environment, isolating fish as they age may adversely affect behavior or physiology that may alter research results.19 To date, environmental enrichment has not been evaluated in zebrafish housed singly; previous studies have only evaluated the effect of environmental enrichment on group-housed fish.6,11,21 An evaluation of single compared with group housing, with and without enrichment, would be useful for the laboratory animal and scientific community, because it would help elucidate best practices for housing zebrafish.
Adult zebrafish have undergone various behavioral testing paradigms similar to those performed by using rodents.1-5,7,8,10,14-16,19,20-23,25,26,29,30 Behavioral tests offer a robust, noninvasive method for evaluating animal responses to various housing conditions. Behavioral tests for anxiety-like behavior, such as the novel tank and light–dark tests, allows experimenters to evaluate how well zebrafish adapt to various stressors.2-4,7,8,10,15,16,19,22,23,24,29,30 The novel-tank test utilizes the zebrafish's natural geotaxic instinct to dive to the bottom of the tank and explore its environment.4,7,13,26 The light–dark test assesses anxiety-like behavior by evaluating the amount of time spent in either a white or black compartment;2,3,16,23,30 zebrafish are scototaxic and therefore exhibit a natural preference for the dark compartment.2,16,23,30 The-place preference test provides fish a choice between 2 different environments.1,14 The preferred choice may provide information regarding the preferred condition for housing laboratory zebrafish.
Our study sought to determine whether adding an artificial plant as enrichment influenced anxiety-like behaviors and whether adult zebrafish preferred enrichment over conspecifics. We hypothesized that providing an artificial plant would reduce anxiety-like behavior in adult zebrafish, and this effect would have a greater effect on single-housed than group-housed fish because it could serve as a hiding place and therefore provide a measure of security in an otherwise barren tank.
Materials and Methods
Animals and housing.
Animals were housed in an AAALAC -accredited facility in compliance with the Guide for the Care and Use of Laboratory Animals.12 All research procedures were approved by The Rockefeller University's IACUC. Ten breeding pairs of adult AB fish (n = 20; age unknown) were obtained from Carolina Biologicals (Burlington, NC) and maintained in mixed-sex groups of 10 fish in 2.5-L tanks on a recirculating quarantine system (Marine Biotech, Apopka, FL). Four weeks after receipt, pairwise breeding was performed and fertilized embryos collected. Embryos were bleached (50 ppm bleach, 10 min) within 3 h after fertilization, hatched, and maintained in culture dishes; at 7 d after fertilization, they were transferred to the main recirculating system (Marine Biotech). The system was supplied with carbon-filtered municipal tap water filtered through a 20-μm pleated particulate filter (Pentair Aquatic Eco-Systems, Apopka, FL) and exposed to a 40-W UV light. The system water was maintained at 28 °C at a conductivity of 510 to 600 μS, pH 7.3 to 7.7, hardness of 80 ppm, alkalinity of 80 ppm, dissolved oxygen greater than 6 mg/L, ammonia 0 ppm, nitrate 0 to 0.5 ppm, and nitrite 0 ppm in a room with a 14:10-h light:dark cycle. Fish were raised at a density of 10 to 25 per 2.5-L tank. Fish were fed a commercial diet (Larval AP100, 150 to 250 μm, Zeigler Bros, Gardners, PA) supplemented with spirulina (Spirulina Powder, Brine Shrimp Direct, Ogden, UT) 3 times daily.
At 5 mo of age (4 wk prior to study initiation), 120 mixed-sex zebrafish were randomly selected for experimental use and housed at a density of 10 per 2.5-L tank. Fish were fed a commercial pelleted diet (Zeigler Adult Zebrafish Complete Diet, Zeigler Bros, Gardners, PA and an artificial Artemia nauplii replacement diet (Golden Pearls Reef and Larval Fish Diet 300 to 500 μm, Brine Shrimp Direct) twice daily and with brine shrimp (Premium Grade Brine Shrimp Eggs, Brine Shrimp Direct) once daily.
Fish were free of Pseudoloma neurophilia, Pseudocapillaria tomentosa, and pathogenic Mycobacterium spp. as determined by twice-yearly gross and histopathologic evaluation of 10 colony fish per rack.
Experimental housing.
Fish were randomly assigned to 1 of 4 experimental housing environments for 3 wk: single-housed enriched (n = 30), single-housed barren (n = 30), group-housed enriched (n = 30), and group-housed barren (n = 30). Group-housed fish were mixed-sex groups of 5 fish per 2.5-L tank. Single-housed male fish were housed individually in 2.5-L tanks; female fish were not housed individually due to concerns regarding the potential for egg binding. All experimental groups were housed on a single rack of the main recirculating system on 2 adjacent rows. Each row held 14 tanks. Each enriched experimental tank contained a 17-cm, red and green, artificial plant (Petco Green and Red Hairgrass Foreground Plastic Aquarium Plant, Petco Animal Supplies, San Diego, CA). Barren tanks were devoid of environmental enrichment.
Behavioral testing.
Fish were randomly selected from each experimental housing environment for behavioral testing. Experimental housing tanks were transported once to the behavioral testing room. Group-housed fish from the same tank were all tested in the same recording session. Individual fish were removed from the tank for testing. Immediately after behavioral testing, fish were placed into a fresh home tank to prevent selecting the same fish twice. No fish was tested in more than one behavioral test. Fish were acclimated to the behavioral testing room for 1 h while in their experimental housing tanks. Behavioral testing occurred between 1000 and 1300 h. Test tanks were filled with water acquired from the main recirculating system; the water was changed after every 5th behavioral trial and maintained at 24 to 26 °C in a testing room with ambient light at 300 lx. All behavioral trials were videotaped (Handycam DCR-SX60, Sony, Tokyo, Japan) at a distance of 50 cm from the test tank. Testing was performed by the same person, who was seated 1 m from the test apparatus during all behavioral trials. Videos were assigned random numbers to disguise trial groups. Videotaped behaviors were hand-scored by a single blinded reviewer.
Novel-tank test.
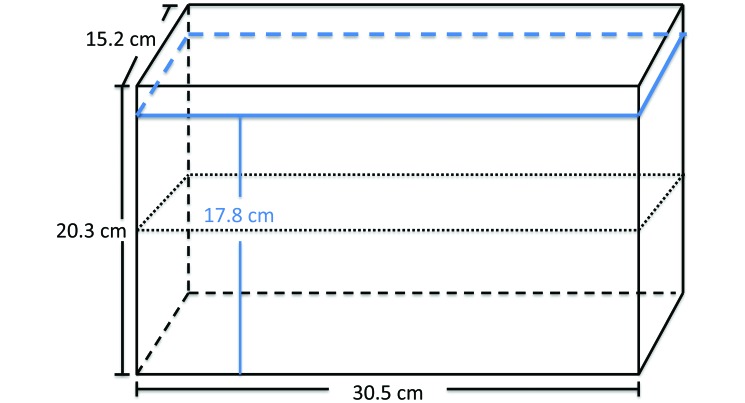
Fish (n = 10 randomly selected from each experimental housing condition) were placed into a 30.5 cm × 15.2 cm × 20.3 cm 9.5-L clear glass aquarium (Aqueon, Franklin, WI) filled to within 2.5 cm of the top (Figure 1). The tank was divided in half horizontally by using a straight black line drawn on the tank exterior. Behavioral parameters measured included the number of transitions to the upper half of the tank, total time spent in the upper half of the tank, number and duration of freezing bouts, and number of erratic movements.2,4,14 Freezing bouts were defined as periods of no body movement for at least 1 s on the bottom of the tank. Erratic movements were defined as sharp, rapid changes of direction or darting. Behaviors were scored over 6 min.
Figure 1.
Diagram of the novel-tank test tank (30.5 cm × 15.2 cm × 20.3 cm) 9.5-L clear glass aquarium, which was filled within 2.5 cm of the top. Water level is indicated in blue. The tank was divided in half horizontally by using a straight black line drawn on the tank exterior.
Light–dark test.
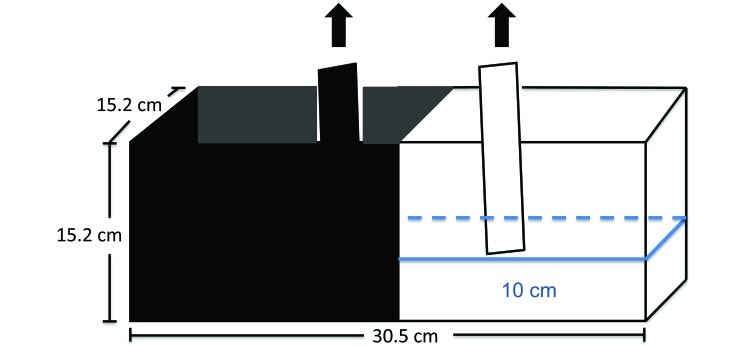
Fish (n = 10 randomly selected from each experimental housing condition) were placed into a 30.5 cm × 15.2 cm × 15.2 cm high, 3-compartment, 5.7-L clear, glass aquarium (Model 3 Betta Black Trim, Deep Blue Professional, Brentwood NY) filled to a height of 10 cm (Figure 2). The tank was divided in half vertically. One exterior half was painted with nontoxic white paint and the other half with nontoxic black paint (Glass Marker, Rust-Oleum, Vernon Hills, IL). On one longitudinal side, a 10-cm window at the transition point of the light and dark compartments was left clear and divided into 2 with a vertical line, to allow video recording. The tank was split internally into 3 compartments through the use of 2 clear, glass separators. Fish were placed in the center compartment for an acclimation period of 3 min. At test start, the 2 clear glass separators were removed allowing fish to freely swim into the white and black compartments. Behavioral parameters measured included the number of transitions to the white compartment, total time spent in the white compartment, and number of shuttling events.4,16,18 Shuttling events were defined as short, less than 1 s crossings into the white compartment.13 Behaviors were scored over 15 min.
Figure 2.
Diagram of the light–dark test tank (30.5 cm × 15.2 cm × 15.2 cm high) 5.7-L clear, glass aquarium filled to a height of 10 cm. Water level is indicated in blue. The tank was divided in half vertically. One exterior half was painted with nontoxic white paint and the other half with nontoxic black paint. The tank was split internally into 3 compartments by using 2 clear, glass separators. The central compartment was used for acclimation.
Place-preference test.
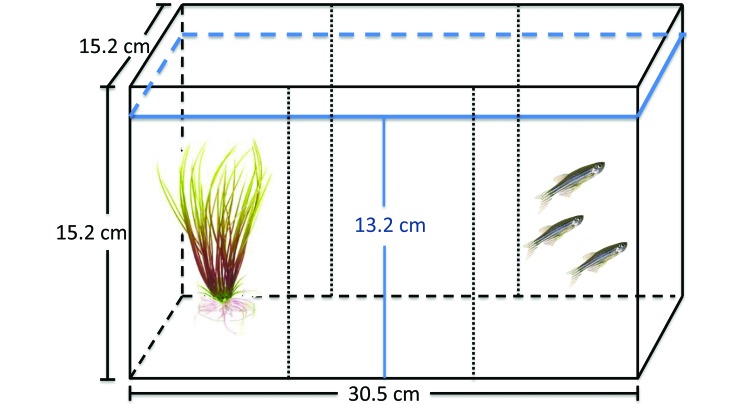
Individual fish (n = 10 randomly selected from each experimental housing condition) were placed in the center compartment of a 30.5 cm × 15.2 cm × 15.2 cm, 3-compartment, 5.7-L clear, glass aquarium (Model 3 Betta Black Trim, Deep Blue Professional) divided internally into 3 vertical compartments (2 outer compartments and one central compartment) by using 2 black lines drawn on the tank exterior (Figure 3). One of the outer compartments contained a 17-cm artificial plant identical to experimental housing enrichment. The opposite outer compartment included a clear plastic bag with multiple pinpoint holes containing 3 mixed-sex, age-matched conspecifics. The same 3 conspecifics were used for all place-preference tests. Test fish were placed into the central compartment and had unrestricted access to both compartments. Behavioral parameters measured included the number of transitions to the compartment containing the artificial plant, number of transitions to the compartment containing the conspecifics, time spent in each compartment, number of erratic movements, and number of freezing bouts and their duration.1,11 Behaviors were scored over 15 min.
Figure 3.
Diagram of the place-preference test tank (30.5 cm × 15.2 cm × 15.2 cm) divided internally into 3 vertical compartments using 2 black lines drawn on the tank exterior. Water level is indicated in blue. One compartment contained a 17-cm artificial plant. The other compartment contained 3 mixed-sex, age-matched conspecifics.
Statistical analysis.
Analysis using the Shapiro–Wilks normality test revealed that data were not normally distributed. Because the data could not be normalized by log transformation, nonparametric tests were used for data analysis. Parameters measured in novel-tank, light–dark, and place-preference tests were analyzed by using the Kruskal–Wallis test followed by a Bonferroni test to determine significant differences between specific groups. Significant differences within groups in the place preference test were determined using the Wilcoxon Signed-Ranks test. All tests were performed by using statistical analysis software (STATA, StataCorp, College Station, TX). A P value less than or equal to 0.05 was used to define statistical significance.
Results
Novel-tank test.
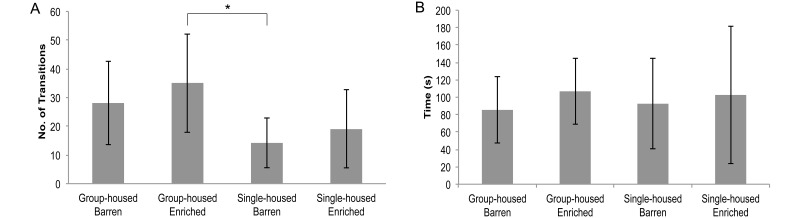
Single-housed barren fish had significantly fewer transitions to the upper half of the tank than did group-housed enriched fish (14 compared with 35; P < 0.05). Single-housed enriched fish trended to have fewer transitions to the upper half of the tank when compared with group-housed enriched fish (35 compared with 19; P = 0.08). The total time spent in the upper half of the tank did not differ between groups (Figure 4). No significant differences between groups were found for latency to enter the upper half of the tank, the number of erratic movements, or the number and duration of freezing bouts.
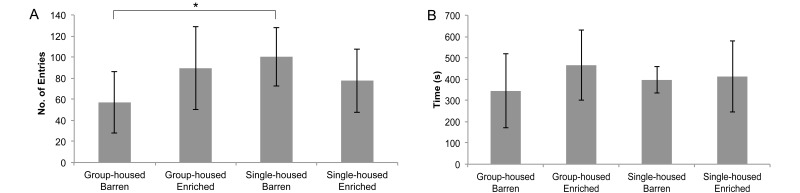
Figure 4.
Novel-tank test, 6-min trial (n = 10 per group; mean ± 1 SD). (A) The number of transitions to the upper half of the tank. (B) Time spent in the upper half of the tank (s). *, Significant differences determined by using the Kruskal–Wallis test (P < 0.05) followed by a Bonferroni test (P < 0.05).
Light–dark test.
Single-housed barren fish had significantly more entries to the white compartment than did group-housed barren fish (100 compared with 57; P < 0.05). The total time spent in the white compartment did not differ between groups (Figure 5). No differences between groups were identified for latency to enter the white compartment and the number of shuttling events.
Figure 5.
Light–dark test, 6-min trial (n = 10 per group; mean ± 1 SD). (A) Number of transitions to the white compartment. (B) Time spent in the white compartment. *, Significant differences determined by using the Kruskal–Wallis test (P < 0.05) followed by a Bonferroni test (P < 0.05).
Place-preference test.
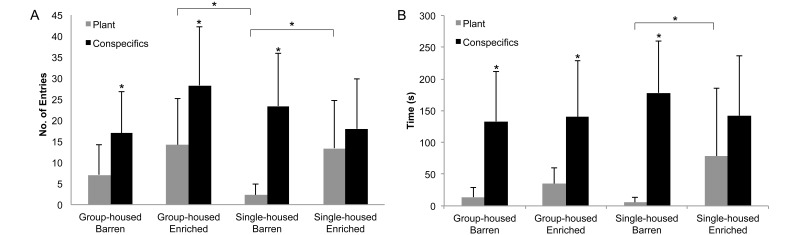
Group-housed barren, group-housed enriched, and single-housed barren fish had significantly more entries into the conspecifics compartment than the artificial plant compartment (17 compared with 7; 28 compared with 14; 23 compared with 2, respectively; P < 0.05). Single-housed barren fish had significantly fewer entries into the plant compartment than did group-housed enriched and single-housed enriched fish (2 compared with 14, P < 0.05; 2 compared with 13, P < 0.05, respectively). Group-housed barren, group-housed enriched, and single-housed barren fish spent more time in the conspecifics compartment than the plant compartment (133 compared with 14, 141 compared with 35, 178 compared with 6, respectively; P < 0.05). Single-housed barren fish spent significantly less time in the plant compartment than did single-housed enriched fish (6 compared with 78; P < 0.05). The time that single-housed enriched fish spent in the artificial plant compartment was similar to that spent in the compartment containing conspecifics (Figure 6). Neither the number of erratic movements nor the number and duration of freezing bouts differed between groups.
Figure 6.
Place-preference test, 15-min trial (n = 10 per group; mean ± 1 SD). (A) Numbers of transitions to the plant- and fish-containing compartments. (B) Time spent in compartments containing an artificial plant or 3 conspecifics. *, Significant differences determined by using the Kruskal–Wallis test (P < 0.05) followed by a Bonferroni test (P < 0.05).
Discussion
In the current study evaluating zebrafish, we used 3 different behavioral tests, because previous work revealed that different tests evoke different behaviors and may stimulate different neurologic pathways.4,16 In addition, previous research has shown that no single behavioral testing paradigm for anxiety-like behavior adequately captures the necessary information to interpret the results.4,16 We used the novel-tank and light–dark tests to evaluate anxiety-like behaviors because one benefit of enrichment may be reduced anxiety.4,8,16 We used the place preference behavioral test to evaluate the type of enrichment—artificial plant or conspecifics—that the zebrafish prefer.
When first exposed to a novel environment, zebrafish have a tendency to linger at the sides and bottom of the tank during the novel-tank test. This behavior has been compared to thigmotaxis in rodents.8,15 Typical vertical exploratory behaviors in zebrafish are gradual and tend to increase over time.2,7 In the novel tank-test, a prolonged latency to enter the upper half of the tank, reduced time spent in the upper half of the tank, and increased frequency of erratic movements and freezing bouts have been associated with greater anxiety-like behaviors.2,7,26 A previous study found that group-housing compared with single-housing affected zebrafish response to the novel-tank test.19 Specifically, group-housed fish demonstrated higher levels of anxiety-like behavior than did single-housed fish in this test, as evidenced by more time spent on the bottom of the novel tank.19 In addition, single-housed and group-housed fish reacted differently to exposure to an anxiolytic and to a water change prior to testing; the authors suggested that fish housed singly or in groups may develop different coping methods depending on their housing condition.19 Our results demonstrate that, compared with fish that were group-housed or provided enrichment or both, single-housed fish maintained in a barren environment exhibited significantly fewer exploratory behaviors when tested by using the novel-tank test as evidenced by fewer transitions to the upper of half of the tank. This finding indicates that single-housing affects the fish, consequently perhaps influencing behaviors displayed in this testing paradigm. However, the total time spent in the upper half of the tank did not differ among experimental groups. As did previous studies, we found that the number of erratic movements, number and duration of freezing bouts, and number of shuttling events did not differ between any of the experimental groups.2,9 The results of the novel-tank test suggest that housing fish singly in a barren environment influences exploratory behavior, as indicated by fewer exploratory transitions to the upper compartment in the novel-tank test, and therefore these fish may exhibit mild anxiety-like behavior.
Adult zebrafish are known to be scototaxic.17 Therefore an increased number of entries into the white compartment suggests greater exploratory behavior, whereas greater time spent in a dark compartment indicates an increased tendency toward anxiety-like behaviors.2,23 Ambient light levels can influence the outcome of the light–dark test, with fish showing higher levels of white avoidance in brightly lit environments (450 lx) compared with low lighting (250 lx).3 In our study, the lighting levels were standardized at 300 lx across all behavioral groups. Like the novel-tank test, the light–dark test revealed altered behavior in single-housed barren fish. Whereas single-housed barren fish entered the white compartment almost twice as often as did the group-housed barren fish (100 entries versus 57 entries), total time spent in the white compartment was equal across groups. These results indicate that the exploratory behavior of the single-housed barren fish was altered in that single-housed fish remained in the white compartment for only brief exploratory bouts. This pattern was evidenced by the darting behavior that the fish displayed as they exited the white compartment;13 this escape behavior has been described as an expression of anxiety-like behavior.10 Again, we observed an overall disassociation between this test and the novel-tank test, given that latency to explore the white compartment did not differ between any of the groups. Explanations for this lack of difference are unclear. Group-housed fish had fewer entries into the white compartment but tended to explore that compartment for longer time intervals than did single-housed fish in each entry. Fish singly housed in a barren environment, therefore, appear to demonstrate mild alterations in anxiety-like behavior as indicated by abbreviated exploratory sessions and darting behavior in the light–dark test.
In a previous place-preference experiment, adult group-housed zebrafish were offered a choice between complex and barren environments.14 The results suggested that zebrafish prefer a complex environment to a barren environment, although study design did not evaluate the behavioral influence of group housing with conspecifics or the behavioral effects of housing fish singly.14 Our experimental design sought to further examine the behavior of zebrafish in a place-preference choice experiment as we independently isolated the behavioral effects of environmental complexity and social housing. Our results demonstrate that all fish, except for those housed singly with an enrichment device, preferred to associate with conspecifics. Fish housed singly in the enriched environment spent as much time with the artificial plant as with conspecifics, indicating no preference between the 2 choices. This behavior sharply contrasts the place-preference choice behaviors observed when fish were single-housed in a barren tank environment. Single-housed barren fish entered the artificial plant compartment only twice on average over the 15-min observation period. In contrast, comparably single-housed enriched and group-housed enriched fish entered the artificial plant compartment an average of 14 and 13 times, respectively, over the same observation period. In addition, single-housed barren fish spent far less time in the plant compartment (average of 6 s) than did single-housed enriched fish (average of 78 s). These results support the findings noted in both the novel-tank and light–dark tests, suggesting that housing fish singly with no enrichment may alter anxiety-like behaviors. In the place preference test this is evidenced as decreased or altered exploratory behaviors when presented with novel environments. Because the single-housed barren fish were housed in a barren tank, this group perceives the addition of an artificial plant as a novel event. Perhaps the enriched groups spent more time in the plant compartment because they were habituated to the enrichment device, as compared with the barren groups. All experimental fish had previous exposure to conspecifics prior to the initiation of our study, therefore the presence of conspecifics would not have been perceived as novel by the single-housed fish. Although novelty and habituation may have influenced behavior of the fish in regard to response to enrichment, we expect all fish to express similar behaviors toward conspecifics. Given that single-housed fish spent approximately equal amounts of time in each of the 2 compartments, whereas all other study groups spent significantly more time associating with conspecifics, enrichment apparently influences the behavioral preferences of zebrafish.
Zebrafish have a brisk habituation response to testing paradigms, particularly when these tests are held in similar conditions to their home environment.8,29 Fish singly housed in the enriched environment may not have demonstrated a preference during the place-preference test because they had previously been exposed to the artificial plant used as an enrichment device for a habituation period of 3 wk.8,29 Because we placed the same device in the home tanks that we used in the place-preference test, habituation may have influenced the results of this test. In future work, the effects of habituation or novelty might be evaluated by exposing fish to a novel enrichment device. The results of our place-preference behavioral test contrast with a previous study, which found that group-housed female zebrafish in large aquaria spent 99% of their time in areas with artificial plants compared with open spaces.6 That study found that female zebrafish preferred to remain near the artificial plants rather than associate with conspecifics. The behavioral differences noted in that study may have been related to the sex of the fish or to a testing condition that might have led to environmental habituation.8,22,29,30 Because those fish were tested in their home tank, they may have habituated to their housing environment; this habituation might have affected their test behavior also. This difference may explain the difference in results between the 2 studies. The fish in the previously cited study were continuously group housed during the behavioral assessment, whereas fish in our study were housed according to the experimental treatment and were tested individually.9
Our research focused on behavioral testing and did not attempt to correlate behavioral measurements with physiologic data, evaluate sex-associated differences related to behavioral responses, strain-specific differences, or measures of increased aggression.8,20,22,30 Additional studies attempting to examine the effects of environmental variables and social housing on plasma cortisol levels in zebrafish have yielded conflicting results;19,27 some of these findings contradict those predicted under the assumption that variables such as enrichment or group housing reduce physiologic markers of stress. One group found that fish group-housed in barren environments had lower whole-body cortisol than did those housed in enriched environments.27 Other researchers found that group-housed fish had higher levels of whole-body cortisol compared with those in individually housed fish19 Future work should attempt to compare whole-body cortisol between various housing conditions. Marked sex- and strain-specific differences in zebrafish behavior have been reported.7,10
Sex-associated differences may have influenced our behavioral results. Because the groups in our study were intended to replicate the most common groupings of zebrafish in a research environment; experimental groups typically contained both sexes. We chose not to single-house female zebrafish due to the possibility of egg-binding leading to morbidity within the study period; this would have been a confounding variable for our study. Future work should include the analysis of sex-associated differences to determine the influence of sex on zebrafish behavior. In addition, zebrafish have been reported to display aggression toward each other by biting and chasing.8,9 A previous study recommended using plants as structures in the environment because they provide protection from aggressive fish.14 Intragroup aggression may explain the increased levels of whole-body cortisol in group-housed fish; however we expect that the addition of enrichment would lessen this effect. Evaluating the effect of enrichment on aggression in standardized housing conditions with or without the addition of enrichment might help to determine whether enrichment may have other benefits for zebrafish.
In conclusion, our study demonstrates that single-housing adult zebrafish in barren environments alters exploratory behaviors. Single-housed fish appear to benefit from the addition of an artificial plant as an enrichment device. We conclude that using artificial plants as enrichment devices may promote the wellbeing of zebrafish that are singly housed. According to our findings, adding an artificial plant does not enhance wellbeing when zebrafish are group-housed. Additional studies are needed to elucidate the long-term effect of social housing variables on zebrafish in the presence or absence of artificial enrichment devices.
Acknowledgments
This work was funded by a grant from the Johns Hopkins Center for Alternatives to Animal Testing. Portions of this work were presented at the National AALAS meeting in Baltimore. In addition, we thank Janelle Monnas, Nathan McKenney, and Adedeji Afolalu for their help in caring for the fish used in this study. We also thank Drs Gillian Braden, Yamina Berchiche, Michal Zimmermann, and Neil Lipman for their critical review of this manuscript.
References
- 1.Avdesh A, Martin-Iverson MT, Mondal A, Chen M, Verdile G, Martins RN. 2010. Natural colour preference in the zebrafish (Danio rerio), p 155–157. In: Spink AJ, Grieco F, Krips OE, Loijens LWS, Noldus LPJJ, Zimmerman PH. Proceedings of Measuring Behavior 2010. Wageningen (The Netherlands): Noldus. [Google Scholar]
- 2.Blaser RE, Chadwick L, McGinnis GC. 2010. Behavioral measures of anxiety in zebrafish (Danio rerio). Behav Brain Res 208:56–62. [DOI] [PubMed] [Google Scholar]
- 3.Blaser RE, Peñalosa YM. 2011. Stimuli affecting zebrafish (Danio rerio) behavior in the light–dark preference test. Physiol Behav 104:831–837. [DOI] [PubMed] [Google Scholar]
- 4.Blaser RE, Rosemberg DB. 2012. Measures of anxiety in zebrafish (Danio rerio): dissociation of black–white preference and novel tank test. PLoS ONE 7:e36931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brydges NM, Braithwaite VA. 2009. Does environmental enrichment affect the behaviour of fish commonly used in laboratory work? Appl Anim Behav Sci 118:137–143. [Google Scholar]
- 6.Delaney M, Follet C, Ryan N, Hanney N, Lusk-Yablick J, Gerlach G. 2002. Social interaction and distribution of female zebrafish (Danio rerio) in a large aquarium. Biol Bull 203:240–241. [DOI] [PubMed] [Google Scholar]
- 7.Egan RJ, Bergner Cl, Hart PC, Cachat JM, Canavello PR, Elegante MF, Elkhayat SI, Bartels BK, Tien AK, Tien DH, Mohniot S, Beeson E, Glasgow E, Amri H, Zukowska Z, Kalueff AV. 2009. Understanding behavioral and physiological phenotypes of stress and anxiety in zebrafish. Behav Brain Res 205:38–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gerlai R, Lahav M, Guo S, Rosenthal A. 2000. Drinks like a fish: zebra fish (Danio rerio) as a behavior genetic model to study alcohol effects. Pharmacol Biochem Behav 67:773–782. [DOI] [PubMed] [Google Scholar]
- 9.Griffin G. [Internet] 2012. Evaluating environmental enrichment is essential. Enrich Rec 12:29–33. [Cited 23 May 2014] Available at: http://enrichmentrecord.com/wp-content/uploads/2012/07/er12-0712.pdf [Google Scholar]
- 10.Grossman L, Utterback E, Stewart A, Gaikwad S, Chung KM, Suciu C, Wong K, Elegante M, Elkhayat S, Tan J, Gilder T, Wu N, Dileo J, Cachat J, Kalueff AV. 2010. Characterization of behavioral and endocrine effects of LSD on zebrafish. Behav Brain Res 214:277–284. [DOI] [PubMed] [Google Scholar]
- 11.Harper C, Lawrence C. 2011. The laboratory zebrafish. Boca Raton (FL): Taylor and Francis. [Google Scholar]
- 12.Institute for Laboratory Animal Research. 2011. Guide for the care and use of laboratory animals, 8th ed. Washington (DC): National Academies Press. [Google Scholar]
- 13.Kalueff AV, Gebhardt M, Stewart AM, Cachat JM, Brimmer M, Chawla JS, Craddock C, Kyzar EJ, Roth A, Landsman S, Gaikwad S, Robinson K, Baatrup E, Tierney K, Shamchuk A, Norton W, Miller N, Nicolson T, Braubach O, Gilman CP, Pittman J, Rosemberg DB, Gerlai R, Echevaria D, Lamb E, Neuhauss SC, Weng W, Bally-Cuif L, Schneider H, Zebrafish Neuroscience Research Consortium. 2013. Towards a comprehensive catalog of zebrafish behavior 1.0 and beyond. Zebrafish 10:70–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kistler C, Hegglin D, Würbel H, König B. 2011. Preference for structured environment in zebrafish (Danio rerio) and checker barbs (Puntius oligolepis). Appl Anim Behav Sci 135:318–327. [Google Scholar]
- 15.Levin ED, Bencan Z, Cerutti DT. 2007. Anxiolytic effects of nicotine in zebrafish. Physiol Behav 90:54–58. [DOI] [PubMed] [Google Scholar]
- 16.Maximino C, Benzery R, Oliveira KRM, Batista EDJO, Herculano AM, Rosmeberg DB, de Oliveria DL, Blaser R. 2012. A comparison of the light–dark and novel tank tests in zebrafish. Behaviour 149:1099–1123. [Google Scholar]
- 17.Maximino C, Marcues de Brito T, Dias CA, Gouveia A, Jr, Morato S. 2010. Scototaxis as anxiety-like behavior in fish. Nat Protoc 5:209–216. [DOI] [PubMed] [Google Scholar]
- 18.Newberry RC. 1995. Environmental enrichment: increasing the biological relevance of captive environments. Appl Anim Behav Sci 44:229–243. [Google Scholar]
- 19.Parker MO, Millington ME, Combe FJ, Brennan CH. 2012. Housing conditions differentially affect physiological and behavioural stress responses of zebrafish, as well as the response to anxiolytics. PLoS ONE 7:e34992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Raymond J, Chanin S, Stewart AM, Kyzar E, Gaikwad S, Roth A, Bruce I, Fryar C, Varga D, Enriquez J, Bagawandoss S, Pham M, Zapolsky I, Green J, Desmond D, Hester J, Kalueff AV. 2012. Assessing habituation phenotypes in adult zebrafish: intra- and intertrial habituation in the novel tank test, p 273–285. In: Kalueff AV, Stewart AM. Zebrafish protocols for neurobehavioral research. Neuromethods, vol 66. New York (NY): Humana Press. [Google Scholar]
- 21.Reed B, Jennings M. 2010. Guidance on the housing and care of zebrafish Danio rerio. West Sussex (UK): Research Animals Department, Science Group, RSPCA. [Google Scholar]
- 22.Sackerman J, Donegan JJ, Cunningham CS, Nguyen NN, Lawless K, Long A, Benno RH, Gould GG. 2010. Zebrafish behavior in novel environments: effects of acute exposure to anxiolytic compounds and choice of Danio rerio line. Int J Comp Psychol 23:43–61. [PMC free article] [PubMed] [Google Scholar]
- 23.Serra EL, Medalha CC, Mattioli R. 1999. Natural preference of zebrafish (Danio rerio) for a dark environment. Braz J Med Biol Res 32:1551–1553. [DOI] [PubMed] [Google Scholar]
- 24.Simon P, Dupuis J, Costentin J. 1994. Thigmotaxis as an index of anxiety in mice. Influence of dopaminergic transmission. Behav Brain Res 61:59–64. [DOI] [PubMed] [Google Scholar]
- 25.Stewart A, Maximino C, Marques de Brito T, Herculano AM, Gouveia A, Jr, Morato S, Cachat J, Gaikwad S, Elegante M, Hart P, Kalueff AV. 2011Neurophenotyping of adult zebrafish using the light–dark box paradigm, p 157–167. In: Kalueff AV, Cachat JM. Zebrafish neurobehavioral protocols. Neuromethods, vol 51. New York (NY): Humana Press. [Google Scholar]
- 26.Stewart A, Gaikwad S, Kyzar E, Green J, Roth A, Kalueff AV. 2012. Modeling anxiety using adult zebrafish: a conceptual review. Neuropharmacology 62:135–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.von Krogh K, Sørensen C, Nilsson GE, Øverli Ø. 2010. Forebrain cell proliferation, behavior, and physiology of zebrafish, Danio rerio, kept in enriched or barren environments. Physiol Behav 101:32–39. [DOI] [PubMed] [Google Scholar]
- 28.Williams TD, Readman GD, Owen SF. 2009. Key issues concerning environmental enrichment for laboratory-held fish species. Lab Anim 43:107–120. [DOI] [PubMed] [Google Scholar]
- 29.Wong K, Elegante M, Bartels B, Elkhayat S, Tien D, Roy S, Goodspeed J, Suciu C, Tan J, Grimes C, Chung A, Rosenberg M, Gaikwad S, Denmark A, Jackson A, Kadri F, Chung KM, Stewart A, Gilder T, Beeson E, Zapolsky I, Wu N, Cachat J, Kalueff AV. 2010. Analyzing habituation responses to novelty in zebrafish (Danio rerio). Behav Brain Res 208:450–457. [DOI] [PubMed] [Google Scholar]
- 30.Wong RY, Perrin F, Oxendine SE, Kezios ZD, Sawyer S, Zhou L, Dereje S, Godwin J. 2012. Comparing behavioral responses across multiple assays of stress and anxiety in zebrafish (Danio rerio). Behaviour 149:1205–1240. [Google Scholar]